Note: Descriptions are shown in the official language in which they were submitted.
CA 02225927 1997-12-29
1
Recovery Process in a Pul~ Mill
The present invention relates to an environmental-friendly process for
reducing the
content of potassium ions in a liquid inventory of a chemical pulp mill.
In the production of a chemical pulp, chips of lignocellulose-containing
material are
cooked in an alkaline or acid aqueous solution. This cooking liquid contains
inorganic pulping
chemicals to improve the dissolution of lignin. The cooking is normally
carried out at a tempera-
ture above 100°C to reduce the residence time for the pulp produced.
Therefore, the cooking is
carried out in a pressure vessel known as a digester.
In the production of sulphate pulp, soda pulp and sulphite pulp with an alkali
metal as
a base, normally sodium, it is possible to recover the inorganic pulping
chemicals in the spent
liquor leaving the digester. It is vital both to economy and environment to
recover these pulping
chemicals to the largest possible extent. This is achieved in the pulping
chemical recovery
system, which essentially transfers the used inorganic pulping chemicals into
a chemical state,
where they can be re-used for cooking.
An essential part of the recovery system is the recovery boiler, where the
spent liquor
is burned. Normally, make-up chemicals are added to the spent liquor before
the recovery
boiler to make up for the chemicals lost during cooking and recovery. The
spent liquor is
sprayed into the lower part of the boiler, previously at a relatively low
temperature to remove
free water. Modem recovery boilers operate at a high temperature to reduce the
content of
sulphur in the flow gases leaving the boiler. Higher up in the boiler, gases
and vapours of light
hydrocarbons and decomposition products are volatilized. This is known as
pyrolysis. Then,
the pyrolysis products are burned after mixing with air or oxygen. The solid
carbon-based
residue which remains after complete pyrolysis of the organics is then
heterogeneously
burned. The solid particles formed are collected as a dust in precipitators at
the top of the
recovery boiler, to reduce the release of solid material to the surrounding
atmosphere.
A substantial and increasing problem with the pulping chemical recovery
system, is
the presence of chloride and potassium in the spent liquor entering the
recovery boiler. These
elements tend to reduce the capacity of the recovery boiler to produce useful
chemicals. Thus,
chloride and potassium increase the stickiness of carryover deposits and dust
particles to the
recovery boiler tubes, which accelerate fouling and plugging in the upper part
of the recovery
boiler. Chloride also tend to increase the corrosion rate of superheater
tubes.
Chloride and potassium are concentrated in the dust formed during the
combustion of
spent liquor in the recovery boiler. The dust is collected in dry-bottom or
wet-bottom
electrostatic precipitators. The dust mainly consists of sodium and potassium
salts, where
sulphate, carbonate and chloride are the dominant anions. The amount of dust
corresponds to
CA 02225927 1997-12-29
2
from about 5 up to about 15% by weight of the sodium entering the recovery
boiler, which
corresponds to from about 50 up to about 150 kg dust per tonne pulp, if the
dust is calculated
as sodium sulphate.
Today, normally all of the precipitator dust collected and withdrawn from the
recovery
boiler is recycled to the flow of spent liquor to be burned in the boiler.
When the concentration
of chloride or potassium is too high, a portion of the precipitator dust is
withdrawn from the
system and discharged or deposited.
The largest source of potassium is the wood, and the intake will depend on the
wood
source generally varying from about 0,5 to 5 kg per tonne pulp. The hardwood
species usually
contains larger amounts of potassium than softwood species. Besides, the
content of chloride
in the spent liquor can be very high for coastal mills, if the raw material
consists of logs floated
in seawater. As the environmental legislation becomes more stringent regarding
pulp mill
discharges to air and water, the degree of system closure increases. This
means that even a
small input of chloride and potassium becomes a severe problem, unless the
content can be
controlled by purging the system in some environmentally acceptable way.
Several methods have been proposed to overcome the problem with chloride and
potassium build-up in pulping chemical recovery systems. The use of organic
ion exchangers
have been proposed as a unit operation for treatment of precipitator dust, but
this has mainly
been for softening purposes, e.g. to reduce the content of multivalent metals
that would harm
membranes in subsequent electrolysis of the precipitator dust. Chloride and
potassium removal
are preferably carried out in a common waste water treatment. Chloride can be
removed
efficiently by e.g. electrodialysis, while potassium still is difficult to
remove efficiently
electrochemically.
For instance, WO-A1-9404747 discloses a process, in which the content of
chloride in
a recovery system for pulping chemicals can be reduced. The process comprises
collecting
precipitator dust, dissolving the dust in water to produce an aqueous solution
of precipitator
dust, whereupon said aqueous solution is electrolysed in a cell for production
of chlorine or
hydrochloric acid in the anolyte. Use of ion exchange is suggested as a
pretreatment before
the electrolyses, chiefly to remove divalent ions such as Ca2+ and Mg2+.
Caron J. R. et al, °Metals management in a closed kraft mill bleach
plant", Pulping
Conference, TAPPI (1995), p. 1155-1160, have investigated metals removal from
recycled
chlorine dioxide bleach plant filtrate with ion exchange resins.
The present invention relates to a process by which the content of potassium
ions in a
recovery system for pulping chemicals can be reduced. The process comprises
bringing spent
liquor to a recovery boiler, burning said spent liquor, collecting
precipitator dust formed, forming
CA 02225927 1997-12-29
3
a solution by dissolving the precipitator dust in a liquid, where the solution
of precipitator dust is
subjected to a treatment with an inorganic ion exchange material in order to
remove at least a
part of the potassium therein.
An advantage of the present process is the possibility to reduce the content
of
potassium in the liquid inventory and more particularly in the spent liquor
entering the recovery
boiler. By the present process, the problem of sticky deposits in the recovery
boiler can be
substantially reduced. This means an improved energy efficiency as well as a
higher degree of
recovery of the pulping chemicals.
The present invention can be used in the production of a chemical pulp and
especially
for production of a sulphate pulp, soda pulp or sulphite pulp with an alkali
metal as base. A
kraft pulp is a special type of sulphate pulp, where the pulp is under-cooked
to produce a dark
coloured pulp of exceptional strength. The present invention can also be used
in the production
of sulphate, soda or sulphite pulps with an alkali metal as base, where the
cooking processes
have been modified, combined or extended compared to the normal pulping
techniques.
Suitably, the present process is applied where the recovery system for pulping
chemicals is a
kraft recovery system.
A liquid inventory is the total quantity of various liquids in a mill, with
varying contents
of active or activatable cooking liquid components. The liquid inventory of a
sulphate mill,
mainly consists of white liquor, black liquor, green liquor and spent liquor
entering the recovery
boiler. The spent liquor to be burned in the present process, is a used
cooking liquid withdrawn
from a digester, optionally with added make-up chemicals. Potassium and sodium
are alkali
metals present in the spent liquors.
The amount of precipitator dust formed depends mainly on the temperature in
the
boiler, the ratio between sodium and sulphur in the spent liquor and the raw
material and
sulphidity of the cooking process. A high temperature in the lower part of the
boiler to reduce
the sulphur content in the flow gases, increases the amount of dust formed.
With the present process, all or a portion of the precipitator dust collected
and with-
drawn from the recovery system is treated with an inorganic ion exchange
material.
The composition of precipitator dust formed in recovery boilers vary
considerably
depending on type and origin of wood, cooking process, purity of make-up
chemicals, tempe
rature in the boiler, type of precipitator etc. However, irrespective of these
factors the dust
mainly consists of sodium and potassium salts, where sulphate, carbonate and
chloride are the
dominant anions. A typical composition of precipitator dust from a kraft
recovery system is
NazS04 80-85% by weight, Na2C03 2-8% by weight, NaCI 2-8% by weight, NaHS04 +
NaZS20~ < 2% by weight, K2S04 5-10% by weight, KZC03 0.5-1 % by weight, KCI <
1 % by
CA 02225927 2001-07-24
4
weight, metal ions such as Ca; Fe, Mg, P, Si, Mn, Zn, Mo < 1 % by weight and
organic
material < 1 °/o by weight.
Natural as well as synthetic inorganic ion exchange material can be used.
Suitable
inorganic ion exchange materials are aluminosilicates, hydrous oxides, acid
salts of
polyvalent metals or salts of het:eropolyacids. Preferably use is made of
aluminosilicates such
as zeolites, and most preferably zeolites are used as ion exchange material.
Zeolites are inorganic crystalline compounds mainly consisting of SiOz and
A1203 in
tetrahedral co-ordination. In the present invention, zeolites also relate to
other crystalline
compounds of zeolite structure; such as aluminium phosphates. Such crystalline
compounds
of zeolite structure which cm be used in the present invention are defined in
W.M. Meier et
al, Atlas of zeolite structure types, sec, ed., Butterworths, London, 1987.
Many zeolites occur
naturally, but most c;ommcncially used zeolites are synthetically produced.
These zeolites
function as adsorbents or molecular sieves and rnay, depending on the size of
the cavities and
the nature of the zeolite surfac e, be used to increase or decrease the taking-
up of specific
chemical compounds. In the present invention, a very essential property of the
zeolites is their
selectivity towards potassium relative to sodium.
A suitable zeolite can he selected from the group consisting of mordenite,
chabazite,
clinoptilolite, zeolite A and zeolite Y. Preferably use is made of mordenite.
A wide variety of
zeolites are available on the market. For instance, Wessalith P is a A-zeolite
manufactured by
Degussa. Zeolite Y EY250, Zeolite Y N3S, Zeolite BMH and Zeolite Sodium
Mordenite EM
120 is manufactured by Eka (:he~micals AB.
Exan-aple of hydrous oxides which can be used are hydrous titanium oxide, iron
hydroxide, hydrous stannic oxide, hydrous zirconium oxide, silica gel ete.
Crystalline
antimonic acid is known to exhibit the following selectivity series
Li>K>Rb>Na.
Suitable acid salts of polyvalent metals used as ion exchange material may be
zirconium phosphate crystal or titanium phosphate crystals.
The amount of inorganic ion exchange material used may vary within wide
limits.
Thus, the amount of inorganic ion exchange material used may be up to 1
tonne/tonne of dry
precipitator dust and e.g. lie in rh.e range of from about 1 kg/tonne up to
about 1000 kg/tonne
of dry precipitator dust, suitably in the range of from about 10 kg/tonne up
to about 1000
kg/tonne of dry precipitator dust and preferably in the range of from about
100 kg/tonne up to
about 500 kg/tonne of dry precipitator dust. Suitably, the amount of inorganic
ion exchange
material used is based upon the ion exchange capacity of the actual inorganic
material and
CA 02225927 1997-12-29
the amount of potassium in the precipitator dust. The inorganic ion exchange
material is
preferably used in excess of the stoicheometric amount of potassium.
The potassium and chloride containing solution is treated with an inorganic
ion
exchange material. The ion exchanged material, enriched on potassium, is then
preferably
5 filtered and washed with water, whereafter the spent ion exchanger material
can be
deposited in a land fill. The solution depleted on potassium and chloride, can
be recycled in
the pulp mill or forwarded to another step in the treatment of precipitator
dust for mixing or
dilution. The separation and washing can preferably take place in a
centrifuge, a filter press
or a vacuum filter.
Alternatively, after separating the ion exchanged slurry from the solution, it
can be
regenerated with a sodium rich aqueous solution. This could for example be a
solution of the
Na2S04 salt cake obtained from a chlorine dioxide generator (or from a
crystallisator, or a
chloride concentrate from an electrochemical cell). Separation, washing,
regeneration and
dewatering then may take place in the same piece of equipment operating in a
continuos
mode. Even the ion exchange may take place in the separation equipment. This
can be done
batchwise in a filter press or continuos eg. in a centrifuge or a rotating
filter of vacuum or
pressure type.
In an embodiment of the present invention, the inorganic ion exchange material
is
made in the form of granules or pellets and are placed as a fixed bed in a
column, thus
forming an ion exchanger. The precipitator dust solution, rich on potassium,
is forced to flow
through the bed of ion exchange material to which the potassium is adsorbed.
The reaction
zone proceeds down the column as the upper layers of ion exchange material
reaches
equilibrium with the solution. At the end of the work cycle when the ion
exchanger becomes
exhausted, the ion exchange material is backwashed (regenerated) with a sodium
electrolyte. The flow of potassium rich solution is simultaneously switched to
another column.
A number of columns can be arranged in parallel so that the continuos
operation is ensured.
The advantage with this embodiment, columns arranged in parallel, over the
previously
described is that the operation is continuous and that a more efficient ion
exchange can be
achieved as multiple equilibrium stages are obtained (cf. McKabe-Thiele
diagram).
The amount potassium removed in a precipitator dust solution subjected to a
treatment with an inorganic ion exchange material, can be above about 40 %,
suitably above
about 50 %, preferably above about 60 % and most preferred above about 70 %.
A potassium free or potassium depleted stream which has undergone the
inorganic
ion exchange, is preferably recycled to the weak black liquor or may, in the
cases where the
CA 02225927 1997-12-29
6
water balance allows, be mixed with the strong black liquor and fed directly
into the recovery
boiler in the recovery system.
A solution of precipitator dust will also commonly have a pH between about 7
and
about 11, within which range most ion exchange material are stable and thus
preferred.
However, the pH is not critical since many ion exchange material work
satisfactory outside
the pH range 7-11.
The ion exchange is suitably performed in the range from above 0°C up
to about
100°C and preferably from about 20°C up to about 60°C.
The residence time for the suitable batchwise ion exchange is preferably at
least
about 1 minute. Suitably the residence time is at least about 1 hour,
preferably at least about 2
hours and most preferred at least about 5 hours. The upper residence time is
not critical, but
have to be set by process-technical reasons. The flow rate for the suitable
continuous
operation of the ion exchange is suitably at least from about 0.1 up to 20
BV/h (Bed Volume
per hour), preferably from about 1 up to 10 BVIh and most preferred from about
2 up to 6 BVIh.
The ion exchange is preferably carried out by a continuous operation.
In one embodiment of the invention the potassium concentration of the solution
is
increased by leaching the precipitator dust with a liquor. The added liquid
may comprise of
water, or water solutions of sulphate or carbonate. Added sulphate may be
alkali metal,
preferably sodium sulphate, suitably at least a part derives from a
recirculated solution,
depleted of chloride and potassium, for instance from a suitable
electrochemical treatment or
recrystallisation. If water is added, it can be either fresh water or purified
process water. The
potassium enriched leach solution is separated from the solid phase of the
leached precipitator
dust, by e.g. filtration, centrifugation, sedimentation etc..
The concentrate in the slurry obtained from the leaching step may comprise
from
about 1 gll up to about 60 g/l potassium, and will be saturated with sulphate.
The solution depleted of potassium obtained from the ion exchange may comprise
from 0 g/l up to about 60 g/l potassium (counted as e.g. K+, K2S04, KCI).
The inorganic ion exchange according to the present invention is preferably
combined with another process. This could for example be a process for
leaching and
electrodialysis of precipitator dust. The ion exchange can also be combined
with processes
where precipitator dust is split in electrolysis cell or a electrodialysis
cell with bipolar
membranes. The advantage is, especially when removing the potassium prior to
electrolysis,
that NaOH with little or no potassium impurities can be prepared.
The inorganic ion exchange according to the present invention can also be
combined with a process where sodium sulphate is re-crystallised from the
precipitator dust.
CA 02225927 1997-12-29
7
In another embodiment, the inorganic ion exchange according to the present
invention can
be advantageously combined with a process where chloride ions are ion
exchanged from the
precipitator dust (for instance the Precipitator Dust Purification System,
PDPT"").
Another possible application is to use the inorganic ion exchange according to
the
present invention on bleach filtrates recovered back to the chemical recovery.
Embodiments of the process of the present invention will now be described in
more
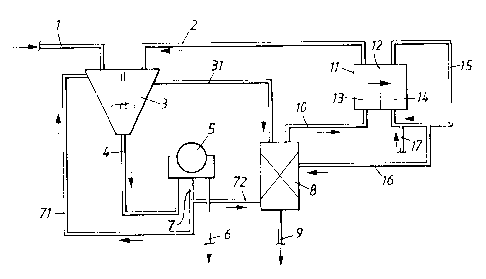
detail with reference to figures. Figure 1 shows a schematic description of
the use of an
inorganic ion exchanger in a process for treatment of precipitator dust
applying a combination
of leaching and electrodialysis treatments. Figure 2 shows the application of
an inorganic ion
exchanger in yet another embodiment in combination with a recrystallisation of
sodium
sulphate from precipitator dust.
In the embodiment of fig. 1, precipitator dust (1) can be mixed with a near
saturated
solution of sodium sulphate (2) in a leach tank (3) where the mixed solution
can be subjected
to a leaching treatment. The chloride and potassium compounds in the
precipitator dust are
leached out and the potassium and chloride enriched solution (31 ) is brought
to an ion
exchange column (8). A slurry (4) is separated on a filter (5), a centrifuge
(not shown) or the
similar. The filter cake (6), virtually free of chloride and potassium, is
recovered to the strong
black liquor. The filtrate (7) is recycled (71 ) to the leach tank (3) or
forwarded (72) to the ion
exchange column (8) where potassium ions are removed (9), and the potassium
depleted
filtrate, still containing a high concentration of chloride ions (10), can be
further brought to
the diluate chambers) (13) of an electrodialysis stack (11 ) holding mono-
anion selective
membranes (12). When an electric field is applied to the electrodialysis stack
the chloride
ions are removed by migration through the anion selective membranes to the
concentrate
(brine) chambers) (14). The remaining chloride and potassium depleted sodium
sulphate
solution (2) can be recycled for use as leachant in the first step (3). The
electrodialysis stack
(11) may also comprise cation selective membranes through which cations such
as sodium
and potassium are removed by migration. The concentrated sodium chloride
solution
prepared in the electrodialysis step (15) can be recirculated back to the
concentrate
chambers) of the cell, but a part may be purged and used for regenerating (16)
the ion
exchange material. In addition, water can be added to dilute the concentrated
chloride
solution (17).
The advantage with implementing potassium ion exchange in the process
described
in fig. 1 is obvious. Chloride and sulphate ions can efficiently be separated
in the
electrodialyser as the divalent sulphate ions will be repelled by the mono-
anion selective
membranes. Unfortunately, cation exchange membranes cannot exhibit a similar
selectivity
CA 02225927 1997-12-29
8
for potassium versus sodium. Consequently, only a minor part of the potassium
will be
removed in the electrodialysis step and without any selective potassium
removal the
concentration of potassium will increase in the solution until the solubility
limit for glacerite
(NaK3(S04)Z) is reached. As a consequence, the main part of the potassium will
follow the
cake resulting in a low removal efficiency for potassium. With the ion
exchange the
potassium concentration can be kept below solubility and potassium removal
efficiency is
improved.
In fig.2, an embodiment is shown where an ion exchanger is combined with a
process where sodium sulphate is re-crystallised from the precipitator dust.
Precipitator dust
(21 ) can be mixed with water (22) (preferably condensate from the
crystallisator (28)) in a
dissolving tank (23). The dissolved solution (24) is forwarded to an ion
exchange column
(25), where potassium, are removed (26) and the potassium depleted filtrate
(27), may
further be brought to a crystallisator (28) where sodium sulphate is re-
crystallised from the
precipitator dust. The solution (29) containing mother liquor and sodium
sulphate crystals is
brought to a separation step with conventional technique, for instance a
vacuum drum filter
(30) and an addition of water (31 ), where the sodium sulphate crystals (32)
(NaZS04) is
separated from the mother liquor (33). Condensed water (22) from the re-
crystallisation (28)
may be recycled to the dissolving step (23). The sodium sulphate crystals can
be recycled to
the strong black liquor, alternatively can a small portion of the crystals be
dissolved in water
and used for regeneration of the ion exchanger. The mother liquor is
preferably discharged.
The process shown in fig.2, purges a stream of the mother liquor to control
the
potassium and chloride levels. As described in connection to fig 1. above, the
limiting factor
is the solubility of glacerite (NaK3(S04)2). When the potassium level is kept
down, either by
ion exchanging the feed solution or a small side stream of mother liquor, the
chloride levels
in the mother liquor can be increased and the purge flow can be reduced. In
other words
less sodium and sulphate are lost with the purge. The concentration of
potassium in the
precipitator dust solution may vary between 0 and about 35 g/l. After ion
exchange, the
solution fed to the crystallizator may have a potassium concentration down to
0 g/I.
The invention and its advantages are illustrated in more detail by the
following
examples which, however, are only intended to illustrate the invention and not
to limit the
same. The percentages and parts used in the description, claims and examples,
refer to
percentages by weight and parts by weight, unless otherwise specified.
A 20 wt% ash solution was prepared from a kraft mill precipitator dust, with a
content in the ash of 32 wt% Na, 42 wt% S04, 3.0 wt% K, 3.4 wt% CI, 16 wt% C03
and
CA 02225927 2001-07-24
9
impurities < 4 wt%. The pH o:f the solution was adjusted by 50% H~S04 to pH 7
or 9 and
filtered through an OOH filter paper. After that the solution contained about
7.5 g/1
potassium. The ash solution was mixed with the zeolite and left for stirring
for at least 10
minutes. The samples that were; run at 50°C the solution was heated and
stirred at the same
time. When the samples reached 50°C they were stirred for 5 minutes
more. For filtration of
the solution, a Millipore (Trademark) equipment and a Nylon (Trademark) filter
paper with a
pore size of 0.45 um were used. The zeolites used in the examples were of two
different types.
The Wessalith P (Trademark) is a zeolite manufactured by Degussa. Sodium
Mordenite
EM120 manufactured by Eka Chemicals in Bohus, Sweden. The tested zeolites were
powder
form. Analyses of potassium content were done on the ash, the start solution
and on the
filtrate. The experiments were nan at pH 7, and at room temperature with
different amounts of
zeolites. In tables I and II below the results of the removal of potassium in
% for each are
shown. The amount of zeolite/1.00 ml ash solution has been recalculated as dry
zeolite. The
removal of potassium is calculated on the concentration in the start solution
and the filtrate as
follows:
K in start solution (g/1) - K in filtrate g/1) x 100 = % Removal of K in the
K in start solution (,gi 1) precipitator dust
Table I shows the results from the experiments with Wessalith P.
Table I
Test: 1 2 3 4 5
Amount zeolite [g]/100 3,9 5,8 7,8 31 38,8
ml solution
K in start solution, 7,2 7,2 7,2 7,4 7,4
[g/1]
K after treatment, [g/1]6,5 6,1 5,9 3,9 3,7
Removal of K:+ [%] 10% 15% 18% 47% 50%
As evident from table I, the removal of potassium with Wessalith P was
efficient and
increases with increasing addition of the zeolite.
Table II shows the results from the experiments with Zeolite Sodium Mordenite
EM 120.
CA 02225927 2001-07-24
est: 1 2 3 4 5 6
mount zeolite [g] 100 3,9 7,8 15,5 23,3 31 38,8
ml solution
in start solution, 7,7 7,7 7,7 7,2 7,2 7,2
[g/1] 6,2 6,0 4,3 3,3 2,8 2,4
after treatment, [g/1]
emoval of K~ [%] --- 19% ~-22% 44% 54% 61% 67/~
As shown in table II, the removal of potassium with Zeolite Sodium Mordenite
EM120 was further
improved, resulting in about 15 '% higher removal rate than with the Wessalith
P zeolite.
Example 2
A 20 wt% ash solution was prepared from the precipitator dust used in example
1.
The pH of the solution was adjusted by 50% H~S04 to pH 8. After that the
solution contained
about 7.7 g/1 potassium. The aslz solution was mixed with the zeolite and left
for stirring for 5
hours at a temperature of 80°C. The zeolite used was Sodium Mordenite
EM 120
manufactured by Eka Chemicals, in Bohus, Sweden. The zeolite was in powder
form. 100m1
solution/20g zeolite was used in this test. The start concentration of
potassium is 7,7g/1.
Analyses of potassium content and the calculation of amount potassium removed
were done
in accordance with example. L . Samples were taken once every hour from the
solution with the
zeolite. The stirrer was turned off and sample was taken from the clear phase.
In table III
below the results of the removal of potassium in % for each hour is shown.
Table III
ime [h] : 1 2 3 4 5
emoval of K+ [%]: 32.5 33.8 35.1 36.4 40.3
_ __.
.As evident from table III, the removal of potassium with Zeolite Sodium
Mordenite EM 120 increasf:d with time.
Example 3
For comparative reasons, Amberlite IRC-718 (Trademark for a cationic
exchange resin) manufactured by Rohm and Haas was used in an experiment.
Before start the
resin was regenerated as follows:
1. 300m1 Amberlite was added to 1200m14% HCI and stirred for 30min.
2. Wash with water (Filter paper and funnel).
3. The Amberlite was added to 3000m14% NaOH and stirred for 30min.
4. Wash with water (water and Amberlite were mixed and stirred for some
minutes and
CA 02225927 1997-12-29
11
wash of the Amberlite as in 2, this was repeated several times). pH 10,9 after
3,5 hour.
A 20 wt% ash solution was prepared from the precipitator dust used in example
1. The pH of the solution was adjusted by 50% HZS04 to pH 10. The solution
contained after
that about 7,5 g/I potassium. The ash solution was mixed with the wet ion
exchanger and
stirred for 30min at 40 °C. After that the solution was filtered.
Analyses of potassium content
and the calculation of amount potassium removed were done in accordance with
example 1.
In table IV below the results of the removal of potassium in % for each are
shown. The
amount of zeolite/100 ml ash solution has been recalculated as dry zeolite.
Table IV
Test 1 2 3
Amount zeolite 25 37,5 50
g / 100 ml solution
K in start solution7,5 7,5 7,5
[g/Il
K after treatment6,0 5,5 5,1
[9/Il
Removal of K 20 26 32
[%]
As evident from table IV, the removal of potassium with Amberlite IRC-718 is
poor, compared to the inorganic ion exchange material used in examples 1 and
2.
Example 4
In this experiment a continuous ion exchange was carried out. The equipment
used were a glass column, a conduit for continuously separating a bleed from
the top of the
column to a balance, a balance with the feed solution in connection to a pump
for
continuously feeding the solution from the bottom of the column and a pump
control unit. In
each trial the column was packed with fresh zeolite granules. Potassium were
analysed by
AAS (Atomic Absorption Spectra). In trials no 1-3 a real precipitator dust
solution were used.
A 5 wt % solution was prepared from Weyerhaeuser precipitator dust (see
example 1 ). The
pH was adjusted by 50 % H2S04 to pH of 7 and the solution was filtered through
a OOH filter
paper. A new solution was prepared in each trial. Table V shows the
precipitator dust
solution content.
CA 02225927 1997-12-29
12
Table V
K g/l Na gll CI g/l S04 gll pH
1,5 --16 ~1,7 ~21 7
The used regeneration solutions has been 3 M NaAc and 3M NH4 CI. The
variables can be seen in table VI. Rex = regeneration. IEX = ion exchange.
BV=Bed Volume.
Trial no.1 is Wessalith MS 330, no.2 is Ammonium Mordenite and no.3 is Sodium
mordenite.
Table VI
TrialAmount Flow IE Temp Performed seriesRegeneration
No. Zeolite [BVI time [ C] (Re),
[g] h] (min] Solution, time
1 30.1 18 100 25 Reo IE~ Rep NH4CI 90 minutes
IEZ
2 27 18 100 25 Reo IE~ Rep NaAc 90 minutes
IEZ
3 31 18 100 25 Reo IE~ Rep NH4C1 90 minutes
IEZ
In table VII below, the continuous ion exchange on precipitator dust solution
is
shown.
Table VII
Zeolite type IE- Rem % K Rem % K Rem % K Rem % K
trial No stage 20 minutes50 minutes70 minutes100 minutes
Wessalith MS 330 IE~ 41,1 26,9 22,5 18,2
/ trial no.1
IE2 39,9 26,7 21,9 17,7
Ammonium MordeniteIE~ 50,4 35,8 30,9 25,7
EM 032 / trial
no. 2
IE2 45,1 33,2 28,4 23,0
Sodium mordenite IE~ 65,2 52,8 44,5 36,2
EM 120 / trial
no.3
IE2 76,5 44,3 37,2 29,6
As evident from table VII above, it is possible to achieve a high removal of
potassium from a precipitator dust solution when using a continuous operation.
The results
from these trials also show that the zeolites can be regenerated and used
again.