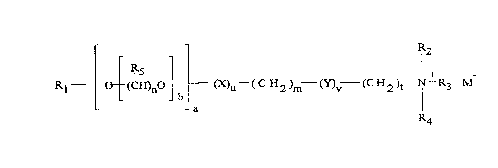Note: Descriptions are shown in the official language in which they were submitted.
CA 02226621 1998-01-12
W O 97/03164 PCTrUS96/11298
Detc~ composition~
Technical field
The present invention relates to a three component surfactant system
suitable for incorporation in de~ergellt compositions clesi~ned for use in
laundry and dish washing methods.
Back~ u,ld to the invention
The s~ti~f~ctory removal of greasy soils/stains, that is soils/stains having
a high proportion of triglycerides or fatty acids, is a challenge faced by
the form~ tor of detergent compositions for use in machine laundry and
dishwashing methods. Surf~ct~n~ components have traditionally been
employed in deter~ellt products to facilitate the removal of such greasy
soils/stains. In particular, surfactant ~y~lellls comprising cationic esters
have been described for use in greasy soil/stain removal.
For example, EP-B-21,491 discloses detergent compositions cont~inin~ a
nonionic/cationic surfactant mixture and a builder mixture comprising
aluminosilicate and polycarboxylate builder. The cationic surf~ct~nt may
be a cationic ester. Improved particulate and greasy/oily soil removal is
o described.
~ US-A-4,228,042 discloses biodegradable cationic surfactants, including
cationic ester surfactants for use in detergent compositions to provide
greasy/oily soil removal. The combination of these cationic surfactants
with nonionic surfactants in compositions designed for particulate soil
CA 02226621 1998-01-12
W O 97/03164 PCTrUS96tll298
removal is also described. Anionic surfactants are disclosed as optional
components of the compositions, but are present at Iow levels relative to
the cationic surfactant component.
US-A-4,239,660 discloses laundry detergent compositions cont~ining
cationic ester surfactant and nonionic surfactant at defined weight ratios.
Anionic surf~ct~nt~ are disclosed as optional components.
US-A-4,260,529 discloses laundry detergent compositions having a pH of
no greater than 11 cont~inin~ cationic ester surfactant and nonionic
surfactant at defined weight ratios. Anionic surfactants are disclosed as
optional components of the compositions, but are present at low levels
relative to the cationic ester surfactant component.
Anionic sulfate surfactants, particularly aL~yl sulfate and aL~cyl
ethoxysulfate surfactants are also known to be useful components of
deterge,lt compositions designed for greasy soil/stain removal. For
example, WO 93/18124 discloses compositions cont~inin~ a mixed alkyl
sulfate and alkyl ethoxysulfate surfactant ~yslelll.
It is desirable to combine the greasy soil/stain removal capability of
anionic sulfate and cationic ester surfactants in a single surfactant system.
The Applicants have however, now found that a problem in combining
cationic ester surfactants with anionic sulfate surfactants is the tendency
for insoluble cationic:anionic sulfate complexes to form. This in fact, can
lead to a marked reduction in greasy soil/stain removal ptlro~mance of
the combined surfactant system.
Surprisingly, the Applicants have found that if hydrophilic nonionic
surf~ct~nt, particularly hydrophilic alkoxylated nonionic surfactant is
added to the combined surf~t~n~ syslelll the aforementioned problem may
be ameliorated and significant greasy soil/stain removal performance
benefits re~ e~l. By contrast, the addition of a hydrophobic alkoxylated
surfactant does not ameliorate the problem.
All documents cited in the present description are, in relevant part,
incorporated herein by reference.
CA 0222662l l998-0l-l2
W O 97/03164 PCTrUS96/11298
~ Summ~ry of the Invention
~ According to the present invention there is provided a surfactant system
comprising
(a) an anionic sulfate surfactant component;
(b) a cationic ester surfactant; and
(c) a hydrophilic alkoxylated nonionic surfactant having a HLB value of at
least 9. 1
wherein the weight ratio of anionic sulfate surfactant component to
cationic ester surf~ct~nt is from 2.5:1 to 20:1 and the weight ratio of
anionic sulfate surfactant component to hydrophilic alkoxylated nonionic
surfactant is from 1:5 to 5:1.
In one ~refel~ed aspect, the anionic sulfate component contains both alkyl
sulfate and alkyl ethoxysulfate at a weight ratio of from 2:1 to 19:1.
In another prerelred aspect, the cationic ester surf~ct~nt is selected from
those having the formula:
R2
IR5 - I +
Rl - C3 (CH)no (~u--(CH2)m--(Y)v--(CH2)t--N--R3 M
~4
wherein R1 is a Cs-C31 linear or branched alkyl, alkenyl or alkaryl chain
or M-. N+(R6R7Rg)(CH2)S; X and Y, independently, are selected from
the group consisting of COO, OCO, O, CO, OCOO, CONH,NHCO,
OCONHNHCOO and CON(RgOR1o)Z, and CONR11Z wherein at least
one of X or Y is a COO, OCO, OCOO, OCONH or NHCOO group; R2,
R3, R4, R6, R7, R8 and R11 are independently selected from the group
CA 0222662l l998-0l-l2
WO97/03164 PCTrUS96/11298
consisting of alkyl, alkenyl, hydroxyaL~yl, hydroxy-alkenyl and alkaryl
groups having from 1 to 4 carbon atoms; Rg and R1o are independently
selected from the group con.~i.ctin~ of linear or branched, saturated or
~1n~ rated carbon chains having from 1 to 8 carbon atoms; Z is a
polyhydroxyhydrocarbyl moiety; and Rs is independently H or a C1-C3
alkyl group; wherein the values of m, n, s and t indepen~ent~y lie in the
range of from 0 to 8, the value of b lies in the range from 0 to 20, and the
values of a, u and v independently are either 0 or 1 with the proviso that
at least one of u and v is l; and wherein M is a counter anion.
In a further preferred aspect, the hydrophilic alkoxylated nonionic
surfactant comprises ethoxylated alcohol surfactant having a degree of
ethoxylation of at least 4.
According to another aspect of the present invention there is provided a
surfactant system comprising
(a) an anionic sulfate component;
(b) a cationic ester surfactant; and
(c) a hydrophilic nonionic surfactant system comprising a plurality of
nonionic surfactants having a HLB value of at least 9.1
wherein the weight ratio of anionic sulfate surf~ct~nt component to
cationic ester surfactant is from 2.5:1 to 20:1 and the weight ratio of
anionic sulfate surfactant component to hydrophilic nonionic surfactant
sy~lelll is from 1:5 to 5:1.
In a preferred embodiment of this further aspect of the present invention
the hydrophilic nonionic surfactant system preferably comprises
aLkoxylated nonionic and polyhydroxy fatty acid amide surfactant.
CA 02226621 1998-01-12
W O 97/03164 PCTrUS96/11298
s
Detailed description of the invention
~ The surfactant ~y~Lell-s of the invention are suitable for incorporation into
various detergellt compositions, especially those designed for use in
~ laundry and m~chin~ dishwashing.
Anionic sulfate surfactant
The first essenti~l element of the surfactant systems of the invention is a
an anionic sulfate surf~c~nt.
Anionic sulfate surfactants suitable for use herein include the linear and
branched primary and secondary alkyl sulfates, alkyl ethoxysulfates, fatty
oleoyl glycerol sulfates, alkyl phenol ethylene oxide ether sulfates, the
C5-C17 acyl-N-(C1-C4 alkyl) and -N-(Cl-C2 hydroxyalkyl) gll-c~mine
slllf~es, and sulfates of alkylpolysaccharides such as the slllf~tes of
alkylpolyglucoside (the nonionic nons-llf~te-i compounds being described
herein).
Alkyl sulfate surf~ct~nts are prefelred herein. These are preferably
selected from the linear and branched primary Clo-C1g alkyl sulfates,
more yrefel~bly the Cl1-Cls branched chain alkyl sulfates and the C12-
Clg linear chain alkyl s~llf~tes.
One ~rererred aspect of the invention has Clo-Clg alkyl sulfate as the
only anionic sulfate component.
Alkyl etho~cysulfate surf~t~ntc are also L,referred herein. These are
preferably selected from the group con~is~in~ of the Clo-Clg alkyl
sulfates which have been ethoxylated with from 0.5 to 20 moles of
ethylene oxide per molecule. More preferably, the alkyl ethoxysulfate
surfactant is a Cl1-C1g, most preferably C11-C1s alkyl sulfate which has
been ethoxylated with from 0.5 to 7, preferably from 1 to 5, moles of
ethylene oxide per molecule.
CA 02226621 1998-01-12
W O 97/03164 PCT~US96/11298
A particularly preferred aspect of the invention employs mixtures of allyl
sulfate and alkyl ethoxysulfate surfactants at a weight ratio of from 2:1 to
19:1, preferably from 3:1 to 15:1, most ~lefe.ably from 4:1 to 10:1.
The anionic sulfate surfactant is typically present as a salt, such as a
sodium, pot~ m, ammonium, or subst~ te-l ammonium salt such as the
mono-, di- and triethanol~mine salts.
Cationic ester surfactant
An essential component of the surfactant system is a cationic ester
surfactant. That is, a compound having surfactant properties comprising
at least one ester (ie -COO-) linkage and at least one cationically charged
group.
The weight ratio of anionic sulfate surfactant component to cationic ester
surfactant is from 2.5:1 to 20:1, preferably from 3:1 to 12:1, most
preferably from 5:1 to 10:1.
Suitable cationic ester surf~ct~ntc, including choline ester surfactants,
have for example been disclosed in US Patents No.s 4228042, 4239660
and 4260529.
Preferred water dispersible cationic ester surfactants are those having the
formula:
R2
Rl (}(CH)nO b (X~u (CH2)m (Y)v (C~H2)t N R3 M
- a
R4
wherein Rl is a Cs-C31 linear or branched alkyl, alkenyl or alkaryl chain
or M-. N+(R6R7Rg)(CH2)S; X and Y, independently, are selected from
the group concictin~ of COO, OCO, O, CO, OCOO, CONH, NHCO,
OCONH NHCOO and CON(RgORlo)Z, and CONRllZ wherein at least
CA 02226621 1998-01-12
W O 97/03164 PCTrUS96/11298
one of X or Y is a COO, OCO, OCOO, OCONH or NHCOO group; R2,
R3~ R4~ R6, R7, R8 and Rll are independently selected from the group
consisting of aLkyl, alkenyl, hydroxyalkyl, hydroxy-alkenyl and alkaryl
groups having from 1 to 4 carbon atoms; Rg and Rlo are indepçntlçntly
selected from the group con~icting of linear or br~ncherl, saturated or
m~ rated carbon chains having from 1 to 8 carbon atoms; Z is a
polyhydroxyhydrocarbyl moiety; and Rs is indepen-1ently H or a Cl-C3
alkyl group; wherein the values of m, n, s and t independently lie in the
range of from 0 to 8, the value of b lies in the range from 0 to 20, and the
values of a, u and v independently are either 0 or 1 with the proviso that
at least one of u and v is l; and wherein M is a counter anion.
Preferably R2,R3 and R4 are independently selected from CH3 and
-CH2CH20H.
Z is preferably derived from a reducing sugar in a reductive ~min~tion
reaction, more preferably Z is glycityl moiety. Suitable reducing sugars
include glucose, fructose, maitose, lactose, galactose, m~nn~se and
xylose as well as glyceraldehyde. Z is most preferably selected from the
group conci~tin~ of-CH2-(CHOH)n-CH2O-, -CH(CH2OH)-(CHOH)n l-
CH2O-, -CH2-(CHOH)2(CHOR')(CHOH)-CH2O-, where n = 1-5
inclusive and R' is H or a cyclic mono- or polysaccharide and aLkoxylated
derivatives thereof. Most ~lererred are glycityls wherein n = 4,
particularly -CH2-(CHOH)4-CH2O-.
Preferably M is selected from the group con~i~tin~ of halide, methyl
sulfate, sulfate, and nitrate, more L,lerelably methyl sulfate, chloride,
bromide or iodide.
Prererled water dispersible cationic ester surf~ct~nt~ are the choline esters
having the formula:
1~l CIH3
Rl--C--O--CH2CH2--I--CH3Mr
CH3
wherein Rl is a Cll-Clg linear or branched alkyl chain.
CA 0222662l l998-0l-l2
W O97/03164 PCT~US96/11298
Particularly preferred choline esters of this type include the stearoylcholine ester quaternary methylammonium halides (Rl=C17 alkyl),
palmitoyl choline ester quaternary methyl~mmonium halides (Rl=Cls
alkyl), myristoyl choline ester quaternary methyl~mmonium h~licle~
(Rl=C13 aLkyl), lauroyl choline ester methylammonium halides
(Rl=Cll alkyl), cocoyl choline ester quaternary methyl~mmonium
halides (Rl=Cll C13 alkyl), tallowyl choline ester quaternary
methylammonium halides (Rl=Cls C17 alkyl), and any mixtures thereof.
The particularly preferred choline esters, given above, may be preparedby the direct esterification of a fatty acid of the desired chain length with
rlimethylaminoethanol~ in the presence of an acid catalyst. The reaction
product is then quaternized with a methyl halide, forming the desired
cationic material. They may also be prepared by the direct esterification
of a long chain fatty acid of the desired chain length together with 2-
haloethanol, in the presence of an acid catalyst material. The reaction
product is then quaternized with trimethyl~mine, forming the desired
cationic material.
A particularly preferred gluc~mide betained ester has the formula:
O CH O
Il 1 3 ll
R1--C--N--CH2--(CHOH)4--CH2--O--C--O--CH2N+(CH3)3- M-
wherein Rl is a Cl l-Clg linear or branched alkyl chain.
Other suitable cationic ester surfactants have the structural formulas
below, wherein d may be from 0 to 20.
CA 02226621 1998-01-12
W O 97/03164 PCT~US96/11298
Rl - O - C - ( CH2 )d C - O - CH2CH2- N -CH3 M
- CH3
M CH3--~--CH2--CH2--O--C--~CH2)-- C--O--CH2--CH2--~--CH3M
CH3 ~H3
In a prefelred aspect the cationic ester surfactant is hydrolysable under the
conditions of a laundry wash method.
Hydrophilic alkoxylated nonionic surfactant
An essential component of the surfactant systems of the invention is a
hydrophilic alkoxylated nonionic surfactant. For the purposes of the
present invention hydrophilic is taken to mean having a HLB value of at
least 9.1, prereldbly at least 10.0, more preferably at least 11Ø
A prefef~ed method of ev~ ting HLB value (hydrophilic-lipophilic
balance value) herein is by use of the following formula:
MH
HLB value = 20 x
MH + ML
where MH = formula weight of the hydrophilic portion of the molecule
ML = formula weight of the lipophilic portion of the molecule
Use of this formula is described in Surfactants and Interfacial Phenomena,
M.J.Rosen, Wiley, 1978 at pages 241-245, particularly formula (8.13) of
page 244.
For clarity, an example calculation using this formula for a C14-Cls
alcohol (i.e. C14.s) ethoxylated with 7 moles of ethylene oxide would
proceed as follows:
Hydrophilic portion = CH3(CH2)13.5- , MH = 204
CA 0222662l l998-0l-l2
W O 97/03164 PCTrUS96/11298
Lipophilic portion = -(C2H4O)7-OH , ML = 325
325
HLB value = 20 x = 12 3
204 + 325
The weight ratio of anionic sulfate surf~ct~nt component to hydrophilicaL~oxylated nonionic surf~ct~nt is from l:S to S:l, preferably from 1:4 to
4:1, most preferably from 1:3 to 3 :1.
Essentially any alkoxylated nonionic surfactants having a HLB
(hydrophilic-lipophilic balance) value of at least 9.1 are suitable. The
ethoxylated and propoxylated nonionic surfactants are preferred.
The alkoxylated surfactants can be selected from the classes of the
nonionic condensates of alkyl phenols, nonionic ethoxylated fatty
alcohols, nonionic ethoxylated/propoxylated fatty alcohols, nonionic
ethoxylate/propoxylate con-lenc~tes with propylene glycol, and the
nonionic ethoxylate con~len~tion products with propylene oxide/ethylene
minP adducts.
Nonionic alko~ylated alcohol surfactant
The condensation products of aliphatic fatty alcohols with at least 4,
preferably from 4 to 25 moles of alkylene oxide, particularly ethylene
oxide and/or propylene oxide, are preferred hydrophilic alkoxylated
nonionic surfactants herein. The alkyl chain of the aliphatic fatty alcohol
can either be straight or branched, primary or secondary, and generally
contains from 12 to 24 carbon atoms.
Prere.led are the ethoxylated aliphatic fatty alcohols having a degree of
ethoxylation of at least 4. Particularly ~rere~.ed are the condensation
products of fatty aliphatic alcohols having an alkyl group cont~inin~ from
12 to 20 carbon atoms, ~r~felably from 12 to 16 carbon atoms with from
4 to 10 moles, preferably from 4 to 7 moles of ethylene oxide per mole of
alcohol.
CA 0222662l l998-0l-l2
W O 97/03164 PCTAUS96/11298
Exemplary ethoxylated fatty alcohols herein include the condensation
product of a C14-C1s alcohol with 7 moles of ethylene oxide (hlb =
12.3) and the condensation product of a fatty alcohol derived from
coconut feedstock (typically C12-C14) with 7 moles of ethylene oxide (hlb
= 12.8)
Additional surfactant
The surfactant systems of the invention may contain an additional
surfactant selected from non-sulfate anionic, hydrophobic alkoxylated
nonionic, non-aL~oxylated nonionic, non-ester cationic, ampholytic,
amphoteric and zwitterionic surfactants and mixtures thereof.
The additional surfactant is preferably present only at low levels, typically
of from 0% to 20%, more pr~fel~bly from 0% to 10% by weight of the
surf~ct~nt ~y~lem. Most preferably the surfactant system contains no
additional surfactant.
A typical listing of anionic, nonionic, ampholytic, and zwitterionic
classes, and species of these surf~ct~nt~, is given in U.S.P. 3,929,678
issued to T ~ hlin and Heuring on December 30, 1975. Further examples
are given in "Surface Active Agents and Detergents" (Vol. I and II by
Schwartz, Perry and Berch). A list of suitable cationic surfactants is given
in U.S.P. 4,259,217 issued to Murphy on March 31, 1981.
Where present, ampholytic, amphoteric and z~,vitteronic surfactants are
generally used in combination with one or more anionic and/or nonionic
surfactants.
Additional anionic surfactant
The surf~ct~nt system may contain additional non-sulfate anionic
surfactant. These can include salts (including, for example, sodium,
pot~si~-m, ammonium, and substituted ammonium salts such as mono-,
di- and triethanol~mine salts) of the anionic sulfonate, carboxylate and
sarcosinate surfactants.
CA 02226621 1998-01-12
W O 97/03164 PCTrUS96/11298
12
Other non-sulfate anionic surfactants include the isethionates such as the
acyl isethionates, N-acyl t~UldteS, fatty acid amides of methyl tauride,
alkyl succinates and sulfosuccinates, monoesters of sulfosuccinate
(especially salul~ted and lln~lrated C12-C18 monoesters) diesters of
sulfosuccinate (especially saturated and lln~hlrated C6-C14 diesters), N-
acyl sarcosinates. Resin acids and hydrogenated resin acids are also
suitable, such as rosin, hydrogenated rosin, and resin acids and
hydrogenated resin acids present in or derived from tallow oil.
Anionic sulfonate surfactant
Anionic sulfonate surfactants suitable for use herein include the salts of
Cs-C20 linear alkylbenzene sulfonates, alkyl ester sulfonates, C6-C22
primary or secondary aLkane sulfonates, C6-C24 olefin sulfonates,
sulfonated polycarboxylic acids, alkyl glycerol sulfonates, fatty acyl
glycerol sulfonates, fatty oleyl glycerol sulfonates, and any mixtures
thereof.
Anionic carboxylate surfactant
Suitable anionic carboxylate surfactants include the alkyl ethoxy
carboxylates, the alkyl polyethoxy polycarboxylate surf~et~ntc and the
soaps ('alkyl carboxyls'), especially certain secondary soaps as described
herein.
Suitable alkyl ethoxy carboxylates include those with the formula
RO(CH2CH20)X CH2COO-M+ wherein R is a C6 to Clg alkyl group, x
ranges from O to 10, and the ethoxylate distribution is such that, on a
weight basis, the amount of material where x is 0 is less than 20 ~ and M
is a cation. Suitable alkyl polyethoxy polycarboxylate surfactants include
those having the formula RO-(CHRl-CHR2-O)-R3 wherein R is a C6 to
Clg alkyl group"c is from 1 to 25, Rl and R2 are selected from the
group con~i~tinr~ of hydrogen, methyl acid radical, succinic acid radical,
hydroxysuccinic acid radical, and mixtures thereof, and R3 is selected
from the group con~i~tin~ of hydrogen, substit--te~l or unsubstituted
hydrocarbon having between 1 and 8 carbon atoms, and mixtures thereof.
CA 0222662l l998-0l-l2
W O 97/03164 PCT~US96/11298
13
Suitable soap surfactants include the secondary soap surfactants which
- contain a carboxyl unit connected to a secondary carbon. Preferredsecondary soap surfactants for use herein are water-soluble members
selected from the group con~i~ting of the water-soluble salts of 2-methyl-
1-undecanoic acid, 2-ethyl-1-decanoic acid, 2-propyl-1-nonanoic acid, 2-
butyl-l-octanoic acid and 2-pentyl-1-heptanoic acid. Certain soaps may
also be included as suds suppressors.
Alkali metal sarcosinate surfactant
Other suitable anionic surfactants are the alkali metal sarcosinates of
formula R-CON (R1) CH2 COOM, wherein R is a Cs-C17 linear or
branched alkyl or aLkenyl group, Rl is a Cl-C4 alkyl group and M is an
aLkali metal ion. Preferred examples are the myristyl and oleoyl methyl
sarcosinates in the form of their sodium salts.
Nonionic polyhydroxy fatty acid amide surfactant
Polyhydroxy fatty acid amides suitable for use herein are those having the
structural formula R2CONR1Z wherein: Rl is H, Cl-C4 hydrocarbyl, 2-
hydroxy ethyl, 2-hydroxy propyl, ethoxy, propoxy, or a mixture thereof,
plefeldble C1-C4 alkyl, more preferably C1 or C2 alkyl, most preferably
Cl alkyl (i.e., methyl); and R2 is a Cs-C31 hydrocarbyl, preferably
straight-chain Cs-Clg alkyl or aLkenyl, more preferably straight-chain
Cg-C17 alkyl or aLkenyl, most preferably straight-chain Cl l-C17 alkyl or
alkenyl, or mixture thereof; and Z is a polyhydroxyhydrocarbyl having a
linear hydrocarbyl chain with at least 3 hydroxyls directly connected to
the chain, or an aLkoxylated derivative (preferably ethoxylated or
propoxylated) thereof. Z preferably will be derived from a reducing
sugar in a reductive ~min~tion reaction; more preferably Z is a glycityl.
Nonionic fatty acid amide surf~ct~nt
Suitable fatty acid amide surfactants include those having the formula:
R6CoN(R7)2 wherein R6 is an alkyl group cont~ining from 7 to 21,
prefe~ably from 9 to 17 carbon atoms and each R7 is selected from the
CA 02226621 1998-01-12
W O 97/03164 PCT~US96/11298
14
group consisting of hydrogen, Cl-C4 alkyl, Cl-C4 hydroxyalkyl, and -
(C2H40)XH, where x is in the range of from 1 to 3.
Nonionic alkylpolysaccharide surfactant
Suitable alkylpolysaccharides for use herein are disclosed in U.S. Patent
4,565,647, Llenado, issued January 21, 1986, having a hydrophobic
group cont~inin~ from 6 to 30 carbon atoms and a polysaccharide, e.g., a
polyglycoside, hydrophilic group cont~inin~ from 1.3 to 10 saccharide
units.
Preferred alkylpolyglycosides have the formula
R20(CnH2nO)t(glYC~sYl)x
wherein R2 is selected from the group con~isting of alkyl, alkylphenyl,
hydroxyalkyl, hydroxyalkylphenyl, and mixtures thereof in which the
alkyl groups contain from 10 to 18 carbon atoms; n is 2 or 3; t is from O
to 10, and x is from 1.3 to 8. The glycosyl is preferably derived from
glucose.
Amphoteric surfactant
Suitable amphoteric surf~ct~nt~ for use herein include the amine oxide
surf~ct~nt~ and the alkyl amphocarboxylic acids.
Suitable amine oxides include those compounds having the formula
R3(0R4)XNO(R5)2 wherein R3 is selected from an alkyl, hydroxyalkyl,
acyl~midopropoyl and alkyl phenyl group, or mixtures thereof, cont~inin~
from 8 to 26 carbon atoms; R4 is an alkylene or hydroxyalkylene group
cont~ining from 2 to 3 carbon atoms, or mixtures thereof; x is from O to
5, ~refel~Lbly from O to 3; and each RS is an alkyl or hydroxyalkyl group
cont~inin~ from 1 to 3, or a polyethylene oxide group cont~inin~ from 1
to 3 ethylene oxide groups. Preferred are Clo-Clg alkyl dimethyl~min~
oxide, and C10 18 acyl~mi~lo aLkyl dimethyl~mine oxide.
CA 02226621 1998-01-12
W O 97/03164 PCTrUS96/11298
A suitable example of an alkyl aphodicarboxylic acid is Miranol(TM)
C2M Conc. m~nllf~ctured by Miranol, Inc., Dayton, NJ.
CA 0222662l l998-0l-l2
W O 97/03164 PCTrUS96/11298
16
Zwitterionic surf~ct~nt
Zwitterionic surf~ct~nt~ can also be incorporated into the deteLgellt
compositions hereof. These surfactants can be broadly described as
derivatives of secondary and tertiary amines, derivatives of heterocyclic
secondary and tertiary amines, or delivatives of quaternary ammonium,
quaternary phosphonium or tertiary sulrulliulLI compounds. Betaine and
slllt~ine surfactants are exemplary zwitterionic surfactants for use herein.
Suitable bet~ines are those compounds having the formula
R(R')2N+R2COO- wherein R is a C6-Clg hydrocarbyl group, each Rl
is typically Cl-C3 alkyl, and R2 is a Cl-Cs hydrocarbyl group. Plerel~ed
betaines are C12 18 dimethyl-ammonio hexanoate and the C10-l8
acylamidopropane (or ethane) dimethyl (or diethyl) betaines. Complex
betaine surfactants are also suitable for use herein.
Cationic surfactants
Additional cationic surf~ct~nt~ can also be used in the detergent
compositions herein. Suitable cationic surfactants include the quaternary
ammonium surfactants selected from mono C6-C16~ preferably C6-C10
N-alkyl or alkenyl ammonium surfactants wherein the rem~inin~ N
positions are substitl~te~l by methyl, hydroxyethyl or hydroxypropyl
groups.
Delel~ellt compositions
The surf~ct~nt ~y~le-lls of the present invention may be incorporated into
detergent compositions. Typical levels of incorporation are from 1% to
95%, preferably from 2% to 50%, most preferably from 3% to 30%
surf~c~nt ~y~ by weight of the detergent composition.
The detergent compositions may also contain additional detergent
components. The precise nature of these additional components, and
levels of incorporation thereof will depend on the physical form of the
composition, and the precise nature of the washing operation for which it
is to be used.
CA 02226621 1998-01-12
W O 97/03164 PCT~US96/11298
17
The detergent compositions preferably contain at least one additional
~ detergent component selected from bleaches, builders, organic polymeric
compounds, enzymes, suds ~uyyreSSOrS, lime soap dispersants, soil
suspension and anti-redeposition agents and corrosion inhibitors.
Water-soluble builder compound
The delergellt compositions in accord with the present invention
preferably contain a water-soluble builder compound, typically present at
a level of from 1 % to 80 % by weight, preferably from 10 % to 70 % by
weight, most preferably from 20~o to 60% by weight of the composition.
Suitable water-soluble builder compounds include the water soluble
monomeric polycarboxylates, or their acid forms, homo or copolymeric
polycarboxylic acids or their salts in which the polycarboxylic acid
comprises at least two carboxylic radicals separated from each other by
not more that two carbon atoms, borates, phosphates, and mixt~lres of any
of the foregoing.
The carboxylate or polycarboxylate builder can be momomeric or
oligomeric in type although monomeric polycarboxylates are generally
yrererred for reasons of cost and performance.
Suitable carboxylates cont~inin~ one carboxy group include the water
soluble salts of lactic acid? glycolic acid and ether derivatives thereof.
Polycarboxylates cont~ining two carboxy groups include the water-soluble
salts of succinic acid, malonic acid, (ethylenedioxy) diacetic acid, maleic
acid, diglycolic acid, tartaric acid, tartronic acid and fumaric acid, as well
as the ether carboxylates and the sulfinyl carboxylates. Polycarboxylates
co~ in~ three carboxy groups include, in particular, water-soluble
citrates, aconitrates and citraconates as well as succinate derivatives such
as the carboxymethyloxysuccinates described in British Patent No.
1,379,241, lactoxysuccinates described in British Patent No. 1,389,732,
and aminosuccinates described in Netherlands Application 7205873, and
the oxypolycarboxylate materials such as 2-oxa-1,1,3-propane
tricarboxylates described in British Patent No. 1,387,447.
CA 02226621 1998-01-12
W O 97/03164 PCTrUS96/11298
18
Polycarboxylates cont~inin~ four carboxy groups include oxydisuccinates
disclosed in British Patent No. 1,261,829, 1,1,2,2-ethane
tetracarboxylates, 1,1,3,3-propane tetracarboxylates and 1,1,2,3-propane
tetracarboxylates. Polycarboxylates cont~ining sulfo substituents include
the sulfosuccinate derivatives disclosed in British Patent Nos. 1,398,421
and 1,398,422 and in U.S. Patent No. 3,936,448, and the sulfonated
pyrolysed citrates described in British Patent No. 1,439,000. Preferred
polycarboxylates are hydroxycarboxylates cont~inin~ up to three carboxy
groups per molecule, more particularly citrates.
The parent acids of the monomeric or oligomeric polycarboxylate
chçl~tin~: agents or mixtures thereof with their salts, e.g. citric acid or
citrate/citric acid mixtures are also contemplated as useful builder
components.
Borate builders, as well as builders con~inin~ borate-forming materials
that can produce borate under detergent storage or wash conditions are
useful water-soluble builders herein.
Suitable examples of water-soluble phosphate builders are the alkali metal
tripolyphosphates, sodium, pot~si~lm and ammonium pyrophosphate,
sodium and pot~ lm and ammonium pyrophosphate, sodium and
potassium orthophosphate, sodium polymeta/phosphate in which the
degree of polymerization ranges from about 6 to 21, and salts of phytic
acid.
Partially soluble or insoluble builder compound
The deter~ellt compositions in accord with the present invention may
contain a partially soluble or insoluble builder compound, typically
present at a level of from 1 % to 80% by weight, ~lere.ably from 10% to
70% by weight, most preferably from 20% to 60% weight of the
composition.
F~mples of largely water insoluble builders include the sodium
~l~lminosilicates .
,
CA 0222662l l998-0l-l2
W O 97/03164 PCTAUS96/11298
19
Suitable aluminosilicate zeolites have the unit cell formula
Naz[(AlO2)z(SiO2)y]. xH2O wherein z and y are at least 6; the molar
ratio of z to y is from 1.0 to 0.5 and x is at least 5, plefeldbly from 7.5 to
276, more preferably from 10 to 264. The ~ minosilicate material are in
hydrated form and are preferably crystalline, cont~ining from 10% to
28%, more prefelably from 18% to 22% water in bound form.
The aluminosilicate zeolites can be naturally occurring materials, but are
preferably synthetically derived. Synthetic crystalline ~ minosilicate ion
exchange materials are available under the designations Zeolite A, Zeolite
B, Zeolite P, Zeolite X, Zeolite HS and mixtures thereof. Zeolite A has
the formula - -
Na 12 [A102) 12 (sio2)l2]. xH2O
wherein x is from 20 to 30, especially 27. Zeolite X has the formula
Na86 [(Alo2)86(sio2)lo6~. 276 H2O.
~lk?,linity system
The detergent compositions ~referably contain from 1.5 % to 95 %,
prefefably from 5% to 60%, most prefe~ably from 10% to 40% by weight
of the composition of an ~lk~linity system comprising components capable
of providing ~ linity species in solution. By ~lk~linity species it is
meant carbonate, bicarbonate, hydroxide and the various silicate anions.
Such ~lk~linity species can be formed for example, when ~lk~line salts
selected from aLali metal or ~lk~lin~. earth carbonate, bicarbonate,
hydroxide or silicate, including crystalline layered ~ilic~te, salts and any
mixtures thereof are dissolved in water. ALkali metal percarbonate and
persilicate salts are also suitable sources of ~lk~linity species.
F~mples of carbonates are the ~lk~lin~ earth and alkali metal carbonates,
including sodium carbonate and sesqui-carbonate and any mixtures
thereof with ultra-fine calcium carbonate such as are disclosed in German
Patent Application No. 2,321,001 published on November 15, 1973.
ALkali metal percarbonate salts are also suitable sources of carbonate
CA 02226621 1998-01-12
WO 97/03164 PCT~US96111298
species and are described in more detail in the section 'inorganic
perhydrate salts' herein.
Suitable silicates include the water soluble sodium silicates with an Si02:
Na20 ratio of from 1.0 to 2.8, with ratios of from 1.6 to 2.0 being
ereLled, and 2.0 ratio being most preferred. The silicates may be in the
form of either the anhydrous salt or a hydrated salt. Sodium silicate with
an SiO2: Na20 ratio of 2.0 is the most preferred silicate. Alkali metal
persilicates are also suitable sources of silicate herein.
Piefe~red crystalline layered silicates for use herein have the generalformula
NaMSix02x+ 1 ~YH20
wherein M is sodium or hydrogen, x is a number from 1.9 to 4 and y is a
number from 0 to 20. Crystalline layered sodium silicates of this type are
disclosed in EP-A-0164514 and methods for their preparation are
disclosed in DE-A-3417649 and DE-A-3742043. Herein, x in the general
formula above piefelably has a value of 2, 3 or 4 and is preferably 2. The
most preferred material is ~-Na2Si20s, available from Hoechst AG as
NaSKS-6.
The crystalline layered silicate material is preferably present in granular
deler~ ent compositions as a particulate in intim~t~ admixture with a solid,
water-soluble ionisable material. The solid, water-soluble ionisable
material is selected from organic acids, organic and inorganic acid salts
and mixtures thereof.
Organic peroxyacid bleachin~ ~yslem
A prere"ed feature of dele,gell~ compositions in accord with the invention
is an organic peroxyacid bleaching system. In one prefel-ed execution the
bleaching system contains a hydrogen peroxide source and an organic
peroxyacid bleach precursor compound. The production of the organic
peroxyacid occurs by an in situ reaction of the precursor with a source of
hydrogen peroxide. PreferLed sources of hydrogen peroxide include
CA 02226621 1998-01-12
W O 97/03164 PCTrUS96/11298
21
inorganic perhydrate bleaches. In an alternative preferred execution a
pLefolllled organic peroxyacid is incorporated directly into the
~ composition. Compositions cont~ininp mixtures of a hydrogen peroxide
source and organic peroxyacid precursor in combination with a plefolllled
organic pero~cyacid are also envisaged.
CA 0222662l l998-0l-l2
W O 97/03164 PCTAUS96/11298
22
Inor~anic perhydrate bleaches
Inorganic perhydrate salts are a prererled source of hydrogen peroxide.These salts are normally incorporated in the form of the alkali metal,
preferably sodium salt at a level of from 1% to 40% by weight, more
preferably from 2% to 30% by weight and most ~l-er~ bly from 5% to
2~ % by weight of the compositions.
Fx~mples of inorganic perhydrate salts include perborate, percarbonate,perphosphate, persulfate and persilicate salts. The inorganic perhydrate
salts are normally the alkali metal salts. The inorganic perhydrate salt
may be included as the crystalline solid without additional protection. For
certain perhydrate salts however, the ~referred executions of such
granular compositions utilize a coated form of the material which
provides better storage stability for the perhydrate salt in the granular
product. Suitable coatings comprise inorganic salts such as alkali metal
silicate, carbonate or borate salts or mixtures thereof, or organic
materials such as waxes, oils, or fatty soaps.
Sodium perborate is a prer~,~ed perhydrate salt and can be in the form of
the monohydrate of nominal formula NaB02H202 or the tetrahydrate
NaB02H202-3H20-
AL~cali metal percarbonates, particularly sodium percarbonate arepreferred perhydrates herein. Sodium percarbonate is an addition
compound having a formula corresponding to 2Na2C03.3H202, and is
available commercially as a crystalline solid.
Pot~si~lm peroxymonopersulfate is another inorganic perhydrate salt of
use in the de~e~ellt compositions herein.
Peroxyacid bleach precursor
Peroxyacid bleach precursors are compounds which react with hydrogen
pe~oxide in a perhydrolysis reaction to produce a peroxyacid. Generally
peroxyacid bleach precursors may be represented as
CA 02226621 1998-01-12
W O 97/03164 PCTnUS96/11298
23
o
X-C--L
where L is a leaving group and X is essentially any functionality, such
~ that on perhydroloysis the structure of the peroxyacid produced is
o
Il
X-C-OOH
Peroxyacid bleach precursor compounds are preferably incorporated at a
level of from 0.5 % to 20% by weight, more preferably from 1 % to 15 %
by weight, most preferably from 1.5% to 10% by weight of the detergent
compositions.
Suitable peroxyacid bleach precursor compounds typically contain one ormore N- or O-acyl groups, which precursors can be selected from a wide
range of classes. Suitable classes include anhydrides, esters, imides,
lactams and acylated derivatives of imi~ oles and oximes. Examples of
useful materials within these classes are disclosed in GB-A-1586789.
Suitable esters are disclosed in GB-A-836988, 864798, 1147871, 2143231
and EP-A-0170386.
Leavln~ ~roups
The leaving group, hereinafter L group, must be sufficiently reactive for
the perhydrolysis reaction to occur within the optimum time frame (e.g.,
a wash cycle). However, if L is too reactive, this activator will be
difficult to stabilize for use in a bleaching composition.
rlerelled L groups are selected from the group con~ictin~ of:
CA 02226621 1998-01-12
W O 97/03164 PCTnUS96/11298
24
~Y ~3 ~R3Y
--N--C--R1 _N N --N--C--C H--R4
1 3 L~ ' i
y
IR3 r
--O--C H=C--C H=C H2 --O--C H--C--C H=C H2
-~11--R1 & H7-C Y 11
O O
--O--C--CHR4 and N--S--CH--R4
1 3 11
and mixtures thereof, wherein Rl is an alkyl, aryl, or alkaryl group
cont~inin~ from 1 to 14 carbon atoms, R3 is an alkyl chain cont~inin~
from 1 to 8 carbon atoms, R4 is H or R3, and Y is H or a solubilizing
group. Any of Rl, R3 and R4 may be substituted by essentially any
functional group including, for example alkyl, hydroxy, alkoxy, halogen,
amine, nitrosyl, amide and ammonium or alkyl ~mmmonium groups
The pr~feLl~d solubilizing groups are -S03-M +, -C02-M +, -S04-M ~,
-N + (R3)~X- and O < --N(R3)3 and most preferably -S03-M + and
-C~2 M wherein R is an alkyl chain cont~inin~ from 1 to 4 carbon
atoms, M is a cation which provides solubility to the bleach activator and
X is an anion which provides solubility to the bleach activator.
Preferably, M is an aLkali metal, ammonium or substi~uted ammonium
CA 0222662l l998-0l-l2
W O 97/03164 PCTrUS96/11298
cation, with sodium and pot~ m being most preferred, and X is a
halide, hydroxide, methylsulfate or acetate anion.
Alkyl percarboxylic acid bleach precursors
Alkyl percarboxylic acid bleach precursors form percarboxylic acids on
perhydrolysis. Plerelled precursors of this type provide peracetic acid on
perhydrolysis.
Pleferled allyl percarboxylic precursor compounds of the imide type
include the N-,N,NlNl tetra acetylated alkylene rli~min~s wherein the
alkylene group contains from 1 to 6 carbon atoms, particularly those
compounds in which the alkylene group contains 1, 2 and 6 carbon atoms.
Tetraacetyl ethylene ~ mine (TAED) is particularly ~referled.
Other ~refelled alkyl percarboxylic acid precursors include sodium 3,5,5-
tri-methyl hexanoyloxyben~ene sulfonate (iso-NOBS), sodium
nonanoyloxybenzene sulfonate (NOBS), sodium acetoxybenzene sulfonate
(ABS) and pent~cetyl glucose.
Amide substituted alkyl peroxyacid precursors
Amide substit~lte~ alkyl peroxyacid precursor compounds are suitable
herein, including those of the following general formulae:
R1 C--N--R2 C L R1--N C R2 C- L
Il l 11 1 11 11
O R5 0 or R5 0 0
wherein R1 is an alkyl group with from 1 to 14 carbon atoms, R2 is an
aLkylene group cont~inin~ from 1 to 14 carbon atoms, and R5 is H or an
alkyl group cont~ining 1 to 10 carbon atoms and L can be essentially any
leaving group. Amide substit~lte~l bleach activator compounds of this type
are described in EP-A-0170386.
CA 02226621 1998-01-12
W O 97/03164 PCT~US96/11298
26
Perbenzoic acid precursor
Perbenzoic acid precursor compounds provide perbenzoic acid on
perhydrolysis. Suitable O-acylated perbenzoic acid precursor compounds
include the substituted and unsub~liLIl~e~1 benzoyl oxybenzene sulfonates,
and the benzoylation products of sorbitol, glucose, and all saccharides
with benzoylating agents, and those of the irnide type including N-benzoyl
succinimide, tetrabenzoyl ethylene ~ min~ and the N-benzoyl substituted
ureas. Suitable imitl~7ole type perbenzoic acid precursors include N-
benzoyl imicl~7ole and N-benzoyl ben7imi~ ole. Other useful N-acyl
group-cont~inin~ perbenzoic acid precursors include N-benzoyl
pyrrolidone, dibenzoyl taurine and benzoyl pyro~lllt~mic acid.
Cationic peroxyacid precursors
Cationic peroxyacid precursor compounds produce cationic peroxyacids
on perhydrolysis.
Typically, cationic peroxyacid precursors are formed by substi~ltin~ the
peroxyacid part of a suitable peroxyacid precursor compound with a
positively charged functional group, such as an ammonium or alkyl
~mmmonium group, preferably an ethyl or methyl ammonium group.
Cationic peroxyacid precursors are typically present in the solid detergent
compositions as a salt with a suitable anion, such as a halide ion.
The peroxyacid precursor compound to be so cationically substituted maybe a perbenzoic acid, or substit lte~l derivative thereof, precursor
compound as described hereinbefore. .Altern~tively, the peroxyacid
precursor compound may be an alkyl percarboxylic acid precursor
compound or an amide substitll~e~l alkyl peroxyacid precursor as
described hereinafter
Cationic peroxyacid precursors are described in U.S. Patents 4,904,406;4,751,015; 4,988,451; 4,397,757; 5,269,962; 5,127,852; 5,093,022;
5,106,528; U.K. 1,382,594; EP 475,512, 458,396 and 284,292; and in
JP 87-318,332.
CA 02226621 1998-01-12
W O 97/03164 PCTnUS96/11298
27
Examples of ~rert;lLed cationic peroxyacid precursors are described in
UK Patent Application No. 9407944.9 and US Patent Application Nos.
08/298903, 08/2986~0, 08/298904 and 08/298906.
- Suitable cationic peroxyacid precursors include any of the ammonium or
alkyl ammonium substi~lte~ alkyl or benzoyl oxyben7ene sulfonates, N-
acylated caprolactams, and monobenzoyltetraacetyl glucose benzoyl
peroxides. Prefe~red cationic peroxyacid precursors of the N-acylated
caprolactam class include the trialkyl ammonium methylene benzoyl
caprol~ct~mc and the trialkyl ammonium methylene alkyl caprolactams.
Benzoxazin or~anic peroxyacid precursors
Also suitable are precursor compounds of the benzoxazin-type, as
disclosed for example in EP-A-332,294 and EP-A~82,807, particularly
those having the formula:
[~N"C R~
wherein Rl is H, alkyl, alkaryl, aryl, or arylalkyl.
Preformed or anic peroxyacid
The organic peroxyacid ble~ching system may contain, in addition to, or
as an alternative to, an organic peroxyacid bleach precursor compound, a
preformed organic peroxyacid, typically at a level of from 1 % to 15% by
weight, more l,ref~,~bly from 1 % to 10% by weight of the composition.
A preferred class of organic peroxyacid compounds are the amide
substituted compounds of the following general formulae:
CA 02226621 1998-01-12
W O 97/03164 PCT~US96/11298
28
R1 C--N--R2--C OOH R1 N C R2--C OOH
Il l 11 1 11 11
O R5 0 or R5 0 0
wherein Rl is an alkyl, aryl or aLkaryl group with from 1 to 14 carbon
atoms, R2 is an alkylene, arylene, and alkarylene group cont~inin~ from 1
to 14 carbon atoms, and R5 is H or an alkyl, aryl, or alkaryl group
cont~inin~ 1 to 10 carbon atoms. Amide substituted or~anic peroxyacid
compounds of this type are described in EP-A-0170386.
Other organic peroxyacids include diacyl and tetraacylperoxides,
especially diperoxydodecanedioc acid, diperoxytetr~(lec~nedioc acid and
diperoxyhex~lec~ne-lioc acid. Mono- and diperazelaic acid, mono- and
diperbrassylic acid and N-phthaloylaminoperoxicaproic acid are also
suitable herein.
Bleach catalyst
The detelgellt compositions optionally contain a transition metal
cont~ininE~ bleach catalyst. One suitable type of bleach catalyst is a
catalyst ~y~em comprising a heavy metal cation of defined bleach
catalytic activity, such as copper, iron or m~n~nese cations, an auxiliary
metal cation having little or no bleach catalytic activity, such as zinc or
ahlmimlm cations, and a sequestrant having defined stability con~t~ntc for
the catalytic and ~llxili~ry metal cations, particularly
ethylenP~ min~tetraacetic acid,
ethylen~ minetetra(methylenephosphonic acid) and water-soluble salts
thereof. Such catalysts are disclosed in U.S. Pat. 4,430,243.
Other types of bleach catalysts include the m~n~nese-based complexes
disclosed in U.S. Pat. 5,246,621 and U.S. Pat. 5,244,594. Plerelred
examples of these catalysts include MnIV2(u-o)3(l ,4,7-trimethyl-1,4,7-
triazacyclononane)2-(PF6)2, MnLII2(u-O)l(u-OAc)2(1,4,7-trimethyl-
1,4,7-triazacyclononane)2-(C104)2, MnIV4(u-O)6(1,4,7-
triazacyclononane)4-(C104)2, MnIIIMnIV4(u-O)1(u-OAc)2 (1,4,7-
trimethyl-1,4,7-triazacyclononane)2-(ClO4)3, and mixtures thereof.
Others are described in European patent application publication no.
CA 02226621 1998-01-12
W O 97103164 PCT~US96/11298
29
549,272. Other li~nfls suitable for use herein include 1,5,9-trimethyl-
1,5,9-triazacyclododecane, 2-methyl-1,4,7-triazacyclononane, 2-methyl-
1,4,7-triazacyclononane, 1,2,4,7-tetramethyl-1,4,7-triazacyclononane,
and mixtures thereof.
For examples of suitable bleach catalysts see U.S. Pat. 4,246,612 and
U.S. Pat. 5,227,084. See also U.S. Pat. 5,194,416 which teaches
mononuclear m~nganp~se (IV) complexes such as Mn(1,4,7-trimethyl-
1,4,7-triazacyclononane)(OCH3)3 (PF6). Still another type of bleach
catalyst, as disclosed in U.S. Pat. 5,114,606, is a water-soluble complex
of m~n~nese (III), and/or (IV) with a ligand which is a non-carboxylate
polyhydroxy compound having at least three consecutive C-OH groups.
Other examples include binuclear Mn complexed with tetra-N-dentate and
bi-N-dentate ligands, including N4MnIII(u-0)2MnIVN4)+and
[13ipy2MnIII(u-0)2MnIVbipy2]-(clo4)3 .
Further suitable bleach catalysts are described, for example, in European
patent application No. 408,131 (cobalt complex catalysts), European
patent applications, publication nos. 384,503, and 306,089 (metallo-
porphyrin catalysts), U.S. 4,728,455 (m~ng~nese/multiclent~te ligand
catalyst), U.S. 4,711,748 and European patent application, publication
no. 224,952, (absorbed m~n~nese on aluminosilicate catalyst), U.S.
4,601,845 (~lllminosilicate support with manganese and zinc or
m~pnt~sium salt), U.S. 4,626,373 (m~ng~nese/ligand catalyst), U.S.
4,119,557 (ferric complex catalyst), German Pat. specification 2,054,019
(cobalt chelant catalyst) ~n~ n 866,191 (transition metal-cont~ining
salts), U.S. 4,430,243 (chelants with m~n~nPse cations and non-catalytic
metal cations), and U.S. 4,728,455 (m~n~n~se gluconate catalysts).
Heavy metal ion sequestrant
The detergellt compositions in accord with the invention preferably
contain as an optional component a heavy metal ion sequestrant. By heavy
metal ion sequestrant it is meant herein components which act to sequester
(chelate) heavy metal ions. These components may also have calcium and
m~nPsium chelation capacity, but preferentially they show selectivity to
binding heavy metal ions such as iron, m~n~~nese and copper.
CA 0222662l l998-0l-l2
W O 97/03164 PCT~US96/11298
Heavy metal ion sequestrants are generally present at a level of from
0.005 % to 20 ~, ~rerelably from 0.1 ~ to 10 %, more preferably from
0.25 ~ to 7.5 % and most prererably from 0.5% to 5~ by weight of the
compositions.
Suitable heavy metal ion sequestrants for use herein include organic
phosphonates, such as the amino alkylene poly (alkylene phosphonates),
alkali metal ethane l-hydroAy disphosphonates and nitrilo trimethylene
phosphonates.
Preferled among the above species are diethylene triamine penta
(methylene phosphonate), ethylene ~ mine tri (methylene phosphon~te)
hexamethylene ~ mine tetra (methylene phosphonate) and hydroxy-
ethylene 1,1 diphosphonate.
Other suitable heavy metal ion sequestrant for use herein include
nitrilotriacetic acid and polyaminocarboxylic acids such as
ethylen~Ai~minotetracetic acid, ethylenetri~mine pent~cetic acid,
ethylen~i~mine disuccinic acid, ethyl~n~ mine diglutaric acid, 2-
hydroAy~ro~ylenP~ mine disuccinic acid or any salts thereof. Especially
preferred is ethylene~ mine-N~N~-disuccinic acid (EDDS) or the alkali
metal, ~lk~line earth metal, ~mmcmillm, or substituted ammonium salts
thereof, or mixtures thereof.
Other suitable heavy metal ion sequestrants for use herein are
iminodiacetic acid derivatives such as 2-hydroxyethyl diacetic acid or
glyceryl imino rli~retic acid, described in EP-A-317,542 and EP-A-
399,133. The imino~ cetic acid-N-2-hydroAyplo~yl sulfonic acid and
aspartic acid N-carboAymethyl N-2-hydroAy~rupyl-3-sulfonic acid
sequestrants described in EP-A-516,102 are also suitable herein. The ~3-
~l~nine-N~Nl-diacetic acid, aspartic acid-N,N'-diacetic acid, aspartic acid-
N-monoacetic acid and iminodisuccinic acid sequestrants described in EP-
A-509,382 are also suitable.
EP-A476,257 describes suitable amino based sequestrants. EP-A-
510,331 describes suitable sequestrants derived from collagen, keratin or
CA 02226621 1998-01-12
W O 97/03164 PCTrUS96/11298
31
casein. EP-A-528,859 describes a suitable alkyl iminodiacetic acid
sequestrant. Dipicolinic acid and 2-phosphonobutane-1,2,4-tricarboxylic
acid are alos suitable. Glycin~mi~le-N,N'-disuccinic acid (GADS),
ethylen~ mine-N-N'-~liElllt~ric acid (EDDG) and 2-
- hydro~ypropylene li~minP--N-N'-disuccinic acid (HPDDS) are also
suitable.
Enzyme
Another preferred ingredient useful in the detergent compositions is one
or more additional enzymes.
Preferred additional enzymatic materials include the commercially
available lipases, cutinases, amylases, neutral and ~lk~line proteases,
esterases, endogl~lc~n~es, cellulases, pectinases, l~ct~es and peroxidases
conventionally incorporated into detergellt compositions. Suitable
enzymes are discussed in US Patents 3,519,570 and 3,533,139.
rlerelled commercially available protease enzymes include those sold
under the tr~clen~me~ Alcalase, Savinase, Primase, Durazym, and
Esperase by Novo rnrlnstries A/S (Denmark), those sold under the
tradename Maxatase, Maxacal and Maxapem by Gist-Brocades, those
sold by Genencor International, and those sold under the tradename
Opticlean and Optimase by Solvay Enzymes. Protease enzyme may be
incorporated into the compositions in accordance with the invention at a
level of from 0.0001% to 4~ active enzyme by weight of the
composition.
Prer~rled amylases include, for example, a-amylases obtained from a
special strain of B licheniformis, described in more detail in GB-
1,269,839 (Novo). rrefelled commercially available amylases include
for example, those sold under the tradename Rapidase by Gist-Brocades,
and those sold under the tr~-len~me Termamyl and BAN by Novo
Tn~ tries A/S. Amylase enzyme may be incorporated into the
composition in accordance with the invention at a level of from 0.0001%
to 2% active enzyme by weight of the composition.
CA 02226621 1998-01-12
WO 97/03164 PCTrUS96/11298
32
Lipolytic enzyme may be present at levels of active lipolytic enzyme offrom 0.0001% to 2% by weight, preferably 0.001% to 1~ by weight,
most ~rerel~bly from 0.001% to 0.5 % by weight of the compositions.
The lipase may be fungal or bacterial in origin being obtained, for
example, from a lipase producing strain of Humicola sp., Thermomyces
sp. or Pseudomonas sp. including Pseudomonas pseudoalcali~enes or
Pseudomas fluorescens. Lipase from chemically or genetically modified
mllt~nt~ of these strains are also useful herein. A preferred lipase is
derived from Pseudomonas pseudoalcali~enes, which is described in
Granted European Patent, EP-B-0218272.
Another preferred lipase herein is obtained by cloning the gene from
Humicola l~ml~inosa and expressing the gene in Aspergillus orvza, as
host, as described in European Patent Application, EP-A-0258 068, which
is commercially available from Novo Industri A/S, Bagsvaerd, Denmark,
under the trade name Lipolase. This lipase is also described in U.S.
Patent 4,810,414, Huge-Jensen et al, issued March 7, 1989.
Alcalase is a ~lereLled enzyme component of bleach-free detergent
compositions ~iesignp~l for the washing of coloured or delicate fabrics,
particularly in combination with a crystal growth inhibitor (e.g. HEDP)
component
Or~anic polymeric compound
Organic polymeric compounds are preferred additional components of the
deler~ellt compositions in accord with the invention, and are preferably
present as components of any particulate components where they may act
such as to bind the particulate component together. By organic polymeric
compound it is meant herein essçnti~lly any polymeric organic compound
commonly used as dispersants, and anti-redeposition and soil suspension
agents in detergent compositions, including any of the high molecular
weight organic polymeric compounds described as clay flocc~ ting
agents herein.
CA 02226621 1998-01-12
W O 97/03164 PCTrUS96/11298
33
Organic polymeric compound is typically incorporated in the detergent
compositions of the invention at a level of from 0.1 % to 30%, l,refe~ably
fromO.5% to 15%, most~refelablyfrom 1% to 10% byweightofthe
compositions.
-
Examples of organic polymeric compounds include the water solubleorganic homo- or co-polymeric polycarboxylic acids or their salts in
which the polycarboxylic acid comprises at least two carboxyl radicals
separated from each other by not more than two carbon atoms. Polymers
of the latter type are disclosed in GB-A-1,596,756. Examples of such
salts are polyacrylates of MWt 2000-5000 and their copolymers with
maleic anhydride, such copolymers having a molecular weight of from
20,000 to 100,000, especially 40,000 to 80,000.
The polyamino compounds are useful herein including those derived from
aspartic acid such as those disclosed in EP-A-305282, EP-A-305283 and
EP-A-351629.
Terpolymers cont~ining monomer units selected from maleic acid, acrylic
acid, polyaspartic acid and vinyl alcohol, particularly those having an
average molecular weight of from 5,000 to 10,000, are also suitable
herein.
Other organic polymeric compounds suitable for incorporation in the
deler~ellt compositions herein include cellulose derivatives such as
methylcellulose, carboxymethylcellulose, hydroxypropylmethylcellulose
and hydroxyethylcellulose.
Further useful organic polymeric compounds are the polyethylene glycols,
particularly those of molecular weight 1000-10000, more particularly
2000 to 8000 and most ~referably about 4000.
Suds ~uypressin~ ~y~lelll
The delergellt compositions in accord with the invention, when formulated
for use in machine washing compositions, preferably comprise a suds
~uppressing ~y~lem present at a level of from 0.01 % to 15 5~, preferably
CA 0222662l l998-0l-l2
W O 97/03164 PCT~US96/11298
34
from 0.05% to 105~, most preferably from 0.1% to 5% by weight of the
composition.
Suitable suds suppressing systems for use herein may comprise essentially
any known antifoam compound, including, for example silicone antifoam
compounds and 2-alkyl alcanol antifoam compounds.
By antifoam compound it is meant herein any compound or mixtures of
compounds which act such as to depress the foaming or sn-l~ing produced
by a solution of a detergent composition, particularly in the presence of
agitation of that solution.
Particularly ~refelred antifoam compounds for use herein are silicone
antifoam compounds defined herein as any antifoam compound including
a silicone component. Such silicone antifoam compounds also typically
contain a silica component. The term "silicone" as used herein, and in
general throughout the in(i~ctry~ encompasses a variety of relatively high
molecular weight polymers cont~ining siloxane units and hydrocarbyl
group of various types. Preferred silicone antifoam compounds are the
siloxanes, particularly the polydimethylsiloxanes having trimethylsilyl end
blocking units.
Other suitable antifoam compounds include the monocarboxylic fatty
acids and soluble salts thereof. These materials are described in US
Patent 2,954,347, issued September 27, 1960 to Wayne St. John. The
monocarboxylic fatty acids, and salts thereof, for use as suds suppressor
typically have hydrocarbyl chains of 10 to 24 carbon atoms, preferably 12
to 18 carbon atoms. Suitable salts include the alkali metal salts such as
sodium, potassium, and lithi~lm salts, and ammonium and
aLkanol~mmonium salts.
Other suitable antifoam compounds include, for example, high molecular
weight fatty esters (e.g. fatty acid triglycerides), fatty acid esters of
monovalent alcohols, aliphatic Clg-C40 ketones (e.g. stearone) N-
alkylated amino tri~7in~s such as tri- to hexa-alkylmel~min~s or di- to
tetra alkylc1i~mine chlortri~7inPc formed as products of cyanuric chloride
with two or three moles of a primary or secondary amine cont~ining 1 to
CA 02226621 1998-01-12
W O 97/03164 PCT~US96/11298
24 carbon atoms, propylene oxide, bis stearic acid amide and monostearyl
di-alkali metal (e.g. sodium, potassium, lithium) phosphates and
phosphate esters.
- A ~rertr~d suds ~up~ressing system comprises
(a) antifoam compound, ~refer~bly silicone antifoam compound, most
preferably a silicone antifoam compound co~ ising in
combination
(i) polydimethyl siloxane, at a level of from 50% to 99%,
preferably 75 % to 9S % by weight of the silicone antifoam
compound; and
(ii) silica, at a level of from 1% to 50%, yrefe~dbly 5% to 25%
by weight of the silicone/silica antifoam compound;
wherein said silica/silicone antifoam compound is incorporated at a level
of from 5% to 50%, preferably 10% to 40% by weight;
(b) a dispersant compound, most preferably comprising a silicone
glycol rake copolymer with a polyoxyalkylene content of 72-78 %
and an ethylene oxide to propylene oxide ratio of from 1:0.9 to
1:1.1, at a level of from 0.5% to 10%, preferably 1% to 10% by
weight; a particularly ~referred silicone glycol rake copolymer of
this type is DCOS44, commercially available from DOW Corning
under the tr~len~me DCOS44;
(c) an inert carrier fluid compound, most preferably comprising a C16-
Clg ethoxylated alcohol with a degree of ethoxylation of from S to
50, prefelably 8 to lS, at a level of from 5% to 80%, preferably
10% to 70%, by weight;
A highly ~re~elled particulate suds suppressing system is described in EP-
A-0210731 and comprises a silicone antifoam compound and an organic
carrier material having a melting point in the range 50~C to 85~C,
wherein the organic carrier material comprises a monoester of glycerol
CA 0222662l l998-0l-l2
W O97/03164 PCT~US96/11298
36
and a fatty acid having a carbon chain cont~ining from 12 to 20 carbon
atoms. EP-A-0210721 discloses other preferred particulate suds
suppressing systems wherein the organic carrier material is a fatty acid or
alcohol having a carbon chain cont~ining from 12 to 20 carbon atoms, or
a mixture thereof, with a melting point of from 45~C to 80~C.
CA 02226621 l998-0l-l2
W O 97/03164 PCTrUS96/11298
37
Clay softenin~ system
The delergent compositions may contain a clay softening ~y~e
comprising a clay mineral compound and optionally a clay flocc~ tin~
agent.
The clay mineral compound is preferably a smectite clay compound.
Smectite clays are disclosed in the US Patents No.s 3,862,058,
3,948,790, 3,954,632 and 4,062,647. European Patents No.s EP-A-
299,575 and EP-A-313,146 in the name of the Procter and Gamble
Company describe suitable organic polymeric clay flocc~ tin~ agents.
Polymeric dye transfer inhibitin~ a~ents
The delergent compositions herein may also comprise from 0.01 % to 10
%, preferably from O.OS % to 0.5 % by weight of polymeric dye transfer
in_ibiting agents.
The polymeric dye transfer inhibiting agents are preferably selècted from
polyamine N-oxide polymers, copolymers of N-vinylpyrrolidone and N-
vinylimicl~7ole, polyvinylpyrrolidonepolymers or combinations thereof.
It has been found that bleachfree delergent compositions cont~inin~
polymeric dye transfer inhibiting agents, crystal growth inhibitors (e.g.
HEDP) and high (>7.5%) levels of nonionic surfactant suprisingly
provide excellent whiten~s m~in~eIl~nce, in addition to reduced tendency
to fade coloured fabrics when used in the l~lln-lering of mixed fabric
loads.
a) Polyamine N-oxide polymers
Polyamine N-oxide polymers suitable for use herein contain units havingthe following structure formula:
CA 0222662l l998-0l-l2
W O 97/03164 PCTrUS96/11298
38
(I) I
wherein P is a polymerisable unit, and
00 0
Il 11 11
A is NC, CO, C, -O-, -S-, -N-; x.is O or1;
R are aliphatic, ethoxylated aliphatics, aromatic, heterocyclic or alicyclic
groups or any combination thereof whereto the nitrogen of the N-O group
can be ~tt~chP-l or wherein the nitrogen of the N-O group is part of these
groups.
The N-O group can be represented by the following general
structures:
0
(R1 ) X - I -(R2)y
(R3)z or = N-(R1 )x
wherein Rl, R2, and R3 are aliphatic groups, aromatic, heterocyclic or
alicyclic groups or combinations thereof, x or/and y or/and z is 0 or 1 and
wherein the nitrogen of the N-O group can be attached or wherein the
nitrogen of the N-O group forms part of these groups. The N-O group can
be part of the polymerisable unit (P) or can be attached to the polymeric
backbone or a combination of both.
Suitable polyamine N-oxides wherein the N-O group forms part of the
polymerisable unit comprise polyamine N-oxides wherein R is selected
from aliphatic, aromatic, alicyclic or heterocyclic groups. One class of
said polyamine N-oxides comprises the group of polyamine N-oxides
wherein the nitrogen of the N-O group forms part of the ~-group.
-
CA 02226621 1998-01-12
W O 97/03164 PCTrUS96/11298
39
Preferred polyamine N-oxides are those wherein R is a heterocyclic group
such as pyrridine, pyrrole, imirl~7ole, pyrrolidine, piperidine, quinoline,
~ acridine and derivatives thereof.
Other suitable polyamine N-oxides are the polyamine oxides whereto the
N-O group is attached to the polymerisable unit. A prererl-ed class of
these polyamine N-oxides comprises the polyamine N-oxides having the
general formula (I) wherein R is an aromatic,heterocyclic or alicyclic
groups wherein the nitrogen of the N-O functional group is part of said R
group. Fx~m~les of these classes are polyamine oxides wherein R is a
heterocyclic compound such as pyrridine, pyrrole, imi~ ole and
derivatives thereof.
The polyamine N-oxides can be obtained in almost any degree of
polymerisation. The degree of polymerisation is not critical provided the
material has the desired water-solubility and dye-suspending power.
Typically, the average molecular weight is within the range of 500 to
1000,000.
b) Copolymers of N-vinylpyrrolidone and N-vinylimidazole
Suitable herein are coploymers of N-vinylimitl~7ole and N-
vinylpyrrolidone having an average molecular weight range of from 5,000
to 50,000. The prefe~red copolymers have a molar ratio of N-
vinylimi~7ole to N-vinylpyrrolidone from 1 to 0.2.
c) Polyvinylpyrrolidone
The detergent compositions herein may also utilize polyvinylpyrrolidone
("PVP") having an average molecular weight of from 2,500 to 400,000.
Suitable polyvinylpyrrolidones are commercially vailable from ISP
Corporation, New York, NY and Montreal, C~n~ under the product
names PVP K-15 (viscosity molecular weight of 10,000), PVP K-30
(average molecular weight of 40,000), PVP K-60 (average molecular
weight of 160,000), and PVP K-90 (average molecular weight of
360,000). PVP K-15 is also available from ISP Corporation. Other
CA 0222662l l998-0l-l2
W O97/03164 PCTrUS96/11298
suitable polyvinylpyrrolidones which are commercially available from
BASF Cooperation include Sokalan HP 165 and Sokalan HP 12.
CA 02226621 1998-01-12
W O 97/03164 PCTnUS96/11298
d) Polyvinyloxazolidone
- The detergent compositions herein may also utilize polyvinyloxazolidones
as polymeric dye transfer inhibiting agents. Said polyvinyloxazolidones
have an average molecular weight of from 2,500 to 400,000.
e) Polyvinylimidazole
The detergellt compositions herein may also utilize polyvinylimicl~7ole as
polymeric dye transfer inhibiting agent. Said polyvinylimid~7oles
~rererably have an average molecular weight of from 2,500 to 400,000.
Optical bri~htener
The de~el~ellt compositions herein also optionally contain from about
0.005% to 5~o by weight of certain types of hydrophilic optical
bri~hten~rs.
Hydrophilic optical briPhte-ners useful herein include those having the
structural formula:
Rl R2
N~ ~ IH IH~NH~N(~N
R2 SO3M SO3M Rl
wherein Rl is selected from ~nilino, N-2-bis-hydroxyethyl and NH-2-
hydroxyethyl; R2 is selected from N-2-bis-hydroxyethyl, N-2-
hydroxyethyl-N-methyl~mino, morphilino, chloro and amino; and M is a
salt-forming cation such as sodium or potassium.
When in the above formula, Rl is ~nilino, R2 is N-2-bis-hydroxyethyl
and M is a cation such as sodium, the brightener is 4,4',-bis[(4-anilino-6-
(N-2-bis-hydroxyethyl)-s-triazine-2-yl)amino]-2,2'-stilbenedisulfonic acid
and disodium salt. This particular brightener species is commercially
CA 0222662l l998-0l-l2
W O 97/03164 PCTrUS96/11298
42
marketed under the tradename Tinopal-UNPA-GX by Ciba-Geigy
Corporation. Tinopal-UNPA-GX is the preferred hydrophilic optical
brigh~P-ner useful in the deterge~t compositions herein.
When in the above formula, Rl is anilino, R2 is N-2-hydroxyethyl-N-2-
methyl~mino and M is a cation such as sodium, the brightener is 4,4'-
bis[(4-anilino-~(N-2-hydroxyethyl-N-methyl~ mino)-s-triazine-2-
yl)amino]2,2'-stilben~ llfonic acid disodium salt. This particular
brightener species is commercially marketed under the tr~-len~me Tinopal
SBM-GX by Ciba-Geigy Corporation.
When in the above formula, R1 is anilino, R2 is morphilino and M is a
cation such as sodium, the bri~htener is 4,4'-bis[(4-anilino-6-morphilino-
s-triazine-2-yl)amino]2,2'-stilbenedisulfonic acid, sodium salt. This
particular briPhten~r species is commercially marketed under the
tradename Tinopal AMS-GX by Ciba Geigy Corporation.
Cationic fabric softenin~ a~ents
Cationic fabric softening agents can also be incorporated into
compositions in accordance with the present invention. Suitable cationic
fabric softening agents include the water insoluble tertiary amines or
dilong chain amide m~teri~lc as disclosed in GB-A-1 514 276 and EP-B-0
011 340.
Cationic fabric softening agents are typically incorporated at total levels
of from 0.5 % to 15 % by weight, normally from 1 % to 5 % by weight.
Other optional inPredients
Other optional ingre~ients suitable for inclusion in the delerge~lt
compositions in accord with the invention include perfumes, colours and
filler salts, with sodium sulfate being a preferred filler salt.
pH of the compositions
CA 02226621 1998-01-12
W O 97/03164 PCTnUS96/11298
43
The delergent compositions preferably have a pH measured as a 1%
solution in distilled water of at least 10.0, preferably from 10.0 to 12.5,
- most pre~idbly from 10.5 to 12 Ø
Form of the compositions
Solid detergent compositions in accordance with the invention can take a
variety of physical forms including granular, bar and tablet forms. The
detergent compositions can also be in liquid form. The compositions are
particularly the so-called concentrated granular detergent compositions
adapted to be added to a washing machine by means of a dispensing
device placed in the machine drum with the soiled fabric load.
The mean particle size of the components of granular compositions in
accordance with the invention should prefelably be such that no more that
5 % of particles are greater than 1 .7mm in diameter and not more than 5 %
of particles are less than 0.15mm in diameter.
The term mean particle size as defined herein is calculated by sieving a
sample of the composition into a number of fractions (typically 5
fractions) on a series of Tyler sieves. The weight fractions thereby
obtained are plotted ~g~in~t the aperture size of the sieves. The mean
particle size is taken to be the aperture size through which 50% by weight
of the sample would pass.
The bulk density of granular detergent compositions in accordance with
the present invention typically have a bulk density of at least 600 g/litre,
more preferably from 650 g/litre to 1200 g/litre.BuLk density is measured
by means of a simple funnel and cup device con~i~ting of a conical funnel
moulded rigidly on a base and provided with a flap valve at its lower
extremity to allow the contenl~ of the funnel to be emptied into an axially
~ligne-l cylindrical cup disposed below the funnel. The funnel is 130 mm
high and has internal diameters of 130 mm and 40 mm at its respective
~ upper and lower extremities. It is mounted so that the lower extremity is
140 mm above the upper surface of the base. The cup has an overall
height of 90 mm, an internal height of 87 mm and an internal diameter of
84 mm. Its nominal volume is 500 ml.
CA 02226621 1998-01-12
W O 97/03164 PCT~US96/11298
44
To carry out a me~ rement~ the funnel is filled with powder by hand
pouring, the flap valve is opened and powder allowed to overfill the cup.
The filled cup is removed from the frame and excess powder removed
from the cup by p~.sin~ a straight edged implement eg; a knife, across its
upper edge. The filled cup is then weighed and the value obtained for
the weight of powder doubled to provide a bulk density in g/litre.
Replicate m~sllrements are made as required.
Surfactant a~glomerate particles
The surfactant system herein is preferably present in granular
compositions in the form of surf~ct~nt agglomerate particles, which may
take the form of flakes, prills, marumes, noodles, ribbons, but ~>refel~bly
take the form of granules. The most ~lefelled way to process the particles
is by agglomerating powders (e.g. ~lllminosilicate~ carbonate) with high
active surf~ct~nt pastes and to control the particle size of the resultant
agglomerates within specified limits. Such a process involves mixing an
effective amount of powder with a high active surfactant paste in one or
more agglomelatols such as a pan agglomerator, a Z-blade mixer or more
preferably an in-line mixer such as those m~nllf~ctllred by Schugi
(Holland) BV, 29 Chroomstraat 8211 AS, Lelystad, Netherlands, and
Gebruder Lodige Maschinenbau GmbH, D4790 Paderborn 1,
Elsenerstrasse 7-9, Postfach 2050, Germany. Most pLefelably a high
shear mixer is used, such as a Lodige CB (Trade Name).
A high active surf~ct~nt paste comprising from 50% by weight to 95% by
weight, ~rer~ably 70% by weight to 85% by weight of surfactant is
typically used. The paste may be pumped into the agglomerator at a
temperature high enough to m~int~in a pumpable viscosity, but low
enough to avoid degradation of the anionic surfactants used. An operating
temperature of the paste of 50~C to 80~C is typical.
Laundry w~hing method
Machine laundry methods herein typically comprise treating soiled
laundry with an aqueous wash solution in a washing machine having
CA 0222662l l998-0l-l2
W O 97/03164 PCTrUS96/11298
dissolved or dispensed therein an effective amount of a machine laundry
d~tel~ent composition in accord with the invention. By an effective
- amount of the detergent composition it is meant from 40g to 300g ofproduct dissolved or dispersed in a wash solution of volume from 5 to 65
litres, as are typical product dosages and wash solution volumes
commonly employed in conventional machine laundry methods.
In a ~referled use aspect a dispen~in~ device is employed in the washing
method. The dispensing device is charged with the detergent product, and
is used to introduce the product directly into the drum of the washing
machine before the commencement of the wash cycle. Its volume
capacity should be such as to be able to contain sufficient detergent
product as would normally be used in the washing method.
Once the washing machine has been loaded with laundry the dispensing
device cont~inin~ the deter~ellt product is placed inside the drum. At the
commencement of the wash cycle of the washing machine water is
introduced into the drum and the drum periodically rotates. The design of
the dispensing device should be such that it permits cont~inment of the
dry d~tel~ellt product but then allows release of this product during the
wash cycle in response to its agitation as the drum rotates and also as a
result of its contact with the wash water.
To allow for release of the delergent product during the wash the device
may possess a number of openings through which the product may pass.
~ltern~tively, the device may be made of a material which is permeable
to liquid but impermeable to the solid product, which will allow release of
dissolved product. Preferably, the delerge-lt product will be rapidly
released at the start of the wash cycle thereby providing tr~n~ient
localised high concentrations of product in the drum of the washing
machine at this stage of the wash cycle.
Prefelled dispensing devices are reusable and are designed in such a way
that cont~iner integrity is m~int~inP~l in both the dry state and during the
wash cycle. Especially prefeiied dispen~in~ devices for use with the
composition of the invention have been described in the following patents;
GB-B-2, 157, 717, GB-B-2, 157, 718, EP-A-0201376, EP-A-0288345
CA 02226621 1998-01-12
WO 97/0316~ PCTrUS96/11298 46
and EP-A-0288346. An article by J.Bland published in ~nllf~cturing
Chemist, November 1989, pages 4146 also describes especially ~rerelled
dispensing devices for use with granular laundry products which are of a
type commonly know as the "granulette". Another preferred dispensing
device for use with the compositions of this invention is disclosed in PCT
Patent Application No. WO94/11562.
Especially preferred dispensing devices are disclosed in European Patent
Application Publication Nos. 0343069 & 0343070. The latter Application
discloses a device comprising a flexible sheath in the form of a bag
extending from a support ring defining an orifice, the orifice being
adapted to admit to the bag sufficient product for one washing cycle in a
washing process. A portion of the washing medium flows through the
orifice into the bag, dissolves the product, and the solution then passes
outwardly through the orifice into the washing me~lium. The support ring
is provided with a m~kin~ arrangemnt to prevent egress of wetted,
undissolved, product, this arrangement typically comprising radially
ext.Qn~lin~ walls e~tPn~linE from a central boss in a spoked wheel
configuration, or a ~imil~r structure in which the walls have a helical
form.
Alternatively, the dispensing device may be a flexible container, such as a
bag or pouch. The bag may be of fibrous construction coated with a
water impermeable protective material so as to retain the contents, such
as is disclosed in European published Patent Application No. 0018678.
Alternatively it may be formed of a water-insoluble synthetic polymeric
material provided with an edge seal or closure designed to rupture in
aqueous media as disclosed in European published Patent Application
Nos. 0011500, 0011501, 0011502, and 0011968. A convenient form of
water frangible closure comprises a water soluble adhesive disposed along
and sealing one edge of a pouch formed of a water impermeable
polymeric film such as polyethylene or polypropylene.
Packa~ing for the compositions
Commercially marketed executions of the bleaching compositions can be
packaged in any suitable container including those constructed from
-
CA 0222662l l998-0l-l2
W O 97/03164 PCT~US96/11298
47
paper, cardboard, plastic materials and any suitable 1~min~tes. A
~refel~ed pacl~ in~ execution is described in European Application No.
- 94921505.7.
-
CA 02226621 1998-01-12
PCTnUS96/11298
W O 97/03164
48
Abbreviations usçd in Examples
In the detergellt compositions, the abbreviated component identifications
have the following me~nin~.c:
LAS : Sodium linear C12 alkyl ben7ene sulfonate
TAS : Sodium tallow alkyl sulfate
C45AS : Sodium C14-Cls linear alkyl sulfate
CxyEzS : Sodium Clx-Cly branched alkyl sulfate
condensed with z moles of ethylene oxide
C45E7 : A C14 15 predomin~ntly linear primary alcohol
condensed with an average of 7 moles of
ethylene oxide (HLB = 12.3)
C25E3 : A C12 15 branched primary alcohol condensed
with an average of 3 moles of ethylene oxide
(HLB = 8.8)
C25E5 : A C12 15 branched primary alcohol condensed
with an average of 5 moles of ethylene oxide
(HLB = 11.1)
CEQ : RlCOOCH2CH2.N+(CH3)3 with Rl = Cll-
C13
l TCEQ : R2R3COOCH2CH2.N + (CH3)3 with R2 = C8
and R3 = C6
QAS : R2.N+(CH3)2(C2H4OH) with R2 = C12 ~ C14
Soap : Sodium linear alkyl carboxylate derived from an
80/20 mixture of tallow and coconut oils.
TFAA : C16-Clg alkyl N-methyl ~lllc~mide
TFAA2 : Hydrophilic C12-C14 alkyl N-methyl gl~lc~mi~le.
TPKFA : C12-C14 topped whole cut fatty acids
STPP : Anhydrous sodium tripolyphosphate
Zeolite A : Hydrated Sodium Aluminosilicate of formula
Nal2(A1~2Si~2)12. 27H20 having a primary
particle size in the range from 0.1 to 10
micrometers
NaSKS-6 : Cryst~lline layered silic~te of formula
~ -Na2Si205
Citric acid : Anhydrous citric acid
-
CA 02226621 1998-01-12
W O 97/03164 PCTnUS96/11298
49
Carbonate : Anhydrous sodium carbonate with a particle size
between 200~1m and 900)1m
Bicarbonate : Anhydrous sodium bicarbonate with a particle
size distribution betvveen 400~m and 1200~Lm
Silicate : Amorphous Sodium Silicate (SiO2:Na2O; 2.0
ratio)
Sodium sulfate: Anhydrous sodium sulfate
Citrate : Tri-sodium citrate dihydrate of activity 86.4%
with a particle size distribution between 425~m and
850 llm
MA/AA : Copolymer of 1:4 maleic/acrylic acid, average
molecular weight about 70,000.
CMC : Sodium carboxymethyl cellulose
Protease : Proteolytic enzyme of activity 4KNPU/g sold by
NOVOTn~netries A/S under the tradename
Savinase
Alcalase : Proteolytic enzyme of activity 3AU/g sold by
NOVOTn~lstries A/S
Cellulase : Cellulytic enzyme of activity 1000 CEVU/g sold
by NOVOTn~stries A/S under the tr~en~m~
Carezyme
Amylase : Amylolytic enzyme of activity 60KNU/g sold by
NOVOTn~lletries A/S under the tradename
Termamyl 60T
Lipase : Lipolytic enzyme of activity 100kLU/g sold by
NOVO Tn~l~l.stries A/S under the tr~-len~me
Lipolase
Endolase : Endogllln~.~e enzyme of activity 3000 CEVU/g
sold by NOVOTn~llstries A/S
PB4 : Sodium perborate tetrahydrate of nominal
formula NaBO2.3H2O-H2o2
PBl : Anhydrous sodium perborate monohydrate
bleach of nominal formula NaBO2.H2O2
Percarbonate : Sodium Pelcarl~onate of nominal formula
2Na2C03.3H202
NOBS : Nonanoyloxybenzene sulfonate in the form of the
sodium salt.
CA 02226621 1998-01-12
W O 97/03164 PCTAUS96/11298
TAED : Tetraacetylethylenefli~mine
DTPMP : Diethylene tri~mine penta (methylene
phosphonate), marketed by Monsanto under the
Trade name Dequest 2060
Photoactivated: Sulfonated Zinc Phthlocyanine encapsulated in
bleach dextrin soluble polymer
Brightener 1 : Disodium 4,4'-bis(2-sulphostyryl)biphenyl
Brightener 2 : Disodium 4,4'-bis(4-anilino-6-morpholino-1.3.5-
triazin-2-yl)amino) stilbene-2: 2 ' -disulfonate .
HEDP : 1, l-hydroxyethane diphosphonic acid
PVNO : Polyvinylpyridine N-oxide
PVPVI : Copolymer of polyvinylpyrolidone and
vinylimicl~ole
SRP 1 : Sulfobenzoyl end capped esters with oxyethylene
oxy and terephtaloyl backbone
SRP 2 : Diethoxylated poly (1, 2 propylene terepht~l~te)
short block polymer
Silicone antifoam: Polydimethylsiloxane foam controller with
siloxane-oxyalkylene copolymer as dispersing
agent with a ratio of said foam controller to said
dispersing agent of 10:1 to 100:1.
In the following F~mrles all levels are quoted as % by weight of the
composition:
Example 1
Comparative performance test protocol - stain removal
Two white polyester cotton sheets were prewashed in a non-biological
bleach-free heavy duty detergent. Two sets of eight test swatches of size
6cm x 6cm were cut from each sheet. SBK sebum stains were then evenly
applied using a paint brush to one set of swatches, and lipstick stains to
the second set.
Each of the eight swatches of each swatch set was subjected to one washcycle in an Atlas (tr~-len~me) launderometer. The swatches were then
CA 02226621 1998-01-12
W O 97/03164 PCTrUS96/11298
51
assessed for removal of the various fatty stains by a four person grading
panel using the well-known four-point Scheffé scale.
In more detail, an Atlas ~ n~lerometer was employed, and a 60~C, 45
mimlte wash cycle employed. Water of 10~ Clark hardness ( = 1.5 mmol
Ca2+/litre) was used. Defined levels of deler~ellt base powder and
surfactant system were employed in the wash solutions.
The detergent base powder was made up with the following composition:
Base powder
Zeolite A 13.0
Na SKS-6/citric acid (79:21) 13.5
Carbonate 9.6
TAED 6.6
Percarbonate 29.0
DETPMP 1. 1
Protease 0.8
Lipase 0.18
Cellulase 0.32
Amylase 0.30
MA/AA 3.7
CMC 0.5
PVNO 0-04
Granular suds ~u~ )ressor 1.9
Misctmoisture to 100%
-
CA 02226621 1998-01-12
W O 97/03164 PCT~US96tll298
52
Comparative testin~ 1 - wash solutions
The above stain removal test protocol was followed in comparing the
efficiency of four dirrerent wash solutions A to D in removing fatty soils.
Wash solution B was derived by a~r~liate dissolution of a surf~ct~nt
sy~le~ in accord with the invention, in combination with the deter~;ellt
base powder. Wash solutions A, C and D are comparative solutions.
The composition of each of the wash solutions was as follows:
A B C D
C45AS 280 ppm 280 ppm 400 ppm 400 ppm
C35AE3S 100 ppm 100 ppm 100 ppm 100 ppm
C45E7 - 400 ppm - 400 ppm
C25E3 400 ppm - 400 ppm
CEQ 120 ppm 120 ppm
Base powder5000 ppm 5000 ppm 5000 ppm 5000 ppm
Comparative testin~ 1 - results
B vs A B vs D A vs C
Sebum removal (PSU) +1.3s +0.9s -0.1
Lipstick removal +1.5s +2.3s -0.5
(PSU)
s = significant at 95% confi~lence level
The comparisons show that:
1. When the hydrophobic C25E3 nonionic of A is replaced by the
hydrophilic C45E7 nonionic of B stain removal performance is enhanced.
2. When a portion of the C45AS of D is replaced by CEQ in the presence
of hydrophilic C45E7 stain removal pelrolmance is enh~nced.
CA 02226621 1998-01-12
W O 97/03164 PCTrUS96/11298
3. When a portion of the C45AS of C is replaced by CEQ in the presence
of hydrophobic C25E3 stain removal pelrc,llllance, by contrast is reduced.
Comparative testin~ 2 - wash solutions
The above stain removal test protocol was followed, with two adjustments
namely that the wash temperature was 30~C and the sebum soil was
replaced by a dirty motor oil soil, in comparing the efficiency of two
further wash solutions E and F in removing fatty soils. Wash solution F
was derived by ~ropliate dissolution of a surfactant system in accord
with the invention. Wash solution E is comparative.
The composition of each of the wash solutions was as follows:
E F
C45AS 360 ppm 360 ppm
C35AE3S 90 ppm 90 ppm
C45E7 - 340 ppm
C25E3 340 ppm
TTCEQ 140 ppm 140 ppm
Base powder 5000 ppm 5000 ppm
Comparative testin~ 2 - results
F vs E
Dirty motor oil removal (PSU) +0.9s
Lipstick removal (PSU) + 1.5s
s = si~nificant at 95% confi-le.nce level
CA 02226621 1998-01-12
W O 97/03164 PCTrUS96/11298
54
The comparison shows that:
1. When the hydrophobic C25E3 nonionic of E is replaced by the
hydrophilic C45E7 nonionic of F stain removal performance is enhanced.
CA 02226621 1998-01-12
W O 97/03164 PCTnUS96111298
Example 2
- The following laundry detel-gellt compositions A to F were prepared in
accord with the invention:
A B C D E F
C45AS 8.0 8.0 8.0 8.0 8.0 8.0
C45E7 3.4 4.4 5 0 3 0 7 0 3 0
CEQ 1.0 0.8 1.2 1.0 0.8 0.6
Zeolite A 18.1 18.1 18.1 18.1 18.1 18.1
Carbonate 13.0 13.0 13.0 27.0 27.0 27.0
Silicate 1.4 1.4 1.4 3.0 3.0 3.0
Sodium sulfate 26.1 26.1 26.1 26.1 26.1 26.1
PB4 9.0 9.0 9.0 9.0 9.0 9.0
TAED 1.5 1.5. 1.5 1.5 1.5 1.5
DETPMP 0.25 0.25 0.250.25 0.250.25
HEDP 0.3 0.3 0.3 0.3 0.3 0-3
Protease 0.26 0.26 0.260.26 0.260.26
Amylase 0.1 0.1 0.1 0.1 0.1 0.1
MA/AA 0.3 0.3 0.3 0.3 0.3 0.3
CMC 0.2 0.2 0.2 0.2 0.2 0.2
CA 02226621 1998-01-12
W O 97/03164 PCTrUS96/11298
56
Photoactivated 15 15 15 15 15 15
bleach (ppm) ppm ppm ppm ppm ppmppm
Brightener 1 0.09 0.09 0.09 0.09 0.09 0.09
Perfume 0.3 0.3 0.3 0 3 0 3 0 3
Silicone antifoam 0.5 0.5 0.5 0.5 0.5 0.5
Misc/minors to
100%
Density in g/litre 630 670 670 500 670 670
~lk~linity 6.8 6.8 6.8 18.5 18.5 18.5
CA 02226621 1998-01-12
W O 97/03164 PCTrUS96/11298
57
Ex~mple 3
The following granular laundry detergellt compositions G to I of buLk
density 750 g/litre were prepared in accord with the invention:
G H
TAS 1.2 1.8 1.6
C45AS 5.2 7.0 8.0
C25AE3S - 0.8 1.2
C45E7 3.25 5.5 5.0
CEQ 0.8 1.0 2.0
STPP 10.7
Zeolite A - 19.5 19.5
NaSKS-6/citric acid - 10.6 10.6
(79:21)
Carbonate 16.1 21.4 21.4
Bicarbonate - 2.0 2.0
Silicate 6.8
Sodium sulfate 39.8 - 14.3
PB4 5.0 12.7
TAED 0.5 3.1
CA 02226621 1998-01-12
W O 97/03164 PCTAUS96/11298
58
DETPMP 0.25 0.2 0.2
HEDP - 0.3 0.3
Protease 0.26 0.85 0.85
Lipase 0.15 0.15 0.15
- Cellulase 0.28 0.28 0.28
Amylase 0.1 0.1 0.1
MA/AA 0.8 1.6 1.6
CMC 0.2 0.4 0.4
Photoactivated bleach 15 ppm 27 ppm 27 ppm
~)pm)
Bri~htçner 1 0.08 0.19 0.19
Bri ~htçner 2 - 0.04 0.04
Perfume 0.3 0.3 0.3
Silicone antifoam 0.5 2.4 2.4
Minors/misc to 100%
CA 02226621 1998-01-12
W O 97/03164 PCT~US96/11298
59
Example 4
The following delergent formulations, according to the present invention
were ~r~ared, where J is a phosphorus-cont~inin~ deterge,lt
composition, K is a zeolite-cont~inin~ detelgent composition and L is a
compact deleLgelll composition:
J K L
Blown Powder
STPP 14.0 - 14.0
Zeolite A - 20.0
C45AS 9.0 12.0 8.0
MA/AA 2.0 4.0 2.0
LAS 6.0 - 9.0
TAS 2.0
CEQ 1.5 3.0 1.5
Silicate 7.0 8.0 8.0
CMC 1.0 1.0 0.5
Bri~htener 2 0.2 0.2 0.2
Soap 1.0 1.0 1.0
DTPMP 0.4 0.4 0.2
Spray On
C45E7 5.0 5.0 4.0
Silicone antifoam 0.3 0.3 0.3
Perfume 0.3 0.3 0-3
Dry additives
Carbonate 26.0 23.0 25.0
PB4 18.0 18.0 10
PBl 4.0 4.0 0
TAED 3.0 3.0 1.0
Photoactivated bleach 0.02 0.02 0.02
Protease 1.0 1.0 1.0
Lipase 0.4 0.4 0-4
Amylase 0.25 0.30 0.15
Dry mixed sodium 3.0 3.0 5.0
sulfate
CA 02226621 1998-01-12
W O 97/03164 PCTAUS96/11298
Balance (Moisture & 100.0 100.0 100.0
Miscellaneous)
Density (g/litre) 630 670 670
CA 0222662l l998-0l-l2
W O 97/03164 ~CT~US96/11298
61
Example ~;
The following nil bleach-cont~ining delergellt formulations of particular
use in the washing of colored clothing, according to the present invention
were prepared:
M N O P
Blown Powder
ZeoliteA 15.0 15.0 - 15.0
Sodium sulfate 0.0 5.0 - 0.0
CEQ 2.0 1.5 1.3 2.0
DTPMP 0.4 0.5 - 0.4
CMC 0.4 0.4 - 0.4
MA/AA 4.0 4.0 - 4.0
LAS - - - 3.0
Agglomerates
LAS - - - 6.0
C45AS 8.0 7.0 11.0
TAS 3.0 2.0 - 3.0
Silicate 4.0 4.0 - 4.0
Zeolite A 10.0 15.0 13.0 10.0
CMC - - 0.5
MA/AA - - 2.0
Carbonate 9.0 7.0 7.0 9.0
Spray On
Perfume 0.3 0.3 0.5 0.3
C45E7 4.0 6.0 4.0 6.0
C25E3 2.0 - 2.0 3.0
Dry additives
HEDP - - - 0.5
MA/AA - - 3.0
NaSKS-6 - - 12.0
Citrate 10.0 - 8.0 10.0
Bicarbonate 7.0 3.0 5.0 6.0
Carbonate 8.0 5.0 7.0 6.0
PVPVI/PVNO 0.5 0.5 0.5 0.5
Alcalase 0.5 0.3 0.9 0.5
~ ir~e 0.4 0.4 0.4 0.4
CA 02226621 1998-01-12
W O 97/03164 PCT~US96/11298
62
Amylase 0.6 0.6 0.6 0.6
Cellulase 0.6 0.6 0.6 0.6
Silicone alltifoam 5.0 5.0 5.0 5.0
Dry additives
Sodium sulfate 0.0 9.0 0.0 0.0
R~l~nce (Moisture and 100.0 100.0 100.0 100.0
Miscellaneous)
Density (g/litre) 700 700 700 700
CA 02226621 1998-01-12
W O 97/03164 PCTrUS96/11298
63
Example 6
~ The following detergent formulations, according to the present invention
were prepared:
Q R S T
C45AS 12.0 12.0 12.0 10.0
- QAS 0.7 1.0 - 0.7
TFAA - 1.0 - -
C25E5 4.0 5.0 5.0 5 0
C45E3S - 2.5
CEQ 2.0 1.5 1.0 1.0
STPP 30.0 18.0 15.0
Silicate 9.0 7.0 10.0
Carbonate 15.0 10.5 15.0 25.0
Bicarbonate - 10.5
DTPMP 0.7 1.0
SRP 1 0.3 0.2 - 0.1
MA/AA 2.0 1.5 2.0 1.0
CMC 0.8 0.4 0.4 0.2
Protease 0.8 1.0 0.5 0.5
Amylase 0.8 0.4 - 0.25
~ .ir~e 0.2 0. 1 0.2 0. 1
Cellulase 0.15 0.05
Photoactivated70ppm 45ppm - 10ppm
bleach (ppm)
Brightener 1 0.2 0.2 0.08 0.2
PBl 6.0 2.0
NOBS 2.0 1.0
R~l~nce 100 100 100 100
(Moisture and
Miscellaneous)
= ~
CA 02226621 1998-01-12
W O97/03164 PCT~US96/11298
64
Example 7
The following deterg~l1t formulations, according to the present invention
were prepared:
U V W
Blown Powder
Zeolite A10.0 15.0 6.0
Sodium sulfate 19.0 5.0 7.0
MA/AA 3.0 3.0 6.0
C45AS 14.0 13.0 14.0
C E Q 2.0 2.0 2.0
Silicate - 1.0 7.0
Soap - - 2.0
Bri~ht~nPr 1 0.2 0.2 0.2
Carbonate 28.0 26.0 20.0
DTPMP - 0.4 0.4
Spray On
C45E7 3.0 3.0 3.0
Dry additives
PVPVI/PVNO 0.5 0.5 0.5
Protease 1.0 1.0 1.0
Lipase 0.4 0.4 0.4
Amylase 0.1 0.1 0.1
Cell~ e 0.1 0.1 0.1
NOBS - 6.1 4.5
PB1 1.0 5.0 6.0
Sodium sulfate - 6.0
R~l~nce (Moisture 100 100 100
and Miscellaneous)
-
CA 02226621 1998-01-12
W O 97/03164 PCTrUS96/11298
Example 8
The following high density and bleach-cont~inin~ detergent formulations,
according to the present invention were prepared:
X Y Z
Blown Powder
Zeolite A 15.0 15.0 15.0
Sodim sulfate 0.0 5.0 0.0
45AS 4.0 4.0 4.0
QAS - 1.5 1.5
CEQ 1.0 1.0 0.5
DTPMP 0.4 0.4 0.4
CMC 0.4 0.4 0-4
MA/AA 4.0 2.0 2.0
Agglomerates
LAS 4.0
TAS 2.0 2.0 1.0
Silicate 3.0 3.0 4.0
Zeolite A 8.0 8.0 8.0
Carbonate 8.0 8.0 6.0
Spray On
Perfume 0.3 0.3 0.3
C45E7 4.0 4.0 4.0
Dry additives
Citrate 5.0 - 2.0
Bicarbonate - 3.0
Carbonate 8.0 15.0 10.0
TAED 6.0 2.0 5.0
PB1 14.0 7.0 10.0
Polyethylene oxide of MW - - 0.2
5,000,000
Bento.~ile clay - - 10.0
Protease 1.0 1.0 1.0
Lipase 0.4 0.4 0.4
Amylase 0.6 0.6 0.6
Cellulase 0.6 0.6 0.6
CA 0222662l l998-0l-l2
WO 97/03164 PCTrUS96/11298
66
Silicone antifoam5.0 5.0 5.0
Dry additives
Sodium sulfate 0.0 3.0 0.0
Balance (Moisture and 100.0 100.0 100.0
Miscellaneous)
Density (g/litre) 850 850 850
CA 02226621 1998-01-12
W O 97/03164 PCTrUS96/11298
67
Example 9
The following _igh density detergellt formulations, according to the
present invention were ~r~ared:
AA AB AC
Agglomerate
C45AS 11.0 14.0 12.0
CEQ 3.0 3.5 3.5
Zeolite A 15.0 6.0 6.0
Carbonate 4.0 8.0 8.0
MA/AA 4.0 2.0 2.0
CMC 0.5 0.5 0.5
DTPMP 0.4 0.4 0.4
Spray On
C25E5 5.0 5.0
C25E3 - 5 o
TFAA2 - - 1.0
Perfume 0.5 0.5
Dry Adds
HEDP 0.5 0.3 0.3
SKS 6 13.0 10.0 10.0
Citrate 3.0 1.0 1.0
TAED 5.0 7.0 7.0
Percarl,onate 20.0 20.0 20.0
SRP 1 0.3 0.3 0.3
Protease 1.4 1.4 1.4
Lipase 0.4 0.4 0.4
Cellulase 0.6 0.6 0.6
Amylase 0.6 0.6 0.6
Silicone antifoam 5.0 5.0 5.0
Bri~htenPr 1 0.2 0.2 0.2
Bri~htenPr 2 0.2
R~l~nce (Moisture and 100 100 100
Miscellaneous)
Density (g/litre) 850 850 850
CA 02226621 1998-01-12
W O 97/03164 P ~ rUS96/11298
68
Example 10
The following liquid dele~e,l~ formulations, according to the present
invention were prepared:
AD AE AF AG AH AI AJ AK
C45AS 10.11.0 9.0 - 13.0 - - -
C25AS 4.0 1.0 2.0 10. - 11.0 15.0 15.0
O
C25E3S 1.0 - - 3.0 - - 2.0 4.0
C25E7 6.0 8.0 11. 2.5 10.0 2.0 4.0 4.0
o
TFAA - - - 4.5 - 6.0 8.0 8.0
C12-14 ally1 - - - - 3
~lim~thylhydroxy ethyl
ammonium chloride
CEQ 0.5 1.5 1.0 0.7 2.0 1.5 1.8 2.0
TPKFA 2.0 - 11. 2.0 - 13.0 7.0 7.0
o
Rapeseed fatty acids - - - 5.0 - - 4.0 4.0
Citric acid 2.0 3.0 1.0 1.5 1.0 1.0 1.0 1.0
Dodecenyl/tetradecenyl 12. 10.0 - - 15.0
succinic acid 0
Oleic acid ; 4.0 2.0 1.0 - 1.0
Fth~nnl 4.0 4.0 7.0 2.0 7.0 2.0 3.0 2.0
1,2 Propanediol 4.0 4.0 2.0 7.0 6.0 8.0 8.0 13.0
Mono Ethanol Amine - - - 5.0 - - 9.0 9.0
Tri Ethanol Amine - - 8.0
NaOHup topH 8.0 8.0 7.6 7.7 8.0 7.5 8.0 8.2
Ethoxylated 0.5 - 0.5 0.2 - - 0.4 0.3
tetraethylene
pen~mine
DTPMP 1.0 1.0 0.5 1.0 2.0 1.2 1.0
SRP 2 0.3 - 0.3 0.1 - - 0.2 0.1
PVNO - - - - - - - 0.10
CA 0222662l l998-0l-l2
W O 97/03164 PCT~US96/11298
69
Protease 0.5 0.5 0.4 0.2 - 0.5 0.3 0.6
Alcalase - - - - 1.5
Lipase - 0.10 - 0.0 - - 0.15 0.15
Amylase 0.2 0.25 0.6 0.5 0.25 0.9 0.6 0.6
s
Cellulase - - - 0.0 - - 0.15 0.15
s
Endolase - - - 0.1
0 0.07
Boric acid 0.1 0.2 - 2.0 1.0 1.5 2.5 2.5
Na formate - - 1.0 - - - - - -
Ca chloride - 0.015 - 0.0
Bentonite clay - - - - 4.0 4.0
Suspending clay SD3 - - - - 0.6 0.3
B~l~nce (water and 100 100 100 100 100 100 100 100
Miscellaneous)