Note: Descriptions are shown in the official language in which they were submitted.
CA 02226747 1998-02-11
W O 97/06835
PCl'tGB96/U1959
CF.-~ CUTTUR~PRODUCTS
T~is invention relates to the c~lturing of
mara~ n anchorage dependent cells onto a con~orma~le
carrier. ~ore particularly ~he invention relates to the
format~on of wound dressings suita~le for treating
wounds such as burns, skin graft donor sites and ulcers
e.g. venous and decu~itus ulcers and to systems for use
in the preparation of such dressings.
~ Am~l i an cells that are ;n~r~h1e of
proliferating in suspended liquid culture but can be
~ade to proliferate on the surface of a carrier are
said to be anchorage-dependent.
Epithelial cells, such as keratinocytes, are
anchorage dependent. Such c~lls cultured in the
presence of a carrier which is non-inhibitory and
non-cytotoxic will multiply in stratified colonies and
eventually produce a confluent layer. Cell cultures of
this t~?e are used to investigate skin growth and have
been used as skin grafts. Various terhn;cal papers have
been published which describe in vitro t~chniques for
growing skin cells and their subse~uent use in the
treatment of full-thic~ness wounds. For example E. Bell
et al (J Invest Derm 81; 2s~lOs 1983); E. Bell et al
(Science 211; lOS2-1054 1981); D. Asselineau and M.
Pruneiras (Br J Derm 1984 III, Supplement 27, 219-22~)
and J.F, Bur~e et al (Ann Surg 94; 413-4Z8 1981).
T~le ability of cells to anchor to a particular
carrier is dependent on the properties of the carrier,
the cult:urin~ conditions e.g. temperature, and the
components of the culture medium. Culturing is usually
CA 02226747 1998-02-11
WO 97/06835 PCT/GB96/01959
carried out in hard plastic flasks made from a material
which is substantially inert to the growth medium and
is non-cytotoxic to the cells. Polystyrene is a
commonly used material for culture flasks.
one of the problems in using hard plastic flasks
for the culture of epithelial cells for use as skin
grafts is that the sheet of cells normally has to reach
confluence before they can be harvested. The time taken
to reach confluence may be long. Furthermore the layer
of cells is not very strong mech~ni cally and can easily
be damaged when the unsupported cells are dislodged
e.g. by using the enzyme dispase and handled
unsupported.
International Patent Application No. W089/03228
discloses a synthetic surgical dressing coated with
collagen having human epidermal cells attached thereto.
The method of producing the composite involves
incubating the synthetic surgical dressing in a
solution of collagen and evaporating the water to give
a collagen coating on the surface of the synthetic
dressing. The evaporation step is prefera~ly conducted
under sterile conditions to impede bacterial
cont~min~tion.
It is known that the sheet of cells can be
supported after it has been dislodged from the surface
in order to facilitate handling and transfer to the
wound surface. However this techni~ue does not overcome
the problems associated with hard surface cultures or
the risks associated with dislodgement of the cells.
It is an object of the present invention, to
overcome the above problems, by growing cells on a
readily manageable, conformable carrier comprising a
CA 02226747 1998-02-11
W O 97/06835 PCT/GB96/01959
hydrophilic, non-inhibitory to cell growth and non-
cytotoxic polymer. The polymex of the present
~ i~vention enables the transfer of a cell layer to the
site of treatment before it has reached confluence.
According to the present invention a wound
dressing comprises a conformable carrier havinq a
wound-facing surface to which a layer of cultured
mammalian cells is anchored, tLle carrier comprising a
synthetic polymer layer which has a water uptake of
at least 16w/w % and is non-cytotoxic and non-
inhibitory to cell growth.
The present invention also provides a system for
producing a wound dressing comprising a conformable
carrier having a wound-facing surface to which a
layer of cultured mammalian cells is anchored, the
carrier being a synthetic polymer which is non-
inhibitory to cell growth and non-cytotoxic and
having a water uptake of at least 16 w/w %, together
with a means for maintaining a aqueous culture medium
containing said cells in contact with the wound
facing surface of the carrier, the carrier being
maintained in a submerged position in the culture
medium.
The present invention further provides a method
of wound treatment comprising the step of applying a
wound dressing comprising a conformable carrier
having a wound-facing surface to which a layer of
cultured mammalian cells is anchored, the carrier
being a synthetic polymer layer which is non-
inhibitory to cell growth and llon-cytotoxic and
having a water uptake of at least 16 w/w %, to the
area to be treated.
In further embodiment of the present invention
there is provided a kit comprising a conformable
SUBSTITUTE SHEET (RIJI E 26)
CA 02226747 1998-02-11
W O 97/06835 PCT/GB96101959
carrier having a wound facing surface, the carrier
being a synthetic ploymer layer, which is non-
inhibitory to cell growth and non-cytotoxic and
having a water uptake of at least 16 w/w %, a
container comprising a nutrient solution or growing
mammalian cells and a vessel for containing the
carrier and the nutrient solution.
The kit may additionally comprise a sample of
mammalian cells.
The mammalian cells employed in the present
invention are anchorage-dependent i.e. they re~uire a
surface on to which they can bind before they are
able to proliferate.
By the term 'conformable' we mean that the
dressing will conform to changes in contours of the
body portion to which the dressing is applied.
Preferably the synthetic polymer layer is a
synthetic polymer film.
In one form, the carrier comprises a single
layer of polymer. In another form the carrier
comprises a laminate comprising two synthetic polymer
layers, at least one polymer of said at least two
layers being non-inhibitory to cell growth, non-
cytotoxic and having a water uptake of at least 16
w/w%. The use of a laminate may be beneficial if the
hydrophilic polymer swells or otherwise becomes
fragile or difficult to handle after exposure to
culture medium.
Where the wound dressing comprises a polymer
layer having a water uptake of less then 16 w/w %,
the cell layer may be anchored to this polymer layer.
SUBSTITUTE SHEET tRULE 2~)
CA 02226747 1998-02-11
W O 97/06835 PCT/GB96101959
Alternatively the cell layer may be anchored to the
polymer layer having a water uptake of at least 16~.
- In order for the carrier to have desirable
surface properties which allows the anchorage of the
cells, it may be surface treated. A suitable form of
surface treatment is corona-discharge treatment.
Corona discharge treatment increases the surface
energy of a material and may provide improved
conditions for cell anchorage. The effect of corona-
discharge treatment can be assessed by measuring the
contact angle of water on the treated material. A
method for measuring contact angle will be described
hereinafter. Aptly the contact angle should be
reduced by at least 10~, favourably by at least 15
and preferably by at least 20~. Other suitable
treatments which can be applied to the carrier
include glow discharge or plasma treatment, chemical
etching and flame treatment.
Thus in one embodiment of the present invention
the wound dressing comprises a conformable carrier
the carrier being a laminate o~ a polymer layer
having a water uptake of at least 16 wjw % and a
hydrophobic polymer layer, the polymers being non-
inhibitory to cell growth and non-cytotoxic.
By hydrophobic polymer we mean that the polymer
has a water uptake less than 16 w/w ~.
Percentage water uptake o~ the polymer layer is
assessed by the following method. A sample of the
polymer layer is weighed in its dry state. This is
then immersed in excess distilled water and left for
a period of 24 hours at 20~C. The hydrated sample is
then removed, excess water is removed from the
surface of the sample and the sample re-weighed. The
percentage increase in weight obtained, is the
SUB~lTUrE SHEET (RULE 26)
CA 02226747 1998-02-11
W O 97/06835 PCT/GB96/01959
percentage water uptake w/w%. The following e~uation
may be used to calculate the percent water uptake
(w/w % ) .
w/w% water uptake = (mass of sample + water) - mass
of sam~le)x 100
mass of sample
The percentage water uptake w/w % of the carrier
may be assessed by the above described method by
taking a sample of the carrier rather than a sample
of the polymer layer.
Preferably the carrier is transparent thus
allowing the cells on the surface to be ~A ined by a
mi-croscopy. However it is not essential that the
cells be viewed during the period of cell culture and
therefore non-transparent or opaque carriers may also
be used in the dressing of the present invention.
The hydrophilic polymer may be a hydrogel or a
hydrocolloid. Hydrogels are preferred as they are
transparent.
The carrier should not be inhibitory to cell
growth. A measure of such inhibition can be
expressed as a percentage cell growth reduction as
measured against cells allowed to grow in the absence
of a test carrier as hereinafter described. Aptly
the carrier should not result in more than 50%
reduction in cell growth. More aptly it should not
result in more than a 40% reduction in cell growth.
Favourably it should not result in more than a 30%
reduction in cell growth and preferably not result in
more than a 20% reduction in cell growth.
The carrier should be non-cytotoxic. Cytotoxicity can
be measured against a non-cytotoxic control material
by a method as hereinafter described. Aptly
SUBSmUTE S~ EET tRULE 26)
CA 02226747 1998-02-11
W O 97/0683~ PCT/GB96/01959
the cytoxicity of the carrier will not ~ e~ 30%. More
aptly the cytotoxicity of the carrier will not ~c~
20%. Preferably the cytotoxicity of the carrier will
not exceed 15%.
Th,e carrier may be a ~ in~te of at least two
films or at least two sheets or a laminate comprising a
film and a sheet.
Suitable synthetic polymers include hydrophilic
polyurethane, polyetherpolyester (also known as a
thermoplastic ether ester elastomer or a copolyester
elastomer) for e.g. HYTREL (Trade Mark),
polyetherpolyamide, e.g. PEB~X (Trade Mark),
polyacrylamides and polyethylene oxide. Thus an apt
laminate may comprise a polyurethane film coated with a
material which allows attach~ent of A~horage dependent
mamm~ n cells such as e.g. ethylene vinyl acetate.
The carrier may comprise a hydrogel, a
hydrocolloid or other suitable polymer. Examples of
suitable hydrogels include polyhydroxyethylmethacrylic
acid (po:Ly ~EMA), cross-linked polyvinylacrylic acid
(PVA), polyacrylic acid cross-linke~ with
triallylsucrose (carbopol) and polyvinylpyrrolidone.
Examples of suitable hydrocolloid materials include
car~oxymethylcellulose eg sodium
carboxymethylcellulose. The carboxyurethylcellulose may
be cross-linked or non cross-linked. The hydrocolloid
may further comprise a polyisobutylene which serves to
hold the hydrocolloid together. The hydrogel may be
in the form of a film or a sheet. The sheet may be
self-supporting. Alternatively the sheet may be
suppor~ed by for example a suitable support layer e.g.
a polymeric film or polymer net. Where the sheet is
supported by a support layer the sheet and support
CA 02226747 1998-02-11
W O ~7/06835 PCT/GB96/01959
layer comprise the carrier.
Where the carrier comprises a hydrophobic polymer
layer it may desirably be apertured to enable it to
handle any exudate from the site of application c
particularly in the case of highly extruding wounds.
Thus a continous polymer layer may be adapted to be
perforated after the cell layer has reached the desired
degree of confluency. Examples of such films, are the
bi~ lly oriented films disclosed in for example in
EP-0141592, GB-1055963 and GB-914489.
Aperturing the carrier as above described
ensures that there is no build up of exudate in e.g.
highly P~ll~ i ng wounds, since exudate can readily escape
through the apertures and therefore does not build up
under the dressings.
Perforation of the carrier may be carried out
either before or after attachment of the cell layer
using any suitable method such as hot pin perforation
or slitting. Perforation after cell growth should be
carried out with ~;ni~ll~ cell disruption and loss.
Perforation should be such as to provide an adequate
open area for escape of wound exudate. The minimum
open area will depend on the moisture vapour
permeability of the film or the lAmi~te.
Where the carrier is a 1 A i nAte of two polymer
films or nets in order that it should have the required
flexibility and con~ormability, it should suitably have
a thickness not excPP~lng 0.075mm. More suitably it
should not have a thickness PxcPp~; ng 0.05mm, and
favourably not PXcPp~ing 0.04mm. More favourably the
film will have a thickness between 0.005 and 0~03mm and
preferably between 0.01 and 0.025mm for example,
~ =
CA 02226747 1998-02-11
WO 97/06835 PCT/GB96tO1959
0.015mm or 0.020mm. Where the carrier comprises a
- sheet and polymeric film or net it may have a thickness
of the range of from 0.075mm up to about 5mm, the
polymeric film or net having the above defined
cions.
Mech;ng is conventionally carried out before
grafting of a skin graft, to increase the surface area
of the graft. In the same way, where the wound
dressing of the present invention consists of polymeric
films it may be conveyed through a conventional mesh -
grafting device.
Films suitable for serving as the carrier may be
flat or contoured. The contours may be produced for
example by embossing. Suitably contoured films may also
have apertures. Such films are described in W090/00398.
Aptly the carrier is per~eable to moisture
vapour, oxygen and carbon dioxide. In this way a
dressing when in place on the wound will provide moist
conditions allowing for the cells to remain viable
while the wound heals. The carrier, whilst desirably
being impe~vious to liquid water should have an upright
moisture vapour tr~nC~;csion rate (MVTR) of at least
300c~m 2 24 hrs 1. Aptly the carrier should have an
upright MVTR of less than 2000 gm 2 24 hrs 1. The
carrier should have an inverted MVTR of at least 2500
gm 2 24 hrs 1. Suitably the inverted MVTR should not
exceed 25000 gm 2 24 hrs l. A method for determining
the upright: and inverted MVTR of a substrate is given
as follows:
The moisture vapour transmission rate (MVTR) may
be measured by the Payne Cup method. This method uses a
cup 1.5cm deep which has a flanged top. The inner
CA 02226747 1998-02-11
W O 97/06835 PCT/GB96/01959
-- 10 --
diameter of the flange provides an area of locm2 of
test material through which moisture vapour may pass.
In this method lOml of distilled water is added to the
cup and a sample of the test material, large enough to
completely cover the flange, is clamped over the cup.
When the test material has an adhesive surface it is
clamped with the adhesive surface facing into the cup.
The complete assembly is then weighed and placed in a
fan assisted electric oven where the temperature and
relative humidity are maintained at 37~C and 10%
respectively. The relative humidity within the oven is
maintained at 10% by placing lkg of anhydrous 3-8 mesh
calcium chloride on the floor of the oven. After a
suitable period of time, for example 17 hours, the cup
is removed from the oven and allowed to cool for 20
minutes to reach room temperature. Ater reweighing the
mass of water lost by vapour transmission is
calculated. The moisture vapour permeability (MVP) is
expressed in units of gm 2 24 hrs 1 at 37OC, 100% to
10% relative humidity difference. This is the MVP when
the test material is in contact with moisture vapour.
To calculate the MVTR, the reading is corrected for
st~n~Ard thickness of test material i.e. a thickness of
1 thou. The MVP when the material is in contact with
water may be measured using the same apparatus and
making the necessary adjustments for thickness of test
material, by simply placing the Payne cup in an
inverted position in the oven so that liquid water (and
not moisture vapour) is in contact with the test
material.
In a modification of the invention the carrier
may be formed from knitted, woven or non-woven
synthetic polymers to form a tight web of small mesh
size. After cell culture the web may be stretched to
form a web having a larger mesh size without loss of
CA 02226747 1998-02-11
W O 97/06835 PCT/GB96/01959
cells.
It is preferred to use autologous cultivated
epithelial cells since these have little or no
immunological rejection problems when applied to the
host (patient). However it may also be possible to use
non-autologous cells e.g. to produce an allograft or
xenograft~ Preferably the cells are keratinocytes. we
have found that it is desira~le not to allow the cell
layer to reach confluence before transferring the wound
dressing onto the wound site, thus enabling a suitable
wound dre~ising to be produced within a few hours. It
will be understood that as the cell layer is
sub-confluent it will comprise a monolayer of cells.
The carrier may be sterilised by any suitable
known meth,ods of sterilisation. Suitable forms of
sterilisation include ethylene oxide (allowing the
required time for de-gassing) gamma-irradiation or
steam sterilisation. The carrier should be washed
after sterilisation, to remove any low molecular weight
contaminants, for example where the carrier is a
synthetic polymer to remove any unpolymerised monomer
as such monomers can be cytotoxic. The washing process
may comprise several sequential washes using sterile
de-ionised water in sequential steps.
Alternatively the carrier may be aseptically
produced.
In the system of the invention the carrier on
which the cells are grown shou]d preferably be easily
removable from the other parts of the system, namely
the means ~or maintaining the aqueous culture medium
containing said cells in contact with the wound facing
surface of the film. For example, where the carrier
CA 02226747 1998-02-11
W O 97/06835 PCT/GB96/ol959
- 12 -
forms a wall of the vessel cont~i ninq the culture
medium, it should be readily removable from the other
parts of the vessel. The carrier may form a part of all
of the container in which the cell culture is grown.
The other parts of the container or culture vessel may
be formed from suitable materials conventionally used
for the manufacture of tissue culture vessels. High
impact polystyrene is preferred.
In an alternative embodiment the carrier may be
laid down within a flask of an appropriate design
adapted to allow the removal of the substrate. Where
the carrier is an integral part of the culture flask,
it may be remova~ly sealed to the other parts of the
flask for example by heat sealing or by means of an
ahdesive. Preferably the carrier will form a flat
surface and will aptly form the base of the flask.
Where the carrier is to be contained within the
flask, the flask will be provided with a closeable
opening having dimensions sufficient to enable the
carrier to be readily removed without disruption of the
cells anchored thereto.
Where apertured carrier are used to form an
integral part of the culture flask these may be
overlaid by e.g. a continuous film to keep the flask
water tight and to maintain sterility. If the carrier
is laid down within the flask it may be ret~;ne~ by,
for example, a pre-sterilised stainless steel ring or,
alternatively, by coating the carrier on one side with
a layer of non-cytotoxic adhesi~e which is capable of
maintaining tack in the presence of tissue culture
medium.
Nutrients, growth factors or medicaments such as
-
CA 02226747 1998-02-11
W O 97/06835 PCT/GB96/01959
- 13 -
antibiotics or antiflammatories may be incorporated
into the a~ueous medium in which the cell culture is
grown. The nature and weight and/or volume of such
additional ingredients are co~ventionally well known.
J
The wound dressing of this invention may be used
for a variety of wounds. The dressing of the invention
is particlllarly suitable for treating partial-thickne~
wounds that is those where only the epidermis and
possibly part of the dermis is lost. Such woun~s
include for example skin graft donor sites, first or
possibly second degree burns, shallow leg ulcers or
pressure sores. The dressing is also suitable for
treating ~ull thickness wounds eg. venous ulcers.
Continuous polymeric film carriers aptly act as
barriers t:o bacteria whilst being sufficiently
permea~le to moisture vapour, oxygen and carbon dioxide
to allow wound healing to occur at a desira~le rate. If
the substrate is perforated, a secondary dressing could
be appliecl to maintain the desired degrees of moisture
vapour, oxygen and car~on dioxide permeabilities. A
suitable material is a polyurethane film dressing such
as OPSITE (Trade Mark) which may be placed over the
wound to create the same conditions. The dressing can
suitably be left in place on the wound for a suitable
period to allow the wound to become from 30-90%
re-epithelialised, (healed) depen~;nq on the nature of
the particular wound and the condition of the patient.
At this time the dressing can be removed and replaced
with a conventional wound dressing.
In 1975 Green et al proposed the use of
transformed cell line 3T3 cells derived from the mouse
as a feeder layer system in order to expand skin
cultures. 3T3 cells synthesize factors which are
essential for the growth of keratinocytes which are
CA 02226747 1998-02-11
W O 97/06835 PCT/GB96/01959
- 14 -
seeded at very low densitites. Furthermore 3T3 cells
inhibit unwanted fibroblast growth. Skin cell
preparations cont~;ni ng 3T3 cells first have to be
g-irradiated (typically about 6000 rads) to inhibit
cell division yet not kill the cells. Such irradiated
cells survive for several days and during that time
will synthesize and supply materials for the host
keratinocyte cells. Eventually the 3T3 cells are
expelled from the skin cell layer. ~owever inevitably
some mouse cells remain and thus will be grafted with
the host keratinocytes.
In the dressings of the present invention the
feeder cells such as 3T3 may be seeded into a medium in
contact with the reverse side of the carrier. These
cells will then synthesize materials for the host
cells. Since the feeder cells do not come into contact
with the host cells there is no need to irradiate them.
When the carrier is removed from the culture flask, the
reverse side may be washed to free floating feeder
cells.
Where a feeder cell layer is employed with an
apertured carrier, the apertures should be large enough
to allow free ~ch~nge of the culture medium, but not
large enough to allow cells to pass through. Aptly the
aperture size should not be larger than 5~ across its
largest dimensions. Suitably the aperture size will be
from 0.5 to 2~.
The dressings of the present invention may be
prepared by suitably qualified personnel at the
location where they are to be applied to the host. Thus
suitably the dressing may be prepared according to the
above disclosed method by a cell-culturist in a
hospital cell-culture laboratory. Alternatively the
CA 02226747 1998-02-11
WO 97/06835 PCT/GB96~1959
dressing may be prepared at a location remote from the
location ~.g. hospital where it is to be applied. In
the latter case, the dressing ~ay be transported under
suitable conditions e.g. under a precisely controlled
t,--,~~ature. Thus the dressin~ may be cryopreserved
ie. maintained at - 190~C and thereafter brought back
to room temperature just prior to use. Alternatively
the dressing may be transported in a ready-to-use
state. Thus the dressing may be tra~sported in a
suitable ;ncllh~tor set at the desire~ temperature.
Contact angles are measured by the Wilhelmy Plate
dynamic contact angle mea~u~ nt system using a Cahn
DCA-322 Dynamic Contact Angle Analyser. Both advancing
and rPC~; ng contact angles may be measured. The test
li~uid used may be water (HPLC grade). An
immersion/withdrawal speed of 150 micron/sec may be
used, Samples of films may be prepared by sticking the
film onto a glass coverslip approx. 24mm by 30mm or by
dip coatinq a slide from solution. T~e testing may be
carried out: at 23 deg. C/50% RH.
A suitable method for measuring cell growth
reduction is the method disclosed in W0 91/13638.
The method of measuring percentage cellular
cytotoxicity of the substrates is as disclosed in
W0 91/13638.
Embo~imPnts of the dressing systems of the
invention will be illustrated by reference to the
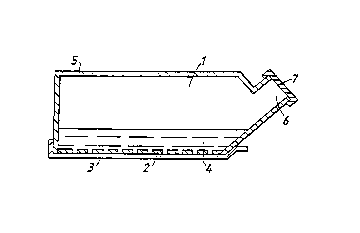
accompanying drawings. Referring to Figure 1, one wall
the base, of a culture flask 1 comprises a laminate of
an aperture~ film 2 and a continuous film 3. The edges
of the ~aminate are removably bonded to the other wall
portions 5 of the flask 3. Culture media 4 and donor
CA 02226747 1998-02-11
W O 97/06835 PCT/GB96/01959
- 16 -
cells can be introduced into flask 3 through neck 6 and
sealed therein by stopper 7.
After the system has been incu~ated under
suitable cell culture conditions, the laminate 2, 3 may
be removed by peeling from flask 1.
Figure 2 illustrates a structure2, 3 formed by
casting a first film 2 over a second film 3, film 3
having a plurality of raised portions 8. The resulting
film 2, has a plurality of thin 9 and thirk~ne~ 10
areas. On separation of film 2 from film 3, the thin
areas 9 ru~L~Le to form apertures 12 as shown in Figure
3. The resulting apertured from which may subsequently
be l~ in~ted to a polymer layer to form a laminate,
suitable for use as a carrier in the dressing of the
present invention.
Referring to Figure 3, the culture flask 1 is
divided into compartments 21, 31 by a carrier
comprisiny a hydrophilic polymer layer 14 and a
perforated hydrophobic film 2.
Culture medium 4 is introduced into the flask and
occupies both compartments 21, 31. Skin cells are
seeded into romp~rtment 21 through neck 6 whilst feeder
cells such as 3T3 cells are seeded into compartment 31
through access port 11.
Nutrients, growth factors or medicaments such as
anitbiotics or antiinflammatories may be incorporated
into the aqueous medium in which the cell culture is
grown. The nature of weight and/or volume of such
additional ingredients are conventionally well known.
After the layer of skin cells has reached the
re~uired degree of confluence, the carrier is detached
CA 02226747 l998-02-ll
W O 9710683~ PCT/GB96tO1959
- 17 -
from the wall portion 5 of the flask and removed from
the access port 13.
The cell layer is preferably sub-confluent.
Alternatively it may be confluent.
The invention may be illustrated by using any of
the polymers given in the following table.
Product Polymer Water
Uptake
w/w %
Aquasorb 'pnlyviny_py~~o_idone) 4~0
ConCeel Ulcus ca~boxymethv_cellulose) ~o
Elastogel po~yacry~.am_~e; 2 o
~,e._perm po_yacry_am ee/agar) 1 n
C-ranu~lex E cmc)
:PU ~ po~yurethane) n
:PU ~ po_yurethane)
~u-ge_ pvp)
Cutinova Hydro ~polyurethane)
SUBSTITUTE SHEET (RULE 26)
CA 02226747 1998-02-11
W O 97/06835 PCT/GB96/O1959
- 18 -
Exam~les
Human epithelial cells (keratinocytes) were
grown on films and laminates comprising a polymer
having a water uptake of greater than 16%. The
materials and method used are detailed below.
The cells used were from the SCaBER cell line,
supplied by American Type Culture Collection of
Maryland USA.
The cells were labelled with a dye marker in
order to facilitate their detection by optical
microscopy. This was necessary because the cells
were difficult to detect against the non-homogenous
surfaces of some samples. The cells were labelled
using a Sigma Immuno Chemicals PKH26 red fluorescent
general cell linker kit supplied by Sigma Chemical
Company of St Louis USA, according to the
instructions suplied with the kit. The cells were
then washed with serum-free culture medium before
being re-suspended in fresh medium for culture on the
samples.
Control experiments were performed in order to
determine whether the fluorescent dye marker was
taken up by the polymer substrates. The results
showed that the dye was not absorbed by the polymers
and was specific to the cells, so fluorescence
observed on the experimental samples could be
attributed to cells attached to the polymer surfaces.
The culture medium used was Earle's ~;n; ~tl~
Essential Medium (MEM) + 10% foetal calf serum made
up as follows:-
348 ml Earle's MEM (without L - glutamine),
supplied by Gibco BRL.
CA 02226747 1998-02-11
W O 97/06835 PCT/GB96/01959
- 19 -
o,
40 ml heat-inactivated foetal calf serum
4 ml penicillin/streptomyci.n (5000IU/ml-
5000 ~g/ml), supplied by Gibco BRL.
4 ml non-essential amino acids
4 ml L-glutamine (100x) 200 mM, supplied by
Gibco BRL.
The cells were seeded at a density of 106 cells
per 3ml of medium.
The culture medium contai~ing cells at the above
concentrati.on was placed in a petri-dish in contact
with the sample film being tested and incubated at
37~C for 24 hours initially and then for further
periods of 24 hours up to 3 days. The polymer
substrate was then removed from the petri dish,
carefully washed and then ~A~; ned for the presence
of attached. cells under ultra-violet light using a
NIKON inverted microscope fitted with a rhodium
filter to d.etect the labelled red-fluorescing cells.
The polymer layers used were all thin films and
were laid on the bottom of the petri dishes using
metal rings to keep the film flat and make subsequent
handling easier.
Polymer layers used for carrier were samples of
hydrophilic films which are used in wound dressing
constructions and so are known to be suitable for
application to skin for medical purposes.
Example 1
Thermoplastic polyether-polyurethane extruded to
form a film approximately 20~m thick, having a water
uptake of 64% as measured by the method described
SUBSmUTE SHEET (RlJLE 26)
CA 02226747 1998-02-11
W O 97/06835 PCT/G B96/01959
- 20 -
above, was used as the carrier layer. The film was
laid on the base of a petri dish and culture medium
containing cells was introduced onto the upper
surface of the film. After incubation for 24 hours
the_carrier was removed and washed. Observation
under u.v. light showed that cells had attached to
the film surface. The film had swollen by absorbtion
of culture medium.
Exam~le 2
A film of thermoplastic polyether - polyurethane
20~m thick was laminated to a 50~m film of ethylene-
vinyl acetate (EVA) to form a carrier layer. The EVA
material had a water uptake of about 0.3%. The
culture medium and cells were placed on the polyether
polyurethane side of the laminate. Some cells were
observed to have attached after 24 hours, with
increasing numbers of cells attaching after 2 and 3
days.
The sample-appeared to have remained generally flat
and ridges were seen in the surface of the film, due
probably to the swelling of the hydrophilic film in
the culture medium.
Example 3
The laminate described in Example 2 was used as the
carrier layer and the cells were grown on the EVA
side of the laminate. Cells were observed to have
attached and grown on the film after 24 hours.
SUBSTITUTE SHEET tRULE 26)
CA 02226747 1998-02-11
W O 97/06835 PCT/GB96/01959
Example 4
The carrier used was a commercial dressing sold under
the trademark "OPSITE IV3000" by Smith & NepheW
Healthcare Ltd, which comprises a backing layer of a
hydrophilic polyurethane film and a layer of acrylic
pressure-se!nsitive adhesive attached to the wound-
facing side thereof. The water-uptake of the
dressing was measured as appro~imately 68%.
Cells were observed to colonise the non-adhesive,
polyurethane side of the dressing.
~y~mnle 5
The carrier was the dressing used in example 4 with
the adhesive side of the dressing used as the cell-
carrying layer. Cells were observed to attach and
proliferate on the adhesive.
Exam~le 6
The carrier used was a laminate of two 20 micrometre
thermoplastic polyether-polyurethane films as used in
Example 1. As expected, cells were observed to be
well disper,sed over the film area in contact with the
medium and attached to the film surface. The sample
had a bubbled appearance after contact with the
medium but this did not appear to unduly affect the
attachment of cells to the surface.
SUBSTITUTE SHEET (RULE 26)