Note: Descriptions are shown in the official language in which they were submitted.
CA 02226491 1998-01-07
W O 97/028S0 PCT~196/00045
HEART ASSIST SYSTEM
FIELD OF T~IE INVENTION
The present invention relates generally to devices and systems for ~u~ ;lID cardiac
output, and specifically to intra-ventricular cardiac assist pumps.
BACKGROUND OF TE{E INVENTION
Intra-aortic and intra-ventricular cardiac assist devices are well known in the art. These
devices are ~enerally used to reduce the heart's work load a~cer insult or surgery. They may
also be used to increase blood flow from the le~ ventricle of the heart into the aorta in cases of
insufficient cardiac output due, for example, to acute or chronic heart ~ilmçnt5 or to
interference with normal cardiac function during surgery.
One of the best-known and most widely-used intra-aortic pump systems is the Intra-
Aortic Balloon Pump (IABP), comprisino a catheter, having an inflatable balloon at its distal
end, which is inserted throuoh an arterv into the aorta. The balloon is then alternately inflated
and deflated by an external pump drive, so as to alternately block and unblock blood flow
through the aorta, in synchrony with the beating of the heart, in order to assist the left ventricle
in propelling blood into the arterial system. The IABP, however, provides only limited
~t-c,m~nt~tion ofthe heart's natural, unassisted output, and is not adequate for o~el~o~ g heart
failure.
U.S. patent 4,014,317, which is incorporated herein by reference, describes a
2 0 cardiocirculato}v assist cannula with a balloon pump and cardiac pacing electrode. The cannula
is inserted percutaneously through the aorta so that its distal end is inside the left vemricle of
the heart. L~uring systole, inlet valves on the cannula inside the left ventricle open, and the
contraction of the ventricle forces blood to flow into the c~nn~ Then, during diastole, the
blood flows out, into the aorta, throu(Jh one or more outlet valves along the cannula
do~,l~Ll~al" from the inlet valve. A gas-filled balloon, similar in function to the IABP
described above, is connected to the cannula downstream of the outlet valves. The balloon is
typically inflated durin, diastole and deflated during systole, to assist in perfusion of the
coronary arteries. The cannula has a small stroke volume, however, and relies on the
contractile force of the heart to pump the blood. It is therefore of limited us~.fi-lnPss in
3 0 ~lom~ntino the blood output of a weakened or failin2 heart.
U.S. patent 4,906,2''9, which is also incorporated herein by reference, describes a high-
frequency transvalvular axisymmetric blood pump. The pump includes a small internal volume,
s which may be alternately expanded and reduced by pneumatic or hydraulic pressure which is
exerted via a flexible membrane radially surrounding the volume. The volume has intake and
3 5 outlet ends, with one-way axial valves at both of the ends, so that blood can flow only from the
heart into the aorta. The pump is connected via the one-way intake valve to a c~nnlll~ which is
CA 02226491 1998-01-07
W 097/02850 PCTnL96/00045
inserted into the left ventricle of the heart through the aortic valve. When the internal vo1ume is
e~p~n(le(l~ blood flows into the pump from the ventricle. The volume is then reduced, and the
blood is ejected into the aorta through the outlet end. This pump is d~ci~ned to operate at a
frequency of 600 to l,000 cycles per minute. Since the stroke volume of the pump is typically
5 only about 3-5 cc, these high cycie rates are needed in order to provide adequate perfusion.
In the Hemopump Cardiac Assist System, distributed by Johnson & Johnson
Interventional Systems, a cannula cont~ining a special, minialure rotor pump me~l~A..;.~ is
inserted into the aorta. The pump is driven by a drive unit outside the body, to pump blood
continuously from the aorta into the rest of the arterial system, thereby suppl~omPntin~ the
10 heart's natural output rate. A system of this type is similarly described in U.S. patent
5,092,844, which is incorporated herein by reference. While continuous-flow devices are useful
for short-term au~mentation or' cardiac output, it is believed that pulsatile pumps provide more
effective long-term support. since they approximate more closely the natural pump action of the
heart.
CA 02226491 1998-01-07
W O 97/02850 PCT~L96/00045
SUMMARY OF TEIE INVENTION
It is an object of the present invention to provide an intraventricular cardiac assist pump
having a sufficiently large internal volume and improved valve structure, so that a~leqll~te
pulsatile perfusion ofthe body may be dependably m~int~inPcl
'' 5 In one aspect of the present invention, wherein the pump comprises a ~nmll~ one-way
valve structures are provided in the sides of the cannula so as to reliablv control the alternate
inflow and outflow of blood therefrom.
In plerel.~:d embodiments of the present invention, an intraventricular cardiac assist
pump comprises a cannula, whose distal end is inserted through the aorta in~o the left ventricle,
and a pulsatile drive unit, coupled to the cannula at the proximal end thereof. The cannula
col..~lises an outer sheath, defining and enclosing an internal lumen, ha~ing at least one intake
valve, adj~ct~nt to the cannula's distal end, and one or more outlet valves~ disposed radially
along the length of the cannula. downstream from the intake valve. The pulsatile drive unit
alternately reduces and increases the fluid pressure in the t~nn~ 'hen the pressure is
15 reduced, the at least one intal;e valve opens, while the one or more outlet vaives are closed, and
blood flows through the intake valve into the lumen of the c:~nn~ The pressure in the cannula
is then increased, causing the intake valve to close and the outlet valves to open, so that blood
flows out of the lumen into the aorta.
In some preferred embodiments of the present invention, the pulsa~ile drive unit in~ es
2 0 a fluid reservoir, comprising first and second chambers, separated by a fle~ible diaphragm, each
chamber havin, a fluid port. The fluid port of the first chamber is connected to the p-o~imal
end of the c~nn~ , so that blood may flow between the chamber and the cannula. The fluid
port of the second chamber is connected to a hydraulic drive, which alternately increases and
decreases the pressure, and hence the volume, of a control fluid in the second chamber. The
?5 flexible diaphragm couples pressure changes from the second to the first chamber, without
direct contact between the fluid in the second chamber and the blood in the first chamber,
thereby controlling the flow of blood into and out of the lumen of the cannula, as described
above. The use of the hydraulic drive enables substantially greater volumes of blood to be
pumped, with greater efficienc~, than pneumatic pump drive mech~nismc that are commonly
3 0 used in other cardiac assist pumps known in the art.
It will be appreciated that in preferred embodiments of the present invention, as
described above, the blood being pumped remains entirely inside the cannula and in the first
chamber of the fluid reservoir connected thereto, without circulating substantially outside the
body. Preferably, the cannula and fluid reservoir are disposable, intended for a single use, so as
to reduce the likelihood of infection.
In p- efe. I t:d embodiments of the present invention, the cannula is capable of pumping at
- least 50 cc, and preferably up to g0 cc of blood, in each stroke of the pulsatile drive unit. It will
be appreciaLed, however, that depending on clinical requirements, the cardiac assist pump may
- -
CA 02226491 1998-01-07
W O 97/02850 PCT~LS.~ 4'
be adjusted to pump a smaller volume in each stroke, for example 20 cc. The pulsatile dri-~e
unit is preferably operated substantially at the rate of the human heart beat, and adjusted so that
adequate perfusion of the arterial system is m~int~ine~ The drive is preferably syllclllolli~ed
with the heart beat, so as to draw blood into the lumen of the cannula during systole and eject
the blood into the aorta during diastole.
Alternatively, the drive may be counter-synchronized, so as to draw blood into the
lumen during diastole and eject it during systole, or the drive may be operated asvnchronously,
independent of the heart beat.
In preferred embodiments of the present invention, the cannula comprises a flexible,
resilient tube having a rii~met~r in the range of l5-~0 French (5-lO mm). It is preferably
inserted percutaneously throuoh the femoral artery, into the aorta. and then through the aortic
valve into the left ventricle of the heart. Alternatively~ the cannula may be inserted elsewhere
into the arterial system through a suitable surgical incision.
In some preferred embodiments of the present invenfion the at least one intake valve of
the internal lumen ofthe cannula comprises a one-wa~ mechanic.~l flap valve or leatlet valve, as
are known in the art. The at least one intake valve may comprise either an axial opening or one
or more radial openin~s.
In some preferred embodiments of the present invention the one or more outlet valves
similarly comprise mechanical flap valves, which open radialbt outward when tne pressure
2 0 inside the lumen of the cannula increases~ and close substan~iall- flush with the outer surface of
the cannula when the pressure inside the lumen is reduced.
In other preferred embodiments of the present in- ention, the cannula contains arotatable inner sleeve, inside the outer sheath and radially enciosing the lumen. Preferably the
sleeve extends along at least the portion of the len~th of the cannula along which the outlet
valves are disposed. E.~ch of the one or more outlet valves comprises a first opening in the
inner sleeve and a corresponding second openin, in the outer sheath. To open the outlet valve,
the first and second openings are aligned, by suitably rotating the sleeve relative to the sheath.
To close the valve. the sleeve is counter-rotated, so as to disalign the first and second openings.
Preferably, the sleeve is constructed so that blood flo-vin(~ into the lumen causes a
torque to be exerted on the sleeve, so that the sleeve rotates and the outlet valves are closed.
When the direction of flow of the blood in the lumen is reversed. the sleeve rotates back to its
previous orientation, in which the outlet valves are open, and the blood can flow out.
Preferably the sleeve includes small wings or rotor blades fixed to its inner surface, for the
purpose of converting the force of the blood flow into the torque e~certed on the sleeve.
Alternatively, an externally-driven mechanical rotation device is coupled to the sleeve
and/or the sheath so as to effect the desired relative rotation to open and close the one or more
outlet valves.
CA 0222649l l998-0l-07
W O 97/02850 PCTALg5/~C~1'
In preferred embodiments of the present invention in which the cannula includes the
rotatable inner sleeve, the at least one intake valve may comprise a merh~rlical flap or leaflet
valve, as described above. Alternatively, the intake valve may comprise first and second
openings, similar in function to the first and second openings of the outlet valves, except that
5 when the first and second openings of the outlet valves are aligned, those of the inlet valve are
disaligned, and vice-versa.
In other preferred embodiments of the present invention, the cannula contains a sliding
element, held inside the lumen, ~jacent to the distal end thereof, in such a manner that the
sliding element can slide axially along the lumen but cannot rotate therein. The sliding element
0 inc~ 5 radial and axial openings through which blood can flow. The cannula further includes
an axial opening, serving as an intake valve into the lumen, ~ Cçnt to the cannula's distal end,
and one or more radial openings, serving as outlet valves from the lumen, along the length of
the cannula proximal to the intake valve.
When the pressure inside the lumen is increased, the sliding element slides in Ihe distal
15 direction, therebv ~ng~(Sin(g and substan~ially closing the a~cial (intake) opening. When the
sliding element is in this position, the radial openings of the sliding element are aligned with the
ràdial openings in the (~nn~ , SO that blood may flow out of the lumen.
When the pressure inside the lumen is reversed, i.e., reduced to a negative plCS:iUlC
relative to the blood pressure outside the lumen, the sliding element slides proximally, away
2 o from the distal end of the cannula. so that blood may flow into the lumen through the reopened
axial openings of the cannula and the sliding element. In the proximal position, the radial
openings of the sliding element are disaligned with the radial openings in the c~nn~ so that
the outlet valves are effectively closed.
Alternatively, in other preferred embodiments of the present invention, which operate
25 similarly tO those just described. the cannula includes one or more radial intake openings, to
serve as intake valves in place of the axial opening described above. The sliding element
similarly includes radial intake openings, in place of the axial openings described above. When
the pressure inside the lumen is reversed, i.e., reduced to a negative pressure relative to the
blood pressure outside the lumen, the radial intake openings in the sliding element align with
30 the radial intake openings in the lumen, so that blood may flow into the lumen. When the
pressure inside the lumen is increased, the intake vah/es close, and the outlet valves open, as
described above.
In still other preferred embodiments of the present invention, the outlet valves of the
cannula comprise radial openings along the length thereof, which are covered and closed by a
3 5 flexible, elastic outer sheath, preferably made of biocompatible rubber. The sheath is preferably
held in place bv a squeeze ring along a portion of its length. The at least one intake valve may
comprise a mechanical flap, leaflet or other valve type described above or otherwise known in
the art.
CA 02226491 1998-01-07
W O 97/02850 PCT~L9~/~CC'5
Alternatively, the at least one intake valve may similarly comprise a flexible, elastic inner
sheath and operate in a manner similar to the outlet valves, as will be described below.
Normally, the elasticity of the outer sheath covering the outlet valves causes it to cling
radially to the outer surface of the c~nn~ thereby closing the outlet valves. When the
5 pressure inside the lumen of the cannula is increased, however, the pressure of the blood exerts
an outward force on the sheath through the radial openin~,s. This force causes the sheath to
stretch out~ards, allo-~ing the blood to flow out ofthe lumen.
There is therefore provided, in accordance with a preferred embodiment of the present
invention, a cardiac assist pump, including:
a ç~nn~ including an outer sheath, which defines and encloses a lumen therein, the
cannula having a distal end and a proximal end, wherein the cannula is inserted through the
aorta of a subject so that the distal end is inside a ventricle of the heart of the subject;
at least one intalie valve~ adjacent to the distal end of the cannula, through which blood
enters the lumen from fne ventricle:
at least one o~let valve disposed radially alon~g, the sheath of the c~nn~ through
which blood e.Yits the lumen into the aorta:
a fluid reservoir. having a ~ariable fluid volume, connected to the proximal end of the
c~nn~ such that blood mav flow between the lumen and the rese~voir; and
a pump drive. c;)upled to the fluid reservoir and controllino the fluid volume in said
2 O reservoir,
wherein the pump drive alternatelv increases and decreases the fluid volume in the
reservoir to produce a pulsatile pumping"~ction of blood through the cannula.
Preferablv, the reservoir has a minimum and a ma~imum volume, the difference
therebetween defining a reser~oir stroi;e volume, wherein the cardiac assist pump has a stroke
25 volume substantially derined b~ the stroke volume of the fluid reservoir. Preferably, the pump
has a maximum stroiie volume or' a~ least ~0 cc, and more preferablv, appro~cimately 80 cc.
Preferablv, the pump drive is hydraulically coupled to the fluid reservoir and is
synchronized with the beating ot'the heart.
Preferably, the intal;e and outle~ valves include at least one one-way valve. Additionally
30 or alternatively, the intaiie and outlet valves include at least one mechanical flap valve, and/or
the intake valve includes a leaflet valve.
Additionallv or alternativelv, the pump includes a rotatable inner sleeve, situated within
the lumen, ~vherein rotation of the inner sleeve relative to the sheath opens and shuts at least
one of the intake and outlet valves. Preferably, at least one of the intake and outlet valves
35 includes a first radial openin<J, in the sheath and a second, correspondino, radial opening in the
inner sleeve, wherein rotation of the inner sleeve relative to the sheath causes the at least one
valve to open by bringing the respective first and second radial openings substantially into
mutual alignment. Preferabiy, a torque-coupling device is coupled to the inner sleeve, which
-
CA 02226491 1998-01-07
W O 97/02850 PCT~L96/00045
device preferably includes winglets fi~ced to the sleeve and causes the inner sleeve to rotate in
response to blood flow in the lumen.
Alternatively or additionallv, the pump inf~lud~-s an inner sliding e!ement, situated within
the lumen, which moves axially inside the lumen to alternately open and close the intake and
5 outlet valves. Preferably, at least one of the intake and outlet valves includes a first radial
opening in the sheath and a second~ corresponding radial opening in the inner sliding element,
~ and axial movement of the sliding element in the lumen causes the at least one valve to open by
bringing the respective first and second radial openings thereof s~ t~nti~lly into mutual
~lionmtont
Alternatively or additionallv, the at least one intake valve includes an axial opening in
the ç~nnul~ and the inner slidino element moves axially away from the axial opening in the
cannula to open the intake valve.
Further alternatively or additionally, the pump includes an elasric outer sleeve, which
clings elastically to an outer, radial surface of the cannula to ciose the outlet valves, wherein the
; 5 elastic outer sleeve st}etches outw-ard in response to a pressure of the blood inside the c~nn
thereby opening the outlet valves.
Moreover, alternativelv or additionally, the pump includes an elaslic inner sleeve, which
clings elastically to an inner, radial surface of the cannula to close the intake valves, wherein
when pressure of the blood inside the cannula is reduced, the elastic inner sleeve deforms
inward in response to pressure of the blood outside the c~nn~ there~v opening the intake
valves.
There is further provided~ in accordance with a preferred embodiment of the present
invention, a method for augmentino the blood output of the heart, includino:
connecting a cannula, having distal and proximal ends and having intake and outlet
valves, to a fluid reservoir at the pro~cimal end of the cannula;
inserting the cannula throuoh an artery, so that the distal end of rhe cannula is inside the
left ventricle of the heart;
drawing blood from the ventricle, through the intake valve of the cannula and into the
fluid reservoir, by reducing a fluid pressure in the reservoir; and
ejecting the blood from the reservoir through the outlet valve or the cannula and into
the artery, by increasin, the fluid pressure in the reservoir.
Preferably, drawing blood and ejecting blood are performed repeatedly, in alternation,
wherein in each alternation, between 20 and 80 cc of blood are drawn and ejected. Preferably
drawing and ejectin~3 blood include applying hydraulic forces to the reservoir.
Preferably, the method includes sensing a heart beat signal, wherein drawing blood and
ejecting blood comprise drawing and injecting blood in synchrony with the heart beat, wherein
blood is drawn either during systole or during diastole.
Preferably, this method is carried out using a cardiac assist pump as described above.
CA 02226491 1998-01-07
W O 97/02850 PCT~L96/00045
There is further provided, in accordance with a preferred embodiment of tke present
invention, a one-way valve for use in a heart-assist device, which valve incl~ldes:
an outer sheath, defining an enclosing a lumen therein, and inclu-iinv a first radial
opening;
an inner sleeve, rotatably held inside the outer sheath, and incl~ltling a second radial
opening, which is alignable with the first radial opening by rotation of the sleeve, such that
when the first and second radial openings are mutually aligned, the valve is open; and
a torque coupling device, coupled to the inner sleeve,
wherein in response to flow of a fluid in the lumen in a first flow direction, the torque
lC coupling device causes the sleeve to rotate in a first rotational direction, therebv altering the
alignment ofthe first and second radial openings.
Preferably, in response to flow of the fluid in the lumen in a second flow direction,
generally opposite to the first flow direction, the torque couplin~r device causes the sleeve to
rota~e in a direction opposite to the first rotational direction. Preterablv rotation of the sleeve
in the firs~ rotational direction causes the valve to open. and rotation of the sleeve in the
opposite direction causes the valve to close.
Preferably, the torque coupling device includes winglets. ~i~ed to the sleeve.
There is also provided, in accordance with a preferred embodiment of the presentinvention, a one-way valve assemblv. including an inta};e ~alve and an outlet valve. for use in a
2 0 heart-assist device, w-hich assemblv includes:
an outer sheath~ defining and enclosing a lumen therein. and includin~ a first intake
opening and a first outlet opening; and
an inner sliding element, held inside the lumen and a.Yially movable therein, and
including a second intaL;e opening and a second outlet opening. respectivelv alignable with the
first intake opening and the frst outlet opening by axial movemen~ of the sliding element, such
that when the slidina element is in a first aYial position, the first and second intake openings are
aligned, so that the intal;e valve is open, and when the sliding element is in a second axial
position, the first and second outlet openings are aligned, so that the outlet valve is open,
wherein in response to changes of a fluid pressure inside the lumen, the inner sliding
3 0 element moves a.Yially in the sheath between the first and second axial positions.
Preferably, for any position of the sliding element intermedi~e the first and second axial
positions, no more than one of the intake and outlet valves is open.
Preferably, in response to an increase of the fluid pressure inside the lumen, the inner
sliding element moves to the second axial position, thereby opening the outlet valve, and in
3 5 response to a decrease of the fluid pressure inside the lumen, the inner sliding element moves to
the first aYi~l position, thereby opening the intake valve.
There is additionally provided. in accordance with a preferred embodiment of thepresent invention, a one-wav valve for use in a heart-assist device, w hich valve includes:
-
CA 02226491 1998-01-07
W O 97/02850 PCT~196/00045
an outer sheath, defininsg and enclosing a lumen therein, and including a radial opening;
and
an elastic outer sleeve, which clings elastically to an outer, radial surface of the sheath
to cover the radial opening, thereby closing the valve,
wherein in response to an increase of a fluid pressure inside the lumen, the outer sleeve
stretches outward, thereby openin~3 the valve.
Moreover, in accordance with another preferred embodiment of the present invention,
there is provided a one-way valve for use in a heart-assist device, said valve including:
an outer sheath, defining and enclosing a lumen therein, and including a radial opening;
l 0 and
an elastic inner sleeve, which clings elastically to an inner, radial surface of the sheath to
cover the radial opening, thereby closing the valve,
wherein in response to a decrease of a fluid pressure inside the lumen, the inner sleeve
deforms inward. thereby opening the valve.
Preferabiy, such one-way valves including an elastic sleeve also include a retaining rin_,
circull~r~ Liallv engaging a portion of the sleeve, which ring holds the sleeve in place relative
to the sheath.
The present invention will be more fully understood from the following detailed
description of ~he preferred embodiments thereof. taken together with the drawings in which:
CA 0222649l l998-0l-07
W O 97t02850 PCT~L~5/~
BRIEF DESCR~PTION OF THE DRAWINGS
Fig. 1 is a schematic, sectional repl~senLalion of a cardiac assist pump, in accordance
with a preferred embodiment of the present invention;
Fig. 2A is a schematic representation of a cannula in accordance with a ~I~Çe;ll~d
embodiment of the present invention, illustrating the insertion of the cannula into the heart;
Fig. 2B is a schematic representation of a cannula in accordance with another pl~rc:lled
embodimen~ of the present invention, illustrating the insertion of the cannula into the heart;
Fig. ~ is a schematic~ sectionah isometric 1 ep~ ese,ltaLion of a valve assembly in
accordance with a preferred embodiment of the present invention;
Fig. 4A is a sectional representation of a valve assemblv in accordance with a preferred
embodiment of the present invention, including intake and outlet valves, shown in a first
position in which the intal;e ~alves are open and the outlet valves are closed;
Fig. ~B is a sectional representation of the valve assemblv of Fig. ~A. shown in a second
posilion in which the inta~;e valves are closed and the outlet valves are open;
Fig SA is a sectional representation of a valve assembly in accordance with another
preferred embodiment ot' the present invention, including intake and outlet valves, shown in a
first position in which the intal;e v alves are open and the outlet valves are closed;
Fig. SB is a sectional re?resentation of the valve assembly of Fig. 5.~, shown in a second
2 0 position in which the intalie v alves are closed and the outlet vaives are open;
Fig. 6A is a schema~ic~ partlv sectional representation of a valve assembly in accordance
with a preferred embodiment ot' the present invention, shown in a first position in which the
valves are closed;
Fig. 6B is a schematic. partlv sectional representation of the valve assembly of Fig. 6A,
2 5 shown in a second position in ~vhich the valves are open;
Fig. 7A is a schematic. sectional representation of another valve assembly in accordance
with a preferred embodiment of the present invention~ shown in a first position in which the
valves are closed; and
Fig. 7B is a schematic~ sectional representation of the valve assemblv of Fig. 7A, shown
in a second position in tvhich the valves are open.
CA 02226491 1998-01-07
W O 97/02850 rCT~ .'C_15
DETAILED DESCR~PTION OF PREFERRED EMBOD~ENTS
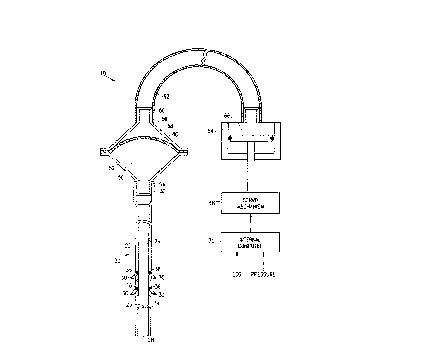
Reference is now made to Fig. 1, which is a schematic, sectional ~ s~ nn of a
cardiac assist pump system lg, in accordance with a preferred embodiment of the present
invention. The system comprises an intra-aortic cannula 20, having an outer sheath 22, which
defines and encloses an inner lumen 24. Preferably cannula 20 has a r7i~m~rer in the range of
15-30 French (5-10 mm) and is made of flexible, resilient material, for example, polyurethane
reinforced with stainless steel wire, so that it may be inserted into and passed through major
arteries of the human body. Cannula 20 further inciudes an intake valve '6, preferably axially
disposed, ~(ijacr nt to its distal end 28, and one or more outlet valves 30, radially disposed along
sheath 22 of the cannula. The intake and outlet valves are preferably made of stainless steel or
stiffplastic material, such as polycarbonate, or other suitable materials l;no~n in the art.
Intake valve 76 and outlet valves 30 are preferably one-way valves. so that blood may
flow into and out of cannula 20 substantially only in a sin~le direction: ente7ing through intake
valve 6 and exiting through ou71et valves 30 (corresponding to the direction of blood flow in
the body, as will be described below). In the preferred embodiment of the present invention
shown in Fig. 1, the intake and outlet valves comprise mechanical flap ~aives, which rotate
about respective hin~es ~4 and 36 to open and shut as desired. A though hinge 34 of intake
valve ~6 is shown to be located along a central axis of the valve, it mav similarly be located at
one side of the valve, like hinges ~6 of outlet valves 30.
Alternatively, intake val~,e 26 may comprise any other suitable tvpe of one-way valve,
for example a leaflet valve. Such leaflet valves are known in the art for use in heart-assist
devices, as described, for example~ in a PCT patent application entitled. "~lethod for Producing
Heart Valves and Heart Valves Produced by the Method," filed on even d~te with the present
application, which is assigned to the assignee of the present invention and ~~hose disclosure is
? 5 incorporated herein by reference.
In other preferred embodiments of the present invention, as w,ill be described below,
other types of intake and outlet valves may similarly be used.
Fig. ~A shows, schematically, the use of cannula 20 in a human heart ~0. Preferably the
cannula is inserted percutaneously~ through an incision into a peripheral arterv ~2, for example
the femoral artery, and passed upstream through aorta 44 into left ventricle '6 of heart 40. The
method of insertion is substantially similar to methods for insertion of other types of cardiac
cz7nnl71~e known in the art. T7ne len~th of cannula 20 is preferably approximately 60 cm, which
is generallv sufficient so that when distal tip 28 is positioned in ventricle d.6~ proximal end 32
remains outside the body, adjacent to the incision. Alternatively, the cannula may be inserted
3 5 surgically through a suitable incision elsewhere in the arterial system, and in such cases may be
shorter than 60 cm, depending on the distance from the incision to the heart.
~ Once cannula 20 is in place. intake valve 26 is opened, and blood flows from ventricle
46 into lumen 24. Preferably outlet valves 30 are kept closed while the blood fills the lumen.
11
CA 02226491 1998-01-07
W O 97/02850 PCTAIS6.~
Proximal end 32 may be temporarily opened, to vent out air or fluid that was inside cannula 20
before its insertion. Then intake valve 26 is closed and outlet valves 30 are opened, so that the
blood may flow out of the lumen and into aorta 44.
As illustrated in Fig. 1, intake valve 26 and outlet valves 30 preferably open and shut in
5 response to pressure exerted through pump system 18 to cannula ~0, in the following manner.
Proximal end 32 of cannula 20 is connected to a first chamber 50 of a fluid reservoir 52 through
a first fluid port 54. Fluid reservoir 5~ further includes a second chamber 56, which is
separated from first chamber 50 by a flexible diaphragm ~8. Diaphragm 58, which is preferably
made of flexible pol,vurethane, deforms to alter the respective volumes of chambers 50 and 56,
10 so as to substantially equalize the fluid pressures in the t~vo chambers, but prevents minolino of
the fluids in the first and second chambers.
Second chamber ~6 preferably contains a substantiall- incompressible liquid, such as
water or, alternatively. anv other suitable fluid, such as normal saline solution. Chamber 56 is
coupled via a second fluid port 60 throucJh a tube 6~ to a pump drive 64. .~ piston 66 in pump
15 drive 64 moves alternately up and do-vn to correspondingly increase and decrease the fluid
pressure in reservoir 5~. thereby pumping blood out of and into lumen ~4.
~ It will be appreciated that the maximum volume of blood that may be pumped in a
single stroke of piston 66 is roughly determined by the volume of reser~oir ~0. Preferably this
maximum single strol;e pumping volume is at least 50 cc~ and more preferablv up to 80 cc,
20 although piston 66 ma!- also be operated with a shorter stro~e to pump a smaller volume of
blood if desired. Preferably, the stroke is adjusted so that when pump drive 64 is operated at or
about the heart's natural rate~ sufficient blood can be pumped to perfuse substantially all of the
person's body.
It will further be appreciated that blood mav enter cannula ~0 and flow into first
25 chamber 50 only up to diaphragm ~ ~rO blood flo~s through tubing 6~ or into pump drive
64. Preferablv, cannula ~0 and reservoir 5'' are disposable and made for single use only, to
prevent transfer of infections and contamination.
Pump drive 64 is driven by a servo mechanism 6~ under the control of an internalcomputer 70~ which regulates the rate and stroke volume of piston 66. Preferably, computer 70
30 receives physiological si_nal inputs, such as ECG and blood pressure signals, and uses these
signals in optimally controlling pump drive 64, preferably to dri~e piston 66 at the rate of the
heart beat.
Preferably, computer 70 adjusts the delay of the piston stroke relative to the systolic
stroke of the heart. This delay may be adjusted so that c~nnula 0 pumps blood out
3 5 synchronously with the heart's systole; countersynchronously, during diastole; or at any suitable
phase therebetween. Alternatively, the rate of piston 66 may be set to be independent of the
heart rate, for example in order to rn~int~in steady perfusion during arrhythmia or fibrillation.
CA 02226491 1998-01-07
O 97102850 PCTnL96/00045
Fig. 2B illustrates, srh~m~tically, an alternative preferred embodiment of the present
invention, in which cannula 20, shown inserted into human heart 40, has a plurality of intake
valves 26, radially disposed along the length of the cannula. Radial intake valves 26 may be
flap valves, like valves 30 shown in Fig. 1 but opening inward, or one-way valves of other types
described below or otherwise known in the art. It will be appreciated that the cannula shown in
Fig. 2B functions in a substantially identical manner to that described above and illustrated in
Figs. 1 and 2A.
Fig. 3 illustrates sch~ tically an alternative construction of outlet valves 30, in
accordance with another preferred embodiment of the present invention. As shown in Fig. 3,
cannula 20 contains an inner sleeve 72, rotatably mounted inside outer sheath 22 and enclosing
lumen 24. Inner sleeve 72 extends axially along at least the portion of cannula 20 in~ ling
outlet valves 30. Each outlet valve 30 comprises an outer opening 74 in outer sheath 22 and an
inner openin_ 76 in inner sleeve 7'7. To open outlet valves 30, inner sleeve 72 is rotated so that
inner openings 76 are aligned with outer openings 74. When the inner and outer openings are
disaligned, the valves are closed.
Preferably, a plurality of winglets ~0 are fixed to the inner surface of sleeve 7Z and
cause the sleeve ~o rotate in response to blood flow through the lumen. When piston 66 is
drawn back in pump drive 64, as shown in Fig. 1, blood will flow through lumen 24
substantially in the direction indicated in Fig. 3 by an arrow S2. The force of this flow against
winglets 80 exerts a torque on sleeve 72, causing it to rotate in a clockwise direction, as
indicated in the figure bv an arrow 84. thus closing outlet valves 30. When a desired volume of
blood has been drawn into reservoir 50, piston 66 is pushed forward, so that blood flows in the
lumen in the direction opposite to arrow 82. Sleeve 7~ then rotates in the counterclockwise
direction, so that outlet valves 30 open.
Alternatively, sleeve 7~ or sheath 22 may be coupled proximally to a me~h~nic~l
rotation drive, of any suitable type known in the art, so as to effect the desired relative rotation
to open and close outlet valves 30
In the preferred embodiment of the present invention utilizing the outlet valves shown in
Fig. 3, intake valve ''6 (not shown in the figure) may be a mechanical flap valve or leaflet valve,
as described above. Alternatively, the intake valve may comprise a pair of alignable openings in
sheath 22 and sleeve 7'', which open and shut by the rotation of the sleeve relative to the
sheath, in a manner similar to the operation of openings 74 and 76. The sheath and sleeve are
constructed, however, so that when the pair of intake valve openings are aligned, to open
intake valve 26, openings 74 and 76 are rlic~ligne~l, to close outlet valves 30. Similarly, when
the outlet valve openings are aligned, the intake valve openings are ~liC~ligne~l, and thus shut.
Figs. 4A and 4B show still another preferred embodiment of the present invention, in
which a sliding element 90 inside lumen 24 alternately opens and shuts intake valve 26 and
outlet valves 30. Preferably, at least one axial tongue 91, fixed on the inner surface of cannula
13
CA 02226491 1998-01-07
WO 97/02850 PCT~lg6/00045
sheath 2, engages a m~chino groove 93 on the outer surface of sliding element 90, so that the
sliding element may move up and down inside the lumen, but may not rotate about its axis.
In Fig. 4A, the pressure in lumen 24 has been reduced below the blood pressure at the
proximal end of cannula 20, preferably by means of pump drive 64, as described above with
5 reference to Fig. 1. The relatively greater pressure of the blood at the distal end of cannula 20,
inside the left ventricle of the heart, forces sliding element 90 upward, opening intake valve 26.
Blood flows into lumen 24 through valve 26, via sliding element front openings 92.
Disalignment of sliding element side openings 94 with cannula radial openings 96 closes outlet
valves 30.
In Fig. 4B, the pressure in lumen 24 is increased, forcing sliding element 90 downward
and closing intake valve ~6. Openings 94 and 96 are now mutually ali2ned, thus opening outlet
valves 30, through which blood flows out into the aorta.
Figs. 5A and SB illustrate another preferred embodiment of the present invention,
substantially similar in operation to that shown in Figs. 4A and 4B. In Figs. SA and SB,
however, intake valves 26 are radiall~v disposed along sheath ~'' of cannula ~0, like outlet valves
30. A sliuing stopper element 97 inside lumen '74 comprises at least two sets of radial
openings: intake openin~Js ~ and ou~let openings 99. In Fig. 5A, increased pressure inside
lumen 24 causes sliding element 97 to move downward, so that outlet openings 99 are aligned
to open outlet valves i0. In Fig. ~B, reduced pressure in the lumen causes the sliding element
to move upward, ali~ning intal;e openings 98 with intake valves 26. A slot 100 in sliding
element 97 engages a pin 101 ~i~ed in sheath ''2~ so as to prevent rotation of the sliding
element. Other methods of preventing rotation, as are known in the art, may also be used.
Figs. 6A and 6B illustrate still another preferred embodiment of the present invention, in
which a fle~ible, elastic outer sleeve 102 covers and closes radial openings 104 in sheath 22 of
cannula ''0. which openings serve as outlet valves 30. Intake valve ~6 (not shown in these
figures) mav comprise a mechanical ~lap valve or leaflet valve or any other suitable type
described herein and/or l;no-~n in the art. Sleeve 102, which is preferably made of latex,
silicone, or other biocompatible rubber, is preferably held in place by squeeze ring 106.
Alternatively, sleeve 10'' mav be glued in place or otherwise secured.
In Fig. 6A, the pressure in lumen ''4 has been reduced so that blood may be drawn in
through the intake valve, as described above with reference to Fig. 1 The elasticity of sleeve
102 causes it to clin~7 radially to the outer surface of cannula '~0, so that outlet valves 30 remain
closed.
In Fig. 6B, however, the pressure of the blood inside lumen 24 has been increased. This
pressure exerts an outward force on sleeve 10'7 through openings 104, causing the sleeve to
stretch outward, and thus opening outlet valves 30.
As illustrated in Figs. 7A and 7B, in a further preferred embodiment of the present
invemion, a flexible, resilient inner sleeve 110 covers and closes radial openings 112 in sheath
14
CA 02226491 1998-01-07
W O 97/02850 PCT~196/00045
22 of cannula 20, which openings servé as intake valves 26. Sleeve 110 preferably co,.,~u,ises
biocompatible rubber, as described above, and is preferably held in place by a substantially rigid
expander ring 114. Alternatively, sleeve 110 may be glued in place or otherwise secured.
When the pressure inside lumen ~4 is greater than the blood pressure outside cannula 20, sleeve
110 is pressed outwards, closing valves 26, as shown in Fig. 7A. When the pressure inside the
lumen is reduced, the pressure of the blood outside cannula 20, exerted through openings 112,
causes sleeve 110 to deform inward, as shown in Fig. 7B, opening valves ~6.
It will be appreciated that the preferred embodiments described above are cited by way
of example, and the full scope of the invention is limited only by the claims.