Note: Descriptions are shown in the official language in which they were submitted.
CA 02225760 1997-12-23
IDAOKGR4uHD OF THE INVENTION
Field of the Invention
The invention is generally directed to medical catheters
and more specifically to the placement of catheters into a
patient.
Descrintion of Related Art
Catheters are commonplace in the medical field, finding
importance in a variety of uses. The term ~catheter" is commonly
used to identify a tubular instrument that is inserted into a body
cavity or orifice, naturally or surgically opened. Vascular
catheters, for example, come in many different forms and have many
different uses. A vascular catheter typically consists of a hub
and tubing or cannula through which fluid flows. The hub may
include a flash chamber that allows the individual placing the
catheter to see blood in the flash chamber that indicates the
catheter has entered a vein. There are different types of
vascular catheters. The primary types include the short
peripheral, which is typically placed only a short distance (e.g.,
2-3 inches) in a vein in the hand or arm cf the patient. There
are also venous catheters that are longer and include a midline
catheter that is placed approximately 6-8 inches in the vein of a
patient, and a peripherally inserted central catheter ("PICC")
which is placed peripherally, e.g., in the arm of a patient, and
fed a significant distance, e.g., to the superior vena cava.
Still another type of vascular catheter is the c=ntral venous
-1- 056301.P004
CA 02225760 1997-12-23
catheter which is typically placed in the internal jugular or
subclavien vein, implanted under the skin, tunneled underneath the
skin, etc.
One common method of placing a vascular catheter in a
patient is through an introducer. In this case, an introducer
including a needle, is placed in a patient and into a vein. The
needle is then removed, and the catheter is inserted through the
introducer into the vein.
A second method of placing a catheter in a patient,
often referred to as an Over-The-Needle ("OTN") insertion places
the catheter cannula directly over the needle used to introduce
the catheter into the vein. The needle is typically connected to
a wire or stylet that allows the needle to be removed from the
distal or hub end of the catheter cannula once the proximate end
of the catheter cannula is placed in the vein. with the needle
removed, the catheter is advanced in the vein and placed where
desired. The OTN catheter permits the use of a smaller outside
diameter needle for a given size outside diameter catheter
cannula. The clinical benefit of the OTN approach is the
maximization of the size of the catheter placed compared to the
size of the needle used. A smaller needle means one can more
easily access veins and impart less pain to the patient.
Figures 1 and 2 illustrate the different types of
vascular catheter introduction methods. Figure 1 illustrates a
Through-The-Introducer ("TTI") insertion method and Figure 2
illustrates an OTN insertion method. In the TTI type insertion,
an introducer sheath 20 and needle 30 are inserted into the vein
-2- 056301.P004
CA 02225760 1997-12-23
of a patient. Once inserted, the needle 30 is removed, and the
catheter cannula 10 is advanced through the introducer sheath 20.
When the catheter cannula is placed through the introducer, the
maximum outside diameter of the catheter cannula that is placed is
slightly smaller than the inside diameter of the introducer, or,
slightly smaller than the outside diameter of the needle. In
Figure 1, for example, at least a 19 gauge needle 30 is needed to
place a 20 gauge catheter cannula 10.
In the OTN introduction method, a needle 40 is inserted
into a patient, the needle 40 being inside a catheter cannula 10
and having a wire or stylet 50 coupled to a hub 60 at the distal
end of the catheter cannula 10. Once the needle 40 and a portion
of the catheter cannula 10 are inserted into the patient, the
needle 40 is removed from the patient by pulling the hub 60 away
from the patient. Once the needle 40 is removed, the catheter
cannula 10 is advanced to the desired placement in the patient.
Figure 2 illustrates that a smaller outside diameter needle, e.g.,
22 gauge, may be used to place a larger outside diameter catheter
cannula, e.g., 20 gauge.
-3- 056301.P004
CA 02225760 1997-12-23
SUMMARY OF THE INV$NTION
A catheter, catheter kit, and a method of placing a
catheter is disclosed. The catheter includes a substantially
tubular body portion having an outside diameter no less than the
inside diameter of an opening in a catheter introducer adapted to
insert a portion of the catheter into a patient's body. The
catheter also includes a transition member portion adjacent to a
first end of the tubular body portion. The transition member
portion has an outside diameter that is less than the outside
diameter of the tubular body portion and less than the inside
diameter of the opening in the catheter introducer. The invention
contemplates that the transition member can be coupled to the end
of the tubular body portion, integrally formed with the body
portion, or extend through the introductory end of the body
portion and be removable from a distal end of the body portion. A
benefit of the catheter including a transition member portion
having an outside diameter that is less than the inside diameter
of the opening in the catheter introducer is that it allows a
method for placing a TTI catheter which is larger than the inside
diameter of the introducer, by first placing the transition member
portion through the introducer. This provides the benefit of OTN
insertion with regard to needle size, but allows for a larger
catheter to be placed in the vein by the subsequent removal of the
introducer once the transition member portion is placed in the
vein.
-4- 056301.P004
CA 02225760 1997-12-23
Additional features and benefits of the invention will
become apparent from the detailed description, figures, and claims
as set forth below.
-5- 056301.P004
CA 02225760 1997-12-23
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 is a planar side view of a through the
introducer catheter introduction configuration.
Figure 2 is a planar side view of an OTN catheter
introduction configuration.
Figure 3 is a planar side view of the catheter of the
invention with a TTI configuration.
Figures 4-9 illustrate the method of the invention of
placing a catheter into a patient wherein the transition member
portion of the catheter is integral or coupled to the end of the
tubular body portion of the catheter.
Figure 10 illustrates a planar cross-sectional view of
the transition member portion of the catheter of the invention
taken through line A-A of Figure 9 wherein the transition member
portion expands to substantially match the cross-sectional area of
the tubular body portion of the catheter.
Figure 11 illustrates a perspective side view of the
catheter of the invention including a transition member portion
wherein the transition member portion expands to substantially
match the diameter of the tubular body portion of the catheter.
Figure 12 illustrates a planar cross-sectional view of
the transit.ion member portion of the catheter of the invention
taken through line A-A of Figure 9 wherein the transition member
portion unfolds to substantially match the cross-sectional area of
the tubular body portion of the catheter.
-6- 056301.P004
CA 02225760 1997-12-23
Figure 13 is a perspective side view of the catheter of
the invention wherein the transition member portion is folded to
reduce the cross-sectional area of that portion of the catheter.
Figure 14 illustrates a perspective side view of the
catheter of the invention wherein the transition member portion is
in an unfolded state to substantially match the cross-sectional
area of the tubular body portion of the catheter.
Figurea 15-20 illustrate a method of inserting the
catheter of the invention into a vein of a patient wherein the
catheter includes a transition member portion that is separate
from the tubular body portion of the catheter and can be removed
after or during placement of the tubular body portion.
Figure 21 is a planar side view of an embodiment of the
catheter of the invention wherein the transition member portion is
comprised of a solid material.
Figure 22 is a planar side view of the catheter of the
invention wherein the transition member portion is transitioned in
steps of decreasing diameter or cross-sectional area.
Figure 23 is a planar side view of an embodiment of the
catheter of the invention wherein the transition member portion is
a metal or synthetic wire.
Figure 24 is a planar side view of an embodiment of the
catheter of the invention wherein the transition member portion is
a braided or twisted wire.
-7- 056301.P004
CA 02225760 1997-12-23
DETAILED DESCRIPTION OF THE INVENTION
The invention relates to a catheter, a catheter kit, and
a method of placing a catheter that includes a substantially
tubular body portion and a transition member portion. The
substantially tubular body portion has an outside diameter no less
than the inside diameter of an opening in a catheter introducer
adapted to insert a portion of the catheter into a patient's body.
The transition member portion is positioned adjacent to a first
end of the tubular body portion. The transition member portion
has an outside diameter that is less than the outside diameter of
the tubular body portion and less than the inside diameter of the
opening in the catheter introducer. The method includes placing
an introducer into a patient, providing a catheter with a
substantially tubular body portion and a transition member
portion, inserting the transition member portion through the
opening in the body of the introducer, removing the introducer
from the patient, and placing a portion of the tubular body
portion of the catheter into the patient. In the following
detailed description, specific embodiments of the invention are
described with reference to particular materials. Numerous
specific details are set forth such as specific materials,
configurations, and methods of placing a catheter. It will be
obvious, however, to one skilled in the art, that these specific
details need not be employed to practice the invention. In other
instances, well known materials or methods have not been described
in detail in order to avoid unnecessarily obscuring the invention.
-8- 056301.P004
CA 02225760 1997-12-23
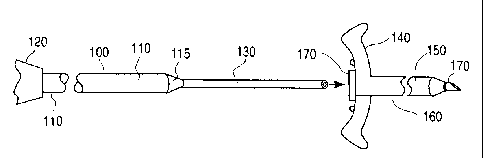
Figure 3 illustrates a planar side view of the catheter
configuration of the invention. Figure 3 shows a catheter 100
made up of a substantially tubular body portion 110 and a
transition member portion 130. The substantially tubular body
portion or cannula 110 is coupled to a hub 120 at the distal end
of the catheter cannula 100. The catheter 100 is configured to be
placed in a patient through an introducer 160. The introducer is
comprised of handles 140 and a sheath 150. The introducer 160
further includes a removable needle 170. To place the catheter
100 in a patient, the introducer 160 and needle 170 are first
placed in the desired location, e.g., the vein, of a patient in a
conventional manner. Next, the needle 170 is removed from the
distal end of the introducer 160, leaving only the introducer
sheath 150 in the patient. Next, the catheter of the invention is
inserted through the introducer sheath 150 into the vein. As
noted, the catheter 100 includes a transition member portion 130,
that has an outside diameter that is less than the inside diameter
of the opening in the introducer sheath 150. The transition
member portion 130 of the catheter 100 is fed into the vein
through the introducer 160. Once the transition member portion
130 is securely placed through the introducer 160 and into the
vein, the introducer 160 is removed from the patient and peeled
apart or divided and separated from the location near the patient
as is common in the prior art. It should be evident that the
division of the introducer 160 can be accomplished while the
introducer 160 is being removed, for example, to accommodate a
shorter transition member portion 130. The tubular body portion
-9- 056301.P004
CA 02225760 1997-12-23
110 of the catheter may then be advanced into the patient by
following transition member 130 through the opening made by the
needle 170 and the introducer sheath 150. To aid insertion of the
tubular body portion 110, the tubular body portion 110 optionally
includes a tapered leading edge 115 that gradually opens the
existing cut made by the needle 170 in the patient's skin.
The cut made by needle 170 in a patient is large enough
to insert the introducer sheath 150, therefore the invention
contemplates that the cut will be large enough to accommodate the
tubular body portion 110 of the invention. The tubular body
portion 110 of the invention has an outside diameter that is no
less than the inside diameter of the opening in the catheter
introducer 160 or no less than the outside diameter of the needle
170. In one embodiment, the outside diameter or gauge of the
tubular body portion 110 of the catheter cannula 100 is
approximately the same as the outside diameter of the introducer
sheath 150 or the needle 170. In another embodiment, the
invention takes advantage of the elastic nature of the skin. The
elasticity of the skin allows an opening in the skin, i.e., a
needle cut, to stretch approximately 2 gauge sizes to accept an
object of a larger diameter than the opening. The invention
contemplates, for example, that a cut made by a 22 gauge needle to
insert a catheter can accept a 20 gauge catheter using the
technique described by the invention.
Figures 4-9 illustrate a method of inserting the
catheter of the invention wherein the transition member portion
130 of the catheter is integral to or coupled to the end of the
-10- 056301.P004
CA 02225760 1997-12-23
tubular body portion 110 of the catheter 100. First, as shown in
Figure 4, a needle 170 and introducer 160 including an introducer
sheath 150 are placed through the skin 200 of a patient and into a
vein 210. Once properly located in the vein 210, the needle 170
is removed from the introducer 160 through the introducer body
140. Next, as shown in Figure 5, the catheter 100 is placed in
the vein 210. The catheter 100 of the invention includes a
transition member portion 130 with an outside diameter that is
less than the outside diameter of the tubular body portion 110 of
the catheter and less than the inside diameter of the introducer
sheath 150. For example, the invention contemplates that for a 22
gauge introducer sheath, the invention contemplates that the
transition member portion 130 of the catheter 100 has an outside
diameter smaller than the inside diameter of the sheath 150. Once
placement of the transition member portion 130 of the catheter 100
in the vein 210 is secure, the introducer 160, including the
introducer sheath 150, is removed from the vein 210 and the skin
200 of the patient as shown in Figure 6.
Once the introducer 160 is removed from the skin, Figure
7 illustrates that the introducer is peeled away from the catheter
100. It is to be noted that the introducer 160 may be peeled away
from the catheter 100 while the introducer 160 is being removed,
for example, to accommodate a catheter 100 with a shorter
transition member portion 130. To facilitate the peeling away and
removal of the introducer 160, the introducer handles 140 and
sheath 150 may be made of a durable material of varying thickness
and a pair of opposed wall portions thinner than the remainder of
-11- 056301.P004
CA 02225760 1997-12-23
the of the introducer 160. In this manner, the introducer 160 is
divided at the opposing thinner side walls as shown in Figure 7.
Alternatively, the introducer handles 140 and sheath 150 may be
scored or sliced along opposing sides and divided along the scored
or sliced section. Similarly, the sheath 150 is made of a
sufficiently thin material so that the sheath 150 may be easily
separated when the introducer 160 is divided.
Once the introducer 160 is removed from the target site
and no longer encompasses the transition member 130 of the
catheter 100, the catheter 100 is further advanced so that the
tubular body portion 110 can be inserted into the skin 200 and the
vein 210 of the patient. Figure 8 illustrates the introduction of
the tubular body portion 110 into the skin 200 and vein 210 of the
patient. The tubular body portion 110 has an outside diameter no
less than the inside diameter of the opening in the introducer
160. Taking advantage of the elastic property of the skin 200,
the invention contemplates that the tubular portion 110 may be
sized approximately 2 gauge sizes larger than the cut made by the
needle 170 (and introducer sheath 150). Thus, for a 22 gauge
needle 170 opening in the skin 200, the invention contemplates
that the tubular body portion 110 of the catheter 100, in one
embodiment, have an outside diameter equivalent to a 20 gauge
size. The tubular body portion 110 is advanced into the vein to
its proper location_
At this point, the invention contemplates different
types of transition member portions 130 coupled to the tubular
body portion 110 of the catheter 100. In its simplest form, the
-12- 056301.P004
CA 02225760 1997-12-23
transition member portion 130 is simply a reduced cross-sectional
area/diameter section of the cannula 100 either integrated or
coupled to the tubular body portion 110 and made of conventional
cannula material including, but not limited to, polypropylene,
polyethylene, polyvinyl chloride, polyurethane (PU), TEFLON
(produced by E.I. DuPont de Nemours and Company, Wilmington,
Delaware), C-FLEX (produced by Concept, Incorporated, Largo,
Florida), or silicone. Once the catheter 100 is placed, the
transition member portion 130 remains a section of reduced
diameter lumen at the end of the catheter cannula body. It is to
be noted, however, that properties of fluid flow dictate that the
fluid flow through the catheter with the reduced lumen portion 130
is better than the fluid flow through a catheter cannula in which
the entire lumen has the same dimension as the transition member
portion 130. Thus, more fluid can be delivered to the patient
through this transitioned catheter than with a catheter with an
entire lumen of the dimension of the transition member.
Figure 9 illustrates an embodiment of the invention
wherein after the transition member portion 130 of the catheter
100 is inserted in the patient's body fluid, e.g., blood, the
transition member portion 130 assumes a lumen with a cross-
sectional area substantially equivalent to the cross-sectional
area of the lumen tubular body portion 110. In this case, since
the entire cannula 100 will have a cross-sectional area equivalent
to the lumen of the tubular body portion 110, the fluid flow
through the catheter is improved. In other words, the invention
-13- 056301.P004
CA 02225760 1997-12-23
contemplates no reduction in flow capacity through the entire
length of the catheter cannula.
Figures 10-11 illustrate the embodiment wherein the
transition member portion 130 expands to substantially match the
cross-sectional area of the tubular body portion 110. The
transition member portion 130 is made of a material that swells or
expands upon exposure to body fluid. Such a material is
Aquavene , a registered trademark of Menlo Care Corporation of
Palo Alto, California. Aquavene is a composite of a hydrogel and
an elastomer. This material is described in U.S. Patent Nos.
4,883,699 and 4,911,691, assigned to Menlo Care. Other materials
include thermally-activated hydrogel, hydrogel derivatives,
hydrophilic polyurethane, and certain memory polymers.
Figure 10 shows a transition member portion 130 having a
lumen of cross-sectional area of diameter 250 that expands to a
cross-sectional area with diameter 260 upon exposure to body
fluid. The range of expansion of the catheter area is typically
at least two gauge sizes. Figure 11 illustrates a perspective
side view of the catheter cannula having a lumen of the transition
member portion 130 that has expanded to substantially match the
cross-sectional area of the tubular body portion 110.
Another embodiment of the catheter with a transition
member portion that expands, as shown in Figure 9, to a lumen with
a cross-sectional area that substantially matches the cross-
sectional area of the lumen of the tubular body portion is shown
in Figures 12-14. Figures 12-14 illustrate an embodiment wherein
the transition member 130 includes a folded state 270 and an
-14- 056301.P004
CA 02225760 1997-12-23
unfolded state 280. In the folded state 270, the transition
member portion 130 has an area of reduced diameter. In the
unfolded state 280, the transition member portion 130 has an
outside diameter substantially equivalent to the tubular body
portion 110. The folded state 270 is accomplished by
longitudinally folding a portion of the transition member 130
inward to reduce the effective outside diameter of the cannula.
Figure 13 illustrates a perspective side view of the transition
member in the folded state. Figure 14 illustrates a perspective
side view of the transition member 130 in the unfolded state with
an outside diameter substantially equivalent to the outside
diameter of the tubular body portion 110.
Figures 15-20 illustrates a placement method of a
catheter embodiment of the invention wherein the transition
portion extends through the lumen of the tubular body portion of
the catheter. Figure 15 illustrates the insertion of an
introducer 160 into the vein 210 of a patient. The introducer is
placed with a needle 170 so that the introducer sheath 150 and the
needle 170 cut the patient's skin and enter the vein 210. Once
the needle 170 and introducer sheath 150 are in the patient's vein
210, the needle 170 is removed and the introducer 160 remains.
Next, as illustrated in Figure 16, the catheter 300 of
the invention is inserted through the introducer body 140 and
sheath 150 and into the patient's vein 210. In this case, the
catheter 300 includes a transition member portion 330 that has an
outside diameter that is less than the outside diameter of the
tubular body portion 310 and less than the inside diameter of the
-15- 056301.P004
CA 02225760 1997-12-23
opening in the catheter introducer 140 and catheter sheath 150.
In this embodiment, the transition member portion 330 is not
coupled to or integral with the tubular body portion 310, but is
connected to a transition member hub 350 and extends through the
lumen of the body portion 310. The transition member 330 further
extends a significant distance from an end of the tubular body
portion 310 to allow the transition member portion 330 to be
properly placed in the vein 210 of a patient. Once the transition
member portion 330 is properly placed in the patient's vein 210,
the introducer 160 is removed from its location in the patient as
shown in Figure 17. Next, the introducer 160 is divided and
removed from the area as illustrated in Figure 18.
Figure 19 shows the introduction of the tubular body
portion 310 into the patient's vein 210. The tubular body portion
310 includes a tapered section 315 at its end to facilitate
insertion into the skin 200 and vein 210. As in the example
described with reference to Figures 4-9, the invention
contemplates that the skin 200 can accommodate approximately a two
gauge size larger catheter cannula than the initial needle
opening.
Once the tubular body portion 310 is securely placed in
the patient's vein 210, the transition member portion 330 is
removed from the vein by retracting the transition member portion
330 from a distal end at the catheter hub 320 with the transition
member hub 350 as shown in Figure 20. At this point, the tubular
body porcion 310 may be further advanced if needed for proper
placement.
-16- 056301.P004
CA 02225760 1997-12-23
The above-described embodiment allows a catheter cannula
of a single cross-sectional area with an outside diameter larger
than the needle (or introducer sheath) opening to be introduced in
a patient's vein. This embodiment ensures the full flow benefits
of a larger diameter cannula discussed above.
Figures 21-24 illustrate various embodiments of catheter
assemblies of the invention. Figure 21 illustrates an embodiment
with a tubular body portion 400 and a transition member portion
410 that is a solid material that may be coupled to the tubular
body portion 400 or may be separate from the tubular body portion
400 and be removable from the catheter hub. The transition member
portion 410 has a rounded tip to facilitate insertion.
Contemplated materials for this solid transition member portion
410 include any standard flexible, non-toxic material used for
catheter production, including, but not limited to, polyurethane,
TEFLON , polypropylene, polyethylene, polyvinyl chloride, or
silicone. The surface of the transition portion 410 may further
be coated with a low-friction material such as, for example, a
hydrogel or TEFLON to aid insertion.
Copending U.S. Patent Application titled "Thermally
Softening Stylet", by Robert Bley, filed December 23, 1996, and
given Attorney's Docket No. 56301.P006, describes a thermally
softening polymer stylet or guidewire that has sufficient
stiffness or column strength to advance through an introducer and
suport the guidance of the catheter, but softens as it is exposed
to body temperature to provide more patient comfort. The catheter
of the invention contemplates that the transition member portion
-17- 056301.P004
CA 02225760 1997-12-23
610, particularly in the embodiment wherein the transition member
portion extends through the lumen of the tubular body portion, may
be made of a similar material to impart the same properties to the
catheter.
Figure 22 iliustrates an embodiment wherein the
transition member portion is transitioned in steps for increased
flexibility at the tip of the catheter. In Figure 22, the
transition member portion includes the transition member portions
of decreasing cross-sectional area 515, 520, and 525, extending
from an end of the tubular body portion 500. Once again, the
invention contemplates that the stepped transition portions 515,
520, and 525 may be integrated with or coupled to the end of
tubular body portion 500 or may be separate from tubular body
portion 500 and extend through the lumen of the tubular body
portion 500 and be removed through the catheter hub. Further, it
is contemplated that transition member portions 515, 520, and 525
can be a solid piece as described with reference to Figure 21 or
be axially-aligned hollow tubes. In the embodiment wherein
transition member portions 515, 520, and 525 are hollow tubes, the
invention further contemplates that the transition member portions
can remain smaller than the tubular body portion 500 or be made of
a material which swells upon exposure to body fluid. Finally, it
is to be understood that a varying number of transition steps may
make up the stepped-transition portion. Figure 22 illustrates
three portions of varying cross-sectional area for illustrative
purposes, but more or less portions may also be employed.
-18- 056301.P004
CA 02225760 1997-12-23
Figure 23 illustrates a catheter having a transition
member portion 610 that is a guidewire or stylet. The catheter
illustrated in Figure 23 is separate from the tubular body portion
600 and extends through the tubular body portion 600 and may be
removed from the hub of the catheter. The catheter illustrated in
Figure 23 is preferably used in the method described above with
reference to Figures 15-20 and the accompanying text. Further,
the end of the transition member portion may be tapered to impart
increased flexibility to the transition member portion 710.
Finally, the invention contemplates that the transition member
portion 610 may be made of various non-toxic wire material and
have a cross-section of varying shape.
Figure 24 illustrates a further embodiment of the
catheter of the invention, wherein the transition member portion
710 is in the form of a guidewire that is a helically-wound wire
wrapped around a core and soldered on each end to the core. The
helically-wound wire/core transition member portion 710 allows the
transition member portion 710 to easily flex and bend which
facilitates the placement of the catheter in a patient. Finally,
the helically-wound wire/core may be coated with a polymer
material that minimizes friction of the catheter in a patient's
body. Examples of coatings include, but are not limited to,
silicone lubricant, hydrogel, and TEFLON .
In the preceding detailed description, the invention has
been described particularly with reference to vascular catheters.
It is to be appreciated, however, that the invention is compatible
with other types of catheters that are introduced into the body.
-19- 056301.P004
CA 02225760 1997-12-23
Thus, the catheter of the invention and the method of placement of
the catheter are intended to be applicable to replace any of the
various types of catheter uses, including, but not limited to,
vascular, neurological, and urinary drainage catheter uses.
In the preceding detailed description, the invention is
described with reference to specific exemplary embodiments
thereof. It will, however, be evident that various modifications
and changes may be made thereto without departing from the broader
spirit and scope of the invention as set forth in the claims. The
specification and drawings are, accordingly, to be regarded in an
illustrative rather than a restrictive sense.
-20- 056301.P004