Note: Descriptions are shown in the official language in which they were submitted.
CA 02224118 1997-12-OS
WO 96!39199 PCT/US96/09150
1
PLASMA WATER VAPOR STERILIZER
AND METHOD
Field of the Invention
This invention relates to relatively low
temperature sterilization of articles with gaseous
species. In particular this invention relates to a
method for sterilizing articles with neutral active
1o species of a gas plasma generated from water vapor at
temperatures below about 100°C.
Background of the Invention
Methods using steam and disinfecting gases,
particularly ethylene oxide, have been widely used for
sterilizing medical products ranging from pharmaceutical
preparations to surgical instruments.
A sterilizing method must effectively render
all microbial organisms non-viable without damage to the
article or goods being sterilized or its packaging.
However, many disinfecting gases which meet this
criteria, such as ethylene oxide, have been recognized
to expose workers and the environment to safety hazards.
Recent legislation has severely restricted the amount of
hazardous gases such as ethylene oxide (a known
carcinogen) in the working environment, or the use of
any system or method which produces toxic residues or
exhaust products. This has created a major crisis in
hospitals and other areas of the health industry.
Further, although steam sterilization is inexpensive and
CA 02224118 1997-12-OS
WO 96/39199 PCT/US96/09150
2
effective, it is too hot for many applications,
particularly those involving sterilization of plastics
with low melting points. Irradiation is a cooler
process, but the large and expensive facilities required
for irradiation are impractical for hospital uses.
The use of plasma in sterilizing methods has
been suggested. Plasma is ionized or partially ionized
gas which may be generated by the application of
electromagnetic energy which may be obtained from
l0 different sources. The ionized gas will contact
microorganisms on the surfaces of the items to be
sterilized and effectively destroy the microorganisms.
Among the variety of gases attempted for use
as sterilizing plasmas has been water vapor. However,
these prior attempts have been reported as unsuccessful,
or as being not particularly effective. Thus, U.S.
Patent 4,643,876, issued February 17, 1987, inventors
Jacobs and Lin, describes a plasma sterilization process
and apparatus where the item to be sterilized has plasma
generated around the item. Attempts to use a water
plasma treatment by itself in this method of sterili-
zation led to no significant sporicidal activity.
Similarly, U.S. Patent 3,701,628, issued October 31,
1972, inventors Ashman, describes generating a plasma
from sterilizing gas that is introduced into the
sterilizing chamber. Plasmas formed of water vapor were
reported as not being particularly effective.
U.S. Patent 5,115,166, issued May 19, 1992,
inventors Campbell and Moulton, describes the use of
3o electrically neutral active species generated in a gas
plasma to sterilize articles. In this system, articles
to be sterilized are placed in a vacuum chamber which is
attached to one or more plasma generators and a vacuum
pump. The plasma generators use a source of microwave
energy such as a magnetron to create the required
CA 02224118 1997-12-OS
W O 96139199 PCT/US96/09150
3
electromagnetic field. The energy is conducted to the
~ gas via a rectangular waveguide. The chamber is sealed
and the vacuum pump is used to evacuate air from the
~ ' chamber. valves are then opened which allow gas to flow
from a source container, through the plasma generators,
through a gas distribution system, then through the
sterilization chamber, and out through the vacuum pump.
The gas mixture is usually oxygen, hydrogen, or a
mixture of oxygen, hydrogen, and an inert gas. A strong
electromagnetic field in the plasma generators interacts
with the gases flowing through the plasma generators and
generates a glow discharge. A variation of such an
apparatus is described in U.S. Patent 5,184,046, issued
February 2, 1993, inventor Campbell, where the plasma
generators incorporate a cylindrical wave guide.
Both these apparatus of Patents '166 and '046
confine the electromagnetic field generating the plasma
within the plasma generator. Once the gas flows out of
the region with a high electromagnetic field, plasma is
2o no longer created. Since highly reactive plasma
components either react or decay quickly, relatively
non-reactive components enter the sterilization chamber.
Charged particles recombine in the gas distribution
system to form electrically neutral components. Thus,
the components entering the sterilization chamber
include neutral active species which accomplish
sterilization.
Summary of the Invention
A method aspect of this invention for plasma
3o sterilization comprises exposing an article to be
sterilized in an evacuated sterilization chamber to the
neutral active species of a plasma generated from water
vapor where the electromagnetic field generating the
plasma is confined to the plasma generator (which can be
CA 02224118 1997-12-OS
WO 96/39199 PCT/US96/09150
4
a plurality of plasma generators). The plasma-induced
gas sterilization is preferably carried out at a
temperature of less than about 100°C and a pressure of
from 0.1 to 150 torr, preferably about 0.1 to 40 torr.
Practice of the inventive method provides
effective sterilization with the inexpensive and readily
available water source and eliminates heavy, costly and
potentially hazardous compressed gas cylinders as are
necessary with many previously used sterilizing methods.
l0 Brief Description of the Drawings
Figure 1 is a top view of a plasma sterilizer
of this invention;
Figure 2 is a cross-sectional view of the
plasma sterilizer embodiment of Fig. 1;
Figure 3 is a schematic illustration of a
component of the Fig. 1 apparatus;
Figure 4 is a cross-sectional view of another
plasma sterilizer embodiment;
Figure 5 is a side sectional view of the
plasma sterilizer according to another embodiment of the
invention; and,
Figure 6 is a detailed, sectional view of the
plasma generator as a component of the plasma sterilizer
shown in Fig. 5.
Detailed Description of the Invention
Hospitals originally relied on disinfectants
and steam autoclaves for sterilizing implements. In
more recent years, ethylene oxide gas sterilization has
made possible the sterilization of articles in heat
sensitive packaging, thermolabile drugs, and heat
sensitive medical supplies, and hospitals are highly
dependent upon these procedures. However, ethylene
oxide has been found to be a dangerous carcinogen and a
CA 02224118 1997-12-OS
WO 96!39199 PCT/US96/09150
number of new state laws protecting worker safety and
the environment are restricting further use of ethylene
oxide sterilizers in hospital environments. In
. addition, ethylene oxide is known to be a dangerous
5 material from several other aspects. In its pure form
it is explosive and flammable and therefore requires
that all equipment must be so designed as to be
classified as explosive proof. The most popular form of
the diluted or explosive proof mixtures contains
fluorocarbons (Freon), which are no longer
environmentally acceptable. Also, because it is a
carcinogen, state and federal authorities have imposed
stringent regulations for the protection of workers and
concerning emissions to the environment. This has
placed further burdens and restrictions on the use of
ethylene oxide sterilizers in all applications.
Gas sterilizers suitable for practicing this
invention produce a plasma from a gas mixture consisting
essentially of water vapor. The exhaust gas products of
the gas mixture after use in the sterilization process
fully satisfy current environmental and worker safety
concerns, as the exhaust is almost entirely water vapor
with traces of carbon dioxide and other gases arising
from the interaction of plasma components with organic
material in the articles being sterilized.
The plasma is produced as a result of applying
an electric or electromagnetic field to the water vapor.
The electromagnetic field can cover a broad frequency
range, and can be produced by a magnetron, klystron, or
3o RF coil. The present invention is usefully practiced
' with apparatus such as described by U.S. Patent
5,115,166. In this system, articles to be sterilized
' are placed in a vacuum chamber which is attached to one
or more plasma generators and a vacuum pump. The
chamber is sealed and the vacuum pump is used to
CA 02224118 1997-12-OS
WO 96/39199 PCT/US96/09150
6
evacuate air from the chamber. Next, valves are opened
which allow gas to flow from a source container, through '
the plasma generators, through a gas distribution
system, through the sterilization chamber, and out
through the vacuum pump. The gas is usually oxygen,
hydrogen, or a mixture of oxygen, hydrogen, and an inert
gas. Next, electrical power is supplied to circuitry
which creates a strong electromagnetic field in the
plasma generators. This field interacts with the gases
l0 flowing through the plasma generators and generates a
glow discharge.
In the discharge, charged particles are
accelerated by the field and gain kinetic energy. When
these particles collide with other particles, energy
transfer may result in the formation of more charged
particles (ions and electrons) or excited atoms or
molecules. Molecules may be broken into fragments such
as atoms or radicals. The particles created in the
collisions may also react with each other or with the
2o feed gas to form yet more products. Light is also
generated as particles in high energy states decay to
lower energy states.
The apparatus is designed to confine the
electromagnetic field which generates the plasma within
the plasma generators. When the gas flows out of the
plasma generators into the gas distribution system,
where the field is vanishingly small, the acceleration
of charged particles and the production of ions and
electrons ceases. The most reactive of the components
created in the discharge relax or react quickly, and the
charged particles formed in the discharge recombine
rapidly to form electrically neutral particles. The gas
distribution system of the apparatus is designed so such
recombination and relaxation processes will be essen-
tially completed before the gas enters the sterilization
CA 02224118 1997-12-OS
w0 96/39199 PCT/US96/09150
7
chamber. Thus, relatively nonreactive components enter
the sterilization chamber. These include the active
species which accomplish sterilization. The active
species are active in that they themselves, or other
species formed by their interactions with other
components present, can interact with microorganisms and
inactivate them. However, the active species are not so
reactive that they will be substantially depleted by
reactions with or on surfaces they may encounter en
route to a microorganism.
Plasma generation and sterilization are
generally performed at reduced pressures, usually on the
order of 0.1 to 100 torr. The lower end of the reduced
pressure range is usually determined by the size (and
cost) of the vacuum pump, whereas the upper end is
usually determined by processing time considerations
because the rate at which microbes are killed typically
decreases as the pressure increases. It is possible
that higher pressures could be used with more efficient
2o plasma generators or with improved gas distribution
systems. The vacuum pump is used to maintain these low
pressures during the sterilization process. When the
gas flows into the vacuum pump, it is compressed before
being exhausted. The compression and concomitant
heating accelerate the reactions of the active species
to form innocuous compounds such that the pump's exhaust
stream contains only nontoxic components.
The term "sterilization" connotes a process by
which all viable forms of microorganisms are destroyed
or removed from an object. In practice, it is
impossible to make an absolute determination of the
destruction or removal of microorganisms, so it has
become customary to define sterility in terms of
"probability of survivors." The practical goal of a
sterilization process is therefore measured as a
CA 02224118 2005-07-22
8
probability ( a . g . , 10-', 10-6, 10-'~ ) , the probability
indicating the lethal effect of a particular sterilizing
dose or regimen. It is usual to assume increased time
of exposure to a set of sterilizing conditions will
decrease the probability of survivors accordingly.
Doubling the sterilizing time of identical conditions
would result in a squaring of the probability term, for
example 10-6 would become 10-'~ .
Broadly, the present invention can be viewed
to as essentially requiring a plasma generator, a steriliz
ing chamber, and a source of water vapor in fluid
communication with the plasma generator. Although
particularly preferred apparatus for practicing the
invention is illustrated by U.S. Patent 5,115,166 (and
by U.S. Patent 5,184,046), it should be
understood that variations in the preferred apparatus
components are within the scope of this invention. For
example, U.S. Patent 5,244,629, issued September 14,
1993, describes a sterilization treatment in which
describes a sterilization treatment in which
the article to be sterilized is alternatively exposed to
an anti-microbial agent and to the neutral active
species of a gas plasma.
Turning to Fig. 1, a top view of a first
plasma sterilizer embodiment suitable for use with this
invention is illustrated. The plasma sterilizer has one
or more means for generating plasma 2 (as illustrated by
this embodiment, there are three plasma generating tubes
3o 10, 12, 14) and a sterilizing chamber 4. The plasma
generating means 2 comprises an electromagnetic field
generator, such as a magnetron 6 and a waveguide 8 which
directs the electromagnetic field. This gas plasma is
generated from the water vapor, as is hereinafter
further described and exemplified.
CA 02224118 1997-12-OS
WO 96!39199 PCT/US96/09150
9
The water vapor, which is the plasma source
gas, is directed into plasma generating tubes 10, 12,
and 14 by gas delivery lines 16, 18, and 20 leading from
the flow controller 22. The operation of flow
controller 22 is controlled by a central processing unit
(CPU) 28 by standard procedures. Flow controller 22 and
CPU 28 can be any of the conventional, standard devices
used for gas flow control in plasma generating
equipment.
1o The water vapor can originate from water
reservoir 24, which is in fluid communication with the
flow controller 22. It is important to prevent
condensation of the vapor in the piping between the
water reservoir 24 and the inlets 26, 16, 18, and 20 to
the means for plasma generation 2. Condensation
prevention is well known to persons skilled in the art,
and typically simply requires that the temperature in
the piping is kept high enough to maintain the vapor
pressure in the piping along the fluid flow path.
2o Water vapor may be generated by various
methods. For example, liquid water can be flowed from
a source through a metering valve and into a vaporizer.
In the vaporizer, water can be flowed over heated
surfaces and evaporated, with the water vapor then being
flowed into the plasma generators. Alternatively, the
flow of water vapor itself can be regulated, as
illustrated by the above described embodiments.
The sterilizing chamber 4 may comprise top
plate 30, side plates 32 and 34, bottom plate 36 (not
shown), back plate 37, and front sealing door 38 through
which articles or materials to be sterilized are placed
in the chamber. The plates are shown attached together
in a sealed relationship to form a vacuum chamber, such
as by welding. The door 38 is secured in a sealed
relationship with the sterilizing chamber.
CA 02224118 1997-12-OS
WO 96/39199 PCT/US96/09150
Thus, plasma generation occurs in one chamber,
and the activated gas is then fed via an indirect
passageway to the sterilizing chamber. This indirect
passageway is of a construction sufficient to prevent
5 direct impingement of nascent plasma generated in the
plasma generating chamber onto the article being
sterilized. This indirect passageway may be constructed
from a restrictor and a plasma distributor.
The plates and door of the sterilizing chamber
l0 can be made of any material having the strength required
to withstand the external atmospheric pressure when the
chamber is evacuated. Stainless steel or aluminum
plates and door can be used. The internal surface
material of the chamber may affect the concentrations)
of killing species available in the chamber. One useful
material is pure (98~) aluminum which can be applied
either as a liner or as a flame-sprayed coating on all
internal walls of the stainless steel chamber. An
alternate material is nickel. However, we prefer to
2o coat the chamber interior with an inert polymer coating
(e. g. PTFE).
The gases are exhausted from the sterilizing
chamber through exhaust outlet port 42 to a conventional
vacuum pump system (not shown).
Fig. 2 is a top cross-sectional view of the
plasma sterilizer embodiment of Fig. 1. Each of the
plasma generators 10, 12, and 14 comprise an inlet cap
44 with a gas inlet port 48 leading to a respective
plasma generator tube 51, 52, or 53 leading through the
3o waveguide 8. In the waveguide 8, the water vapor in
tubes 51, 51, and 53 is energized and converted to a
plasma.
The plasma generator tube directs the plasma
flow into the gas distribution tubes 54, 56, and 58 from
which the plasma is fed into the evacuated sterilizing
CA 02224118 1997-12-OS
WO 96/39199 PCT/US96/09150
11
chamber 60. The preferred plasma generating tubes and
plasma distributing tubes are made of quartz. However,
any other materials with the necessary physical,
chemical, and electrical properties for plasma genera-
tion in an electromagnetic field can be used for the
plasma generating tubes. Similarly, the conduits and
tubing used for transport of plasma from the plasma
generator to the sterilizing chamber can be any solid
material which has the requisite shape and strength and
1o which is resistant to chemical action and degradation
by
the plasma gases. Suitable transport conduit materials
include quartz and other plasma corrosion resistant
glasses, stainless steel and other oxidation resistant
metals, and oxidation resistant plastics such as
fluorocarbon polymers, e.g. PTFE and the like, and
siloxane polymers.
In a particularly preferred embodiment the
plasma is fed into a plenum portion of chamber 60, with
the plenum defined by a perforated lower plenum plate,
or baffle plate (not illustrated). The plenum plate can
be made of any of the materials used for constructing
the sterilizing chamber 60, with PTFE coating on metal
being a preferred embodiment. The size, number, and
placement of the perforations in the plenum plate may
vary. The size and distribution of holes in the plate
should provide sufficiently uniform gas flow through the
sterilization chamber and may also be chosen to keep
residual electromagnetic energy from entering the
sterilization chamber.
3o The plasma generator tubes are enclosed in
tubular metal cooling tubes 62 and 64. The caps 44 and
the cooling tubes 62 and 64 are preferably provided with
groves or cooling fins (not shown) in a conventional
manner to increase their efficiency in removing heat
from plasma generator tubes. The length and inner
CA 02224118 1997-12-OS
WO 96/39199 PCT/US96/09150
12
diameter of the cooling tubes are chosen to attenuate
the electromagnetic field in the waveguide to keep
microwave energy from entering the gas distributor and
sterilization chamber. As a consequence, the only
places where the field is strong enough to generate a
plasma are within the plasma generating tubes in regions
within or near the waveguide. The distal ends of the
gas distribution tubes 54, 56, and 58 are supported by
spring-biased end supports 66 mounted on sideplate 32,
but could be modified for gas distributor plenum
designs, as known in the art.
The door 38 is held in sealing engagement by
atmospheric pressure against the O-ring seal 40 mounted
in the flange 41 extending from the side plates 32 and
34, and the top and bottom plates 30 and 36 (not shown).
Optionally, additional conventional closure clamp or
latch devices can be used to insure closure of the door
before chamber evacuation is initiated.
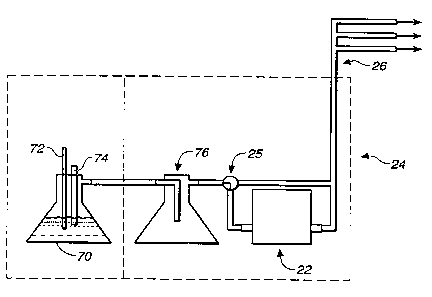
Turning to Fig. 3, a schematic detail of a
water reservoir 24 is illustrated, as was used for
experiments hereinafter more fully described. A
container 70 capable of withstanding vacuum was
partially filled with distilled or deionized water. A
thermocouple 72 and an electric heater 74 were immersed
in the water. The thermocouple 72 and heater 74 were
connected to a control unit (not illustrated) which
regulated electrical power to the heater to maintain the
temperature of the water at 50°C. The container 70 was
placed in an insulated chamber which was held at 50°C.
A tube ran from the container to the flow controller 22
in a second chamber, which was held at 60°C. A
condensation trap 76 was placed in the line between the
flow controller 22 and the container 70 to prevent any
condensation from reaching the flow controller 22. A
three-way valve 25 was inserted in the line between the
CA 02224118 1997-12-OS
WO 96/39199 PCT/US96109150
13
trap 76 and the flow controller 22 to allow vacuum to be
applied directly to the upstream side of the flow
controller 22 to aid in removing any accumulated
condensation during maintenance operations. The flow
controller 22 need not be electronic, but could be a
variety of flow control means, such as a needle valve,
and the trap 76 is preferred but optional.
The embodiment described above has been
presented with three plasma generating units. The
1o number of generating units is not critical, being
selected to provide a good plasma distribution in the
particular sterilizing chamber used. Any desired number
of plasma generators can be used with each sterilizing
chamber and are intended to be included within the scope
of this invention. It will be also be readily apparent
that any number of gas plasma tubes can be positioned to
interact with the electromagnetic field generated from
a single magnetron and that various waveguide
configurations can be used to achieve this effect.
2o Turning to Fig. 5, a side sectional view of
the plasma sterilizer according to another embodiment of
the invention is illustrated. The plasma sterilizer
comprises one or more plasma generators 12' connected to
a sterilizing chamber 4'. In a preferred embodiment,
there are three plasma generators mounted on top of the
sterilizing chamber to provide a uniform and adequate
distribution of sterilizing gas mixture into the
sterilizing chamber.
Fig. 6 is a detailed, sectional view of the
3o plasma generator. Each plasma generator 12' comprises
a housing 62' that is mounted onto a top portion of the
sterilizing chamber. The housing supports a plasma
generator tube 52' that is preferably a quartz tube
transparent to microwave. One end of the plasma
generator tube 52' is coupled to a gas inlet 48' for
CA 02224118 1997-12-OS
WO 96/39199 PCT/US96/09150
14
receiving a gas or gas mixture from outside the housing
62'. The other end of the plasma generator tube is
coupled to an outlet manifold 49' at the bottom of the
housing that allows the gas mixture to flow from the '
generator tube to the sterilizing chamber 4'.
The housing 62' also supports a waveguide 8',
a portion of which intersects the plasma generator tube
52'. The waveguide serves to transmit microwave energy
from a microwave source, such as a magnetron 6', to the
to portion of the plasma generator tube inside the
waveguide. Typically, the plasma generator tube is
positioned at a crest of the standing waves in the
waveguide. The initiation of the plasma is facilitated
by a striker 102' near the gas inlet. The striker is
connected to a high voltage source (not shown). In this
way, the gas mixture that flows through the plasma
generator tube is energized in the waveguide and
converted into a plasma.
The housing 62' and the waveguide 8' assembly
are preferably constructed out of a good conductor such
as aluminum and designed to minimize microwave leakage
outside the assembly. The housing is also able to
establish good thermal contact with the plasma generator
tube so that it can dissipate heat generated in the
plasma. In a preferred embodiment, cooling fins 104'
near the top portion of the housing help to improve heat
dissipation.
As the gas mixture flows through the generator
tube it is converted into a nascent plasma. It then
exits via the outlet manifold into the sterilizing
chamber. During that passage the nascent plasma is
transformed into a cooler gas mixture of essentially
neutral species. The conversion is facilitated by
routing the gas mixture through a restrictor 99' and the
outlet manifold 49'.
CA 02224118 1997-12-OS
WO 96/39199 PCT/US96/09150
The restrictor 99' helps to define the plasma
' generating tube 52' as a plasma generating chamber
distinct from the sterilizing chamber 4'. In a
- preferred embodiment, the restrictor is formed by a
5 special termination of the plasma generator tube. Near
the outlet manifold 49 ' , the generator tube is formed
into a dual-wall tube, with the inner tube terminating
into a smooth surfaced venturi restriction 96' of
reduced cross-sectional area. The outer wall has the
10 same cross-sectional area as the rest of the generator
tube. An O-ring 106' around the dual wall portion
secures the plasma generator tube in sealed engagement
with the housing near the outlet manifold. The dual-
wall construction has the advantage of insulating the O-
15 ring from the heat of the plasma flowing in the inner
tube.
The restrictor 99', by virtue of its reduced
aperture, serves several important functions. First,
different optimal pressures can be maintained in the two
different chambers. Typically, the plasma generating
chamber is maintained at a higher pressure than the
sterilizing chamber, the former being optimized for
plasma generation and sustenance and the latter being
optimized for uniform dispersion of the sterilizing gas.
Secondly, and especially in combination with the outlet
manifold, the restrictor increases the probability of
plasma components colliding into a surface. This
physical structure thus promotes the conversion or
recombination of charged particles in the plasma into
3o neutral species. Thirdly, harmful ultra-violet ("UV")
r
radiation generated in the plasma in the generator tube
only has a small opening through which to escape into
the sterilizing chamber.
Referring back to Fig. 5, the sterilizing
chamber 4' comprises a sterilizing enclosure 37' with a
CA 02224118 1997-12-OS
WO 96/39199 PCT/US96/09150
16
sealing door 38'. An inlet port 112' at the top of the
enclosure is coupled to the outlet manifold 49' of the
plasma generator. An exhaust port 114' at the bottom of
the sterilizing enclosure is coupled to an external
vacuum pump system (not shown). The articles to be
sterilized may be placed in baskets 116' inside the
sterilizing enclosure. Alternatively, a perforated
exhaust panel may be mounted across the enclosure and
above the exhaust port to form a platform for supporting
to articles to be sterilized. Near the top of the
sterilizing enclosure and below the inlet port 112', a
perforated gas distribution panel 118' is mounted to
distribute the gas mixture including the neutral species
entering through the inlet port 112' uniformly
throughout the sterilizing enclosure. The perforated
gas distribution panel is preferably made of rigid and
inert material, such as PTFE, tempered glass, stainless
steel or stainless steel coated with PTFE. Furthermore,
the material should be opaque to Uv light. In general,
the perforated gas distribution panel 118' should
comprise a self-supporting structure, the main function
of which is to distribute the in-flowing gas mixture in
a uniform manner into the sterilizing enclosure 37'. In
this embodiment, the structure from the base of the
plasma generator tube 52' to the perforated gas
distribution panel 118' can be regarded as forming the
outlet manifold 49'.
A second inlet port 39' on a side wall of the
sterilizing enclosure allows antimicrobial additives to
3o be introduced as a vapor or liquid from an external
source (not shown).
During operation of the plasma sterilizer, the
nascent plasma generated in the plasma generating tube
52' emerges via the outlet manifold 49' into the
sterilizing chamber 4'. As described before, virtually
CA 02224118 1997-12-OS
w0 96!39I99 PC'f/US96/09150
17
all of the charged particles generated in the plasma are
converted into neutral species as they pass through the
restrictor 99' and the outlet manifold 49' (see also
Fig. 6). Similarly, the Uv radiation generated in the
plasma is greatly reduced by the restrictor 99' and the
outlet manifold 49'. Thereafter, the gas mixture is
made to negotiate through the perforated gas
distribution panel 118' before it enters the sterilizing
chamber 4' and acts on the article 120' to be
1o sterilized. In a preferred embodiment a disk 122' made
of inert material such as PTFE or glass is placed on the
top surface of the perforated gas panel directly below
the inlet port 112'. The perforated gas distribution
plate, especially in combination with the disk 112',
serves several functions. Primarily, it cools and
disperses the gas mixture uniformly in the sterilizing
enclosure. Secondly, it helps to block what little
remaining UV radiation that may. have been admitted into
the outlet manifold 49'. Finally, it provides an
2o additional surface for conversion of the few remaining
charged particles in the gas mixture to neutral species.
In this manner, by the time the gas mixture reaches the
article to be sterilized, the main sterilizing agent
contained therein is essentially neutral species
generally devoid of undesirable charged particles and UV
radiation.
The apparatus of this invention generates a
sterilizing species derived from water vapor, as
exemplified hereinafter. The sterilization is carried
out in the sterilization chamber at a vacuum pressure of
a
from about 0.1 to 150 torr and preferably from about 0.1
to about 40 torr. The temperature in the sterilizing
chamber is maintained below 100°C, and preferably is
from about 35°C to about 82°C. Under these conditions,
effective sterilization is effected without significant
CA 02224118 1997-12-OS
WO 96/39199 PCT/US96/09150
18
deterioration of packaging materials in which articles
to be sterilized may be placed.
The method of this invention for plasma
sterilization comprises exposing an article to be
sterilized to a plasma generated from water vapor at
temperatures of less than 100°C, a pressure of from 0.1
to 150 torr, and an effective treatment time. The
treatment time efficacy will vary depending on the
article being sterilized, whether or not it is wrapped
or packaged, and the type of wrap or packaging. For
instance, articles with no lumens or no relatively
inaccessible features can be sterilized more rapidly
than those articles with lumens. Articles with several
layers of wrapping may require more time than those with
a single layer of wrapping. Effective treatment times
may be determined empirically.
In an optimum method of sterilizing, the
articles to be sterilized are placed in the sterilizing
chamber, supported in conventional fixtures (e. g. wire
2o baskets) which permit the activated gas to reach all
surfaces of the articles. The chamber is closed, the
sterilizing chamber is evacuated, plasma generation is
initiated, and the activated gas is directed into and
through the sterilizing chamber.
when the activated gas is compressed and
heated in the vacuum pump, the activated species in the
gas react very quickly to form non-toxic gases which may
be exhausted with minimal treatment.
Plasma sterilization apparatuses of the
invention are readily adapted to perform other
sterilizing cycles in addition to plasma sterilization,
particularly such as steam sterilization (autoclaving).
With reference to Fig. 4, another embodiment
100 of the invention is schematically illustrated where
a means for plasma generation 102 is in communication
CA 02224118 1997-12-OS
WO 96!39199 PCT/US96/09150
19
with sterilizing chamber 104 with valve 103 interposed
so as to control communication of plasma generated in
the plasma generator 102 from the sterilizing chamber
104. The source of the water vapor is water reservoir
124. water flows into heater 126 and is converted into
water vapor. Heater 126 is controlled by temperature
controller 156. Water vapor then flows through valve
130, flow controller 122, and into plasma generator 102,
where it is converted into a plasma. The activated gas
then flows through valve 103, through sterilizing
chamber 104, through valve 132, and out through vacuum
pump 140. Alternatively, steam can be generated by
adjusting heater 126 to heat the water to a much higher
temperature. Steam would then flow through valve 128
into chamber 104, with valves 103 and 130 closed.
Still with reference to Fig. 4, in operation
of this embodiment as a steam autoclave, valves 103,
128, 130, 132, 134, and 148 would be initially closed.
An article to be sterilized 136 would be loaded into
2o chamber 104 and the door 138 closed and sealed. One
could, if desired, open valve 132 to evacuate air from
the chamber 104 through vacuum pump 140 while monitoring
chamber pressure on gauge 142. Then valve 132 would be
closed and valve 128 and 134 would be opened. Steam
generated by heater 126, adjusted to an appropriate
temperature by controller 156, would flow through valve
128 and into the sterilizing chamber 104. The
temperature of the system would be monitored by sensor
144 in the drain line 158, and thermostat valve 146
would regulate the temperature (and pressure) of steam
in the system. After a prescribed time, valves 128 and
134 would be closed and valve 132 would be opened.
Steam would be evacuated from the sterilizing chamber
104 through vacuum pump 140. Then valve 132 would
close, valve 148 would open, and the chamber would be
CA 02224118 1997-12-OS
WO 96/39199 PCT/US96/09150
brought to atmospheric pressure with sterile air. Door
138 would be opened and the sterilized article 136 would
be removed.
In the operation of this embodiment as a
5 plasma sterilizer, valves 103, 128, 130, 132, 134, and
148 would be initially closed. An article to be
sterilized 136 would be loaded into chamber 104 and the
door 138 would be closed and sealed. Valves 103 and 132
would then be opened and air would be evacuated from the
1o sterilizing chamber 104 and the plasma generator 102
through vacuum pump 140. Then valve 130 would open and
the plasma generator 102 would be activated. Water
vapor generated by heater 126, adjusted to an
appropriate temperature by controller 156, would flow
15 through valve 130 and flow controller 122 into plasma
generator 102, where it would be converted into a
plasma. The activated gas would flow through valve 103
into a gas distribution manifold 154, through the
sterilizing chamber 104, and out through valve 132 and
2o vacuum pump 140. After a prescribed period of time,
valve 130 would be closed, the plasma generator 102
would be turned off, and remaining gases would be pumped
out of the sterilizing chamber and plasma generator.
Then valves 132 and 103 would be closed. Valve 148
would open, and the chamber would be brought to
atmospheric pressure with sterile air. Door 138 would
be opened and the sterilized article 136 would be
removed.
Such a multiple function sterilizing apparatus
100 provides increased operating flexibility since
plasma sterilization or steam sterilization can be
accomplished with the same apparatus.
CA 02224118 1997-12-OS
WO 96139199 PCT/US96/09150
21
EXAMPLE 1
Biological indicators are characterized
preparations of specific microorganisms resistant to a
particular sterilization process. They are used to
assist in the qualification of the physical operation of
sterilization apparatus and to validate a sterilization
process for a particular article. In this example, the
biological indicators were Bacillus circulars spores
inoculated onto filter paper carriers and sealed in
1o pouches made of porous polyethylene (Tyvek) and Mylar.
Packages for the biological indicators were obtained
from Baxter Laboratories as "Plastipeel Pouches."
Filter paper disks (1/4 inch diameter Schleicher &
Schuell 740E) were used as carriers for spores. Each
disk was inoculated with about 2 x 106 spores of B.
circulars.
The biological indicators were placed into the
sterilizer apparatus and exposed to a plasma cycle.
During the plasma cyc 1e , water vapor was f lowing at a
2o rate of about 0.8 standard L/min. The sterilization
chamber pressure was about 0.2 torr.
After exposing the biological indicators to
the sterilizing water vapor treatment for 78 minutes,
the indicators were removed and tested for sterility.
The paper disks were aseptically transferred
into growth medium (tryptic soy broth) and incubated at
37°C for 7 days. No growth was observed, indicating
that all the bacteria spores on the disks were
inactivated.
CA 02224118 1997-12-OS
WO 96/39199 PCT/US96/09150
22
EXAMPLE 2
Three pieces of 14 gauge stainless steel
tubing were inoculated with about 2 x 105 B. circulars -
spores each. Each piece was placed in the center of a
length of polyvinyl chloride tubing 1.8 m long and 1.27
mm ID (Tygon tubing, made by Norton Performance Plastics
Corp.). Each piece of PvC tubing was coiled and sealed
in a Tyvek/Mylar sterilization pouch, as described in
Example 1. The three assemblies were placed in the
sterilizer and treated for six hours. The inoculated
stainless steel pieces were recovered, transferred into
growth medium, and incubated for three days. No growth
was observed.
EXAMPLE 3
The plasma sterilization system was equipped
with a temperature control system which caused the
electrical power to the magnetrons to be interrupted
when a thermocouple in the sterilization chamber reached
a temperature of 180°F. The power was restored when the
temperature dropped to about 177°F. This modification
is similar to the one put forth in U.S. Patent
5,186,893, issued February 16, 1993.
The tests were run twice. Between the tests,
a slight modification was made to the exhaust line
between the sterilizing chamber and the vacuum pump. As
a result, the pressure in the sterilizing chamber for
the second test was slightly higher than for the first
(200 mTorr v. 170 mTorr).
The articles sterilized were "test packs"
similar (but not identical) to those used to qualify
ethylene oxide sterilization processes. The packs were
constructed as follows: Disks of filter paper about 5
CA 02224118 1997-12-OS
WO 96!39199 PCT/US96/09150
23
mm in diameter were inoculated with about 2x106 Bacillus
circulans spores. Ten disks were placed in two
Tyvek/Mylar sterilization pouches (five disks per pouch)
and the pouches were sealed using a heat sealer. Each
pouch was placed in the barrel of a plastic 20 cc
syringe with the Tyvek side facing the inside of the
syringe barrel. The syringe plungers were inserted but
did not touch the pouches. There were no tip caps on
the ends of the syringes. The syringes were placed in
the center of a stack of two Huck towels. (A "Ruck"
towel is a woven cotton towel commonly used in hospital
procedures.) An oral airway was placed on one side of
the syringes and a 10 inch piece of latex tubing was
placed on the other side of the syringes. Two more Huck
towels were placed on the instruments. This stack was
then wrapped in two pieces of cotton/polyester
sterilization wrapping material, 27 in. by 27 in. each.
The pack was placed in the sterilization
chamber and the thermocouple was placed under the top
fold of the outermost wrapper. The chamber was sealed
and the vacuum pump was activated. The chamber was
evacuated for about 20 minutes to allow this rather
absorptive load to outgas. The water vapor flow was
started and the plasma was initiated. The treatment
lasted for four hours in all. The thermocouple reached
its setpoint (180F) about 85 minutes after the plasma
was started; in all, the plasma was on about 185 minutes
during the four hour exposure.
After exposure, the packs were disassembled
and the disks were transferred into growth medium. No
growth was observed after either test.
It is to be understood that while the
invention has been described above in conjunction with
CA 02224118 1997-12-OS
WO 96/39199 PCT/US96/09150
24
preferred specific embodiments, the description and
examples are intended to illustrate and not limit the
scope of the invention, which is defined by the scope of
the appended claims.