Note: Descriptions are shown in the official language in which they were submitted.
64680-1022
CA 02223404 2000-06-19
_1_
~JOVEL CRYSTAL. FORM OF ANHYDROUS 7-(f 1 a.5v.6a1r6-AMA
AZABICYCLOf 3.1.01 HEX-3-YLL-6-FLUORO-1-(2.4-DIFLUOROPHENYL)-1,4-
r
DIHYDRO-4-OXO-1,8-NAPHTHYRIDINE-3-CARBOXYLIC ACID,
METHAPJESULFONIC ACID SALT
Background of the Invention
The invention is directed to a novel crystal form of anhydrous 7-([1 v,5v,6a]-
6-
amino-3-azabicyclo [3.1.0]h~ax~-yl)-6-fluoro-1-(2,4-difluorophenyl)-1,4-
dihydro-4-oxo-1, 8-
naphthyridine-3-carboxylic acid, methanesulfonic acid salt, a method of using
the
compound in the treatment of a bacterial infection in mammals, especially
humans, and
to pharmaceutical compositions useful therefor.
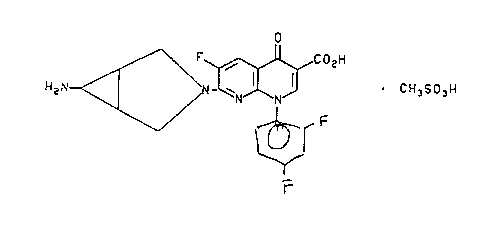
United States Patent No. 5,229,396,
discloses 7-([la,5a,6aJ-6-amino-3-azabicyclo[3.1.0]hex-3-yl)-6-fluoro-1-(2,4-
difluorophenyl)-1,4-dihydro-4-oxo-1,8-naphthyridine-3-carboxylic acid,
methanesulfonic
acid salt of the formula
CI
2~ F C02H
,.
~ . CH3S03H
'N N
wherein Y is o,p-diiluorophenyl and R~ is
H2N _
II
having antibacterial activity.
CA 02223404 1997-12-03
WO 96/39406 PCT/US95/07211
-2-
Summary of the Invention
The invention is directed to a novel crystal form of anhydrous 7-([1 a,5a,6a]-
6- '
amino-3-azabicyclo[3.1.0]hex-3-yl)-6-fluoro-1-(2,4-difluorophenyl)-1,4-dihydro-
4-oxo-1,8-
naphthyridine-3-carboxylic acid, methanesulfonic acid salt which possesses
valuable
and nonobvious properties. Since the anhydrate is substantially
hydrophobically stable,
formulation problems of the active ingredient during tableting or capsulation
operations
are alleviated.
Detailed Description of the Invention
The 7-([la,5a,6a]-6-amino-3-azabicyclo[3.1.0]hex-3-yl)-6-fluoro-1-(2,4
difluorophenyl)-1,4-dihydro-4-oxo-1,8-naphthyridine-3-carboxylic acid,
methanesulfonic
acid salt referred to in United States Patent No. 5,229,396 characterized by
the major
peaks in the following X-ray powder diffraction pattern
Peak 1 2 3 4 5 6 7 8 9 10
no.
28( ) 5.0 9.8 13.014.8 19.7 20.9 22.0 23.0 28.1 29.3
Cu
d space 17.9 9.0 6.8 6.0 4.5 4.2 4.0 3.9 3.2 3.0
is substantially hygroscopic and can pick up water from the atmosphere to form
a
monohydrate. The monohydrate is characterized by the major peaks in the
following
X-ray powder diffraction pattern
Peak no. 1 2 3 4 5 6 7 8
28() Cu 4.7 9.4 12.4 13.1 13.6 14.2 17.0 17.9
d space 8.7 9.4 7.1 6.7 6.5 6.3 5.2 5.0
Peak no. 9 10 11 12 12 14 15
28() Cu 8.7 21.0 22.0 24.2 24.2 26.6 27.2
d space 4.7 4.2 4.0 3.7 3.7 3.5 3.3
The novel crystal form of 7-([i a,5a,6a]-6-amino-3-azabicyclo[3.1.0]hex-3-yl)-
6-
fluoro-1-(2,4-difluorophenyl)-1,4-dihydro-4-oxo-1,8-naphthyridine-3-carboxylic
acid,
methanesulfonic acid salt (hereinafter the anhydrate") is hydrophobically
stable and
characterized by the major peaks in the following X-ray powder diffraction
pattern.
CA 02223404 1997-12-03
WO 96139406 PCT/US95/07211
Peak no. 1 2 3 4 5 6 7 8
28() Cu 4.5 7.7 9.1 13.6 15.0 18.2 18.6 22.8
d space 9.5 11.5 9.7 6.5 5.9 4.9 4.8 3.9
The anhydrite may be prepared by heating 7-([1 a,5a,6a]-6-amino-3-
azabicyclo[3.1.0]hex-3-yl)-6-fluoro-1-(2,4-difluorophenyl)-1,4-dihydro-4-oxo-
1,8-
naphthyridine-3-carboxylic acid, methanesulfonic acid salt or its derived
monohydrate
in an organic solvent or a mixture thereof with an aprotic co-solvent, such as
isopropanol, dimethylsulfoxide, n-propanol, tetrahydrofuran or n-butanol,
preferably
n-butanol or tetrahydrofuran/n-butanol, to reflux or to a temperature between
about
70°C to about 90°C, preferably about 85°C. Depending on
the reaction temperature
and other conditions, the reaction time generally ranges from about 1 hour to
about 20
hours, preferably about 2 hours to about 16 hours.
The crystal slurry formed is cooled to a temperature between about
20°C to
about 30°C, preferably about 25°C, for a time period between
about 2 hours to about
24 hours, preferably about 2 hours to about 12 hours. The crystalline product
is then
filtered from the mother liquid and dried under vacuum until all the solvent
has been
removed.
The anhydrite may be administered as an antibacterial agent as described in
above-mentioned United States Patent No. 5,229,396. Administration to a
subject may
be alone, but the anhydrite will generally be administered in admixture with a
pharmaceutical carrier selected with regard to the intended route of
administration and
standard pharmaceutical practice. For example, it can be administered orally
or in the
form of tablets containing such excipients as starch or lactose, or in
capsules either
alone or in admixture with excipients, or in the form of elixirs or
suspensions containing
flavoring or coloring agents. In the case of animals, it is advantageously
contained in
an animal feed.
The invention also provides pharmaceutical compositions comprising an
antibacterially effective amount of the anhydrite together with a
pharmaceutically
acceptable diluent or carrier.
The anhydrite can be administered to humans for the treatment of bacterial
diseases by either the oral or parenteral routes, and may be administered
orally at
dosage levels of about 0.1 to 500 mg/kg/day, advantageously 0.5-50 mg/kg/day
given
CA 02223404 1997-12-03
WO 96/39406 PCT/US95/07211
in a single dose or up to 3 divided doses. For intramuscular or intravenous
administration, dosage levels are about 0.1-200 mg/kg/day, advantageously 0.5-
50 '
mg/kg/day. While intramuscular administration may be a single dose or up to 3
divided
doses, intravenous administration can include a continuous drip. Variations
will -
necessarily occur depending on the weight and condition of the subject being
treated
and the particular route of administration chosen as will be known to those
skilled in
the art.
The antibacterial activity of the anhydrate is shown by testing according to
the
Steer's replicator technique which is a standard in vitro bacterial testing
method
described by E. Steers et al., Antibiotics and Chemotherapy, 9, 307 (1959).
The hydration properties were determined gravimetrically over a range of
relative
humidities using a VTI microbalance system for moisture sorption studies
(Model
MB300W).
PREPARATION A
7-(f1 d,5a,6a1-6-amino-3-azabicyclo~3.1.Olhex-3-yl)-6-fluoro-1-
2,4-difluorophenyl)-1.4-dihydro-4-oxo-1.8-naphthyridine-3-carboxylic acid.
methanesulfonic acid salt
7-([i a,5a,6a]-6-tert-butyloxycarbonylamino-3-azabicyclo]3,1.O]hex-3yl)-6-
fluoro
1 (2,4-difluorophenyl)-1,4-dihydro-4-oxo-1,8-naphthyridine-3-carboxylic acid,
ethyl ester,
(25 g) and methanesulfonic acid (11 g) was added to a mixture of water (250
mL) and
tetrahydrofuran (250 mL). The resultant slurry was heated to reflux (about
66°C)
temperature and held at this temperature for 20 hours after which time a clear
solution
was obtained. The solution was cooled to 35-40°C and concentrated under
reduced
pressure to about half its original volume. The resultant crystal slurry was
cooled slowly
to room temperature (about 20°C) and then further stirred at
10°C for 2 hours. The
crystalline product 7-([la,5a,6a]-6-amino-3-azabicyclo[3.1.0]hex-3-yl)-6-
fluoro-1-(2,4-
difluorophenyl)-1,4-dihydro-4-oxo-1,8-naphthyridine-3-carboxylic acid,
methanesulfonic
acid salt was isolated by filtration and washed with a mixture of
tetrahydrofuran (12.5
mL) and water (12.5 mL). The crystals were dried under vacuum at 30-35°
until the
residual water content of the crystals was below 0.2°~. Yield 21.2 g,
90°~6.
The crystals of 7-([la,5a,6a]-6-amino-3-azabicyclo[3.1.0]hex-3-yl)-6-fluoro-
CA 02223404 1997-12-03
WO 96139406 PCTlUS95107211
-5-
1-(2,4-difluorophenyl)-1,4-dihydro-4-oxo-1,8-naphthyridine-3-carboxylic acid,
methanesulfonic acid salt are characterized by the major peaks in the
following X-ray
powder diffraction pattern.
Peak 1 2 3 4 5 6 7 8 9 10
no.
2B() 5.0 9.8 13.0 14.8 19.7 20.9 22.0 23.0 28.1 29.3
Cu
d space 17.9 9.0 6.8 6.0 4.5 4.2 4.0 3.9 3.2 3.0
The crystals of 7-([ia,5a,6a]-6-amino-3-azabicyclo[3.1.0]hex-3-yl)-6-fluoro-
1-(2,4-difluorophenyl)-1,4-dihydro-4-oxo-1,8-naphthyridine-3-carboxylic acid,
methanesulfonic acid salt can pick up water from the atmosphere and form a
monohydrate. The monohydrate is characterized by the major peaks in the
following
X-ray powder diffraction pattern.
Peak no. 1 2 3 4 5 6 7 g
28() Cu 4.7 9.4 12.4 13.1 13.6 14.2 17.0 17.9
d space 8.7 9.4 7.1 6.7 6.5 6.3 5.2 5.0
Peak no. 9 10 11 12 12 14 15
28( ) 8.7 21.0 22.0 24.2 24.2 26.6 27.2
Cu
d space 4.7 4.2 4.0 3.7 3.7 3.5 3.3
Example 1
7-(~la. 5a. 6a1-6-amino-3-azabicyclo~3 1 0lhex 3y) 6 fluoro 1 (2 4
difluorophenyl)-1.4-dihydro-4-oxo-1 8-naphthyridine 3 carboxylic acid
methanesulfonic acid salt anhydrous
7-([la,5a,6a]-6-amino-3-azabicyclo[3.1.0]hex-3-yl)-6-fluoro-1-(2,4-
difluorophenyl)-
1,4-dihydro-4-oxo-1,8-naphthyridine-3-carboxylic acid, methanesulfonic acid
salt or its
monohydrate (20 g) was stirred with isopropanol (220 ml). The crystal
suspension was
_ refluxed for 16 hours or until microscopic examination had shown that the
crystal form
had changed to a hexagonal form. The crystal slurry was cooled to 20-
25°C and
stirred at this temperature for about 1 hour. The crystalline product was
filtered from
the mother liquor, washed with isopropanol (about 50 mL) and dried under
vacuum at
40°C until all the solvent had been removed. Yield 98°.6.
CA 02223404 1997-12-03
WO 96/39406 PCT/US95/07211
-6-
The product is a new polymorphic form of 7-([1 a,5a,6a]-6-amino-3-
azabicyclo[3.1.0]hex-3-yl)-6-fluoro-1-(2,4-difluorophenyl)-1,4-dihydro-4-oxo-
1,8- '
naphthyridine-3-carboxylic acid, methanesulfonic acid salt, anhydrous,
characterized by
the following major peaks in its X-ray powder diffraction pattern.
Peak no. 1 2 3 4 5 6 7 8
28() Cu 4.5 7.7 9.1 13.6 15.0 18.2 18.6 22.8
d space 9.5 11.5 9.7 6.5 5.9 4.9 4.8 3.9
Example 2
7-(yia, 5a. 6a1-6-amino-3-azabicycloP3.l.Olhex-3y )-6-fluoro-1-(2 4-
difluorophenyt)-1.4-dihydro-4-oxo-1.8-naphthyridine-3-carboxylic acid
methanesulfonic acid salt, anhydrous
7-([1 a,5a,6a]-6-amino-3-azabicyclo[3.1.0]hex-3-yl)-6-fluoro-1-(2,4-
difluorophenyl)
1,4-dihydro-4.-oxo-1,8-naphthyridine-3-carboxylic acid, methanesulfonic acid
salt or its
monohydrate (7 g) was dissolved in dimethylsulfoxide, DMSO (21 mL) by heating
to 80
85°C until complete solution was obtained. Isopropanol (150 mL) was
added dropwise
to the solution at about 85°C to induce crystallization. The crystal
suspension was held
at reflux temperature about 85°C for 2-16 hours or until microscopic
examination had
shown that the crystal form had changed to a hexagonal form. The resultant
crystal
slurry was cooled to 20-25°C. The crystalline product was filtered from
the mother
liquor, washed with isopropanol (about 50 mL) and dried under vacuum at
50°C until
all the solvents had been removed. Yield 77°~6.
The product is the same as in F~cample 1.
Example 3
7-((ia. 5a. 6a1-6-amino-3-azabicyclor3.l.Olhex-3yr)-6-fluoro-1-(2 4-
difluorophenyl)-1,4-dihydro-4-oxo-1.8-naphthyridine-3-carboxylic acid
methanesulfonic acid salt, anhydrous
7-([1 a,5a,6a]-6-amino-3-azabicyclo[3.1.0]hex-3-yl)-6-fluoro-1-(2,4-
difluorophenyl)-
1,4-dihydro-4-oxo-1,8-naphthyridine-3-carboxylic acid, methanesulfonic acid
salt or its
t
monohydrate (55.6 g) was dissolved in dimethylsulfoxide, DMSO (159 mL) by
heating
to 80-85°C until complete solution was obtained. The solution was
cooled to 20-25°C
and stirred for 2 hours until a crystal slurry formed. Dichloromethane (1200
mL) was
CA 02223404 1997-12-03
WO 96/39406 PCT/US95107211
-7-
added dropwise to the solution at about 25 ° C to fully induce
crystallization. The crystal
suspension was held at room temperature overnight or until microscopic
examination
had shown that the crystal form had changed to a hexagonal form. The
crystalline
' product was filtered from the mother liquor, washed with dichloromethane (3
x 119 mL)
and dried under vacuum at 50°C until all the solvent had been removed.
Yield 9196.
The product is the same as in Example 1.
Example 4
7-(~1 a. 5a 6a1-6-amino-3-azabicycloP3 1 0lhex 3v) 6 fluoro 1 (2 4
difluorophenvl)-1,4-dihvdro-4-oxo-1.8-naahthyridine 3 carboxylic acid
methanesulfonic acid salt anhydrous
7-([1 a,5a,6a]-6-amino-3-azabicyclo [3.1.0]hex-3-yl)-6-fluoro-1-(2,4-
difluorophenyl)-
1,4-dihydro-4.-oxo-1,8-naphthyridine-3-carboxylic acid, methanesulfonic acid
salt or its
monohydrate (1 g) was stirred with n-propanol (44 mL). The crystal suspension
was
refluxed for 3 hours or until microscopic examination had shown that the
crystal form
had changed to a hexagonal form. The crystal slurry was cooled at 20-
25°C and
stirred overnight. The crystalline product was filtered from the mother
liquor, washed
with n-propanol (about 10 mL) and dried under vacuum at 50-55°C until
all the solvent
had been removed. Yield 6896.
The product is the same as in Example 1.
Example 5
7- 1 a. 5a. 6a1-6-amino-3-azabicyclof3 1 Olhex 3y) 6 fluoro 1 (2 4
difluoroahenyl)-1 4-dihydro-4-oxo-1 8-naahthyridine 3 carboxylic acid
methanesulfonic acid salt anhydrous
7-([i a,5a,6a]-6-amino-3-azabicyclo[3.1.0]hex-3-yl)-6-fluoro-1-(2,4-
difluorophenyl)
1,4-dihydro-4.-oxo-1,8-naphthyridine-3-carboxylic acid, methanesulfonic acid
salt or its
monohydrate (70 g) was stirred with a mixture of tetrahydrofuran (175 mL) and
a n
butanol (525 mL). The crystal suspension was heated for 16 hours or until
microscopic
examination had shown that the crystal form had changed to a hexagonal form.
The
crystal slurry was cooled to 20-25 ° C and stirred overnight. The
crystalline product was
filtered from the mother liquor, washed with a mixture of tetrahydrofuran (25
mL) and
n-butanol (75 mL) and dried under vacuum at 80°C until all the solvent
had been
removed. Yield 95°.6.
The product is the same as in Example 1.
CA 02223404 1997-12-03
WO 96/39406 PCT/US95/07211
-g-
Example 6
7-(fl a, 5a. 6a1-6-amino-3-azabicyclof3.l.Olhex-3y)-6-fluoro-1-(2,4-
difluorophenyl)-1.4-dihydro-4-oxo-1.8-naphthyridine-3-carboxyrlic acid,
methanesuifonic acid salt, anhydrous
7-([la,5a,6a]-6-amino-3-azabicyclo[3.1.0]hex-3-yl)-6-fluoro-1-(2,4-
difluorophenyl)-
1,4-dihydro-4-oxo-1,8-naphthyridine-3-carboxylic acid, methanesulfonic acid
salt or its
monohydrate (5 g) was stirred with n-butanol containing up to 196 water (220
mL). The
crystal suspension was heated to reflux for 5 hours or until microscopic
examination
had shown that the crystal form had changed to a hexagonal form. The crystal
slurry
was cooled to 20-25°C and stirred overnight. The crystalline product
was filtered from
the mother liquor, washed with n-butanol (about 20 mL) and dried under vacuum
at 50-
55°C until all the solvent had been removed. Yield 92°~.
The product is the same as in Example 1.
f