Note: Descriptions are shown in the official language in which they were submitted.
CA 022233=,6 1997-12-03
SPECIFICATION
A CONTINUOUS CASTING NOZZLE FOR CASTING MOLTEN STEEL
FIELD OF THE INVENTION
The present invention relates to a continuous casting nozzle for permitting
effective prevention of narrowing, clogging of the nozzle bore or metal sticking to the
nozzle bore through which molten steel passes in performing continuous casting of the
molten steel cont~ining ~ minllm such as aluminum-killed steel.
THE RELATED ART
A continuous casting nozzle for casting molten steel is used for the following
purposes.
As for continuous casting molten steel, a continuous casting nozzle is used for
such purpose of preventing the molten steel from being oxidized by contacting with the
open air and from splashing when the molten steel is poured from a tundish to a mold,
and rectifying the flow of the molten steel poured for preventing non-metallic inclusion
and slag present near or on the mold surface from being entrapped in the cast steel strand.
Material of a conventional continuous casting nozzle of molten steel comprises
such material as graphite, alumina, silica, silicon carbide and recently zirconia.
However, there are following problems in the case of casting alllmim~m-killed steel and
the like.
As for the alumin-lm-killed steel and the like, alllminllm, which is added as a de-
oxidizer, reacts with oxygen existing in the molten steel to produce non-metallic
inclusion such as ~-alumina. Therefore, in casting the alllminllm-killed steel and the
like, the non-metallic inclusion such as~-alumina adheres and accum~ tes onto the
surface of the bore of the continuous casting nozzle, so that the bore is narrowed or
clogged up in the worst case, which makes stable casting to be difficult. Furthermore,
the non-metallic inclusion such as c~-alumina adhered or accumulated onto the surface
of the bore peels off or falls down, and is entrapped in the cast steel strand, thus
- 1 -
CA 022233~6 1997-12-03
degrading the quality of the cast steel strand.
For the purpose of preventing the above-mentioned reduction or clogging of the
bore caused by the non-metallic inclusion such as ~-alumina, there is a commonlyused method for preventing the non-metallic inclusion such as o~-alumina existing in the
molten steel from adhering or accumulating on the surface of the bore of the nozzle by
ejecting inert gas from the inner surface of the nozzle bore toward the molten steel
flowing through the bore (for example, Japanese Patent Publication No. Hei 6-
59533/1994)
However, there are problems as described below for the above-mentioned
method wherein inert gas is ejected from the inner surface of the nozzle.
A large amount of the ejected inert gas causes enll ~plllent of bubbles producedby the inert gas into the cast steel strand, resulting in defects based on pinholes. On the
other hand, a small amount of the ejected inert gas causes adhesion and accllm~ tion of
the non-metallic inclusion such as o~ -alumina onto the surface of the bore of the nozzle,
thus causing narrowing or clogging, in the worst case, of the bore.
Additionally, it is constructionally difficult to uniformly eject the inert gas from
the inner surface of the nozzle bore toward the molten steel flowing through the bore.
And in the case that the casting is performed in a long period of time, a stable control of
the amount of ejected inert gas becomes gradually more difficult as the composition and
the structure of the material consisting of the continuous casting nozzle degrades. And
moreover, it becomes difficult to eject inert gas uniformly from the inner surface to the
nozzle bore. As a result, the non-metallic inclusion such as~-alumina adhere andaccumulate onto the surface of the bore of the nozzle so that the bore is narrowed or
clogged up in the end.
It is thought that the clogging of the nozzle by the non-metallic inclusion,
specially by the alumina inclusion is caused as described below.
(I) Alumina inclusion is produced from alllminllm in the steel by secondary oxidation,
such as oxidation by entrapped air passing through a refractory junction and refractory
structure or oxidation by supplying oxygen obtained from reduction of silica in a
graphite- or carbon-cont~ining refractory.
CA 022233=,6 1997-12-03
(2) Alumina inclusion is produced by diffusion and cohesion of the alumina produced
in the above process.
(3) Graphite on the surface of the nozzle bore vanishes and the surface of the bore
becomes rough and thus the alumina inclusion is apt to accumulate on the rough surface
of the bore.
On the other hand, as a counterplan in view of nozzle material, a nozzle in
which a non-oxide raw material (SiC, Si3N4, BN, ZrB2, SIALON etc.) that has low
reactivity with alllminllm oxide is added to alumina-graphite or a nozzle consisting of the
non-oxide material itself is proposed (for example, Japanese Patent Publication No. Sho
61-38158/1986).
However, this counterplan is not practical in the case of the alumina-graphite
nozzle because the adhesion preventing effect is not recognized and further corrosion
resistance is decreased unless much of the non-oxide material is added.
Also, the nozzle consists of only the non-oxide material is not suitable for
practical use in view of material cost and manufacturing cost, although a substantial
effect is expected.
A nozzle consisting of graphite-oxide raw material cont~ining CaO is proposed
for producing low-melting-point material by a reaction of CaO in an oxide raw material
cont~ining CaO (CaO ZrO2, CaO SiO2, 2CaO SiO2 etc.) with Al2O3 and forming the
low-melting-point material in the steel (for example, Japanese Patent Laid-Open
Publication No. Sho 62-56101/1987).
However, reactivity of CaO with Al2O3 is apt to be influenced by a temperature
condition of the molten steel in casting, and there is a case that amount of CaO is not
sufficiently secured for satisfying spalling resistance and corrosion resistance when plenty
of Al2O3 inclusion is contained in the steel.
SUMMARY OF THE INVENTION
The object of the present invention is to provide a continuous casting nozzle
having features of forming a glass layer at the surface of the bore of the nozzle when the
nozzle is used, thereby preventing air from being entrapped through refractory structure,
CA 022233~6 1997-12-03
smoothing the bore surface of the nozzle and preventing the accumulation of alumina.
Also, the object of the present invention is to provide a continuous casting nozzle which
prevents erosion by products having a low-melting point on account of a reactionbetween an aggregate in a refractory and alumina in the steel, and to provide the nozzle
which is not influenced by a temperature of the molten steel in casting, and which is able
to prevent the bore from narrowing or clogging economically, comparatively easy and
stable.
In the present invention, the surface layer of the bore of a continuous casting
nozzle contacting with molten steel is formed of a refractory comprising graphite from
10 to 35 wt%, an aggregate of 10 to 60 wt% selected from from alumina matter, zirconia
matter, zircon matter, or alumina-silica matter and roseki containing the pyrophyllite
(Al2O3 4SiO2 H2O) as the main component as the rest part of the above mentioned
materials.
In anotherr embodiment of the present invention, the surface layer of the bore of
a continuous casting nozzle contacting with molten steel is formed of a refractory
comprising graphite from 10 to 35 wt%, an aggregate of 10 to 60 wt% selected from
from alumina matter, zirconia matter, zircon matter, or alumina-silica matter and roseki
cont~ining the pyrophyllite (Al203 4SiO2 H20) as the main component as the rest part of
the above mentioned materials, the said refractory being added binder, kneaded, formed,
and sintered in the anti-oxidizing atmosphere.
It is preferable that the roseki cont~ining the pyrophyllite as the main
component is calcinated at a temperature equal to or more than 800~C so as to vanish
crystal water and contain alkaline component from 1 to 5 wt%. As for the roseki
having above mentioned component, it is preferable that a mixing weight ratio of roseki
with an average grain diameter equal to or less than 250,~m is equal to or less than 60%
relative to the whole of the roseki content.
As for roseki having above mentioned component, it is preferable that the rosekicont~ining the pyrophyllite as the main component is calcinated at a temperature equal to
or more than 800~C so as to vanish crystal water and contains alkaline component from
1 to5wt%.
CA 022233~6 1997-12-03
Furthermore, the mixing weight ratio of roseki whose average grain diameter equal to or
less than 250~1m, is equal to or less than 60% relative to the whole of the roseki content.
And as for the binder a thermosetting resin, for example, phenol resin is preferable
selected. With respect to forming process CIP (Cold isostatic process) should bepreferably selected.
BRIEF DESCRIPTION OF THE DRAWINGS
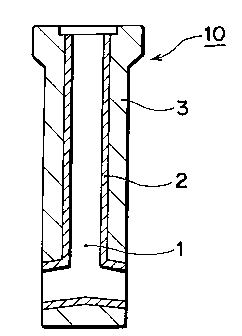
FIG. 1 shows a longitudinal cross section of a nozzle according to the present
invention comprising a invented refractory at the surface layer of the bore of the nozzle.
FIG. 2 shows a longitudinal cross section of a nozzle according to the present
invention comprising a invented refractory at the surface layer of the bore of the nozzle
and the lower part (a part immersed in the molten steel) of the nozzle.
EMBODIMENTS OF THE INVENTION
A major characteristic of a continuous casting nozzle of the present invention is
that the main component of a refractory of the surface layer of the bore of the nozzle is
roseki. During usage, when silica in the above mentioned refractory coexisting with
graphite or carbon, the following reactions are usually caused.
SiO2(S) + C(S) = SiO(g) + CO(g)
3SiO(g) + 2AI = Al203(S) + 3Si
3CO(g) + 2AI = Al203 (S) + 3C
As shown in the above reactions, decomposition of the silica produces SiO(g)
and CO(g), which react with aluminum in the steel to form Al203 and it becomes the
source of oxygen to the steel.
However, as for the roseki, the roseki particles do not decompose even if it is
coexisting with graphite or carbon, namely SiO2 in pyrophyllite (Al203 4SiO2H20)which is the main mineral of the roseki is stable. This fact is found from the facts that the
particles do not decay and bubbles are not produced, which is confirmed by means of a
microscope observation after forming a briquette consisting of the roseki, resin powders
and carbon powders and performing heat-treatment at a temperature of 1500~C for 24
CA 022233~6 1997-12-03
hours with burying it in a coke breeze.
The half-melting temperature of the roseki is about 1500~C, so that it melts at
the bore surface contacting with the molten steel to form a glass coat for smoothing the
structure of the surface of the bore and for preventing air from being entrapped through
a refractory structure.
This is found from the fact that the permeability is decreased such that the
permeability after performing heat-treatment at a temperature of 1500~C for 1 hours is as
small as about 9.5x10-5 darcy, in contrast the permeability after performing heat-
treatment at a temperature of 1000~C for 1 hours is about 9.5x10-4 darcy.
Although the mixing amount of the roseki is the rest part of the mixing amount
of other components, a mixing weight ratio of the roseki is equal to or more than 30
wt% in order to actively form the glass coat on the surface of the bore in use as
continuous casting nozzle, preferably. Also, it is preferably that the mixing weight ratio
of the roseki is equal to or less than 80 wt% because degree of softening deformation is
large with a range of over 80 wt%.
The most preferable mixing weight ratio of the roseki is from 30 wt% to 60 wt%.
In this case the aggregate of roseki particles does not decompose even coexisting with
graphite.
As for kinds of roseki, it is possible to use three kinds of roseki, that is
pyrophyllite matter roseki, kaolin matter roseki, and sericite matter roseki.
The pyrophyllite matter roseki with refractoriness from SK29 to SK32 (SK(Seger cone)
is a Japanese Standard for refractoriness ) is suitable, considering formation of a glass
layer and erosion resistance against the molten steel, as the surface of the bore contacting
with the molten steel is half-molten in use. Both of the kaolin matter roseki and the
sericite matter roseki is not preferable because the kaolin matter roseki has a greater
refractoriness from SK33 to SK36, and the sericite matter roseki has a smaller
refractoriness from SK26 to SK29.
As for the binder for forming the nozzle body a thermosetting resin, for examplephenol resin, is preferably used and the mixing ratio is preferably 5 to 15 wt%. And the
most preferable process of the mixed material is CIP(cold isostatic pressing) to produce
CA 022233~6 1997-12-03
the nozzle having a high heat resistance.
Sintering of the formed body is preferably performed in the nonoxidizing
atmosphere to minimi7e the burning loss of the graphite mixed in the material, which is
mixed to enhance the erosion resistance and oxidation resistance and the sintering
temperature is preferably 1000 to 1200 ~C to obtain a sufficient strength ofthe nozzle.
The reason for using the roseki calcinated at a temperature equal to or more
than 800~C to vanish crystal water is that the crystal water is released from the roseki at
a temperature in a range of from 500 to 800~C in sintering and the refractory cracks by
virtue of an unusually large coefficient of thermal expansion in this range. The alkaline
component of the roseh from 1 to 5 wt% is preferable to control the melting point of
roseki adequdately
It is preferable that a mixing weight ratio of roseki with an average grain
diameter equal to or less than 250,um is equal to or less than 60% relative to the whole
of the roseki content because, in the range of over 60%, structural defects such as
l~min~tion are apt to be produced in molding and softening deformation of rosekiparticles is apt to happen when used in a continuous casting nozzle.
The half-melting temperature of the roseki is about 1500~C, and it melts at the
bore surface contacting with the molten steel to form a glass coat for smoothing the
structure of the surface of the bore and for preventing air from being entrapped through
a refractory structure, so that it has the effect to depress the adherence of A12~3 and
metal.
To prevent the softening deformation and to m~int~in heat-impact resistance of
the roseki, preferably, a mixing weight ratio of the graphite is equal to or more than 10
wt%. Also, it is preferably that the mixing weight ratio of the graphite is equal to or
less than 35 wt% from the view point of manufacturing of the nozzle because the volume
ratio of the graphite relative to the roseki is too large so that structural defects such as
l~min~tion are apt to be produced in the range of over 35 wt%. Considering thermal
conductivity and oxidation resistance, natural graphite is suitable as the graphite to be
mixed.
As for the aggregate to be mixed, an aggregate of 10 to 60 wt% selected from
CA 022233~6 1997-12-03
from alumina matter, zirconia matter, zircon matter, or alumina-silica matter should be
selected. to obtain a suff1cient erosion resistance of the nozzle against molten steel.
The continuous casting nozzle for steel according to the present invention will
be described in detail with reference to the accompanying drawings of nozzle forcontinuous casting.
As shown in FIG. 1, a surface layer 2 of the bore 1, through which the molten
steel flows, of the immersion nozzle 10 consists of a refractory having the chemical
composition as described above. The rest part of the nozzle 3 is composed of regular
refractory, for example, of alumina-graphite which is already known in public. The
dimensions of the nozzle are about l,OOOmm in total length, about 60mm in diameter of
the bore, 160mm in outer diameter, and about 50mm in thickness.
FIG. 2 shows another embodiment of the invention, a nozzle comprising a
refractory according to the present invention at the surface layer of the bore of the nozzle
and the lower part (a part immersed in the molten steel) of the nozzle. In the bore 1 of
the nozzle for continuous casting, the adherence and accllm~ tion of non-metallic
inclusion such as the (x -alumina are depressed.
EXAMPLES
The present invention is explained with examples as described below.
The samples Nos. 1 to 5 (hereinafter referred to as the "sample of the present invention")
having the chemical compositions within the scope of the present invention, and the
samples Nos. 6 to 8 (hereinafter referred to as "sample for comparison") having chemical
compositions out of the scope of the present invention were prepared as shown in Table
1, and phenol resin in the state of powder and liquid was added in an amount within a
range of from 5 to 10 wt% to each of the mixed materials. From the mixed materials
above, the following formed bodies were prepared.
A first formed body (hereinafter referred to as the "formed body 1") with
dimensions of 30mm by 30mm by 230mm for ex~mining an amount of adhesion of non-
metallic inclusion such as alumina and corrosion resistance against the molten steel, a
second formed body (hereinafter referred to as the "formed body 2") with dimensions of
CA 022233~6 1997-12-03
50mm~by 20mm for ex~mining permeability, and a third formed body (hereinafter
referred to as the "formed body 3") with dimensions of 100mm in outer diameter, 60mm
in inner diameter and 250mm in length for ex~mining spalling resistance, were
respectively prepared, and then the bodies were sintered in reducing atmosphere at a
temperature in a range from 1000 to 1200~C and samples 1 to 8 were prepared.
Physical properties (porosity and bulk density) for each of the above-mentioned
samples of the present invention Nos. 1 to 5 and the samples for comparison Nos. 6 to 8
are shown in Table 1.
The spalling resistance of each of the sintered formed bodies 3 of the samples of
the present invention Nos. 1 to 5 and the samples for comparison Nos. 6 to 8 were
examined after heating at a temperature of 1500~C for 80 minutes in an electric furnace
and then rapidly cooling by water. The results are shown in Table 1.
An erosion ratio (%) and an amount of adhesion of non-metallic inclusion such
as alumina of each of the sintered formed bodies 1 of the samples of the presentinvention Nos. 1 to 5 and the samples for comparison Nos. 6 to 8 were examined after
immersing in molten steel, which contains alllmim~m in a range from 0.02 to 0.05 wt%,
at a temperature of 1550~C for 180 minutes. The results are shown in Table 1.
The permeability for each of the sintered formed bodies 2 of the samples of the
present invention Nos. 1 to 5 and the samples for comparison Nos. 6 to 8 were examined
after heating at a temperature of 1500~C for 60 minutes in an electric furnace and then
cooling. The results are shown in Table 1.
It is easily understood from Table 1 that the samples of the present invention are
superior in the spalling resistance so the nozzle is not destroyed at the beginning of
casting. Also, the non-metallic inclusion such as alumina does not adhere in spite of the
low erosion ration, thereby effectively preventing narrowing or clogging of the
continuous casting nozzle of the molten steel.
And also, it is possible for the samples of the present invention to prevent airfrom being entrapped through the refractory in practical use because of small
permeability.
On the other hand, it is obvious that the sample for comparison No. 6 is
CA 022233~6 1997-12-03
remarkably inferior in the spalling resistance and the corrosion resistance against the
molten steel, although a small amount of alumina adheres due to much roseki content.
As for the sample for comparison No. 7, the amount of adhesion of alumina is
remarkably large, because it contains Al203 and SiO2, which decomposes to supplyoxygen in the steel, instead of the roseki.
As for the sample for comparison No. 8, it does not contain SiO2 instead of
roseki and contains only Al203 and it has high permeability and the amount of adhesion
of alumina is remarkably large, although it contains no mineral source of oxgen to the
steel.
Therefore, with the use of the continuous casting nozzle for casting steel
according to the present invention, it is possible to perform stable casting with
preventing narrowing or clogging of the bore caused by the non-metallic inclusion such
as alumina without deterioration of the refractory structure.
According to the present invention, approximately 300 ton of a low carbon
aluminum killed steel of 5 to 7 charges is continuously cast with one nozzle without
clogging by 2 strand slab caster in real operation, though with conventional nozzle,
clogging up in the nozzle were occurred within 2 to 4 charges under same condition.
- 10 -
[Table 1]
Sample No. of the Present Invention Sample No. for Comparison
2 3 4 5 6 7 8
Mixing Composition Graphite 10 10 10 20 35 5 25 25
(wt%) Roseki (0.5-lmm) 35 25 20 15 15 35
Roseki (-0.25mm) 45 35 20 15 15 50
Al203 10 30 50 50 35 10 50 70
sio2 25
Physical Properties Porosity (%) 13.713.8 13.6 13.5 16.2 13.5 12.8 16.4
Bulk density 2.192.21 2.30 2.31 2.05 2.22 2.30 2.56
Modulus of Rupture (MPa) 8.8 9.2 9.5 9.5 7.0 8.5 12.1 8.0 D
Erosion to Molten Steel (%) 10 7 3 3 5 20 3
Permeability (xlO~5darcy) 4.0 7.5 10.0 13.0 12.0 3.0 65 95
after Heat-treatment 1500~C- 1 hr
SpallingResistance No crack No crack No crack No crack No crack Crack No crack Crack
occurrence occurrence ~
Amount of Adhesion of Alumina ~ . O ~ . O ~ . 0.5 ~ . 1.0 ~ . 0.5 5 15 10 o