Note: Descriptions are shown in the official language in which they were submitted.
CA 02226740 1998-01-13
PIPERAZINE DERIVATIVE AND ITS USES
5TECHNICAL FIELD
The present invention relates to a cysteine protease
inhibitor composition comprising a piperazine derivative or a
salt thereof as an active ingredient.
10BACKGROUND TECHNOLOGY
Cysteine protease is a protease having a cysteine
residue in the activity centerofthe enzyme molecule and includes
such species as cathepsin B, H, and L and dipeptidyl peptidase,
all of which are lysosomal enzyme fractions, and calpain which
15exists in the cytoplasm, among others. Though much remains to
be explored about the physiological roles of these enzymes, a
considerable amount of body has been cast on their roles in recent
years. For example, calpain is known to be a protease ubiquitous
in life, which is activated by calcium ions and has the optimum
20pH in the neighborhood of neutral. As elucidated to this day,
it takes part in degradation of the skeletal protein of cells,
activation of inert cell precursors such as protein kinase C, and
degradation of receptor proteins. It has also been shown that
the abnormality of this enzyme activity is involved in many
25diseases. For example, its involvement in refractory diseases
such as cerebral apoplexy (stroke), subarachnoid hemorrhage,
CA 02226740 1998-01-13
Alzheimer's disease, ischemic diseases, myodystrophy, cataract,
platelet aggregation disorder, arthritis, and osteoporosis,
among other diseases. [Trends in Pharmacological Sciences, 15,
412, 1994].
As inhibitors of such cysteine proteases, several
peptide compounds inclusive of an epoxysuccinic acid peptide
derivative (JP-B 1-54348, JP-A 55-153778, etc.), a
peptidoaldehyde derivative (JP-B 45-17154, JP-B 46-22012, etc.),
a peptidohalomethane derivative (JP-B 6-29229), and a
peptidohalohydrazide derivative [Eur. J. Med. Chem., 28, 297-311,
1993] have been reported. As enzymes having calpain-inhibitory
activity among various kinds of cysteine proteases, several
peptide compounds such as a peptide aldehyde derivative (JP-A
6-287167), a peptidodiazomethane derivative [Biochem, J., 253,
751-758, 1988, J. Med. Chem., 35, 216-220, 1992], a
peptidodisulfide derivative [Chem. Lett., 191-194, 1990], etc.
and several non-peptide compounds such as an isocoumarin
derivative (WO 92/11850), KP-1241 (JP-A 6-41067), etc. have also
been reported. Moreover, as inhibitors of cathepsin L and B, an
aldehyde derivative (JP-A 7-101924) and an epoxysuccinic acid
derivative (JP-A 8-104683, WO 95/32954) have been reported.
However, many of these known inhibitors are not fully
satisfactory in transferability to the cell and/or in vivo
stability, while others are not as effective as desired, with the
result that there is not available a clinically useful inhibitor.
CA 02226740 1998-01-13
DISCLOSURE OF THE INVENTION
The inventors of the present invention did much research
to develop a drug substance which would show high cysteine
protease-inhibitory activity and be highly membrane-permeable
and comparatively stable in the in vivo environment.
Consequently they discovered that a piperazine derivative of the
following general formula (I) has potent cysteine
protease-inhibitory activity and have perfected the present
invention.
The present invention, therefore, is directed to a
compound of the following formula (I) inclusive of its
salt.
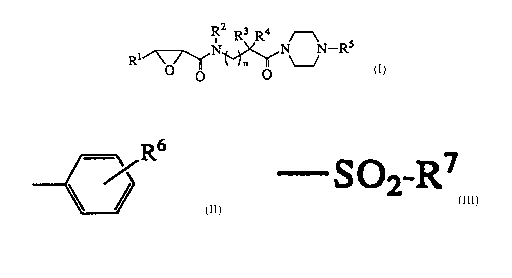
R2 R3 R4 ~--~
Rl~\ /~N~N~N--Rs
[wherein Rlrepresents carboxywhich maybe esterified or amidated
carboxy which may be substituted; R2 represents hydrogen or lower
alkyl and may be linked to either R3 or R4 to form a ring; R3 and
R4may be the same or different and each represents hydrogen, lower
alkyl which may be substituted or a sulfide group which may be
substituted; R3 and R4 may conjoinedly form a ring; Rs represents
a substituted phenyl group of formula (II)
CA 02226740 1998-01-13
R6
~ (II)
(wherein R6 represents halogen or alkoxy) or a substituted
sulfonyl group of formula (III)
- So2-R7 (III)
(wherein R7representseitheraryloptionallysubstitutedbylower
alkyl or amino which may be substituted); n is O or 1].
The present invention is further directed to a
pharmaceutical composition comprisingthe above compoundand more
particularly to a cysteine protease inhibitor composition
comprising said compound.
Referring to the above general formula (I), the optionally
esterified carboxy represented by R1 includes but is not limited
to carboxy and alkoxycarboxy. The alkoxy moiety of said
alkoxycarboxy may for example be Cl6 alkoxy and preferably C14
alkoxy, such as methoxy, ethoxy, propoxy, isopropoxy, butoxy,
isobutoxy, sec-butoxy, and tert-butoxy. Particularly preferred
is ethoxy.
The substituent for said optionally substituted
amidated carboxyfor R1includeshydroxy, alkoxy (methoxy, ethoxy,
propoxy, etc.), and aralkyloxy (benzyloxy etc.). Preferred are
hydroxy and benzyloxy.
The lower alkyl for R2 includes C16 straight-chain or
CA 02226740 1998-01-13
branched-chain alkyl or preferably Cl4 alkyl, such as methyl,
ethyl, n-propyl, isopropyl, n-butyl, isobutyl, sec-butyl,
tert-butyl, n-pentyl, isopentyl, neopentyl, tert-pentyl, n-hexyl,
isohexyl, 4-methylpentyl, 1,1-dimethylbutyl, 2,2-dimethylbutyl,
3,3-dimethylbutyl, and 2-ethylbutyl. Preferred is hydrogen or
methyl.
The ring that may be formed conjoinedly by R2 and either
R3 or R4 includes but is not limited to aziridine, azetidine,
pyrrolidine, and piperidine. Particularly preferred is
pyrrolidine.
The optionally substituted lower alkyl for R3and R4includes
C16straight-chain or branched-chain alkyl such as methyl, ethyl,
n-propyl, isopropyl, n-butyl, isobutyl, sec-butyl, tert-butyl,
n-pentyl, isopentyl, neopentyl, tert-pentyl, n-hexyl,
4-methylpentyl, 1,1-dimethylbutyl, 2,2-dimethylbutyl,
3,3-dimethylbutyl, and 2-ethylbutyl. Preferred are methyl,
ethyl, isobutyl, andsec-butyl. Thesubstituent groupoptionally
present on said alkyl includes an aromatic ring and carbamoyl.
The aromatic ring mentioned just above includes aromatic
carbocycles such as benzene ring and aromatic heterocycles such
as indole ring. Particularly preferred is benzene ring.
The sulfide group of said optionally substituted sulfide
group for R3 or R9 includes alkylthioalkyl groups and preferably
Cl4 alkyl-thio-Cl4 alkyl, such as dimethyl sulfide, diethyl
sulfide, dipropyl sulfide, dibutyl sulfide, dipentyl sulfide,
CA 02226740 1998-01-13
dihexyl sulfide, methylethyl sulfide, methylpropyl sulfide, and
ethylbutylsulfide, amongothers. Preferredaredimethylsulfide
and methylethyl sulfide. The substituent optionally present on
said sulfide group includes acylamino. The acylamino includes
but is not limited to formylamino, acetylamino, propionylamino,
butyrylamino, isobutyrylamino, valerylamino, isovalerylamino,
pivaloylamino, and n-hexanoylamino. Preferred is acetylamino.
The ring optionally formed conjoinedly by R3 and R4 includes
cyclopropane, cyclobutane, cyclopentane, cyclohexane and
cycloheptane, etc. Particularly preferred is cyclopentane.
Referring to the substituent R6 for said substituted
phenyl group of formula (II), the halogen includes but is not
limited to fluorine, chlorine, bromine, and iodine. Preferred
are fluorine and chlorine. The halogen may be situated in any
of meta, para, and ortho positions.
Referring further to the substituent R6for said substituted
phenyl group of formula (II), the alkoxy includes C16 alkoxy and
preferably C14 alkoxy, such as methoxy, ethoxy, propoxy,
isopropoxy, butoxy, isobutoxy, sec-butoxy, and tert-butoxy.
Particularly preferred is methoxy.
Referring to the substituent R7for the substituted sulfonyl
group of formula (III), the aryl optionally substituted by lower
alkyl includes but is not limited to phenyl and naphthyl. The
lower alkyl optionally substituting said aryl includes methyl,
CA 02226740 1998-01-13
ethyl, propyl, isopropyl, butyl, etc. and may be situated in any
position of the aryl group.
Referring further to the substituent R' for said substituted
sulfonyl group of formula (III), the amino includes amino mono- or
5 di-substituted by Cl 6 straight-chain, branched-chain, or cyclic
alkyl, such as methylamino, dimethylamino, ethylamino,
diethylamino, propylamino, dipropylamino, isopropylamino,
diisopropylamino, butylamino, dibutylamino, cyclohexylamino,
etc. Particularly preferred is dimethylamino.
In the context of the present invention, the salt of the
compound of general formula (I) is preferably a physiologically
acceptable salt, thus including salts with inorganic bases, salts
with organic bases, salts with inorganic acids, salts with organic
acids, and salts with basic or acidic amino acids. The preferred
15 inorganic base salt includes alkali metal salts such as sodium
salt and potassium salt, alkaline earth metal salts such as
calcium salt andmagnesium salt, aluminum salt, and ammonium salt.
The preferred organic base salt includes salts with
trimethylamine, pyridine, picoline, ethanolamine,
20 diethanolamine, triethanolamine, dicyclohexylamine, and
N,N-dibenzylethylenediamine, among others. The preferred
inorganic acid salt includes salts with hydrochloric acid,
hydrobromic acid, nitric acid, sulfuric acid, and phosphoric acid,
among others. The preferred organic acid salt includes salts with
25 formic acid, acetic acid, trifluoroacetic acid, fumaric acid,
CA 02226740 1998-01-13
oxalic acid, tartaric acid, maleic acid, citric acid, succinic
acid, malicacid, methanesulfonicacid, benzenesulfonic acid, and
p-toluenesulfonic acid, among others. The preferred salt with
abasicaminoacidincludessaltswitharginine, lysine, ornithine,
etc., while the preferred salt with an acidic amino acid includes
salts with aspartic acid and glutamic acid, among others.
The compound of general formula (I) according to the present
invention can be produced in accordance with the following
reaction scheme.
~ R3 R4 /--\
R~ OH 2RNH~N N--R5
O
(IV) (V)
R2 R3 4
Rl ~N~,N~ N--R5
(I)
(wherein each symbol has the meaning defined hereinbefore). In
this process, a compound of general formula (IV) [hereinafter
sometimes referred to as compound (IV)] or a reactive derivative
in the carboxyl function thereof, or a salt thereof, is reacted
CA 02226740 1998-01-13
- _ 9 _
with a compound of general formula (V) [hereinafter sometimes
referred to as compound (V)] or a reactive derivative thereof,
or a salt thereof, to provide compound (I).
The above reaction can be carried out by the routine
liquid-phaseorsolid-phase (stationary) technique knowntothose
skilledinpeptidesynthesis. Astosuch knownroutineprocedures
and analogous procedures, the descriptions in the following
literature are incorporated herein by reference: Izumiya, Nobuo
et al.: Peptide Gosei no Kiso to Jikken (Fundamentals and
Experiments inPeptideSynthesis), Maruzen, 1985; Yajima, Haruaki
& Sakakibara, Shumpei: Seikagaku Jikken Koza 1 (Biochemical
- Experiment Series 1), Japanese Biochemical Society (ed.), Tokyo
Kagaku Dojin, 1977; Kimura, Toshiya: Zoku Seikagaku Jikken Koza
1 (New Biochemical Experiment Series 1, Japanese Biochemical
Society (ed.), Tokyo Kgaku Dojin, 1987; Suzuki, Nobuo: Jikken
Kagaku Koza (4th Edition) 22, Yuki Gosei IV (Experimental
Chemistry Series (Edition IV) 22, Organic Synthesis IV), The
Chemical Society of Japan (ed.), Maruzen, 1992.
The preferred reactive derivative in the carboxyl function
- of compound (IV) includes the acid halide, acid anhydride,
activated amide, and activated ester. The acid halide includes
but is not limited to the acid chloride. The acid anhydride
includes mixed acid anhydrides with various acids such as
substituted phosphoric acid (dialkylphosphoric acid,
phenylphosphoric acid, diphenylphosphoric acid,
CA 02226740 1998-01-13
--10-
dibenzylphosphoric acid, halophosphoric acid, etc.),
dialkylphosphorous acid, sulfurous acid, thiosulfuric acid,
sulfuric acid, sulfonic acids (methanesulfonic acid, etc.),
aliphatic carboxylic acids (acetic acid, propionic acid, butyric
acid, isobutyricacid, pivalicacid, pentanoicacid, isopentanoic
acid, trichloroacetic acid, etc.), and aromatic carboxylic acids
(benzoic acid etc.) as well as the symmetric acid anhydride. The
preferredactivatedamideincludesbutisnotlimitedtoimidazole,
4-substituted imidazole, dimethylpyrazole, triazole, and
tetrazole. The preferred activated ester includes but is not
limited to the cyanomethyl ester, methoxymethyl ester,
dimethyliminomethyl ester, vinyl ester, propargyl ester,
p-nitrophenyl ester, trichlorophenyl ester, pentachlorophenyl
ester, methylphenyl ester, phenylazophenyl ester, phenylthio
ester, p-nitrophenylthio ester, p-cresylthio ester,
carboxymethylthio ester, pyranyl ester, pyridyl ester,
~-quinolylthio ester, etc. and esters with N-hydroxy compounds
such as N,N-dimethylhydroxylamine, 1-hydroxy-2-(lH)-pyridone,
N-hydroxysuccinimide, N-hydroxyphthalimide, 1-hydroxy-lH-
benzotriazole, etc. The preferred salt of compound (IV) or areactive derivative thereof includes salts with inorganic bases
suchasalkalimetalsalts, e.g.sodiumsalt, potassiumsalt, etc.,
alkaline earth metal salts such as calcium salt, magnesium salt,
etc., aluminum salt, and ammonium salt, as well as salts with
organic bases such as trimethylamine salt, triethylamine salt,
CA 02226740 1998-01-13
pyridine salt, picoline salt, ethanolamine salt, diethanolamine
salt, triethanolamine salt, dicyclohexylamine salt,
N,N-dibenzylethylenediamine salt, etc. The kind of reactive
derivative can be selected according to the type of compound (IV).
The preferred reactive derivative in the amino function of
compound (V) includes Schiff base type imino and enamine tautomers
available on reaction of compound (V) with carbonyl compounds such
as aldehydes and ketones, silyl derivatives available on reaction
of compound (V) with silyl compounds such as
bis(trimethylsilyl)acetamide, mono(trimethylsilyl)acetamide,
bis(trimethylsilyl)urea, etc., and derivatives available on
reaction of compound (V) with phosphorus trichloride or phosgene.
The preferred salt of said reactive derivative of compound (V)
includes salts with inorganic acids, such as hydrochloride,
hydrobromide, nitrate, sulfate, phosphate, etc. and salts with
organic acids, such as formate, acetate, trifluoroacetate,
fumarate, oxalate, tartrate, maleate, citrate, succinate, malate,
methanesulfonate, benzenesulfonate, p-toluenesulfonate, etc.
These reactive derivatives can be selectively used according to
the type of compound (V).
The reaction between compounds (IV) and (V) is generally
conducted in the common solvent, e.g. water, alcohol (e.g.
methanol, ethanol, etc.), acetone, dioxane, acetonitrile,
chloroform, methylene chloride, ethylene chloride,
25 tetrahydrofuran, ethyl acetate, N,N-dimethylformamide, and
CA 02226740 1998-01-13
pyridine, although the reaction can be carried out in any other
organic solvent that does not interfere with the reaction. The
common organic solvent mentioned above may be used in admixture
with water. When compound (IV) is used either in the free form
or in the form of a salt in the above reaction, the reaction is
preferably conducted in the presence of the common condensing
agent such as N,N'-dicyclohexyl-carbodiimide,
N-cyclohexyl-N'-morpholinoethylcarbodiimide,
N-cyclohexyl-N'-(4-diethylaminocyclohexyl)carbodiimide,
N,N'-diethylcarbodiimide, N,N'-diisopropylcarbodiimide,
N-ethyl-N'-(3-dimethylaminopropyl)carbodiimide,
N,N'-carbonyl-bis(2-methylimidazole), pentamethyleneketene-
N-cyclohexylimine, diphenylketene-N-cyclohexylimine,
ethoxyacetylene, l-alkoxy-l-chloroethylene, trimethyl
phosphite, ethyl polyphosphate, isopropyl polyphosphate,
phosphorus oxychloride, diphenylphosphorylazide, thionyl
chloride, oxalyl chloride, haloformic acid lower alkyl esters
(e.g. ethyl chloroformate, isopropyl chloroformate, etc.),
triphenylphosphine, N-hydroxybenzotriazole,
1-(p-chlorobenzenesulfonyloxy)-6-chloro-lH-benzotriazole,
Vilsmeier reagents prepared by reacting N,N-dimethylformamide
with thionyl chloride, phosgene, trichloromethyl chloroformate,
phosphorus oxychloride, or the like. The reaction may be carried
out in the presence of an inorganic or organic base, e.g. alkali
metal hydrogen carbonate, tri(lower)alkylamine, pyridine,
CA 02226740 1998-01-13
N-(lower)alkylmorpholine, N,N-di(lower)alkylbenzylamine, etc.
The reaction temperature is not so critical and the reaction can
be generally carried out under cooling, at ambient temperature,
or under mild heating.
5The structural formulas of the compounds synthesized
- in the examples which appear hereinafter are
shown below.
R,2 R3 R4 ~ ~R6
~N~,N~N~
CA 02226740 l998-0l-l3
--14--
Table 1-1
EX No. n R' RZ R3 R~ R6
1 0 -COOEt H benzyl H 4-fluoro
2 0 -COOEt H benzyl H 2-fluoro
3 0 -COOEt H isobutyl H 4-fluoro
~ 4 0 -COOEt H isobutyl H H
7 0 -COOEt H isobutyl H 2-chloro
8 0 -COOEt H isobutyl H 3-chloro
9 0 -COOEt H isobutyl H 4-chloro
0 -COOEt H isobutyl H 4-methoxy
11 0 -COOEt H isopropyl H 2-chloro
12 - O -COOEt H H H 2-chloro
13 0 -COOEt H methyl H 2-chloro
14 0 -COOEt H sec-butyl H 2-chloro
1 -COOEt H H H 2-chloro
16 0 -COOEt methyl H H 2-chloro
17 0. -COOEt pyrrolidinyl H 2-chloro
18 0 -COOEt H -CH2-S-CH2NHCOCH3 . H 2-chloro
19 0 -COOEt H -CH2CH2-S-CH3 H 2-chloro
0 -COOEt H -CH2CH2CONH2 H 2-chloro
21 0 -COOH H benzyl H 4-chloro
22 0 -COOH H benzyl H 2-fluoro
23 0 -COOH H isobutyl H 4-fluoro
24 0 -COOH H isobutyl H H
27 0 -COOH H isobutyl H 2-chloro
28 0 -COOH H isobutyl H 3-chloro
29 0 -COOH H isobutyl H 4-chloro
CA 02226740 1998-01-13
Table 1-2
Ex No. n R' R2 R' R' R6
O -COOH H isobutyl H 4-methoxy
31 O -COOH H isopropyl H 2-chloro
-32 O -COOH H H H 2-chloro
33 O -COOH H methyl H 2-chloro
34 O -COOH H sec-butyl H 2-chloro
1 -COOH H H H 2-chloro
- 36 O -C~OH methyl H H 2-chloro
37 O -COOH pyrrolidinyl H 2-chloro
38 O -COOH H -CH2-S-CH2NHCOCH3 H 2-chloro
39 O -COOH H -CH2CH2-S-CH3 H 2-chloro
O -COOH H -CH2CH2CONH~ H 2-chloro
41 O -COOH H cyclopentyl 4-fluoro
Rl ~ \ O ~ ~ N N - S o2-R7
O O
CA 02226740 1998-01-13
Table 2
Ex No. n . R' R2 R3 R~ R'
O -OOOEt H isobutyl H - N(CH~)2
6 O -OOOEt H isobutyl H ~ CH3
O 'CD0H H isobutyl H - N(CH~)2
26 O -COOH H isobutyl H ~ CH3
42 o - CONHOCH~ ~ H isobutyl H ~ CH3
43 O -CONHOH H isobutyl H ~ CH3
CA 02226740 1998-01-13
The biological activity of the compound of the present
invention is now described. The compound of general formula (I)
or its salt according to the present invention has thiol
protease-inhibitory activity. The inhibitory activity of the
compound against calpain, cathepsin L, papain, and trypsin which
- is a serine protease was determined. The results are shown in
Tables 3 and 4.
Assay of ~-calpain-inh;hitory ~ct;vity
The activity of ~-calpain (Nakarai Tesque) was assayed
in accordancewiththeproceduredescribedinthe literature [Anal
Biochem., 2n~, 387-392 (1993)]. Thus, to a solution containing
0.5 mg/ml casein, 50 mM Tris-HCl (pH 7.4), 20 mM dithiothreitol,
and 4 mM calcium chloride was added 2.5 ~l of a dimethyl sulfoxide
solution containing a varying concentration of the test drug as
-well as 0.03 unit of~-calpaintoinitiatethe reaction. The final
liquid volume was 250 ~l. After 60 minutes of reaction at 30~C,
100 ~l of the reaction mixture was transferred to another vessel,
to which 50 ~l of purified water and 100 ~l of 50% Coumassie
brilliant blue solution were added. The mixture was allowed to
stand at room temperature for 15 minutes and the absorbance was
measured at 595 nm. As a control, 2.5 ~l of dimethyl sulfoxide
not contaiping the test drug was added and the mixture was treated
in the same manner as above. The absorbance value thus found was
used as the control value. Similarly, the value found by adding
0.2 mM EDTA in lieu of 4 mM aqueous calcium chloride solution was
CA 02226740 1998-01-13
--18--
used as the blank value. The inhibition rate was calculated by
means of the-following equation and plotted against concentration
on log paper and the amount necessary for 50% inhibition (IC50)
was determined. E64 which is a known cysteine protease inhibition
5 compound was used as a reference drug.
- Inhibition rate (%)
Measured value - blank value
( 1 - ) x 100
Control value - blank value
Assay of catheps;n T-inhih;tory ~ct;v;ty
The activity of cathepsin L (Cosmo Bio), a cysteine protease,
was assayed by the method described in the literature [Methods
inEnzymology, 80, 535-561, 1981]. Thus, toasolutioncontaining
-85 mM acetate buffer (pH 5.5), 2 mM dithiothreitol, 1 rnM EDTA,
2 ~lg cathepsin L, and a varying concentration of the test compound
was added 20 ~M carbobenzoxy-L-phenylalanyl-L-arginine-4-
methyl-coumaryl-7-amide (Z-Phe-Arg-MCA) to initiate the reaction
at the final liquid volume of 200 111. After 20 minutes of reaction
at 30~C, 20 111 of 1 M Tris-HCl (pH 8.0) was added so as to stop
the reaction. The amount of liberated 4-methyl-7-
aminocoumarin was determined with a fluorospectrometer at an
excitation wavelength of 360 nm and a fluorescent emission
wavelength of 450 nm. Using the value found without addition of
the test drug as control and the value found without addition of
CA 02226740 1998-01-13
the enzyme as blank, IC50 was determined in the same manner as
above. E64 was used as a reference drug.
A.ss~y of p~p~;n- ~n~ tryps;n-;nhih;tory ~ctiv;ty
The activity of papain which is a cysteine protease
and of trypsin (Sigma) which is a serine protease was assayed in
- accordance with the method described in the literature [Anal.
Biochem., 2Q~, 387-392, 1993]. Thus, to a solution containing
0.5 mg/ml casein, 50 mM Tris-HCl (pH 8.0), 20 mM dithiothreitol!
and 0.2 mM EDTA was added 2.5 ~1 of dimethyl sulfoxide containing
a varying concentration of the test drug as well as 0.03 unit of
papain or trypsin to initiate the reaction. The final liquid
volume was adjusted to 250 ~l. After 60 minutes of reaction at
30~C, 100 ~l of the reaction mixture was transferred to another
vessel and following addition of 50 ~1 of purified water and 100
~l of 50% Coumassie brilliant blue solution, the mixture was
allowed to stand at room temperature for 15 minutes. The
absorbance of the mixture was then measured at 595 nm. Using the
value found similarly by adding 2.5 ~l of dimethyl sulfoxide not
containing the test drug as control and the value found without
addition of the enzyme as blank, ICso was determined in the same
manner as above. E64 and leupeptin were used as reference drugs.
CA 02226740 1998-01-13
-20- .
Table 3
Calpain 50% inhibitory concentration (ICso)
Test drug (~M) Test drug (~M)
E-64 0.66 Example 31 0.95
Example 347.00 Example 32 17.50
Example 212.90 Example 33 5.10
Example 230.81 Example 34 0.84
Example 240.78 Example 35 41.00
Example 251.10 Example 37 2400
Example 260.64 Example 38 3.90
Example 270.35 Example 39 0.60
Example 280.63 Example 40 6.00
Example 290.49 Example 41 145
Example 301.20 Example 43 1.90
CA 02226740 1998-01-13
-21-
Table 4
50% Inhibitory concentration (IC50)
S Test drug Cathepsin L Papain Trypsin
E-64 0.015 0.032 >300
Leupeptin 7-4
Example 21 0.012 0.110 >3000
Example 23 0.029 0.079 >3000
Example 26 0.082 0.210 >3000
Example 27 0.027 0.062 >3000
Example 32 37.60 4.00 >3000
Example 34 0.009 0-047 >3000
CA 02226740 1998-01-13
--22--
Having inhibitory activity against cysteine proteases
such as calpain, cathepsin L, and papain and showing no activity
against serine protease (trypsin), the compound of general
formula (I) or its salt according to the present invention is of
value as a prophylactic or therapeutic agent for a variety of
~ cysteine protease-associated diseases, for example ischemic
diseases, inflammatory diseases, myodystrophy, immune diseases,
essential hypertension, Alzheimer's disease, subarachnoid
hemorrhage, andosteoporosis, inmammals (e.g.mouse, rat, rabbit,
dog, cat, bovine, swine, and man).
The compound of general formula (I) and its salt
according to the present invention can be administered
systemically orlocally. Systemic administration maybe made not
only orally but also by the intravenous, subcutaneous,
intramuscular and other routes. Local administration can be made
transdermally, transmucosally, intranasally or intraocularly.
The compound of general formula (I) or its salt
according to the present invention can be formulated into a
- pharmaceutical composition. The composition that can be
administered orally to man includes powders, granules, tablets,
capsules, syrups, and elixirs. In the manufacture of said
composition in a powdery, granular ortablet form, pharmaceutical
carrierssuitedforsoliddosage forms, suchas excipients (starch,
glucose, fructose, sucrose, etc.), -lubricants (magnesium
stearate etc.), disintegrators (starch, crystalline cellulose,
CA 02226740 1998-01-13
-2~
etc.), binders (starch, gum arabic, etc.), etc. can be employed.
Such dosage forms may be coated with a coating agent (gelatin,
sucrose, etc.). For the manufacture of said composition in the
form of a syruporanelixir, such additivesas stabilizers (sodium
edetate etc.), suspending agents (gum arabic,
~ calboxymethylcellulose, etc.), corrigents (simple syrup, glucose,
etc.), andperfumes canbe selectively employed. The composition
for parenteral administration includes injections and
suppositories. For the manufacture of an injection, such
auxiliary agents as solvents (distilled water for injection),
stabilizers (sodium edetate etc.), isotonizing agents (sodium
chloride, glycerin, mannitol, etc.), pH control agents
(hydrochloric acid, citric acid, sodium hydroxide, etc.), and
suspending agents (methylcellulose etc.) can be employed. For
the manufacture of suppositories, suppository bases (e.g. cacao
butter, macrogols, etc.) and others can be selectively employed.
~ The composition for external application includes ointments,
creams, lotions, nasal drops, and eye-drops. Such compositions
for external application may contain, in addition to compound (I)
of the present invention, an assortment of known substances such
as ointment bases (petrolatum, lanolin, etc.), solvents (saline,
purified water), stabilizers (sodiumedetate, citricacid, etc.),
wetting agents (glycerin etc.), emulsifiers
(polyvinylpyrrolidone etc.), suspending agents
(hydroxypropylmethylcellulose, methylcellulose, etc.),
CA 02226740 1998-01-13
--2 4--
surfactants (polysorbate 80, polyoxyethylene-hydrogenated
castor oil etc.), preservatives (benzalkonium chloride,
p-hydroxybenzoates, chlorobutanol, etc.), buffers (boric acid,
borax, sodium acetate, citrate buffer, phosphate buffer, etc.),
isotonizing agents (sodium chloride, glycerin, mannitol, etc.),
pH control agent (hydrochloric acid, sodium hydroxide, etc.), and
so on.
The dosage of the compound of general formula (I) or
a salt thereof according to the present invention is dependent
on the disease to be treated, symptoms, recipient, administration
method, etc. However, the therapeutic unit oral dosage is
generally 1-500 mg and preferably 10-200 mg and the therapeutic
unit injection dose is generally 0.1-100 mg and preferably 1-50
mg.
EXAMPLES
The following reference, working, and formulation
examples are all intended to describe the present invention in
further detail and should by no means be construed as defining
the scope of the invention.
Reference ~x~m~le 1
To a solution of N-tert-butoxycarbonylphenyl
alanine (53 g, 0.2 mol) and p-nitrophenol (27.8 g, 0.2 mol) in
ethyl acetate (200 ml) on an ice-water bath was added a solution
of N,N'-dicyclohexylcarbodiimide (41.2 g, 0.2 mol) in ethyl
CA 02226740 1998-01-13
acetate (100 ml) dropwise and the mixture was stirred under
cooling for 3 hours and then at room temperature for 20 hours.
The precipitated byproduct N,N'-dicyclohexyl-carbodiurea was
filtered off and the filtrate was concentrated under reduced
pressure. The residue thus obtained was recrystallized from
~ ethyl acetate-hexane to provide
N-tert-butoxycarbonylphenylalanine p-nitrophenyl ester (61.7 g,
80%).
Reference F.x~m~l e 2
To a solution of N-tert-butoxycarbonylleucine (6.94
g, 30 mmol) and N-hydroxysuccinimide (3.45 g, 30 mmol) in dioxane
(50 ml) on an ice-water bath was added a solution of
N-ethyl-N'-(3-dimethylaminopropyl)carbodiimide hydrochloride
(5.75 g, 30 mmol) in dioxane dropwise and the mixture was stirred
under cooling for 20 minutes and then at room temperature for 24
hours. This reaction mixture was poured into cold water and
~extracted with ethyl acetate. The extract was washed with 10~
aqueous citric acid solution, 10% aqueous sodium hydrogen
carbonate solution, and saturated saline in the order mentioned
and the organic layer was dried over anhydrous magnesium sulfate
and concentrated under reduced pressure. The residue was
recrystallized from isopropyl ether to provide
N-tert-butoxycarbonylleucineN-hydroxysuccinimideester (7.77 g,
78.9%)-
Reference Fx~m~le 3
CA 02226740 1998-01-13
-26-
To a solution of 1-(4-fluorophenyl)piperazine
dihydrochloride (2.53 g, 10 mmol) in N,N-dimethylformamide (40
ml) were added triethylamine (2.8 ml, 20 mmol) and
N-tert-butoxycarbonylphenylalanine p-nitrophenyl ester (2.65 g,
10 mmol) in the order mentioned and the mixture was stirred at
room temperature overnight. This reaction mixture was poured
into cold water and extracted with ethyl acetate. The extract
was washed with 1% aqueous ammonia, saturated saline,
O.lN-hydrochloric acid, saturated saline, saturated aqueous
sodium hydrogen carbonate solution, and saturated saline in the
- order mentioned and the organic layer was dried over anhydrous
magnesiumsulfateandfurtherconcentratedunderreducedpressure
The residue was purified by silica gel column chromatography
using chloroform-methanol (50:1) to provide 1,1-dimethylethyl
2-(4-(4-fluorophenyl)-
1-piperazinyl)-2-oxo-1-(phenylmethyl)ethylcarbamate (2.7 g,
92.2%) as colorless oil.
Reference F.x~mple 4
Usingl-(o-fluorophenyl)piperazinemonohydrochloride
in lieu of 1-(4-fluorophenyl)piperazine dihydrochloride, the
procedure of Reference Example 3 wasotherwise repeatedtoprovide
1,1-dimethylethyl 2-(4-(2-fluorophenyl)-1-
piperazinyl)-2-oxo-1-(phenylmethyl)ethylcarbamate (1.89 g,
88.4%).
Reference F.x~le 5
CA 02226740 1998-01-13
To a solution of 1-(4-fluorophenyl)piperazine
dihydrochloride (0.91 g, 3 mmol) and N-tert-
butoxycarbonylleucine N-hydroxysuccinimide ester (0.99 g, 3
mmol) in dichloromethane (50 ml) was added triethylamine (1.3 ml,
9 mmol) and the mixture was stirred at room temperature for 20
~ hours. This reaction mixture was washed with O.lN-hydrochloric
acid, saturatedaqueoussodiumhydrogen carbonate solution, water,
and saturated saline in the order mentioned and the organic layer
was dried over anhydrous magnesium sulfate and concentrated under
reduced pressure. The residue was purified by silica gel column
chromatography using ethyl acetate-hexane (1:1) to provide
1,1-dimethylethyl
2-(4-(4-fluorophenyl)-1-piperazinyl)-2-oxo-1-(2-methylpropyl)
ethylcarbamate (1.05 g, 89.0%) as colorless oil.
Reference F.x~m~l e 6
Using 4-phenylpiperazine in lieu of
1-(4-fiuorophenyl)piperazine dihydrochloride, the procedure of
Reference Example 5 was otherwise repeated to provide
1,1-dimethylethyl 2-(4-phenyl-1-piperazinyl)-2-oxo-1-
(2-methylpropyl)ethylcarbamate (7.99 g, 99%).
Reference Fx~m~l e 7
Using 1-dimethylsulfamoylpiperazine in lieu of
1-(4-fluorophenyl)piperazine dihydrochloride, the procedure of
Reference Example 5 was otherwise repeated to provide
1,1-dimethylethyl 2-(4-dimethylsulfamoyl-1-
CA 02226740 1998-01-13
piperazinyl)-2-oxo-1-(2-methylpropyl)ethylcarbamate (7.19 g,
88.4%).
Reference F.x~TT~le 8
Using p-toluenesulfonylpiperazine in lieu of
5 1-(4-fluorophenyl)piperazine dihydrochloride, the procedure of
Reference Example 5 was otherwise repeated to provide
1,1-dimethylethyl 2-(4-(4-methylphenylsulfonyl) -1-
piperazinyl)-2-oxo-1-(2-methylpropyl)ethylcarbamate (6.95 g,
79.4%)-
Reference F.X~T~1 e 9
Using 1-(2-chlorophenyl)piperazine in lieu of
1-(4-fluorophenyl)piperazine dihydrochloride, the procedure of
Reference Example 5 was otherwise repeated to provide
1,1-dimethylethyl 2-(4-(2-chlorophenyl) -1-piperazinyl)-
2-oxo-1- (2-methylpropyl)ethylcarbamate (5.70 g, 95.5%).
Reference F.x~lT~le 10
Using 1-(m-chlorophenyl)piperazine monohydrochloride
in lieu of 1-(4-fluorophenyl)piperazine dihydrochloride, the
procedure of Reference Example 5 was otherwise repeated to provide
1,1-dimethylethyl 2-(4-(3-chlorophenyl)-1-
piperazinyl)-2-oxo-1-(2-methylpropyl)ethylcarbamate (2.63 g,
88.4%).
Reference F.x~ le 11
Using 1-(4-chlorophenyl)piperazine monohydrochloride
in lieu of 1-(4-fluorophenyl)piperazine dihydrochloride, the
CA 02226740 1998-01-13
-29- .
procedureof Reference Example5wasotherwise repeatedtoprovide
1,1-dimethylethyl 2-(4-(4-chlorophenyl)-1-
piperazinyl)-2-oxo-1-(2-methylpropyl)ethylcarbamate (2.83 g,
94.8%).
Reference F.x~m~l e 1~
Using N-(p-methoxyphenyl)piperazine succinate
hydrochloride in lieu of 1-(4-fluorophenyl)piperazine
dihydrochloride, the procedure of Reference Example 5 was
otherwise repeated to provide l,1-dimethylethyl
2-(4-(4-methoxyphenyl)-1-piperazinyl)-2-oxo-1-(2-
methylpropyl)ethylcarbamate (2.73 g, 92.3%).
Reference F.x~m~l e 13
To a solution of 1,1-dimethylethyl
2-(4-(4-fluorophenyl)-l-piperazinyl)-2-OXo-l-(phenylmethyl)et
hylcarbamate (2.7 g, 6.3 mmol) in ethyl acetate (20 mmol) on an
ice bath was added 4N-HCl/ethyl acetate (20 ml) dropwise and the
mixture was stirred at room temperature overnight. The resulting
crystals were recovered by filtration and recrystallized from
ethanol-diethyl ether to provide
1-(2-amino-1-oxo-3-phenylpropyl)-4-(4-fluorophenyl)piperazine
- hydrochloride (2.2g, 96.1%) as pale yellow crystals.
Reference F.x~mrl e 14
Using 1,1-dimethylethyl 2-(4-(2-fluorophenyl)-1-
piperazinyl)-2-oxo-l-(phenylmethyl)ethylcarbamate in lieu of
1,1-dimethylethyl 2-(4-(4-fluorophenyl)-1-
CA 02226740 1998-01-13
-30- -
piperazinyl)-2-oxo-1-(phenylmethyl)ethylcarbamate, the
procedure of Reference Example 13 was otherwise repeated to
provide 1-(2-amino-1-oxo-3-phenylpropyl)-4-(2-
fluorophenyl)piperazine hydrochloride (1.3 g, 99.1%) as white
crystals.
Reference Fx~m~le 15
Using 1,1-dimethylethyl 2-(4-(2-fluorophenyl)-1-
piperazinyl)-2-oxo-1-(2-methylpropyl)ethylcarbamate in lieu of
1,1-dimethylethyl 2-(4-(4-fluorophenyl)-1-
piperazinyl)-2-oxo-1-(phenylmethyl)ethylcarbamate, the
procedure of Reference Example 13 was otherwise repeated to
provide 1-(2-amino-4-methyl-1-oxopentyl)-4-
(4-fluorophenyl)piperazine hydrochloride (0.56 g, 70.4%) as
white crystals.
Reference Fx~le 16
Using 1,1-dimethylethyl 2-(4-phenyl-1-
piperazinyl)-2-oxo-1-(2-methylpropyl)ethylcarbamate in lieu of
1,1-dimethylethyl 2-(4-(4-fluorophenyl)-1-
piperazinyl)-2-oxo-1-(phenylmethyl)ethylcarbamate, the
procedure of Reference Example 13 was otherwise repeated to
provide 1-(2-amino-4-methyl-1-oxopentyl)-4-phenylpiperazine
hydrochloride (6.5 g, 99.2%) as white crystals.
Reference Fx~mple 17
Using 1,1-dimethylethyl 2-(4-dimethylsulfamoyl-1-
piperazinyl)-2-oxo-l-(2-methylpropyl)ethylcarbamate in lieu of
CA 02226740 1998-01-13
-31- -
1,1-dimethylethyl 2-(4-(4-fluorophenyl)-1-
piperazinyl)-2-oxo-1-(phenylmethyl)ethylcarbamate, the
procedure of Reference Example 13 was otherwise repeated to
provide 1-(2-amino-4-methyl-1-oxopentyl)-4-
dimethylsulfamoylpiperazine hydrochloride (5.0 g, 83.3%) as
- white crystals.
Reference F.x~m~l e 18
Using 1,1-dimethylethyl 2-(4-(4-
methylphenylsulfonyl)-1-piperazinyl)-2-oxo-1-(2-methylpropyl)
ethylcarbamate in lieu of 1,1-dimethylethyl
2-(4-(4-fluorophenyl)-1-piperazinyl)-2-oxo-1-(phenylmethyl)et
hylcarbamate, the procedure of Reference Example 13was otherwise
repeated to provide 1-(2-amino-4-methyl-1-
oxopentyl)-4-(4-methylphenylsulfonyl)piperazine hydrochloride
(4.83 g, 78.4%) as white crystals.
Reference F.x~m~l e 19
Using 1,1-dimethylethyl 2-(4-(2-chlorophenyl)-1-
piperazinyl)-2-oxo-1-(2-methylpropyl)ethylCarbamate in lieu of
1,1-dimethylethyl 2-(4-(4-fluorophenyl)-1-
piperazinyl)-2-oxo-1-(phenylmethyl)ethylcarbamate, the
procedure of Reference Example 13 was otherwise repeated to
provide 1-(2-amino-4-methyl-1-oxopentyl)-4-(2-
chlorophenyl)piperazine hydrochloride (1.54 g, 62.6%) as white
crystals.
Reference F.x~m~l e 70
CA 02226740 1998-01-13
Using 1,1-dimethylethyl 2-(4-(3-chlorophenyl)-1-
piperazinyl)-2-oxo-1-(2-methylpropyl)ethylcarbamate in lieu of
1,1-dimethylethyl 2-(4-(4-fluorophenyl)-1-
piperazinyl)-2-oxo-1-(phenylmethyl)ethylcarbamate, the
procedure of Reference Example 13 was otherwise repeated to
provide 1-(2-amino-4-methyl-1-oxopentyl)-4-(3-
chlorophenyl)piperazine hydrochloride (1.40 g, 65.7%) as white
crystals.
Reference Fx~ple 21
Using 1,1-dimethylethyl 2-(4-(4-chlorophenyl)-1-
piperazinyl)-2-oxo-1-(2-methylpropyl)ethylcarbamate in lieu of
1,1-dimethylethyl 2-(4-(4-fluorophenyl)-1-
- piperazinyl)-2-oxo-1-(phenylmethyl)ethylcarbamate, the
procedure of Reference Example 13 was otherwise repeated to
provide 1-(2-amino-4-methyl-1-oxopentyl)-4-(4-
chlorophenyl)piperazine hydrochloride (1.50 g, 65.3%) as white
crystals.
Reference Fx~m~le 22
Using 1,1-dimethylethyl 2-(4-(4-methoxyphenyl)-1-
piperazinyl)-2-oxo-1-(2-methylpropyl)ethylcarbamate in lieu of
1,1-dimethylethyl 2-(4-(4-fluorophenyl)-1-
piperazinyl)-2-oxo-1-(phenylmethyl)ethylcarbamate, the
procedure of Reference Example 13 was otherwise repeated to
provide 1-(2-amino-4-methyl-1-oxopentyl)-4-(4-
methoxyphenyl)piperazine hydrochloride (2.21 g, 87.4%) as white
CA 02226740 1998-01-13
-33-
crystals.
Reference F,x~m~l e ~3
To a solution of N-tert-butoxycarbonyl-L-valine (2.27
g, 10 mmol) and 1-(2-chlorophenyl)piperazine (2.00 g, 10 mmol)
ln N,N-dimethylformamide (50 ml) on an ice bath was added
1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride
(2.2 g, 11 mmol) and 1-hydroxybenzotriazole (1.5 g, 11 mmol) in
dichloromethane (50 ml) dropwise and the mixture was stirred at
room temperature for 15 hours. The dichloromethane was then
distilledoffunder reducedpressure andthe residue was extracted
with ethyl acetate (200 ml). The ethyl acetate layer was serially
washed with 10% aqueous citric acid solution, saturated aqueous
sodium hydrogen carbonate solution, and saturated saline in the
order mentioned and dried over anhydrous sodium sulfate. The
solvent was then distilled off and the residue was purified by
silica gel chromatography. Elution with ethyl acetate-n-hexane
(1:2, v/v) gave 1,1-dimethylethyl 2-(4-(2-chlorophenyl~-
piperazinyl)-2-oxo-(s)-1-(2-propyl)ethylcarbamate. After this
colorless oil was dissolved in ethyl acetate (50 ml), 4N HCl/ethyl
acetate (50 ml) was added dropwiseunderice-cooling. Themixture
was stirred at room temperature for 3 hours. The reaction product
was filtered and washed with ethyl acetate-n-hexane (1:1, v/v)
to provide 1-((s)-2-amino 3-methyl-1-oxobutyl)-4-(2-
chlorophenyl)piperazine hydrochloride (3.32 g, 95.8%) as
colorless crystals.
CA 02226740 1998-01-13
-34-
Reference Fx~le 24
Starting with N-tert-butoxycarbonylglycine, the
procedure of Reference Example 23 was otherwise repeated to
provide 1-(2-amino-1-oxoethyl)-4-(2-chlorophenyl)piperazine
hydrochloride (2.4 g, 90.4%) as colorless crystals.
Reference F.x~m~l e 25
Starting with N-tert-butoxycarbOnyl-L-alanine, the
procedure of Reference Example 23 was otherwise repeated to
provide 1-((s)-2-amino-1-oxopropyl)-4-(2-chlorophenyl)-
piperazine hydrochloride (1.7 g, 58.8%) as colorless crystals.
Reference Fx~m~le 26
Starting with N-tert-butoxycarbonyl-L-isoleucine,
- the procedure of Reference Example 23 was otherwise repeated to
provide 1-((s)-2-amino-3-methyl-1-oxopentyl)-
- 4-(2-chlorophenyl)piperazine hydrochloride (3.4 g, 90.2%) as
colorless crystals.
Reference Fx~m~le ~7
Starting with N-tert-butoxycarbonyl-~-alanine, the
procedure of Reference Example 23 was otherwise repeated to
provide 1-(3-amino-l-oxopropyl)-4-(2-chlorophenyl)piperazine
hydrochloride (2.9 g, 90.0%) as colorless crystals.
Reference Fx~m~le ~8
Starting with N-tert-butoxycarbonylsarcosine, the
procedure of Reference Example 23 was otherwise repeated to
provide 1-(2-methylamino-1-oxoethyl)-4-(2-
CA 02226740 1998-01-13
chlorophenyl)piperazine hydrochloride (3.0 g, 93.3%) as
colorless crystals.
Reference Fx~m~le 29
Starting with N-tert-butoxycarbonyl-L-proline, the
procedure of Reference Example 23 was otherwise repeated to
provide 1-(1-(2-pyrrolidinyl)-1-oxomethyl)-4-(2-
chlorophenyl)piperazine hydrochloride (4.3 g, 98.0%) as
colorless crystals.
Reference Fx~m~le 30
Starting with N-tert-butoxycarbonyl-(s-
acetamidomethyl)-L-cysteine, the procedure of Reference Example
23 was otherwise repeated to provide 1-((s)-2-amino-3-
- (acetylaminomethylthio)-1-oxopropyl)-4-(2-chlorophenyl)-
piperazine hydrochloride (4.0 g, 95.9~) as colorless crystals.
Reference F.x~m~l e 31
Starting with N-tert-butoxycarbonyl-L-methionine,
the procedure of Reference Example 23 was otherwise repeated to
provide 1-((s)-2-amino-4-methylthio-1-oxobutyl)-
4-(2-chlorophenyl)piperazine hydrochloride (3.7 g, 97.1~) as
colorless crystals.
Reference Fx~m~le 32
Starting with N-tert-butoxycarbonyl-L-glutamine, the
procedure of Reference Example 23 was otherwise repeated to
provide 1-((s)-2-amino-4-carbamoyl-1-oxobutyl)-
4-(2-chlorophenyl)piperazine hydrochloride (2.7 g, 60.7%) as
CA 02226740 1998-01-13
-36-
colorless crystals.
F.x~rr~l e 1
To a solution of 1-(2-amino-1-oxo-3-phenylpropyl)-
4-(4-fluorophenyl)piperazine hydrochloride (1.82 g, 5 mmol) in
S N,N-dimethylformamide (20 ml) was addedtriethylamine (0.697 ml,
~ 5 mmol) and the mixture was stirred at room temperature for 10
minutes. To this reaction mixture was added ethyl p-nitrophenyl
L-trans-epoxysuccinate, synthesized in accordance with the
method of Tamai et al. [Chem. Pharm. Bull., 35, 1098 (1987)] (1.41
g, 5 mmol), and the mixture was stirred at room temperature for
20 hours. This reaction mixture was poured into cold water and
extracted with ethyl acetate. The extract was serially washed
with l%aqueous ammonia, saturatedsaline,O.lN-hydrochloricacid,
saturated saline, saturated aqueous sodium hydrogen carbonate
solution, and saturated saline in the order mentioned and the
organic layer was dried over anhydrous magnesium sulfate and
concentrated under reduced pressure. The residue was subjected
to silicagel chromatography, elutionbeingcarriedoutwith ethyl
acetate-hexane (1:1). The procedure provided ethyl
(2s,3s)-3-[[[[(ls)-1-[[4-(4-fluorophenyl)-1-piperadinyl]carbo
nyl]-2-phenyl]ethyl]amino]carbonyl]oxiranecarboxylate (1.2 g,
51. 1%) .
H NMR (CDC13) ~: 1.31 (t, 3H, J=7.0 Hz), -C-CH3),
2.42-2.50 (m, lH, piperazine ring), 2.83-2.94 (m,
2H, piperazine ring), 3.00 (d, 2H, J=7.6 Hz,
CA 02226740 1998-01-13
-37-
ph-CH2-C-), 2.97-3.07 (m, lH, piperazine ring),
3.14-3.22 (m, lH, piperazine ring), 3.35 (d, lH,
J=l.9 Hz, epoxy ring), 3.43-3.52 (m, lH, piperazine
ring), 3.64 (d, lH, J=l.9 Hz, epoxy ring), 3.71
(t, 2H, J=5.4 Hz, piperazine ring), 4.25 (dq, 2H,
J=7.3, 2.6 Hz, -O-CH2-C), 5.18 (q, lH, J=5.3 Hz,
-N-CH-CO), 6.75-6.83 (m, 2H, aromatic), 6.91-7.04
(m, 2H, aromatic, lH, NH), 7.17-7.34 (m, 5H,
aromatic).
Fx~m~le 2
Using 1-(2-amino-1-oxo-3-phenylpropyl)-4-
(2-fluorophenyl)piperazine hydrochloride in lieu of
~ 1-(2-amino-l-oxo-3-phenylpropyl)-4-(4-fluorophenyl)piperazine
hydrochloride, the procedure of Example 1 was otherwise repeated
to provide ethyl (2s, 3s)-3-[[[[(ls)-l-[[4-(2-
fluorophenyl)-l-piperazinyl]carbonyl]-2-phenyl]ethyl]amino]ca
rbonyl]oxiranecarboxylate (0.83 g, 58.6%).
H NMR (CDC13) ~: 1.31 (t, 3H, J=7.1 Hz, -C-CH3),
2.42-2.49 (m, lH, piperazine ring), 2.72-2.90 (m,
2H, piperazine ring), 3.02 (d, 2H, J=8.3 Hz,
ph-CH2-C-), 2.94-3.10 (m, lH, piperazine ring),
3.17-3.29 (m, lH, piperazine ring), 3.36 (d, lH,
J=1.7 Hz, epoxy ring), 3.44-3.57 (m, lH, piperazine
ring), 3.65 (d, lH, J=1.3 Hz, epoxy ring), 3.66-
3.80 (m, 2H, piperazine ring), 4.25 (dq, 2H,
CA 02226740 1998-01-13
-38-
J=7.1, 2.3 Hz, -O-CH2-C), 5.19 (q, lH, J=7.6 Hz,
-N-CH-CO), 6.8 (t, lH, J=8.3 Hz, -NH-), 6.93-7.11
(m, 4H, aromatic), 7.18-7.34 (m, 5H, aromatic).
Fx~m~le 3
Using 1-(2-amino-4-methyl-1-oxopentyl)-4-(4-
fluorophenyl)piperazine hydrochloride in lieu of
1-(2-amino-l-oxo-3-phenylpropyl)-4-(4-fluorophenyl)piperazine
hydrochloride, the procedure of Example 1 was otherwise repeated
to provide ethyl (2s,3s)-3-[[[[(ls)-1-[[4-(4-
fluorophenyl)-1-piperazinyl]carbonyl]-3-methyl]butyl]amino]ca
rbonyl]oxiranecarboxylate (0.30 g, 45.6%).
lH NMR (CDC13) ~: 0.93 (d, 3H, J=6.3 Hz, -C-CH3), 1.00
- (d, 3H, J=6.3 Hz, -C-CH3), 1.32 (t, 3H, J=7.3 Hz,
-C-CH3), 1.40-1.63 (m, 3H, -C-CH2-CH-C2), 3.06-
- 3.15 (m, 4H, piperazine ring), 3.49 (d, lH, J=1.7
Hz, epoxy ring), 3.60-3.89 (m, 4H, piperazine
ring), 3.68 (d, lH, J=1.7 Hz, epoxy ring), 4.26
(dq, 2H, J=7.3, 3.3 Hz, -O-CH2-C), 5.03 (dt, lH,
J=8.91, 4.3 Hz, -N-CH-CO), 6.85-7.03 (m, 5H,
aromatic and -NH).
F.x~n~l e 4
Using 1-(2-amino-4-methyl-1-oxopentyl)-4-phenyl-
piperazine hydrochloride in lieu of 1-(2-amino-1-oxo-3-
phenylpropyl)-4-(4-fluorophenyl)piperazine hydrochloride, the
procedure of Example 1 was otherwise repeated to provide ethyl
CA 02226740 1998-01-13
--39-
(2s, 3s) -3- [ [ [ [ (ls) -1- [ (4-phenyl-1-piperazinyl) carbonyl] -3-
methyl]butyl] amino] carbonyl] oxiranecarboxylate (3. 96 g, 63. 39~) .
lH NMR (CDC13) ~: 0. 93 (d, 3H, J=6. 3 Hz, -C-CH3), 1. 00
(d, 3H, J=6. 3 Hz, -C-CH3), 1. 32 (t, 3H, J=7 . 3 Hz,
-C-CH3), 1. 40-1. 63 (m, 3H, -C-CH2-CH-C2), 3 .16-
3 . 24 (m, 4H, piperazine ring), 3 . 49 (d, lH, J=1 . 7
Hz, epoxy ring), 3 . 60-3. 89 (m, 4H, piperazine
ring), 3 . 68 (d, lH, J=l . 7 Hz, epoxy ring), 4 . 26
(dq, 2H, J=7 . 3, 3 . 3 Hz, -O-CH2-C), 5 . 03 (dt, lH,
J=8 . 9, 4 . 3 Hz, -N-CH-CO), 6. 90-6. 95 (m, 4H,
aromatic and -NH), 7 . 25-7 . 33 (m, 2H, aromatic) .
F~x~ e 5
Using 1- (2-amino-4-methyl-1-oxopentyl) -4-dimethyl-
sulfamoylpiperazine hydrochloride in lieu of 1- (2-amino-1-
oxo-3-phenylpropyl ) -4- ( 4 -fluorophenyl ) piperazine hydrochlo-
ride, the procedure of Example 1 was otherwise repeated to provide
ethyl (2s, 3s) -3- [ [ [ [ (ls) -1- [ (4-dimethylsulfamoyl-
l-piperazinyl ) carbonyl ] -3-methyl ] butyl ] amino ] carbonyl ] oxirane
carboxylate (3.7 g, 82. 6%) .
H NMR (CDC13) ~: 0 . 92 (d, 3H, J=6 . 3 Hz, -C-CH3), 0 . 89
(d, 3H, J=6. 3 Hz, -C-CH3), 1 . 32 (t, 3H, J=7 . 3 Hz,
-C-CH3), 1 . 38-1 . 60 (m, 3H, -C-CH2-CH-C2), 2 . 85 (s,
6H, -N-CH3), 3.15-3. 38 (m, 4H, piperazine ring),
3 . 48 (d, lH, J=1. 7 Hz, epoxy ring), 3 . 52-3 . 68 (m,
CA 02226740 1998-01-13
-40- -
3H, piperazine ring), 3.67 (d, lH, J=1.7 Hz, epoxy
ring), 3.79-3.87 (m, lH, piperazine ring), 4.27
(dq, 2H, J=i.3, 4.0 Hz, -O-CH2-C), 4.96 (dt, lH,
J=8.9, 4.3 Hz, -N-CH-CO), 6.90 (d, lH, J=8.6 Hz,
-NH-).
~ ~x~le 6
Using 1-(2-amino-4-methyl-1-oxopentyl)-4-(4-methyl-
phenylsulfonyl)piperazine hydrochloride in lieu of 1-(2-amino-
l-oxo-3-phenylpropyl)-4-(4-fluorophenyl)piperazine hydrochlo-
ride, the procedure of Example 1 was otherwise repeated to provide
ethyl (2s,3s)-3-[[[[(2s)-1-[[4-(4-methylphenyl-sulfo-
nyl)-l-piperazinyl]carbonyl]-3-methyl]butyl]-amino]-carbon-
yl]oxirane carboxylate (4.31 g, 95.1%).
'H NMR (CDC13) ~: 0.87 (d, 3H, J=6.3 Hz, -C-CH3), 0.94
(d, 3H, J=6.3 Hz, -C-CH3), 1.30 (t, 3H, J=7.3 Hz,
-C-CH3), 1.31-1.55 (m, 3H, -C-CH2-CH-C2), 2.45 (s,
6H, -ph-CH3), 2.70-2.84 (m, 2H, piperazine ring),
3.22-3.54 (m, 4H, piperazine ring), 3.43 (d, lH,
J=1.7 Hz, epoxy ring), 3.61 (d, lH, J=2.0 Hz,
epoxy ring), 3.68-3.78 (m, lH, piperazine ring),
3.96-4.06 (m, lH, piperazine ring), 4.25 (dq, 2H,
J=7.3, 4.0 Hz, -O-CH2-C), 4.87 (dt, lH, J=9.2, 4.0
Hz, -N-CH-CO), 6.81 (d, lH, J=8.6 Hz, -NH-), 7.35
(d, 2H, J=7.9 Hz, aromatic), 7.63 (d, 2H, J=8.3
Hz, aromatic).
CA 02226740 1998-01-13
-41-
Fx~mple 7
Using 1-t2-amino-4-methyl-1-oxopentyl)-4-(2-
chlorophenyl)piperazine hydrochloride in lieu of
1-(2-amino-1-oxo-3-phenylpropyl)-4-(4-fluorophenyl)piperazine
hydrochloride, the procedure of Example 1 was otherwise repeated
to provide ethyl (2s,3s)-3-[[[[(ls)-1-[[4-(2-
chlorophenyl)-1-piperazinyl]carbonyl]-3-methyl]butyl]amino]ca
rbonyl]oxiranecarboxylate (0.65 g, 35.9%).
lH NMR (CDC13) ~: 0.93 (d, 3H, J=6.3 Hz, -C-CH3), 1.01
(d, 3H, J=6.3 Hz, -C-CH3), 1.32 (t, 3H, J=7.3 Hz,
-C-CH3), 1.41-1.61 (m, 3H, -C-CH2-CH-C2), 2.96-
3.10 (m, 4H, piperazine ring), 3.49 (d, lH, J=2.0
Hz, epoxy ring), 3.61-3.81 (m, 3H, piperazine
ring), 3.68 (d, lH, J=2.0 Hz, epoxy ring), 3.90-
3.98 (m, lH, piperazine ring), 4.27 (dq, 2H,
J=7.3, 4.0 Hz, -O-CH2-C), 5.03 (dt, lH, J=8.9, 4.3
Hz, -N-CH-CO), 6.91 (d, lH, J=8.6 Hz, -NH-),
7.00-7.06 (m, 2H, aromatic), 7.21-7.27 (m, lH,
aromatic), 7.37-7.41 (m, lH, aromatic).
F.x~m~l e 8
Using 1-(2-amino-4-methyl-1-oxopentyl)-4-(3-
chlorophenyl)piperazine hydrochloride in lieu of
1-(2-amino-1-oxo-3-phenylpropyl)-4-(4-fluOrOphenyl)piperazine
hydrochloride, the procedure of Example 1 was otherwise repeated
to provide ethyl (2s,3s)-3-[[[[(ls)-1-[[4-(3-
CA 02226740 1998-01-13
-42-
chlorophenyl)-1-piperazinyl]carbonyl]-3-methyl]butyl]amino]-
carbonyl]oxiranecarboxylate (1.24 g, 68.4%).
H NMR (CDC13) ~: 0.93 (d, 3H, J=6.3 Hz, -C-CH3), 1.00
(d, 3H, J=6.3 Hz, -C-CH3), 1.32 (t, 3H, J=7.3 Hz,
-C-CH3), 1.42-1.63 (m, 3H, -C-CH2-CH-C2), 3.17-
3.25 (m, 4H, piperazine ring), 3.48 (d, lH, J=2.0
Hz, epoxy ring), 3.60-3.90 (m, 4H, piperazine
ring), 3.67 (d, lH, J=2.0 Hz, epoxy ring), 4.27
tdq, 2H, J=7.3, 4.0 Hz, -O-CH2-C), 5.02 (dt, lH,
J=8.9, 4.3 Hz, -N-CH-CO), 6.77-6.81 (m, lH,
aromatic), 6.86-6.89 (m, 3H, aromatic and -NH),
7.16-7.22 (m, lH, aromatic).
F.Xj~T~1 e 9
Using 1-(2-amino-4-methyl-1-oxopentyl)-4-(4-
- chlorophenyl)piperazine hydrochloride in lieu of
1-(2-amino-1-oxo-3-phenylpropyl)-4-(4-fluorophenyl)piperazine
hydrochloride, the procedure of Example 1 was otherwise repeated
to provide ethyl (2s,3s)-3-[[[[(ls)-1-[[4-(4-
chlorophenyl)-1-piperazinyl]carbonyl]-3-methyl]butyl]amino]-
carbonyl]oxiranecarboxylate (0.55 g, 27.1%).
H NMR (CDC13) ~: 0.93 (d, 3H, J=6.3 Hz, -C-CH3), 1.0
(d, 3H, J=6.3 Hz, -C-CH3), 1.32 (t, 3H, J=7.3 Hz,
-C-CH3), 1.42-1.63 (m, 3H, -C-CH2-CH-C2), 3.12-
3.20 (m, 4H, piperazine ring), 3.48 (d, lH, J=2.0
Hz, epoxy ring), 3.60-3.90 (m, 4H, piperazine
CA 02226740 1998-01-13
-43-
ring), 3.67 (d, lH, J=2.0 Hz, epoxy ring), 4.27
(dq, 2H, J=7.3, 4.0 Hz, -O-CH2-C), 5.02 (dt, lH,
J=8.9, 4.3 Hz, -N-CH-CO), 6.83-6.87 (m, 2H,
aromatic), 6.90 (d, lH, J=9.9 Hz, -NH), 7.21-7.3
(m, 2H, aromatic).
F.x~m~l e 10
Using 1-(2-amino-4-methyl-1-oxopentyl)-4-(4-
methoxyphenyl)piperazine hydrochloride in lieu of
1-(2-amino-1-oxo-3-phenylpropyl)-4-(4-fluorophenyl)piperazine
hydrochloride, the procedure of Example 1 was otherwise repeated
to provide ethyl (2s,3s)-3-[[[[(ls)-1-[[4-(4-
methoxyphenyl)-l-piperazinyl]carbonyl]-3-methyl]butyl]amino]c
- arbonyl]oxiranecarboxylate (0.94 g, 65.2%).
'H NMR (CDC13) ~: 0.92 (d, 3H, J=6.6 Hz, -C-CH3), 1.00
15 - (d, 3H, J=6.3 Hz, -C-CH3), 1.32 (t, 3H, J=7.3 Hz,
-C-CH3), 1.40-1.60 (m, 3H, -C-CH2-CH-C2), 3.03-
3.11 (m, 4H, piperazine ring), 3.49 (d, lH, J=2.0
Hz, epoxy ring), 3.60-3.88 (m, 4H, piperazine
ring), 3.67 (d, lH, J=2.0 Hz, epoxy ring), 3.78
(s, 3H, -O-CH3), 4.27 (dq, 2H, J=7.3, 4.0 Hz,
-O-CH2-C), 5.03 (dt, lH, J=8.9, 4.3 Hz, -N-CH-CO),
6.83-6.96 (m, 5H, aromatic and -NH).
F,x~m~l e 11
Using 1-((s)-2-amino-3-methyl-1-oxobutyl)-4-(2-
25 chlorophenyl)piperazine hydrochloride in lieu of
CA 02226740 1998-01-13
-44-
1-(2-amino-1-oxo-3-phenylpropyl)-4-(4-fluorophenyl)piperazine
hydrochloride, the procedure of Example 1 was otherwise repeated
to provide ethyl (2s,3s)-3-[[[[(ls)-1-[[4-(2-
chlorophenyl)-1-piperazinyl]carbonyl]-2-methyl]propyl]amino]c
arbonyl]oxiranecarboxylate (1.3 g, 57.4%) as colorless oil.
F.x~m~l e 1~
Using 1-(2-amino-1-oxoethyl)-4-(2-chlorophenyl)-
piperazine hydrochloride in lieu of 1-(2-amino-1-oxo-3-
phenylpropyl)-4-(4-fluorophenyl)piperazine hydrochloride, the
procedure of Example 1 was otherwise repeated to provide ethyl
(2s,3s)-3-[[[[4-(2-chlorophenyl)-1-piperazinyl]carbonyl]-
methyl]amino]carbonyl]oxiranecarboxylate (1.75 g, 53.5%) as
~ colorless oil.
F.X~pl e 13
Using 1-((s)-2-amino-1-oxopropyl)-4-(2-
chlorophenyl)-piperazine hydrochloride in lieu of 1-(2-amino-
1-oxo-3-phenylpropyl)-4-(4-fluorophenyl)piperazine hydrochlo-
ride, the procedure ofExample 1 was otherwise repeatedto provide
ethyl (2s,3s)-3-[[[(ls)-1-[[4-(2-
chlorophenyl)-1-piperazinyl]carbonyl]ethyl]amino]carbonyl]-
oxiranecarboxylate (1.23 g, 55.0%) as colorless oil.
F.X~pl e 14
Using 1-((s)-2-amino-3-methyl-1-oxopentyl)-4-(2-
chlorophenyl)piperazine hydrochloride in lieu of
1-(2-amino-1-oxo-3-phenylpropyl)-4-(4-fluorophenyl)piperazine
CA 02226740 1998-01-13
-45-
hydrochloride, the procedure of Example 1 was otherwise repeated
to provide ethyl (2s,3s)-3-[[[[(ls)-1-[[4-(2-
chlorophenyl)-1-piperazinyl]carbonyl]-2-methyl]butyl]amino]ca
rbonyl]oxiranecarboxylate (1.48 g, 56.6%) as colorless oil.
~x~m~le 15
Using 1-(3-amino-1-oxopropyl)-4-(2-chlorophenyl)-
piperazine hydrochloride in lieu of 1-(2-amino-1-oxo-3-
phenylpropyl)-4-(4-fluorophenyl)piperazine hydrochloride, the
procedure of Example 1 was otherwise repeated to provide ethyl
(2s,3s)-3-[[[2-[[4-(2-chlorophenyl)-1-piperazinyl]carbonyl]-
ethyl]amino]carbonyl]oxiranecarboxylate (1.16 g, 52.9%) as
colorless oil.
F,x~m~l e 16
Using 1-(2-methylamino-1-oxoethyl)-4-(2-chloro
phenyl)piperazine hydrochloride in lieu of 1-(2-amino-1-oxo-
3-phenylpropyl)-4-(4-fluorophenyl)piperazine hydrochloride,
the procedure of Example lwas otherwise repeated to provide ethyl
(2s,3s)-3-[[[[N-[[4-(2-chlorophenyl)-1-piperazinyl]-
carbonyl]methyl]-N-methyl]amino]carbonyl]oXiranecarboxylate
(1.55 g, 76.7%) as colorless oil.
F.X~ e 17
Using 1-(1-(2-pyrrolidinyl)-1-oxomethyl)-4-(2-
chlorophenyl)piperazine hydrochloride in lieu of
1-(2-amino-1-oxo-3-phenylpropyl)-4-(4-fluorophenyl)piperazine
hydrochloride, the procedure of Example 1 was otherwise repeated
CA 02226740 1998-01-13
-46-
to provide ethyl (2s,3s)-3-[[(2s)-2-[[4-(2-
chlorophenyl)-l-piperazinyl]carbonyl]-l-pyrrolidinyl]carbon
]oxiranecarboxylate (1.42 g, 49.9%) as colorless oil.
F.X~pl e 18
Using 1-((s)-2-amino-3-(acetylaminomethylthio)-1-
oxopropyl)-4-(2-chlorophenyl)piperazine hydrochloride in lieu
of 1-(2-amino-1-oxo-3-phenylpropyl)-4-(4-fluorophenyl)-
piperazine hydrochloride, the procedure of Example 1 was
otherwise repeated to provide ethyl (2s,3s)-3-[[[[(ls)-1-
[[4-(2-chlorophenyl)-1-piperazinyl]carbonyl]-2-acetylamino-
methylthio]ethyl]amino]carbonyl]oxiranecarboxylate (1.19 g,
43.7%) as colorless oil.
Fx~m~le 19
Using 1-((s)-2-amino-4-methylthio-1-oxobutyl)-4-
(2-chlorophenyl)piperazine hydrochloride in lieu of
l-(2-amino-1-oxo-3-phenylpropyl)-4-(4-fluorophenyl)piperazine
hydrochloride, the procedure of Example 1 was otherwise repeated
to provide ethyl (2s,3s)-3-[[[[(ls)-1-[[4-(2-
chlorophenyl)-1-piperazinyl]carbonyl]-4-methylthio]butyl]-
amino]carbonyl]oxiranecarboxylate (1.54 g, 61.5%) as colorless
oil.
Fx~m~le 20
Using 1-((s)-2-amino-4-carbamoyl-l-oxobutyl)-4-(2-
chlorophenyl)piperazine hydrochloride in lieu of
1-(2-amino-1-oxo-3-phenylpropyl)-4-(4-fluorophenyl)piperazine
CA 02226740 1998-01-13
-47-
hydrochloride, the procedure of Example 1 was otherwise repeated
to provide ethyl (2s,3s)-3-[[[[(ls)-1-[[4-(2-
chlorophenyl)-l-piperazinyl]carbonyl]-3-carbamoyl]propyl]-
amino]carbonyl]oxiranecarboxylate (0.2g, 5.8%) ascolorlessoil.
- F.x~m~l e 21
To a solution of ethyl (2s,3s)-3-[[[[(ls)-1-[[4-
(4-fluorophenyl)-1-piperazinyl]carbonyl]-2-phenyl]ethyl]-
amino]carbonyl]oxiranecarboxylate (0.5 g, 1.06 mmol) in ethanol
(20 mi) on an ice-water bath was added O.lN-sodium
hydroxide/ethanol (16 ml) and the mixture was stirred at room
temperature for 20 hours. This reaction mixture was poured into
cold water and acidified with lN-hydrochloric acid and the
resultingwhiteprecipitatewasrecoveredbyfiltrationanddried.
This precipitate was recrystallized from ethyl acetate-hexane
to provide (2s,3s)-3-[[[[(ls)-1-[[4-(4-
fluorophenyl)-l-piperazinyl]carbonyl]-2-phenyl]ethyl]amino]-
carbonyl]oxiranecarboxylic acid (0.36 g, 77.8%).
1H NMR (CDC13) ~: 2.34-2.41 (m, lH, piperazine ring),
2.82-2.96 (m, 2H, piperazine ring), 2.99-3.08 (m,
lH, piperazine ring), 3.06 (d, 2H, J=7.3 Hz,
ph-CH2-C-), 3.16-3.24 (m, lH, piperazine ring),
3.49-3.58 (m, lH, piperazine ring), 3.55 (d, lH,
J=1.7 Hz, epoxy ring), 3.57 (d, lH, J=1.7 Hz,
epoxy ring), 3.71 (t, 2H, J=5.1 Hz, piperazine
CA 02226740 1998-01-13
-48-
ring), 4.5-6.0 (br d, lH, -COOH), 5.23 (q, lH,
J=7.9 Hz, -N-CH-CO), 6.74-6.82 (m, 2H, aromatic),
6.91-7.00 (m, 2H, aromatic), 7.20-7.35 (m, 5H,
aromatic), 8.23 (d, lH, J=8.6 Hz, -NH-).
F.x~m~l e ~
~ Using ethyl (2s,3s)-3-[[[[(ls)-1-[[4-(2-fluoro-
phenyl)-l-piperazinyl]carbonyl]-2-phenyl]ethyl]amino]-
carbonyl]oxiranecarboxylate in lieu of ethyl (2s,3s)-3-
[[[[(ls)-1-[[4-(4-fluorophenyl)-1-piperazinyl]carbonyl]-2-
phenyl]ethyl]amino]carbonyl]oxiranecarboxylate, the procedure
of Example 21 was otherwise repeated to provide
((2s,3s)-3-[[[[(ls)-1-[[4-(2-fluorophenyl)-1-piperazinyl]-
carbonyl]-2-phenyl]ethyl]amino]carbonyl]oxiranecarboxylic
acid (0.1 g, 29.6%).
lH NMR (CDC13) ~: 2.38-2.43 (m, lH, piperazine ring),
2.83-2.93 (m, 2H, piperazine ring), 2.95-3.08 (m,
lH, piperazine ring), 3.06 (d, 2H, J=7.6 Hz,
ph-CH2-C-), 3.20-3.28 (m, lH, piperazine ring),
3.49-3.66 (m, lH, piperazine ring), 3.55 (d, lH,
J=1.7 Hz, epoxy ring), 3.58 (d, lH, J=1.3 Hz,
epoxy ring), 3.67-3.80 (m, 2H, piperazine ring),
4-.0-6.0 (br d, lH, -COOH), 5.23 (q, lH, J=7.9 Hz,
-N-CH-CO), 6.77-6.87 (m, lH, aromatic), 6.93-7.09
(m, 3H, aromatic), 7.20-7.36 (m, 5H, aromatic),
8.23 (d, lH, J=8.6 Hz, -NH-).
CA 02226740 1998-01-13
--49--
Fxi~rr~le 23
Using ethyl (2s,3s)-3-[[[[(ls)-1-[[4-(4-fluoro-
phenyl)-1-piperazinyl]carbonyl]-3-methyl]butyl]amino]-
carbonyl]oxiranecarboxylate in lieu of ethyl (2s,3s)-3-
[[[[(ls)-1-[[4-(4-fluorophenyl)-1-piperazinyl]carbonyl]-2-
phenyl]ethyl]amino]carbonyl]oxiranecarboxylate, the procedure
of Example 21 was otherwise repeated to provide (2s,3s)-3-
[[[[(ls)-1-[[4-(4-fluorophenyl)-1-piperazinyl]carbonyl]-3-
methyl]butyl]amino]carbonyl]oxiranecarboxylic acid (0.13 g,
69.5%).
H NMR (CDCl3) ~: 0.96 td, 3H, J=6.6 Hz, -C-CH3), 0.99
(d, 3H, J=6.59 Hz, -C-CH3), 1.42 (ddd, lH, J=14.1,
10.5, 3.6 Hz, -C-CH2-C), 1.6-1.82 (m, lH, -C-CH-
C2), 1.69 (ddd, lH, J=14.5, 10.7, 4.23 Hz, -C-CH2-
C), 3.08-3.26 (m, 4H, piperazine ring), 3.55 (d,
lH, J=1.7 Hz, epoxy ring), 3.60-3.92 (m, 4H,
piperazine ring), 3.62 (d, lH, J=1.8 Hz, epoxy
ring), 5.08 (ddd, lH, J=10.6, 8.6, 3.6 Hz, -N-CH-
CO), 5.2-6.4 (br d, lH, -COOH), 6.84-7.03 (m, 4H,
aromatic), 8.18 (d, lH, J=8.6 Hz, -NH-).
Fx~Tr~le 74
Using ethyl (2s,3s)-3-[[[[(ls)-1-[(4-phenyl-1-
piperazinyl)carbonyl]-3-methyl]butyl]amino]carbonyl]-
oxiranecarboxylate in lieu of ethyl (2s,3s)-3-[[[[(ls)-
1-[[4-(4-fluorophenyl)-1-piperazinyl]carbonyl]-2-phenyl]-
CA 02226740 1998-01-13
--50--
ethyl]amino]carbonyl]oxiranecarboxylate, the procedure of
Example 21 was otherwise repeated to provide (2s,3s)-3-
[[[[(ls)-1-[(4-phenyl-1-piperazinyl)carbonyl]-3-methyl]-
butyl]amino]carbonyl]oxiranecarboxylic acid (1.06 g, 29.1%).
S lH NMR (CDC13) ~: 0.96 (d, 3H, J=6.6 Hz, -C-CH3), 0.99
(d, 3H, J=6.26 Hz, -C-CH3), 1.37-1.47 (m, lH,
-C-CH-C2), 1.64-1.80 (m, 2H, -C-CH2-C-), 3.17-3.36
(m, 4H, piperazine ring), 3.55 (d, lH, J=1.7 Hz,
epoxy ring), 3.62 (d, lH, J=1.3 Hz, epoxy ring),
3.70-3.90 (m, 4H, piperazine ring), 5.10 (m, lH,
-N-CH-CO), 6.5-7.5 (br d, lH, -COOH), 6.87-6.96
(m, 3H, aromatic), 7.27-7.33 (m, 2H, aromatic),
8.20 (d, lH, J=8.6 Hz, -NH-).
F.X~ e 25
Using ethyl (2s,3s)-3-[[[[(ls)-1-[(4-dimethyl-
sulfamoyl-1-piperazinyl)carbonyl]-3-methyl]butyl]-
amino]carbonyl]oxiranecarboxylate in lieu of ethyl
(2s,3s)-3-[[[[(ls)-1-[[4-(4-fluorophenyl)-1-piperazinyl]-
carbonyl]-2-phenyl]ethyl]amino]carbonyl]oxiranecarboxylate,
20 the procedure of Example 21 was otherwise repeated to provide
(2s,3s)-3-[[[[(ls)-1-[(4-dimethylsulfamoyl-1-piperazinyl)carb
onyl]-3-methyl]butyl]amino]carbonyl]oxiranecarboxylic acid
(1.28 g, 39.0%).
lH NMR (CDC13) ~: 0.94 (d, 3H, J=6.3 Hz, -C-CH3), 0.96
(d, 3H, J=5.6 Hz, -C-CH3), 1.36-1.44 (m, lH,
CA 02226740 1998-01-13
--51--
-C-CH-C2), 1.61-1.68 (m, 2H, -C-CH2-C-), 2.85 (s,
6H, -N-CH3), 3.17-3.35 (m, 4H, piperazine ring),
3.48-3.60 (m, 2H, piperazine ring), 3.58 (d, lH,
J=1.7 Hz, epoxy ring), 3.62 (d, lH, J=1.7 Hz,
epoxy ring), 3.70-3.80 (m, lH, piperazine ring),
3.83-3.95 (m, lH, piperazine ring), 4.95-5.05 (m,
lH, -N-CH-CO), 7.7-8.1 (br d, lH, -COOH), 7.94 (d,
lH, J=8.6 Hz, -NH-) .
F.X~l e 26
Using ethyl (2s,3s) -3- [ [ [ [ (ls) -1- [ [4- (4-methyl-
phenylsulfonyl) -l-piperazinyl] carbonyl] -3-methyl] -
butyl] amino] carbonyl] oxiranecarboxylate in lieu of ethyl
(2s,3s) -1- [ [ [ [ (ls) [ [4- (4-fluorophenyl) -1-piperazinyl] -
carbonyl] -2-phenyl] ethyl] amino] carbonyl] oxiranecarboxylate,
the procedure of Example 21 was otherwise repeated to provide
(2s,3s) -3- [ [ [ [ (ls) -1- [ [4- (4-methylphenylsulfonyl) -1-
plperazinyl ] carbonyl ] -3-methyl ] butyl ] amino] carbonyl ] oxiraneca
rboxylic acid (2.8 g, 70.0%) .
1H NMR (CDC13) ~: 0.90 (d, 3H, J=6.6 Hz, -C-CH3), 0.93
(d, 3H, J=6.6 Hz, -C-CH3), 1.23-1.33 (m, lH,
-C-CH-C2), 1.53-1.67 (m, 2H, -C-CH2-C-), 2.45 (s,
3H, -ph-CH3), 2.73-2.91 (m, 2H, piperazine ring),
3.28-3.59 (m, 4H, piperazine ring), 3.45 (d, lH,
J=1.7 Hz, epoxy ring), 3.48 (d, lH, J=1.7 Hz,
epoxy ring), 3.70-3.83 (m, lH, piperazine ring),
CA 02226740 1998-01-13
3.98-4.08 (m, lH, piperazine ring), 4.85-4.97 (m,
lH, -N-CH-CO), 7.35 (d, 2H, J=7.9 Hz, aromatic),
7.63 (d, 2H, J=8.3 Hz, aromatic), 7.97 (d, lH,
J=8.6 Hz, -NH-).
F.X~pl e 77
Using ethyl (2s,3s)-3-[[[[(ls)-1-[[4-(2-chloro-
phenyl)-l-piperazinyl]carbonyl]-3-methyl]butyl]amino]-
carbonyl]oxiranecarboxylate in lieu of ethyl (2s,3s)-3-
[[[[(ls)-1-[[4-(4-fluorophenyl)-1-piperazinyl]carbonyl]-2-
phenyl]ethyl]amino]carbonyl]oxiranecarboxylate, the procedure
of Example 21 was otherwise repeated to provide (2s,3s)-
3-[[[[(ls)-1-[[4-(2-chlorophenyl)-1-piperazinyl]carbonyl]-
3-methyl]butyl]amino]carbonyl]oxiranecarboxylic acid (0.41 g,
67.2%).
lH NMR (CDC13) ~: 0.97 (d, 3H, J=6.9 Hz, -C-CH3), 0.98
(d, 3H, J=7.3 Hz, -C-CH3), 1.38-1.47 (m, lH,
-C-CH-C2), 1.65-1.77 (m, 2H, -C-CH2-C-), 2.98-3.19
(m, 4H, piperazine ring), 3.55 (d, lH, J=1.7 Hz,
epoxy ring), 3.61-3.77 (m, 2H, plperazine ring),
3.64 (d, lH, J=1.7 Hz, epoxy ring), 3.77-3.89 (m,
lH, piperazine ring), 3.89-4.15 (m, lH, piperazine
~ ring), 5.04-5.18 (m, lH, -N-CH-CO), 7.00-7.06 (m,
2H, aromatic), 7.21-7.28 (m, lH, aromatic),
7.37-7.41 (m, lH, aromatic), 8.25 (d, lH, J=8.9
Hz, -NH-).
CA 02226740 1998-01-13
-53-
Fx~le ~8
Using ethyl (2s,3s)-3-[[[[(ls)-1-[[4-(3-chloro-
phenyl)-1-piperazinyl]carbonyl]-3-methyl]butyl]amino]-
carbonyl]oxiranecarboxylate in lieu of ethyl (2s,3s)-3-
[[[[(ls)-l-[[4-(4-fluorophenyl)-1-piperazinyl]carbonyl]-2-
phenyl]ethyl]amino]carbonyl]oxiranecarboxylate, the procedure
of Example 21 was otherwise repeated to provide (2s,3s)-3-
[[[[(ls)-1-[[4-(3-chlorophenyl)-1-piperazinyl]carbonyl]-3-
methyl]butyl]amino]carbonyl]oxiranecarboxylic acid (0.39 g,
67.2%).
H NMR (CDC13) ~: 0.96 (d, 3H, J=6.6 Hz, -C-CH3), 0.99
(d, 3H, J=6.6 Hz, -C-CH3), 1.36-1.47 (m, lH,
-C-CH-C2), 1.64-1.80 (m, 2H, -C-CH2-C-), 3.18-3.36
(m, 4H, piperazine ring), 3.53 (d, lH, J=1.7 Hz,
lS epoxy ring), 3.60-3.92 (m, 4H, piperazine ring),
3.62 (d, lH, J=1.7 Hz, epoxy ring), 5.04-5.12 (m,
lH, -N-CH-CO), 5.5-6.5 (br d, lH, -COOH), 6.77-
6.82 (m, lH, aromatic), 6.88-6.90 (m, 2H, aroma
tic), 7.17-7.23 (m, lH, aromatic), 8.21 (d, lH,
J=8.6 Hz, -NH-).
Fx~m~le 29
Using ethyl (2s,3s)-3-[[[[(ls)-1-[[4-(4-chloro-
phenyl)-1-piperazinyl]carbonyl]-3-methyl]butyl]amino]-
carbonyl]oxiranecarboxylate in lieu of ethyl (2s,3s)-3-
[[[[(ls)-1-[[4-(4-fluorophenyl)-1-piperazinyl]carbonyl]-2-
CA 02226740 1998-01-13
--54--
phenyl] ethyl] amino] carbonyl] oxiranecarboxylate, the procedure
of Example 21 was otherwise repeated to provide (2s,3s) -3-
[ [ [ [ (ls) -1- [ [4- (4-chlorophenyl) -1-piperazinyl] carbonyl] -3-
methyl ] butyl ] amino] carbonyl ] oxiranecarboxylic acid (0.33 g,
5 63.4%).
H NMR (CDC13) ~: 0.96 (d, 3H, J=6.6 Hz, -C-CH3), 0.99
(d, 3H, J=6.59 Hz, -C-CH3), 1.36-1.47 (m, lH,
-C-CH-C2), 1.64-1.80 (m, 2H, -C-CH2-C-), 3.10-3.31
(m, 4H, piperazine ring), 3.54 (d, lH, J=1.7 Hz,
epoxy ring), 3.58-3.93 (m, 4H, piperazine ring),
3.62 (d, lH, J=1.7 Hz, epoxy ring), 5.04-5.12 (m,
lH, -N-CH-CO), 4.8-6.5 (br d, lH, -COOH), 6.82-
6.88 (m, 2H, aromatic), 7.21-7.26 (m, lH, aroma
tic), 8.18 (d, lH, J=8.9 Hz, -NH-) .
F.Xj~T~)1 e 30
Using ethyl (2s,3s) -3- [ [ [ [ (ls) -1- [ [4- (4-methoxy-
phenyl) -1-piperazinyl] carbonyl] -3-methyl] butyl] amino] -
carbonyl] oxiranecarboxylate in lieu of ethyl (2s,3s) -3-
[ [ [ [ (ls) -1- [ [4- (4-fluorophenyl) -1-piperazinyl] carbonyl] -2-
20 phenyll ethyl] amino] carbonyl] oxiranecarboxylate, the procedureof Example 21 was otherwise repeated to provide (2s,3s) -3-
[ [ [ [ (ls) -1- [ [4- (4-methoxyphenyl) -1-piperazinyl] carbonyl] -3-me
thyl ] butyl ] amino ] carbonyl ] oxiranecarboxylic acid (0.49 g,
56.7% ) -
lH NMR (CDC13) ~: 0.95 (d, 3H, J=6.3 Hz, -C-CH3), 0.99
CA 02226740 1998-01-13
-55-
(d, 3H, J=6.6 Hz, -C-CH3), 1.38-1.46 (m, lH,
-C-CH-C2), 1.63-1.80 (m, 2H, -C-CH2-C-), 3.05-3.19
(m, 4H, piperazine ring), 3.55 (d, lH, J=1.7 Hz,
epoxy ring), 3.60-3.90 (m, 4H, piperazine ring),
3.62 (d, lH, J=1.7 Hz, epoxy ring), 5.06-5.13 (m,
lH, -N-CH-CO), 4.8-5.8 (br d, lH, -COOH), 6.84-
7.00 (m, 4H, aromatic), 8.14 (d, lH, J=8.6 Hz,
-NH-).
~x~ple 31
Using ethyl (2s,3s)-3-[[[[(ls)-1-[[4-(2-chloro-
phenyl)-1-piperazinyl]carbonyl]-2-methyl]propyl]amino]-
carbonyl]oxiranecarboxylate in lieu of ethyl (2s,3s)-3-
[[[[(ls)-1-[[4-(4-fluorophenyl)-1-piperazinyl]carbonyl]-2-
phenyl]ethyl]amino]carbonyl]oxiranecarboxylate, the procedure
of Example 21 was otherwise repeated to provide (2s,3s)-3-
[[[[(ls)-1-[[4-(2-chlorophenyl)-1-piperazinyl]carbonyl]-2-
methyl]propyl]amino]carbonyl]oxiranecarboxylic acid (1.09 g,
89.5%) as colorless crystals.
lH NMR (CDC13) ~: 0.98 (d, 3H, J=6.6 Hz, -C-CH3), 1.01
(d, 3H, J=6.6 Hz, -C-CH3), 2.10 (m, lH, -CH-C2),
2.98-3.14 (m, 4H, piperazine ring), 3.64 (d, J=1.6
Hz, lH, epoxy ring), 3.66 (d, lH, J=1.6 Hz,-epoxy
ring), 3.68-4.01 (m, 4H, piperazine ring), 4.98
(dd, lH, J=8.9, 5.9 Hz, -N-CH-CO), 7.00-7.06 (m,
2H, aromatic), 7.24 (m, lH, aromatic), 7.39 (m,
CA 02226740 1998-01-13
-56-
lH, aromatic), and 8.29 (d, lH, J=8.9 Hz, -NH).
F.x~m~l e 32
Using ethyl (2s,3s)-3-[[[[[4-(2-chlorophenyl)-1-
piperazinyl]carbonyl]methyl]amino]carbonyl]oxiranecarboxylate
in lieu of ethyl (2s,3s)-3-[[[[(ls)-1-[[4-(4-fluorophenyl)-
1-piperazinyl]carbonyl]-2-phenyl]ethyl]amino]carbonyl]-
oxiranecarboxylate, the procedure of Example 21 was otherwise
repeated to provide (2s,3s)-3-[[[[[4-(2-chlorophenyl)-
1-piperazinyl]carbonyl]methyl]amino]carbonyl]oxiranecarboxyli
c acid (1.08 g, 66.5%) as colorless crystals.
H NMR (CDCl3) ~: 3.02-3.09 (m, 4H, piperazine ring),
3.63 (m, 2H, piperazine ring), 3.66 (d, lH, J=1.6
Hz, epoxy ring), 3.78 (d, lH, J-1.6 Hz, epoxy
ring), 3.80 (m, 2H, piperazine ring), 4.11 (dd,
lH, J=17.0, 5.4 Hz, -N-CH-CO), 4.33 (dd, lH,
J=17.0, 5.4 Hz, -N-CH-CO), 6.99-7.05 (m, 2H,
aromatic), 7.23 (m, lH, aromatic), 7.38 (m, lH,
aromatic), and 8.67 (br d, lH, -NH).
F.x~m~l e 33
Using ethyl (2s,3s)-3-[[[(ls)-1-[[4-(2-chloro-
phenyl)-1-piperazinyl]carbonyl]ethyl]amino]carbonyl]-
oxiranecarboxylate in lieu of ethyl (2s,3s)-3-[[[[(ls)-
1-[[4-(4-fluorophenyl)-1-piperazinyl]carbonyl]-2-phenyl]-
ethyl]amino]carbonyl]oxiranecarboxylate, the procedure of
Example 21 was otherwise repeated to provide (2s,3s)-3-
CA 02226740 1998-01-13
[[[(ls)-1-[[4-(2-chlorophenyl)-1-piperazinyl]carbonyl]-
ethyl]amino]carbonyl]oxiranecarboxylic acid (0.87 g, 77.5%) as
colorless crystals.
lH NMR (CDC13) ~: 1.41 (d, 3H, J=6.8 Hz, -C-CH3),
2.98-3.13 (m, 4H, piperazine ring), 3.61 (d, lH,
J=1.6 Hz, epoxy ring), 3.63 (d, lH, J=1.6 Hz,
epoxy ring), 3.67-3.84 (m, 3H, piperazine ring),
3.95 (m, lH, piperazine ring), 5.06 (dq, lH,
J=8.4, 6.8 Hz, -N-CH-CO), 7.00-7.06 (m, 2H,
aromatic), 7.24 (m, lH, aromatic), 7.39 (m, lH,
aromatic), and 8.13 (d, lH, J=8.4, -NH).
F~xi~ e 34
Using ethyl (2s,3s)-3-[[[[(ls)-1-[[4- (2-chloro-
phenyl) -1-piperazinyl]carbonyl]-2-methyl]butyl]amino]-
carbonyl]oxiranecarboxylate in lieu of ethyl (2s,3s)-3-
[[[[(ls)-1-[[4-(4-fiuorophenyl)-1-piperazinyl]carbonyl]-2-
phenyl]ethyl]amino]carbonyl]oxiranecarboxylate, the procedure
or Example 21 was otherwise repeated to provide (2s,3s)-3-
[[[[(ls)-1-[[4-(2-chlorophenyl)-1-piperazinyl]carbonyl]-2-
20 methyl]butyl]amino]carbonyl]oxiranecarboxylic acid (1.07 g,
77.4%) as colorless crystals.
H NMR (CDC13) ~: 0.92 (t, 3H, J=7.3 Hz, -C-CH3), 1.00
(d, 3H, J=6.8 Hz, -C-CH3), 1.22 (m, lH, -CH-C2-),
1.54 (m, lH, -CH-C), 1.84 (m, lH, -CH-C), 2.97-
3.17 (m, 4H, piperazine ring), 3.60 (d, lH, J=1.6
CA 02226740 1998-01-13
-58-
Hz, epoxy ring), 3.64 (d, lH, J=1.6 Hz, epoxy
ring), 3.65-3.79 (m, 2H, piperazine ring), 3.85-
4.05 (m, 2H, piperazine ring), 4.98 (dd, lH,
J=9.2, 6.3 Hz, -N-CH-C0), 7.00-7.06 (m, 2H,
aromatic), 7.24 (m, lH, aromatic), 7.39 (m, lH,
~ aromatic), and 8.29 (d, lH, J=9.2 Hz, -NH).
F.x~m~l e 35
Using ethyl (2s,3s)-3-[[[2-[[4-(2-chlorophenyl)-
1-piperazinyl]carbonyl]ethyl]amino]carbonyl]oxiranecarboxylat
e in lieu of ethyl (2s,3s)-3-[[[[(ls)-1-[[4-(4-fluorophenyl)-
1-piperazinyl]carbonyl]-2-phenyl]ethyl]amino]carbonyl]-
oxiranecarboxylate, the procedure of Example 21 was otherwise
repeated to provide (2s,3s)-3-[[[2-[[4-(2-chlorophenyl)-
1-piperazinyl]carbonyl]ethyl]amino]carbonyl]oxiranecarboxylic
acid (0.85 g, 78.9%) as colorless crystals.
H NMR (DMS0-d6) ~: 2.56 (t, 2H, J=6.9 Hz, -C-CH2-CO),
2.91-2.98 (m, 4H, piperazine ring), 3.35 (td, 2H,
J=6.9, 5.6 Hz, N-CH2-C), 3.49 (d, lH, J=2.0 Hz,
epoxy ring), 3.59 (d, lH, J=1.6 Hz, epoxy ring),
3.56-3.64 (m, 2H, piperazine ring), 3.80 (m, 2H,
piperazine ring), 7.07 (td, lH, J=7.9, 1.7 Hz,
aromatic), 7.15 (dd, lH, J=7.9, 1.7 Hz, aromatic),
7.43 (dd, lH, J=7.9, 1.7 Hz, aromatic), 7.31 (m,
lH, aromatic), 8.40 (t, lH, J=5.6 Hz, -NH), and
13.50 (br d, lH, -COOH).
CA 02226740 1998-01-13
-59-
F.x~m~l e 36
Using ethyl (2s,3s)-3-[[[[N-[[4-(2-chlorophenyl)-
1-piperazinyl]carbonyl]methyl]-N-methyl]amino]carbonyl]-
oxiranecarboxylate in lieu of ethyl (2s,3s)-3-[[[[(ls)-1-
[[4-(4-fluorophenyl)-1-piperazinyl]carbonyl]-2-phenyl]-
ethyl]amino]carbonyl]oxiranecarboxylate, the procedure of
Example 21 was otherwise repeated to provide (2s,3s)-3-
[[[[N-[[4-(2-chlorophenyl)-1-piperazinyl]carbonyl]methyl]-
N-methyl]amino]carbonyl]oxiranecarboxylic acid (1.08 g, 74.8%)
as colorless crystals.
H NMR (CDC13) ~: 3.01-3.10 (m, 6H, piperazine ring),
3.27 (s, 3H, -NCH3), 3.63-3.71 (m, 2H, -N-CH2-CO),
3.75 (d, lH, J=1.9 Hz, epoxy ring), 3.78-3.90 (m,
2H, piperazine ring), 4.02 (d, lH, J=1.9 Hz, epoxy
ring), 6.98-7.05 (m, 2H, aromatic), 7.24 (m, lH,
aromatic), and 7.36 (m, lH, aromatic).
F.x~m~l e 37
Using ethyl (2s,3s)-3-[[(2s)-2-[[4-(2-chloro-
phenyl)-1-piperazinyl]carbonyl]-1-pyrrolidinyl]carbonyl]-
oxiranecarboxylate in lieu of ethyl (2s,3s)-3-[[[[(ls)-
1-[[4-(4-fluorophenyl)-1-piperazinyl]carbonyl]-2-
phenyl]ethyl]amino]carbonyl]oxiranecarboxylate, the procedure
of Example 21 was otherwise repeated to provide
(2s,3s)-3-[[(2s)-2-[[4-(2-chlorophenyl)-1-piperazinyl]-
carbonyl]-1-pyrrolidinyl]carbonyl]oxiranecarboxylic acid (0.94
CA 02226740 1998-01-13
--60--
g, 7.07%) as colorless crystals.
H NMR (CDCl3) ~: 1.94-2.11 (m, 2H, pyrrolidine ring),
2.17-2.30 (m, 2H, pyrrolidine ring), 3.06-3.20 (m,
4H, piperazine ring), 3.63-3.76 (m, 2H, piperazine
ring), 3.81-3.85 (m, 5H), 4.00 (dt, lH, J=13.7,
4.4 Hz, pyrrolidine ring), 4.96 (dd, lH, J=7.8,
4.3 Hz, pyrrolidine ring), 6.97-7.04 (m, 2H,
aromatic), 7.24 (m, lH, aromatic), and 7.37 (m,
lH, aromatic).
0 F.X;3T~)1 e 38
Using ethyl (2s,3s)-3-[[[[(ls)-1-[[4-(2-chloro-
phenyl)-1-piperazinyl]carbonyl]-2-acetylaminomethyl-
thio]ethyl]amino]carbonyl]oxiranecarboxylate in lieu of ethyl
(2s,3s)-3-[[[[(ls)-1-[[4-(4-fluorophenyl)-1-piperazinyl]-
carbonyl]-2-phenyl]ethyl]amino]carbonyl]oxiranecarboxylate,
the procedure of Example 21 was otherwise repeated to provide
(2s~3s)-3-[[[[(ls)-l-[[4-(2-chlorophenyl)-l-piperazinyl]
carbonyl]-2-acetylaminomethylthio]ethyl]amino]carbonyl]-
oxiranecarboxylic acid (0.88 g, 77.9%) as colorless crystals.
1H NMR (CDC13) ~: 2.05 (s, 3H, -COCH3), 2.85 (dd, lH,
J=13.9, 8.3 Hz, -C-CH-S), 2.96-3.16 (m, 5H), 3.69
(d, lH, J=1.6 Hz, epoxy ring), 3.78 (d, lH, J=1.6
Hz, epoxy ring), 3.71-3.89 (m, 4H, piperazine
ring), 4.39 (d, 2H, J=8.3 Hz, -S-CH2-N), 5.21 (m,
lH, -N-CH-CO), 6.97-7.02 (m, 2H, aromatic), 7.22
CA 02226740 1998-01-13
-61-
(m, lH, aromatic), 7.36 (m, lH, aromatic), 7.80
(d, lH, J=8.3, -NH), and 9.00 (br d, lH, -NH).
F.x~m~l e 39
Using ethyl (2s,3s)-3-~[[[(ls)-1-[[4-(2-chloro-
phenyl)-1-piperazinyl]carbonyl]-3-methylthio]propyl~
~ amino]carbonyl]oxiranecarboxylate in lieu of ethyl
(2s,3s)-3-[[[[(ls)-1-[[4-(4-fluorophenyl)-1-piperazinyl]-
carbonyl]-2-phenyl]ethyl]amino]carbonyl]oxiranecarboxylate,
the procedure of Example 21 was otherwise repeated to provide
(2s,3s)-3-[[[[(ls)-1-[[4-(2-chlorophenyl)-1-piperazinyl]-
carbonyl]-3-methylthio]propyl]amino]carbonyl]oxiranecarboxyli
c acid (1.17 g, 80.7%) as colorless crystals.
H NMR (CDC13) ~: 1.98 (dd, 2H, J=6.9, 6.6 Hz,
-CH-C-C), 2.12 (s, 3H, -SCH3), 2.57 (dt, 2H,
J=6.9, 2.3 Hz, -C-CH2-C-S), 3.05-3.19 (m, 4H,
piperazine ring), 3.63 (d, lH, J=1.9 Hz, epoxy
ring), 3.65 (d, lH, J=1.9 Hz, epoxy ring), 3.71-
3.94 (m, 4H, piperazine ring), 5.26 (m, lH,
-N-CH-CO), 7.00-7.06 (m, 2H, aromatic), 7.24 (m,
lH, aromatic), 7.38 (m, lH, aromatic), and 8.18
(d, lH, J=8.6, -NH).
- Fx~mple 40
Using ethyl (2s,3s)-3-[[[[(ls)-1-[[4-(2-chloro-
phenyl)-l-piperazinyl]carbonyl]-3-carbamoyl]propyl]amino]-
2S carbonyl]oxiranecarboxylate in lieu of ethyl (2s,3s)-3-
CA 02226740 1998-01-13
-62-
[[[[(ls)-1-[[4-(4-fluorophenyl)-l-piperazinyl]carbonyl]-2-
phenyl]ethyl]amino]carbonyl]oxiranecarboxylate, the procedure
of Example 21 was otherwise repeated to provide (2s,3s)-3-
[[[[(ls)-1-[[4-(2-chlorophenyl)-1-piperazinyl]carbonyl]-3-
carbamoyl]propyl]amino]carbonyl]oxiranecarboxylicacid (0.14 g,
~ 74.5%) as colorless crystals.
H NMR (CDC13) ~: 1.85 (br d, 2H, -NH2), 2.14 (m, lH,
-C-CH-C-CO-), 2.36-2.53 (m, 3H, -CH-CH2-C-CO-),
2.89-3.06 (m, 4H, piperazine ring), 3.57-3.79 (m,
6H, piperazine and epoxy ring), 5.02 (m, lH,
-N-CH-CO), 6.96-7.02 (m, 2H, aromatic), 7.21 (m,
lH, aromatic), 7.37 (m, lH, aromatic), and 7.88
(br d, lH, -NH).
F.x~m~l e 41
Using ethyl (2s,3s)-3-[[[(ls)-1-[[4-(4-fluoro-
phenyl)-1-piperazinyl]carbonyl]-1-cyclopentyl]amino]-
~carbonyl]oxiranecarboxylate in lieu of ethyl (2s,3s)-3-
[[[[(ls)-1-[[4-(4-fluorophenyl)-1-piperazinyl]carbonyl]-2-
phenyl]ethyl]amino]carbonyl]oxiranecarboxylate, the procedure
of Example 21 was otherwise repeated to provide (2s,3s)-3-
[[[(ls)-1-[[4-(4-fluorophenyl)-1-piperazinyl]carbonyl]-1-
cyclopentyl]amino]carbonyl]oxiranecarboxylic acid (0.52 g,
55.6~) as colorless crystals.
'H NMR (DMSO-d6) ~: 1.61 (m, 4H, cyclopentyl), 1.87 (m,
2H, cyclopentyl), 2.22 (m, 2H, cyclopentyl), 2.97
CA 02226740 1998-01-13
-63-
(m, 4H, piperazine), 3.45 (d, lH, J=1.6 Hz, epoxy
ring), 3.58 (d, lH, J=2.1 Hz, epoxy ring), 3.60
(m, 4H, piperazine ring), 6.95-7.20 (m, 4H,
aromatic), 8.89 (s, lH, -NH) and 13.4 (br d, lH,
-COOH).
- F.x~m~l e 42
To a solution of the (2s,3s)-3-[[[[(ls)-1-[[4-(4-
methylphenylsulfonyl)-1-piperazinyl]carbonyl]-3-methyl]-
butyl]amino]carbonyl]oxiranecarboxylic acid (0.935 g, 2 mmol)
obtained in Example 26 in dichloromethane (15 ml) were added-
o-benzylhydroxylamine (0.638 g, 4.0 mmol) and N-methyl-
morpholine (0.405 g, 4.0 mmol). Then, a solution of
dicyclohexylcarbodiimide (0.619 g, 3.0 mmol) in dichloromethane
(5 ml) was added dropwise under ice-cooling. The mixture was
stirred at room temperature for 24 hours, at the end of which time
it was filtered. The precipitate was washed with dichloromethane
(20 ml) and the washes and the filtrate were pooled and washed
with water. The organic layer was dried over anhydrous magnesium
sulfate and concentrated under reduced pressure and the residue
was chromatographed on silica gel. Elution was carried out with
ethyl acetate-hexane (2:1) to provide
(2s,3s)-3-[[[[(ls)-1-[[4-(4-methyl-
phenylsulfonyl)-1-piperazinyl]carbonyl]-3-methyl]butyl]-
amino]carbonyl]oxiranecarbobenzyloxamide (0.86 g, 75.1%).
lH NMR (CDC13) ~: 0.84 (d, 3H, J=6.2 Hz, -C-CH3), 0.91
CA 02226740 1998-01-13
--64-- -
(d, 3H, J=6.5 Hz, -C-CH3), 1.23-1.31 (m, lH,
-C-CH2-C), 1.36-1.58 (m, 2H, -C-CH-C, -C-CH-C2),
2.43 (s, 3H, -ph-CH3), 2.72-2.86 (m, 2H, piperazine
ring), 3.14-3.27 (m, 2H, piperazine ring), 3.31-
3.51 (m, 2H, piperazine ring), 3.40 (d, lH, J=1.4
Hz, epoxy ring), 3.43 (d, lH, J=1.7 Hz, epoxy
ring), 3.63-3.74 (m, lH, piperazine ring), 3.84-
3.98 (m, lH, piperazine ring), 4.80-4.90 (m, lH,
-N-CH-CO), 4.87 (s, 3H, -O-CH2-ph), 7.30-7.40 (m,
8H, aromatic, -NH-), 7.56-7.66 (m, 2H, aromatic),
9.05 (s, lH, -NH-).
F.X;~l e 43
To a solution of the (2s,3s)-3-[[[[(ls)-1-[[4-(4-
methylphenylsulfonyl)-1-piperazinyl]carbonyl]-3-methyl]-
butyl]amino]carbonyl]oxiranecarbobenzyloxamide (0.57 g, 1 mmol)
obtained in Example 42 in methanol (25 ml) was added a catalyst
amount of palladium-on-carbon and catalytic reduction was carried
out. After completion of the reaction, the palladium-on-carbon
was filtered off and the filtrate was concentrated and
chromatographed on silica gel. Elution was carried out with ethyl
acetate to provide (2s,3s)-3-
[[[[(ls)-1-[[4-(4-methylphenylsulfonyl)-1-piperazinyl] -
carbonyl]-3-methyl]butyl]amino]carbonyl]oxiranecarbohydroxami
c acid (0.18 g, 37.396).
lH NMR (CDC13) ~: 0.84 (d, 3H, J=5.9 Hz, -C-CH3), 0.90
CA 02226740 1998-01-13
-65-
(d, 3H, J=5.9 Hz, -C-CH3), 1.24-1.33 (m, lH,
-C-CH2-C), 1.50-1.64 (m, 2H, -C-CH-C, -C-CH-C2),
2.42 (s, 3H, -ph-CH3), 2.90-3.20 (m, 4H, piperazine
ring), 3.44-3.80 (m, 3H, piperazine ring), 3.51
(s, lH, epoxy ring), 3.68 (s, lH, epoxy ring),
~ 4.56-4.66 (m, lH, piperazine ring), 4.76-4.90 (m,
lH, -N-CH-CO), 7.33 (d, 2H, J=7.8 Hz, aromatic),
7.62 (dd, 2H, J=7.8, 1.7 Hz, aromatic), 7.84-7.94
(br d, lH, -NH-), 9.80-10.40 (br d, lH, -OH).
Formul~tion F,x~m~l e 1
Tablets
Compound of Example 30 80 mg
Starch 17 mg
Magnesium stearate 3 mg
The above components per tablet are compressed into tablets
in the routine manner. Where necessary, the tablets can be
sugar-coated.
Formul~tion F.X~l e
Capsules
Compound of Example 25 50 mg
Lactose 100 mg
Starch 30 mg
Magnesium stearate 10 mg
The abovè components per tablet are mixed and filled in
gelatin capsule shells.
CA 02226740 1998-01-13
-66-
For~ tion Fx~mrle 3
Injection
Compound of Example 28 2.5 mg
Sodium chloride 900 mg
lN-sodium hydroxide q.s.
- Distilled water for injection to make 100 ml
The above components are mixed in the routine manner to
provide an injection.
Formul~t;on F.x~m~l e 4
Ophthalmic solution
Compound of Example 25 50 mg
Boric acid 700 mg
Borax q.s.
Sodium chloride 500 mg
Sodium edetate 0.05 mg
Benzalkonium chloride 0.005 mg
~ Sterilized pure water to make 100 ml
The above components are mixed in the routine manner to
provide an ophthalmic solution.
EFFECT OF THE INVENTION
The compound of general formula (I) according to the
present invention has cysteine protease inhibitory activity and
is, therefore, canbe usedas a therapeutic drug for myodystrophy,
amyotrophy, cerebral infarction, stroke (cerebral apoplexy),
CA 02226740 1998-01-13
Alzheimer's disease, disturbance of consciousness or dyskinesia
associated with head trauma, multiple sclerosis, peripheral
neuropathy, cataract, inflammation, allergy, fulminanthepatitis,
osteoporosis, hypercalcemia, breast cancer, prostate cancer,
S prostatic hypertrophy, etc. or a cancer growth inhibitor or
~ antimetastatic agent, or a platelet aggregation inhibitor.