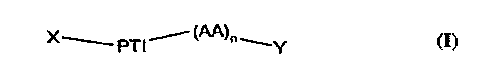Note: Descriptions are shown in the official language in which they were submitted.
CA 02227~16 1998-01-21
W O 97/08193 P~T~EP96/03473
ACYLATED OLIGOPEPTIDE DERIYATIVES HAVING CELL SIGNAL INHIBITING ACTIVITY
The present invention relates to pharmaceutically active compounds comprising a N-acyl
peptide structure, processes for the preparation of said compounds, pharmaceutical
preparations comprising said compounds, the compounds for the use in the therapeutic
(including prophylactic) or diagnostic treatment of the animal or especially human body, and
the use of said compounds for the therapeutic or diagnostic treatment of the animal or
especially human body or for the manufacture of pharmaceutical preparations.
Background of the invention
The search for new classes of compounds for the therapy and prophylaxis of proliferative
diseases, cancer and metabolic deregulation is one of the most important tasks for
pharmaceutical research. These diseases affect a large portion of the population, leading to
suffering and often being the cause for the death of the individuals stricken therewith.
Signal transduction is the process of relaying extracellular messages, e.g. chemical
messages in the form of growth factors, hormones and neurotransmitters, via receptors,
e.g. cell-surface I ece,uLur~, to the interior of the cell. At the heart of this biological
communication are the protein-tyrosine kinases. These enzymes, found, for example, as
either transmembrane growth factor receptors or as nuclear or cytosolic non-receptor
proteins, catalyze the phosphorylation of specific tyrosine residues. This class of enzymes
includes, but is not limited to, the PDGF receptor, the FGF receptor, the HGF receptor,
members of the EGF receptor family such as the EGF receptor, erb-B2, erb-B3 and erb-B4,
the src kinase family, Fak kinase and the Jak kinase family. The tyrosine-phosphorylated
proteins are involved in a range of metabolic processes, from proliferation and growth to
differentiation. Protein-tyrosine phosphorylation is known to be involved in modulating the
activity of some target enzymes as well as in generating specific complex networks involved
in signal transduction via various proteins containing a specific amino acid sequence called
a Src Homology Region or SH2 domain (for review see Proc. Natl. Acad. Sci. USA 90, 5891
'~ (1990)). A malfunction in this protein-tyrosine phosphorylation through tyrosine kinase
overexpression or deregulation is manifested by various oncogenic and (hyper-)proliferative
CA 02227~16 1998-01-21
WO 97/08193 PCT~EP96/03473
disorders such as cancer, inflammation, autoimmune disease, hyperproliferative skin
disorders, such as psoriasis, and allergy/asthma.
SH2- and/or SH3-co~ risi.lg proteins that play a role in cellular signaling and transforma-
tion include, but are not limited to, the following: Src, Lck, Fps, ras GTPase-activating
protein (GAP), phospholipase C, phosphoinositol-3 (Pl-3) kinase, Fyn, Lyk, Fgr, Fes, ZAP-
70, Sem-5, p85, SHPTP1, SHPTP2, corkscrew, Syk, Lyn, Yes, Hck, Dsrc, Tec, AtklBpk,
ItklTsk, Arg, Csk, tensin, Vav, Emt, Grb2, BCR-Abl, Shc, Nck, Crk, CrkL, Syp, Blk,113TF,
91TF, Tyk2, JAK1, and JAK2, especially Src, phosholipase C, phosphoinositol-3 (Pl-3)
kinase, Grb2, BCR-Abl, Shc, Nck, Crk and CrkL.
A direct link has been established between activated receptor kinases and Ras with the
finding that the mammalian Grb2 protein, a 26 kilodalton protein col ~ " ising a single SH2
and two SH3 domains, directly couples receptor tyrosine kinases to the Ras guanine
nucleotide exchange factor Sos in mammals and also Drosophila. The Grb2 SH2 domain
binds to specific tyrosine phosphorylated sequences, e.g. in receptor tyrosine kinases, while
the Grb2 SH3 domains bind to proline-rich sequences present in the Sos exchange factor.
The significance of ras-regulatory pr~L~i. ,s in human tumors is also highlighted by the critical
role of GRB2 in BCR-Abl mediated oncogenesis (J. Exp. Med.,179(1),167-175 (1994)).
Recently, DNA sequences within the chromosomal locus 17q22-qter, which harbors the
GRB2 gene, were shown by comparative genomic hybridization to exhibit a high frequency
of amplification in both human breast cancer cell lines and tumors (Proc. Natl. Acad. Sci.
USA 91, 2156-2160 (1994)).
In a study of GRB2 gene expression in human breast cancer cell lines, Northern Blot
analysis also revealed that 7/19 breast cancer cell lines exhibited more than 2 fold
overexpression of GRB2 mRNA relative to normal breast epithelial cells. In MCF-7, MDA-
MB-361, and -453 cells, the overexpression of GRB2 mRNA was accompanied by a 10-20
fold increase in the amount of GRB2 protein (Oncogene 9, 2723 (1994)).
SH2 domains represent recognition motifs for specific tyrosine-phosphorylated peptide .i
sequences. Short, conserved motifs, primarily 3 to 6 amino acids on the carboxy-terminal
side of a phosphotyrosine residue, carry the sequence-specific information for SH2-
CA 02227~16 1998-01-21
W O 97/08193 PCTAEP96/03473
recognition. This concept has been supported by the mapping of separate sites for binding
of SH2 domains from different signaliing molecules on various receptors [see, e.g., Cell 69,
413 (1992); Proc. Natl. Acad. Sci. USA89, 678 (1992); Mol. Cell. Biol.12, 991 (1992);
EMBO J.11,1365 (1992); EMBO J.11, 559 (1992); EMBO J.11, 3911 (1992); Cell 73, 321
(1993)]. Degenerate peptide libraries have also been used to predict the specifity of
individual SH2 domains (src family members, Abl, Nck, Sem5, phosholipase C-y, p85
subunit of Pl-3 kinase, and HCP (amino terminal SH2) [see Cell 72, 767 t1993); Mol. Cell.
Biol.14, 2777 (1994)]). High-resolution crystallographic analysis and nuclear magnetic
resonance of the SH2 domains of Src, Lck, PLC-y C-terminal, p85 N-terminal, Abl, Syp C-
terminal have also revealed that the region on the carboxyl side of the phosphotyrosine
carries the sequence-specific information for SH2 recognition. Each of these SH2containing proteins controls a cellular pathway involved in the biological response to a
growth factor. Activation of a particular pathway can thus be inhibited by designing a small
molecule that specifically disrupts a phosphoprotein/SH2 domain interaction.
The approach of selectively eliminating a mitogenic pathway by a point mutation of the
tyrosine kinase or a tyrosine-phosphorylated protein has been successful. For example,
tyrosine 317 is the major site for SHC tyrosine phosphorylation and is the sole high-affinity
binding site for Grb2 SH2. Mutant SHC proteins with suhstitl Ition of tyrosine 317 by
phenylalanine loose the capacity to be highly phosphorylated on tyrosine upon growth
factor activation, to bind Grb2 and to induce neoplastic tran~l~r",dLion ~see Oncogene 9,
2827 (1994)]. An FGR receptor with a point mutation at tyrosine 766 does not bind
phospholipase C-y (an SH2-containing protein). lt abolishes phosphatidylinositol turnover
and calcium flux but not mitogenesis [see Nature 358, 678 (1992)]. For Epidermal Growth
Factor Receptor (EGFR) it has been shown that tyrosine 1068 is the binding site for Grb2
SH2 (see Buday et al., Cell 73, 611 -620 (1993)). A phosphopeptide based on the
surrounding sequence, Pro-Val-Pro-Glu-Tyr(PO3H2)-lle-Asn-Gln-Ser, was shown to inhibit
the interaction of phosphorylated EGFR and Grb2.
It has also been demonstrated that uncoupling a tyrosine kinase from signal transduction
pathways results therapeutically in antitumor activity. Antitumor activity for tyrosine kinase
inhibitors has been demonstrated both in vitro and in vivo [see J. Antibiot. 39,170 (1986);
Eur. J. Cancer 26(6), 722 (1990); J. Med. Chem. 34, 2328 (1991); Cancer Res. 51, 4430
CA 02227~16 1998-01-21
W O 97/08193 PCTAEP96/03473
(1991); J. Med. Chem. 34, 2328 (1991); Helv. Chim. Acta 75, 696 (1992); Cancer Res. 52,
4492 (1992); Science 265, 1093 (1994); Science 267,1782 (1995)]. For example, it has
been shown that a small molecule called PD 153035 rapidly suppressed autophosphoryla-
tion of the EGF receptor at low concentrations in human epidermoid carcinoma cells and
selectively blocked EGF-mediated cellular processes including mitogenesis, early gene
expression and oncogenic transformation ~see Science 265,1093 (1994)]. In addition, it has
been shown that tyrosine kinase inhibitors RS-13022 and 14620 supressed EGF-stimulated
prolireldlion of HER-14 cells (transfected NIH 3T3 cells) and MH-85 cells in vitro. The MH-
85 tumor is a human squamous cell ca,.ii"or"a associated with three paraneoplastic
syndromes: hypercalcemia, leukocytosis and cachexia. The well-characterized cells show
overex~ression of endogenous EGF receptor tyrosine kinase and are dependent on the
EGF receptor signal transduction pathway for growth in vitro and in nude mice. In vivo, the
compounds suppressed the growth of MH-85 tumors in nude mice as well as the expression
of the paraneoplastic syndromes. An increase in life span of 75% was observed for RG-
13022-treated tumor bearing mice ~see Cancer Res. 51~ 4430 (1991)].
4,5-Dianilinophthalimides inhibit the growth of human tumor cells that overexpress EGFR or
HER2-ErbB2 and exhibit good antitumor activity in mice in which these tumors are grown as
xenografts (see Buchdunger et al., Proc. Natl. Acad. Sci USA 91, 2334 (1994) and Trinks et
al., J. Med. Chem. 37,1015 (1994)).
immunologic downregulation of the p185neU receptor in transgenic mice that express the
rat neu oncogene (neuT) in mammary epithelial cells can also effectively prevent breast
tumor development (Nature Medecine,1 (7), 1995).
Anilinoquinazolines also represent a class of compounds which exhibit promising anti-
cancer activity. They were shown to inhibit the EGF-stimulated growth of human KB
nasopharyngeal cells in vitro at concentrations of 1 -10 ~lM.
These results show that the inhibition of regulatory pathways by way of inhibition of protein
tyrosine kinases results in therapeutically useful effects. lt is therefore reasonable that
inhibition at the level of interaction of protein tyrosine kinases with other, e.g. regulatory,
proteins, for example those with SH2 domains, will result in similar therapeutic usefulness.
CA 02227~16 1998-01-21
W O 97/08193 PCT~P96/03473
Not all phosphoproteins bind the same SH2-binding proteins. The divergent residues of
individual domains can be shown to confer specificity for binding to structural variants within
each ligand binding site ~for SH2 domains, ligands with different amino acids surrounding
the respective phosphotyrosine residue]. Various synthetic peptides derived from these
ligand binding sites and as small as five amino acids in length have been shown to interfere
specifically with these interactions in vitro [Mol. Cell. Biol.11 (2),1125 (1991); Mol. Cell. Biol.
12(4),1451 (1992); Cell 69, 413 (1992); and Cell 72, 767-778 (1993)]
It is a goal of the present invention to prt:se~ ~l small organic molecules that, due to their
ability to mimic the structure of the phosphotyrosine peptide binding site, have the ability to
disrupt the interaction between SH2 domains of (e.g. regulatory) proteins, for example that
of Grb2, and proteins with phosphorylated moieties, especially phosphorylated tyrosine
moieties, for example phosphorylated protein tyrosine kinase receptors. The effect is to
inhibit the association of SH2 containing (e.g. regulatory) prc tei"s with a protein tyrosine
kinase in order to inhibit downstream signalling through one or more specifically targeted
effector proteins.
Summary of the Invention
Surprisingly, it has been found that the compounds of the present invention show very
favourable and valuable characteristics for pharmaceutical application, especially with
regard to the therapeutic (including, in a broader sense, prophylactic) and/or diagnostic
treatment of diseases that depend on the downstream signal transduction pathways,
especially those mediated by an interaction of a protein co",prisil ,g a SH2 domain with a
tyrosine phosphorylated protein, such as a phosphorylated tyrosine protein kinase; proteins
comprising one or more SH2 domains that are effective in cellular signalling andtransformation include, but are not limited to, the following: Src, Lck, Fps, ras GTPase-
activating protein (GAP), phospholipase C, phosphoinositol-3 (Pl-3) kinase, Fyn, Lyk, Fgr,
Fes, ZAP-70, Sem-5, p85, SHPTP1, SHPTP2, corkscrew, Syk, Lyn, Yes, Hck, Dsrc, Tec,
Atk/Bpk, Itk/Tsk, Arg, Csk, tensin, Vav, Emt, Grb2, BCR-Abl, Shc, Nck, Crk, CrkL, Syp, Blk,
113TF,91TF, Tyk2, JAK1, and JAK2.
Especially, very good inhibition is already found in vitro with the compounds of formula 1.
' CA 02227~16 1998-01-21
-6--
The new peptides of this invention preferably show selective inhibition of the binding of
SH2-comprising proteins, such as Grb2, to phosphorylated proteins, especially activated
growth factor receptor tyrosine kinases like EGF receptor tyrosine protein kinase, or Shc.
The compounds of formula I disrupt the interaction between the SH2-comprising protein and
the phosphoprotein, such as protein tyrosine kinase, and thus blocks the ability of the
tyrosine protein kinases to initiate regulatory events depending on the SH2-comprising
proteins, thus resulting in inhibition of specific downstream signal transduction pathways
utilized in some hyperproliferative diseases, such as tumor diseases and psoriasis and the
other diseases mentioned above and below, by uncoupling of the respective protein
tyrosine kinase(s) from the respective SH2-containing effector protein.
One feature of the present invention is the positive effect of the moieties X as defined
below on the inhibitory action of the compounds of the present invention on the interaction
of a broad variety of phosphoproteins, especially phosphotyrosine-comprising proteins, to
SH2-comprising proteins (e.g. those mentioned below in the definition of the bivalent radical
-(AA)n-). These moieties X are even able to allow for large seauence variability in the pep-
tide derivatives of formula 1. Many of the compounds of formula I show inhibition if n in for-
mula I given below is 3, 2 and even 1 or O. In addition, the C- and especially the N-terminal
modification even allows that PTI in formula I given below is simply tyrosine without any
phosphono group or an analogue thereof; even then very active compounds can be
obtained.
Detailed description of the invention
The invention relates to an acylated peptide, namely a compound of the formuia 1,
X~ PTI (AA)n (I)
wherein
n is O to 4,
A~lEND~D SH~
o CA 02227~16 1998-01-21
X is arylcarbonyl, cycloalkylcarbonyl, tricycloalkyicarbonyl, arylsulfonyl, heterocyclylcarbonyl,
heterocyclylsulfonyl, carbamoyl-lower alkanoyl, aryl-lower alkylcarbonyl, cycloalkyl-lower
alkylcarbonyl, aryl-lower alkylsulfonyl, heterocyclyl-lower alkylcarbonyl, heterocyclyl-lower
alkylsulfonyl with the proviso that in any of the lower alkyl radicals mentioned a methylene
group may be replaced with oxa, aza or thia; heterocyclyl-lower alkenylcarbonyl or aryl-
lower-alkenylcarbonyl; or, if Y is a secondary or tertiary amino group, i-s one of the morieties
X mentioned above or lower alkanoyl, halo-lower alkanoyl, lower-alkoxycarbonyl, aryl-lower
alkoxycarbonyl or cycloalkyl-lower alkoxycarbonyl;
PTI is the bivalent radical OT phosphotyrosine or a phosphotyrosine mimic,
AA stands for a bivalent radical of a natural or unnatural amino acid, and
Y is a primary, secondary or tertiary amino group,
or a compound with the name indole-~-ylcarbonyl-Tyr-lle-Asn-Gln-NH2 (Si-Q ID NO: 114);
or a salt thereof.
Unless indicated otherwise, the general terms and names used in the description of the
present invention preferably have the following meanings:
The term "lower" defines a moiety with up to and including maximally 7, especially up to and
including maximally 4, carbon atoms, said moiety being branched or straight-chained. Lower
alkyl, for example, is methyl, ethyl, n-propyl, sec-propyl, n-butyl, isobutyl, sec-butyl, tert-
butyl, n-pentyl, n-hexyl or n-heptyl.
In the case of lower alkenyl, the term "lower" designates a residue with 2 to 7 carbon atoms,
preferably with 2 to 4 carbon atoms, such as vinyl, allyl or 1- or 2-butenyl.
The compounds of formula I with one or more centers of asymmetry, such as one or more
asymmetric carbon atoms, may be present in the form of isomeric mixtures or pure isomers;
for example, a compound of formula I with one center of asymmetry may be present in the
form of a pure enantiomer or a mixture of enantiomers, e.g. a racemate, while a compound
Al~/;ENDED Sll~T
CA 02227S16 1998-01-21
-8-
of formula I with two or more centers of asymmetry may be present in the form of a pure
isomer (enantiomer) or in the form of diastereomeric mixtures, e.g. mixtures of epimers.
A double bond in a compound of formula I may be present in the cis (Z) or trans (E) form.
In a compound of formula I with a center of asymmetry and a double bond, the respective
compound may be present as a mixture of isomers or as a pure isomer.
Generally, pure isomers of compounds of formula I are preferred over isomeric mixtures.
n is 1 to 4, preferably 1 to 3 and most preferably 2 or especially 3.
Aryl has preferably from 6 to 14 ring carbon atoms, such as in phenyl (which is especially
preferred), naphthyl (which is especially preferred), such as 1-naphthyl or 2-naphthyl,
indenyl, indanyl, anthryl, phenanthryl (which is especially preferred), acenaphthyl or
fluorenyl (which is preferred), and may be unsubstituted or preferably mono- to trl--
substituted, especially by amino, mono- or di-lower alkylamino, lower alkanoylamino, such
as acetylamino, amino-lower alkyl, mono- or di-loweralkylamino-lower alkyl, lower alkanoyl-
amino-lower alkyl, hydroxy, lower alkoxy, such as methoxy, carboxy, lower-alkoxycarbonyl,
such as methoxycarbonyl, phenyl-, naphthyl- or fluorenyl-lower alkoxycarbonyl, such as
benzyloxycarbonyl, lower alkanoyl, cyano, lower alkyl, for example methyl, ethyl or propyl,
halo-lower alkyl, for example trifluoromethyl, phenyl, 1- or 2-naphthyl, halogen, for example
fluorine, chlorine or bromine, mercapto, lower-alkylthio, such as methylthio, lower alkyl-sul-
finyl, such as methylsulfinyl (CH3-S(=0)-), sulfo, lower alkanesulfonyl, for example metha-
nesulfonyl (CH3-S((0)2)-), carbamoyl, mono- or di-lower alkylcarbamoyl, sulfamoyl, mono- or
di-lower alkylaminosulfonyl, and /or by nitro; more preferably substituted by one or two
substituents selected independently from amino, mono- or di-lower alkylamino, lower alka-
noylamino, such as acetylamino, amino-lower alkylamino, mono- or di-loweralkylamino-lo-
wer alkyl, lower alkanoylamino-lower alkyl, hydroxy, lower alkoxy, such as methoxy, carb-
oxy, lower-alkoxycarbonyl, such as methoxycarbonyl, cyano, halogen, such as chloro or
bromo, lower-alkylthio, such as methylthio, and !ower alkyl-sulfinyl, such as methylsulfinyl
(CH3-S(=0)-); most preferably mono- or disubstituted by amino or hydroxy.
L' S~FF--
_
CA 02227~l6 l998-0l-2l
W O 97/08193 PCTrEP96/03473
Cycloalkyl preferably has from 3 to 10 ring carbon atoms, preferably from 4 to 7 carbon
atoms, and is unsl Ihstitl Ited or preferably mono- to tri-substituted, especially by amino,
mono- or di-lower alkylamino, lower alkanoylamino, such as acetylamino, amino-lower alkyl,
mono- or di-loweralkylamino-lower alkyl, lower alkanoylamino-lower alkyl, hydroxy, lower
alkoxy, such as methoxy, carboxy, lower-alkoxycarbonyl, such as methoxycarbonyl, phenyl-,
naphthyl- or fluorenyl-lower alkoxycarbonyl, such as benzyloxycarbonyl, lower alkanoyl,
cyano, lower alkyl, for example methyl, ethyl or propyl, halo-lower alkyl, for example tri-
fluoromethyl, phenyl, 1- or 2-naphthyl, halogen, for example fluorine, chlorine or bromine,
mercapto, lower-alkylthio, such as methylthio, lower alkyl-sulfinyl, such as methylsulfinyl
(CH3-S(=0)-), sulfo, lower alkanesulfonyl, for example methanesulfonyl (CH3-S((0)2)-),
carbamoyl, mono- or di-lower alkylcarbamoyl, sulfamoyl, mono- or di-lower alkylamino-
sulfonyl, and /or by nitro; more preferably mono- or di-sl Ihstitl Ited by a sl Ih5tjtl lent selected
independently from amino, mono- or di-lower alkylamino, lower alkanoylamino, such as ace-
tylamino, amino-lower alkylamino, mono- or di-loweralkylamino-lower alkyl, lower alkanoyl-
amino-lower alkyl, hydroxy, lower alkoxy, such as methoxy, carboxy, lower-alkoxycarbonyl,
such as methoxycarbonyl, cyano, halogen, such as chloro, lower-alkylthio, such as methyl-
thio, and lower alkyl-sulfinyl, such as methylsulfinyl (CH3-S(=0)-); most preferably mono- or
disubstituted by amino. Cycloalkyl may also be annelated with a benzo ring, such as in
1 ,2,3,4-tetrahydronaphthyl .
Tricycloalkyl preferably has 8 to 16 carbon atoms and is, for example, tricyclo[5.2.1 .0261dec-
8-yl or especially adamantyl, preferably 1-adamantyl.
Heterocyclyl is preferably a single or double ring system having from 3 to 10 ring atoms, is
bonded preferebly via a carbon atom or also via a nitrogen atom and contains up to 3
hetero atoms selected from oxygen, sulfur, sulfur linked to 1 or 2 oxygen atoms and, most
preferably, nitrogen; which in addition may also be fused with 1 or 2 phenyl radicals or with
1 or 2 cycloalkyl radicals, cycloalkyl preferably having from 5 to 7 ring atoms; and which
may be unsaturated or partially or fully saturated, for example thienyl, furyl, pyrrolyl, imid-
azolyl, such as imidazole-4-yl, pyrazolyl, oxazolyl, thiazolyl, such as 4- or 5-thiazolyl, tetraz-
olyl, pyridyl, such as pyridin-3- or pyridin-4-yl, pyrazinyl, such as pyrazin-2-yl, pyrimidinyl, py-
ridazinyl, indolyl, such as indole-2-yl, -3-yl or -5-yl, indolinyl, such as indolin-2-yl, benzimid-
azolyl, such as 5-benzimidazolyl, quinolinyl, such as quinoline-8-yl, -6-yl, -4-yl, -3-yl or-2-yl,
CA 02227~16 1998-01-21
W O 97/08193 PCT~EP96/03473
-10-
isoquinolyl, such as isoquinoline-1-yl, benzofuranyl, isobenzofuranyl, 2,3-dihydrobenzo-fu-
ranyl, such as 2,3-dihydrobenzofuran-5-yl, chromanyl, cyclohexa[b]pyrrolyl, cyclohexa[blpy-
ridyl, cyclohexa[b]pyrazinyl, cyclohexa[b]pyrimidinyl, pyrrolidinyl, pyrrolinyl, ill -J~701 dyl~
piperidyl, piperazinyl, morpholinyl, thiomorpholinyl, S,S-dioxo-thiomorpholinyl, 4,5,6,7--
tetrahydroindolyl, 1,2,3,4-tetrahydroquinolyl or 1,2,3,4-tetrahydroisoquinolyl, or (alternatively
or in addition to the group of moieties mentioned so far) chromenyl (= 1 H- or 2H-benzo-
pyranyl), such as 2H-chromen-3-yl; with heterocyclyl, for example one of the last-mentioned
r~dic~ls, being unsllhstihlted or 5llhstihlted by one or more, pre~r~bly one or two,
substituents independently selected from lower alkyl, for example methyl, phenyl, 1- or 2-
naphthyl, phenyl-lower alkyl, for example benzyl, hydroxy-lower alkyl, for example hydro-
xymethyl or 2-hydroxyethyl, hydroxy, lower alkoxy, for example methoxy or ethoxy, amino,
lower alkylamino, for example methyl-, ethyl- or tert-butyl-amino, di-lower alkylamino, for
example dimethyl- or diethyl-amino, carboxy, lower alkoxycarbonyl, for example methoxy-,
isopropoxy-, sec-butoxy- or tert-butoxy-carbonyl, phenyl- or naphthyl-lower alkoxycarbonyl,
for example benzyloxycarbonyl, halogen, for example fluorine, chlorine, bromine or iodine,
especially chlorine or bromine, lower alkanoyl, for example acetyl or pivaloyl, nitro, oxo and
cyano; more preferably heterocyclyl being selected from ill~id~ulyl, such as i",ida~ole-4-yl,
thiazolyl, such as 4- or 5-thiazolyl, pyridyl, such as pyridin-3- or pyridin-4-yl, pyrazinyl, such
as pyrazin-2-yl, indolyl, such as indole-2-yl, -3-yl or -5-yl, indolinyl, such as indolin-2-yl,
bell~il"id~olyl, such as 5-ben~i,l,:da~olyl, quinolinyl, such as quinoline-8-yl, -6-yl, -4-yl, -3-yl
or-2-yl, isoquinolyl, such as isoquinoline-1-yl, and 2,3-dihydrobenzofuranyl, such as 2,3-
dihydrobenzofuran-5-yl, or (alternatively or in addition to the group of moieties mentioned
just before) chromenyl (=1 H- or2H-benzopyranyl), such as 2H-chromen-3-yl, these radicals
being un~l Ihstitl Ited or sl Ihstitl Ited as above; most preferably from imidazolyl, such as
imidazole-4-yl, thiazolyl, such as 4- or s-thiazolyl, pyridyl, such as pyridin-3- or pyridin-4-yl,
pyrazinyl, such as pyrazin-2-yl, indolyl, such as indole-2-yl, -3-yl or-5-yl, indolinyl, such as
indolin-2-yl, ben~i" ' 7-1yl, such as 5-benzimidazolyl, quinolinyl, such as quinoline-8-yl, -6-
yl, -4-yl, -3-yl or-2-yl, isoquinolyl, such as isoquinoline-1-yl, and 2,3-dihydrobenzofuranyl,
such as 2,3-dihydrobenzofuran-5-yl, each of which is unsubstituted or substituted by lower
alkyl, such as methyl, or amino, or (alternatively or in addition to the group of moieties
mentioned just before) chromenyl, such as 2H-chromen-3-yl, that is unsuhstituted by 1 to 3
substitutents selected from oxo and hydroxy, especially 7,8-dihydroxy-2-oxo-2H-
(benzopyran) -3-yl .
CA 02227~16 1998-01-21
WO 97/08193 PCTA~P96/03473
In aryl-lower alkylcarbonyl, aryl-lower alkylsulfonyl, heterocyclyl-lower alkylcarbonyl and
heterocyclyl-lower alkylsulfonyl with the proviso that in any of the lower alkyl radicals men-
tioned a methylene group may be replaced with aza, thia or (preferably) oxa, preferably the
oxa being bound to the respective aryl or heterocylyl or to the carbonyl group in formula 1,
the lower alkyl or the lower alkyl wherein a methylene group may be replaced with oxa, ~a
or thia can be linear or branched and is preferably selected from methyl (forming methyle-
ne), 1,2-ethyl (forming 1,2-ethylene), 1 ,1-ethyl (forming methyl-methylene), methoxy whe-
rein the methyl is bound to the aryl or heterocyclyl and the oxygen is bound to the carboxy
group or the methyl is bound to the carbonyl and the oxygen is bound to the aryl or hete-
rocyclyl group (forming 1-oxaethylene = -CH2-O-; or 2-oxaethylene = -O-CH2-),1,3-propyl
fforming a 1 ,3-propylene = -CH2-CH2-CH2-), ethoxy wherein the terminal methylene is bound
to the aryl or heterocyclyl and the oxygen is bound to the carbonyl group or ethoxy wherein
the terminal methylene is bound to the carbonyl and the oxygen is bound to the aryl or hete-
rocylyl (forming 1-oxa-3-propylene = -CH2-CH2-O- or 3-oxa-1-propylene = -O-CH2-CH2-).
The radicals given in parenthesiws are to be regarded in the following way: The bond on the
left of each radical is to be regarded to be directed to the N-terminus in formula 1, the bond
on the right is to be regarded to be directed to the C-terminus of formula 1.
In arylcarbonyl X, the aryl moiety is preferably defined as above; more preferably, arylcar-
bonyl is selected from benzoyl or naphthoyl and, even more preferably, from benzoyl sub-
stituted with amino; lower alkylamino; amino-lower alkyl; hydroxy; lower alkoxy; amino and
hydroxy; amino and lower alkoxy; carboxy; lower-alkoxycarbonyl; cyano; halogen, especially
chloro; lower-alkylthio; or lower alkylsulfinyl; or (alternatively or in addition to the group of
moieties mentioned just before) selected from naphthoyl or hydroxy-naphthoyl, such as
naphthalene-2-yl-carbonyl or 6-hydroxy-naphthalene-2-yl-carbonyl, and, less preferably,
from fluorenylcarbonyl, such as fluoren-9-ylcarbonyl; especially from 4-aminobenzoyl, 3-
aminobenzoyl, 2-aminobenzoyl, 4-lower alkylamino-benzoyl, such as 4-methylamino-benzoyl, 4-(amino-lower alkyl)-benzoyl, such as 4-(methylamino)-benzoyl, 4-hydroxy-
benzoyl, 4-lower alkoxy-, such as 4-methoxybenzoyl, 4-amino-2-hydroxy-benzoyl, 4-amino-
3-lower alkoxy-benzoyl, such as 4-amino-3-methoxy-benzoyl, 4-carboxybenzoyl, 4-lower
alkoxycarbonyl-benzoyl, such as 4-methoxycarbonyl-benzoyl, 4-cyanobenzoyl, 4-lower
alkylthio-benzoyl, such as 4-methylthiobenzoyl, and 4-lower alkylsulfinyl-benzoyl, such as 4-
lower methylsulfinyl-benzoyl, or (alternatively or in addition to the group of moieties
CA 02227~16 1998-01-21
W O 97/08193 PCT/EP96/03473
- 12-
mentioned just before) from naphthalene-2-yl-carbonyl or 6-hydroxy-naphthelene-2-yl-
carbonyl; and most preferably from 3- or especially 4-aminobenzoyl, or (altematively or in
addition to the group of moieties mentioned just before) 6-hydroxy-naphthalen-2-yl-
carbonyl.
In cycloalkylcarbonyl X, cycloalkyl is preferably as defined above; more preferably, cyclo-
alkylcarbonyl is C3-C7-, especi~lly C4- C5- or C6-cycloalkylcarbonyl, such as cyclohexyl-car-
bonyl, and is unsuhstitllted or substituted by amino or annelated to a benzo ring; most pre-
ferably cyclohexylcarbonyl, 1,2,3,4-tetrahydronaphthylcarbonyl, such as 1,2,3,4-tetrahydro-
naphthyl-2-carbonyl, or especially 1-amino-cyclohexylcarbonyl or 1-amino-cyclopentylcarbo-
nyl. Cyclohexylcarbonyl and especially 1,2,3,4-tetrahydronaphthyl-2-carbonyl are most pre-
ferred.
In Tricycloalkylcarbonyl, tricycloalkyl preferably has 8 to 16 carbon atoms and is, for ex-
ample, tricyclo[5.2.1.02~1dec-8-yl or especially adamantyl, preferably 1-adamantyl. 1-
Adamantylcarbonyl is especially preferred.
In arylsulfonyl X [= aryl-(SO2)-], the aryl moiety is preferably defined as above; more pre-
ferably, arylsulfonyl is 2-or 3-napthylsulfonyl which is substituted with amino or mono- or di-
lower alkylamino, such as dimethylamino, especially 5-dimethylamino-naphthalenesulfonyl.
In heterocyclylcarbonyl, the heterocyclyl moiety is preferably as defined above; more pre-
ferably, heterocyclylcarbonyl is selected from pyridylcarbonyl which is unsllhstitllted or sub-
stituted with amino, such as pyridin-4-yl- or pyridin-3-ylcarbonyl, or amino-pyridin-3-yl-car-
bonyl, such as 2- or 6-amino-pyridin-3-ylcarbonyl, benzimidazolylcarbonyl, such as benz-
imidazol-5-ylcarbonyl, quinolinyl-carbonyl, such as quinoline-2-, quinoline-3- or quinoline-6-
ylcarbonyl, 2,3-dihydrobenzofuranylcarbonyl, such as 2,3-dihydrobenzofuran-5-ylcarbonyl,
and indolylcarbonyl, such as indole-5-yl-, indole-3-yl- or indole-2-yl-carbonyl, or (alternatively
or in addition to the group of moieties mentioned just before) chromenyl, such as 2H-
chromen-3-yl, that is unsuhstitllted by 1 to 3 5llhstitlltents selected from oxo and hydroxy,
especially 7,8-dihydroxy-2-oxo-2H-(benzopyran)-3-yl; most preferably from quinoline-6-yl-
carbonyl and especially from indolylcarbonyl, such as indole-3- and indole-5-ylcarbonyl, or
(alternatively or in addition to the group of moieties mentioned just before) 7,8-dihydroxy-2-
oxo-2H-(benzopyran)-3-yl.
CA 02227~16 1998-01-21
W O 97/08193 PCT~EP96/03473
In heterocyclylsulfonyl X [= heterocyclyl-(SO2)-]; the heterocyclyl moiety is preferably as
defined above; more preferably, heterocyclylsulfonyl is selected from quinolinylsulfonyl,
such as quinoline-8-ylsulfonyl.
In carbamoyl-lower alkanoyl, lower alkanoyl is especially propionyl. r,er~r,ed is 3-
carbamoylpropionyl.
In aryl-lower alkylcarbonyl X, with the proviso that in the lower alkyl radical mentioned a
methylene group may be replaced with oxa, aza or thia (the latter two being less prt:rt:r,ed
than oxa), the aryl moiety is preferably as defined above for aryl (most preferably lower
alkylaminophenyl, such as 2-, 3- or 4-acetylaminophenyl, or especially hydroxyphenyl, such
as 3-hydroxyphenyl, or (in the sequence of growing preference) 2-amino-, 3,5-diamino-, 4-
amino- or 3-aminophenyl); or (alternatively or in addition to the group of moieties mentioned
just before) amino-lower alkylphenyl, such as aminomethyl-phenyl, or preferably 3,4-
dihydroxyphenyl; and the lower alkyl or the lower alkyl wherein a methylene group may be
replaced with oxa, aza or thia can be linear or branched and is preferably selected from
methyl (forming methylene), 1,2-ethyl (forming 1,2-ethylene), 1,1-ethyl (forming methyl-
methylene), methoxy wherein the methyl is bound to the aryl and the oxygen is bound to the
carboxy group or the methyl is bound to the carbonyl and the oxygen is bound to the aryl
group fforming 1-oxaethylene = -CH2-O-; or 2-oxaethylene = -O-CH2-), 1,3-propyl (forming a
1 ,3-propylene = -CH2-CH2-CH2-), ethoxy wherein the terminal methylene is bound to the aryl
and the oxygen is bound to the carbonyl group or ethoxy wherein the terminal methylene is
bound to the carbonyl and the oxygen is bound to the aryl (forming 1-oxa-3-propylene = -
CH2-CH2-O- or 3-oxa-1-propylene = -O-CH2-CH2-); more p,~r,~d is a moiety selected from
phenyl-methoxycarbonyl wherein the phenyl residue is unsllhstitllted or 5llh5tituted with
amino in the (in growing preference) 2-, 3- and 5-, 4- or 3-position of the phenyl ring; (+)-,
(+) or (-)-1-(3-aminophenyl)ethyloxycarbonyl; hydroxybenzyloxy- or hydroxyphenyl-2-etho-
xycarbonyl, such as 3-hydroxybenzyloxycarbonyl or 3-hydroxyphenyl-2-ethoxycarbonyl;
(with less preference) lower alkanoylamino-phenyloxymethylcarbonyl, such as 2-, 3- or
especially 4-acetylaminophenyloxymethylcarbonyl, aminophenyloxymethylcarbonyl, such as
3- or 4-aminophenyloxymethylcarbonyl; aminophenyl-lower alkylcarbonyl, such as 4-ami-
~ nophenyl-acetyl or 3-(3-aminophenyl)-propionyl; and (with still less preference) benzyloxy-
carbonyl or phenylacetyl. More preferred is also (alternatively or in addition to the group of
CA 02227~16 1998-01-21
W O 97/08193 PCT~EP96/03473
-14-
moieties mentioned just before) 3-(3,4-dihydroxyphenyl)-propionyl or 2-(3,4-dihydroxy-
phenyl)-acetyl. -
In cycloalkyl-lower alkylcarbonyl X, cycloalkyl is preferably as defined above; more prefer-
ably, cycloalkyl-lower alkylcarbonyl is C3-C7-, especially C4- C5- or C6-cycloalkyl-C1-C4-car-
bonyl, such as 3-cyclohexylpropanoyl, and is unsubstituted or 5llhstihlted by amino; most
preferably 1-amino-cyclohexylcarbonyl or 1-amino-cyclopentylcarbonyl; or (alternatively or in
addition to the group of moieties mentioned just before) 3-(cyclohexyl)-propionyl.
In aryl-lower alkylsulfonyl X, with the proviso that in the lower alkyl radical mentioned a
methylene group may be replaced with oxa, ~a or thia; aryl and the lower alkyl or lower
alkyl wherein a methylene group is replaced with oxa, aza or thia are preferably as
described above.
In heterocyclyl-lower alkylcarbonyl X, with the proviso that in the lower alkyl radical mentio-
ned a methylene group may be replaced with oxa, aza or thia; the heterocyclyl moiety is
preferably as defined above, more preferably being selected from unsl ~hstjtllted or amino
or lower alkyl-sllhstihlt~qd thi~olyl, such as 4-methyl-thiazol-5-yl or 2-amino-thi~ole-4-yl,
indolyl, such as indole-3-yl, and benzimid~olyl, such as benzimid~ol-~-yl; and the lower
alkyl or the lower alkyl wherein a methylene group may be replaced with oxa, ~a or thia
can be linear or branched and is preferably selected from methyl (forming methylene), 1,2-
ethyl fforming 1,2-ethylene), 1,1-ethyl (forming methyl-methylene), methoxy wherein the
methyl is bound to the heterocyclyl and the oxygen is bound to the carboxy group or the
methyl is bound to the carbonyl and the oxygen is bound to the heterocyclyl group (forming
1-oxaethylene = -CH2-O-; or 2-oxaethylene = -O-CH2-), 1,3-propyl (forming a 1,3-propylene
= -CH2-CH2-CH2-), ethoxy wherein the terminal methylene is bound to the heterocyclyl and
the oxygen is bound to the carbonyl group or ethoxy wherein the ler",il,al methylene is
bound to the carbonyl and the oxygen is bound to the heterocylyl (forming 1-oxa-3-propyle-
ne = -CH2-CH2-O- or 3-oxa-1-propylene = -O-CH2-CH2-). More pr~fell~d is unsubstituted or
lower alkyl-substituted 2-(thi~olyl)-ethoxycarbonyl, such as 2-(4-methyl-thiazol-5-yl)-ethoxy-
carbonyl, unsubstituted or amino-substituted thiazolyl-lower alkylcarbonyl, such as 2-amino-
thiazole-5-ylacetyl, or especially indolyl-lower alkylcarbonyl, such as indole-3-yl-acetyl, 3-
(indole-3-yl)propionyl or 4-(indole-3-yl)butyroyl.
CA 02227~l6 l998-0l-2l
W O 97/08193 PCT~EP96/03473
-15-
ln heterocyclyl-lower alkylsulfonyl with the proviso that in the lower alkyl radical mentioned a
methylene group may be replaced with oxa, aza or thia, the heterocyclyl moiety and the
lower alkyl or the lower alkyl radical wherein a methylene group is repl~ced with oxa, aza or
thia are preferably as defined above, respectively.
In aryl-lower alkenylcarbonyl X, aryl is preferably as defined above, especially being selec-
ted from phenyl which is substituted by 1 to 2 moieties independently selected from lower
alkoxy, pr~rdbly methoxy, and especially hydroxy, while the lower alkenyl radical prefer-
ably is linear and has one double bond and 2 to 7 carbon atoms, preferably being a 1,2-
vinyl radical. r~ led is cinnamoyl substituted by 1 to 2 moieties selected independently
from methoxy and especially hydroxy, e~pec~ y p-hydroxycinnamoyl (= 4-hydroxycinna-
moyl), m-hydroxy-p-methoxy-cinnamoyl (= 3-hydroxy-4-methoxycinnamoyl) or preferably
m,p-dihydroxy-cinnamoyl (= 3,4-dihydroxycinnamoyl).
In heterocyclyl-lower alkenylcarbonyl X, the heterocyclyl moiety is pl~:r~rdbly as defined
above, especially being selected from imidazolyl, such as 4-ill~ 7r~1yl, and from indolyl,
such as indole-3-yl, while the lower alkenyl radical preferably is linear and has one double
bond and 2 to 7 carbon atoms, preferably being a 1,2-vinyl radical; more preferably, hete-
rocyclyl-lower alkenylcarbonyl is selected from imidazolyl-lower alkenylcarbonyl, such as
imidazole-4-yl-lower alkenylcarbonyl, especially i~,lid~ole-4-yl-acryloyl, and indolyl-lower
alkenylcarbonyl, such as indole-3-ylacryloyl.
For X, arylcarbonyl; heterocyclylcarbonyl; aryl-lower alkylcarbonyl, heterocyclyl-lower
alkylcarbonyl with the proviso that in the lower alkyl radical mentioned a methylene group
may be replaced with oxa, aza or thia (the latter two being lesss preferred than oxa); and
heterocyclyl-lower alkenylcarbonyl are especially preferred, the respective substituents
being marked as preferred in the definitions given above being more prer~rled. In addition
or alternatively to this group of moieties, carbamoyl-lower alkanoyl, cycloalkyl-carbonyl and
aryl-lower alkylcarbonyl are especially preferred.
Lower alkanoyl X, which may be present only if Y is a secondary or tertiary amino group, is
especially acetyl, but may also be pivaloyl.
CA 02227~l6 l998-0l-2l
-16-
Halo-lower alkanoyl preferably has one to three halogen substituents, preferably selected
from chloro and fluoro, and is for example trifluoroacetyl or trichloroacetyl.
Lower-alkoxycarbonyl X, which may be present only if Y is a secondary or te~iary amino
aroup, is especially methoxycarbonyl, ethoxycarbonyl or tert-butoxvcarbonyl.
Aryl-lower alkoxycarbonyl X, which may be present only if Y is a secondary or tertiary amino
group, has an aryl moiety as defined above, especially phenyl or fluorenyl, and is especially
benzyloxycarbonyl or fluoren-9-ylmethoxycarbonyl.
Cycloalkyl-lower alkoxycarbonyl X, which may be present only if Y is a seccndary or tertiary
amino group, has a cycloalkyl moiety as defined above and is especially cyclohexyl-lower
alkoxycyrbonyl, such as cyclohexylmethyl-oxycarbonyl.
PTI is a bivalent radical of phosphotyrosine or a phosphotyrosine mimic.
A bivalent radical of phosphotyrosine is especially (D,L), (D)- or preferably (L)-4-(0-
Phosphono)-Tyr [= (O-PO3H2)Tyr] (bound N-terminally via the imino group resulting from the
a-amino group and C-terminally via the carbonyl group resulting from its a-carboxy group),
preferably the radical of the formula A
o~ ,OH
/ '
(A)
-HN CO-
A bivalent radical of a phosphotyrosine mimic PTI is defined as any radical that is able to
replace a phosphotyrosine radical which resembles, but is structurally different from the
respective phosphotyrosine radical and which cannot lose its phosphono-group too easily
CA 02227~16 1998-01-21
W O 97/08193 PCTrEP96/03473
respective phosphotyrosine radical and which cannot lose its phosphono-group too easily
due to hydrolysis. Preferably, such a mimic is selected from the respective bivalent radical
(which is bound N-terminally via the imino group resulting from the ~-amino group and C-
terminally via the carbonyl group resulting from its a-carboxy group) of an amino acid selec-
ted from phosphonomethyl-phenylalanine, especially 4-phosphonomethyl-phenylalanine,
phosphono-(a-fluoro)methyl-phenylalanine, especially 4-phosphono-(a-fluoro)methyl-phe-
nylalanine, phosphono-(a,a-difluoro)methyl-phenylalanine, especially 4-phosphono-(a,a-
difluoro)methyl-phenylalanine, phosphono-(a-hydroxy)methyl-phenylalanine, especially 4-
phosphono-(a-hydroxy)methyl-phenylalanine, O-sulfo-tyrosine, such as 4-(O-sulfo)tyrosine,
dicarboxymethoxy-phenylalanine (= (HOOC)2-CH2-O-phenylalanine), especially p-
(dicarboxymethoxy)-phenylalanine; (less preferably) phosphonophenylalanine, such as 4-
phosphonophenylalanine; aspartic acid, glutamic acid, phosphoserine and
phosphothreonine, each of which is presenL in the (D,L)-, D- or preferably the L-form.
For PTI, a bivalent radical of phosphotyrosine and especially a bivalent radical of phos-
phono-(a,a-difluoro)methyl-phenylalanine, especially 4-phosphono-(a,a-difluoro)methyl-
phenylalanine, or a bivalent radical of phosphonomethyl-phenylalanine, especially 4-
(phosphonomethyl)-phenylalanine, is most pl~rerled.
AA stands for a natural or unnatural amino acid, and is preferably a bivalent radical of an a-
or ,~-amino acid which is preferably bonded N-terminally by way of its a- or ,~-amino group
and C-terminally by way of its carboxy group and is preferably selected from the group
comprising a bivalent radical of a natural a- amino acid having the L-configuration, such as
those normally occurring in proteins, or an epimer of such an amino acid, that is to say
having the unnatural D-configuration, or a (D,L)-isomeric mixture thereof; or a homologue of
such an amino acid, for example a ~-amino acid or an a-amino acid wherein the amino acid
side chain has been shortened by one or two methylene groups or lengthened to up to 10
carbon atoms, such as an a-aminO alkanoic acid with 5 up to and including 10 carbon
atoms in a linear chain, an unsubstituted or substituted aromatic (a-aryl or a-aryl lower alkyl)
amino acid wherein the aryl radical has from 6 to 14 carbon atoms, for example ahomophenylalanine or a substituted phenylalanine or phenylglycine wherein the phenyl may
be mono- or poly-sllbstittlt~d by lower alkyl, for example methyl, lower alkoxy, for example
CA 02227~l6 l998-0l-2l
PCT~EP96/03473
W 097/08193
methoxy, lower alkanoyloxy, for example acetoxy, amino, lower alkylamino, for example
methylamino, di-lower aikylamino, for example dimethylamino, lower alkanoylamino, for
example acetylamino or pivaloylamino, lower alkoxycarbonylamino, for example tert-butoxy-
carbonylamino, arylmethoxycarbonylamino wherein aryl preferably has from 6 to 14 carbon
atoms, for example benzyloxycarbonylamino or 9-fluorenylmethoxycarbonylamino, halogen,
for example fluorine, chlorine, bromine or iodine, carboxy and/or by nitro, a benzo-fused
phenylalanine or phenylglycine, such as ~-naphthylalanine, and a hydrogenated phenyl-
alanine or phenylglycine, such as cyclohexylalanine or cyclohexylglycine, or an a-amino
heterocyclyl-lower alkanoic acid wherein heterocyclyl pre~erably is a single or double ring
system having from 3 to 10 ring atoms, is bonded via a carbon atom or via a nitrogen atom
and contains up to 3 further hetero atoms selected from oxygen, nitrogen, sulfur, and sulfur
linked to 1 or 2 oxygen atoms, and may be unsaturated or partially or fully saturated, for
example furyl, pyrrolyl, py"~lilinyl, morpholinyl, pyridyl or indolyl, a cyclic ~-amino-(a,a-
lower alkylene)-carbonic acid; or an a-amino-~(C6-C8)-bicyclo]-carbonic acid; each being
present in the L-, D- or (D,L)-con~iguration and in unprotected or amino-, carboxy- or
sulfhydryl-protected form.
Especially preferred is the bivalent radical, bonded via its a-amino and its a- or ,~-carbonyl
group, of an amino acid selected from glycine (H-Gly-OH), alanine (H-Ala-OH), ,~-alanine (H-
,~Ala-OH), valine (H-Val-OH), norvaline (a-aminovaleric acid), leucine (H-Leu-OH), iso-
leucine (H-lle-OH), norleucine (a-aminohexanoic acid, H-Nle-OH), a-amino-n-decanoic acid,
serine (H-Ser-OH), homoserine (a-amino-y-hydroxybutyric acid), threonine (H-Thr-OH),
methionine (H-Met-OH), cysteine (H-Cys-OH), S-acetylaminomethyl-cysteine (H-Cys(Acm)-
OH), proline (H-Pro-OH), trans-3- and trans-4-hydroxyproline, phenylalanine (H-Phe-OH),
tyrosine (H-Tyr-OH), 4-aminophenylalanine, 4-nitrophenylalanine, 4-chlorophenylalanine, 4-
carboxyphenylalanine, ,~-phenylserine (~-hydroxyphenylalanine), phenylglycine, a-
naphthylalanine (H-Nal-OH), cyclohexylalanine (H-Cha-OH), cyclohexylglycine, tryptophan
(H-Trp-OH), indoline-2-carboxylic acid, 1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid,
aspartic acid (H-Asp-OH), asparagine (H-Asn-OH), aminomalonic acid, aminomalonic acid
monoamide, glutamic acid (H-Glu-OH), glutamine (H-Gln-OH), histidine (H-His-OH), arginine
(H-Arg-OH), Iysine (H-Lys-OH), N~-benzyl-N'-methyl-lysine, N',N'-dibenzyl-lysine, ~-hydroxy-
lysine, ornithine (a,~-diaminovaleric acid), a-amino-cyclopentane carboxylic acid (H-Ac5c-
CA 02227~16 1998-01-21
W O 97/08193 PCT~P96/03473
OH), a-amino-cyclohexane carboxylic acid (H-Ac6c-OH), a-amino cycloheptane carboxylic
acid (H-Ac7c-OH), ~-(2-amino-2-norbornane)-carboxylic acid (H-Nbo-OH), a,y-
diaminobutyric acid and a"l3-diaminopropionic acid; or (alternatively or in addition to the
group of moieties mentioned just before) homophenylalanine (H-Hph-OH = 2-amino-4-
phenyl-butyric acid) and a-tert-butylglycine (H-Tle-OH = tert-leucine); it being pOSai''~ for
each of the mentioned amino acids (with the exception of glycine or any other amino acid
without asymmetric carbon atom) to be in the D-, L- or (D,L)-form, preferably in the L- or in
the D-form. H-DHph-OH is D-homophenylalanine.
More preferably, the bivalent radical -(AA)n- in formula I is an analogue of an SH2 domain
binding site of a protein with phosphotyrosine of a mammal, especially a human, for
example one of the binding sites mentioned in Songyang et al., Cell 72, 767-778 (1993),
e.g. (i) in the case of the Src family SH2 binding prolt i"s, especially an analogue of the
human CD3 ~ chain following Tyr 110 (that is, -Asn-Glu-Leu-Gln-Lys-Asp-Arg Mct Al~-Glu-
Ala-) or Tyr 122 (that is, -Ser-Glu-lle-Gly-Met-), an analogue of the human Rb-~soc~ d rb
110 chain following Tyr 321 (that is, -Glu-Glu-lle-Tyr-Leu-), an analogue of the human vav
oncogene chain following Tyr 126 (that is, -Glu-Asp-Leu-Met-Arg-), an analogue of the
human ErbB3 chain following Tyr 1270 (that is, -Glu-Glu-Met-Arg-Ala-), an analogue of the
human T cell CD7 chain following Tyr 222 (that is, -Glu-Asp-Met-Ser-His-) or an analogue of
the human G2 cyclin b chain following Tyr 255 (that is, -Glu-Asp-Met-Ser-His-), (ii) in the
case of the Abl SH2 binding proteins, especially an analogue of the human B cell CD19
chain following Tyr 409 (that is, -Glu-Glu-Pro-Asp-Ser-) or Tyr 439 (that is, -Glu-Asn-Pro-
Glu-Asp-), an analogue of the human B cell CD 72 chain following Tyr 39 (that is, -Glu-Asn-
Val-Gln-Val-), an analogue of the human colony-stimulating factor 1 receptor chain following
Tyr 923 (that is, -Thr-Asn-Leu-Pro-Ser-), an analogue of the human JunB chain following
Tyr 182 (that is, -Thr-Asn-Leu-Ser-Ser-), an analogue of the human protein kinase C ,6-1
chain following Tyr 662 (that is, -Thr-Asn-Pro-Glu-Phe-), (iii) in the case of Crk SH2 binding
proteins, especially an analogue of the human Fer tyrosine kinase chain following Tyr 615
(that is, -Asp-His-Pro-Asn-lle-), (iv) in the case of Nck SH2 binding proteins, especially an
analogue of the human cell cylce gene 1 protein chain following Tyr 139 (that is, -Asp-Glu-
Asp-Asp-Tyr-), (v) in the case of Sem-5/Grb2 SH2 binding proteins, especially an analogue
of the human EGF receptor chain following Tyr 1092 (that is, -lle-Asn-Gln-Ser-Val-), an
analogue of the human EGF receptor chain following Tyr 1138 (that is, -Leu-Asn-Thr-Val-
CA 02227~16 1998-01-21
WO 97/08193 PCTAEP96/03473
-20-
Gln-), an analogue of the human SHC chain following Tyr 317 (that is, -Val-Asn-Val-Gln-
Asn, an analogue of the human HGF receptor chain following Tyr 1374 (that is, -Val-Asn-
Val-Leu-Cys-), an analogue of the human ErbB2 chain following Tyr 1139 (that is, -Val-Asn-
Gln-Pro-Asp-), an analogue of the human ErbB3 chain following Tyr 1200 (that is, -Met-Asn-
Arg-Arg-Arg-) or Tyr 1262 (that is, -Met-Asn-Arg-Gln-Arg-), an analogue of the human IGF-1
receptor chain following Tyr 1125 (that is, -Leu-Asn-Ala-Asn-Lys-), an analogue of the
human Fit tyrosine kinase chain following Tyr 1213 (that is, -Val-Asn-Ala-Phe-Lys-), an
analogue of the human insulin receptor chain following Tyr 1149 (that is, -Leu-Asn-Ala-Lys-
Lys-), an ana-logue of the human CD45 PTPase chain following Tyr 706 (that is, -lle-Asn-
Ala-Ser-Tyr-), or Tyr 1015 (that is, -lle-Asn-Ala-Ser-Phe-), (vi) in the case of p85 N-terminal
SH2, especially an analogue of the human PDGF receptor ,~ chain following Tyr 740 (that is,
-Met-Asp-Met-Ser-Lys-Asp-Glu-Ser-Val-Asp-) or Tyr 751 (that is, -Val-Pro-Met-Leu-Asp-), an
analogue of the human c-K~t chain following Tyr 721 (that is, -Met-Asp-Met-Lys-Pro-), an
analogue of the human ErbB3 chain following Tyr 1257 (that is, -Ala-Ala-Met-Gly-Ala-Cys-
Pro-Ala-Ser-Glu-Gln-Gly-), Tyr 1270 (that is, -Glu-Glu-Met-Arg-Ala-), Tyr 1241 (that is, -Glu-
Met-Asn-Arg-Gln-), Tyr 1257 (that is, -Ala-Ala-Met-Gly-Ala-), Tyr 922 (that is, -Met-Val-Met-
Val-Lys-), Tyr 1035 tthat is, -Met-Pro-Met-Asn-Gln-), Tyr 1,178 (that is, -Glu-Tyr-Met-Asn-
Arg-), or Tyr 1203 (-Glu-Tyr-Met-Asp-Val-), (vii) in the case of p85 C-terminal SH2,
especially the same sites as for p85 N-ter",i"al SH2, (vii) in the case of PLC-y C-terminal
SH2, especially an analogue of the human PDGF receptor ,3 chain following Tyr 1021 (that
is, -lle-lle-Pro-Leu-Pro-), an analogue of the human PDGF receptor a chain following Tyr
1018 (that is, -lle-lle-Pro-Leu-Pro-), an analogue of the human ErbB2 chain following Tyr
1127 (that is, -Val-Ala-Pro-Leu-Thr-), (viii) in the case of PLC-y N-terminal SH2 binding
proteins, especially an analogue of the human basic FGF receptor 1, Il, ll, IV chain following
-Tyr 766 (that is, -Leu-Asp-Leu-X-X-), an analogue of the human EGF receptor chain
following Tyr 978 (that is, -Leu-Val-lle-Gln-Gly-) or Tyr 1197 (that is, -Leu-Arg-Val-Ala-Pro-),
an analogue of the human ErbB2 chain following Tyr 1248 (that is, -Leu-Gly-Leu-Asp-Val-),
and (ix) in the case of SHPTP2 N-terminal SH2 binding proteins, especially an analogue of
the human PDGF receptor ,~ chain following Tyr 1009 (that is, -Thr-Ala-Val-Gln-Pro-) or Tyr
1021 (that is, -lle-lle-Pro-Leu-Pro-), an analogue of the human PDGF receptor a chain
following Tyr 1018 (that is, -lle-lle-Pro-Leu-Pro-), Tyr 988 (that is, -lle-Gly-Val-Thr-Tyr-) or
Tyr 720 (that is, -Val-lle-Leu-Ser-Phe-), an analogue of the human ErbB3 chain following
Tyr 1159 (that is, -Val-Met-Pro-Asp-Thr-) or Tyr 471 (that is, -Val-lle-Val-Glu-Tyr-), an
CA 02227~16 1998-01-21
W O 97/08193 . PCT~EP96/03473
-21-
analogue of the human Fit receptor type tyrosine kinase chain following Tyr 1169 (that is, -
lle-Pro-lle-Asn-Ala-), an analogue of the human Kit receptor type tyrosine kinase chain
following Tyr 568 (that is, -Val-Tyr-lle-Asp-Pro-), an analogue of the human Ros ~ece~,lur
type tyrosine kinase chain foliowing Tyr 234 (that is, -lle-lle-Leu-Glu-Leu-), an analogue of
the human HGF receptor (Met) chain following Tyr 1367 (that is, -Val-His-Val-Asn-Ala-) or
Tyr 1374 (that is, -Val-Asn-Val-Leu-Cys-);
and (x) most especially an analogue of the Epidermal Growth Factor Receptor (EGFR)
sequence following Tyr 1068, the binding site for GrbZ SH2 (see Buday et al., Cell 73, 611 -
620 (1993)), that is the sequence lle-Asn-Gln-Ser-Val-Pro-Lys-Arg Pro-Ala-Gly-Ser-Val-Gln-
Asn, preferably -lle-Asn-Gln-Ser-; wherein in each case one or more amino acids are
replaced by analogues which still allow for binding to the respective SH2-co"".ri~i"g protein,
especially Grb2, or 1 or more amino acids are deleted, preferably 1 or more amino acids of
the sequence -lle-Asn-Gln-Ser-, more prt:r~rably 1, 2 or up to 3 amino acids of the
sequence -lle-Asn-Gln- being deleted. Preferably, the -Asn- in position 2 of the mentioned
sequence following Tyr 1068 in EGFR is present as such, while the amino acids in the other
positions may be replaced with one of the other amino acids mentioned above or (as far as
the C-terminal amio acid(s) following the Asn are concerned) may be deleted.
More preferably, -(AA)n- has one of the following meanings:
~ A bivalent radical of a tripeptide of the formula -(AA1)-(AA2)-(AA3)- wherein -(AA1)- is
preferably selected from -lle-, -Ac5c-, -AC6C-, -Asp-, -Gly- and -Phe- or (alternatively or in
addition to the group of moieties mentioned just before) from -AC7C-, -Nbo-, -Met-, -Pro-,
-,~Ala-, -Gln-, -Glu-, -DHph-, -HPh- and -tLe-, most preferably -lle-, -Acsc-, -AC6C-, -Asp-
or -Gly- or (alternatively or in addition to the group of moieties mentioned just before) -
Ac7c-, -Nbo- and -Gln-; -(AA2~- is pr~r~rably selected from -Asn-, and also from -,6Ala-
and -Gly-, or (alternatively or in addition to the group of moieties mentioned just before),
from -lle-, -,~Ala- and Gln; most preferably -Asn-; and -(AA3)- is preferably selected from
-Val-, and -~-Ala, and also from -Gly-, -Gln-, -Val-, -Asp- and -Acsc~; or, less preferably,
~ a bivalent radical of a dipeptide of the formula -(AA')-(AA2)- wherein -(AA1)- and -(AA2)-
preferably have the meanings given above, preferably -(AA')- being -lle- or -Ac6c-, or
(alternatively or in addition to the group of moieties mentioned just before) -Ac7c-, -Nbo-
and -Gln-; and -(AA2)- being -Asn- (preferred) or also -,~Ala-;
CA 02227~16 1998-01-21
W O 97/08193 PCT~EP96/03473
- 22 -
~ or (even less preferably) simply a bivalent radical of an amino acid selected from the
amino acids mentioned above, especially -lle- or (alternatively or in addition to the group
of moieties mentioned just before) -Ac6c-, -Nbo- and Acsc~.
A C-terminal protecting group Y is preferably an esterifying group, thus leading to an
esterified C-terminal carboxy group. More preferred is a lower alkoxy group that is
preferably branched in the 1 -position of the lower alkoxy group or sl Ih5tituted in the 1- or 2-
position of the lower alkoxy group by (one) suitable sl Ihstituent(s).
A lower alkoxy group that is branched in the 1-position of the lower alkoxy group is, for
example, tert-lower alkoxy, for example tert-butoxy.
A lower alkoxy group that is substituted in the 1- or 2-position of the lower alkoxy group by
(one) suitable sllhstitl lent(s) is, for ~xd,l,ple, arylmethoxy having one or two aryl radicals,
wherein aryl is preferably phenyl that is unsubstituted or mono-, di- or tri-substituted, for
example, by lower alkyl, for example tert-lower alkyl, such as tert-butyl, lower alkoxy, for
example methoxy, hydroxy, halogen, for example chlorine, and/or by nitro, for e,xdrllple
benzyloxy, benzyloxy substituted by the mentioned substituents, for example 4-nitro-
benzyloxy or 4-methoxybenzyloxy, diphenylmethoxy or diphenylmethoxy sl Ihstitl Ited by the
mentioned substituents, for example di(4-methoxyphenyl)methoxy; 1-lower alkoxy-lower
alkoxy, for example methoxymethoxy, 1-methoxyethoxy or 1-ethoxyethoxy, 1-lower alkyl-
thio-lower alkoxy, for example 1-methylthiomethoxyl or 1-ethylthioethoxy, aroylmethoxy
wherein the aroyl group is preferably benzoyl that is unsubstituted or sl Ihstituted~ for
example, by halogen, such as bromine, for example phenacyloxy, 2-halo-lower alkoxy, for
example 2,2,2-trichloroethoxy, 2-bromoethoxy or 2-iodoethoxy, as well as 2-(tri-substituted
silyl)-lower alkoxy wherein the substituents are each independently of the others selected
from lower alkyl, phenyl-lower alkyl, cycloalkyl or phenyl each of which is unsubstituted or
sl Ihstih ~ted as above, for example 2-tri-lower alkylsilyl-lower alkoxy, such as 2-tri-lower alkyl-
silylethoxy, for example 2-trimethylsilylethoxy or 2-(di-n-butyl-methyl-silyl)-ethoxy, or tri-
phenylsilylethoxy.
A C-terminal prul~olirlg Y group can furthermore be an organic silyloxy group.
CA~CELLE~ / ANNt 'L~
CA 02227~16 1998-01-21
- 22 + 23--
~ or (even less preferably) simply a bivalent radical of an amino acid selected from the
amino acids mentioned above, especially -lle- or (alternatively or in addition to the group
of moieties mentioned just before) -Ac6c-, -Nbo- and Acsc-.
A primar~, seconda ,~ or tertiarV amino group Y is preferably a free amino group! a mono- or
disubstituted amino group the substituents of which are preferably selected from the group
comprising lower alkyl, such as methyl, ethyl; isobutyl or 3-methylbutyl; octyl, such as 2-
ethyl-hexyl; aryloxy-lower alkyl, especially halonaphthyloxy-lower alkyl, such as 2-(1-bromo-
naphthalen-2-yloxy)-ethyl, or naphthyloxy-lower alkyl, such as 2-(naphthalen-2-yloxy or
naphthalen-1-yloxy)-ethyl; aryl-lower alkyl, such as phenyl-lower alkyl, e.g. benzyl, 3-
phenylpropyl, di-phenyl-lower alkyl, such as 2,2-diphenyl-ethyl or 3,3-diphenyl-propyl,
(mono- or di-halo-phenyl)-lower alkyl, such as 2-(4-chlorophenyl)ethyl or 3-(2,4-
dichlorophenyl)-propyl, naphthalenyl-lower alkyl, such as 3-naphthtalen-1-ylpropyl or 3-
naphthalen-2-ylpropyl, hydroxy-naphthalenyl-lower alkyl, such as 3-(2-hydroxy-naphthalen-
1-yl)-propyl, or phenanthrenyl-lower alkyl, such as 3-phenanthren-9-yl-propyl; heterocyclyl-
lower alkyl, such as pyrrolidinyl-lower alkyl, e.g. 2-(1-pyrrolidinyl)-ethyl, pyridyl-lower alkyl,
e.g. 2-(2-pyridyl)-ethyl, furyl-lower alkyl, e.g. 2-furylmethyl, morpholinyl-lower alkyl, e.g. 2-(4-
morpholinyl)-ethyl, and indolyl-lower alkyl, e.g. 2-(3-indolyl)-ethyl; cycloalkyl, such as
cyclohexyl; and cycloalkyl-lower alkyl, such as cyclohexylmethyl. A disubstituted amino
group may also be N-containing heterocyclyl bonded via its nitrogen atom, such as e.g. 1-
pyrrolidinyl or 4-morpholinyl.
In one preferred aspect of the invention, a primary, secondary or tertiary amino group Y is a
free amino group, a mono- or disubstituted amino group the substituents of which are pre-
ferably selected from the group comprising lower alkyl, e.g. methyl or ethyl, aryl-lower alkyl,
such as phenyl-lower alkyl, e.g. benzyl, or heterocyclyl-lower alkyl, such as pyrrolidinyl-lower
alkyl, e.g. 2-(1-pyrrolidinyl)-ethyl, pyridyl-lower alkyl, e.g. 2-(2-pyridyl)-ethyl, furyl-lower alkyl,
e.g. 2-furylmethyl, morpholinyl-lower alkyl, e.g. 2-(4-morpholinyl)-ethyl, and indolyl-lower
alkyl, e.g. 2-(3-indolyl)-ethyl. A disubstituted amino group may also be N-containing hetero-
cyclyl bonded via its nitrogen atom, such as e.g. 1-pyrrolidinyl or 4-morpholinyl.
A~ '~r ' ~ ~
-
CA 02227~16 1998-01-21
W O 97/08193 PCTAEP96/03473
- 24 -
Preferably, Y is a primary, secondary or tertiary amino group as defined above, most pre-
ferabiy amino (-NH2) or monosllhstihlted amino selected from lower alkylamino, such as
methylamino, ethylamino; isobutylamino or 3-methylbutylamino; octylamino, such as 2-ethyl-
hexyl-amino; aryloxy-lower alkylamino, especially halonaphthyloxy-lower alkylamino, such
as 2-(1-bromo-naphthalen-2-yloxy)-ethylamino, or naphthyloxy-lower alkylamino, such as 2-
(naphthalen-2-yloxy or naphthalen-1-yloxy)-ethylamino; aryl-lower alkylamino, such as
phenyl-lower alkylamino, e.g. benzylamino, 3-phenylpropylamino, di-phenyl-lower alkyl-
amino, such as 2,2-diphenyl-ethylamino or 3,3-diphenyl-propylamino, (mono- or di-halo-
phenyl)-lower alkylamino, such as 2-(4-chlorupllenyl)ethylamino or 3-(2,4-dichlorophenyl)-
propylamino, naphthalenyl-lower alkylamino, such as 3-naphthtalen-1-ylpropylamino or 3-
naphthalen-2-ylpropylamino, hydroxy-naphthalenyl-lower alkylamino, such as 3-(2-hydroxy-
naphthalen-1-yl)-propylamino, or phenanthrenyl-lower alkylamino, such as 3-phenanthren-
9-yl-propylamino; cycloalkylamino, such as cyclohexylamino; and cycloalkyl-loweralkylamino, such as cyclohexylmethylamino.
Salts of compounds of formula I are especi~lly acid addition salts, salts with bases or,
where several salt-forming groups are present, can also be mixed salts or internal salts.
Salts are especially pharmaceutically acceptable salts of compounds of formula 1.
Such salts are formed, for example, from compounds of formula I having an acid group, for
example a carboxy group, a sulfo group, or a phosphoryl group sl Ihstitl Ited by one or two
hydroxy groups, and are, for example, salts thereof with suitable bases, such as non-toxic
metal salts derived from metals of groups la, Ib, lla and llb of the Periodic Table of the
Elements, especially suitable alkali metal salts, for example lithium, sodium or potassium
salts, or alkaline earth metal salts, for example magnesium or calcium salts, also zinc salts
or ammonium salts, as well as salts formed with organic amines, such as unsubstituted or
hydroxy-substituted mono-, di- or tri-alkylamines, especially mono-, di- or tri-lower alkyl-
amines, or with quaternary ammonium compounds, for example with N-methyl-N-ethyl-
amine, diethylamine, triethylamine, mono-, bis- or tris-(2-hydroxy-lower alkyl)amines, such
as mono-, bis- or tris-(2-hydroxyethyl)amine, 2-hydroxy-tert-butylamine or tris(hydroxy-
methyl)methylamine, N,N-di-lower alkyl-N-(hydroxy-lower alkyl)-amines, such as N,N-
dimethyl-N-(2-hydroxyethyl)-amine or tri-(2-hydroxyethyl)-amine, or N-methyl-D-glucamine,
CA 02227~l6 l998-0l-2l
WO 97/08193 PCTAEP96/03473
-25-
-
or quaternary ammonium salts, such as tetrabutylammonium salts. The compounds offormula I having a basic group, for example an amino group, can form acid addition salts,
for example with inorganic acids, for example hydrohalic acids, such as hydrochloric acid,
sulfuric acid or phosphoric acid, or with organic carboxylic, sulfonic, sulfo or phospho acids
or N-s~ Ihstitl Ited sulfamic acids, for example acetic acid, propionic acid, glycolic acid,
succinic acid, maleic acid, hydroxymaleic acid, methylmaleic acid, fumaric acid, malic acid,
tartaric acid, gluconic acid, glucaric acid, glucuronic acid, citric acid, ben~oic acid, cinnalllic
acid, mandelic acid, salicylic acid, 4-aminosalicylic acid, 2-phenoxybenzoic acid, 2-
acetoxybenzoic acid, embonic acid, nicotinic acid or isonicc Li"-~ acid, as well as with amino
acids, for example the a-amino acids mentioned hereinbefore, especially glutamic acid and
aspartic acid, and with methanesulfonic acid, ethanesulfonic acid, 2-hydroxyethanesulfonic
acid, ethane-1,2-disulfonic acid, benzenesulfonic acid, 4-methylbenzene-sulfonic acid,
naphthalene-2-sulfonic acid, 2- or 3-phosphoglycerate, glucose-6-phosphate, N-cyclohexyl-
sulfamic acid (forming cyclamates) or with other acidic organic compounds, such as
ascorbic acid. Compounds of formula I having acid and basic groups can also form internal
salts.
For isolation or purification purposes, it is also possible to use pharmaceutically inaccept-
able salts, for example a perchlorate or picolinate salt.
The compounds of the invention have useful, in particular pharmacologically useful, proper-
ties. Surprisingiy, it has been found that the compounds of formula I are able to inhibit the
interaction between SH2 domains of downstream regulatory proteins, such as Grb2, and
phosphoproteins, especially phosphorylated protein tyrosine kinases, such as a phospho-
protein containing a -Tyr(PO3H2)-X-Asn- motif, preferably phosphorylated EGFR protein ty-
rosine kinase (EGFR = Epidermal Growth Factor Receptor) or modified derivatives thereof,
but in a broader sense also other phospho-proteins such as SHC or modified derivatives
thereof, in a favourable way. They are thus useful in the treatment or prevention of diseases
that respond to such inhibition.
The ability to inhibit the interaction between the SH2 domain of Grb2 and phosphorylated
EGFR can be shown by the following type of assay: The principle of the assay is that a full-
length or truncated phosphotyrosine protein immobilized on a solid phase is incubated with
a chimeric SH2-GST (GST = glutathione S-transferase) protein or a full length SH2-contai-
CA 02227~16 1998-01-21
W O 97/08193 PCTAEP96/03473
ning protein-GST fusion protein capable of binding to it, in the presence of a test substance ~,
(for review, see Proc. Natl. Acad. Sci. USA 91, 83-87 (1994)). For example, the following
procedure is used for the screening of inhibitors with regard to the interaction between Grb2
- for exampie, full-length (sequence: see Lowenstein et al., Cell 70, 431-42 (1992)) or SHZ
alone - and phosphorylated EGFR ffull-length cytoplasmic tyrosine kinase or a fusion pro-
duct obtained from Maltose Binding Protein and the carboxy-terminal part of the EGF recep-
tor ("tail" EGFR-MBP fusion protein)) (for EGFR sequence, see Nature 309, 418-425 (1984);
for purified recombinant intr~cell~ r domain (ICD), see Eur. J. Biochem. 207, 265-75
(1992); the EGFR-MBP fusion protein is made as follows: Oligonucleotides flanking the en-
tire carboxy-terminal half (nucleotides 3112 to 3816) of the EGFR and containing enginee-
red EcoRI-Hindlll restriction sites are used to amplify the appropriate DNA fragment by
PCR. The amplified DNA fragment is recombined with purified EcoRI-Hindlll-digested
pMALc2 vector (New England riol~hs~ Inc., Beverly, USA) do~ Lrean~ from and in the
same reading frame as the malE gene, which encodes maltose-binding protein (MBP). The
vector containing the fused gene is transformed in E. Coli, and the fusion protein is expres-
sed from the PtaC promoter. A crude cell extract is prepared and passed over a column of
amylose resin. The fusion protein is then eluted with neutral buffer, containing maltose. Ali-
quots are frozen in liquid nitrogen and stored at -70 ~C).
Wells of polystyrene microtiter plates (e.g. Nunc-lmmuno Plate MaxiSorpTM) are coated
overnight at 4 ~C in incubation buffer (20 mM Tris pH 7.5) with phosphorylated EGFR or
"tail" EGFR-MBP fusion protein (phosphorylation conditions: 0.5 mg/ml of purified
recombinant EGFR-ICD, or EGFR-MBP (+0.03mg/ml EGFR-ICD) is phosphorylated by theaddition of 10 mM MnCi2, 10 mM MgC12, 40 ~lM ATP in 20 mM Tris buffer pH 7.5 for 45 min).
with a working solution containing 0.5 mg/ml of phosphorylated EGFR or MBP-EGFR. 11
ng/ml Grb2-SH2-GST [obtainable, e.g., from Santa Cruz Biotech, California, USA, or as
follows: a cDNA clone encoding human Grb2 SH2 domain (e.g., aa 45-164) is amplified by
polymerase chain reaction (PCR), using nucleotides with appropriate linkers, e.g. with
BamH1 (5').3' EcoRI linkers; the purified (e.g. BamH1-EcoRI) fragments from PCR products
are then subcloned in-frame into the appropriate (e.g. BamH1-EcoRI) restriction sites of an
apprupli~L~ vector (e.g. pGEX-3X, Pharmacia, Uppsala, Sweden) - comparable results are
also obtained when using full length Grb2, see also Embo ~1. 13, 4011-21 (1994)] are then
added to coated phosphorylated EGFR or "tail" EGFR-MBP fusion protein for 2 h at room
temperature in the absence or presence of test substance of formula I dissolved in buffer +
dimethyl sulfoxide (maximally 25%). Bound SH2 is detected with polyclonal rabbit anti-GST
CA 02227~16 1998-01-21
WO 97/08193 PCTAEP96/03473
- 27 -
antibody (obtained by immunisation of rabbits with glutathione-S-transferase) for 1 h at
room temperature. Bound antibody is then detected after the addition of peroxydase-
conjugated goat-antirabbit IgG (EIA Grade Affinity purified Goat Anti-Rabbit IgG (H+L) -
Horseradish Peroxydase Conjugate from Bio-Rad, Hercules, USA) and incubation for 1 h at
room temperature. Peroxydase activity is then monitored at 655 nm on a plate reader by the
addition of a TMB substrate (Single Component TMB Peroxidase EIA Substrate Kit from
Bio-Rad, Hercules, USA; TMB = 3,3',5,5'-tetramethylbenzidine solution).
With this type of assay, IC50 values (= the concentrations where half-maximal inhibition of
the interaction is found) in the range of 104 to 1 o-10 M, preferably 10-5 to 1 o~l~ M, more
preferably in the range of 10 -6 to 10 ~'~ M) are found with test compounds of formula 1.
This type of assays is not limited to the EGF receptor - it can also be used analogously with
erb-B2 or other protein tyrosine kinases. Furthermore, it is possible to use other SH2
domains instead of Grb2 SH2. For example, the interactions of the SH2-comprising pruL~,i"s
and phosphotyrosine comprising proteins mentioned above in the definition of a bivalent
radical -(AA)n- in formula I as an analogue of an SH2 domain binding site of a protein with
phosphotyrosine of a mammal can be tested.
In addition, it can be shown by methods known in the art that injection of a compound of
formula I into mammalian cells leads to a stop of cell growth (the test can be made in
analogy to the well-known method of microinjection of antibodies into mammalian cells in
which it has been shown that microinjection of antibodies against Grb2 blocks the induction
of S phase entry in response to EGF and PDGF, see EMBO J. 12, 3467-73 (1993); or to the
well-known methods for cell perme~h~ tion where a) a phosphorylated peptide
encompassing Tyr 716 (an autophosphorylation site) in the PDGFR kinase insert has been
shown to significantly inhibit the activation of Ras by PDGF in permeabilized PAE cells as
well as fibroblasts (see Mol. and Cell. Biol., 6715-26 (1994)), or b) the effect of various
phosphopeptides on the activation of guanine nucleotide exchange on Ras by EGF and
NGF has been studied in permeabilised 6-24 cells, the EGFR-Tyr 1068 phosphopeptide
corresponding to an autophosphorylation site for Grb2 on the EGFR showing inhibition of
EGF and NGF stimulation of Ras by 96% and 98%, respectively, at 50 ~M concentration
(Oncogene 9(12), 3483-3491 (1994)) .
CA 02227~16 1998-01-21
W O 97/08193 PCTIEP96/03473
- 28 -
The compounds of the invention, due to their ability to uncouple a phosphorylated protein,
especially a protein tyrosine kinase, e.g. EGF receptor, from a respective SH2 containing
protein, e.g. the SH2-containing Grb2, are able to inhibit subsequent cellular signal trans-
duction pathways important for diseases such as viral, inflammatory, allergic, autoimmune,
cardiovascular and especially ptc,lir~r~Live diseases, such as for malignant hyperprolifera-
tive diseases, e.g. tumor diseases, preferably breast cancer, chronic myelogenous leukemia
(eML), thyroid carcinoma and osteosarcoma, or for hyperproliferation of epithelial cells, e.g.
psoriasis, are appropriate for the treatment and prophylaxis of said diseases.
Therefore, the compounds of the present invention are useful for the treatment of diseases
that respond to inhibition of the interaction of (a) protein(s) comprising (an) SH2 domain(s)
and a phosphoprotein, preferably a protein tyrosine kinase or a modified version thereof,
more preferably of Grb2 SH2 with EGFR or modified derivatives thereof. The term "modified
version" or "modified derivative" means mainly a derivative that is causative or active in the
establishment of diseases, e.g. truncated versions, virus derived analogues, etc.
The treatment can also, e.g. in the case of hematopoietic cell proliferative disorders, such
as leukemias, be used in conjunction with autologous bone marrow transplantation and
chemotherapy techniques. For example, an aliquot of bone marrow cells (even one cell or
some single cells, which can be treated by microinjection of a compound of formula I as
described above) are obtained from a patient, e.g. from the pelvis. The cells are then
cultured in the presence of a compound of formula I (which may also be applied by
microinjection) which is able to disrupt the protein tyrosine kinase/SH2-comprising protein-
interaction. Thus blocking the signal transduction pathway of those bone marrow cells
capable of forming complexes resulting from such interaction, it is possible to select against
the presence of clonal daughter cells derived from these cells and to purge the culture(s) of
these cells from those responsible for the hematopoietic cell proliferative disorder being
treated. After chemotherapeutic and/or radiotherapeutic treatment of the patient from whom
the cells have been obtained, the patient can receive an autologous infusion of cultured
bone marrow cells resulting from the above purging procedure.
Due to their high binding affinity to SH2 domains, such as those of Grb2, the compounds of
formula I can also be bound covalently to chromatographic materials, thus making it
CA 02227~16 1998-01-21
WO 97/08193 PCTAEP96/03473
-29-
possible to produce chromatographic materials for the affinity purification of natural or
recombinant SH2-domains or SH2-comprising proteins from the cells of living organisms.
For example, a compound of formula I with an appropriate free functional group (e.g. -NH2,
-SH, -OH and/or -COOH) can be attached covalently to activated or activatable matrices
appropriate for chromatography, e.g. cyanogen bromide activated matrices, epoxy-activated
matrices, nitrophenyl chloroformate and N-hydroxysuccinimde chloroformate.
polyacrylhydrazido agarose, oxirane acrylic beads, bromoacetyl-cellulose, epichlorohydrin-
activated matrices, tresyl-chloride-activated agarose, vinylsulfone-activated agarose, and
the like. P~:r~r,~d activated or activatable coupling gels for affinity chromatography include
but are not limited to
a) for coupling of compounds of formula I with an -NH2 group e"~ ,rcd for binding:
cyanogen bromide activated Sepharose 4B; ECH Sepharose 4B (carbodiimide couplingmethod used most often in analogy to process for preparation of compounds of formula I as
described below); or activated CH Sepharose 4B;
b) for coupling of compounds of formula I with an -NH2 and/or an -SH group: Tresyl-
activated Sepharose 4B;
c) for coupling of compounds of formula I with an -NH2,-OH and/or -SH group: epoxy-
activated Sepharose 6B; and
d) for coupling of compounds of formula I with a -COOH group: EAH Sepharose 4B
(carbodiimide method for coupling most often used in analogy to process for preparation of
compounds of formula I as described below).
Sepharose stands for agarose derived chromatographic materials and is a trademark from
Pharmacia, Uppsala, Sweden, from where the mentioned gels are available.
In the following definitions of preferred compounds, general terms may be rep'~~ed by their
more specific (more preferred) definitions as given above in order to obtain more preferred
compounds.
One preferred embodiment of the invention relates to a compound of formula 1, wherein
n is 1 to 15,
X is arylcarbonyl, cycloalkylcarbonyl, arylsulfonyl, heterocyclylcarbonyl, heterocyclylsulfonyl;
aryl-lower alkylcarbonyl, cycloalkyl-lower alkylcarbonyl, aryl-lower alkylsulfonyl, heterocyclyl-
lower alkylcarbonyl, heterocyclyl-lower alkylsulfonyl with the proviso that in any of the lower
CA 02227~16 1998-01-21
- 30 -
alkyl radicals mentioned a methylene group may be replaced with oxa, aza or thia; or
heterocyclyl-lower alkenylcarbonyl,
PTI is the bivalent radical of phosphotyrosine or a phosphotyrosine mimic,
AA stands for a bivalent radical of a natural or unnatural amino acid, and
Y is a primary, secondary or tertiary amino group,
or a salt thereof if at least one salt-forming group is present.
Preferred is a compound of formula I wherein
n is 1 to 4, more preferably 1 to 3, most preferably 2 or especially 3;
X is selected from
(i) benzoyl or, even more preferably, from benzoyl substituted with amino; loweralkylamino; amino-lower alkyl; hydroxy; lower alkoxy; amino and hydroxy; amino and lower
alkoxy; carboxy; lower-alkoxycarbonyl; cyano; halogen, especially chloro; lower-alkylthio; cr
lower alkylsulfinyl; especially from 4-aminobenzoyl, 3-aminobenzoyl, 2-aminobenzoyl, .-
lower alkylamino-benzoyl, such as 4-methylamino-benzoyl, 4-(amino-lower alkyl)-benzoyl,
such as 4-(methylamino)-benzoyl, 4-hydroxy-benzoyl, 4-lower alkoxy-, such as 4-
methoxybenzoyl, 4-amino-2-hydroxy-benzoyl, 4-amino-3-lower alkoxy-benzoyl, such as 4-
amino-3-methoxy-benzoyl, 4-carboxybenzoyl, 4-lower alkoxycarbonyl-benzoyl, such as 4-
methoxycarbonyl-benzoyl, 4-cyanobenzoyl, 4-lower alkylthio-benzoyl, such as 4-
methylthiobenzoyl, 4-lower alkylsulfinyl-benzoyl, such as 4-lower methylsulfinyl-benzoyl; and
most preferably from 3- or especially 4-aminobenzoyl;
(ii) naphthoyl or hydroxy-naphthoyl, such as naphthalene-2-yl-carbonyl or especially 6-
hydroxy-naphthalene-2-yl-carbonyl, or (less preferably) fluorenylcarbonyl, such as fluoren-9-
ylcarbonyl;
(iii) cyclohexylcarbonyl or especially 1,2,3,4-tetrahydronaphthyl-2-carbonyl
(iv) tricyclo[5.2.1.025ldec-8-ylcarbonyl or especially adamantoyl, preferably 1-adamantoyl
...(v) pyridylcarbonyl which is unsubstituted or substituted with amino, such as pyridin-4-yl-
or pyridin-3-ylcarbonyl, or amino-pyridin-3-yl-carbonyl, such as 2- or 6-amino-pyridin-3-
-
CA 02227~16 1998-01-21
W O 97/08193 PCT/EP96/03473
ylcarbonyl, bell~i",.~ lylcarbonyl, such as ben~ ,ida~ol-5-ylcarbonyl, quinolinyl-carbonyl,
such as quinoline-2-, quinoline-3- or quinoline-6-ylcarbonyl, 2,3-dihydrobenzofuranylcar-
bonyl, such as 2,3-dihydrobenzofuran-5-ylcarbonyl, or indolylcarbonyl, such as indole-5-yl-,
indole-3-yl- or indole-2-yl-carbonyl; most preferably from quinoline-6-ylcarbonyl and
especially from indolylcarbonyl, such as indole-3- and indole-5-ylcarbonyl;
(vi) chromenylcarbonyl, such as 2H-chromen-3-ylcarbonyl, that is unsubstitllted by 1 to 3
sllhstitlltents selected from oxo and hydroxy, especially 7,8-dihydroxy-2-oxo-2H-
(benzopyran) -3-yl-carbonyl;
(vii) carbamoyl-lower alkanoyl, wherein lower alkanoyl is especially propionyl, especially 3-
carbamoylpropionyl;
(vii) phenyl-methoxycarbonyl wherein the phenyl residue is unsl Ih5tjh Ited or substituted
with amino in the (in growing p, er~rence) 2-, 3- and 5-, 4- or 3-position of the phenyl ring;
(+)-, (+) or (-)-1-(3-aminophenyl)ethyloxycarbonyl; hydroxybenzyloxy- or hydroxyphenyl-2-
ethoxycarbonyl, such as 3-hydroxybenzyloxycarbonyl or 3-hydroxyphenyl-2-ethoxy-
carbonyl; (with less pre~rence) lower alkanoylamino-phenyloxymethylcarbonyl, such as 2-,
3- or especially 4-acetylaminophenyloxymethylcarbonyl, aminophenyloxymethylcarbonyl,
such as 3- or 4-aminophenyloxycarbonyl; aminophenyl-lower alkylcarbonyl, such as 4-
aminophenyl-acetyl or 3-(3-aminophenyl)-propionyl; or (with still less preference) benzyl-
oxycarbonyl or phenylacetyl;
(viii) dihydroxyphenyl-lower alkylcarbonyl, especially 3-(3,4-dihydroxyphenyl)-propionyl or
2-(3,4-dihydroxyphenyl)-acetyl;
(ix) cyclohexyl-lower alkylcarbonyl, especially 3-(cyclohexyl)-propionyl;
(x) unsubstituted or lower alkyl-substituted 2-(thi~olyl)-ethoxycarbonyl, such as 2-(4-
methyl-thiazol-s-yl)ethoxycarbonyl, unsl Ihstitl It~d or amino-sl Ihstituted thi~olyl-lower
alkylcarl,onyl, such as 2-amino-thi~ole-5-ylacetyl, or especially indolyl-lower alkylcarbonyl,
such as indole-3-yl-acetyl, 3-(indole-3-yl)propionyl or 4-(indole-3-yl)butyroyl;(xi) imid~olyl-lower alkenylcarbonyl, such as i"lida~ole-4-yl-lower alkenylcarbonyl,
especially inlid~ole-4-yl-acryloyl, or indolyl-lower alkenylcarbonyl, such as indole-3-yl-
acryloyl;
(xii) cinnamoyl substituted by 1 to 2 moieties selected independently from methoxy and
especially hydroxy, especially p-hydroxycinnamoyl, m-hydroxy-p-methoxy-cinnamoyl or
preferably m,p-dihydroxy-cinnamoyl; and
- (xiii) if Y is secondary or tertiary amino, also from lower alkanoyl, especially acetyl,
CA 02227~16 1998-01-21
WO 97/08193 PCTAEP96/03473
-32-
PTI is a bivalent radical of tyrosine or (preferably) a bivalent radical of phosphotyrosine (in
the D-, L- or less preferably the (D,L)-form) or of a phosphotyrosine mimic in the form of a
bivalent radical (which is bound N-terminally via the imino group resulting from the a-amino
group and C-terminally via the carbonyl group resulting from its a-carboxy group) of an
amino acid selected from phosphonomethyl-phenylalanine, especially 4-phosphono-methyl-
phenylalanine, phosphono-(a-fluoro) methyl-phenylalanine, especially 4-phosphono-(a-
fluoro)methyl-phenylalanine, phosphono-(a,a-difluoro)methyl-phenylalanine, e.speci~lly 4-
phosphono-(a,a-difluoro)methyl-phenylalanine, phosphono-(a-hydroxy)methyl-phenyl-
alanine, especially 4-phosphono-(a-hydroxy)methyl-phenylalanine, O-sulfo-tyrosine, such
as 4-(0-sulfo)tyrosine, dicarboxymethoxy-phenylalanine (= (HOOC)2-CH2-O-phenylalanine,
especially 4-(dicarboxymethoxy)-phenylalanine aspartic acid, glutamic acid, phosphoserine
and phosphothreonine, each of which is present in the (D,L)-, D- or preferably the L-form;
-(AA)n- has one of the following meanings:
~ A bivalent radical of a tripeptide of the formula -(AA1)-(AA2)-(AA3)- wherein -(AAl)- is
preferably selected from -lle-, -AC5C-, -Ac6c-, -Asp-, -Gly- and -Phe- or (alternatively or in
addition to the group of moieties mentioned just before) from -Ac7c-, -Nbo-, -Met-, -Pro-,
-,I~Ala-, -Gln-, -Glu-, -DHph-, -HPh- and -tLe-, most preferably -lle-, -Ac5c-, -Ac6c-, -Asp-
or -Gly- or (alternatively or in addition to the group of moieties mentioned just before) -
Ac7c-, -Nbo- and -Gln-; -(AA2)- is pl~r~rdbly selected from -Asn-, and also from -~Ala-
and -Gly-, or (alternatively or in addition to the group of moieties mentioned just before),
from -lle-, -~Ala- and Gln; most preferably -Asn-; and -(AA3)- is preferably selected from
-Val-, and -~-Ala, and also from -Gly-, -Gln-, -Val-, -Asp- and -Acsc-; or, less preferably,
~ a bivalent radical of a dipeptide of the formula -(AA1)-(AA2)- wherein -(AAl)- and -(AA3-
preferably have the meanings given above, pr~r~rdbly -(AA1)- being -lle- or -Ac6c-, or
(alternatively or in addition to the group of moieties mentioned just before) -Ac7c-, -Nbo-
and -Gln-; and -(AA2)- being -Asn- (preferred) or also -,~Ala-;
~ or (even less preferably) simply a bivalent radical of an amino acid selected from the
amino acids mentioned above, especially -lle- or (alternatively or in addition to the group
of moieties mentioned just before) -Ac6c-, -Nbo- and Ac5c-;
CA 02227~16 1998-01-21
W O 97/08193 PCTAEP96/03473
- 33 -
and
Y is amino (-NH2) or monosllhstitllted amino selected from lower alkylamino, such as
methylamino, ethylamino; isobutylamino or 3-methylbutylamino; octylamino, such as 2-ethyl-
hexyl-amino; aryloxy-lower alkylamino, especially halonaphthyloxy-lower alkylamino, such
as 2-(1-bromo-naphthalen-2-yloxy)-ethylamino, or naphthyloxy-lower alkylamino, such as 2-
(naphthalen-2-yloxy or naphthalen-1-yloxy)-ethylamino; aryl-lower alkylamino, such as
phenyl-lower alkylamino, e.g. benzylamino, 3-phenylpropylamino, di-phenyl-lower
alkylamino, such as 2,2-diphenyl-ethylamino or 3,3-diphenyl-propylamino, (mono- or di-halo-
phenyl)-lower alkylamino, such as 2-(4-chlorophenyl)ethylamino or 3-(2,4-dichlorophenyl)-
propylamino, naphthalenyl-lower alkylamino, such as 3-naphthtalen-1-ylpropylamino or 3-
naphthalen-2-ylpropylamino, hydroxy-naphthalenyl-lower alkylamino, such as 3-(2-hydroxy-
naphthalen-1-yl)-propylamino, or phenanthrenyl-lower alkylamino, such as 3-phenanthren-
9-yl-propylamino; cycloalkylamino, such as cyclohexylamino; and cycloalkyl-loweralkylamino, such as cyclohexylmethylamino;
or a salt thereof where at least one salt-forming group is p, ~senL.
r~ r-ed is also a compound of formula I wherein
n is 1 to 4, more preferably 1 to 3, most preferably 2 or especially 3;
X is selected from
(i) benzoyl or, even more preferably, from benzoyl substituted with amino; loweralkylamino; amino-lower alkyl; hydroxy; lower alkoxy; amino and hydroxy; amino and lower
alkoxy; carboxy; lower-alkoxycarbonyl; cyanc; halogen, especially chloro; lower-alkylthio; or
lower alkylsulfinyl; especially from 4-aminobenzoyl, 3-aminobenzoyl, 2-aminobenzoyl, 4-
lower alkylamino-benzoyl, such as 4-methylamino-benzoyl, 4-(amino-lower alkyl)-benzoyl,
such as 4-(methylamino)-benzoyl, 4-hydroxy-benzoyl, 4-lower alkoxy-, such as 4-
methoxybenzoyl, 4-amino-2-hydroxy-benzoyl, 4-amino-3-lower alkoxy-benzoyl, such as 4-
amino-3-methoxy-benzoyl, 4-carboxybenzoyl, 4-lower alkoxycarbonyl-benzoyl, such as 4-
methoxycarbonyl-benzoyl, 4-cyanobenzoyl, 4-lower alkylthio-benzoyl, such as 4-
methylthiobenzoyl, 4-lower alkylsulfinyl-benzoyl, such as 4-lower methylsulfinyl-benzoyl; and
most preferably from 3- or especially 4-aminobenzoyl;
(ii) pyridylcarbonyl which is unsubstituted or sl Ihstituted with amino, such as pyridin-4-yl-
or pyridin-3-ylcarbonyl, or amino-pyridin-3-yl-carbonyl, such as 2- or 6-amino-pyridin-3-
CA 02227~l6 l99X-0l-2l
W O 97/08193 PCT/EP96/03473
-34-
ylcarbonyl, benzimidazolylcarbonyl, such as benzimidazol-5-ylcarbonyl, quinolinyl-carbonyl,
such as quinoline-2-, quinoline-3- or quinoline-6-ylcarbonyl, 2,3-dihydrobenzofuranyl-
carbonyl, such as 2,3-dihydrobenzofuran-5-ylcarbonyl, or indolylcarbonyl, such as indole-5-
yl-, indole-3-yl- or indole-2-yl-carbonyl; most preferably from quinoline-6-ylcarbonyl and
especially from indolylcarbonyl, such as indole-3- and indole-5-ylcarbonyl;
(iii) phenyl-methoxycarbonyl wherein the phenyl residue is unsl Ihstitl Itf~d or suhstih ~ted
with amino in the (in growing preference) 2-, 3- and 5-, 4- or 3-position of the phenyl ring;
(+)-, (+) or (-)-1-(3-aminophenyl)ethyloxycarbonyl; hydroxybenzyloxy- or hydroxyphenyl-2-
ethoxycarbonyi, such as 3-hydroxybenzyloxycarbonyl or 3-hydroxyphenyl-2-ethoxy-
carbonyl; (with less preference) lower alkanoylamino-phenyloxymethylcarbonyl, such as 2-,
3- or especially 4-acetylaminophenyloxymethylcarbonyl, aminophenyloxymethylcarbonyl,
such as 3- or 4-aminophenyloxycarbonyl; aminophenyl-lower alkylcarbonyl, such as 4-
aminophenyl-acetyl or 3-(3-aminophenyl)-propionyl; and (with still less p.t~r~rence) benzyl-
oxycarbonyl or phenylacetyl;
(iv) unsubstituted or lower alkyl-sl ~hstituted 2-(thi~olyl)-ethoxycarbonyl, such as 2-(4-
methyl-thiazol-5-yl)ethoxycarbonyl, uncllhstitllt~d or amino-suhstitllt~d thi~olyl-lower
alkylcarbonyl, such as 2-amino-thi~ole-5-ylacetyl, or especially indolyl-lower alkylcarbonyl,
such as indole-3-yl-acetyl, 3-(indole-3-yl)propionyl or 4-(indole-3-yl)butyroyl; and
(v) imidazolyl-lower alkenylcarbonyl, such as imidazole-4-yl-lower alkenylcarbonyl,
especially i--,id-~ol~ 1-yl-acryloyl, or indolyl-lower alkenylcarbonyl, such as indole-3-yl-
acryloyl;
PTI is a bivalent radical of phosphotyrosine (in the D-, L- or less pr~r~.~bly the (D,L)-form)
or of a phosphotyrosine mimic in the form of a bivalent radical (which is bound N-terminally
via the imino group resulting from the a-amino group and C-terminally via the carbonyl
group resulting from its c~-carboxy group) of an amino acid selected from phosphonomethyl-
phenylalanine, especially 4-phosphono-methyl-phenylalanine, phosphono-(a-fluoro)methyl-
phenylalanine, especially 4-phosphono-(a-fluoro)methyl-phenylalanine, phosphono-(a,a-
difluoro)methyl-phenylalanine, especially 4-phosphono-(a,a-difluoro)methyl-phenylalanine,
phosphono-(a-hydroxy)methyl-phenylalanine, especially 4-phosphono-(a-hydroxy)methyl-
phenylalanine, O-sulfo-tyrosine, such as 4-(0-sulfo)tyrosine, dicarboxymethoxy-
phenylalanine (= (HOOC)2-CH2-O-phenylalanine, especially 4-(dicarboxymethoxy)-
CA 02227~16 1998-01-21
WO 97/08193 PCT/EP96/03473
phenylalanine aspartic acid, glutamic acid, phosphoserine and phosphothreonine, each of
which is present in the (D,L)-, D- or preferably the L-form;
r
-(AA)n- has one of the following meanings:
~ A bivalent radical of a tripeptide of the formula -(AAl)-(AA2)-(AA3)- wherein -(AA1)- is pre-
ferably selected from -lle-, -Ac5c-, -Ac6c-, -Asp-, -Gly- and -Phe-, most preferably -lle-,
-Ac5c-, -Ac6c-, -Asp- or -Gly-; -(AA2)- is preferably selected from -Asn-, and also from -
,~Ala- and -Gly-, most preferably -Asn-; and -(AA3)- is preferably selected from -Val-, and
-,3-Ala, and also from -Gly-, -Gln-, -Val-, -Asp- and -Ac5c-;
~ a bivalent radical of a dipeptide of the formula -(AAl)-(AA2)- wherein -(AAl)- and -(AA2)-
preferably have the meanings given above, preferably -(AAl)- being -lle- or -Ac6c- and
-(AA2)- being -Asn- (pl~rt:r.ed) or also -~Ala-;
~ or (less preferably) simply a bivaient radical of an amino acid selected from the amino
acids mentioned above, especially -lle-;
and
Y is a free amino group (prer~ d), a mono- or disubstituted amino group the sl Ihstitl lents
of which are preferably selected from the group comprising lower alkyl, e.g. methyl or ethyl,
phenyl-lower alkyl, e.g. benzyl, pyrrolidinyl-lower alkyl, e.g. 2-(1-pyrrolidinyl)-ethyl, pyridyl-
lower alkyl, e.g. 2-(2-pyridyl)-ethyl, furyl-lower alkyl, e.g. 2-furylmethyl, morpholinyl-lower
alkyl, e.g. 2-(4-morpholinyl)-ethyl, and indolyl-lower alkyl, e.g. 2-(3-indolyl)-ethyl; or is 1-
pyrrolidinyl or 4-, - lor~c holinyl,
or a salt thereof.
rl~re--ed is also a compound of formula I wherein
n is 1 to 4, more prer~rdbly 1 to 3, most preferably 2 or especially 3;
X is selected from
(i) naphthoyl or hydroxy-naphthoyl, such as naphthalene-2-yl-carbonyl or especially 6-
hydroxy-naphthalene-2-yl-carbonyl, or (less preferably) fluorenylcarbonyl, such as fluoren-9-
ylcarbonyl;
CA 02227~l6 l998-0l-2l
WO 97/08193 PCTAEP96/03473
-36-
(ii) cyclohexylcarbonyl or especially 1,2,3,4-tetrahydronaphthyl-2-carbonyl
(iii) tricyclo[5.2.1.0~6]dec-8-ylcarbonyl or especially adamantoyl, preferably 1-adamantoyl
(iv) chromenylcarbonyl, such as 2H-chromen-3-ylcarbonyl, that is unsl Ihstih It~sd by 1 to 3
substitutents selected from oxo and hydroxy, especially 7,8-dihydroxy-2-oxo-2H-
(benzopyran)-3-yl-carbonyl;
(v) carbamoyl-lower alkanoyl, wherein lower alkanoyl is especially propionyl, especially 3-
carbamoylpropionyl;
(vi) dihydroxyphenyl-lower alkylcarbonyl, especially 3-(3j4-dihydroxyphenyl)-propionyl or
2-(3 ,4-dihydroxyphenyl)-acetyl;
(vii) cyclohexyl-lower alkylcarbonyl, especially 3-(cyclohexyl)-propionyl; and
(viii) cinnamoyl sl Ihstitl Ited by 1 to 2 moieties selected independently from methoxy and
especially hydroxy, especially p-hydroxycinnamoyl, m-hydroxy-p-methoxy-cinnamoyl or
preferably m,p-dihydroxy-cinnamoyl; and
(ix) if Y is secondary or tertiary amino, also from lower alkanoyl, especially acetyl,
PTI is a bivalent radical of tyrosine or (preferably) a bivalent radical of phosphotyrosine (in
the D-, L- or less preferably the (D,L)-form) or of a phosphotyrosine mimic in the form of a
bivalent radical (which is bound N-terminally via the imino group resulting from the a-amino
group and C-terminally via the carbonyl group resulting from its a-carboxy group) of an
amino acid selected from phosphonomethyl-phenylalanine, espe~ lly 4-phosphono-methyl-
phenylalanine, phosphono-(a-fluoro)methyl-phenylalanine, especially 4-phosphono-(a-
fluoro)methyl-phenylalanine, phosphono-(a,a-difluoro)methyl-phenylalanine, especially 4-
phosphono-(a,a-difluoro)methyl-phenylalanine, phosphono-(a-hydroxy)methyl-phenyl-
alanine, especially 4-phosphono-(a-hydroxy)methyl-phenylalanine, O-sulfo-tyrosine, such
as 4-(0-sulfo)tyrosine, dicarboxymethoxy-phenylalanine (= (HOOC)2-CH2-O-phenylalanine,
especially 4-(dicarboxymethoxy)-phenylalanine aspartic acid, glutamic acid, phosphoserine
and phosphothreonine, each of which is present in the (D,L)-, D- or preferably the L-form;
-(AA)n- has one of the following meanings:
~ A bivalent radical of a tripeptide of the formula -(AAl)-(AA2)-(AA3)- wherein -(AA1)- is
preferably selected from -lle-, -Ac5c-, -AC6C-, -Asp-, -Gly- and -Phe- or (alternatively or in
addition to the group of moieties mentioned just before) from -Ac7c-, -Nbo-, -Met-, -Pro-,
-,~Ala-, -Gln-, -Glu-, -DHph-, -HPh- and -tLe-, most preferably -lle-, -Ac5c-, -Ac6c-, -Asp-
CA 02227~16 1998-01-21
WO 97/08193 PCT~EP96/03473
or -Gly- or (alternatively or in addition to the group of moieties mentioned just before) -
Ac7c-, -Nbo- and -Gln-; -(AA2)- is preferably selected from -Asn-, and also from -3Ala-
and -Gly-, or (alternatively or in addition to the group of moieties mentioned just before),
from -lle-, -i3Ala- and Gln; most preferably -Asn-; and -(AA3)- is pr~rt:r~bly selected from
-Val-, and -i3-Ala, and also from -Gly-, -Gln-, -Val-, -Asp- and -Ac5c-; or, less preferably,
~ a bivalent radical of a dipeptide of the formula -(AA1)-(AA2)- wherein -(AA1)- and -(AA2)-
preferably have the meanings given above, preferably -(AA')- being -lle- or -Ac6c-, or
(alternatively or in addition to the group of moieties mentioned just before) -Ac7c-, -Nbo-
and -Gln-; and -(AA2)- being -Asn- (prei~,-ed) or also -~Ala-;
~ or (even less preferably) simply a bivalent radical of an amino acid selected from the
amino acids mentioned above, especially -lle- or (alternatively or in addition to the group
of moieties mentioned just before) -Ac6c-, -Nbo- and Ac5c-,
and
Y is amino (-NH2) or monosubstituted amino selected from lower alkylamino, such as
methylamino, ethylamino; isobutylamino or 3-methylbutylamino; octylamino, such as 2-ethyl-
hexyi-amino; aryloxy-lower alkylamino, especially halonaphthyloxy-lower alkylamino, such
as 2-(1-bromo-naphthalen-2-yloxy)-ethylamino, or naphthyloxy-lower alkylamino, such as 2-
(naphthalen-2-yloxy or naphthalen-1-yloxy)-ethylamino; aryl-lower alkylamino, such as
phenyl-lower alkylamino, e.g. benzylamino, 3-phenylpropylamino, di-phenyl-lower
alkylamino, such as 2,2-diphenyl-ethylamino or 3,3-diphenyl-propylamino, (mono- or di-halo-
phenyl)-lower alkylamino, such as 2-(4-chlorophenyl)ethylamino or 3-(2,4-dichlorophenyl)-
propylamino, naphthalenyl-lower alkylamino, such as 3-naphthtalen-1-ylpropylamino or 3-
naphthalen-2-ylpropylamino, hydroxy-naphthalenyl-lower alkylamino, such as 3-(2-hydroxy-
naphthalen-1-yl)-propylamino, or phenanLI ,renyl-lower alkylamino, such as 3-phenanthren-
9-yl-propylamino; cycloalkylamino, such as cyclohexylamino; and cycloalkyl-loweralkylamino, such as cyclohexylmethylamino;
or a salt thereof where at least one salt-forming group is present.
,.
More pr~r,ed is a compound of formula I wherein
':
n is 1, 2 or 3, especially 2 or 3;
CA 02227~16 1998-01-21
WO 97/08193 PCT/EP96/03473
- 38 -
X is selected from 4-aminobenzoyl, 3-aminobenzoyl, 4-amino-2-hydroxy-benzoyl, 4-lower
alkoxycarbonyl-benzoyl, such as 4-methoxycarbonyl-benzoyl, quinolinyl-carbonyl, such as
quinoline-2-, quinoline-3- or quinoline-6-ylcarbonyl, indolylcarbonyl, such as indole-5-yl-,
indole-3-yl- or indole-2-yl-carbonyl; phenyl-methoxycarbonyl wherein the phenyl residue is
unsubstitl Ited or sl Ihstitl Ited with amino in the (in growing prer~rence) 2-, 3- and 5-, 4- or 3-
position of the phenyl ring; (+)-, (+) or (-)-1-(3-aminophenyl)ethyloxycarbonyl; hydroxy-
benzyloxy- or hydroxyphenyl-2-ethoxycarbonyl, such as 3-hydroxybenzyloxycarbonyl or 3-
hydroxyphenyl-2-ethoxy-carbonyl; unsl Ibstitl ~ted or lower alkyl-sl Ihstitl Ited 2-(thiazoiyl)-
ethoxycarbonyl, such as 2-(4-methyl-thiazol-5-yl)ethoxycarbonyl, uncllhstitllt~d or amino-
substituted thiazolyl-lower alkylcarbonyl, such as 2-amino-thiazole-5-ylacetyl, indolyl-lower
alkylcarbonyl, such as indole-3-yl-acetyl, 3-(indole-3-yl)propionyl or 4-(indole-3-yl)-butyroyl,
naphthoyl or hydroxy-naphthoyl, such as naphthalene-2-yl-carbonyl or especially 6-hydroxy-
naphthalene-2-yl-carbonyl, cyclohexylcarbonyl, 1,2,3,4-tetrahydronaphthyl-2-carbonyl
(pr~r~r,ed), adamantoyl, preferably 1-adamantoyl, 7,8-dihydroxy-2-oxo-2H-(benzopyran)-3-
yl-carbonyl, carbamoyl-lower alkanoyl, especially 3-carbamoylpropionyl, dihydroxyphenyl-
lower alkylcarbonyl, especially 3-(3,4-dihydroxyphenyl)-propionyl or 2-(3,4-dihydroxyphenyl)-
acetyl, and cinnamoyl substituted by 1 to 2 moieties selected independently from methoxy
and especially hydroxy, especially p-hydroxycinnamoyl, m-hydroxy-p-methoxy-cinnamoyl or
preferably m,p-dihydroxy-cinnamoyl; and, if Y is monosubstituted amino, also from lower
alkanoyl, especially acetyl;
PTI is a bivalent radical of phosphotyrosine (in the D-, L- (most preferred) or (less
preferably) the (D,L)-form) or of a phosphotyrosine mimic of the phosphono-(a,a-difluoro)methyl-phenylalanine or the phosphonomethyl-phenylalanine type, especially 4-
phosphono-(a,a-difluoro)methyl-phenylalanine
-(AA)n- has one of the following meanings:
~ A bivalent radical of a tripeptide of the formula -(AAl)-(AA2)-(AA3)- wherein -(AA1)- is
preferably selected from -lle-, ~Acsc-, -Ac6c-, -Asp-, -Gly- and -Phe- or (alternatively or in
addition to the group of moieties mentioned just before) from -Ac7c-, -Nbo-, -Met-, -Pro-,
-,3Ala-, -Gln-, -Glu-, -DHph-, -HPh- and -tLe-, most preferably -lle-, -ACsc-~ -Ac6c-, -Asp-
or -Gly- or (alternatively or in addition to the group of moieties mentioned just before) -
Ac7c-, -Nbo- and -Gln-; -(AA2)- is preferably selected from -Asn-, and also from -~Ala-
CA 02227~16 1998-01-21
WO 97/08193 PCTAEP96/03473
.
and -Gly-, or (alternatively or in addition to the group of moieties mentioned just before),
from -lle-, -,~Ala- and Gln; most preferably -Asn-; and -(AA3)- is preferably selected from
-Val-, and -~-Ala, and also from -Gly-, -Gln-, -Val-, -Asp- and -Acsc-; or, less preferably,
~ a bivalent radical of a dipeptide of the formula -(AAl)-(AA2)- wherein -(AA1)- and -(AA2)-
preferably have the meanings given above, preferably -(AA')- being -lle- or -Ac6c-, or
(alternatively or in addition to the group of moieties mentioned just before) -Ac7c-, -Nbo-
and -Gln-; and -(AA2)- being -Asn- (pr~r~lled) or also -,~Ala-;
~ or (even less preferably) simply a bivalent radical of an amino acid selected from the
amino acids mentioned above, especially -lle- or (alternatively or in addition to the group
of moieties mentioned iust before) -Ac6c-, -Nbo- and Acsc-,
and
Y is amino (-NH2) or monosubstituted amino selected from lower alkylamino, such as
methylamino, ethylamino; isobutylamino or 3-methylbutylamino; octylamino, such as 2-ethyl-
hexyl-amino; aryloxy-lower alkylamino, especi~lly halonaphthyloxy-lower alkylamino, such
as 2-(1-bromo-naphthalen-2-yloxy)-ethylamino, or naphthyloxy-lower alkylamino, such as 2-
(naphthalen-2-yloxy or naphthalen-1-yloxy)-ethylamino; aryl-lower alkylamino, such as
phenyl-lower alkylamino, e.g. benzylamino, 3-phenylpropylamino, di-phenyl-lower
alkylamino, such as 2,2-diphenyl-ethylamino or 3,3-diphenyl-propylamino, (mono- or di-halo-
phenyl)-lower alkylamino, such as 2-(4-chlorophenyl)ethylamino or 3-(2,4-dichlorophenyl)-
propylamino, naphthalenyl-lower alkylamino, such as 3-naphthtalen-1-ylpropylamino or 3-
naphthalen-2-ylpropylamino, hydroxy-naphthalenyl-lower alkylamino, such as 3-(2-hydroxy-
naphthalen-1-yl)-propylamino, or phenanthrenyl-lower alkylamino, such as 3-phenanthren-
9-yl-propylamino; cycloalkylamino, such as cyclohexylamino; and cycloalkyl-loweralkylamino, such as cyclohexylmethylamino;
or a salt thereof where at least one salt-forming group is present.
More preferred is also a compound of formula I wherein
n is20r3;
.~
CA 02227~l6 l99X-0l-2l
O 97/08193 PCTAEP96/03473
- 40 -
X is seiected from 4-aminobenzoyl, 3-aminobenzoyl, 4-amino-2-hydroxy-benzoyl, 4-lower
alkoxycarbonyl-benzoyl, such as 4-methoxycarbonyi-benzoyl, quinolinyl-carbonyl, such as
quinoline-2-, quinoline-3- or quinoline-6-ylcarbonyl, indolylcarbonyl, such as indole-5-yl-,
indole-3-yl- or indole-2-yl-carbonyl; phenyl-methoxycarbonyl wherein the phenyl residue is
unsubstituted or 5llhstihlted with amino in the (in growing pr~er~nce) 2-, 3- and 5-, 4- or 3-
position of the phenyl ring; (+)-, (+) or (-)-1-(3-aminophenyl)ethyloxycarbonyl; hydroxy-
benzyloxy- or hydroxyphenyl-2-ethoxycarbonyl, such as 3-hydroxybenzyloxycarbonyl or 3-
hydroxyphenyl-2-ethoxy-carbonyl; unsl Ihstjtl Ited or lower alkyl-sl Ihstitl Ited 2-(thi~olyl)-
ethoxycarbonyl, such as 2-(4-methyl-thiazol-5-yl)ethoxycarbonyl, unsllhstitllted or amino
substituted thiazolyl-lower alkylcarbonyl, such as 2-amino-thiazole-5-ylacetyl, and indolyl-
lower alkylcarbonyl, such as indole-3-yl-acetyl, 3-(indole-3-yl)propionyl or 4-(indole-3-yl)-
butyroyl;
PTI is a bivalent radical of phosphotyrosine (in the D-, L- (most prt:r~:"ed) or (less
preferably) the (D,L)-form) or of a phosphotyrosine mimic of the phosphono-(a,a-difluoro)methyl-phenylalanine type, especially 4-phosphono-(a,a-difluoro)methyl-phenylalanine
-(AA)n- has one of the following meanings:
~ a bivalent radical of a tripeptide of the formula -(AA1)-(AA2)-(AA3)- wherein -(AA')- is
selected from -lle-, -AC5C-, -AC6C-, -Asp-, -Gly- and -Phe-, most preferably -lle-, -Acsc~,
-Ac6c-, -Asp- or -Gly-; -(AA2)- is selected from -Asn-, and also from -~Ala- and -Gly-,
most preferably -Asn-; and -(AA3)- is selected from -Val-, and -,~-Ala, and also from
-Gly-, -Gln-, -Val-, -Asp- and -Acsc-; or
~ a bivalent radical of a dipeptide of the formula -(AA1)-(AA2)- wherein -(AA')- is-lle- or
-Ac6c- and -(AA2)- is-Asn- (preferred) or also -~Ala-; and
Y is a free amino group (-NH2),
or a salt thereof.
More preferred is also a compound of formula I wherein
CA 02227~16 1998-01-21
WO 97/08193 PCT/EP96/03473
n is 1, 2 or 3, especiaily 2 or 3;
;
X is selected from naphthoyl or hydroxy-naphthoyl, such as naphthalene-2-yl-carbonyl or
especially 6-hydroxy-naphthalene-2-yl-carbonyl, cyclohexylcarbonyl, 1,2,3,4-
tetrahydronaphthyl-2-carbonyl (preferred), adamantoyl, preferably 1-adamantoyl, 7,8-
dihydroxy-2-oxo-2H-(benzopyran)-3-yl-carbonyl, carbamoyl-lower alkanoyl, especially 3-
carbamoylpropionyl, dihydroxyphenyl-lower alkylcarbonyl, especially 3-(3,4-
dihydroxyphenyl)-propionyl or 2-(3,4-dihydroxyphenyl)-acetyl, and cinnamoyl substituted by
1 to 2 moieties selected independently from methoxy and especi~lly hydroxy, especially p-
hydroxycinnamoyl, m-hydroxy-p-methoxy-cinnamoyl or preferably m,p-dihydroxy-cinnamoyl;
and,-if Y is monosubstituted amino, also from lower alkanoyl, especially acetyl;
PTI is a bivalent radical of phosphotyrosine (in the D-, L- (most plefer,~d) or (less
preferably) the (D,L)-form) or of a phosphotyrosine mimic of the phosphono-(a,~-difluoro)methyl-phenylalanine or the phosphonomethyl-phenylalanine type, especially 4-
phosphono-(a,a-difluoro)methyl-phenylalanine
-(AA)n- has one of the following meanings:
A bivalent radical of a tripeptide of the formula -(AA')-(AA2)-(AA3)- wherein -(AA')- is
preferably selected from -lle-, -AC5C-, -AC6C-, -Asp-, -Gly- and -Phe- or (alternatively or in
addition to the group of moieties mentioned just before) from -Ac7c-, -Nbo-, -Met-, -Pro-,
-i3Ala-, -Gln-, -Glu-, -DHph-, -HPh- and -tLe-, most preferably -lle-, -Ac5c-, -Ac6c-, -Asp-
or -Gly- or (alternatively or in addition to the group of moieties mentioned just before) -
Ac7c-, -Nbo- and -Gln-; -(AA2)- is pr~:rel~bly selected from -Asn-, and also from -i3Ala-
and -Gly-, or (alternatively or in addition to the group of moieties mentioned just before),
from -lle-, -i3Ala- and Gln; most preferably -Asn-; and -(AA3)- is preferably selected from
-Val-, and -i3-Ala, and also from -Gly-, -Gln-, -Val-, -Asp- and -Ac5c-; or, less preferably,
a bivalent radical of a dipeptide of the formula -(AA1)-(AA2)- wherein -(AAl)- and -(AA2)-
preferably have the meanings given above, preferably -(AA1)- being -lle- or -Ac6c-, or
(alternatively or in addition to the group of moieties mentioned just before) -Ac7c-, -Nbo-
and -Gln-; and -(AA2)- being -Asn- (preferred) or also -i3Ala-;
CA 02227~16 1998-01-21
W O 97/08193 PCTAEP96/03473
- 42 -
~ or (even less preferably) simply a bivalent radical of an amino acid selected from the
amino acids mentioned above, especially -lle- or (alternatively or in addition to the group
of moieties mentioned just before) -Ac6c-, -Nbo- and Ac5c-,
and
Y is monosl ~bstitl Ited amino selected from lower alkylamino, such as methylamino,
ethyiamino; isobutylamino or 3-methylbutylamino; octylamino, such as 2-ethyl-hexyl-amino;
aryloxy-lower alkylamino, especially halonaphthyloxy-lower alkylamino, such as 2-(1-bromo-
naphthalen-2-yloxy)-ethylamino, or naphthyloxy-lower alkylamino, such as 2-(naphthalen-2-
yloxy or naphthalen-1-yloxy)-ethylamino; aryl-lower alkylamino, such as phenyl-lower
alkylamino, e.g. benzylamino, 3-phenylpropylamino, di-phenyl-lower alkylamino, such as
2,2-diphenyl-ethylamino or 3,3-diphenyl-propylamino, (mono- or di-halo-phenyl)-lower alkyl-
amino, such as 2-(4-chlorophenyl)ethylamino or 3-(2,4-dichlorophenyl)-propylamino,
naphthalenyl-lower alkylamino, such as 3-naphthtalen-1-ylpropylamino or 3-naphthalen-2-
ylpropylamino, hydroxy-naphthalenyl-lower alkylamino, such as 3-(2-hydroxy-naphthalen-1-
yl)-propylamino, or phena.,Ll,r~nyl-lower alkylamino, such as 3-phenanll,ren-9-yl-
propylamino; cycloalkylamino, such as cyclohexylamino; and cycloalkyl-lower alkylamino,
such as cyclohexylmethylamino;
or a salt thereof where at least one salt-forming group is present.
Especially preferred is a compound mentioned in the examples, or a (preferably
pharmaceutically acceptable) salt thereof.
Most preferred is a compound of formula I being selected from the following compounds:
3-aminobenzyloxycarbonyl-Tyr(P03H2)-lle-Asn-NH2
(SEQ ID NO: 3),
3-aminobenzyloxycarbonyl-Tyr(P03H2)-Ac5c-Asn-Gln-NH2
(SEQ ID NO: 6),
3-aminobenzyloxycarbonyl-Tyr(P03H2)-Ac6c-Asn-Gln-NH2
(SEQ ID NO: 7),
4-aminobenzoyl-Tyr(P03H2)-lle-Asn-Gln-NH2
(SEQ ID NO: 24),
CA 02227~16 1998-01-21
W O 97/08193 PCTrEP96/03473
-43-
4-aminobenzoyl-Tyr(P03H2)-Asp-Asn-Gln-NH2
(SEQIDNO:51),
4-aminobenzoyl-Tyr(P03H2)-Gly-Asn-Gln-NH2
(SEQ ID NO:52),
4-aminobenzoyl-Tyr(P03H2)-lle-Asn-~Ala-NH2
(SEQ ID NO:58),
Indole-5-ylcarbonyl-Tyr(P03H2)-lle-Asn-Gln-NH2
(SEQ ID NO:61)
Indole-5-ylcarbonyl-Tyr(P03H2)-lle-Asn-NH2
(SEQ ID NO:62)
Indole-5-ylcarbonyl-Tyr(P03H2)-Ac6c-Asn-Gln-NH2
(SEQ ID N 0:63); and
3-aminobenzyloxycarbonyl-L-F2Pmp-lle-Asn-Gln-NH2
(SEQ ID N 0:64)
Most pre~e,.ed is also a compound of formula I being selected from the followingcompounds:
trans-3,4-dihydroxy-cinnamoyl-Tyr(PO3H2)-lle-NH2
(SEQ ID NO:70);
trans-3 ,4-dihydroxy-cinnamoyl-Tyr(P03H2)-AC6C-N H2
(SEQ ID NO:71);
trans-3,4-dihydroxy-cinnamoyl-Tyr(PO3H2)-AC6C-Asn-NH2
(SEQ ID NO:72) (very preferred);
trans-3,4-dihydroxy-cinnamoyl-Tyr(PO3H2)-Met-Asn-NH2
(SEQ ID NO:74) (very pr~rer,ed);
3-(3,4-dihydroxyphenyl)-propionyl-Tyr(PO3H2)-lle-Asn-NH2
(SEQ ID N 0:76);
3~4-dihydroxyphenyl-acetyl-Tyr(po3H2)-lle-Asn-NH2
(SEQ ID N 0:77);
trans-3,4-dihydroxy-cinnamoyl-Tyr(PO3H2)-Ac7c-Asn-NH2
(SEQID N 0:80) (very preferred);
trans-3,4-dihydroxy-cinnamoyl-Tyr(PO3H2)-Nbo-Asn-NH2
(SEQ ID N 0:81) (very preferred);
CA 02227~16 1998-01-21
W O 97/08193 PCTAEP96/03473
trans-3,4-dihydroxy-cinnamoyl-Tyr(PO3H2)-Nbo-NH2 (epimer 1 )
(SEQIDNO:82);
trans-3,4-dihydroxy-cinnamoyl-Tyr(PO3H2)-Nbo-NH2 (epimer 2)
(SEQIDNO:83);
trans-4-hydroxy-cinnamoyl-Tyr(P03H2)-lle-Asn-NH2
(SEQIDNO:84);
6-hydroxy-2-naphthoyl-Tyr(PO3H2)-lle-NH2
(SEQIDNO:85);
trans-3~4-dihydroxy-cinnamoyl-Tyr(po3H2)-Gln-NH2
(SEQIDNO:86);
trans-3,4-dihydroxy-cinnamoyl-Tyr(P03H2)-GIU-NH2
(SEQIDNO:87);
1 -adamantoyl-Tyr(P03H2)-lle-Asn-NH2
(SEQIDNO:93);
cyclohexanoyl-Tyr(P03H2)-lle-Asn-NH2
(SEQIDNO:94);
succinamoyl-Tyr(P03H2)-lle-Asn-NH2
(SEQID NO: 101);
trans-3,4-dihydroxy-cinnamoyl-Tyr(P03H2)-lle-Asn-NH2
(SEQIDNO:105) (very preferred);
trans-3,4-dihydroxy-cinnamoyl-F2Pmp-lle-Asn-NH2
(SEQIDNO:109) (very preferred);
trans-3,4-dihydroxy-cinnamoyl-F2Pmp-lle-NH2
(SEQIDNO:110);
3-aminobenzyloxycarbonyl-Tyr(P03H2)-Met-ASn-NH2
(SEQIDNO:111);
indole-5-ylcarbonyl-L-F2Pmp-lle-Asn-NH2
(SEQIDNO: 115) (very preferred);
3-aminobenzyloxycarbonyl-Tyr(PO3H2)-Gln-Asn-NH2
(SEQIDNO:117) (very pr~r~l,ed);
3-Aminobenzyloxycarbonyl-Tyr(P03H2)-AC6C-Asn-NH2
(SEQIDNO:120) (very preferred);
acetyl-Tyr(PO3H2)-lle-Asn-NH-(3-naphthalen-1 -yl-propyl)
CA 02227~16 1998-01-21
W O97/08193 PCT~EP96/03473
(SEQ ID NO: 121);
acetyl-Tyr(P03H2)-lle-Asn-NH-[3-(2-hydroxy-naphthalen-1 -yl)-propyl]
(SEQ ID NO: 122);
acetyl-Tyr(P03H2)-lle-Asn-NH-(3-naphthalen-2-yl-propyl)
(SEQ-ID-NO: 123);
3-aminobenzyloxycarbonyl-Tyr(P03H2)-lle-Asn-NH-(3-naphthalen-1 -yl-propyl)
(SEQ ID NO: 140);
3-aminobenzyloxycarbonyl-Tyr(P03H2)-lle-Asn-NH-[3-(2-hydroxy-naphthalen-1 -yl)-propyl]
(SEQ ID NO: 141) (very pr~r~:l,ed);
3-aminobenzyloxy.;arl,onyl-Tyr(P03H2)-lle-Asn-NH-(3-phenanthren-9-yl-propyl)
(SEQ ID NO: 143);
3-aminobenzyloxycarbonyl-Tyr(P03H2)-lle-Asn-NH-(3-methyl-butyl)
(SEQ ID NO: 145); and
3-aminobenzyloxyca.L,onyl-Tyr(P03H2)-lle-Asn-NH-cyclohexyl
(SEQ ID NO: 146);
or a pharmaceutically acceptable salt thereof if a salt-forming group is present.
Most preferred is also indole-5-ylcarbonyl-Tyr-lle-Asn-Gln-NH2
(SEQ ID NO: 112), or a pharmaceutically acceptable salt thereof.
The compounds of the present invention can be synthesized according to known
procedures, especially by a process comprising reacting a fragment of a compound of
formula 1, which has a free carboxy group or a reactive derivative thereof, or, in the case of
the introduction of X, a free carboxy or sulfo group, or a reactive derivative thereof, with a
complementary fragment that has an amino group with at least one free hydrogen atom, or
with a reactive derivative thereof, with formation of an amide bond; in the mentioned
fragments free functional groups with the exception of those that parLici~ale in the reaction
if required being present in protected form; and removing any protecting groups present;
and, if desired, transforming a compound of formula I into a different compound of formula
I; transforming a salt of an obtainable compound of formula I into the free compound or a
different salt or an obtainable free compound of formula I into a salt; and/or separating
obtainable mixtures of isomers of compounds of formula I into the individual isomers.
CA 02227516 1998-01-21
WO 97/08193 PCT/EP96/03473
- 46 - _
In the following, more detailed description of the preferred process conditions, X, PTI,
-(AA)n-, n and Y have the meanings given for compounds of the formula 1, if not mentioned
otherwise.
Detailed descri~tion of ~referred reaction conditions
The compounds of the present invention preferably can be readily prepared according to
well-est~hliched, standard liquid or, preferably, solid-phase peptide synthesis methods,
general desc,i~lions of which are broadly available (see, for example, in J.M. Stewart and
J.D. Young, Solid Phase Peptide Synthesis, 2nd edition, Pierce Chemical Company,Rockford, Illinois (1984), in M. Bodanzsky and A. Bodanzsky, The Practice of Peptide
Synthesis, Springer Verlag, New York (1984); and Applied Biosystems 430A Users Manual,
ABI Inc., Foster City, California), or they may be prepared in solution, by the liquid phase
method or by any combination of solid-phase, liquid phase and solution chemistry, e.g. by
first completing the respective peptide portion and then, if desired and appropriate, after
removal of any protecting groups being present, by introduction of the residue X by reaction
of the respective carbonic or sulfonic acid or a reactive derivative thereof.
A fragment with a free carboxy or sulfonic group can be an amino acid (if required, in
suitably protected form) or a di- or other appropriate oligopeptide or also (in the case of the
introduction of the N-terminal X of compounds of formula I with acylated or sulfonylated
terminal amino group) the acylating carbonic or sulfonic acid. A fragment that has an amino
group with at least one free hydrogen atom (= a group -NH-) can also be a single amino
acid, a di- or other oligopeptide or, in the case of prepald~ion of peptamides (Y = amino or
mono- or disubstituted amino), ammonia or mono- or ~iisuhstituted ammonia.
Reactive derivatives of carbonic or sulfonic acids are preferably reactive esters, reactive
anhydrides or reactive cyclic amides. Reactive carbonic acid or reactive sulfonic acid
derivatives can also be formed in situ.
A reactive derivative of an "amino group with at least one free hydrogen" is preferably
derivatized by the reaction with a phosphite, such as diethyl-chlorophosphite, 1,2-
phenylene-chlorophosphite, ethyl-dichlorophosphite, ethylene-chlorophosphite or tetraethyl-
CA 02227~16 1998-01-21
W O 97/08193 PCTIEP96/03473
pyrophosphite; or is present in the form of a carbamic acid chioride wherein the amino
group participating in the reaction is 5llhtihlted by halocarbonyl, such as chlorocarbonyl.
Preferably, free amino is used instead of a reactive derivative.
The reaction steps required e.g. for the synthesis of amide or sulfonamide bonds usually
depend on the type of activation of the carboxylic or sulfo group participating in the reaction.
The reactions normally run in the presence of a condensing agent or, when activating the
carboxylic or sulfonic acids in the form of anhydrides, of an agent that binds the carboxylic
or sulfonic acid formed. In some cases it is also possible to add chaotropic agents such as
LiF in N-methylpyrrolidin-2-one. The reactions are especially carried out in a temperature
range from -30 to +150 ~C, prt:r~:,ably from +10 to +70 ~C, and, most preferably, from +20
to +50 ~C, if appropriate, in an inert gas atmosphere, e.g. under nitrogen or argon.
If desired or appropriate, unreacted amino groups can be acylated after a reaction cycle,
e.g. by acetylation of unreacted amino groups with an excess of acetic anhydride/pyri-
dine/DMA (1:1:8), thus f~cilitAting later purification of the final product.
In general, a suitably protected amino acid as a ligand is attached via its carboxyl group (-
COOH) to a derivatized, insoluble polymeric support, e.g. a cross-linked polystyrene or
polyamide resin, such as a 4-(2',4'-dimethoxyphenyl-[hydroxy- or amino1methyl)-phenyoxy -
polystyrene resin (the polymer is, e.g., a copolymer of styrene with 1% divinylbenzene, 100-
200 mesh) or a PAL-PEG-PS (synonym: PAL-PEG-MBHA-PS) resin (PAL stands for a
trisalkoxy, especially trismethoxy, benzylamide linker; PEG for polyethyleneglycol; and
MBHA for 4-methylbenzhydrylamine - in this type of resin, polystyrene (PS) supports
u"i~r",ly incor,uo,~l~ a derivatized polyethylene glycol (PEG) spacer between the functional
group on the PS gel bead (e.g. pr~r~" ed particle size in the range of 75 to 150 ,um,
crosslinking by 1% divinylbenzene) and the handle or attachment point of the peptide to be
synthesized (see J. Org. Chem. 55, 3730 (1990) or in "Peptides: Chemistry and Biology,
Proceedings of the Twelfth American Peptide Symposium (Smith, J.A., and Rivier, J.E.,
eds.) ESCOM, Leiden, The Netherlands (1992), p. 603)) by condensation reactions, or,
especially for the synthesis of compounds of formula I with a C-terminal secondary or
tertiary amino group Y, said compound being bound to the resin via a carboxy group
different from the C-terminal a-carboxy group of an amino acid, e.g. the y-carboxy group of
CA 02227~16 1998-01-21
WO 97/08193 PCTAEP96/03473
- 48 -
aspartic acid or the ~-carboxy group of glutamic acid, a 4-(2',4'-dimethoxyphenyl-Fmoc-
aminomethyl)-phenoxyacetamido-norleucyl-4-methyl-benzhydrylamine resin (Novabiochem,
Laufelfingen, Switzerland). "Suitably protected" refers to the presence of protecting groups
on the amino group (e.g. a-NH2 or ~-NH2) and any side-chain functional group (if present) of
the amino acid. Di-, tri- or other oligopeptides can be used instead of the amino acids as
building blocks (fragments).
Synthesis proceeds in a stepwise, cyclical fashion by successively removing the NH2
pruLecling group of the amino group to be reacted next and then coupling an activated
fragment (e.g. an amino acid, di-, tri- or oligopeptide or the carboxylic acid or sulfonic acid of
formula ll,
X-OH (Il)
or a reactive derivative thereof, wherein X has the meanings given under formula 1) to the
deprotected NH2 (e.g. a- or ,3-NH2). Preferably, activation of the COOH group of the amino
acid to be reacted or ( in the case of the introduction of X) the carboxyl or sulfo group of the
acid of of formula ll to be attached by the condensation reaction is effected
(i) directly with a carbodiimide, e.g. dicyclohexylcarbodiimide (DCC), N-ethyl-N'-(3-dimethyl-
aminopropyl)-carbodiimide, N,N'-diethylcarbodiimide or N,N'-diisop,upylcarbodiimide (DICD);
with a carbonyl compound such as carbonylc~ ;d~ole; with 1,2-oxazolium compoundssuch as 2-ethyl-5-phenyl-1,2-oxazolium-3'-sulfonate and 2-tert-butyl-5-methylisoxazolium
perchlorate; with acylamino compounds such as 2-ethoxy-1-ethox~ca,l,onyl-1,2-dihydroqui-
noline; with N-~(dimethylamino)-1 H-1 ,2,3-triazolo[4,5-b]pyridin-1 -ylmethylene]-N-
methylmethanaminium hexafluorophosphate N-oxide (HATU); with an uronium compoundsuch as 2-(1 H-ben~c,l, i~c,l-1 -yl)-1 ,1 ,3,3-tetramethyluronium tetrafluoroborate (HBTU) or 2--
(pyridon-1-yl)-1,1,3,3-tetramethyluronium tetrafluoroborate = 0-(1,2-dihydro-2-oxo-1-pyri-
dyl)-N,N,N',N'-tetramethyluronium-tetrafluoroborate (TPTU); or phosphonium compounds
such as ben~uL~ ul-1-yl-oxy-tris(dimethylamino)-phosphonium hexafluorophosphate (BOP)
or ben~uL~ia~ol-1-yl-oxy-pyrrolidino-phosphonjum hexafluorophusphdle (PyBOP);
,
CA 02227~16 1998-01-21
W O 97/08193 PCTAEP96/03473
.
(ii) via formation of the sylllr"~l~ic anhydride (obtainable, for example, by condensation of
the corresponding acid in the presence of a carbodiimide or 1-diethylaminopropyne;
symmetric anhydrides method), or an asymmetric anhydride, such as the respectivecarbonic or sulfonic acid bromide, chloride or fluoride (obtainable, for example, by treatment
of the respective carbonic or sulfonic acid with thionyl-, phosphopenta- or oxalyl-fluoride,
-chloride or -bromide; acid halide (e.g. chloride) method), or
(iii) by formation of an "active ester", e.g. an amino- or amido ester, such as a 1-hydroxy-
ber,~ulri~ole (HOBT) or N-hydroxysuccinimide ester, or an aryl ester, such as a penta-
fluorophenyl, 4-nitrophenyl or 2,4,5-tetrachlorophenyl ester (obtainable by treatment of the
respective acid with a phenyl with the appropriate substituents, such as 4-nitrophenol or
2,4,5-trichlorophenol, and the like);
or by an appropriate combination of any of the reagents and reactions mentioned under (i)
to (iii).
Useful acid binding agents that can be employed in the condensation reactions are, for
example, alkaline metals, carbonates or bicarbonates, such as sodium or potassium
carbonate or bicarbonate (if appropriate, together with a sulfate), or organic bases such as
sterically hindered organic nitrogen bases, for example tri-lower alkylamines, such as N,N-
diisopropyl-N-ethylamine, pyridine or N-methyl-pyrrolidin-2-one, which can be used alone or
in any appropriate combination.
Reactive groups in the monomers of ligands or in the resin-bound or free intermediates
resulting from one or more coupling steps can be protected by third groups as protecting
groups that are customarily used in peptide synthesis. Examples of prule~;ling groups, their
introduction and their removal are, for example, described in standard works such as
"Protective groups in Organic Chemistry", Plenum Press, London, New York 1973;
"Methoden der organischen Chemie", Houben-Weyl, 4. edition, Vol. 15/1, Georg-Thieme
Verlag, Stuttgart 1974; Th. W. Greene, "Protective Groups in Organic Synthesis", John
Wiley & Sons, New York 1981; Atherton et al., "Solid Phase Peptide Synthesis - A Practical
Approach", IRL Press Oxford University, 1984; Jones, "The Chemical Synthesis of
Peptides", Oxford Science Publications, Clavendon Press Oxford, 1991; and Bodanszky,
"Peptide Chemistry", Springer Verlag Berlin, 1988. The term "protecting groups" comprises
CA 02227~l6 l998-0l-2l
W O 97/08193 PCT~EP96/03473
- 50 -
also resins used for solid phase synthesis, preferably those specifically mentioned above
and below.
Examples for hydroxy protecting groups are acyl radicals, such as tert-lower alkoxycarbonyl
radicals, for example tert-butoxycarbonyl, etherifying groups, such as tert-lower alkyl
groups, for example t-butyl, or silyl- or tin r~ s~ such as tert-butyl-dimethylsilyl or the tri-n-
butyltin radical.
Carboxy groups can be protected by groups as defined above for the C-terminal p~ g
groups Y, preferably by esterifying groups selected from those of the tert-butyl type, from
benzyl, from trimethylsilylethyl and from 2-triphenylsilyl groups, or they can be protected as
lower alkenyl esters, such as allylic esters. .
Amino or guanidino (e.g. in H-Arg-OH) groups can be protected by removable acyl groups
or by arylmethyl, etherified mercapto, 2-acyl-lower alk-1-enyl, a silyl group or an organic
sulfonyl group or tin amino prc.L~ g groups; tert-butoxycarbonyl, allyloxycarbonyl, benzyl-
oxycarbonyl, 4-nitrobenzyloxycarbonyl, 2-chlorobenzyloxycarbonyl, 2-bromobenzyloxy-
carbonyl, diphenylmethoxycarbonyl, nitrophenylsulfenyl, 2,2,2-trichloroethoxycarbonyl,
2,2,5,7,8-pentamethylchroman-6-sulfonyl (PMC - very pr~r~r, ed), 2,2,4,6,7-pentamethyl-
dihydrobenzofuran-5-sulfonyl (Pbf) or 4-methoxy-2,3,6-trimethylbenzenesulfonyl (Mtr) being
especially pr~r~r,ed.
Carbamide groups (for example, in the side chains of asparagine and glutamine) can be
prole.;led at the nitrogen atom by arylmethyl groups, preferably triphenylmethyl (trityl) or
analogues thereof with one or more lower alkoxy, such as methoxy, and/or lower alkyl, such
as methyl, substituents in one or more phenyl rings.
Phosphono groups can be protected in the form of diesters, e.g. in the form of di-lower alkyl
esters, e.g. as diethylphosphonate (-P(=O)(OC2Hs)2) or di-tert-butylphosphonate.
~.
Imino groups (e.g. in imidazole) can be protected by 2,4-dinitrophenyl, trityl, tert-butoxy-
carbonyl or p-toluenesulfonyl, or (e.g. in indole) by formyl or tert-butoxycarbonyl.
CA 02227~l6 l998-0l-2l
WO 97/08193 PCT~EP96/03473
-51-
Mercapto groups can be p-uL~ ed, e.g., by acetamidomethyl, by trityl or by p-methylbenzyl.
A large number of methods of removing protective groups in the final products or any inter-
mediates are known in the art and comprise, inter alia"~-elimination, solvolysis, hydrolysis,
alcoholysis, acidolysis, photolysis, enzymatical removal, treatment with a base or reduction.
The protective groups are usually removed after the complete synthesis of the resin-bound
molecule by conventional methods of peptide chemistry, conveniently by treatment with
95 % trifluoroacetic acid (Fmoc-chemistry). In some cases, strong nucleophiles, such as
dimethyl sulfide and/or 2-ethanedithiol, may be additionally added to capture the generated
compounds resulting from the pr~,le~;Li"g groups, e.g. in a colllbi,lalion such as trimethyl-
silyltrifluoro-methansulfonate/dimethylsulfide/trifluoroacetic acid/ethanedithiol/m-cresol.
Cleavage of phosphonate diester protecting groups is possible in appropriate solvents, such
as acetonitrile, in the presence of tri-lower alkylsilylhalogenides, such as trimethylsilyl-
iodide, and subsequent hydrolysis of the resulting tri-lower alkylsilyl-ester intermediate in the
presence of an acid, especially a lower alkanoic acid, such as acetic acid, in aqueous
solution.
Cleavage of lower-alkenoic esters of carboxy groups, such as allyloxycarbonyl, is preferably
effected in a solution of a lower alkanoic acid, such as acetic acid, and a sterically hindered
base, such as N-methylmorpholine, in an appropriate solvent, such as a halogenated
hydrocarbon, especially chloroform, in the presence of tetrakis-
(triphenylphosphin)palladium, pre~rdbly under inert gas, such as argon.
The two preferred methods of solid phase peptide synthesis are the Boc and the Fmoc
methods, which are named with reference to their use of the tert-butoxycarbonyl (Boc) or 9-
fluorenylmethyloxycarbonyl (Fmoc) group, respectively, to protect the a-NH2 or a-NHR3 of
the amino acid residue to be coupled (see J. M. Stewart, J. D. Young, Solid-Phase Peptide
Synthesis, 2n edn., Pierce, Rockford, Illinois (1984) or G. Barany, R.B. Merrifield, Solid-
~ phase Peptide Synthesis, in: The Peptides, Vol. 2 (E. Gross, J. Meienhofer, eds.),
Academic Press, New York (1979)); and E. Atherton and R.C. Sheppard, in Solid-Phase
CA 02227~16 1998-01-21
PCT~EP96/03473
W O 97/08193
-52-
Peptide Synthesis-A Practical Approach, ed. D. Rickwood and B.D. Hames, IRL Press at
Oxford University Press, Oxford, 1989), respectively).
In the more est~hli~hed Boc method, the acid-lability of the Boc group is exploited and
trifluoroacetic acid (TFA) treatment is used in order to remove the protective group.
Plerell~d third groups as p,~,lecli"g groups (for functional groups in side chains) are
relatively stable in weak acid, e.g. TFA. Most can be cleaved by strong acids such as
hydrofluric acid (HF) or trifluoromethanesulfonic acid. A small number of side chain groups
(e.g. 2,4-dinitrophenyl protected imino in the histidyl side chain) may require a separate
deprotection step, e.g. treatment with thiophenol or ammonolysis. After synthesis, the
product is typically cleaved from the resin and simultaneously deprul~.;led by HF treatment
at low temperature (e.g. around û ~C).
The Fmoc-group can be cleaved off preferably in the presence of a mild nitrogen base,
preferably piperidine, in an inert solvent, preferably dimethyl acetamide, thereby allowing
the use of side-chain protecting groups which are labile to milder treatment, e.g. TFA.
Preferably, an acid labile ether resin such as HMP-resin (p-hydroxymethylphenoxymethyl
polystyrene), 4-(2',4'-dimethoxyphenyl-hydroxymethyl)-phenoxy-polystyrene (Rink-resin), or
a resin with a benzyloxy- or alkyloxy linker (see Wang, J. Amer. Chem. Soc. 95, 1328
(1973); or, for the synthesis of compounds with a C-terminal amino group X (forming a car~
boxamide) which are preferred, 4-(2',4'-dimethoxyphenyl-aminomethyl)-phenoxypolysty-
rene or -phenoxymethyl-polystyrene (Rink et al., Tetrahedron. Lett. 28(33), 3787-9û (1987);
Novabiochem, Laufelfingen, Switzerland); or a PAL-PEG-PS resin (1\~ "i, ore, Bedford, USA)
is used as the solid support, permitting simultaneous cleavage/depruLt ~:lion in TFA.
Additional process steps
Compounds of formula I can be transformed into different compounds of formula 1, e.g. by
oxydation and hydrolysis.
For the preferred oxidation of mercapto groups, e.g. Iower alkyl-mercapto groups, such as
methylthio, which are preferably oxidised to the respective sulfinyl groups, e.g. Iower alkyl
sulfinyl, such as methylsulfinyl, organic or preferably inorganic peroxides, such as hydrogen
-
CA 02227~16 1998-01-21
WO 97/08193 PCT/EP96/03473
peroxide, can be employed. For example, reaction of lower alkylthio compounds with
hydrogen peroxide in concentrations from 2 to 30 volume-% at preferred temperatures from
O to 50 ~C, especially around room temperature, leads to the respective lower alkyl sulfinyl
compounds.
For the preferred hydrolysis of esterified carboxy groups, such as lower alkoxycarbonyl
groups, well-known conditions for hydrolysis are used, for example hydrolysis in the
presence of a base, e.g. a hydroxide of an alkaline metal, such as sodium hydroxide, under
conditions known in the art, e.g. in an aqueous solvent at preferred temperatures between O
and 50 ~C, preferably at room temperature.
Salts of compounds of formula I having at least one salt-forming group may be prepared in
a manner known per se. For example, salts of compounds of formula I having acid groups
may be formed, for example, by treating the compounds with metal compounds, such as
alkali metal salts of suitable organic carboxylic acids, e.g. the sodium salt of 2-ethylhexanoic
acid, with organic alkali metal or alkaline earth metal compounds, such as the corres-
ponding hydroxides, carbonates or hydrogen carbonates, such as sodium or potassium
hydroxide, carbonate or hydrogen carbonate, with corresponding calcium compounds or
with ammonia or a suitable organic amine, stoichiometric amounts or only a small excess of
the salt-forming agent preferably being used. Acid addition salts of compounds of formula I
are obtained in customary manner, e.g. by treating the compounds with an acid or a
suitable anion exchange reagent. Internal salts of compounds of formula I containing acid
and basic salt-forming groups, e.g. a free carboxy group and a free amino group, may be
formed, e.g. by the neutralisation of salts, such as acid addition salts, to the isoelectric
point, e.g. with weak bases, or by treatment with ion exchangers.
Salts can be converted in customary manner into the free compounds; metal and
ammonium salts can be converted, for example, by treatment with sl lit~le acids, and acid
addition salts, for example, by treatment with a suitable basic agent.
Mixtures of isomers obtainable according to the invention can be separated in a manner
known Per se into the individual isomers; diastereoisomers and/or cis/trans-isomers can be
separated, for example, by partitioning between polyphasic solvent mixtures, recrystal-
lisation and/or chromatographic separation, for example over silica gel or by e.g. medium
CA 02227~16 1998-01-21
W O 97/08193 PCT~EP96/03473
-54-
pressure liquid chromatography over a reversed phase column, and racemates can be
separated, for example, by the formation of salts with optically pure salt-forming reagents
and separation of the mixture of diastereoisomers so obtainable, for example by means of
fractional cryst~ s~tion~ or by chromatography over optically active column ",al~,ials.
Startinq materials:
The plese~l invention relates also to novel starting materials and/or interme~i~tes and to
processes for their preparation. The starting materials used and the reaction conditions
selected are preferably those that result in the compounds described as being prefer"ad.
Unless a specific method of synthesis is indicated for starting materials, the starting
materials are known, can be prepared according to processes known per se, especially in
analogy to methods given in the Examples, and/or are commercially available.
For example, suitably protected and/or preactivated D-, (D,L)- or L- amino acids, unnatural
amino acids, di-, tri- or oligopeptides, derivatized and/or preloaded resins, the ancillary
reagents and solvents required for either Boc or Fmoc peptide synthesis are commercially
available from various sl rrpliers or can be prepared readily according to standard
procedures. In addition, di- or other oligopeptoids can be prepared readily according to
standard procedures. In addition, automated peptide synthesizers with optimized,preprogrammed Boc and Fmoc synthesis cycles are available from numerous sources.
The starting materials for the phosphotyrosine mimics and the respective protected
derivatives can be synthesized according to methods known in the art; (e.g., for phosphono-
methyl-phenylalanine, especially 4-phosphonomethyl-phenylalanine, see Synthesis 1991,
1019, Tetrahedron Lett. 32(43), 6061 (1991) Tetrahedron Lett.33(9),1193 (1992) and
SynLett 1994, 233-254; for phosphono-(a-fluoro)methyl-phenylalanine, especially 4-phos-
phono-(a-fluoro)methyl-phenylalanine, see J. Chem. Soc., Perkin Trans.1,1986(1), 913-917
or Cancer Cells 2, 95 (1990) and J. Org. Chem 58,1336-1340 (1993), for phosphono-(a,a-
difluoro)methyl-phenylalanine, especially 4-phosphono-(a,a-difluoro)methyl-phenylalanine,
see Tetrahedron Lett.35, 551-554 (1994), Tetrahedron Lett. _(22), 3543 (1993) and
Tetrahedron Lett. 33(29), 4137 (1992), for phosphono-(a-hydroxy)methyl-phenylalanine,
CA 02227~16 1998-01-21
WO 97/08193 PCTAEP96/03473
especially 4-phosphono~ -hydroxy)methyl-phenylalanine, see Drugs of the Future 17, 119
(1992) and J. Org. Chem. 68, 1336-1340 (1 993), for O-sulfo-tyrosine, such as 4-(O-sulfo)-
tyrosine (= tyrosine sulfate), see Chem. Pharm. Bull. 41, 376-380 (1 993), for dicarboxy-
methoxy-phenylalanine (= (HOOC)2-CH2-O-phenylalanine, especially 4-(dicarLoxymethoxy)-
phenylalanine, see Abstract No. 014.024.114 presented at the 109;h American Ghemical
Society Meetin, April 2-6 (1 995) in Anaheim, California; and for phosphono-phenylalanine,
such as 4-phosphonophenylalanine, see Tetrahedron 46, 7793-7802 (1990)).
General Process conditions
The following applies in general to all processes mentioned hereinbefore and hereinarler,
while reaction conditions specifically mentioned above or below are pr~r,~d:
Functional groups in starting materials the reaction of which is to be avoided, espec~ y
carboxy, amino, hydroxy, mercapto and sulfo groups, can be protected by s~it~'s protect-
ing groups (conventional protecting groups) which are customarily used in the synthesis of
peptide compounds, and also in the synthesis of cephalosporins and penicillins as well as
nucleic acid derivatives and sugars. These protecting groups may already be presenl in the
precursors and are intended to protect the functional groups in question against undesired
secondary reactions, such as acylation, etherification, esterification, oxidation, solvolysis,
etc. In certain cases the protecting groups can additionally cause the reactions to proceed
selectively, for example stereoselectively. lt is characteristic of protecting groups that they
can be removed easily, i.e. without undesired secondary reactions taking place, for example
by solvolysis, reduction, photolysis, and also enzymatically, for example also under physio-
logical conditions, and, especially, that they are not present in the end products.
The protection of functional groups by such protecting groups, the protecting groups them-
selves and the reactions for their removal are described, for example, in standard works
such as J. F. W. McOmie, "Protective Groups in Organic Chemistry", Plenum Press, London
and New York 1973, in Th. W. Greene, "Protective Groups in Organic Synthesis", Wiley,
New York 1981, in "The Peptides", Volume 3 (E. Gross and J. Meienhofer, eds.), Academic
Press, London and New York 1981, in "Methoden der organischen Chemie", Houben-Weyl,
~ 4th edition, Volume 1 ~/I, Georg Thieme Verlag, Stuttgart 1974, in H.-D. Jakubke and H.
Jescheit, "Aminosauren, Peptide, Proteine" ("Amino acids, peptides, proteins"), Verlag
CA 02227~16 1998-01-21
W O 97/08193 PCTAEP96/03473
-56-
Chemie, Weinheim, Deerfield Beach and Basle 1982, and in Jochen Lehmann, "Chemieder Kohlenhydrate: Monosaccharide und Derivate" ("The Chemistry of Carbohydrates:
monosaccharides and derivatives"), Georg Thieme Verlag, Stuttgart 1974.
When several pr~,Lecl~d functional groups are present, if desired the protecting groups can
be so selected that more than one such group can be removed simultaneously, for example
by acidolysis, such as by treatment with trifluoroacetic acid, or with hydrogen and a
hydrogenation catalyst, such as a p~ on-carbon catalyst Conversely, the groups can
also be so selected that they cannot all be removed simultaneously, but rather in a desired
sequence, the cor.~spondi~,g intermediates being obtained.
In view of the close relationship between the compounds of formula I and their salts and
starting materials (starting materials and intermediates) in free form and in the form of their
salts, any reference hereinbefore and hereinafter to a free compound or a salt thereof is to
be understood as meaning also the corresponding salt or free compound, respectively,
where appropriate and expedient.
All the above-mentioned process steps can be carried out under reaction conditions that are
known ~r se, preferably those mentioned specifically, in the absence or, customarily, in the
presence of solvents or diluents, preferably solvents or diluents that are inert towards the
reagents used and dissolve them, in the absence or presence of catalysts, condensation
agents or neutralising agents, for example ion exchangers, such as cation exchangers, e.g.
in the Hi form, depending on the nature of the reaction and/or of the reactants at reduced,
normal or elevated temperature, for exd",ple in a temperature range of from approximately -
1 00~C to app~ uxi" Id~ly 1 90~C, preferably from approximately -80~C to approximately
1 50~C, for example at from -80 to -60~C, at room temperature, at from -20 to 40~C or at
reflux temperature, under atmospheric pressure or in a closed vessel, where appropriate
under pressure, and/or in an inert atmosphere, for example under an argon or nitrogen
atmosphere.
At all stages of the reactions, mixtures of isomers that are formed can be separated into the
individual isomers, for example diastereoisomers or enantiomers, or into any desired mix-
CA 02227~16 1998-01-21
WO 97/08193 PCT/EP96/03473
- 57 -
~, .
tures of isomers, for example racemates or mixtures of diastereoisomers, for example ana-
logously to the methods described under "Additional process steps".
The solvents from which those solvents that are suitable for any particular reaction may be
selected include, for ex~rl,~lE, water, esters, such as lower alkyl-lower alkanoates, for
example ethyl acetate, ethers, such as aliphatic ethers, for example diethyl ether, or cyclic
ethers, for example tetrahydrofuran, liquid aromatic hydrocarbons, such as benzene or
toluene, alcohols, such as methanol, ethanol or 1- or 2-propanol, nitriles, such as aceto-
nitrile, halogenated hydrocarbons, such as methylene chloride, acid amides, such as
dimethylformamide, bases, such as heterocyclic nitrogen bases, for example pyridine,
carboxylic acid anhydrides, such as lower alkanoic acid anhydrides, for example acetic
anhydride, cyclic, linear or branched hydrocarbons, such as cyclohexane, hexane or iso-
pentane, or mixtures of those solvents, for example aqueous solutions, unless otherwise
indicated in the description of the processes. Such solvent mixtures may also be used in
working up, for example by chromatography or partitioning.
The compounds, including their salts, may also be obtained in the form of hydrates, or their
crystals may, for example, include the solvent used for cryst~ s~tion. Different crystalline
forms may be present.
If necessary, prote~;led starting materials may be used in all process steps and the
protecting groups may be removed at suitable stages of the reaction.
The invention relates also to those forms of the process in which a compound obtainable as
intermediate at any stage of the process is used as starting material and the remaining
process steps are carried out, or in which a starting material is formed under the reaction
conditions or is used in the form of a derivative, for example in protected form or in the form
of a salt, or a compound obtainable by the process according to the invention is produced
under the process conditions and processed further in situ. In the process of the present
invention there are preferably used those starting materials which result in the compounds
of formula I described at the beginning as being especially valuable. Special preference is
given to reaction conditions that are analogous to those mentioned in the Examples.
.
CA 02227~l6 l998-0l-2l
W O 97/08193 PCT~EP96/03473
-58-
Pharmaceutical ComPositions:
The invention relates also to pharmaceutical compositions comprising compounds of
formula 1, to their use in the therapeutic (including prophylactic) treatment of the diseases
mentioned above, to the compounds for said use and to the preparation of pharm~celltic~l
prepardlions.
The pharmacologically acceptable compounds of the present invention may be used, for
example, for the preparation of phar",aceutical compositions that comprise an effective
amount of the active ingredient together or in admixture with a sig, li~icant amount of
inorganic or organic, solid or liquid, pharmaceutically acce~.L~ble carriers.
The invention relates also to a pharmaceutical composition that is suitable for acl",i";JI-~Iion
to a warm-blooded animal, especi~lly a human (or to cells or cell lines derived from a warm-
blooded animal, especially a human, e.g. Iymphocytes), for the treatment or preven-tion of
(= prophylaxis against) a disease that responds to diseases that respond to inhibition of the
interaction of p,ul~,;.,s comprising SH2 domains and phosphoproteins, especially a
phosphorylated protein tyrosine kinase or modified versions thereof, pr~erably inhibition of
the interaction of Grb2 SH2 with a phosphoprotein containing a -Tyr(PO3H2)-X-Asn- motif,
such as phosphorylated EGFR protein tyrosine kinase or modified derivatives thereof, but
also other phospho-proteins such as SHC or modified derivatives thereof, comprising an
amount of a com-pound of formula I or a pharmaceutically ~3ccept~hle salt thereof, which is
effective for said inhibi-tion, especially the inhibition of the interaction of Grb2 SH2 with with
a phosphoprutei" con-taining a -Tyr(PO3Hz)-X-Asn- motif, such as phosphorylated EGFR
protein tyrosine kinase or modified derivatives thereof, but also other phospho-proteins
such as SHC, or truncated derivatives thereof, together with at least one pharmaceutically
acceptable carrier.
The pharmaceutical compositions according to the invention are those for enteral, such as
nasal, rectal or oral, or par~llLerdl, such as intramuscular or intravenous, adlllini-;lrdlion to
warm-blooded animals (humans and animals), that comprise an effective dose of the
pharmacologically active ingredient, alone or together with a significant amount of a
pharmaceutically acceptable carrier. The dose of the active ingredient depends on the
CA 02227~16 1998-01-21
WO 97/08193 PCT/EP96/03473
. -59-
species of warm-blooded animai, the body weight, the age and the individual condition,
individual pharmacokinetic data, the disease to be treated and the mode of ad",i~ ldlion.
.
The invention relates also to a method of treating diseases that respond to inhibition of the
interaction of prul~ s comprising SH2 domains and phosphopr~tei"s, especially the phos-
phorylated protein tyrosine kinases or modified versions thereof; prert:ldbly of Grb2 SH2
with a phospho-protein containing a -Tyr(PO3H2)-X-Asn- motif, such as phosphorylated
EGFR protein tyrosine kinase or modified derivatives thereof, but also other phospho-
prul~,;.,s such as SHC or modified derivatives thereof; which comprises ad""nisle,i"g a
prophylactically or especially therapeutically effective amount of a compound of formula I
according to the invention, especially to a warm-blooded animal, for example a human, that,
on account of one of the mentioned diseases, requires such treatment. The dose to be
ad",;"i;,lered to warm-blooded animals, for example humans of approxi",dlely 70 kg body
weight, is from apprc xil"ately 3 mg to approxi, lldLely 30 9, pr~r~rably from approximately 10
mg to approximately 1.5 9, for example approkill,dlely from 100 mg to 1000 mg per person
per day, divided preferably into 1 to 3 single doses which may, for example, be of the same
size. Usually, children receive half of the adult dose.
The pharmaceutical compositions comprise from approximately 1 % to approximately 95%,
preferably from approximately 20 % to approximately 90%, active ingredient. Pharma-
ceutical compositions according to the invention may be, for example, in unit dose form,
such as in the form of ampoules, vials, suppositories, dragées, tablets or capsules.
The pharmaceutical compositions of the present invention are prepared in a manner known
per se, for example by means of conventional dissolving, Iyophilising, mixing, granulating or
confectioning processes.
Solutions of the active ingredient, and also suspensions, and especially isotonic aqueous
solutions or suspensions, are preferably used, it being possible, for example in the case of
Iyophilised compositions that comprise the active ingredient alone or together with a carrier,
for example mannitol, for such solutions or suspensions to be produced prior to use. The
pharmaceutical compositions may be sterilised and/or may comprise excipients, for example
preservatives, stabilisers, wetting and/or emulsifying agents, solubilisers, salts for regulating
the osmotic pressure and/or buffers, and are prepared in a manner known per se, for
CA 02227~16 1998-01-21
WO 97/08193 PCTAEP96/03473
-60-
example by means of conventional dissolving or Iyophilising processes. The said solutions
or suspensions may comprise viscosity-increasing substances, such as sodium carboxy-
methylcellulose, carboxymethylcellulose, dextran, polyvinylpyrrolidone or gelatin.
Suspensions in oil comprise as the oil component the vegetable, synthetic or semi-synthetic
oils customary for injection purposes. There may be mentioned as such especially liquid
fatty acid esters that contain as the acid component a long-chained fatty acid having from 8
to 22, especially from 12 to 22, carbon atoms, for example lauric acid, tridecylic acid,
myristic acid, pentadecylic acid, palmitic acid, margaric acid, stearic acid, arachidic acid,
behenic acid or corresponding unsaturated acids, for example oleic acid, elaidic acid, erucic
acid, brasidic acid or linoleic acid, if desired with the addition of antioxidants, for example
vitamin E, ,~-carotene or 3,~i-di-tert-butyl-4-hydroxytoluene. The alcohol component of those
fatty acid esters has a maximum of 6 carbon atoms and is a mono- or poly-hydroxy, for
example a mono-, di- or tri-hydroxy, alcohol, for example methanol, ethanol, propanol,
butanol or pentanol or the isomers thereof, but especially glycol and glycerol. The following
examples of fatty acid esters are therefore to be mentioned: ethyl oleate, isopropyl
myristate, isopropyl pal~lli~tt:, "Labrafil M 2375" (polyoxyethylene glycerol trioleate,
Gattefossé, Paris), "Miglyol 812" (triglyceride of saturated fatty acids with a chain length of
C8 to C12, Huls AG, Germany), but especially vegetable oils, such as cottonseed oil, almond
oil, olive oil, castor oil, sesame oil, soybean oil and more especially groundnut oil.
The injection compositions are prepared in customary manner under sterile conditions; the
same applies also to introducing the compositions into ampoules or vials and sealing the
containers.
Pharmaceutical compositions for oral administration can be obtained by combining the
active ingredient with solid carriers, if desired granulating a resulting mixture, and process-
ing the mixture, if desired or necessary, after the addition of appropriate excipients, into
tablets, dragée cores or capsules. It is also possible for them to be incorporated into plastics
carriers that allow the active ingredients to diffuse or be released in measured amounts.
Suitable carriers are especially fillers, such as sugars, for example lactose, sàccharose,
mannitol or sorbitol, cellulose preparations and/or calcium phosphates, for example
tricalcium phosphate or calcium hydrogen phosphate, and binders. such as starch pastes
CA 02227~16 1998-01-21
WO 97/08193 PCT~EP96/03473
-61 -
using for example corn, wheat, rice or potato starch, gelatin, tragacanth, methyicellulose,
hydroxypropylmethylcelluiose, sodium carboxymethylcellulose and/or polyvinylpyrrolidone,
and/or, if desired, disintegrators, such as the above-mentioned starches, also carboxy-
methyl starch, crosslinked polyvinylpyrrolidone, agar, alginic acid or a salt thereof, such as
sodium alginate. Excipients are especially flow conditioners and lubricants, for example
silicic acid, talc, stearic acid or salts thereof, such as magnesium or calcium stearate, and/or
polyethylene glycol. Dragée cores are provided with suitable, optionally enteric, coatings,
there being used, inter alia, concentrated sugar solutions which may comprise gum arabic,
talc, polyvinylpyrrolidone, polyethylene glycol and/or titanium dioxide, or coating solutions in
suitable organic solvents, or, for the preparation of enteric coatings, solutions of suitable
cellulose preparations, such as ethylcellulose phthalate or hydroxypropylmethylcellulose
phthalate. Capsules are dry-filled c~psules made of gelatin and soft sealed capsules made
of gelatin and a p!~ticicer~ such as glycerol or sorbitol. The dry-filled c~psl ~les may
co",prise the active ingredient in the form of granules, for example with fillers, such as
lactose, binders, such as starches, and/or glidants, such as talc or magnesium :jL~a-dLe,
and if desired with st~ rs. In soft capsules the active ingredient is preferably di~solved
or suspended in suitable oily excipients, such as fatty oils, paraffin oil or liquid polyethylene
glycols, it being possible also for stabilisers and/or antibacterial agents to be added. Dyes or
pigments may be added to the tablets or dragée coatings or the capsule casings, for
example for idenLiricaLion purposes or to indicate different doses of active ingredient.
ExamPles:
Embodiments of the invention are described in the following specific ex~" Fles which are
not to be construed to be intended to limit the scope of the invention in any way, but serve
merely for illustration:
Temperatures, if not mentioned: room temperature/ambient temperature. In mixtures,
relations of parts of solvent or eluent or reagent mixtures in liquid form are given as volume
relations (v/v), if not indicated otherwise.
Symbols used for amino acids and peptides are in accordance with IUPAC-IUB Co"""ission
on Biochemical Nomenclature. If not mentioned otherwise, amino acids are present in the L-
form. Other abbreviations used are:
CA 02227~16 1998-01-21
O 97/08193 PCTlEP96/03473
-62-
Abbreviations: Acsc: bivalent radical of 1-aminocyclopentane carboxylic acid (Aldrich,
Buchs, Switzerland); Ac6c: bivalent radical of 1-aminocyclo-hexane carboxylic acid (Fluka,
Buchs, Switzerland); Ac7c = bivalent radical of 1-aminocyclopentane carboxylic acid (see N.
Zelinsky and G. Stadnikoff, Chem. Ber., 39, 1722-1732, (1906)); ~AIa: beta Alanyl =
bivalent radical of 3 aminopropionic acid; Boc: tert-Butoxycarbonyl; BOP: ben~uL-i~cle 1-yl-
oxy-tris-(dimethylamino)-phosphoniumhexa-fluorophosphate; DHph = bivalent radical of D-
homophenylalanine (Bachem, Bubendorf, Switzerland) ;Fmoc: fluorenylmethoxy-carbonyl;
Fmoc-PAL-PEG-PS (Millipore, Bedford, USA): a resin used for peptide synthesis; F2Pmp:
4-phosphono(difluoromethyl)-L-phenyl-alanine; HATU = N-[(dimethylamino)-1H-1,2,3-
triazolo[4,5-b~pyridin-1-ylmethylene]-N-methylmethanaminiumhexafluorophosphate N-oxide;
HBTU: 2-(1 H-benzotriazol-1-yl)-1,1 ,3,3-tetramethyluronium tetrafluoroborate; HOBt: N-
hydroxy-be"~ul,ia~ole; Hph = bivalent radical of L-homophenylalanine (Bachem, Bubendorf,
Switzerland); HPLC: high performance liquid chromatography; HPLC System A is defined in
Example 1; HPLC System B is defined in Example 45; HPLC System C is defined in
Example 51; HPLC system D is defined in Example 123; MeCN = acetonitrile; Nbo =
bivalent radical of 2-amino-norbornane carboxylic acid (Aldrich, Buchs, Switzerland); PAL:
tris-alkyl- (= methyl-)-oxybenzylamide linker; PEG: polyethylene glycol; Pmp = bivalent
radical of p-phosphonomethyl-phenylalanine (see Example 121); R~: ratio of fronts in thin
layer chromatography employing silica gel plates (Merck, Darmstadt, FRG); TFA =
trifluoroacetic acid; Tle = bivalent radical of L-a-t-butyl-glycine (= tert-leucine; see Example
120); tR: retention time in HPLC; Tyr(PO3H2): [p-(O-Phosphono)-Tyr].
Examcle 1: 3-Aminobenzyloxycarbonyl-Tyr(PO3H2)-lle-Asn-Gln-NH2 (TFA salt)
(SEQ ID NO: 1 )
The peptide is synthesized manually on a 4-(2',4'-dimethoxyphenyl-aminomethyl)-phenoxy-
resin (Fmoc-protected at the amino group, obtainable from Novabiochem, Laufelfingen,
Switzerland; 0.47 mmol/g, 300 mg), employin~ the fluorenylmethoxycarbonyl (Fmoc)strategy (see E. Atherton and R.C. Sheppard, in Solid-Phase Peptide Synthesis-A Practical
Approach, ed. D. Rickwood and B.D. Hames, IRL Press at Oxford University Press, Oxford,
1989). Fmoc is removed with piperidine/dimethylacetamide (1:4, v/v; 6 x 2 min), followed by
washing with methanol (3 x 1 min), N-methylpyrrolidin-2-one (2 x 1 min), methanol (3 x 1
min), and N-methylpyrrolidin-2-one (3 x 2 min). Coupling is achieved by first dissolving the
CA 02227~16 1998-01-21
WO 97/08193 PCTAEP96/03473
-63-
Fmoc-amino acid (2 equiv.), diisopropylethylamine (2.2 equiv.), and the 2-(2-pyridon-1-yl)-
1,1,3,3-tetramethyluroniu",lelldrluoroborate reagent (TPTU, Senn Chemicals, Dielsdorf,
Switzerland; 2 equiv.) in N-methylpyrrolidin-2-one, then waiting 3 min for preactivation,
adding the mixture to the resin, and finally shaking for at least 45 min. The side chain of
asparagine and glutamine is pl ulecLed with the trityl group. The incorporation of Na-Fmoc-
Tyr(PO3H2)-OH (see Biochemistry 32, 4354 (1993)) (3 equiv.) is acco",plished with
be~ ,~uI~ia~ole-1 -yl-oxy-tris-(dimethylamino)-phosphoniumhexafluorophosphate/N-hydroxyben~ulria,ole (1:1; 3 equiv.; first coupling) in the presence of di;sop,c pylethylamine
(7 equiv.) and N-[(dimethylamino)-1 H-1 ,2,3-triazolo[4,5-b]pyridin-1 -ylmethylene]-N-
methylmethanaminium hexafluorophosphate N-oxide (3 equiv., second coupling) in the
presence of diisopropylethylamine (7 equiv.). 3-N-fert-butoxy-carbonyl-aminobenzyl-4-
nitrophenyl-carbonate (3 equiv.) is coupled to the N-terminal residue of the peptide resin in
the presence of an equimolar amount of diisopropylethyl-amine in N-methylpyrrolidin-2-one
during 17 h at room temperature. The complete peptide resin obtained after the final
coupling reaction is simultaneously deprotected and cleaved by treatment with
trifluoroacetic acid/H2O (95:5, v/v) for 3 h at room temperature. The filtrate from the
cleavage reaction is preciri':~ted in diisopropyl ether-petroleum ether (1:1, v/v, 0 ~C), and
the precipitate is collected by filtration. The crude peptide is purified by medium-pressure
liquid chromatography using a C1 8-column eluted with an acetonitrile-water gradient
containing 0.1 % of TFA (Merck ~LICHROPREP RP-18, 15-25 um bead diameter, reversed
phase HPLC column material based on C,8-derivatized silicagel, Merck, Darmstadt, FRG;
column length 46 cm, diameter 3.6 cm; flow rate 53.3 ml/min; detection at 215 nm). The title
compound is obtained: Mass spectral analysis (matrix-assisted laser-desoption ionization
time-of-flight mass spectrometry, MALDI-TOF) rcvcalcs a molecular mass within 0.1 % of
the expected values (negative-ion mode): 763.7 (calc 763 7, C32H44N8~12P1)- The purity
of the peptide is verified by reversed-phase analytical HPLC on a Nucleosil C1 g-column
(250 x 4 mm, 5 ~lm, 100 A): linear gradient over 10 min of MeCN/0.09% TFA and
H2O/0.1%TFA from 1:49 to 3:2; flow rate 2.0 ml/min, detection at 215 nm; single peak at
tR= 5.22 min (HPLC System A).
The starting materials are prepared as follows:
-
a) 3-N-tert-Butoxycarbonvl-aminobenzvl alcohol
CA 02227~16 1998-01-21
WO 97/08193 PCT~EP96/03473
-64-
Boc2O (1 3.4 ml, 60 mmol) is added to a solution of 3-aminobenzyl alcohol (2.46 g,
20 mmol; Aldrich, Buchs, Switzerland) in 0.5 N NaOH (25 ml) and stirring is continued until
completion of the reaction. The alkaline solution is extracted with petroleum ether (2x10 ml);
the ether extracts are discarded. The aqueous phase is acidified with a 5% solution of citric
acid to pH 2-3 and extracted with ethyl acetate (2x30 ml). The ethyl acetate extracts are
pooled, washed with water (2x30 ml), dried over anhydrous Na2SO4 and evaporated in
vacuo. Rf= 0.72 (chloroform:methanol:water:acetic acid = 750:270:50:5, v/v/v/v).
b) 3-N-tert-Butoxycarbonvl-aminobenzYl-4-nitrophenvl-carbonate
A solution of 3-N-tert-butoxycarbonyl-aminobenzyl alcohol (1.0 9) in anhydrous pyridine
(18 ml) is cooled in an ice-water bath and 4-nitrophenyl chloroformate is added with stirring.
The solution is stirred for 17 h at room temperature and then added to a mixture of
diisopropyl ether/petroleum ether (1 :1, v/v; 200 ml). The ether mixture is extracted with brine
(1x50 ml) and then with water (7x 50 ml), dried over anhydrous Na2SO4 and evaporated in
vacuo. The crude compound is purified by flash chromatography on silica gel, using
dichloromethane as eluent. Rf= 0.66 (chloroform/methanol = 95:5, v/v).
The following examples are obtained in analogy to the title compound of Example 1:
Example 2: 2-Aminobenzyloxycarbonyl-Tyr(PO3H2)-lle-Asn-Gln-NH2 (TFA salt)
(SEQ ID NO: 2)
Title compound: Mass spectral analysis (negative-ion mode): 763.3 (calc. 763.7,
C32H44N8~12P1). tR= 5.50 min (HPLC System A).
The starting material is obtained as follows:
a) 2-N-tert-Butoxwarbonyl-aminobenzyl-4-nitro,~henvl-carbonate
The title compound is synthesized as described for 3-N-tert-butoxycarbonyl-aminobenzyl-4-
nitrophenyl-carbonate (Examples 1 a) and b)). Rf= 0.92 (dichloromethane:methanol:wa-
ter:acetic acid = 850:130:15:5, v/v/v/v), starting from 2-aminobenzyl alcohol (Aldrich, Buchs,
Switzerland).
Example 3: 3-Aminobenzyloxycarbonyl-Tyr(PO3H2)-lle-Asn-NH2 (TFA salt)
CA 02227~16 1998-01-21
W O97/08193 PCTAEP96/03473
- 65 -
(SEQ ID NO: 3)
Title compound: Mass spectral analysis (negative-ion mode): 635.7 (calc. 635.6,
C27H36N6~1 oP1), tR= 5 54 min (HPLC System A).
ExamPle 4:1-(3-Aminophenyl)ethyloxycarbonyl-Tyr(PO3H2)-lle-Asn-Gln-NH2 (TFA salt)
(SEQ ID NO: 4), Diastereomer A:
Title compound: Mass spectral analysis (negative-ion mode): 777.8 (calc. 777.8,
C33H46N8~1 2P1), tR= 5 41 min (HPLC System A).
The starting material is obtained as follows:
a) 1-(3-N-tert-Butoxycarbonyl-aminophenyl)ethvl-4-nitrophenvl-carbonate
The title compound is synthesized as described for 3-N-tert-butoxycarbonyl-aminobenzyl-4-
nitrophenyl-carbonate (Example 1 b)), starting from 1-(3-aminophenyl)ethanol (Aldrich,
Buchs, Switzerland). Rf= 0.54 (chloroform:methanol:water:acetic acid = 900:100:10:5,
v/v/v/v) .
ExamPle 5: 1-(3-Aminophenyl)ethyloxycarbonyl-Tyr(P03H2)-lle-Asn-Gln-NH2 (TFA salt)
(SEQ ID NO: 5) Diastereomer B:
Title compound: Mass spectral analysis (negative-ion mode): 777.2 (calc. 777.8,
C33H46N8~1 2P1), tR= 5.61 min(HPLC System A).
Example 6: 3-Aminobenzyloxycarbonyl-Tyr(PO3H2)-Ac5c-Asn-Gln-NH2 (TFA salt)
(SEQ ID NO: 6)
Title compound: Mass spectral analysis (negative-ion mode): 761.7 (calc. 761.7,
C32H42N8O12P1)~ tR= 4.28 min (HPLC System A).
The starting material is obtained as follows:
a) Fmoc-L-amino-1-cvclopentanecarboxvlic acid
The title compound is synthesized starting from 1-amino-1-cyclopentanecarboxylic acid
(Aldrich, Buchs, Switzerland) according to a procedure known in the art (see E. Atherton et
al., in: Solid-Phase Peptide Synthesis - A Practical Approach; D. Rickwood an B.D. Hames,
CA 02227~16 1998-01-21
WO 97/08193 PCT/EP96/03473
-66- c
IRL Press at Oxford University Press, Oxford, 1989): Rf = 0.79 (chloro-form:methanol:water:acetic acid 750:270:50:5, v/v/v/v).
ExamPle 7: 3-Aminobenzyloxycarbonyl-Tyr(PO3H2)-Ac6c-Asn-Gln-NH2 (TFA salt)
(SEQ ID NO: 7)
Title compound: Mass spectral analysis (negative-ion mode): 775.6 (calc. 775.7,
C33H44N8O12P1), tR= 5.33 min (HPLC System A) .
The starting material is prepared as follows:
a) Fmoc-L-amino-1-cvclohexanecarboxvlic acid
The title compound is synthesized starting from 1-amino-1-cyclohexanecarboxylic acid
(Janssen, Olen, Belgium) according to a procedure known in the art (see E. Atherton et al.,
in: Solid-Phase Peptide Synthesis - A Practical Approach; D. Rickwood an B.D. Hames, IRL
Press at Oxford University Press, Oxford, 1989): Rf = 0.84 (chloro-form:m~lhanol;wa
ter:acetic acid 750:270:50:5, v/v/v/v).
a) Fmoc-L-amino-1-cvclopentanecarboxvlic acid
The title compound is synthesized starting from 1-amino-1-cyclopentanecarboxylic acid
(Aldrich, Buchs, Switzerland) according to a procedure known in the art (see E. Atherton et
al., in: Solid-Phase Peptide Synthesis - A Practical Approach; D. Rickwood an B.D. Hames,
IRL Press at Oxford University Press, Oxford,1989): Rf = 0.79 (chloro-
form:methynol:water:acetic acid 750:270:50:5, v/v/v/v).
Example 8: 2-(4-Methyl-5-thiazolyl)-ethyloxycarbonyl-Tyr(PO3H2)-lle-Asn-Gln-NH2
(SEQ ID NO: 8)
Title compound: Mass spectral analysis (negative-ion mode): 783.7 (calc. 783.8,
C31 H44N8~12P1 S1), tR= 5-09 min(HPLC System A).
The starting material is obtained as follows:
a) 2-(4-Methyl-5-thiazole)-ethyl-4-nitrophenyl-carbonate
CA 02227~16 1998-01-21
W O 97/08193 PCT~EP96/03473
The title compond is synthesized as described for 3-N-tert-butoxycarbonyl-aminobenzyl-4-
nitrophenyl-carbonate (Example 1 b)), starting from 2-(4-methyl-5-thiazolyl)-ethanol (Aldrich,
Buchs, Switzerland). Rf= 0.72 (chloroform:methanol:water:acetic acid = 850:130:15:5,
v/v/v/v) .
Example 9: 2-(3-Hydroxyphenyl)ethyloxycarbonyl-Tyr(PO3H2)-lle-Asn-Gln-NH2
(SEQ ID NO: 9)
In the final reaction step, the N-terminal group is coupled as 2-(3-tert-butoxycarbonyl-oxy-
phenyl)-ethyl-4-nitropheny!-dicarbonate. Title compound: Mass spectral analysis (negative-
ion mode): 778.4 (calc. 778.7, C33H45N7O13P1), tR= 6.44 min (HPLC System A).
The starting material is prepared as follows:
a) 2-(3-tert-ButoxycarbonyloxyPhenyl)-ethanol
A suspension of 2-(3-hydroxyphenyl)ethanol (3.7 9, 29.8 mmol; Fluka, Buchs, Switzerland))
and potassium carbonate (6 9, 43.4 mmol) in dichloromethane (6 ml) is cooled in an ice-
water; di-tert-butyl-dicarbonate (6.54 g, 30 mmol) and 18-crown-6 (Aldrich, Buchs,
Switzerland) are added and the mixture is allowed to warm up to room temperature under
vigorous stirring. Stirring is continued overnight. The solution is extracted with brine, dried
over Na2SO4 and dried in vacuo. The crude compound is subjected to flash chromato-
graphy on silica gel using dichloromethane/methanol (95:5, v/v) as eluent. Rf= 0.66
(ch'3rorurm:methanol:water:acetic acid = 850:130:15:5, v/v/v/v).
b) 2-(3-tert-butoxvcarbonyl-oxyphenvl)ethyl-4-nitroPhenvl-carbonate
The title compound is synthesized in analogy to the method described for 3-N-tert-butoxy-
carbonyl-aminobenzyl-4-nitrophenyl-carbonate (Example 1 b)), starting from 2-(3-tert-
butoxycarbonyloxy-phenyl)ethanol: Rf= 0,81 (chloroform:methanol = 95:5, v/v).
Example 10: 3-Hydroxybenzyloxycarbonyl-Tyr(PO3H2)-lle-Asn-Gln-NH2
(SEQ ID NO: 10)
The N-terminal group is coupled as 3-(tert-butoxycarbonyl-oxy)-benzyl-4-nitrophenyl-
dicarbonate. Title compound: Mass spectral analysis (negative-ion mode): 764.8 (calc.
764 7, C32H43N7O13P1). tR= 6.57 min (HPLC System A).
CA 02227~l6 l998-0l-2l
WO 97/08193 PCT/EP96/03473
- 68 -
The starting material is prepared as follows:
a) 3-O-tert-Butoxycarbonyl-oxybenzvl-4-nitrophenvl-carbonate
3-O-tert-butoxycarbonyi-oxybenzyl-4-nitrophenyl-carbonate is synthesized as described for
3-N-tert-butoxycarbonyl-aminobenzyl-4-nitrophenyl-carbonate (Example 1 b), starting from
3-hydroxybenzyl alcohol (Fluka, Buchs, Switzerland). Rf= 0.81 (cl1lo.ururm:methanol:wa-
ter:acetic acid = 850:130:15:5, v/v/v/v).
Example 11: 3-Aminobenzyloxycarbonyl-Tyr(PO3H2)-Ac6c-~Ala-NH2 (TFA salt)
(SEQ ID NO: 1 1 )
Title compound: Mass spectral analysis (negative-ion mode): 604.1 (calc. 604.6,
c27H35Nsosp1), tR= 5.43 min (HPLC System A).
Example 12: 3-Aminobenzyloxycarbonyl-Tyr(PO3H2)-Ac6c-~ly-NH2 (TFA salt)(SEQ ID NO: 12)
Title compound: Mass spectral analysis (negative-ion mode): 590.5 (calc. 590.6,
C26H33N5O9P1 ), tR= 5.93 min (HPLC System A).
Example 13: Benzyloxyca,L,onyl-Tyr(PO3H2)-lle-Asn-Gln-NH2
(SEQ ID NO: 13)
Title compound: Mass spectral analysis (negative-ion mode): 748.6 (calc. 748.7,
C32H43N7~12P1), tR= 7.37 min (HPLC System A).
Example 14: 3,5-Diaminobenzyloxycarbonyl-Tyr(PO3H2)-lle-Asn-Gln-NH2 (TFA salt)
(SEQ ID NO: 14)
The N-terminai group is coupled as 3,s-di-tert-butoxycarbonyl-diaminobenzyl-4-nitrophenyl-
dicarbonate. Title compound: Mass spectral analysis (negative-ion mode): 778.8 (calc.
778 7, C32H4sNsO1 2P1), tR= 4.69 min (HPLC System A).
The starting material is prepared as follows:
a) 3.5-N,N-di-tert-Butoxvcarbonyl-aminobenzvl-4-nitrophenvl-carbonate
CA 02227~16 1998-01-21
O 97/08193 PCT/EP96/03473
The title compound is synthesized as described for 3-N-tert-butoxycarbonyl-aminobenzyl-4-
nitrophenyl-carbonate (Example 1 b)), starting from 3,5-diaminobenzyl alcohol (Aldrich,
Buchs, Switzerland, 2 HCI form. Rf= 0.71 (chloroform:methanol:water:acetic acid =
850:130:15:5, v/v/v/v).
Example 15: 3-(3-lndolyl)propionoyl-Tyr(PO3H2)-lle-Asn-Gln-NH2
(SEQ ID NO: 15)
3-(3-indole)-propionic acid (Aldrich, Buchs, Switzerland) is coupled with BOP/HOBt (1:1; 10
equiv.) in the presence of diisopropylethylamine (25 equiv.) in N-methylpyrrolidin-2-one,
yielding the title compound: Mass spectral analysis (negative-ion mode): 786.6 (calc. 785.8,
C35H46N8~11 P1), tR= 7 11 min (HPLC System A).
Example 1 6: 3-(N-Acetylamino)phenoxyacetyl-Tyr(PO3H2)-lle-Asn-Gln-NH2
(SEQ ID NO 16)
3-(N-Acetylamino)-phenoxy-acetic acid (2 equiv.) is coupled with BOP/HOBt (1 :1; 2 equiv.)
in the presence of diisopropylethylamine (4 equiv.) in N-methylpyrrolidin-2-one, yielding the
title compound: Mass spectral analysis (negative-ion mode): 805.7 (calc. 805.8,
C34H46N8~13P1), tR= 6.02 min (HPLC System A).
The starting material is prepared as follows:
a) 3-(N-Acetvlamino)phenoxv-acetic acid (= 3-(N-acetamido)Phenoxyacetic acid)
NaOH (2.08 9; 52 mmol; Fluka, Buchs, Swikerland) and chloroacetic acid (2.46 g, 26 mmol)
are added to a solution of m-(acetylamino)-phenol (4.0 g, 26 mmol; Fluka, Buchs,Switzerland) in water (10 ml). The solution is incutl~ted under reflux conditions for 1.5 h,
and a further quantity of NaOH (1.04 g ) and chloroacetic acid (1.23 g) is added. 2.5 h later,
the solution is cooled to room temperature and acidified with a concenlrdled solution of HCI.
The precipitate is filtered and crystallized from water, yielding the title compound: Rf= 0.38
(ethyl acetate:methanol = 4:1 (v/v) with 0.5 % acetic acid).
Example 1 7: 4-(N-Acetylamino)phenoxyacetyl-Tyr(PO3H2)-lle-Asn-Gln-NH2 (TFA salt)
(SEQ ID NO: 17)
CA 02227~16 1998-01-21
WO 97/08193 PCTrEP96/03473
- 70 -
The title compound is prepared using a procedure analogous to Example 16, starting from
4-(N-acetylamino)phenoxy-acetic acid: Mass spectral analysis (negative-ion mode): 805.7
(calc- 805.8~ C34H46N8o13p1)~ tR= 5.73 min (HPLC System A).
The starting material is obtained as follows:
a) 4-(N-Acetvlamino)phenoxv-acetic acid
The title compound is prepared in analogy to the method described for N-(3-acetylamino)-
phenoxy-acetic acid (Example 16 a)): Rf = 0.42 (ethyl acetate: methanol = 4:1 (v/v), with
0.5 % acetic acid).
ExamPle 18: N-(2-Acetylamino)phenoxyacetyl-Tyr(PO3H2)-lle-Asn-Gln-NH2
(SEQ ID NO: 18)
The title compound is prepared using a procedure analogous to the method in Example 16,
starting from 2-(N-acetylamino)phenoxy-acetic acid: Mass spectral analysis (negative-ion
mode): 805.8 (calc. 805.8, C34H46N8O13P1), tR= 6.92 min (HPLC System A).
The starting material is obtained as follows:
a) 2-(N-Acetvlamino)phenoxv-acetic acid
The title compound is synthesized in analogy to the method described for 3-(N-
acetylamino)phenoxy-acetic aicd (Example 16 a)): Rf= 0.47 (ethyl acetate:methanol = 3:2
(v/v) with 0.5 % acetic acid).
Example 19: 4-Aminophenoxyacetyl-Tyr(PO3H2)-lle-Asn-Gln-NH2
(SEQ ID NO: 19)
4-(N-tert-butoxycarbonyl-amino)-phenoxyacetic acid (3 equiv.) is coupled with BOP/HOBt
(1 :1; 3 equiv.) in the presence of diisopropylethylamine (7 equiv.) in N-methylpyrrolidin-2-
one (double coupling). Title compound: Mass spectral analysis (negative-ion mode): 763.5
(calc 763.7~ C32H44N8o12p1)~ tR= 5.15 min (HPLC System A).
The starting material is obtained as follows:
CA 02227~16 1998-01-21
W O 97/08193 PCT/EP96tO3473
t - 71 -
a) 4-(N-tert-Butoxycarbonylamino)phenoxv-acetic acid
A soiution of 4-(N-acetylamino~phenoxy-acetic acid (Example 17 a)) (1.99 g, 8.77 mmol) in
water (8 ml) and concentrated HCI (37 %, 8 ml) is refluxed for 2.5 h. The solution is cooled
in an ice bath, and the precipitate is filtered. Incorporation of of the Boc group at the free
amino group is carried out as described for 3-(N-tert-butoxycarbonyl)aminobenzyl alcohol
(Example 1 a)), yielding the title compound: Rf= 0.58 (ethyl acetate:methanol = 3:1 (v/v) with
0.5 % acetic acid).
ExamPle 20: 3-Aminophenoxyacetyl-Tyr(PO3H2)-lle-Asn-Gln-NH2 (TFA salt)
The title compound is prepared using a procedure analogous to Example 19, starting from
3-(N-tert-butoxycarbonylamino)phenoxy-acetic acid. Mass spectral analysis (negative-ion
mode): 764.0 (calc. 763.7, C32H44N8O12P1), tR= 4.92 min (HPLC System A).
The starting material is obtained as follows:
a) 3-(N-tert-Butoxvcarbonylamino)phenoxy-acetic acid
The title compound is synthesized as described for 4-(N-tert-butoxycarbonylamino)phenoxy-
acetic acid (Example 19 a)), starting from 3-(N-Acetylamino)phenoxy-acetic acid (Example
16 a)): Rf= 0.66 (ethyl acetate:methanol = 3:1 (v/v) with 0.5 % acetic acid).
Example 21: 4-(3-lndolyl)butyrolyl-Tyr(PO3H2)-lle-Asn-Gln-NH2
(SEQ ID NO: 21)
The title compound is prepared using a protocol analogous to Example 16 with 4-(3-
indolyl)butyric acid (Aldrich, Buchs, Switzerland). Mass spectral analysis (negative-ion
mode): 799.4 (calc. 799.8, C36H48N8O11 P1), tR= 7-49 min (HPLC System A).
Example 22: 3-lndolyl-acetyl-Tyr(PO3H2)-lle-Asn-Gln-NH2
(SEQ ID NO: 22)
The title compound is prepared using a protocol analogous to Example 16 with 3-indolyl-
acetic acid (Aldrich, Buchs, Switzerland). Mass spectral analysis (negative-ion mode): 773.0
(calc. 771.8, C34H44NgO11 P1), tR= 6.73 min (HPLC System A).
ExamPle 23: 8enzoyl-Tyr(PO3H2)-lle-Asn-Gln-NH2
CA 02227~16 1998-01-21
WO 97/08193 PCTAEP96/03473
(SEQ ID NO: 23)
The title compound is prepared using a protocol analogous to Example 16. Mass spectral
analysis (negative-ion mode): 718.4 (calc. 718 7, C31 H41 N7O1 1 P1), tR= 6-25 min (HPLC
System A).
ExamPle 24: 4-Aminobenzoyl-Tyr(PO3H2)-lle-Asn-Gln-NH2 (TFA salt)
(SEQ ID NO: 24)
(4-teff-Butoxycarbonyl-amino)-benzoic acid (2 equiv.) is coupled with BOP/HOBt ( 1:1; 2
equiv.) in the presence of diisopropylethylamine (4 equiv.) in N-methylpyrrolidin-2-one.
Title compound: Mass spectral analysis (negative-ion mode): 733.5 (calc. 733.7,
C31 H42N8~11 P1). tR= 4-57 min (HPLC System A).
The starting material is obtained as follows:
a) (4-tert-Butoxvcarbonyl-amino)-benzoic acid
The title compound is synthesized in analogy to the method described for 3-N-tert-butoxy-
carbonylamino-benzyl alcohol (Example 1 a) ), starting from 4-amino benzoic acid (Aldrich,
Buchs, Switzerland): Rf= 0.37 (chloroform:methanol:water:acetic acid = 850:130:15:5,
v/v/v/v) .
Example 25: Benzimid~ol-5-ylcarbonyl-Tyr(PO3H2)-lle-Asn-Gln-NH2
(SEQ ID NO: 25)
5-Be~l~i,llida~ule-carboxylic acid (Aldrich, Buchs, Switzerland) is coupled with BOP/HOBt
(1:1; 3 equiv.; first coupling) in the presence of diisopropylethyiamine (7 equiv.) and with O-
(7-azaben~ulrid~ol-1-yl)-1,1,3,3-bis(tetramethylene)uronium hexafluorophosphdle (3 equiv.;
second coupling) in the presence of diisopropylethylamine (7 equiv.). Title compound: Mass
spectral analysis (negative-ion mode): 758.4 (calc. 758 7, C32H41Ns~11P1)~ tR= 4-91 min
(HPLC System A).
Example 26: 2-Amino-thiazol-4-yl-acetyl-Tyr(PO3H2)-lle-Asn-Gln-NH2 (TFA salt)
(SEQ ID NO: 26)
The compound is prepared using a protocol analogous to Example 16, using 2-amino-
thiazol-4-yl-acetic acid (Aldrich, Buchs, Siwtzerland). Title compound: Mass spectral
CA 02227~16 1998-01-21
WO 97/08193 PCTAEP96/03473
analysis (negative-ion mode): 754.7 (calc 754 8, C29H41 N9~11 P1 S1), tR= 4 51 min
(HPLC System A).
~.
ExamPle 27: 4-Cyanobenzoyl-Tyr(PO3H2)-lle-Asn-Gln-NH2
(SEQ ID NO: 27)
The compound is prepared using a protocol analogous to Example 25, using 4-cyano-
benzoic acid (Fluka, Buchs, Swike~land). Title compound: Mass spectral analysis (negative-
ion mode): 743.9 (calc. 743.7, C32H40N8o11 P1), tR= 6.44 min (HPLC System A).
Example 28: 2,3-Dihydrobenzofuran-5-carbonyl-Tyr(PO3H2)-lle-Asn-Gln-NH2
(SEQ ID NO: 28)
The compound is prepared using a protocol analogous to Example 25, using 2,3-
dihydobenzofuran-5-carboxylic acid (Maybridge, Cornwall, USA). Title compound: Mass
spectral analysis (negative-ion mode): 760.6 (calc- 760-7, C33H43N7~12P1)~ tR= 6-36 min
(HPLC System A).
ExamPle 29: 4-Hydroxybenzoyl-Tyr(PO3H2)-lle-Asn-Gln-NH2
(SEQ ID NO: 29)
The compound is prepared using a protocol analogous to Example 25, using 4-hydroxy
benzoic acid (Fluka, Buchs, Switzerland). Title compound: Mass spectral analysis (negative-
ion mode): 734.6 (calc. 734.7, C31 H41 N7O12P1), tR= 5.49 min (HPLC System A).
ExamPle 30: 4-Methoxybenzoyl-Tyr(PO3H2)-lle-Asn-Gln-NH2
(SEQ ID NO: 30)
The compound is prepared using a protocol analogous to Example 25, using 4-methoxy-
benzoic acid (Fluka, Buchs, Switzerland). Title compound: Mass spectral analysis (negative-
ibn mode): 748.9 (calc. 748.7, C32H43N7O12P1), tR= 6.65 min (HPLC System A).
Example 31: 4-Methylthiobenzoyl-Tyr(PO3H2)-lle-Asn-Gln-NH2
(SEQ ID NO: 31)
The compound is prepared using a protocol analogous to Example 25, using 4-methylthio-
benzoic acid (Fluka, Buchs, Switzerland). Title compound: Mass spectral analysis (negative-
ion mode): 764.6 (calc. 764.8, C32H43N7O11 P1 S1), tR= 7.21 min (HPLC System A).
CA 02227~16 1998-01-21
WO 97/08193 PCT~EP96/03473
Example 32: 4-Methylsulfinyl-benzoyl-Tyr(PO3H2)-lle-Asn-Gln-NH2
(SEQ ID NO: 32)
The compound is obtained by oxidation of the title compound of Example 31 with 6 %
hydrogen peroxide at room temperature; the course of the reaction is ~l~onilured by HPLC
(System A). A~ter 45 min, the starting material has been converted to the sulfoxide deri-
vative. The solution is then concenlldL~d to dryness and Iyophilized from 80 % acetic acid.
Title compound: Mass spectral analysis (negative-ion mode): 780.5 (calc. 780.8,
C32H43N7~12P1 S1), tR= 7 09 min (HPLC System A).
Example 33: trans-(4-lmid~olyl-acryloyl)-Tyr(PO3H2)-lle-Asn-Gln-NH2 (TFA salt)
(SEQ ID NO: 33)
The compound is prepared using a protocol analogous to Example 25, using trans-4-imida-
zoleacrylic acid (Aldrich, Buchs, Swik~l land)). Title compound: Mass spectral analysis (ne-
gative-ion mode): 735.1 (calc. 734.7, C30H41 N9O11 P1), tR= 4.41 min (HPLC System A).
Example 34: 4-(N-Methylamino)benzoyl-Tyr(PO3H2)-lle-Asn-Gln-NH2
(SEQ ID NO: 34)
The compound is prepared using a protocol analogous to Example 25, using 4-(N-methyl-
amino)benzoic acid (Fluka, Buchs, Switzerland). Title compound: Mass spectral analysis
(negative-ion mode): 748.4 (calc. 747.7, C32H44N8O11 P1). tR= 4-97 min (HPLC System
A).
Example 35: 4-(N,N-Dimethylamino)benzoyl-Tyr(PO3H2)-lle-Asn-Gln-NH2
(SEQ ID NO: 35)
The compound is prepared using a protocol analogous to Example 25, using 4-(N,N-di-
methylamino)benzoic acid (Fluka, Buchs, Switzerland). Title compound: Mass spectral
analysis (negative-ion mode): 761.7 (calc. 761.8, C33H46N8O11 P1), tR= 5.41 min (HPLC
System A).
Example 36: Pyridin-4-carbonyl-Tyr(P03H2)-lle-Asn-Gln-NH2
(SEQ ID NO: 36)
CA 02227~16 1998-01-21
WO 97/08193 PCT/EP96/03473
The compound is prepared using a protocol analogous to Example 25, using pyridin-4-
carbonic acid (Fluka, Buchs, Switzerland). Title compound: Mass spectral analysis (nega-
tive-ion mode): 719.9 (calc. 719.7, C30H40N8o11p1)~ tR= 4.21 min (HPLC System A).
ExamPle 37: 4-Aminomethylbenzoyl-Tyr(PO3H2)-lle-Asn-Gln-NH2 (TFA salt)
(SEQ ID NO: 37)
The compound is prepared using a protocol analogous to Example 25, using 4-aminome-
thyl-benzoic acid (Fluka, Buchs, Swikerland). Title compound: Mass spectral analysis (ne-
gative-ion mode): 747.9 (calc. 747.7, C32H44N8O11 P1). tR= 4.39 min (HPLC System A).
Example 38: 4-Amino-3-methoxybenzoyl-Tyr(PO3H2)-lle-Asn-Gln-NH2 (TFA salt)
(SEQ ID NO: 38)
The compound is prepared using a protocol analogous to Example 25, using 4-amino-3-
methoxybenzoic acid (Aldrich, Buchs, Switzerland). Title compound: Mass spectral analysis
(negative-ion mode): 764.4 (calc.763.7, C32H44N8O12P1), tR= 4-59 min (HPLC System A).
ExamPle 39: 4-Aminophenylacetyl-Tyr(PO3H2)-lle-Asn-Gln-NH2 (TFA salt)
(SEQ ID NO: 39)
The compound is prepared using a protocol analogous to Example 25, using 4-aminophenyl
acetic acid (Aldrich, Buchs, Switzerland). Title compound: Mass spectral analysis (negative-
ion mode): 748.4 (calc. 747.7, C32H44N8011 P1), tR= 4.89 min (HPLC System A).
ExamPle 40: 3-Aminobenzoyl-Tyr(PO3H2)-lle-Asn-Gln-NH2 (TFA salt)
(SEQ ID NO: 40)
3-(teff-Butoxycarbonyl-amino)benzoic acid (3 equiv.) is coupled with BOP/HOBt (1 :1; 3
equiv.) in the presence of diisopropylethylamine (7 equiv.) in N-methylpyrrolidin-2-one. Title
compound: Mass spectral analysis (negative-ion mode): 733.3 (calc. 733.7,
C31 H42N8~11 P1), tR= 4.42 min (HPLC System A).
t The starting material is prepared as follows:
a) 3-(tert-Butoxycarbonvl-amino)benzoic acid
The title compound is synthesized in analogy to the method described for 3-N-tert-butoxy-
carbonyl-aminobenzyl alcohol (Example 1 a)), starting from 3-aminobenzoic acid (Fluka,
CA 02227~16 1998-01-21
W O 97/08193 PCT~EP96/03473
- 76-
Buchs, Swi~erland): Rf= 0.48 (chloroform:metanol:water:acetic acid = 850:130:15:5
(v/v/v/v)).
ExamPle 41: Quinoline-6-ylcarbonyl-Tyr(PO3H2)-lle-Asn-Gln-NH2
(SEQ ID NO: 41)
The compound is prepared using a protocol analogous to Example 25, using quinoline-6-
carboxylic acid (Fisher Scientific, Pittsburg, USA). Title compound: Mass spectral analysis
(negative-ion mode): 769.8 (calc. 769.7, C34H42N8O11 P1), tR= 4.88 min (HPLC System
A).
Example 42: 4-Methoxycarbonylbenzoyl-Tyr(PO3H2)-lle-Asn-Gln-NH2
(SEQ ID NO: 42)
The compound is prepared using a protocol analogous to Example 25, using 4-methoxy-
carbonylbenzoic acid (= monomethyl terephthalate, Fluka, Buchs, Switzerland). Title
compound: Mass spectral analysis (negative-ion mode): 776.9 (calc. 776.7,
C33H43N7ol3p1)~ tR= 6.78 min (HPLC System A).
Example 43: 4-Cacboxybenzoyl-Tyr(PO3H2)-lle-Asn-Gln-NH2
(SEQ ID NO: 43)
The compound is obtained by saponification of the title compound of Example 42 with
sodium hydroxide as follows: 4-Methoxycarbonylbenzoyl-Tyr(PO3H2)-lle-Asn-Gln-NH2(SEQ ID NO: 42) (27 mg, 35 ~lmol) is dissolved in 2.7 ml of water and 34.7 ~l of a 1 N solu-
tion of NaOH is added. The course of the reaction is followed by anayltical HPLC. After 2 h,
a further 31 2 ~11 of 1 N NaOH are added. After disappearance of the starting material
(15 min), the solution is neutralized with 2N HCI (1 74 ~11) and injected directly into the
Medium Pressure Liquid Chromatography system described in Example 1 for purification.
Title compound: Mass spectral analysis (negative-ion mode): 762.7 (calc. 762.7,
C32H41N7o13p1)~ tR= 6.81 min (HPLC System A).
Example 44: 4-Amino-2-chlorobenzoyl-Tyr(PO3H2)-lle-Asn-Gln-NH2 (TFA salt)
(SEQ ID NO: 44)
The compound is p,epaled using a protocol analogous to Example 16, using 4-amino-2-
chlorobenzoic acid (Aldrich, Buchs, Switzerland). Title compound: Mass spectral analysis
CA 02227~16 1998-01-21
W O 97/08193 PCTAEP96/03473
- 77 -
(negative-ion mode): 768.1 (calc. 768.2, C31 H41 N8~11 P1 Cl1), tR= 4.59 min (HPLC
System A).
ExamPie 45: 6-Amino-nicotinoyl-Tyr(PO3H2)-lle-Asn-Gln-NH2 (TFA salt)
(SEQ ID NO: 45)
The compound is prepared using a protocol analogous to Example 16, using 6-amino-nicoti-
nic acid (Fluka, Buchs, Switzerland). Title compound: Mass spectral analysis (negative-ion
mode): 734.7 (calc. 734.7, C30H41 N9~11 P1). Purity control is made with reversed-phase
analytical HPLC on a Nucleosil C,8-column (Macherey & Nagel, Duren, FRG; 250 x 4 mm,
5 ~Lm,100 A); linear gradient over 10 min of MeCN/0.09% TFA and H2O/0.1 % TFA from
5:95 to 13:7, flow rate 1.5 ml/min, detection at 215 nm (HPLC System B): tR= 4.77 min.
Example 46: 4-Amino-3-hydroxybenzoyl-Tyr(PO3H2)-lle-Asn-Gln-NH2 (TFA salt)
(SEQ ID NO: 46)
The compound is prepared using a protocol analogous to Example 16, using 4-amino-3-
hydroxybenzoic acid (Fluka, Buchs, Switzerland). Title compound: Mass spectral analysis
(negative-ion mode): 749.5 (calc.749.7, C31 H42N8O12P1), tR= 5.51 min (HPLC System B).
Example 47: 3-(3-Aminophenyl)propionoyl-Tyr(PO3H2)-lle-Asn-Gln-NH2 (TFA salt)
(SEQ ID NO: 47)
3-(3-Aminophenyl)propionic acid (3 equiv.; Trans World Chemicals, Rockville, USA) is
coupled with BOP/HOBt (1 :1; 3 equiv.) in the presence of diisopropylethylamine (7 equiv.) in
N-methylpyrrolidin-2-one. Title compound: Mass spectral analysis (negative-ion mode):
761.8 (calc. 761.8, C33H46N8O11 P1)- tR= 5.22 min (HPLC System B).
ExamPle 48: 4-Aminobenzoyl-Tyr(PO3H2)-lle-NH2 (TFA salt)
(SEQ ID NO: 48)
4-(tert-butoxycarbonyl-amino)benzoic acid (2 equiv.) is coupled with BOP/HOBt (1 :1; 2
equiv.) in the presence of diisopropylethylamine (4 equiv.) in N-methylpyrrolidin-2-one. Title
compound: Mass spectral analysis (negative-ion mode): 491.3 (calc. 491.5,
C22H28N4~7P1), tR= 4.84 min (HPLC System A).
,.
- The starting material is prepared as follows:
CA 02227~16 1998-01-21
W O 97/08193 PCTAEP96/03473
a) 4-ltert-Butoxvcarbonylamino)benzoic acid
The title compound is prepared using a protocol analogous to that for synthesis of 3-N-tert-
butoxycarbonyl-aminobenzyl alcohol (Example 1 a)), starting with 4-amino-benzoic acid
(Fluka, Buchs, Switzerland): Rf= 0.38 (chloroform:methanol:water:acetic acid =
850:130:15:5, v/v/v/v)).
Example 49: trans-(3-lndolyl-acryloyl)-Tyr(PO3H2)-lle-Asn-Gln-NH2
(SEQ ID NO: 49)
trans-3-lndolyl-acrylic acid (Fluka, Buchs, Switzerland) is coupled with BOP/HOBt (1 :1, 3
equiv.; first coupling) and N-t(dimethylamino)-1 H-1,2,3-triazolo[4,5-b]pyridin-1-ylmethylene]-
N-methylmethanaminium hexafluorophosph~Le N-oxide (3 equiv.; second coupling) in the
presence of diisopropylethylamine (6 equiv.).. Title compound: Mass spectral analysis
(negative-ion mode): 783.6 (calc. 783.8, C35H44N8O11 P1), tR= 4.32 min (HPLC System
A).
Example 50: 4-Aminobenzoyl-Tyr(P03H2)-lle-Asn-NH2 (TFA salt)
(SEQ ID NO: 50)
(4-tert-Butoxycarbonyl-amino)benzoic acid (Example 48 a)) is coupled with BOP/HOBt (1 :1,
3 equiv., double coupling) in the presence of diisopropyl ethylamine (6.0 equiv.). Title
compound: Mass spectral analysis (negative-ion mode): 605.3 (calc. 605.6,
C26H34N6OsP1). tR= 4.53 min (HPLC System A).
ExamPle 51: 4-Aminobenzoyl-Tyr(PO3H2)-Asp-Asn-Gln-NH2 (TFA salt)
(SEQ ID NO: 51)
The compound is prepared using a protocol analogous to Example 50. Title compound:
Mass spectral analysis (negative-ion mode): 735.9 (calc. 735.6, C29H36NgO13P1),
tR= 5.15 min (HPLC System C = reversed-phase analytical HPLC on a Nucleosil C,8 column
(250 x 4 mm, 5 ~Lm, 100 A; Macherey & Nagel, Duren, FRG); linear gradient over 10 min of
acetonitrile/0.09% TFA and H20/0.1 % TFA from 0:1 to 23:77, flow rate 2.0 ml/min,
detection at 215 nm).
Example 52: 4-Aminobenzoyl-Tyr(PO3H2)-Gly-Asn-Gln-NH2 (TFA salt)
-
CA 02227~l6 l998-0l-2l
W O 97/08193 PCT~EP96/03473
-79-
~D '
(SEQ ID NO: 52)
The compound is prepared using a protocol analogous to Example 50. Title compound:
Mass spectral anaiysis (negative-ion mode): 678-0 (calc- 677.6,C27H34N8~11P1)~
tR= 4.87 min (HPLC System C).
ExamPle 53: 4-Aminobenzoyl-Tyr(PO3H2)-lle-Asn-Gly-NH2 (TFA salt)
(SEQ ID NO: 53)
The compound is prepared using a protocol analogous to Example 50. Title compound:
Mass spectral analysis (negative-ion mode): 662.7 (calc. 662.6,C28H37N7O10P1),
tR= 4.46 min (HPLC System A).
Example 54: 4-Aminobenzoyl-Tyr(PO3H2)-lle-Asn-Val-NH2 (TFA salt)
(SEQ ID NO: 54)
The compound is prepared using a protocol analogous to Example 50. Title compound:
Mass spectral analysis (negative-ion mode): 705.1 (calc. 704.7,C31H43N7O10P1),
tR= 5.76 min (HPLC System A).
Example 55: 4-Aminobenzoyl-Tyr(PO3H2)-lle-Asn-Asp-NH2 (TFA salt)
(SEQ ID NO: 55)
The compound is prepared using a protocol analogous to Example 50. Title compound:
Mass spectral analysis (negative-ion mode): 721.3 (calc. 720.7, C30H39N7o12p1)~
tR= 4.61 min (HPLC System A).
ExamPle 56: 4-Aminobenzoyl-Tyr(PO3H2)-lle-Asn-Ac5c-NH2 (TFA salt)
(SEQ ID NO: 56)
The compound is prepared using a protocol analogous to Example 50. Title compound:
Mass spectral analysis (negative-ion mode): 717.4 (calc. 716.7,C32H43N7O10P1),
tR= 5.38 min (HPLC System A).
Example 57:4-Aminobenzoyl-Tyr(PO3H2)-lle-Asn-Asn-NH2 (TFA salt)
(SEQ ID NO: 57)
The compound is prepared using a protocol analogous to Example 50. Title compound:
Mass spectral analysis (negative-ion mode): 720.0 (calc. 719.7,C30H40N8O11Pl),
CA 02227~16 1998-01-21
W O 97/08193 PCT~EP96/03473
-80- ~
tR= 4.36 min (HPLC System A). t
Exam~le 58: 4-Aminobenzoyl-Tyr(P03H2)-lle-Asn-,3Ala-NH2 (TFA salt)
(SEQ ID NO: 58)
The compound is prepared using a protocol analogous to Example 50. Title compound:
Mass spectral analysis (negative-ion mode): 676.9 (calc. 676.7, C2gH39N7O1oP1)~
tR= 4.68 min (HPLC System A).
Exampie 59: 4-Aminobenzoyl-Tyr(P03H2)-lle-Gly-Gln-NH2 (TFA salt)
(SEQ ID NO: 59)
The compound is prepared using a protocol analogous to Example 50. Title compound:
Mass spectral analysis (negative-ion mode): 676.4 (calc. 676.6, C2gH39N7O10P1),
tR= 4.81 min (HPLC System A).
Example 60: 4-Aminobenzoyl-Tyr(PO3H2)-lle-Gln-Gln-NH2 (TFA salt)
(SEQ ID NO: 60)
The compound is prepared using a protocol analogous to Example 50. Title compound:
Mass spectral analysis (negative-ion mode): 747.9 (calc 747.7 C32H44N8~11P1)~
tR= 4-45 min (HPLC System A).
Example 61: Indole-5-ylcarbonyl-Tyr(PO3H2)-lle-Asn-Gln-NH2
(SEQ ID NO: 61)
The peptide is synthesized manually on a 4-(2~,4~-dimethoxyphenyl-aminomethyl)-phenoxy
resin, employing a procedure analogous to Example 1. The N-terminal group is incorporated
as N-tert-butoxycarbonyl-indole-5-ylcarboxylic acid (3 equiv.) using ben~uL~ ule-1 yl-N-oxy-
tris-(dimethylamino)-phosphonium hex~fluorophosphate/N-hydroxyben~olli~ole (1:1; 3
equiv.) in the presence of diisopropylethylamine (7 equiv.) in N-methylpyrrolidin-2-one. The
complete peptide resin obtained after the final coupling is simultaneosly deprotected-
cleaved by treatment with trifluoro-acetic acid/ethanedithiol/H2O (76:20:4, v/v/v) for 3 h at
room temperature. The filtrate from the cleavage reaction is precipitated in diisopropyl
ether-petroleum ether (1:1, v/v, 0 ~C), and the precipitate collected by filtration.
Decarboxylation of the resulting N-carboxy of the N-terminal indole ring is carried out in 0.1
M acetic acid (1 h at room temperature; see White, P, in Smith, J.A., Rivier, J.E. (eds.):
CA 02227~16 1998-01-21
W O 97/08193 PCTAEP96/03473
-81 -
Peptides, Chemistry and Biology, ESCOM, Leiden 1992, pp 537-538). The crude peptide is
purified by medium-pressure liquid chromatography as described in Example 1. The title
compound is obtained: Mass spectral analysis (negative-ion mode): 757.3 (calc. 757.7,
C33H42N8~l 1 Pl ), tR= 6 50 min (HPLC System B).
The starting material is prepared as follows:
a) N-tert-Butoxyc-a, L,onyl-indole-5-ylcarboxylic acid
The methyl ester of 5-indolecarboxylic acid is prepared as described in J. Org. Chem. 42,
1286 (1977), having an R~ = 0.66 (chloroform:methanol:water:acetic acid = 850:130:15:5,
v/v/v/v). N-tert-butoxycarbonyl-indole-5-ylcarboxylic acid is prepared as decribed in J. Chem.
Soc., Chem. Commun.1984,1699, the title compound being characterized by an Rf = 0.56
(chloroform:methanol:water:acetic acid = 850:130:15:5, v/v/v/v).
Example 62: Indole-5-ylcarbonyl-Tyr(PO3H2)-lle-Asn-NH2
(SEQ ID NO: 62)
The compound is prepared using a procedure analogous to Example 61. Title compound:
Mass spectral analysis (negative-ion mode): 629.5 (calc 629-6, C28H34N6~gP1)
tR= 6.86 min (HPLC System B).
Example 63: Indole-5-ylcarbonyl-Tyr(PO3H2)-Ac6c-Asn-Gln-NH2
(SEQ ID NO: 63)
The compound is pr~:par~d using a procedure analogous to Example 61. Title compound:
Mass spectral analysis (negative-ion mode): 769 7 (calc 769-7, C34H42N8~11 P1)
tR= 6.53 min (HPLC System B).
Example 64: 3-Aminobenzyloxycarbonyl-L-F2Pmp-lle-Asn-Gln-NH2 (TFA salt)(SEQ ID NO: 64)
The peptide is synthesized manually on a 4-(2',4'-dimethoxyphenyl-aminomethyl)-phenoxy
resin, employing a procedure analogous to Example 1. Incorporation of Na-Fmoc-[4-(0-
diethyl)-phosphono(difluoromethyl)]-L-phenylalanine ffor synthesis see Tetrahedron Lett.
34(22), 3543 (1993)) to the protected peptide resin is carried out with ben~olri~ole-1yl-N-
oxytris-(dimethylamino)-phosphonium hexafluorophosphate/N-hydroxyben~ulri~ole (1:1; 3
equiv.) in the presence of diisopropylethylamine (7 equiv.), 3 h reaction time. After removal
CA 02227~16 1998-01-21
WO 97/08193 PCTAEP96/03473
-82-
of the N-terminal Fmoc group, the peptide resin is treated with 1 M trimethylsilyltrifluoro-
methane sulfonate-2 M dimethylsulfide-trifluoroacetic acid (500 ml to 0.005 mmol of NH2)-
ethanedithiol (100 ml)-rn cresol (25 ml) 30 min at 4 ~C and 3.5 h at room temperature (see
Tetrahedron Lett. 34(44), 7093 (1993). The fiitrate from the cleavage reaction is plec~ f3d
in diisopropyl ether-petroleum ether (1 :1, v/v, O ~C), and the prec;pit~t~ collected by
filtration. The crude peptide is purified by medium-pressure liquid chromatography as
described in Example 1. The crude title compound in Boc-protected form is obtained by
reaction of H-L-F2Pmp-lle-Asn-Gln-NH2 (1 equiv.) with 3-tert-butoxycarbonyl-aminobenzyl-
4-nitrophenyl-carbonate (see Example 1 b)) (3 equiv.) in the presence of
diisopropylethylamine (3 equiv.). The crude product is purified by medium-pressure liquid
chromatography as described in Example 1. The teff-butoxycarbonyl group of the N-
terminal moiety is removed with trifluoroacetic acid/water (95:5, v/v), and the title compound
is obtained: Mass spectral analysis (negative-ion mode): 797.6 (calc. 797.7,
C33H44N8~11 P1 F2), tR= 5.43 min (HPLC System A).
Examle 65: 4-Aminobenzoyl-L-F2Pmp-lle-Asn-Gln-NH2 (TFA salt)
(SEQ ID NO: 65)
The peptide is synthesized manually on a 4-(2~,4~-dimethoxyphenyl-aminomethyl)-phenoxy
resin, employing a procedure analogous to Example 1. Incorporation of N~-Fmoc-[4-(0-di-
ethyl)-phosphono(difluoromethyl)]-L-phenylalanine (reference: see Example 64) to the pro-
tected peptide resin is carried out as in Example 64. (4-tert-butoxycarbonyl-amino)-benzoic
acid (Example 48 a)) (3 equiv.) is coupled with BOP/HOBt (1 :1; 3 equiv.) in the presence of
diisopropylethylamine (7 equiv.) in N-methylpyrrolidin-2-one. After completion of the syn-
thesis, the peptide resin is treated with 1 M trimethylsilyltrifluoro-methane sulfonate-2 M
dimethylsulfide-trifluoroacetic acid (500 ml to 0.005 mmol of NH2)-ethanedithiol (100 ml)-n7
cresol (25 ml) 30 min at 4 ~C and 3.5 h at room temperature. The filtrate from the cleavage
reaction is precipitated in diisopropyl ether-petroleum ether (1 :1, v/v, 0 ~C), and the preci-
pitate collected by filtration. The crude peptide is purified by medium-pressure liquid chro-
matography as described in Example 1. Title compound: Mass spectral analysis (negative-
ion mode): 768.1 (calc. 767.7, C32H42N8O10p1F2)~ tR= 4.86 min (HPLC System A).
ExamPle 66: 4-Aminobenzoyl- Tyr(PO3H2)-Phe-Asn-Gln-NH2 (TFA Salt)
(SEQ ID NO: 66)
CA 02227~16 1998-01-21
WO 97/08193 PCTAEP96/03473
-83-
The title compound is prepared analogously to Example 50: Mass spectral analysis(negative ion mode): 767.9 (calc. 767.7, C34H4ON8O~1P,); t,~ = 5.0 min (HPLC System A).
Example 67: Trans-3,4-dihydroxy-cinnamoyl-Tyr(PO3H2)-lle-Asn-Gln-NH2
(SEQ ID NO: 67)
The peptide is synthesized manually on a 4-(2',4'-dimethoxyphenyl-aminomethyl)-phenoxy-
resin (Novabiochem, Laufelfingen, Switzerland), employing the fluorenylmethox~,carL.onyl
strategy. Fmoc-removal is with piperidine/dimethylacetamide (1 :4, v/v; 6 x 2 min), followed
by washing with methanol (3 x 1 min), N-methylpyrrolidin-2-one (2 x 1 min)""~ll,anol (3 x 1
min), and N-methylpyrrolidin-2-one (3 x 2 min). Coupling is achieved by first dissolving the
Fmoc-amino acid (2 equiv.), diisopropylethylamine (2.2 equiv.), and the 2-(2-pyridon-1-yl)-
1,1,3,3-tetramethyluronium tetrafluoroborate reagent (2 equiv.) in N-methylpyrrolidin-2-one,
then waiting 3 min for preactivation, adding the mixture to the resin, and finally shaking for
at least 45 min. Asparagine and glutamine side chains are protected with the trityl group.
The incorporation of Na-Fmoc-Tyr(PO3H2)-OH (3 equiv.) is accomplished with benzo-
triazole-1 -yl-oxy-tris-dimethylamino)-phosphoniumhexafluorophosphate/N-hydroxybenzo-
triazole (1 :1; 3 equiv.; first coupling) and N-~(dimethylamino)-1 H-1,2,3-triazolo~4,5-b]pyridin-
1-ylmethylene]-N-methylmethanaminium hexafluorophosphate N-oxide (3 equiv.; second
coupling) in the presence of diisopropylethylamine (7 equiv.). trans-3,4-Dihydroxy-cinnamic
acid (2 equiv.; Fluka, Buchs, Switzerland) is incorporated (double coupling) with benzotri-
azole-1 -yl-oxy-tris-dimethylamino)-phosphoniumhexafluorophosphate/N-hydroxybenzo-
triazole (1 :1; 3 equiv.) in the presence of diisopropylethylamine (4 equiv.). The complete
peptide resin obtained after the last coupling step is simultaneosly deprotected and cleaved
by treatment with trifluoroacetic acid/H2O (95:5, v/v) for 3 h at room temperature. The filtrate
from the cleavage reaction is prec;pil~led in diisopropyl ether/petroleum ether (1 :1, v/v, 0
~C), and the precirit~te collected by filtration. The crude peptide is purified by medium-pres-
sure liquid ch(u,,,~lugraphy using a C18-column (Merck LICHROPREP RP-18, 15-25 ~m
bead diameter, reversed phase material based on C18-derivatized silicagel, Merck, Darm-
stadt, FRG; column length 46 cm, diameter 3.6 cm; flow rate 53.3 ml/min; detection at
215 nm) eluted with an acetonitrile-water gradient containing 0.1 % of TFA. The title com-
pound is obtained: Mass-spectral analysis (matrix-assisted laser-desorption ionization time-
of-flight mass spectrometry, MALDI-TOF) reveals a molecular mass within 0.1 % of the
expected value (negative-ion mode): 776.6 (calc. 776.7, C33H43N7O13P1). The purity of the
peptide is verified by reversed-phase analytical HPLC on a Nucleosil C18-column (25û x 4
mm, 5 ~lm, 1ûû A): linear gradient over 10 min of MeCN/0.09% TFA and H20/0.1% TFA
CA 02227~16 1998-01-21
W O 97/08193 PCTrEP96/03473
- 84 -
from 1:49 to 3:2; flow rate 2.0 mL/min, detection at 215 nm (HPLC system A), single peak at
tR= 5.29 min.
In analogy to example 67, the following examples 68 to 122 are obtained, if not described
otherwise:
Example 68: trans-3-Hydroxy-cinnamoyl-Tyr(PO3H2)-lle-Asn-Gln-NH2
(SEQ ID NO: 68)
trans-3-Hydroxy-cinnamic acid is from Fluka (Buchs, Swikerland) and is used without side-
chain protection. Title compound: Mass-spectral analysis (negative-ion mode): 760.8 (calc.
760.7, C33H43N7O12P1). tR= 6.04 (HPLC system A).
Example 69: trans-3-Hydroxy-4-methoxy-cinnamoyl-Tyr(po3H2)-lle-Asn-Gln-NH2
(SEQ ID NO: 69)
trans-3-Hydroxy-4-methoxy-cinnamic acid is from Janssen (Olen, Belgium) and is used
without side-chain protection. Title compound: Mass-spectral analysis (negative-ion mode):
790.1 (calc. 790.7, C34H45N7O13P1). tR= 5.77 (HPLC system A).
Example 70: trans-3~4-Dihydroxy-cinnamoyl-Tyr(po3H2)-lle-NH2
(SEQ ID NO: 70)
trans-3,4-Dihydroxy-cillnall,ic acid (3 equiv.) is incorporated with ben,ullia~olE 1-yl-oxy-tris-
dimethylamino)-phosphonjumhexafluorophosphate/N-hydroxybe,,~ul,ia~ole (1:1; 3 equiv.;
first coupling) and N-[(dimethylamino)-1 H-1 ,2,3-triazolo[4,5-b]pyridin-1 -ylmethylene]-N-
methylmethanaminium hex~fluorophosphate N-oxide (3 equiv.; second coupling) in the
presence of diisopropylethylamine (7 equiv.). Title compound: Mass-spectral analysis
(negative-ion mode): 534.4 (calc. 534.5, C24H2gN3OgP1). tR= 6.45 (HPLC system A).
ExamPle 71: trans-3~4-Dihydroxy-cinnamoyl-Tyr(po3H2)-Ac6c-NH2
(SEQ ID NO: 71)
trans-3,4-Dihydroxy-cinnamic acid (3 equiv.) is incorporated with be~uLria~ole-1-yl-oxy-tris-
dimethylamino)-phosphoniumhexafluorophosphate/N-hydroxyben~ul~ le (1:1; 3 equiv.;
first coupling) and N-[(dimethylamino)-1 H-1 ,2,3-triazolo[4,5-b]pyridin-1-ylmethylene]-N-
methylmethanaminium hexafluorophosphate N-oxide (3 equiv.; second coupling) in the
presence of dii~oprupylethylamine (7 equiv.). Title compound: Mass-spectral analysis
(negative-ion mode): 546.8 (calc. 546.5, C25H2gN3OgP1). tR= 6.18 (HPLC system A).
The starting material is prepared as follows:
CA 02227~16 1998-01-21
WO 97/08193 PCT~EP96/03473
a) Fmoc-1-amino-cyclohexanecarboxylic acid
The title compound is synthesized starting from 1-amino-cyclohexanecarboxylic acid
(Janssen, Olen, Belgium) according to a procedure known in the art (see E. Atherton and
R.C. Sheppard, in Solid-Phase Peptide Synthesis- A Practical Approach, eds.: D. Rickwood
and B.D. Hames, IRL Press at Oxford University Press, Oxford, 1989): Rf= 0.84
(chloroform:methanol:water:acetic acid, 750:270:50:5, v/v/v/v).
ExamPle 72: trans-3,4-Dihydroxy-cinnamoyl-Tyr(PO3H2)-Ac6c-Asn-NH2
(SEQ ID NO: 72)
Mass-spectral analysis (negative-ion mode): 660.4 (calc. 660.6, C2gH3sNsO11P1). tR= 5.86
(HPLC system A).
The starting material is prepared as follows:
a) Fmoc-1-amino-cyclohexanecarboxylic acid
The title compound is synthesized starting from 1-amino-cyclohexanecarboxylic acid
(Janssen, Olen, Belgium) according to a procedure known in the art (E. Atherton and R.C.
Sheppard, in Solid-Phase Peptide Synthesis- A Practical Approach, eds.: D. Rickwood and
B.D. Hames, IRL Press at Oxford University Press, Oxford, 1989): Rf= 0.84
(chloroform:methanol:water:acetic acid, 750:270:50:5, v/v/v/v).
Example 73: 7~8-Dihydroxy-2-oxo-2H-(benzopyran)-3-carboxyl-Tyr(po3H2)-lle-Asn-NH2
(SEQ ID NO: 7)
Mass-spectral analysis (negative-ion mode): 689.7 (calc. 690.6, c2sH33Nso13p1)~ Linear
gradient over 10 min of MeCN/0.09% TFA and H2O/0.1 % TFA from 1 :19 to 13:7; flow rate
2.0 mL/min, detection at 215 nm (HPLC system B), single peak at tR= 6.41.
The starting material 7,8-dihydroxy-2-oxo-2H-(benzopyran)-3-carboxylic acid (3,4-dioxycu-
marin carboxylic acid) is synthesized as described in the literature (see F. Vorsatz, J. Prakt.
Chem.,145, 265-269 (1936)).
Example 74: trans-3~4-Dihydroxy-cinnamoyl-Tyr(po3H2)-Met-Asn-NH2
(SEQ ID NO: 74)
trans-3,4-Dihydroxy-cinnamic acid (3 equiv.) is incorporated with bell~oL~i~ule-1-yl-oxy-tris-
dimethylamino)-phosphoniumhexafluorophosphate/N-hydroxybel ,~ ,ole (1 :1; 3 equiv.;
first coupling) and N-[(dimethylamino)-1 H-1,2,3-triazolo[4,5-b]pyridin-1-ylmethylene]-N-
methylm~Ll,anar"i"ium hexafluorophosphate N-oxide (3 equiv.; second coupling) in the
presence of diisopropylethylamine (7 equiv.). The manual synthesis results in the title
CA 02227~16 1998-01-21
WO 97/08193 PCT/EP96/03473
- 86 -
compound: Mass-spectral analysis (negative-ion mode): 666.6 (calc. 666.6,
c27H33Nso1lp1sl). tR= 5.64 (HPLC system B).
ExamPle 75: trans-3,4-Dihydroxy-cinnamoyl-Tyr(P03H2)-Pro-Asn-NH2
(SEQ ID N0: 75)
The manual synthesis results in the title compound: Mass-spectral analysis (negative-ion
mode): 632.4 (calc. 632.6, C27H31 N5~11 P1). tR= 4.83 (HPLC system A).
Example 76: 3-(3,4-Dihydroxyphenyl)-propionyl-Tyr(PO3H2)-lle-Asn-NH2
(SEQ ID N0: 76)
3-(3,4-Dihydroxyphenyl)-propionic acid is from Fluka (Buchs, S~/.;kerland) and is incorpora-
ted with ben~uL,id~ole-1-yl-oxy-tris-dimethylamino)-phosphoniumhexafluorophosphate/N-hy-
droxybenzotriazole t1 :1; 3 equiv.; first coupling) and N-t(dimethylamino)-1 H-1,2,3-
triazolo[4,5-b]pyridin-1-ylmethylene]-N-methylmethanaminium hexafluorophosphate N-oxide
(3 equiv.; second coupling) in the presence of diisopropylethylamine (7 equiv.). The manual
synthesis results in the title compound: Mass-spectral analysis (negative-ion mode): 650.8
(calc. 650.6, C2gH37NsO11P1). tR= 5.54 (HPLC system A).
ExamPle 77: 3~4-Dihydroxyphenyl-acetyl-Tyr(po3H2)-lle-Asn-NH2
(SEQIDN0:77)
3,4-Dihydroxyphenyl-acetic acid is from Fluka, Buchs, Switzerland and is incorporated with
ber l~ul- i~ole-1 -yl-oxy-tris-dimethylamino)-phosphoniumhexafluorophosphate/N-hydroxy-
berl~ ri~ole (1 :1; 3 equiv.; first coupling) and N-[(dimethylamino)-1 H-1,2,3-triazolo[4,5-
b]pyridin-1-ylmethylene]-N-methylmethanaminium hexafluorophosphdle N-oxide (3 equiv.;
second coupling) in the presence of diisopropylethylamine (7 equiv.). The manual synthesis
results in the title compound: Mass-spe~;lrdl analysis (negative-ion mode): 636.4 (calc.
636-6, c27H3sNso11p1)~ tR= 5.43 (HPLC system A).
Example 78: trans-3~4-Dihydroxy-cinnamoyl-Tyr(po3H2)-Gly-NH2
(SEQ ID N0: 78)
Mass-spectral analysis (negative-ion mode): 478.4 (calc. 478.4, C2oH2lN3ogp1). tR= 4-61
(HPLC system A).
Example 79: trans-3~4-Dihydroxy-cinnamoyl-Tyr(po3H2)-~Ala-NH2
(SEQ ID N0: 79)
CA 02227~16 1998-01-21
W O 97/08193 PCT/EP96/03473
-87-
Mass-spectral analysis (negative-ion mode): 494.4 (calc. 492.4, c21H23N3osp1). tR= 4-74
(HPLC system A).
ExamPle 80: trans-3,4-Dihydroxy-cinnamoyl-Tyr(PO3H2)-Ac7C-ASn-NH2
(SEQ ID NO: 80)
Mass-spectral analysis (negative-ion mode): 674.6 (calc. 674.6, c3oH37Nso1lp1). tR= 6-03
(HPLC system A).
The starting material is prepared as follows:
a) Fmoc-1-amino-cycloheptanecarboxylic acid
The title compound is synthesized starting from 1-amino-cycloheptanecarboxylic acid (see
N . Zelinsky and G. Stadnikoff, Chem. Ber., 39, 1722-1732, (1906)) according to a proce-
dure known in the art (see E. Atherton and R.C. Sheppard, in Solid-Phase Peptide Synthe-
sis- A Practical Approach, eds.: D. Rickwood and B.D. Hames, IRL Press at Oxford Univer-
sity Press, Oxford, 1989): Rf= 0.25 (dichloromethane:methanol, 9:1, v/v).
ExamPle 81: trans-3,4-Dihydroxy-cinnamoyl-Tyr(PO3H2)-Nbo-ASn-NH2
(SEQ ID NO: 81)
Fmoc-2-amino-2-norbornanecarboxylic acid (2 equiv.) is incorporated with beh~uLri~ule-1-
yl-oxy-tris-dimethylamino)-phosphoniumhexafluorophosphate/N-hydroxybe, l~uLI id~ole (1: 1;
2 equiv.) in the presence of diisopropylethylamine (4 equiv.). The manual synthesis yields
the title compound: Mass-spectral analysis (negative-ion mode): 672.4 (calc. 672.6,
C30H3sNso11p1). tR= 5.80 (HPLC system A).
The starting material is prepared as follows:
a) Fmoc-2-amino-2-norbornanecarboxylic acid
The title compound is synthesized starting from 2-amino-2-norbornanecarboxylic acid
(Aldrich, Buchs, Switzerland) according to a procedure known in the art (see E. Atherton
and R.C. Sheppard, in Solid-Phase Peptide Synthesis- A Practical Approach, eds.: D.
Rickwood and B.D. Hames, IRL Press at Oxford University Press, Oxford, 1989): Rf= 0.51
(chloroform:methanol:water:acetic acid, 850:13û:1 5:5, v/v/v/v).
Example 82: trans-3,4-Dihydroxy-cinnamoyl-Tyr(PO3H2)-Nbo-NH2 (epimer 1)(SEQ ID NO: 82)
Fmoc-2-amino-2-norbornanecarboxylic acid (2 equiv.) is incorporated with ben~ulri~ole-1-
yl-oxy-tris-dimethylamino)-phosphoniumhexafluorophosphate/N-hydroxyber ,~ul, id~ule (1: 1;
2 equiv., first coupling) and N-[(dimethylamino)-1 H-1,2,3-triazolo[4,5-b]pyridin-1 -ylmethyle-
ne]-N-methylmethanaminium hexafluorophosphate N-oxide (2 equiv.; second coupling) in
CA 02227~l6 l998-0l-2l
W O 97/08193 PCTAEP96/03473
the presence of diisopropyiethylamine (6 equiv.). Title compound: Mass-spectral analysis
(negative-ion mode): 558.7 (calc. 558.5, C26H2gN30gP1). tR= 6.18 (HPLC system A).
ExamPle 83: trans-3,4-Dihydroxy-cinnamoyl-Tyr(P03H2)-Nbo-NH2 (epimer 2)(SEQ ID NO: 83)
Fmoc-2-amino-2-norbornanecarboxylic acid (2 equiv.) is incorporated with bel~uLrid~ole-1-
yl-oxy-tris-dimethylamino)-phosphoniumhexafluorophosphate/N-hydroxyben~oLIi~ole (1:1;
2 equiv., first coupling) and N-[(dimethylamino)-1 H-1,2,3-triazolo[4,5-b]pyridin-1-ylmethyle-
ne]-N-methyll,.~lllanaminium hexafluorophosphate N-oxide (2 equiv.; second coupling) in
the presence of diisopropylethylamine (6 equiv.). Title compound: Mass-spectral analysis
(negative-ion mode): 558.7 (calc. 558.5, C26H2gN30gP1). tR= 6.21 (HPLC system A).
ExamPle 84: trans-4-Hydroxy-cinnamoyl-Tyr(P03H2)-lle-Asn-NH2
(SEQ ID NO: 84)
trans-4-Hydroxy-cinnamic acid is from Fluka, Buchs, Switzerland and is incorporated (2
equiv.; double coupling) with be,-~ulria~ule-1-yl-oxy-tris-dimethylamino)-phosphoniumhexa-
fluorophosphate/N-hydroxyben~ullid~ole (1 :1; 2 equiv.) in the presence of diisopropylethyl-
amine (4 equiv.). The manual synthesis yields the title compound: Mass-spectral analysis
(negative-ion mode): 632.9 (calc. 632.6, C28H3sN50l0pl) tR= 6.11 (HPLC system A).
Example 85: 6-Hydroxy-2-naphthoyl-Tyr(po3H2)-lle-NH2
(SEQ ID NO: 85)
6-Hydroxy-2-naphthoic acid is from Lancaster, Strasbourg, France and is incorporated (2
equiv.; double coupling) with be,l~ullid~ole-1-yl-oxy-tris-dimethylamino)-phosphoniumhexa-
fluorophosphatelN-hydroxybell~otria~ule (1 :1; 2 equiv.) in the presence of diisopropylethyl-
amine (4 equiv.). The manual synthesis results in the title compound: Mass-spectral analy-
sis (negative-ion mode): 542.2 (calc. 542.5, C26H29N308P1). tR= 7.23 (HPLC system A).
Example 86: trans-3,4-Dihydroxy-cinnamoyl-TYr(P03H2)-GIn-NH2
(SEQ ID NO: 86)
The manual synthesis results in the tilte compound: Mass-spectral analysis (negative-ion
mode): 549.4 (calc. 549.5, C23H26N4010pl)~ tR= 4.74 (HPLC system A).
Example 87: trans-3~4-Dihydroxy-cinnamoyl-Tyr(po3H2)-Glu-NH2
(SEQ ID NO: 87)
CA 02227~16 1998-01-21
WO 97/08193 PCTAEP96/03473
8 9
The side chain of the glutamic acid building block is protected with the tert-butyl group.
Manual synthesis results in the title compound: Mass-spectral analysis (negative-ion mode):
550.4 (calc. 550.5, C23H2sN3O11 P1). tR= 4.87 (HPLC system A).
ExamPle 88: trans-3,4-Dihydroxy-cinnamoyl-Tyr(PO3H2)-Glu-DHph-NH2
(SEQ ID NO: 88)
N~-Fmoc-~homophenylalanine (2 equiv.; double coupling) is incorporated with 2-(2-pyri-
don-1-yl)-1 ,1,3,3-tetramethyluronium-tetrafluoroborate (2 equiv.) in the presence of diiso-
propylethylamine (2.2 equiv.). The side chain of the glutamic acid building block is protected
with the tert-butyl group. The manual synthesis results in the title compound: Mass-spectral
analysis (negative-ion mode): 711.8 (calc. 711.7, C33H36N4O12P1). tR= 7.40 (HPLC system
A).
The starting material is prepared as follows:
a) Na-Fmoc-~homoPhenvlalanine
The title compound is synthesized starting from D-homophenylalanine (Bachem, Bubendorf,
Switzerland) according to a procedure known in the art (see E. Atherton and R.C. Sheppard
in Solid-Phase Peptide Synthesis- A Practical Approach, eds.: D. Rickwood and B.D.
Hames, IRL Press at Oxford University Press, Oxford, 1989): Rf= 0.44 (dichlorometha-
ne:methanol:water:acetic acid, 850:130:15:5, v/v/vlv).
ExamPle 89: trans-3,4-Dihydroxy-cinnamoyl-Tyr(PO3H2)-DHPh-NH2
(SEQ ID NO: 89)
Na-Fmoc-~homophenylalanine (2 equiv.) is incorporated with 2-(2-pyridon-1-yl)-1,1,3,3-
tetramethyluroniu~ rdtluoroborate (2 equiv.) in the presence of diisopropylethylamine (2.2
equiv.). Mass-spectral analysis (negative-ion mode): 582.7 (calc. 582.5, C28H30N3o9p1).
tR= 7.04 (HPLC system A).
Example 90: trans-3,4-Dihydroxy-cinnamoyl-Tyr(PO3H2)-ACsc-NH2
(SEQ ID NO: 90)
Fmoc-1-amino-cyclopentanecarboxylic acid (2 equiv.) is incorporated with ben~uL~ ole-1-
yl-oxy-tris-dimethylamino)-phosphoniumhexafluorophosphate/N-hydroxyber,~uL~ ole (1: 1;
2 equiv.; first coupling) and N-[(dimethylamino)-1 H-1 ,2,3-triazolo[4,5-b]pyridin-1 -ylmethyle-
ne]-N-methyl,llt3l1,a,laminium hexafluorophosphate N-oxide (2 equiv.; second coupling) in
the presence of diisopropylethylamine (6 equiv.). The title compound is obtained after
manual synthesis: Mass-spectral analysis (negative-ion mode): 532.6 (calc. 532.5,
c24H27N3osp1)~ tR= 5.41 (HPLC system A).
CA 02227~16 1998-01-21
WO 97/08193 PCT/EP96/03473
- 90 -
The starting material is obtained as follows:
a) Fmoc-1 -amino-cvcloPentanecarboxvlic acid
The title compound is synthesized from 1-amino-1-cyclopentanecarboxylic acid (Aldrich,
Buchs, Swikerland) accordil19 to a procedure known in the art (see E. Atherton and R.C.
Sheppard, in Solid-Phase Peptide Synthesis- A Practical Approach, eds.: D. Rickwood and
B.D. Hames, IRL Press at Oxford University Press, Oxford, 1989): Rf= 0.79
(chloroform:methanol:water:acetic acid, 750:270:50:5, v/v/v/v).
ExamPle 91: trans-3,4-Dihydroxy-cinnamoyl-Tyr(PO3H2)-Pro-NH2
(SEQ ID NO: 91)
The manual synthesis results in the title compound: Mass-spectral analysis (negative-ion
mode): 518.6 (calc. 518.5, C23H2sN3OgP1). tR= 5.22 (HPLC system A).
ExamPie 92: 2-Naphthoyl-Tyr(PO3H2)-lle-Asn-NH2
(SEQ ID NO: 92)
Mass-spectral analysis (negative-ion mode): 640.5 (calc. 640.6, c3oH3sNsosp1). tR= 8-05
(HPLC system A).
2-Naphthoic acid is from Fluka, Buchs, Switzerland, and is incorporated (2 equiv.) with ben-
~uL, i~ule-1 -yl-oxy-tris-dimethylamino)-phosphoniumhexafluorophosphate/N-hydroxybenzo-
triazole (1 :1; 2 equiv.; double coupling) in the presence of diisopropylethylamine (4 equiv.).
Examole 93: 1-Adamantoyl-Tyr(PO3H2)-lle-Asn-NH2
(SEQ ID NO: 93)
Mass-spectral analysis (negative-ion mode): 648.3 (calc. 648.7, c3oH43Nsosp1). tR= 8-23
(HPLC system A).
1-Adamantoic acid is from Fluka, Buchs, Switzerland, and is incorporated (2 equiv.) with
be, l~uLr i~ole-1 -yl-oxy-tris-dimethylamino)-phosphoniumhexafluorophosphate/N-hydroxy-
benzotriazole (1 :1; 2 equiv.; double coupling) in the presence of diisopropylethylamine (4
equiv.).
ExamPle 94: Cyclohexanoyl-Tyr(PO3H2)-lle-Asn-NH2
(SEQ ID NO: 94)
Mass-spectral analysis (negative-ion mode): 596.7 (calc. 596.6, c26H3sNsosp1). tR= 6-94
(HPLC system A).
Cyclohexanoic acid is from Fluka, Buchs, Switzerland, and is incorporated (2 equiv.) with
ben~uL, i~ule-1 -yl-oxy-tris-dimethylamino)-phosphoniumhexafluorophosphate/N-hydroxy-
CA 02227~16 1998-01-21
W O 97/08193 PCTrEP96/03473
ben~uLrid~ole (1 :1; 2 equiv.; double coupling) in the presence of diisopropylethylamine (4
equiv.).
ExamPle 95: 3-Cyclohexyl-propionyl-Tyr(PO3H2)-lle-NH2
(SEQ ID NO: 95)
Mass-spectral analysis (negative-ion mode): 510.5 (calc. 510.6, C24H37N3~7P1)- tR= 9-17
(HPLC system A).
-Cyclohexyl-propionic acid is from Fluka, Buchs, Switzerland, and is incorporated (2 equiv.)
with ber,~uL,id~ole-1-yl-oxy-tris-dimethylamino)-phosphoniumhexafluorophosphate/N-hydro-
yben~uLri~ole (1 :1; 2 equiv.; double coupling) in the presence of diisopropylethylamine (4
equiv.).
Example 96: 1,2,3,4-Tetrahydro-2-naphthoyl-Tyr(PO3H2)-lle-NH2
(SEQ ID NO: 96)
Mass-spectral anaiysis (negative-ion mode): 530.6 (calc. 530.6, C~6H~3N3O7P1). tR= 8-73
(HPLC system A).
1,2,3,4-Tetrahydro-2-naphthoic acid is from Fluka, Buchs, Switzerland, and is incorporated
(2 equiv.) with ben~uLri~ole-1-yl-oxy-tris-dimethylamino)-phosphoniumhexafluorophos-
phate/N-hydroxyben~oL, id~ole (1 :1; 2 equiv.; double coupling) in the presence of diisopro-
pylethylamine (4 equiv.).
ExamPle 97: Cyclohexanoyl-Tyr(PO3H2)-lle-NH2
(SEQ ID NO: 97)
Mass-spectral analysis (negative-ion mode): 482.4 (calc. 482.5, C22H33N3o7p1). tR= 7-65
(HPLC system A).
Cyclohexanoic acid is from Fluka, Buchs, Switzerland, and is incorporated (2 equiv.) with
bel ,~uL, i~ole-1 -yl-oxy-tris-dimethylamino)-phosphoniumhexafluorophosphate/N-hydroxy-
ben~ul,i~ole (1:1; 2 equiv.; double coupling) in the presence of diisopropylethylamine (4
equiv.).
ExamPle 98: 2-Naphthoyl-Tyr(po3H2)-lle-NH2
(SEQ ID NO: 98)
Mass-spectral analysis (negative-ion mode): 526.6 (calc. 526.5, C26H2gN3O7P1). tR= 8.67
(HPLC system A).
.
CA 02227~16 1998-01-21
WO 97/08193 PCTAEP96/03473
- 92 -
-Naphthoic acid is from Fluka, Buchs, Switzerland and is incorporated (2 equiv.) with ben-
~ul, ia~ole-1 -yl-oxy-tris-dimethylamino)-phosphoniumhexafluorophosphate/N-hydroxybenzo-
triazole (1 :1; 2 equiv.; double coupling) in the presence of diisopropylethylamine (4 equiv.).
Example 99: 1-Adamantoyl-Tyr(PO3H2)-lle-NH2
(SEQIDNO:99)
Mass-spectral analysis (negative-ion mode): 534.7 (calc. 534.6, C2~H3,N3O7P1)~ tR= 9-15
(HPLC system A).
1-Adamantoic acid is from Fluka, Buchs, Switzerland and is incorporated (2 equiv.) with
bel l~oLri~ole-1 -yl-oxy-tris-dimethylamino)-phosphoniumhexafluorophosphate/N-hydroxy-
ber,~oLri~ole (1 :1; 2 equiv.; double coupling) in the presence of diisopropylethylamine (4
equiv.).
Example 1û0: 4-Acetamino-benzoyl-Tyr(PO3H2)-lle-NH2
(SEQ ID NO: 10û)
Mass-spectral analysis (negative-ion mode): 533.5 (calc. 533.5, C24H3oN4o8p1)~ tR= 5-99
(HPLC system A).
4-Acetamino-benzoic acid is from Fluka, Buchs, Switzerland and is incorporated (2 equiv.)
with ber,~oL,ia~ole-1-yl-oxy-tris-dimethylamino)-phosphoniumhexafluorophosphate/N-hydro-
xybell~ulrid~ùle (1 :1; 2 equiv.; double coupling) in the presence of diisopropylethylamine (4
equiv.).
Example 101: Succinamoyl-Tyr(PO3H2)-lle-Asn-NH2
(SEQ ID NO: 101)
Mass-spectral analysis (negative-ion mode): 585.5 (calc. 585.5, C23H34N6o1op1)~ tR= 4-55
(HPLC system A).
Succinamic acid is from Aldrich, Buchs, Switzerland, and is incorporated (2 equiv.) with ben-
~uL, ia~ole-1 -yl-oxy-tris-dimethylamino)-phosphoniumhexafluorophosphate/N-hydroxybenzo-
triazole (1 :1; 2 equiv.; double coupling) in the presence of diisopropylethylamine (4 equiv.).
ExamPle 102: 1,2,3,4-Tetrahydro-2-naphthoyl-Tyr(PO3H2)-lle-Asn-NH2
(SEQ ID NO: 102)
Mass-spectral analysis (negative-ion mode): 644.7 (calc. 644.7, c3oH3sNsosp1) tR= 8 19
(HPLC system A).
1,2,3,4-Tetrahydro-2-naphthoic acid is from Fluka, Buchs, Switzerland, and is incorporated
(2 equiv.) with ben~oLria~ole-1-yl-oxy-tris-dimethylamino)-phosphOniUmheXafluorophospha-
CA 02227~16 1998-01-21
WO 97/08193 PCT~EP96/03473
-93-
te/N-hydroxybenzotriazoie (1 :1; 2 equiv.; double coupling) in the presence of diisopropyl-
ethylamine (4 equiv.).
Exam~le 103: 1,2,3,4-Tetrahydro-2-naphthoyl-Tyr(PO3H2)-lle-lle-Pro-NH2
(SEQ ID NO: 103)
Mass-spectral anaiysis (negative-ion mode): 740.7 (calc. 740.8, C37Hs1NsOgP1). tR= 6.77
(HPLC system A).
1,2,3,4-Tetrahydro-2-naphthoic acid is from Fluka, Buchs, Swikt:rland, and is incorporated
(2 equiv.) with ber,~ul.i~ole-1-yl-oxy-tris-dimethylamino)-phosphoniumhexafluorophospha-
te/N-hydroxyben~ul~ ole (1 :1; 2 equiv.; double coupling) in the presence of diisopropyl-
ethylamine (4 equiv.).
Exam~le 104: trans-3,4-Dihydroxy-cinnamoyl-Tyr(PO3H2)-HPh-NH2
(SEQ ID NO: 104)
Mass-spectral analysis (negative-ion mode): 682.5 (calc. 582.5, C2gl l~gN3OgP1). tR= 6.92
(HPLC system A).
The starting material is prepared as follows:
a) Fmoc-L-homo-phenylalanine
The title compound is synthesized starting from L-homophenylalanine (H-Hph-OH, Bachem,
Bubendorf, Switzerland) according to a procedure known in the art (see E. Atherton and
R.C. Sheppard, in Solid-Phase Peptide Synthesis- A Practical Approach, eds.: D. Rickwood
and B.D. Hames, IRL Press at Oxford University Press, Oxford, 1989): Rf= 0.63 (chloro-
form:methanol:water:acetic acid, 750:270:50:5).
Example 105: trans-3~4-Dihydroxy-cinnamoyl-Tyr(po3H2)-lle-Asn-NH2
(SEQ ID NO: 1û5)
The peptide is synthesized on a Milligen 9050 automated peptide synthesizer (continuous
flow; Millipore, Bedford, MA, USA), starting with an Fmoc-PAL-PEG-PS resin (see Albericio,
F. et al. J. Org. Chem., 55 (1990) 3730-3743) for establishing the C-l~"ni,1al caboxamide,
and using chemical protocols based on the fluorenylmethoxycarbonyl chemistry (see E.
Atherton and R.C. Sheppard, in Solid-Phase Peptide Synthesis- A Practical Approach, eds.:
D. Rickwood and B.D. Hames, IRL Press at Oxford University Press, Oxford, 1989). The
required Fmoc-amino acids (3 equiv.) are coupled using their 2,4,5-trichlorophenyl esters
r (single coupling) with minimum reaction times of 30 min (see 9050 Plus PeptSynthesizer,
User's Guide, Millipore Corporation, Bedford, MA, 1992). The incorporation of N~-Fmoc-
Tyr(PO3H2)-OH (3 equiv.) and trans-3,4-dihydroxy-cinnamic acid are accomplished with
CA 02227~l6 l998-0l-2l
WO 97/08193 PCT/EP96/03473
ben~ulr i~ole-1 -yl-oxy-tris-dimethylamino) -phosphoniumhexafluorophosphate/N-hydroxy-
ben~uLIia~ole (1:1; 3 equiv., first coupling) and N-[(dimethylamino)-1 H-1 ,2,3-triazolo[4,5-
b]pyridin-1-ylmethylene]-N-methylmethanaminium hexafluorophosphate N-oxide (3 equiv.;
second coupling) in the presence of diisopropylethylamine (6 equiv.). The complete peptide
resin obtained after the last coupling step is simultaneously deprotected and cleaved by
treatment with trifluoroacetic acid/H2O (95:5, v/v) for 3 h at room temperature. The filtrate
from the cleavage reaction is precipitated in diisopropyl ether/petroleum ether (1:1, v/v,
O ~C), and the pl eci~c,ilaLe collected by filtration. The crude peptide is purified by medium-
pressure liquid chromatography as described in Example 1. Title compound: Mass-spectral
analysis (negative-ion mode): 648.3 (calc. 648.6, C28H3sNsO11P1). tR= 5.88 (HPLC system
A).
Exam~le 106: trans-3,4-Dihydroxy-cinnamoyl-Tyr(PO3H2)-lle-~Ala-NH2
(SEQ ID NO: 1û6)
Fmoc-,~-alanine is from Novabiochem (Laufelfingen, Switzerland) and is incorporated (3
equiv.) with b~ u~ ole-1-yl-oxy-tris-dimethylamino)-phosphoniumhexafluorophospha-
te/N-hydroxyben~uL,i~ole (1:1; 3 equiv.) in the presence of diisopropylethylamine (6
equiv.). Title compound: Mass-spectral analysis (negative-ion mode): 605.6 (calc. 605.6,
C26H34N4O10P1). tR= 5.85 (HPLC system A).
Example 107: trans-3l4-Dihydroxy-cinnamoyl-Tyr(po3H2)-lle-Gln-NH2
(SEQ ID NO: 107)
Automatic synthesis yields the title compound: Mass-spectral analysis (negative-ion mode):
661.0 (calc. 662.6, C2gH37NsO11P1). tR= 5.87 (HPLC system A).
Example 108: trans-3~4-Dihydroxy-cinnarl~oyl-Tyr(PO3H2)-lle-Gly-NH2
(SEQ ID NO: 108)
Automatic synthesis yields the title compound: Mass-spectral analysis (negative-ion mode):
591.1 (calc. 591.5, C26H32N4O10p1) tR= 6.18 (HPLC system A).
Exam~le 109: trans-3~4-dihydroxy-cinnamoyl-F2pmp-lle-Asn-NH2
(SEQ ID NO: 109)
The peptide is synthesized manually on a 4-(2',4'-dimethoxyphenyl-aminomethyl)-phenoxy
resin, employing a procedure analogous to Example 67. Incorporation of Na-Fmoc-[4-(O-
diethyl)-phophono(difluoromethyl)]-L-phenylalanine (for synthesis see Tetrahedron Lett. 34
(22), 3543 (1993)) to the protected peptide resin is carried out with be"~ul.ia~ole-1 yl-N-
CA 02227~16 1998-01-21
W O 97/08193 PCT~EP96/03473
- 95 -
oxytris-(dimethylamino)-phosphonium hexafluorophosphate/N-hxdroxybenzotriazole (1:1, 3
equiv.) in the presence of diisopropylethylamine (7 equiv.), 3 h reaction time. trans-3,4-
Dihydroxy-cinnamic acid (3 equiv.) is incorporated with beh~ul~i~ole-1-yl-oxy-tris-dimethyl-
amino)-phosphoniumhexafluorophosphate/N-hydroxyben~ul, ia~ole (1 : 1; 3 equiv. , first
coupling) in the presence of diisopropylethylamine (7 equiv.), 2 h reaction time. The
complete peptide resin obtained a~ter the last coupling step is deprotected and cleaved via
a two step process. The peptide is removed from the solid-support and partially deprotected
by treatment with trifluoroacetic acid/H2O (95:5, v/v) for 3 h at room temperature. The
cleavage of the phosphonate diester protecting groups is carried out as follows: The crude
compound is suspended in MeCN (1 ml) and trimethylsilyl-iodide (1 ml) is added dropwise to
the suspension. The course of the reaction is followed by analytical HPLC. A~ter 60 min
complete reaction is achieved. Reaction is ter",i"~led by removal of MeCN followed by
addition of a 10 % solution of aqueous AcOH (12 ml) to hydrolyze the trimetylsilyl ester in-
termediates. The aqueous solution is extracted with diethylether, concentrated and injected
directly in the medium-pressure liquid chror"dlography system (see Example 1). Title com-
pound: Mass-spectral analysis (negative-ion mode): 682.8 (calc. 682.6,
c29H35Nso1oF2p1). tR= 5.65 (HPLC system A).
ExamPle 11 0: trans-3,4-Dihydroxy-cinnamoyl-F2PmP-lle-NH2
(SEQ ID NO: 110)
Manual synthesis results in the title compound: Mass-spectral analysis (negative-ion mode):
568.2 (calc. 568.5, C2~ gN3OgF2P1). tR= 6.63 (HPLC system A).
Example 111: 3-Aminobenzyloxycarbonyl-Tyr(PO3H2)-Met-Asn-NH2 (TFA salt)(SEQ ID NO: 111)
By manual synthesis, the title compound is obtained in analogy to Example 3: Mass spectral
analysis (negative-ion mode): 653.7 (calc. 653.6, C26H34N6O10p1s1)~ tR= 4.88 min(HPLC System A).
Example 112: Indole-5-ylcarbonyl-Tyr-lle-Asn-Gln-NH2
(SEQ ID NO: 112)
In analogy to Example 61, the title compound is obtained by manual synthesis: Mass
spectral analysis (negative-ion mode): 677.8 (calc. 677.7, C33H41NgOg), tR= 7.12 min
(HPLC System A).
.
Example 113: Cyclohexanecarbonyl-Tyr(po3H2)-lle-Asn-NH2
-
CA 02227~16 1998-01-21
WO 97/08193 PCT~EP96/03473
-96-
(SEQ ID NO: 113)
Cyclohexanecarboxylic acid (2 equiv.; Fluka, Buchs, Switzerland) is coupled with BOP/HOBt
(1 :1, 2 equiv.) in the presence of diisopropylethylamine (4 equiv.) in N-methylpyrrolidin-2-
one, yielding the title compound: Mass spectral analysis (negative-ion mode): 596.7 (calc.
596.6, C26H3gNsOgP1), tR= 6.94 min (HPLC System A).
Example 11 4: 2-Naphthylcarbonyl-Tyr(PO3H2)-lle-ASn-NH2
(SEQ ID NO: 114)
2-Naphthoic acid (2 equiv.; Fluka, Buchs, Switzerland) is coupled with BOP/HOBt (1 :1, 2
equiv.) in the presence of diisopropylethylamine (4 equiv.) in N-methylpyrrolidin-2-one, yiel-
ding the title compound: Mass spectral analysis (negative-ion mode): 640.5 (calc. 640.6,
C30H3sNsOgP1), tR= 8.05 min (HPLC System A).
Example 11 5: Indole-5-ylcarbonyl-L-F2Pmp-lle-Asn-NH2
(SEQ ID NO: 115)
In analogy to example 64, the title compound is obtained by manual synthesis: Mass
spectral analysis (negative-ion mode): 663.7 (calc. 663.6, C2sH34N6o8p1 F2), tR= 6-63
min (HPLC System A).
Example 11 6: 9-Fluorenylcarbonyl-Tyr(PO3H2)-lle-Asn-NH2
(SEQ ID NO: 116)
The synthesis proceeds manually. 9-Fluorenecarboxylic acid (3 equiv.; Aldrich, Steinheim,
Germany) is coupled with BOP/HOBt (1 :1, 3 equiv.; first coupling) and HATU (3 equiv.;
second coupling) in the presence of diisopropylethylamine (7 equiv.) in N-methylpyrrolidin-2-
one, yielding the title compound: Mass spectral analysis (negative-ion mode): 678.5 (calc.
678.7, C33H37N~OgP1), tR= 8.01 min (HPLC System A).
Example 11 7: 3-Aminobenzyloxycarbonyl-Tyr(PO3H2)-Gln-Asn-NH2 (TFA salt)
(SEQ ID NO: 117)
Manual synthesis yields the title compound: Mass spectral analysis (negative-ion mode):
650.7 (calc. 650.6, C26H33N7O11 P1), tR= 3.78 min (HPLC System A).
ExamPle 118: 3-aminobenzyloxycarbonyl-Tyr(PO3H2)-Tle-Asn-NH2 (TFA salt)(SEQ ID NO: 118)
Synthesis is manually. Na-Fmoc-L-a-t-butyl-glycine (2 equiv.; Novabiochem, Laufelfingen,
Switzerland) is coupled with BOP/HOBt (1 :1, 2 equiv.) in the presence of diisopropylethyl-
CA 02227~l6 l998-0l-2l
W O 97/08193 PCTAEP96/03473
amine (5 equiv.) in N-methylpyrrolidin-2-one. Title compound: Mass spectral analysis (nega-
tive-ion mode): 635.3 (calc. 635.6, C27H36N6O10pl)~ tR= 5.53 min (HPLC System A).
Example 119: 3-Aminobenzyloxycarbonyl-L-Pmp-lle-Asn-NH2 (TFA)
(SEQ ID NO: 119)
Synthesis is manually. N-a-Fmoc-p-(bisethoxyphosphoryl-methyl)-Phe-OH (1.5 equiv.;
Neosystem Catalogue; See also C. Garbay-Jaureguiberry et al., Int. J. Pept. Prot. Res. 39,
523-527 (1992)) is incorporated with BOP/HOBt 1 :1; 1.5 equiv.) in the presence of diisopro-
pylethylamine (4 equiv.) in N-methylpyrrolidin-2-one. After the addition of the building block,
the Fmoc group is removed and the peptide resin is cleaved with trifluoroacetic acid/water
(95:5, v/v, v/v) for 3 h at room temperature. The filtrate is precirit~ted in diisopropyl ether-
petroleum ether (1 :1, v/v) at 0 ~C, and the p(eG~ ,lt3j5 collected by filtration. The com-
pound (68 mg) is dissolved in acetonitrile (2 ml) and trimethylsilyl iodide (1.7 ml; Fluka,
Buchs, Swike,lal1d) is added. The course of the reaction is monitored by analytical HPLC
using a reversed-phase column. After 1.5 h at room temperature, the solution is concentra-
ted to dryness and the crude compound is dissolved in 10% AcOH in water (5 ml). The so-
lution is extracted with ether (x2) and the ,,-lueous phase is iniected directly to the medium-
pressure liquid cl"~"~Lography system decribed in Example 1, yielding the title compound:
Mass spectral analysis (negative-ion mode): 633.5 (calc. 633.6, C28H88N6o9p1)~
tR= 5.44 min (HPLC System A).
Example 120: 3-Aminobenzyloxycarbonyl-Tyr(PO3H2)-Ac6c-Asn-NH2 (TFA salt)
(SEQ ID NO: 120)
Manual synthesis results in the title compound: Mass spectral analysis (negative-ion mode):
647.7 (calc. 647.6, C28H36N6O10p1)~ tR= 5.38 min (HPLC System A).
Example 121: Acetyl-Tyr(PO3H2)-lle-Asn-NH-(3-naphthalen-1 -yl-propyl)
(SEQ ID NO: 121)
The peptide is sy"ll,esi~ed manually on a 4-(2',4',-dimethoxyphenyl-Fmoc-aminomethyl)-
phenoxyacetamido-norleucyl-MBHA resin (commercially available from Novabiochem,
Laufelfingen, Switzerland, 0.55 mmol/g), employing the Fmoc strategy (see E.Atherton and
R.C.Sheppard, in Solid-Phase Peptide Synthesis- A Practical Approach, ed. D.Rickwood
and B.D. Hames, IRL Press at Oxford University Press, Oxford,1989). Fmoc is removed
with piperidine/ dimethylacetamide (1 :4, v/v; 6 x 2 min) followed by washing with isopropa-
nol (3 x 1 min), dimethylacetamide (2 x 1 min), isopropanol (3 x1 min) and dimethylacet-
amide (2 x 1 min). Coupling is achieved by first dissolving the Fmoc-amino acid (3 equiv.),
CA 02227~l6 l998-0l-2l
W O 97/08193 PCTAEP96/03473
-98-
diisopropylethyl amin (3.3 equiv.), and the 2-(2-pyridon-1-yl)-1,1,3,3-tetramethyluroniumte-
trafluoroborate reagent (TPTU, commercially available from Senn Chemicals, Dielsdorf,
Switzerland; 3 equiv.) in N-methylpyrrolidin-2-one, then waiting 3 min for preactivation, ad-
ding the mixture to the resin, and finally shaking for at least 45 min. Na-Fmoc-aspartic acid
a-allylic ester (Novabiochem, Laufelfingen, Switzerland) is coupled through its side chain to
the resin. The incorporation of Na-Fmoc-Tyr(PO3H)-OH (see Biochemistry 32, 4354 (1993)
(3 equiv.) is accomplished with BOP/ HOBt (1 :1; 2 equiv.; first coupling) in the presence of
di..,opro,l~ylethylamine (6 equiv.) and N-~(dimethylamino)-1 H-1,2,3-triazolo[4,5-b]pyridin-1-
ylmethylene]-N-methylmethanaminium hexafluorophosphate N-oxide (HATU, 2 equiv.,
second coupling) in the presence of diisopropylethylamine (6 equiv.). Acetylation is perfor-
med with acetic anhydride and pyridine in dimethylacetamide (1 :1 :8 v/v) for 2 min followed
by washing with dimethylacetamide and dichloromethane. For the removal of the a-allyl
ester group, the dried peptide resin is re-suspended in a degassed solution of acetic acid
and N-methylmorpholin in chloroform (2:1 :37 v/v) followed by addition of tetrakis-(triphenyl-
phosphine)- p~ m(O) (0.8 equiv.) under an argon atmosphere (see F. Albericio, G.Barany, G.B. Fields, D. Hudson, S.A. Kates, M.H. Lyttle, N.A. Solé, in Peptides 1992, C.H.
Schneider, A.N. Eberle (eds), ESCOM, Leiden, 1993, p.191; S.A. Kates, S.B. Daniels, N.A.
Solé, G. Barany, F. Albericio, in Peptides; Chemistry & Structure, R.S. Hodges, J.A. Smith
(eds), ESCOM, Leiden, 1994, p.113). After treatment for 3h, the resin is washed with chlo-
roform (3 x 1 min), dimethylformamide (3 x 1 min), a solution of sodium diethyldithiocarba-
mate (0.05M) containing 0.5% diisopropylethylamine in dimethylformamide (2 x 1 min), di-
methylrur.,,a, llide (2 x 1 min) and dichloromethane (3 x1 min) and dried. The final incorpo-
ration of 3-naphthalen-1 -yl-propylamine (4 equiv.) is accomplished with TPTU/ HOBt (1 :1, 6
equiv.) in the presence of diisopropylethylamine (9 equiv.) with N-methylpyrrolidin-2-one as
solvent. The complete peptide resin obtained after the final washing steps with dimetylacet-
amide (2 x 1 min), isopropanol (2 x 1 min) dimetylacetamide (2x1 min), dichloromethane (3
x 1 min) is simultaneously deprotected and cleaved by treatment with trifluoroacetic acid/
H2O (95:5, v/v) for 3 h at room temperature. The filtrate from the cleavage reaction is preci-
pitated in tertbutyl-methyl ether/petroleum ether (1:1, v/v, 0 CC), and the pre::ipit~te is col-
lected by centrifugation. The crude peptide is purified by medium-pressure liquid chromato-
graphy using a C18 reversed phase column (Merck ~'LICHROPREP RP-18, 15-25 llm bead
diameter, reversed phase HPLC column material based on C18-derivatized silicagel, Merck,
Darmstadt, FRG; column length 46 cm, diameter 3.6 cm; flow rate 53.3 ml/min; detection at
215nm), eluted with an acetonitrile-water gradient containing 0.1% of TFA. The title com-
pound is obtained: Mass spectral analysis (matrix-assisted laser-desorption ionisation time-
of-flight mass spectrometry, MALDI-TOF and fast atom bombardment mass spectroscopy,
CA 02227~16 1998-01-21
W O 97/08193 PCTAEP96/03473
99
FAB) reveals a molecular mass within 0.1% of the expected values (negative- ion mode:
696.0 [M-H]+, (C34H44N5OgP: 697.73)). The purity of the peptide is verified by reversed-
phase analytical HPLC on a Nucleosil C1g column (250x4 mm, 5 ~m (AB), 100~, fromMacherey-Nagel): linear gradient of acetonitrile/ 0.09% TFA and H2Ot 0.1% TFA from 1:49
to 3:2 over 10 min; flow rate 1.5 ml/min, detection at 215 nm; single peak at tR= 8.56 min
(HPLC System D).
The starting material is obtained as follows:
a) 3-(1-NaPhthyl)-acrylonitril
A mixture of 6.8 ml (7.8 9 = 0.05 mol) 1-naphthaldehyd (Aldrich, Buchs, Switzerland) and
7.9 ml (8.8 9 = 0.05 mol) diethyl cyanomethylphosphonate (Fluka, Buchs, Swikerldnd) is
treated dropwise with 17 ml of a 6 molar potassium carbonate solution (0.1 mol) under
stirring. The ter"pe,dlure is kept at 20~C by cooling in an ice bath. After complete addition,
the mixture is stirred 10 minutes at room temperature. Thin layer chromatography (tolu-
ene/ethyl acetate 1:1) illdiCdl~:S the end of the reaction. After dilution with ethyl acetate, the
reaction mixture is washed with water, dried over sodium sulfate, filtered and evaporated.
The residue, a viscous oil, is purified by flash-chromatography (toluene/petrol ether 1:1).
The title compound is obtained as a colorless oil.
Rf (toluene/petrol ether 1:1) = 0.15
b) 3-(1-NaPhthyl)-1-propylamine hydrochloride
6.8 9 (0.0379 mol) of 3-(1-naphthyl)-acrylonitril are hydrogenated at normal pressure and at
45~C in presence of 2.0 9 of Raney-Nickel (Fluka, Buchs, Switzerland) in 220 ml methanol
containing 5 % a",r"onia. The catalyst is removed by filtration, and the methanol is evapo-
rated. The residue is purified by flashchromatography (ethyl acetate/methanol 9:1 contai-
ning 1% conc. ammonia). 3-(1-naphthyl)-1-propylamine is obtained: Rf (ethyl ~et~te
methanol 1:1 containing 1% conc. ammonia) = 0.10
The free base is dissolved in 40 ml of ethanol and treated with 10 ml of a 10% solution of
hydrogen chloride in ethanol (approximately 0.03 mol). The solution is concentrated and the
product plt:c;~ dled by addition of ether. The product is collected by filtration, yielding the
title compound: Melting point 154-1 56~C.
The following examples 122 to 139 are obtained in analogy to the title compound of
Example 121, if not indicated otherwise:
CA 02227~16 1998-01-21
W O 97/08193 PCTAEP96/03473
-100-
Example 1 22: Acetyl-Tyr(P03H2)-lle-Asn-NH-[3-(2-hydroxy-naphthalen-1 -yl)-propyl]
(SEQ ID NO: 122)
1-(3-Aminopropyl)-naphthol-(2) is synthesized according to known procedures (see H.
Plieninger, Chem. Ber. 87, 232 (1 954)), starting from 1-(2-cyanoethyl)-naphthol-(2)
Title compound: Mass spectral analysis (negative-ion mode): 713.0 lM-H]+,
(C34H44N5010P, calc. 713.73), tR= 8.01 min (HPLC System D).
ExamPle 123: Acetyl-Tyr(PO3H2)-lle-Asn-NH-(3-naphthalen-2-yl-propyl)
(SEQ-ID-N0: 123)
Title compound: Mass spectral analysis (negative-ion mode): 696.7 [M-H]+,
(C34H44N5O9P, calc. 697.73), tR= 8.75 min (HPLC System D).
The starting material is obtained as follows:
a) 3-(2-Naphthvl)-acrylonitril
This product is synthesized as described for 3-(1 -naphthyl)-acrylonitril (Example 123 a).
Starting from 15.6 9 (0.1 mol) 2-naphthaldehyd, of 3-(2-naphthyl)-acrylonitril are obtained as
an oil: Rf (toluene/petrol ether 4:1) = 0.30
b) 3-(2-Naphthyl)-1-propvlamine hydrochloride
This product is Syl lll ,esi~ed as described for 3-(1 -naphthyl)-1 -propylamine hydrochloride
(Example 122 b). Starting from 12.0 9 (0.067 mol) 3-(2-naphthyl)-acrylonitril, 3-(2-naphthyl)-
1-propylamine is obtained: Rf (ethyl :3cet~tP./methanol 9:1 containing 1% conc. ammonia) =
0.10. This ~ L~,ial is converted to the hydrochloride, yielding the title compound: Melting
point 250-253~C.
ExamPle 1 24: Acetyl-Tyr(PO3H2)-lle-Asn-NH-(3-phenanthren-9-yl-propyl)
(SEQ ID N0: 124)
Title compound: Mass spectral analysis (negative-ion mode): 747.0 [M-H]+,
(C38H46NsOsp~ calc 747.79), tR= 9.43 min (HPLC System D).
The starting material is obtained as follows:
a) 3-(Phenanthren-9-yl)-Propylamine
The title compound is synthesized starting with a Grignard reaction between 9-
bromophenanthrene (Fluka, Buchs, S~ike,land) and ethylene oxide (Fluka, Buchs,
Switzerland). The resulting alcohol is converted to the chloride with thionylchloride in the
CA 02227~l6 l998-0l-2l
W O 97/08193 PCTrEP96/03473
-101 -
.
presence of pyridine in chloroform (see D.J. Jakiela, P.Helquist, L.D. Jones, Organic
Synthesis 1983, 62, p.74). Then, conversion of the halide to the nitrile is achieved with
potassium cyanide and a catalytic amount of sodium iodide in dimethylsulfoxide as solvent
(see D.Brett, l.M.Downie, J.B. Lee, J.Org.Chem 1967, 32, p.855). Finally, LiAlH4 reduction
provides 9-phenanthren-9-yl-propylamine. NMR spectra and mass spectra are in agreement
with published data (see F.D.Lewis, G.D. Reddy, B.E. Cohen, Tetrahedron Letters 1994,
35, p.535)-
Example 125: Acetyl-Tyr(PO3H2)-lle-Asn-NH-[2-(1-bromo-naphthalen-2-yloxy)-ethyl](SEQ ID NO: 125)
Title compound: Mass spectral analysis (negative-ion mode): 777.3 [M-H]+,
(C33H41 BrN5O10P, calc. 778.60), tR= 8.79 min (HPLC System D).
The starting material is obtained as follows:
a) (1-Bromo-naphthalen-2-yloxy)-acetonitrile
To a stirred mixture containing 1-Brom-2-naphthol (44.69, 0.2 mol, Aldrich, Buchs, Switzer-
land), potassium carbonate (30.5g, 0.22 mol) and potassium iodide (109) in methyl-ethyl-
ketone chloroacetonitrile is added at 60 ~C. After stirring for 6h at 80 ~C, the reaction is
cooled to room temperature and then filtered. The filtrate is evaporated to dryness. The
residue obtained is partitioned between toluene and water. After separation, the organic
layer is washed with 2N NaOH and water, dried and the solvent evaporated. The crude
product is dissolved in dichloromethane, purified by filtration through silica gel and
crystallized by adding hexane to the solution. Crystalline (1-bromo-naphthalen-2-yloxy)-
acetonitrile is obtained, Rf= 0.37 (ethylacetate/ hexane 1 :3), melting point 96-98 ~C.
b) 2-(1-Bromo-naphthalen-2-vloxy)-ethvlamine hydrochloride
To a boiling solution of (1 -Bromo-naphthalen-2-yloxy)-acetonitrile (15.72 9, 0.06 mol) in
tetrahydrofurane (6ml), borane dimethylsulfide (6.25ml, 0.066 mol) is added dropwise in 20
min. After that, the reaction mixture is kept stirring for additional 20 min at 80 ~C. After
cooling to room temperature,1 M methanolic HCI (70 ml) is slowly added, and the hydrolysis
is completed by refluxing the mixture for 5h. The volatile components are removed by distil-
lation, and the residue is treated with methanol. After evaporating the solvent, the crude
product is dissolved in boiling ethanol, filtered and crystallized by adding diethyl ether to
result in 2-(1-bromo-naphthalen-2-yloxy)-ethylamine hydrochloride, obtained as yellowish
crystals, m.p. 265 ~C (decomposition).
CA 02227~16 1998-01-21
W O 97/08193 PCTAEP96/03473
- 102 -
ExamPle 126: Acetyl-Tyr(PO3H2)-lle-Asn-NH-(3,3-diphenyl-propyl)
(SEQ ID NO: 126)
3,3-Diphenylpropylamine is from Aldrich, Buchs, Switzerland.
Title compound: Mass spectral analysis (negative-ion mode): 722.4 [M-H]+,
(C36H46N5OgP, calc. 723.77), tR= 8.99 min (HPLC System D).
ExamPle 127: Acetyl-Tyr(PO3H2)-lle-Asn-NH-(3-phenyl-propyl)
(SEQ ID NO: 127)
3-Phenylpropylamine is from Fluka, Buchs, S~ike,land.
Title compound: Mass spectral analysis (negative-ion mode): 647.6 [M-H]+,
(C30H42N5O9P, calc. 647.68), tR= 7.62 min (HPLC System D).
Example 128: Acetyl-Tyr(PO3H2)-lle-Asn-NH-[3-(2,4-d;cl1loro-phenyl)-propyl]
(SEQ ID NO: 128)
3-(2,4-dichloro-phenyl)-propylamine is from Fluka, Buchs, S\hikerland.
Title compound: Mass spectral analysis (negative-ion mode): 715.7 [M-H]+,
(C30H40CI2N5OgP, calc. 716.56), tR= 8.91 min (HPLC System D).
Example 129: Acetyl-Tyr(PO3H2)-lle-Asn-NH-(2,2-diphenyl-ethyl)
(SEQ ID NO: 129)
2,2-Diphenylethylamine is from Aldrich, Buchs, Swikt:rland.
Title compound: Mass spectral analysis (negative-ion mode): 709.0 [M-H]+, C3sH44NsOgP,
calc. 709.74), tR= 8.69 min (HPLC System D).
Example 130: Acetyl-Tyr(PO3H2)-lle-Asn-NH-[2-(4-chloro-phenyl)-ethyl]
(SEQ ID NO: 130)
2-(4-Chloro-phenyl)-ethylamine is from Fluka, Buchs, Switzerland.
Title compound: Mass spectral analysis (negative-ion mode): 667.0 [M-H]+,
(C29H39ClN5OgP, calc. 668.04), tR= 7.95 min (HPLC System D).
ExamDle 131: Acetyl-Tyr(PO3H2)-lle-Asn-NH-benzyl
(SEQ IS NO: 131)
Benzylamine is from Fluka, Buchs, Switzerland.
Title compound: Mass spectral analysis (negative-ion mode): 618.2 [M-H]+,
(C28H38NsOgP, calc. 619.62), tR= 6.69 min (HPLC System D).
CA 02227~16 1998-01-21
W O 97/08193 PCTAEP96/03473
- -103- =
ExamPle 132: Acetyl-Tyr(P03H2)-ile-Asn-NH-isobutyl
t (SEQ ID NO: 132)
Isobutylamine is from Fluka, Buchs, Switzerland.
Title compound: Mass spectral analysis (negative-ion mode): 584.7 [M-H]+,
(C2sH40NsOgP, calc. 585.59), tR= 6.27 min (HPLC System D).
Example 133: Acetyl-Tyr~P03H2)-lle-Asn-NH-(3-methyl-butyl)
(SEQ ID NO: 133) Isopentylamine is from Fluka, Buchs, Switzerland.
Title compound: Mass spectral analysis (negative-ion mode): 598.6 [M-H]+,
(C26H42N50gP, calc. 599.62), tR= 6.98 min (HPLC System D).
Example 134: Acetyl-Tyr(P03H2)-lle-Asn-NH-(2-ethyl-hexyl)
(SEQ ID NO: 134)
(+)-2-Ethylhexylamine is from Fluka, Buchs, Switzerland.
Title compound: Mass spectral analysis (negative-ion mode): 641.2 [M-H]+,
(C2gH48N509P, calc. 641.71), tR= 8.85 min (HPLC System D).
ExamPle 135: Acetyl-Tyr(P03H2)-lle-Asn-NH-cyclohexyl
(SEQ ID NO: 135)
Cyclohexylamine is from Fluka, Buchs, Switzerland.
Title compound: Mass spectral analysis (negative-ion mode): 610.3 [M-H]+,
(C27H42N509P, calc. 611.64), tR= 6.93 min (HPLC System D).
Example 136: Acetyl-Tyr(P03H2)-lle-Asn-NH-cyclohexylmethyl
(SEQ ID NO: 136)
Aminomethyl-cyclohexane is from Fluka, Buchs, Switzerland.
Title compound: Mass spectral analysis (negative-ion mode): 624.7 [M-H]+,
(C28H44N509P, calc. 625.67), tR= 7.62 min (HPLC System D).
Example 137: Acetyl-Tyr(P03H2)-Ac6c-Asn-NH-(3-naphthalen-1 -yl-propyl)
(SEQ ID NO: 137)
The title compound is prepared in analogy to Example 121.
?
Example 138: Acetyl-Tyr(P03H2)-Ac6c-Asn-NH-(3-naphthalen-2-yl-propyl)
(SEQ ID NO: 138)
The title compound is prepared in analogy to Example 123.
CA 02227~l6 l998-0l-2l
W O 97/08193 PCTAEP96/03473
-104-
ExamDle 139: Acetyl-Tyr(PO3H2)-Ac6c-Asn-NH-[3-(2-hydroxy-naphthalen-1-yl)-propyl]
(SEQ ID NO: 139)
The title compound is prepared in analogy to Example 122.
ExamPle 140: 3-Aminobenzyloxycarbonyl-Tyr(PO3H2)-lle-Asn-NH-(3-naphthalen-1-yl-
propyl)
(SEQ ID NO: 140)
The peptide is synthesized manually on a 4-(2',4',-dimethoxyphenyl-Fmoc-aminomethyl)-
phenoxyacetamido-norleucyl-MBHA resin (commercially available from Novabiochem, Lau-
felfingen, Switzerland, 0.55 mmol/g), employing the Fmoc strategy (see E.Atherton and
R.C. Sheppard, in Solid-Phase Peptide Synthesis- A Practical Approach, ed. D.Rickwood
and B.D. Hames, IRL Press at Oxford University Press, Oxford,1989). Fmoc is removed
with piperidine/ dimethylac~L~",~ 4, v/v; 6 x 2 min) followed by washing with isopropa-
nol (3 x 1 min), dimethylacetamide (2 x 1 min), isopropanol (3 x1 min) and dimethylacet-
amide (2 x 1 min). Coupling is achieved by first dissolving the Fmoc-amino acid (3 equiv.),
diisopropylethylamine (3.3 equiv.), and the 2-(2-pyridon-1-yl)-1,1,3,3-tetramethyluroniumte-
trafluoroborate reagent (TPTU, commercially available Senn chemicals, Dielsdorf, Switzer-
land; 3 equiv.) in N-methylpyrrolidin-2-one, then waiting 3 min for preactivation, adding the
mixture to the resin, and finally shaking for at least 45 min. Na-Fmoc-asparagine-a-allylic
ester (Novabiochem, Laufelfingen, Switzerland) is coupled through its side chain to the
resin. The incorporation of N~-Fmoc-Tyr(PO3H2)-OH (see Biochemistry 32, 4354 (1993) (3
equiv.) is acco", ' shed with BOP/ HOBt (1 :1; 2 equiv.; first coupling) in the presence of
diisopropylethylamine (6 equiv.) and N-[(dimethylamino)-1 H-1,2,3-triazolo[4,5-b]pyridin-1-
ylmethylene]-N-methylmethana",i"ium hexafluorophosphate N-oxide (HATU, 2 equiv., se-
cond coupling) in the presence of diisoprupylethylamine (6 equiv.). 3-N-tert-Butoxy-carbo-
nyl-aminobenzyl-4-nitrophenyl-carbonate (3 equiv.) is coupled to the N-terminal residue of
the peptide in the presence of an equimolar amount of diisopropylethylamine in N-methyl-
pyrrolidin-2-one during 17h at room temperature. For the removal of the a-allylic ester
group, and the dried peptide resin is re-suspended in a degassed solution of acetic acid
and N-methylmorpholin in chloroform (2:1 :37 v/v) followed by addition of tetrakis-(triphenyl-
phosphne)-p~ um(û) (0.8 equiv.) under an argon atmosphere (see F. Albericio, G.
Barany, G.B. Fields, D. Hudson, S.A. Kates, M.H. Lyttle, N.A. Solé, in Peptides 1992, c.H.
Schneider, A.N. Eberle (eds), ESCOM, Leiden,1993, p.191; S.A. Kates, S.B. Daniels, N.A.
Solé, G. Barany, F. Albericio, in Peptides; chemistry & Structure, R.S. Hodges, J.A. Smith
(eds), ESCOM, Leiden,1994, p.113) . After treatment for 3h, the resin was washed with
CA 02227~16 1998-01-21
W O 97/08193 PCT~EP96/03473
-105-
chloroform (3 x 1 min), dimethylformamide (3 x 1 min), a solution of sodium diethyldithio-
carbamate (0.05M) containing 0.5% diisopropylethylamine in dimethylformamide (2 x 1 min),
dimethylformamide (2 x 1 min) and dichloromethane (3 x1 min) and dried. The final
incorporation of 3-naphthalen-1-yl-propylamine (4 equiv.) is accomplished with TPTU/ HOBt
(1 :1, 6 equiv.) in the presence of diisopropylethylamine (9 equiv.) with N-methylpyrrolidin-2-
one as solvent. The complete peptide resin obtained after the final washing steps with
dimetylacela~ e (2 x 1 min), isopropanol (2 x 1 min) dimetylacetamide (2x1 min), di-
chloromethane (3 x 1 min) is simultaneously deprotected and cleaved by treatment with
trifluoroacetic acid/ H20 (95:5, v/v) for 3 h at room L~mperdlure. The filtrate from the clea-
vage reaction is preci~ l.sd in tert-butyl-methyl ether/petroleum ether (1 :1, v/v, 0 ~c), and
the precipit~te is collected by centrifugation. The crude peptide is purified by medium-pres-
sure liquid chromatography using a c18 reversed phase column (Merck ~LICHROPREP RP-
18,15-25 ,um bead diameter, reversed phase HPLC column material based on C18-deri-
vatized silicagel, Merck, Darmstadt, FRG; column length 46 cm, dia~"~:ler 3.6 cm; flow rate
53.3 ml/min; d~lal;Lion at 215nm), eluted with an acetonilr;lc w~ter gradient containing 0.1%
of TFA. The title compound is obtained: Mass spectral analysis (matrix-~sisted laser-de-
sorption ionis~lion time-of-flight mass spectrometry, MALDI-TOF and electro-spray ionisa-
tion mass spectroscopy, ESI) reveals a molecular mass within 0.1% of the expected values
(negative- ion mode: 803.0 [M-H]+, (C40H49N6O10P, calc. 804.85). The purity of the pep-
tide is verified by reversed-phase analytical HPLC on a Nucleosil C18-column (250x4 mm, 5
~m (AB), 100A, from Macherey-Nagel): linear gradient of acetonitrile/ 0.09% TFA and H2O/
0.1 % TFA from 1 :49 to 3:2 over 10 min; flow rate 1.5 ml/min detection at 215 nm; single
peak at tR= 8.6 min (HPLC System D).
The following examples 141 to 149 are obtained in analogy to the title compound of
Example 140 and Examples 121 to 139 where also starting materials are described, if not
mentioned otherwise:
Example 141: 3-Aminobenzyloxycarbonyl-Tyr(PO3H2)-lle-Asn-NH-[3-(2-hydroxy-
naphthalen- 1 -yl)-propyl]
(SEQ ID NO: 141)
Title compound: Mass spectral analysis (negative-ion mode): 819.7 [M-H]+,
(C40H4gN6O11 P, calc. 820.84), tR= 8.11 min (HPLC System D).
-
ExamPle 142: 3-Aminobenzyloxycarbonyl-Tyr(PO3H2)-lle-Asn-NH-(3-naphthalen-2-yl-
propyl)
CA 02227~16 1998-01-21
W O 97/08193 PCTAEP96/03473
- 106-
(SEQ ID NO: 142)
Title compound: Mass spectral analysis (negative-ion mode): 803.7 [M-H]+,
(C40H4gN6O1 oP, calc. 804.85), tR= 8.81 min (HPLC System D).
Example 143: 3-Aminobenzyloxycarbonyl-Tyr(PO3H2)-lle-Asn-NH-(3-phenanthren-9-yl-propyl)
(SEQ ID NO: 1 43)
Title compound: Mass spectral analysis (negative-ion mode): 853.2 [M-H]+,
(C44H51N6O10P, calc. 854.91), tR= 8.76 min (HPLC System D).
Example 144: 3-Aminobenzyloxycarbonyl-Tyr(PO3H2)-lle-Asn-NH-[2-(1-bromo-naphthalen-
2-yloxy)-ethyl]
(SEQ ID NO: 1 44)
Title compound: Mass spectral analysis (negative-ion mode): 884.5 [M-H]+,
(C3gH46N6O11 P, calc. 885.71), tR= 8.81 min (HPLC System D).
Example 145: 3-Aminobenzyloxycarbonyl-Tyr(PO3H2)-lle-Asn-NH-(3-methyl-butyl)
(SEQ ID NO: 145)
Title compound: Mass spectral analysis (negative-ion mode): 705.0 [M-H]+,
(C32H47N6O1opl calc 706.74), tR= 7.27 min (HPLC System D).
Example 146 3-Aminobenzyloxycarbonyl-Tyr(PO3H2)-lle-Asn-NH-cyclohexyl
(SEQ ID NO: 146)
Title compound: Mass spectral analysis (negative-ion mode): 718.4 [M-H]+,
(C33H47N6O1OP, calc. 718.75), tR= 7.23 min (HPLC System D).
Exam~ole 147: 3-Aminobenzyloxycarbonyl-Tyr(P03H2)-Ac6c-Asn-NH-(3-naphthalen-1-yl-
propyl)
(SEQ ID NO: 147)
The title compound is obtained in analogy to Example 1 21.
Example 148: 3-Aminobenzyloxycarbonyl-Tyr(PO3H2)-Ac6c-Asn-NH-(3-naphthalen-2-yl-propyl)
(SEQ ID NO: 148)
The title compound is obtained in analogy to Example 123.
-
CA 02227~l6 l998-0l-2l
WO 97/08193 PCTAEP96/03473
-107-
Example 149: 3-Aminobenzyloxycarbonyl-Tyr(PO3H2)-Ac6c-Asn-NH-[3-(2-hydroxy-
naphthalen-1-yl)-propyl]
(SEQ ID NO: 149)
The title compound is obtained in analogy to Example 122.
ExamPle 150: Gelatine solution:
A sterile-filtered aqueous solution, with 20 % cyclodextrins as soll Ihjli5er5, of one of the
compounds of formula I mentioned in the preceding Examples (e.g. Example 1) as active
ingredient, is so mixed under aseptic conditions, with heating, with a sterile gelatine solution
containing phenol as preservative, that 1.0ml of solution has the following composition:
active ingredient 3 mg
gelatine 150.0 mg
phenol 4.7 mg
dist. water with 20 % cyclodextrins
as solubilisers 1.0 ml
Example 151: Sterile dry substance for injection:
5 mg of one of the compounds of formula I mentioned in the preceding Examples (e.g.
Example 1 ) as active ingredient are dissolved in 1 ml of an aqueous solution with 20 mg of
mannitol and 20 % cyclodextrins as solubilisers. The solution is sterile-filtered and
introduced under aseptic conditions into a 2 ml ampoule, deep-frozen and Iyophilised.
Before use, the Iyophilisate is dissolved in 1 ml of distilled water or 1 ml of a physiological
saline solution. The solution is ad,llil~ ered intramuscularly or intravenously. This
formulation can also be introduced into a twin-chambered injection ampoule.
Example 1!;2: Nasal spray:
500 mg of finely ground (<5.0 !lm) powder of one of the compounds of formula I mentioned
in the preceding Examples (e.g. Example 1) is suspended as active ingredient in a mixture
of 3.5ml of Myglyol 812/ and 0.08 9 of benzyl alcohol. The suspension is introduced into a
container having a metering valve. 5.0 9 of Freon 12/ are introduced under pressure into the
container through the valve. The "Freon" is dissolved in the Myglyol/benzyl alcohol mixture
by shaking. The spray container contains approximately 100 single doses which can be
ad" ,i~ ered individually.
CA 02227~16 1998-01-21
WO 97/08193 PCTAEP96/03473
-108-
ExamDle 153: Film-coated tablets
The following ingredients are used for the preparation of 10000 tablets each containing 100
mg of active ingredient:
active ingredient 1000 9
corn starch 680 9
colloidal silica 200 9
magnesium stearate 20 9
stearic acid 50 9
sodium carboxymethyl starch 250 9
water quantum satis
A mixture of one of the compounds of formula I mentioned in the preceding Examples (e.g.
Example 1) as active ingredient, 50 g of corn starch and the collcid~l silica is processed
with a starch paste, made from 250 9 of corn starch and 2.2 kg of demineralised water, to
form a moist mass. This is forced through a sieve having a mesh size of 3 mm and dried at
45~ for 30min in a fluidised bed drier. The dry granules are pressed through a sieve having
a mesh size of 1 mm, mixed with a pre-sieved mixture (1 mm sieve) of 330 9 of corn starch,
the magnesium stearate, the stearic acid and the sodium carboxymethyl starch, and
compressed to form slightly biconvex tablets.
Example 154: Incubation of phosphotyrosine protein immobilized on a solid phase with a
chimeric SH2-GST (GST = glutathione S-transferase) protein in the presence of a test
substance
Using the test system mentioned above, and using the phosphorylated "tail" EGFR-MBP
fusion protein as ligand, the following IC50 values are obtained:
Example IC50
0.1
2 0.3
3 0 065
4 0.57
CA 02227~l6 l998-0l-2l
WO 97/08193 PCT~EP96/03473
-109-
0.185
6 0.021
7 0.011
8 0.96
9 0.337
0.97
14 0.177
0.139
17 0.5
21 0.089
22 0.115
24 0.008
26 0.8
34
0.18
41 0.39
42 0.53
46 0.84
47 0.15
0.78
51 0.023
52 0.046
63 0.127
54 0.325
0.148
56 0.1
57 0.105
58 0.027
61 0.002
62 0.0026
v 64 ~-~
66 0.33
67 0.034
CA 02227~l6 l998-0l-2l
WO 97/08193 PCT~EP96/03473
-110-
68 0.68
69 0.54
0.35
71 0.13
72 0.007
73 0.076
0.018
1.96
76 0.1
77 0.14
0.014
81 0.009
82 o 99
83 1.9
84 1.0
5.9
86 ~5
87 27.5
89 8.3
go 18.8
91 1.2
92 0.55
93 0.47
94 2.8
1.5
96 1.1
97 22.5
98 5.5
99 48.5
100 75
101 18.7
102 12.6
103 0 47
CA 02227~l6 l998-0l-2l
W O 97/08193 PCT~EP96/03473
- 111 -
104 3.3
106 4.2
107 0.022
108 1.65
111 0.041
112 1.59
113
114 28.7
115 1.1
116 2.8
117 0.004
118 1.84
119 0.060
120 0.20
121 3.0
122 0.001
123 0.92
124 0.29
125 0.29
126 0-97
128 3.03
129 2.27
130 1.44
131 3.68
132 2.3
133 5.4
134 3.2
135 3.08
136 1.6
138 3.65
142 0.2
143 0.018
145 0.72
CA 022275l6 l998-0l-2l
WO 97/08193 PCTAEP96/03473
-112-
147 0.21
148 0.16
CA 022275l6 l998-0l-2l
WO 97/08193
PCT~EP96/03473
-113-
LISTING
..
(l) OE NER~L INFORMATION:
(i) APPLICANT
(A) NAME: CIBA- OE IGY AG
(B) STREET: Kly~eckstr. 14l
(C) CITY: Basel
(E) C~u l~Y: Switzerland
(F) POSTAL CODE (ZIP): 4002
(G) ~ r~P~ONE: +4l 6l 69 ll ll
(H) TELEFAX: + 41 61 696 79 76
(I) TELEX: 962 991
(ii) TITLE OF lNV~'N'l'l~N: Acylated Oligopeptide Derivatives
(iii) NUMBER OF ~U~N~S: 151
(iv) COMPUTER RT'.~T~ART.R FORM:
(A) MEDIUM TYPE: Floppy disk
(B) COMPUTER: IBM PC compatible
(C) OP~.R~rNG SYSTEM: PC-DOS/MS-DOS
(D) SOFTWARE: PatentIn Release #l.0, Version ~1.30 (EPO)
(2) INFORMATION FOR SEQ ID NO: l:
U~N~ CHARACTERISTICS:
(A) LENGTH: 4 aTnino acids
(B) TYPE: amino acid
(C) STRANI3~ N~:~S: single
(D) TOPOLOG~: linear
(ii) M~R~CTJRT~ TYPE: peptide
(ix) FEATURE:
CA 022275l6 l998-0l-2l
WO 97/08193 PCTAEP96/03473
-114-
(A) NAME/ ~ Y: Modified-site
(B) LOCATION:l
(D) OTHER INFORMATION:/product=
"N-[(3-aminobenzyloxycarbonyl)-(0-P-Tyr)]"
/note= "O-P-Tyr stands for O-ph~sph~n~-L-Tyr
(ix) FEATURE:
(A) NAME/KEY: M~;fie~-site
(B) LOCATION:4
(D) OTHER INFORMATION:/product= "Gln-NH2"
(Xi) ~U~N~ n~.~RTpTIoN: SEQ ID NO: l:
Xaa Ile Asn Xaa
(2) INFORMATION FOR SEQ ID NO: 2:
(i) ~U~N~: CHARACTERISTICS:
(A) LENGTH: 4 amino acids
(B) TYPE: amino acid
(C) S~ N~ l)N~:~cs single
(D) TOPOLOGY: linear
(ii) MnT~ ~ TYPE: peptide
(ix) FEATURE:
(A) NAME/~ Y: M~;f;~-site
(B) IOCATION:l
(D) OTHER INFORMATION:/product=
"N-[(2-~min~hPn~yloxycarbonyl)-(O-P-Tyr)"
/note= "O-P-Tyr stands for O-ph~srh~n~-L-Tyr
(ix) FEATURE:
(A) NAME/KEY: Modified-site
CA 022275l6 l998-0l-2l
WO 97/08193
PCTIEP96/03473
-115-
,.
(B) LCCATION:4
(D) OTHER INFORMATION:/plo~o~- "Gln-NH2"
(xi) ~u~ DESCRIPTION: SEQ ID NO: 2:
Xaa Ile Asn Xaa
(2) INFORMATION FOR SEQ ID NO: 3:
( i ) ~QU ~:N~ ~AR~RISTICS:
(A) LENGTH: 3 amino acids
(B) 'l~Y~E: amino acid
(C) STRAN~ ~S: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: peptide
(ix) FEATURE:
(A) NAME/KEY: M~if;~-site
(B) LOCATION:l
(D) OTHER INFORMATION:/product=
"N-[(3-~m;n~h~n7.yloxycarbonyl)-(o-p-Tyr)]"
/note= "O-P-Tyr stands for O-Phosphono-L-Tyr"
(ix) FEATURE:
(A) NAME/REY: Modified-site
(B) LOCATION:3
(D) OTHER INFORMATION:/product= "Asn-NH2"
..
( Xl ) ~ ~:QU ~:N~ DESCRIPTION: SEQ ID NO: 3:
Xaa Ile Xaa
CA 022275l6 l998-0l-2l
WO 97/08193 PCTAEP96/03473
-116-
(2) INFORMATION FOR SEQ ID NO: 4:
( i ) ~U~N~ CHARACTERISTICS:
(A) T.T~.T~: 4 amino acids
(B) TYPE: amino acid
(C) STRAN~ 1)N ~:.~S: single
(D) TOPOLOGY: linear
( ii ) MnT-T~'~lT-~ TYPE: peptide
(ix) FEATURE:
(A) NAME/KEY: M~ifiF~-site
(B) LOCATION:l
(D) OTHER INFORMATION:/~u~-
"N-[(l-(3-~m; noph~nyl )ethoxy-CO)-(O-P-Tyr)]"
/note= "O-P-Tyr stands for O-ph~srhnn~-L-Tyr~
(ix) FEATURE:
(A) NAMEJREY: Mr~;f;~-site
(B) T~TT~ 4
(D) OTHER INFORMATION:/product= "Gln-NH2"
( Xi ) ~U~N~ DESCRIPTION: SEQ ID NO: 4:
Xaa Ile Asn Xaa
(2) INFORMATION FOR SEQ ID NO: 5:
( i ) ~yu~N~ CHARACTERISTICS:
(A) T.~N~,T~: 4 amino acids
(B) TYPE: amino acid
(C) sTR~Nn~nN~: single
(D) TOPOLOGY: linear
CA 022275l6 l998-0l-2l
W O 97/08193 PCTtEP96tO3473
-117-
(ii) Mc~T~rlT~R TYPE: peptide
(ix) FEATURE:
(A) NAME/KEY: M~;f;~-site
(B) LOCATION:l
(D) OTHER INFORMaTION:/~l~lu~-
"N-[(l-( 3_Am; nnph~nyl ) ethoxy-CO)-(O-P-Tyr)]"
/note= "O-P-Tyr stands _or O-phnsphnn~-Tyr; diastel~v..~L to SEQ
ID:4"
(ix) FEATURE:
(A) NAME/KEY: M~;f;e~-site
(B) LOCATION:4
(D) ~l~R rNFORMATION:/product= "Gln-NH2"
(Xi) ~l~:c~?u~;Nc~; I)R~::c-RTT~TIoN: SEQ ID NO: 5:
Xaa Ile Asn Xaa
(2) INFORMATION FOR SEQ ID NO: 6:
QI l~:NC '~: C-r~AR~l-~FRT~TIcs:
(A) LENGTH: 4 arnino acids
(B) TYPE: amino acid
(C) SlTlRANl)l~ s: single
(D) TOPOLOGY: linear
(ii) ~T~crlT~ TYPE: peptide
-
(ix) FEATURE:
(A) NAME/KEY: Mn~if;~-site
(B) LOCATION:l
CA 022275l6 l998-0l-2l
W O 97/08193 PCTrEP96/03473
-118-
(D) OTHER INFORMATION:/product=
"N-[(3-aminobenzyloxycarbonyl)-(O-P-Tyr)]"
/note= "O-P-Tyr stands for O - Phn phnnn - Tyr~
(ix) FEATURE:
(A) NAME/KEY: Mn~; f;~-site
(B) LOCATION:2
(D) OTHER INFORMATION:/product=
"l-aminocyclopentane-carbonyl"
/note= "R~A;~1 of l-Am;n~cyclopentane carboxylic acid"
(ix) FEATURE:
(A) NAME/KEY: ~;f;e~-site
(B) LOCATION:4
(D) OTHER INFORMATION:/product= "Gln-NH2"
(xi) ~U~N~ DESCRIPTION: SEQ ID NO: 6:
Xaa Xaa Asn Xaa
(2) rNFoRMATIoN FOR SEQ ID NO: 7:
(i) ~U~N~ CHARACTERISTICS:
(A) LENGTH: 4 amino acids
(B) TYPE: a-m-ino acid
(C) ST~N~ N~ S: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: peptide
(ix) FEATURE:
(A) NAME/~ Y: Modified-site
(B) LOCATION:l
(D) OTHER INFORMATION:/product=
CA 022275l6 l998-0l-2l
W O 97/08193 PCTtEP96tO3473
-119-
; "N-[(3-aminobenzyl)oxycarbonyl-]-(O-P-Tyr)"
/note= "O-P-Tyr stands for O-Phosphono-L-Tyr"
(ix) FEATURE:
(A) NAME/KEY: M~; f; ~-site
(B) LOCATION:2
(D) OTHER INFORMATION:/~.~u~L-
"l-aminocyclohexy1c~ yl"
/note= "RA~; ~Al of l-amincy~1~h~An~ carboxylic acid"
(ix) FEATURE:
(A) NAME/REY: M~; f; F~ - site
(B) LOCATION:4
(D) OTHER rNFORMATION:/~.~u~L- "Gln-NH2"
(xi) ~U~N~ n~RTpTIoN: SEQ ID NO: 7:
Xaa Xaa Asn Xaa
(2) INFORMATION FOR SEQ ID NO: 8:
(i) ~u~N~ ~R~T~RT~TICS:
(A) LENGTH: 4 amino acids
(B) TYPE: Amino acid
(C) STRAN~ )N~ : single
(D) TOPOLOGY: linear
(ii) MnT.~crlT.~ TYPE: peptide
(ix) FEATURE:
~ (A) NAME/KEY: ~ f;~-site
(B) LOCATION:l
(D) OTHER INFORMATION:/~.~u~L-
"N-t2-(4-methyl-5-thiazolyl)ethoxy-CO]-PTyr"
CA 02227516 1998-01-21
WO 97/08193 PCT~EP96/03473
-120-
/note= "PTyr stands for O-Phosphono-L-Tyr"
(ix) FEATURE:
(A) NAME/KEY: M~if;~-site
(B) LOCATION:4
(D) OTHER INF~RMA~T~N: /~lUdU~ L- "Gln-NH2"
( xi ) ~U~N~'~ DESCRIPTION: SEQ ID NO: 8:
Xaa Ile Asn Xaa
(2) INFORMATION FOR SEQ ID NO: 9:
(i) SEQUENCE CH~RACTERISTICS:
(A) LENGTH: 4 amino acids
(B) TYPE: amino acid
(C) S~lTRAN~ NI-:'.;,C;: single
(D) TOPOLOGY: linear
(ii) M~T.~lT.T~ TYPE: peptide
(ix) FEATURE:
(A) NAME/REY: M~i f; ~ - site
~~ (B) LOCATION:l
(D) OTHER INFORMATION:/product=
"N-[2-(3-1-ydlu~y~henyl)ethoxycarbonyl]-PTyr"
/note= "PTyr stands for O-E1.o~y~l~o-L-Tyr"
(ix) FEATURE:
(A) NAME/XEY: M~;f;~-site
(B) LOCATION:4
(D) OTHER INFORMATION:/product= "Gln-NH2"
CA 022275l6 l998-0l-2l
W O 97/08193
PCT~EP96/03473
-121 -
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 9:
~.
Xaa Ile Asn Xaa
(2) INFORMATION FOR SEQ ID NO: l0:
?UI~;N~; CE~ RA~ RT~TIcs
(A) LENGTH: 4 amino acids
(B) TYPE: amino acid
(C) STRA N1)1'~ ; single
(D) TOPOLOGY: linear
(ii) -M~T~rlT~ lY~E: peptide
(ix) FEATURE:
(A) NAME/KEY: Mn~;f;~-site
(B) LOCATION:l
(D) OTHER INFORMATION:/product=
"N-[3-llydl~yLenzyloxycarbonyl]-O-P-Tyr"
/note= "O-P-Ty-r stands for O-Phnsphnnn-L-Tyr"
(ix) FEATURE:
(A) NAME/REY: Mn~; f; ~ -site
(B) LOCATION:4
(D) OTHER INFORM~TION:/product= "Gln-NH2"
(Xi) ~U~N~ D~RTPTION: SEQ ID NO: l0:
Xaa Ile Asn Xaa
t2) INFORMATION FOR SEQ ID NO: ll:
~ U~NC~: CHARACTERISTICS:
CA 022275l6 l998-0l-2l
W O 97/08193 PCTAEP96/03473
-122-
(A) LENGTH: 3 amino acids
(B) TYPE: arnino acid
(C) STRAN~ N~ : single
(D) TOPOLOGY: linear
(ii) M~T.~cr~.~ TYPE: peptide
(ix) FEATURE:
(A) NAME/REY.: ~r~; f; ~ - site
(B) LOCATION:1
(D) OTHER INF~RMA~T~:/product=
"N--t3--~m;nt-h~n7.yloxycarbonyl]--(O--P--Tyr)"
/note= "O-P-Tyr stands for O-phn~hnl~n-L-Tyr
(ix) FEATURE:
(A) NAME/REY: M~; f;~ - site
(B) T~I~TTt~N 2
(D) OTHER INFORMATION:/y~uc~-
"1-arninocyclohexyl c~rhonyl"
/note= "Bivalent radical of l-arninocycloh~n~ carboxylic acid"
(ix) FEATURE:
(A) NAME/REY: Mn~i f; ~ - site
(B) LOCATION:3
(D) OTHER INFORMATION:/yl~u~L- "bAla-N~2"
tnote= "kAla stands for beta-Alanyl (-NH-CH2-CH2-(C=O)-)"
(xi) ~U~N~ n~RTpTIoN: SEQ ID NO: 11:
Xaa Xaa Xaa
(2) rNFoRMATIoN FOR SEQ ID NO: 12:
(i) ~u~N~ CHARACTERISTICS:
CA 02227516 1998-01-21
W O 97/08193 PCT/EP96/03473
~ -123-
; (A) LENGTH 3 amino acids
(B) TYPE: am.ino acid
(C) Sl~ANI~ Nl~ S single
(D) TOPOLOGY: linear
(ii) ~T.~CTlT.T~ TYPE peptide
~ix) FEATURE:
(A) NAME/KEY Mn~;f;~ - site
( B ) LOCATION l
(D) OTHER INFORMATION /~l~U~ L -
~N-[ 3-~m;nnhpn~yloxycarbonyl]-(o-p-Tyr)"
/note= "O-P-Tyr stands for O-phnsphnnn-L-Tyr~
(ix) FEATURE
(A) NAME/KEY Mn~; f; ed-site
( B ) LOCATION:2
(D) OTHER INFORMATION:/~r~lu~L -
"l-aminocyclohexylc~honyl"
/note= "Bivalent radical of l-aminocyclohexyl carboxylic
acid"
(ix) FEATURE
(A) NAME/KEY Modified-site
( B ) T~rrT~N: 3
(D) OTHER INFORMATION: /~L ~U~ L - " Gly-NH2"
( Xi ) ~QU~N~ DESCRIPTION: SEQ ID NO 12:
Xaa Xaa Xaa
(2) INFORMATION FOR SEQ ID NO: 13:
( i ) ~ U~N~ CHARACTERISTICS:
CA 022275l6 l998-0l-2l
WO 97/08193 PCTAEP96/03473
-124-
"
(A) LENGTH: 4 amino acids
(B) TYPE: amino acid
(C) STRANn~nN~S: single
(D) TOPOLOGY: linear
( ii ) M~T~crlT~ TYPE: peptide
(ix) FEATURE:
(A) NAME/KEY: Mn~if;~-site
(B) LOCATION:l
(D) ~l~R rNFORMATION:/~l~ucL-
"N-[benzyloxycarbonyl]-(O-P-Tyr)"
/note= "O-P-Tyr stands for O-Phnsrhnno-L-Tyr"
(ix) FEATURE:
(A) NAME/REY: M~; f;~-site
(B) LOCATION:4
(D) OTHER INFORMATION:/product= "Gln-NH2"
(Xi) ~U~:N~ D~-~rRTpTIoN: SEQ ID NO: 13:
Xaa Ile Asn Xaa
(2) INFORMATION FOR SEQ ID NO: 14:
(i) SEQUEN OE CHARACTERISTICS:
(A) LENGTH: 4 amino acids
(B) TYPE: amino acid
(C) sTRANn~nN~.ss: single
(D) TOPOLOGY: linear
( ii ) M~T~cr~T~T~ TYPE: peptide
CA 02227516 1998-01-21
WO 97/08193 PCT/EP96/03473
- 125 -
(ix) FEATURE:
(A) NAME/KEY: M~l;f; ~--site
(B) LOCATION:l
(D) OTE~ER I~lFOR~TION: /~L~lU~: L--
"N--t3,5--~m;nnh~n~yloxycarbonyl]--(PTyr)"
/note= "PTyr stands for O-Phosphono-L-Tyr"
(ix) FEATURE:
(A) NA~OE/KEY: M~l;f;~-site
(B) LOCATION:4
(D) OTHER INEY)RMA~T ~: /~L~lU~: L-- "Gln-NH2"
(Xi) ~ U~;N~; DESCRIPTION: SEQ ID NO: 14:
Xaa Ile Asn Xaa
(2) INFORMATION FOR SEQ ID NO: 15:
;5 ?Uh'N~ FrARA~RT::TIcs:
(A) LENGTH: 4 amino acids
(B) TYPE: amino acid
(C) SrrRANI~ )Nt~ single
(D) TOPOLOGY: linear
(ii) Mf~T~r~crlT~r~ TYPE: peptide
(ix) FEATURE:
(A) NAME/KEY: M~l;f;FYl-site
(B) LOCATION:l
(D) OTHER l:NFORMATION:/product=
"N--~3--(3--indolyl)--propionyl]--(O--P--Tyr)"
/note= "O--P-Tyr stands for O--Ph~sph~n-~-L-Tyr"
(ix) FEATURE:
CA 02227S16 1998-01-21
W O 97/08193
PCTAEP96/03473
-126-
(A) NAME/KEY~ i f; e~-site
(B) LOCATION:4
(D) OTHER INFORMATION:/p.udu. L- "Gln-NH2"
(Xi) ~:~U~N~ DESCRIPTION: SEQ ID NO: 15:
Xaa Ile Asn Xaa
(2) INFORMATION FOR SEQ ID NO: 16:
( i) .~;1~:5)~ :N( 1~: C~ARA~ RlsTIcs
(A) LENGTH: 4 amino acids
(B) TYPE: amino acid
( C ) ST~P-NI)1-:1 )N~ : single
(D) TOPOLOGY: linear
( ii ) M~T~crlT~ TYPE: peptide
(ix) FEATURE:
(A) NAME/KEY: M~ifi~-site
(B) LOCATION:l
(D) OTHER INFORMATION:/product=
"N-[3-(N-acetylamino)phenoxyacetyl]-PTyr"
/note= "PTyt stands for O-ph~sph~n~-L-Tyr~
(ix) FEATURE:
(A) NAME/KEY: M~if i f~-site
(B) LOCATION:4
(D) OTHER INFO~MATION:/product= "Gln-NH2"
(Xi) ~:~U~N~ DESCRIPTION: SEQ ID NO: 16:
Xaa Ile Asn Xaa
CA 022275l6 l998-0l-2l
WO 97/08193
PCT/EP96/03473
- 127-
(2) rNFORMATION FOR SEQ ID NO: 17:
( i ) ~;C,~U~;N(,:~; CHAR~:RT!;TICS:
(A) LENGTH: 4 amino acids
(B) TYPE: amino acid
(C) srR~t~nF:nN~s: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: peptide
(ix) FEATURE:
(A) NAME/KEY: M~l;f;~-l--site
(B) LOCATION:1
(D) OTHER INFORMATION:/product=
"N-t4-(N-acetylamino)phenoxyacetyl]-PTyr"
/note= "PTyr stands for O--Phosphono-L--Tyr"
(ix) FEATURE:
(A) N~ME/KEY: M~;f;~--site
(B) LOCATION:4
(D) OTHER INFORMATION:/product= "Gln-NH2"
( Xi ) ~ U~;N~; DESCRIPTION: SEQ ID NO: 17:
Xaa Ile Asn Xaa
(2) INFORMATION FOR SEQ ID NO: 18:
2U~N(.:~; CH~RACTERISTICS:
(A) LhrNGTH: 4 amino acids
(B) TYPE: amino acid
(C) STRZ~N~ "N~ S: single
CA 02227516 1998-01-21
WO 97/08193
PCT/EP96/03473
- 128-
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: peptide
(ix) FEATURE:
(A) NAME~KEY: M~lif;~l-site
(B) LOCATION:l
(D) OTHER I~FORMATION:/product=
"N--[(2--acetylamino)--phenoxyacetyl]--PTyr"
/note= "PTyr stands for O--Phr~sphon~-- ~r"
(ix) FEATURE:
(A) NAME/KEY: Mrxl;f; ~l-site
(B) LOCATION:4
(D) ~T~;K INFORMATION: /f ~1~lu_:L-- "Gln--NH2"
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 18:
Xaa Ile Asn Xaa
(2) INFORMATION FOR SEQ ID NO: l9:
;S2U~;N~;~; CHARACTERISTICS:
(A) LENGTH: 4 amino acids
(B) TYPE: am~no acid
(C) STRaNDEDNESS: single
(D) TOPOLOGY: linear
( ii ) ~t~T~F~cTlT~F~ TYPE: peptide
(ix) FEATURE:
(A) NAME/REY: M~;f;e-l--site
(B) LOCATION:l
CA 02227516 1998-01-21
WO 97/08193 PCT/EP96/03473
- 129 -
~, ,
~, (D) OTHER INFORMATION:/product=
"N--[4--;~.m;nnphPnn~yacetyl]--(O--P--Tyr)"
~ /note= ~o-p-rryr stands for O--Phn~phnnn--~Tyr"
(ix) E~ATURE:
(A) NAME/REY: Mn~l;f;P-l--site
(B) LOCATION:4
(D) ~fl~;R INFORMATION~ ~lu~ "Gln-NH2"
(xi) ~;~U~N~:~ n~;~'RTT~TIoN: SEQ ID NO: l9:
Xaa Ile Asn Xaa
(2) INFORMATION FOR SEQ ID NO: 20:
( i ) ~;le2U~:~; t'~ARA~IsTIcs:
(A) LENGTEI: 4 ~mi no acids
(B) TYPE: amino acid
( C ) S'rR ~ N ~ 1 )N ~ S: single
(D) TOPOLOGY: linear
(ii) M ~T~ T.~ TYPE: peptide
(ix) E'EATtlRE:
(A) NAME/REY: Active--site
(B) LOCATION:l
(D) OTHER INFORMATION:/product=
"N--t3--aminophenoxyacetyl]--(O-P--Tyr)"
/note= "O-P-Tyr stands for o-phosphono-L-Tyr
.~
(ix) E~:ATURE:
- (A) NAME/REY: ~ ;f;el--site
(B) LOCATION:4
(D) OTEIER INFORM~TION:/product= "Gln--NH2"
CA 02227516 1998-01-21
WO 97/08193 PCT/EP96/03473
- 130 -
( Xi ) ~ ;52U~ ; DF~ 'R TPTION: SEQ ID NO: 20:
Xaa Ile Asn Xaa
(2) INFORMATION FOR SEQ ID NO: 2l:
( i ) ~;Ç~U~;N~; ~'r~R;~ RT~TIcs
(A) LENGTH: 4 amino acids
(B) TYPE: amino acid
( C ) STRAN I )1-:1 )N 1- C~S single
(D) TOPOLOGY: linear
( ii ) ~oT~F~crlT~ TYPE: peptide
(ix) FEATURE:
(A) NAME/KEY: M~rl;fi~--site
(B) LOCATION:l
(D) OTHER INFoRM~rT~N:/product=
"N--~4--(3--indolyl)L,uLyl~yl]--(O--P--Tyr) "
/note= "O--P--Tyr stands :Eor O-Phosphono--L--Tyr"
(ix) FEATURE:
(A) NAME/KEY: M~lifi~--site
(B) IOCATION:4
(D) a~E1ER INFORMATION: /~Jl~UC_: L-- "Gln--NH2"
(xi) SEQUENOE DESCRTPTION: SEQ ID NO: 2l:
Xaa Ile Asn Xaa
(2) INFOR~qATION FOR SEQ ID NO: 22:
CA 022275l6 l998-0l-2l
W 097/08193 PCT~P96/03473
-131 -
(i) SEQUENCE ~ARA~T~RT.STICS:
(A) LENGTH: 4 amino acids
(B) TYPE: amino acid
(C) STRAN~ l)N~:~S: single
(D) TOPOLOGY: linear
( ii ) M~T.~rlT.~ TYPE: peptide
(ix) FEATURE:
(A) NAME/KEY: M~;f;~-site
(B) LOCATION:l
(D) OTHER INFORMA~TO~ ~C L -
"N-[(3-indolyl)-acetyl]-(O-P-Tyr)"
/note= "O-P-Tyr stands ~or O-Phosphono-L-Tyr"
(ix) FEATURE:
(A) NAME/KEY: M~;f;~-site
(B) LOCATION:4
(D) OTHER INFORMATION: /~l~U~ L- "Gln-NH2"
(xi) ~:QIJ~:N(~; n~rRTpTIoN: SEQ ID NO: 22:
Xaa Ile Asn Xaa
(2) INFORMATION FOR SEQ ID NO: 23:
( i ) ~hQUhN~h CHARACTERISTICS:
(A) LENGTH: 4 amino acids
(B) TYPE: amino acid
(C) STRAN,)~ N~:-~S: single
(D) TOPOLOGY: linear
.
( ii ) ~wT~cT~ TYPE: peptide
CA 02227516 1998-01-21
W O 97/08193 PCTAEP96/03473
-132-
(ix) FEATURE:
(A) NAME/KEY: ~;f;~-site
(B) LOCATION:l
(D) OTHER INFOR~A~T~: /product= "N-[benzoyl]-(O-P-Tyr)"
/note= "O-P-Tyr stands for O-Ph~fiph~n~-L-Tyr"
(ix) FEATURE:
(A) NAME/REY: M~ifi~-site
(B) LOCATION:4
(D) ~ln~R rNFORMATION:/~l~U~L- "Gln-N~2"
(xi) ~yU~N~ D~RTT~TION: SEQ ID NO: 23:
Xaa Ile Asn Xaa
(2) INFORMATION FOR SEQ ID NO: 24:
;$,2UISN~ 'T~ARA~'.~R T~TICS:
(A) T .T~,~: 4 amuno acids
(B) TYPE: amino acid
(C) S'T'RANI~ 1 )Nl-~S single
(D) TOPOLOGY: linear
( ii ) M~T.~.~TJT.~ TYPE: peptide
(ix) FEATURE:
(A) NAME/KEY: Modified-site
(B) LOCATION:l
(D) OTHER INFORMATION:/product=
"N-t 4 - Am i n~h~n ~nyl ] - ( O-P-Tyr)"
/note= "O-P-Tyr stands for O-Phosphono-L-Tyr"
CA 02227516 1998-01-21
WO 97/08193 PCTAEP96/03473
-133-
~ (ix) FEATURE:
~ (A) NAME/KEY~ ifi~-site
(B) LOCATION:4
(D) OTHER INFORMATION:/product= "Gln-NH2"
(Xi) ~U~NU~ DESCRIPTION: SEQ ID NO: 24:
Xaa Ile Asn Xaa
(2) INFORMATION FOR SEQ ID NO: 25:
( i ) ~UU~N~ CHARACTERISTICS:
(A) LENGTH: 4 amino acids
(B) TYPE: amino acid
(C) S~RAN~ l)N~ : single
(D) TOPOLOGY: linear
(ii) MnT~cTlT~T~ TYPE: peptide
(ix) FEATURE:
(A) NAME/KEY: ~n~;f;~-site
(B) LOCATION:l
(D) OTUER INFORMATION:/product=
"N-th~n~;m;~ol-5-yl c~rh~nyl] -(O-P-Tyr)"
/note= "O-P-Tyr stands for O-Phnsphnnn-L-Tyr"
(ix) FEATURE:
(A) NAME/KEY: Modified-site
(B) LOCATION:4
(D) Oq~ER INFORMATION:/product= "Gln-N~2"
(Xi) ~:~U~N~ DESCRIPTION: SEQ ID NO: 25:
~ =
CA 02227516 1998-01-21
W O 97/08193 PCTAEP96/03473
-134-
Xaa Ile Asn Xaa
(2) INFORMATION FOR SEQ ID NO: 26:
N( ~: r~ARA~RlsTIcs:
(A) LENGTH: 4 amino acids
(B) TYPB: amino acid
( C ) S~RANI )I~:I )Nl~:.SS: single
(D) TOPOLOGY: linear
(ii) M~T~F~cr7T~ TYPE: peptide
(ix) FEATURE:
(A) NAME/~ Y: M~;f;~-site
(B) LOCATION:l
(D) OT~7ER INFO~MATTO~:/product=
"N-[2-amino-thiazol-4-yl-acetyl]-(O-P-Tyr)"
/note= "O-P-Tyr stands for O-Phosphono-L-Tyr"
(ix) FEATURE:
(A) NAME/REY: ~;fi~-site
(B) LOCATION 4
(D) OT~ER INFnRMA~T~N:/product= "Gln-NH2"
( Xi ) ~U~N~'~ DESCRIPTION: SEQ ID NO: 26:
Xaa Ile Asn Xaa
(2) INFORMATION FOR SEQ ID NO: 27:
U~N~ CHARACTERISTICS:
(A) LENGTH: 4 amino acids
(B) TYPE: amino acid
CA 02227516 1998-01-21
WO 97/08193 PCT/EP96/03473
-13!~ -
(C) Sq~RAN~ S: single
(D) TOPOLOGY: linear
r
(ii) M(~T.~crlT.~ TYPE: peptide
(ix) FEATURE:
(A) NAME/KEY: M~;f;~l-site
(B) LOCATION:l
(D) ClTn~;R INFORMATION:/yL~lu~iL--
"N--t4-~;y~ ~n~oyl]-(O-P-Tyr)"
/note= "O--P--Tyr stands for O-Phnsphnnn-L--Tyr"
(ix) FEATURE:
(A) NAME/KEY: M~l;f;~-site
(B) LOCATION:4
(D) OTE~ R INFORMATION: /~l~lU~ - L-- "Gln--NH2"
( Xi ) ,'-i ~:(,ll l~:N~: T)F~:rRTpTIoN: SEQ ID NO: Z7:
Xaa Ile Asn Xaa
(2) rNFORMATION FOR SEQ ID NO: 28:
(i) ~s.~u~;No~; CHARACTERISTICS:
(A) LENGTH: 4 amino acids
(B) TYPE: amino acid
(C) STRANI)t~:l)N~ S: single
(D) TOPOLOGY: linear
(ii) MoT~Fcr~T~F~ TYPE: peptide
(ix) E~EATURE:
(A) N~ME/KEY: Modified-site
~ = ~ - =
CA 02227516 1998-01-21
WO 97/08193 PCT/EP96/03473
- 136-
(B) LOCATION:l
(D) OTEiER INFORMATION:/product=
"N-[2,3-dihydrobenzofuran-5-yl-CO]-PTyr"
/note= "PTyr stands for P-ph~srh~n~l-L-Tyr
(ix) FEATURE:
(A) NAME/KEY~ f;~-site
(B) LOCATION:4
(D) OTHER INFORMATION:/~l~lu~L-- "Gln--NH2"
(Xi) ~1~;(2U~:N.(~; D~.~rR TPTION: SEQ ID NO: 28:
Xaa Ile Asn Xaa
(2) INFoRMATTnN FOR SEQ ID NO: 29:
U~iN~ ' CHaRACTERISTICS:
(A) LENGTH: 4 amino acids
(B) TYPE: amino acid
(C) STE~AN"~ N~ : single
(D) TOPOLOGY: linear
(ii) M )T.~Tr.F~ TYPE: peptide
(ix) FEATURE:
(A) NAME/KEY: Modified--site
(B) T~ATTc)~: 1
(D) OTE3ER INFORMATION:/product=
"N-[4-hy~ ylJenzoyl]-(o-p-Tyr)"
/note= "O--P--Tyr stands for o-phosphono-L-Tyr
(ix) FEATURE:
(A) NAME/KEY: Modified--site
(B) LOCATION:4
CA 02227516 1998-01-21
WO 97/08193 PCTAEP96/03473
-137-
r (D) OTHER INFORMATION:/product= "Gln-NH2"
r
( Xi ) ~QU~N~ DESCRIPTION: SEQ ID NO: 29:
Xaa Ile Asn Xaa
(2) INFORMATION FOR SEQ ID NO: 30:
( i ) ~(~2U~;N~; CF~P~RA~ RT.~TIcs
(A) LENGTH: 4 amino acids
(B) TYPE: amino acid
(C) S'l'RAN I )~ N1-:- S single
(D) TOPOLOGY: linear
( ii ) M~T.~CrlT.~ TYPE: peptide
(ix) FEATURE:
(A) NAME/KEY: Mr~;f;e~-site
(B) LOCATION:l
(D) OTHER INFORMATION:/~ro~u~L-
"N-[4-meth~yLel1z~yl]-(O-P-Tyr)"
/note= "O-P-Tyr stands for O-Phosphono-L-Ty-r~
(ix) FEATURE:
(A) NAME/KEY: M~;f;~-site
(B) LOCATION:4
(D) OTHER INFORMATION:/product= "Gln-NH2"
(Xi) ~U~N~ DESCRIPTION: SEQ ID NO: 30:
Xaa Ile Asn Xaa
CA 02227516 1998-01-21
WO 97/08193 PCTrEP96/03473
-138-
(2) INFORMATION FOR SEQ ID NO: 3l:
( i ) ~U~N~'~ CHARACTERISTICS:
(A) LENGTH: 4 amino acids
(B) TYPE: amino acid
(C) S'r'R~NI~ )N~ C;: single
(D) TOPOLOGY: linear
( ii ) M~r.~crlT.~ TYPE: peptide
(ix) FEATURE:
(A) NA~E/~ Y: ~.~;f;~-site
(B) T~Z~'T'Tt)N 1
(D) OTHER INFORMATION:/product=
"N-~4-methylthio-benzoyl]-(O-P-Tyr)"
/note= "O-P-Tyr stands for O-P1,o~hono-L-Tyr"
(ix) FEATURE:
(A) NAME/KEY: M~;f;~-site
(B) LOCATION:4
(D) uTHER INFORMATION:/product= "Gln-NH2"
( Xi ) ~ ~:~U~N~ DESCRIPTION: SEQ ID NO: 3l:
Xaa Ile Asn Xaa
(2) INFORMATION FOR SEQ ID NO: 32:
(i) ~Qu~N~: CHARACTERISTICS:
(A) T-FN~T~: 4 amino acids
(B) TYPE: amino acid
(C) sT~Nn~nN~s: single
(D) TOPOLOGY: linear
CA 02227516 1998-01-21
W O 97/08193 PCT/EP96/03473
-139-
(ii) MOLECULE TYPE: peptide
(ix) FEATURE:
(A) NAME/KEY: ~nA; fi~A-site
(B) LCCATION:l
(D) OTHER rNFORMATION:/~r~u~L-
"N-t4-methylsulfinyl-benzoyl]-(O-P-Tyr)"
/note= "O-P-Tyr stands for O-phnsphnnn-L-Tyr~
(ix) FEATURE:
(A) NAME/~ Y: MnA;f;~A-site
(B) LCCATION:4
(D) OTHER INFORMATION: /~LV~U~L - "Gln-NH2"
(Xi) ~itl:(_)lll-:Nl'l': 1')~ )N: SEQ ID NO: 32:
Xaa Ile Asn Xaa
(2) INFORMATION FOR SEQ ID NO: 33:
.?U~;-N~ R z~(~R T~TIcs
(A) LENGTH: 4 a~mino acids
(B) TYPE: amino acid
(C) S'T'RZ~N~ 1)Nl~ : single
(D) TOPOLOGY: linear
( ii ) ~T-~lT.~ TYPE: peptide
(ix) FEATURE:
(A) NAME/KEY: Modified-site
(B) LOCATION:l
(D) OTHER INFORMATION:/product=
"N- [; mi ~ol-4-yl-acryloyl]-(O-P-Tyr)"
CA 022275l6 l998-0l-2l
W O 97/08193 PCT~P96/03473
-140-
/note= "O-P-Tyr stands for O-phnsphono-L-Tyr; trans isomer"
(ix) FEATURE:
(A) NAME/KEY~ ;f;~-site
(B) LOCATION:4
(D) vl~R INFORMATION:/product= "Gln-NH2"
(xi) ~U~N~ DESCRIPTION: SEQ ID NO: 33:
Xaa Ile Asn Xaa
(2) INFORMATION FOR SEQ ID NO: 34:
,)U~;NC~ CE~ARAf~'l~RT~TICS:
(A) T-T~N~: 4 amino acids
(B) TYPE: amino acid
(C) S~RANI)I~:')N'~ S: single
(D) TOPOLOGY: linear
( ii ) M~T.~CTlT.T' TYPE: peptide
(ix) FEATURE:
(A) NAME/KEY: Mn~;f;~A-site
(B) LOCATION:l
(D) OTHER INFORMATION:/product=
"N-t4-(N-methylamino)-benzoyl]-(O-P-Tyr)"
/note= "O-P-Tyr stands for O-phnsphnnn-L-Tyr
(ix) FEATURE:
(A) NAME/KEY: Mr~;f;e~-site
(B) LOCATION:4
(D) OTHER INFORMATION:/product= "Gln-NH2"
CA 022275l6 l998-0l-2l
W O 97/08193 PCT/EP96/03473
-141-
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 34:
.
Xaa Ile Asn Xaa
(2) rNFORMATION FOR SEQ ID NO: 35:
U~;N~ ARp~rq~RT~:TIcs:
(A) LENGTH: 4 amino acids
(B) TYPE: amino acid
(C) STRAN~ l)N~:~S: single
(D) TOPOLCGY: linear
( ii ) ~T~cTlT~ Y~E: peptide
(ix) FEATURE:
(A) NAME/KEY: ~;f;~-site
(B) ~OCATION:l
(D) OTHER INFORMATION:/product=
"N-t4-(N,N-dimethy1;~m; nnhf~n~yl ) ]--(O--P--Tyr ) "
/note= "O-P-Tyr stands for O-ph~ h~ n-L-Tyr~
(ix) FEATURE:
(A) NAME/KEY: Modified-site
(B) LOCATION:4
(D) OTHER INFORMATION:/~uoL- "Gln-NH2"
(Xi) ~U~N~ DESCRIPTION: SEQ ID NO: 35:
Xaa Ile Asn Xaa
(2) INFORMATION FOR SEQ ID NO: 36:
~ U~N~ CHARACTERISTICS:
CA 02227516 1998-01-21
WO 97/08193 PCT~P96/03473
-14~-
(A) LENGTH: 4 amino acids
(B) TYPE: amino acid
(C) sTRANn~nN~s: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: peptide
(ix) FEATURE:
(A) NAME/REY: M~;fi~-site
(B) LOCATION:l
(D) OT~ER INFORM~T~N:/product=
"N-~pyridin-4-car~onyl]-(O-P-Tyr)"
/note= "O-P-Tyr stands for O-Phosphono-L-Tyr"
(ix) FEATURE:
(A) NAME/KEY: Mn~;fi~-site
(B) LCCATION:4
(D) OTHER INF~RMA~T~N:/pL~u~L- "Gln-NH2"
( Xi ) ~OU~N~ DESCRIPTION: SEQ ID NO: 36:
Xaa Ile Asn Xaa
(2) INFORMATION FOR SEQ ID NO: 37:
U~'N~ CHARACTERISTICS:
(A) LENGTH: 4 amino acids
(B) TYPE: amino acid
(C) sTRANn~nNR~s: single
(D) TOPOLOGY: linear
( ii ) ~T~crlT~ TYPE: peptide
CA 02227516 1998-01-21
WO97/08193 PCT~EP96/03473
-143-
(ix) FEATURE:
(A) NAME/~ Y: M~;fied-site
(B) LOCATION:l
(D) OTHER INFORMATION:/product=
"N-[4-~m;n~-thyIbenzoyl]-(O-P-Tyr)"
/note= "O-P-Tyr stands for O-Phosphono-L-Tyr"
(ix) FEATURE:
(A) NAME/REY: Mr~;f;e~-site
(B) LOCATION:4
(D) OTHER INF~RMATT~N:/~lv~ù~L- "Gln-NH2"
(xi) ~:vN~:N~ ~RTPTION: SEQ ID NO: 37:
Xaa Ile Asn Xaa
(2) INF~RM~TT~N FOR SEQ ID NO: 38:
:N~ ~ARA~T~RlsTIcs:
(A) T-~N~T~: 4 amino acids
(B) TYPE: amino acid
(C) STRANI)~:l)N~ : single
(D) TOPOLOGY: linear
( ii ) ~T.~T.~ TYPE: peptide
(ix) FEATURE:
(A) NAME/KEY: M~;f;~-site
(B) LOCATION:l
(D) OT~ER INFO~MATION:/pl~ucL-
~N-[4-amino-3-methoxybenzoyl]-(o-p-Tyr)~
/note= "O-P-Tyr stands ~or O-Phosphono-L-Tyr"
.
(ix) FEATURE:
CA 02227516 1998-01-21
W O97/08193 PCTAEP96/03473
-144-
(A) NP~3E/KEY: Mn~; f i ~ - site
(B) LOCATION:4
(D) OTHER INFORMATION:/~.~u~- "Gln-NH2"
(xi) .~:Q1 I~:N('~: DESCRIPTION: SEQ ID NO: 38:
Xaa Ile Asn Xaa
(2) INFORMATION FOR SEQ ID NO: 39:
(i) .~:3N ~ N( '~ CHARACTERISTICS:
(A) LENGTH: 4 amino acids
(B) TYPE: a~mino acid
(C) STR~Nl)~:l3~ S: single
(D) TOPOLOGY: linear
(ii) M~3T.~CrlT~ TYPE: peptide
(ix) FEATURE:
(A) NAME/KEY: Mn~; f; ~,3-site
(B) LOCATION:l
(D) OTHER INFORMATION:/product= "N-t4-~m;nnph~nylacetyl]
-(O-P-Tyr)"
/note= "O-P-Tyr stands for O-phn~h~ n-L-Tyr~
(ix) FEATURE:
(A) NAME/KEY: Modified-site
(B) LOCATION:4
(D) OTHER INFORMATION:/product= "Gln-NH2"
(Xi) .~ N('~ DESCRIPTION: SEQ ID NO: 39:
Xaa Ile Asn Xaa
CA 02227516 1998-01-21
W O 97/08193 PCT~EP96/03473
-145-
(2) INFORMATION FOR SEQ ID NO: 40:
(i) SEQUENCE C~AR~T~RISTICS:
(A) LENGTH: 4 amino acids
(B) TYPE: amino acid
(C) STR~N~ N~-~S single
(D) TOPOLOGY: linear
( ii ) MnT-T'~lT-~ TYPE: peptide
(ix) FEATURE:
(A) NAME/KEY: M~;f;P~-site
(B) LCCATION:l
(D) OTHER INFORMATION:/product=
"N-[3-~m;n~h~oyl]-(O-P-Tyr)"
/note= "O-P-Tyr stands for O-Ph~sphnn~-L-Tyr"
(ix) FEATURE:
(A) NAME/KEY: Modified-site
(B) LOCATION:4
(D) OTHER INFORMATION: /plUdU~ ~ - " Gln-NH2"
(xi) ~yu~N~ n~.~TFyTIoN: SEQ ID NO: 40:
Xaa Ile Asn Xaa
(2) INFORMATION- FOR SEQ ID NO: 4l:
.
( i ) ~U~N~' CHARACTERISTICS:
(A) LENGTH: 4 amino acids
(B) TYPE: amino acid
(C) STR~Nn~nN~S: single
~ ~ i
CA 02227516 1998-01-21
WO 97/08193 PCT~EP96/03473
-146- ~
(D) TOPOLOGY: linear
( ii ) M~T.~.CTlT.~ TYPE: peptide
(ix) FEAIURE:
(A) NAME/REY: M~; fi~A - site
(B) LOCA~ION:l
(D) OTHER INFORMATION:/~l~u~-
"N_[~lin~l in~o - 6 - yl - car~onyl] - (o - p - Tyr) "
/note= "O-P-Tyr stands for O-Pl,o~h~.lo-L-Tyr"
(ix) FEATURE:
(A) NAME/REY: M~A;fi~A-site
( B ) T~A~7'Tc)N 4
(D) OTHER INFORM~T~N:/~l~u~- "Gln-NH2"
(xi) ~QU~N~ n~cRrpTIoN: SEQ ID NO: 41:
Xaa Ile Asn Xaa
(2) INFORMATION FOR SEQ ID NO: 42:
( i ) ~U~N~: ~R~T~RT~TIcs:
(A) T-F~T~: 4 aTnino acids
(B) TYPE: ~ino acid
(C) S~PN~ )NtI~ ,S: single
(D) TOPOLOGY: linear
( ii ) M~T.F.CT~T.~ TYPE: peptide
(ix) FEATURE:
(A) NAME/KEY: M~i fi~ - site
(B) LOCATION:l
CA 02227516 1998-01-21
W O97/08193 PCTAEP96/03473
-147-
~ (D) OTHER INFORMATION:/product=
- "N-t4-methoxycarbonyl-benzoyl]-(O-P-Tyr)"
/note= "O-P-Tyr stands for O-Phosphono-L-Tyr"
(ix) FEATURE:
(A) NAME/~ Y: Modified-site
(B) LOCATION:4
(D) OTHER INF~RMATTnN:/yludu~- "Gln-NH2"
( Xi ) ~U~N~ ~T~'~RTT'TION: SEQ ID NO: 42:
Xaa Ile Asn Xaa
(2) INFnRMA~T~N FOR SEQ ID NO: 43:
(i) ~Il~:N~. CHARACTERISTICS:
(A) LENGTH: 4 amino acids
(B) TYPE: amino acid
(C) S~RAN~ :~S: single
(D) TOPOLCGY: linear
(ii) MnT~CrlT~ TYPE: peptide
(ix) FEAIURE:
(A) NAME/~ Y: ~r~;f;~-site
(B) LOCATION:l
(D) OTHER INFORMATION:/product=
"N-~4-carboxy-benzoyl]-(O-P-Tyr)"
/note= "O-P-Tyr stands for O-Phosphono-L-Tyr"
(ix) FEATURE:
- (A) NAME/~ Y: ~;f;~-site
(B) LOCATION:4
(D) OTHER INFORMATION:/product= "Gln-NH2"
CA 022275l6 l998-0l-2l
WO 97/08193 PCTAEP96/03473
-148-
(Xi) ~QUh'N~h' DESCRIPTION: SEQ ID NO: 43:
Xaa Ile Asn Xaa
(2) INFORMATION FOR SEQ ID NO: 44:
(i) ~h'(,~Uh'N~:h ~'~ARA~ ~T.sTIcs:
(A) IENGT~: 4 amino acids
(B) TYPE: amino acid
( C ) S'l'R Z~ N 1) 1~:1 ) N t~ single
(D) TOPOIOGY: linear
(ii) M~T.~lT.~ TYPE: peptide
(ix) FEATURE:
(A) NAME/KEY: M~; f;~A-site
(B) LOCATION:l
(D) OTHER INFo~MA~ToN:/~r~u~L-
"N-[4-amino-2-chlorobenzoyl]-(O-P-Tyr)"
/note= "O-P-Tyr stands for O-ph~ hc~ -L-Tyt~
(ix) FEATURE:
(A) NAME/KEY: M~;f;~-site
(B) LOCATION:4
(D) OTHER INFORMATION:/pl~u~L- "Gln-NH2"
( Xi ) ~h~Uh'N~ DESCRIPTION: SEQ ID NO: 44:
Xaa Ile Asn Xaa
..
(2) INFORMATION FOR SEQ ID NO: 45:
-
CA 02227516 1998-01-21
WO 97/08193 PCT/EP96/03473
-149-
( i ) ~U~N~ CHARACTERISTICS:
(A) LENGTH: 4 amino acids
(B) TYPE: amino acid
(C) STRAN~ l)N~:~S: single
(D) TOPOLOGY: linear
( ii ) M~T~crlT~T' TYPE: peptide
(ix) FEATURE:
(A) NAME/REY: Mn~;f;~-site
(B) LOCATION:l
(D) OTHER INFOR~MATION:/~r~u~-L-
"N-~6 . ;nnn;cotinoyl]-(O-P-Tyr)"
/note= "O-P-Tyr stands for O-Ehn~L~h~ n-L-Tyr~
(ix) FEATURE:
(A) NAME/KEY: Mn~;f;~-site
(B) LOCATION:4
(D) OTHER INFORMATION: /~L ~U~ L- "Gln-NH2"
(Xi) ~UU~N~' D~RTPTION: SEQ ID NO: 45:
Xaa Ile Asn Xaa
(2) INFORMATION FOR SEQ ID NO: 46:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 4 amino acids
(B) TYPE: amino acid
(C) STRANI)~:lJN~:~S: single
- (D) TOPOLOGY: linear
(ii) MOLECULE TYPE: peptide
CA 02227516 1998-01-21
W O97/08193 PCT~EP96/03473
-150- -
( ix ) FEATURE:
(A) NAME/KEY: M~;f;~-site
( B ) LOC'ATION: 1
(D) OTHER rNF~RMZ~'T'T~N: /~Lt~~
"N--[4 ~--~;n~--3--l-yd~. ~yL~llzoyl3--(O--P--T5~r) "
/note= "O-P-TyT stands for O-Ph~ph~nn-L-Ty-r~
( ix ) FEA~rtlRE:
(A) NaME/~Y: M~l; f; f~--site
( B ) T~rt~TtT~N 4
(D) OTHER l~lE'nRMA'T'T~N:/~tL~lu~L-- "Gln-- NH2"
(Xi) ~ U~;N(~; nT~ 'RTT~ ON SEQ ID NO: 46:
Xaa Ile Asn Xaa
(2) INFORMATION T;'OR SEQ ID NO: 47:
.~L. ~t~T~RA~T~RT~TIcs:
(A) IENGTH: 4 amino acids
( B ) TYPE: amino acid
(C) S'T'RAN~ N~ .S: single
( D ) 'I'OPOI.OGY: line~
( ii) M )T.T~ T.~.' TYPE: peptide
( ix ) FEATURE:
(A) NAME/KEY: MrAif;i~-site
( B ) ~CATION: 1
(D) OTHER INE~RM~'T'T~)N:/~L~lU~
"N-t3-(3-~;n~rh~nyl)propionyl]-(O-P-Tyr)"
/note= "O-P-Tyr stands for O-ph~rh~n~-L-T
CA 02227516 1998-01-21
WO 97/08193 PCT/EP96/03473
- (ix) FEAT~RE:
(A) NAME/KEY: M~;f; ~-site
(B) LOCATION:4
(D) OTHER INFORM~TION:/product= "Gln-NH2"
(xi) ~ U~N~:~; DESCR~ N: SEQ ID NO: 47:
Xaa Ile Asn Xaa
-
(2) l~JFORMATION FOR SEQ ID NO: 48:
(i) ~;~;~U~;N~ ; r~p~R7~rtl~RT~ TIcs:
(A) LENGTH: 2 amino acids
(B) TYPE: amino acid
(C) S'rR~NI~ NI~ S: single
(D) TOPOLOGY: linear
(ii) M~T ~cr~T~ TYPE: peptide
(ix) FEATURE:
(A) NAME/KEY: M~;f;F~-site
(B) LOCATION:l
(D) OTHER INFOR~5ATION:/product=
"N--t4--i9m;n~h~n~oyl]--(O--P--Tyr)"
/note= "O-P-Tyr stands for O-Phosphono-L-Tyr"
(ix) FEATURE:
(A) NAME/KEY: M~l;f;~d-site
(B) LOCATION:2
(D) OTHER INFORMATION:/pl~lu~-- "Ile-NH2"
-
( Xi ) ~ 2U~;N~ :~; DESCRIPTION: SEQ ID NO: 48:
,
CA 02227516 1998-01-21
W O 97/08193 PCT~EP96/03473
-1~2-
Xaa Xaa
(2) INFORM~TT~N FOR SEQ ID NO: 49:
( i ) ~U~N~ ~r~RA~RISTICS:
(A) T.T~Tr~: 4 amino acids
(B) TYPE: amino acid
(C) S~R~N~ )N~ : single
(D) TOPOLOGY: linear
(ii) M~T~rlT~ TYPE: peptide
(ix) FEATURE:
(A) NAME/REY: M~;f;~-site
(B) T~TON:l
(D) OTHER INFORMATION:/~v~ucL-
"N-[indole-3-yl-acryloyl]-(O-P-Tyr)"
/note= "O-P-Tyr stands for O-Phf~,h~ ~-L-Tyr; trans-isomer"
(ix) FEATURE:
(A) NAME/REY: M~;f;~-site
(B) LOCATION:4
(D) OTHER INFORMATION:/~l~u~--- "Gln-NH2"
(Xi) ~U~'N~ ~RTT~rION SEQ ID NO: 49:
Xaa Ile Asn Xaa
(2) INFORM~TON FOR SEQ ID NO: 50:
(i) ~1':5,~U~;N~; CE~ ~RT~;TICS
(A) LENGTH: 3 amino acids
CA 02227516 1998-01-21
WO 97/08193 PCTAEP96/03473
-153-
(B) TYPE: amino acid
- (C) STRAN~ l)N~:~s: single
(D) TOPOLOGY: linear
( ii ) M~T.T'.CT7T.T<. TYPE: peptide
(ix) FEATURE:
(A) NAME/KEY: M~; fiG~ - site
B) Tl~A'l'TO~
(D) OTHER INFORMATION:/product=
"N-[4-~m;n~h~..7.~yl]-(O-P-Tyr)"
/note= ''O-P-Ty-r stands for O-Ph~sphnn~-L-Tyr"
(ix) FEATURE:
(A) NAME/KEY: M~;f;~ -site
(B) T~A~T~T~N 3
(D) OTHER INFORMATION:/product- "Asn-N~2"
( Xi ) ~OU~N~ nT~RTPTION: SEQ ID NO: 50:
Xaa Ile Xaa
(2) INFORMATION FOR SEQ ID NO: 5l:
(i) ~U~N~: CHARACTERISTICS:
(A) LENGTH: 4 amino acids
(B) TYPE: amino acid
( C ) ST ~AN I )1':1 ~N 1':.~;: single
(D) TOPOLOGY: line OE
.
(ii) MOLECULE TYPE: peptide
(ix) FEATURE:
CA 022275l6 l998-0l-2l
W O 97/08193 PCTAEP96/03473
-154-
(A) NAME/KEY: Modified-site -
(B) LOCATION:l
(D) OTEER INFORMATION:/product=
" N--[ 4--;3m; nnh~n ~oyl ]--( O--P--Tyr ) "
/notea "O-P-Tyr stands for O-Pl.~h-~..n-L-Tyr"
(ix) FEATURE:
(A) NAME/~ Y: M~;f;~-site
(B) T~ATTnN 4
(D) OTEER INF~RMATTnN~ u~- "Gln-NH2"
(Xi) ~h'VUh'N~h n~RTpTIoN: SEQ ID NO: 51:
Xaa Asp Asn Xaa
(2) INFORMATION FOR SEQ ID NO: 52:
( i ) ~hVU~'N~ CHARACTERISTICS:
(A) LENGTH: 4 amlno acids
(B) TYPE: amino acid
(C) ST~ANI)~:l)N~:~S: single
(D) TOPOLOGY: linear
(ii) M~T~cr7T~ TYPE: peptide
(ix) F3ATURE:
(A) NAME/REY: Mn~;f;~-site
(8) LOCATION:l
(D) OTHER rNFORMATION:/product=
"N-[4-~;n~h~n~oyl]-(O-P-Tyr)"
/note= "O-P-Tyr stands for O-phosrhnnn-L-Tyr
(ix) F7~ATURE:
(A) NAME/KEY: ~;fi~-site
-
CA 02227516 1998-01-21
WO 97/08193 PCTAEP96/03473
-155-
(B) LOCATION:4
- (D) OTHER INFORMATION:/product= "Gln-NH2"
(Xi) ~ U~N~'~ DESCRIPTION: SEQ ID NO: 52:
Xaa Gly Asn Xaa
(2) INFnRMA~ToN FOR SEQ ID NO: 53:
( i ) ~;YU~;N.~S r~7~RA-'T1;~RT~;TICS:
(A) LENGTH: 4 amino acids
(B) TYPE: amino acid
(C) S~NI)I':I)N~:-C~: single
(D) TOPOLOGY: linear
(ii) M~r~rRcrlr~ T ~E: peptide
(ix) FEATURE:
(A) NAME/REY: Mn~;f;~-site
(B) LOCAIION:l
(D) OTHER INFORMATION:/~luducL-
"N--t 4_A~li nnh~n~oyl ]--( O--P--Tyr ) "
/note= "O-P-Tyr stands for O-phnsphnnn-L-Tyr
(ix) FEATURE:
(A) NAME/KEY: M~ifi~-site
(B) LOCATION:4
(D) OTHER INFORMATION:/product= "Gly-NH2"
(xi) SEQUENCE D~RTPTION: SEQ ID NO: 53:
Xaa Ile Asn Xaa
,.
CA 02227516 1998-01-21
W O 97/08193 PCT~EP96/03473
-156-
(2) INFORMATION FOR SEQ ID NO: 54:
(i) ~UU~N~: CH~RACTERISTICS:
(A) LENGTH: 4 amino acids
(B) TYPE: amino acid
(C) ST~ANI)~:I)N~:~.~: single
(D) TOPOLOGY: linear
( ii ) M~T.T CTTr.~ TYPE: peptide
(ix) FEATURE:
(A) NAME/KEY: M~;f~ site
(B) LCCATION:l
(D) OTHER INFORMATTON:/product=
"N-[4-~m;..~h~n~.yl]-(O-P-Tyr)"
/note= "O-P-Tyr stands for O-Phosphono-L-Tyr"
(ix) FEAIURE:
(A) h-AME/KEY: Mr~;f;~-site
(B) ~OCATION:4
(D) OTHER INFORMATION:/product= "Val-NH2"
(Xi) ~vN~ N( '~: D~RTPTION: SEQ ID NO: 54:
Xaa Ile Asn Xaa
(2) rNFORMATION FOR SEQ ID NO: 55:
U~N~ ~ARA~T~RISTICS:
(A) LENGTH: 4 amino acids
(B) TYPE: amino acid
(C) STRANn~nN~.~S: single
(D) TOPOLOGY: linear
CA 02227516 1998-01-21
W O97/08193 PCTAEP96/03473
-157-
( ii ) M~T~CT~ TYPE: peptide
~.
(ix) FEATURE:
(A) NAME/~ Y: Mn~;fi~A-site
(B) LOCATION:l
(D) uT~K INF~RMA~T~: /~l~U~ -
"N--t 4--Am; nnh~n~oyl ]--( O--P--Tyr ) "
/note= "O-P-Tyr stands for O-PhnsFhono-L-Tyr"
(ix) FEATURE:
(A) NAME/~ Y: Mn~if;~-site
(B) LCCATION:4
(D) OTHER INFnRMA~T~w:/product= "Asp-NH2"
(Xi) ~U~N~ n~RTpTIoN: SEQ ID NO: 55:
Xaa Ile Asn Xaa
(2) INFORMATION FOR SEQ ID NO: 56:
( i ) ~U~N~ CH~RACTERISTICS:
(A) LENGTH: 4 amino acids
(B) TYPE: amino acid
(C) SrrRANl~l)J~s: single
(D) TOPOLCGY: linear
( ii ) M~T.~lT.~ TYPE: peptide
..
(ix) FEATuRE:
(A) NAME/ ~ Y: Mn~;f;~-site
(B) LOCATION:l
(D) OTHER INFORMATION:/~u~- "N-t4 - Am; noh~n ~syl]-(O-P-Tyr)"
CA 02227516 1998-01-21
W O 97/08193 PCT~EP96/03473
-158-
/note= "O-P-Tyr stands for O-Phosphono-L-Tyr"
(ix) FEATVRE:
(A) NAME/REY: ~if;~-site
(B) LOCATION:4
(D) OTHER INFORMATION:/product=
"l-am~nocyclopentane_cArh~mi~1~"
/note= ~R~ l of l-aminocyclopentane carboxylic acid
with
carboxy-group (COOH) in c~rh~m;~ (CONH2) form"
(xi) ,~:Q~ N(~: n~RTT~TIoN: SEQ ID NO: 56:
Xaa Ile A;sn Xaa
(2) INFORMATION FOR SEQ ID NO: 57:
(i) SEQUENCE r~AR~RISTICS:
(A) IENGTH: 4 amino acids
(B) TYPE: amino acid
(C) STR~NI)~:l )N~ single
(D) TOPOLOGY: linear
(ii) M~T.F.CrrT.F. TYPE: peptide
(ix) FEA~URE:
(A) NAME/KEY: ~;f;e~-site
(B) LOCATION:l
(D) OTHER INFORM~TT~:/product=
"N-[4-aminobenzoyl]--(O-P-Tyr)"
/note= "O-P-Tyr stands for O-Phosphono-L-Tyr"
(ix) FEATURE:
(A) NAME/KEY: Modified-site
CA 02227516 1998-01-21
W O 97/08193 PCTAEP96/03473
-159-
.
(B) LOCATION:4
(D) OTHER INFORMATION:/product= "Asn-NH2"
(Xi) ~U~N~ DESCRIPTION: SEQ ID NO: 57:
Xaa Ile Asn Xaa
(2) INFORMATION FOR SEQ ID NO: 58:
Ul':NC:I~; CHARA~"ITF'RT~:TICS:
(A) LENGTH: 4 amino acids
(B) TYPE: amino acid
(C) STR A N I ) ~ I )N ~:~S S ingle
(D) TOPOLOGY: linear
(ii) MnT.~IT.~ TYPE: peptide
(ix) FEATURE:
(A) NAME/KEY: M~; f; ~ -site
(B) LOCATION:l
(D) OTHER INFORMATION:/~u~L-
"N-[4-~;noh~n~oyl]-(O-P-Tyr)"
/note= "O-P-Tyr stands for O-phnsph~nn-L-Tyr~
(ix) FEATURE:
(A) NAME/KEY: ~; f; ~ - site
(B) LOCATION:4
(D) OTHER INFORMATION:/product= "bAla-NH2"
/note= "radical of beta-alanyl-amide (-NH-CH2-CH2-(C=O)-)"
( Xi ) ~ U~N-~ DESCRIPTION: SEQ ID NO: 58:
Xaa Ile Asn Xaa
CA 02227516 1998-01-21
WO 97/08193 PCTAEP96/03473
-160-
(2) INFORMATION FOR SEQ ID NO: 59:
(i) ~U~:N~ CHARACTERISTICS:
(A) T.~TFT 4 amino acids
(B) TYPE: amino acid
(C) STRAN~ )N~ S: single
(D) TOPOLOGY: linear
( ii ) M~T~cT~T~ TYPE: peptide
(ix) FEATURE:
(A) NAME/KEY: ~n~;f;~d-site
(B) LOCATION:l
(D) ~l~K INFORMA~T~: /~lV~U~ L-
"N-t4-~i n~h~n 70yl ~ - ( O-P-Tyr)"
/note= "O-P-Tyr stands for O-Pho~h~l~o-L-Tyr"
(ix) FEAIURE:
(A) NAME/KEY: M~; fi~-site
(B) LCCATION:4
(D) OTHER INFORMATION:/~u~L- "Gln-NH2"
(Xi) ~U~N~ n~rRTPTION: SEQ ID NO: 59:
Xaa Ile Gly Xaa
(2) INFORMATION FOR SEQ ID NO: 60:
( i ) ~QU~N~ CHARACTERISTICS:
(A) LENGTH: 4 amino acids
(B) TYPE: aTnino acid
(C) sTRANn~nN~s: single
CA 022275l6 l998-0l-2l
WO 97/08193 PCT/EP96/03473
-161 -
..
(D) TOPOLCGY: linear
( ii ) ~nT~CuT~ TYPE: peptide
(ix) FEATURE:
(A) NAME/KEY: M~;f;~-site
(B) LOCATION:l
(D) OTHER INFORMATION:/~l~U~L-
"N-[4-~m;n~h~-~~yl ]-(O-P-Tyr)"
/note= "O-P-Tyr stands for O-ph~srhnn~-L-Tyr
(ix) FEATURE:
(A) NAME/REY: ~;f;G~-site
(B) T~TT~N 4
(D) OTHER INFORMATION:/~.~u~L- "Gln-NH2"
(Xi) ~ U~N~ n~RTT~TION: SEQ ID NO: 60:
Xaa Ile Gln Xaa
(2) INFORMATION FOR SEQ ID NO: 6l:
( i ) ~U~N~ CHARACTERISTICS:
(A) T.~,Tr~ 4 amino acids
(B) TYPE: amino acid
(C) ST~N~ l)N~:~S: single
(D) TOPOLOGY: linear
( ii ) ~T~crlT~T~ TYPE: peptide
.
(ix) FEATURE:
(A) NAME/XEY: Modified-site
(B) LOCATION:l
CA 02227516 1998-01-21
W O 97/08193 PCTAEP96/03473
-162-
(D) OTHER INFORMATION:/product=
"N-tindole-5-yl-carbonyl]-(O-P-Tyr)"
/note= "O-P-Tyr stands for O-Ph~sph~n~-L-Tyr"
(ix) FEATURE:
(A) NAME/KEY: Mr~;f;eA-site
(B) LOCATION:4
(D) OTHER INFORMATION:/product= "Gln-NH2"
(xi) ~U~N~ ~T~RTpTIoN: SEQ ID NO: 61:
Xaa Ile Asn Xaa
(2) INFORMATION FOR SEQ ID NO: 62:
(i) ~QU~N~ CHA~A~RT~TICS:
(A) LENGTH: 3 amino acids
(B) TYPE: amino acid
(C) STR~ S: single
(D) TOPOIOGY: linear
( ii ) M~T~cTTT~ TYPE: peptide
(ix) FEATURE:
(A) NAME/KEY: M~;f;~-site
(B) LOCATION:l
(D) OTHER INFORMATION:/product=
"N-[indole-5-yl-carbonyl]-(O-P-Tyr)"
/note= "O-P-Tyr stands for O-Phosphono-L-Tyr"
(ix) FEATURE:
(A) N-AME/KEy: M~;f;~-site
(B) LOCATION:3
(D) OTHER INFORMATION:/product= "Asn-NH2"
CA 02227516 1998-01-21
WO 97/08193 PCTAEP96/03473
-163-
(xi) ~Qu~N~ ~ ~RTpTIoN: SEQ ID NO: 62:
Xaa Ile Xaa
(2) INFORMATION FOR SEQ ID NO: 63:
( i ) ~U~N~ CHARACTERISTICS:
(A) LENGTH: 4 amino acids
(B) TYPE: amino acid
( C ) STRAN ~ 1 )N~ S: single
(D) TOPOLOGY: linear
( ii ) M~T.~.CrlT.~ TYPE: peptide
(ix) FEATURE:
(A) NAME/KEY: M~;f;~-site
(B) LOCATION:l
(D) OTHER INFORMATION:/product=
"N-[indole-5-ylc~ rh~nyl ] - ( O-P-Tyr)"
/note= "O-P-Tyr stands for O-Pho~phono-L-Tyr"
(ix) FEATURE:
(A) NAME/KEY: M~;f;~-site
(B) LOCATION:2
(D) OTHER INFORMATION:/product=
"l-aminocycl~h~n~-carbonyl"
/note= "R~ l of l-aminocy~l~h~n~ carboxylic acid"
(ix) FEATURE:
(A) NAME/REY: Modified-site
(B) LOCATION:4
(D) OTHER INFORMATION: /~L~U~ L- "Gln-NH2"
CA 022275l6 l998-0l-2l
WO 97/08193 PCT/EP96/03473
-164-
(xi) ~uu~N~: DESCRIPTION: SEQ ID NO: 63:
Xaa Xaa Asn Xaa
(2) rNFORMATT~N FOR SEQ ID NO: 64:
(i) ~5~U~;NC:.~; Cl~z~R;~ ~RT-~TIcs:
(A) T.~N~,TW: 4 amino acids
(B) TYPE: amino acid
(C) STRP~N~ )N~ S single
(D) TOPOLOGY: linear
( ii ) M~T-~JT-~ TYPE: peptide
(ix) FEATURE:
(A) NAME/KEY: MnAif;P~_site
(B) LOCATION:l
(D) OTHER INFORMATION:/product=
"N--[3--;~m;nnhf~n~yloxycarbonyl]--(L--CF2Pmp)"
/note= "L-CF2-Pmp stands for 4 - phosrhnnnA; fl .1~ ~Lhyl-L-Phe"
(ix) FEATURE:
(A) NAME/REY: MnA; f; ~ -site
(B) Tn~A~TnN 4
(D) OTHER INFORM~T~M:/product= "Gln-NH2"
(xi) ~uu~ DESCRIPTION: SEQ ID NO: 64:
Xaa Ile Asn Xaa
(2) INFORMATION FOR SEQ ID NO: 65:
CA 02227516 1998-01-21
WO 97/08193 PCT/EP96/03473
- 165-
( i ) ~ QU~N~:~: CHARACTERISTICS:
(A) LENGTH: 4 amino acids
(B) TYPE: amino acid
(C) STRANn~:n~ S: single
(D) TOPOLOGY: linear
( ii ) Mt T~FC~lT~F TYPE: peptide
(ix) FEATURE:
(A) NAME/KEY: M~if;~l--site
(B) LOCATION:l
(D) OTHER INFORMATION:J~..xlu~ L--
"N--[4--Aminnh~n~oyl]--(L--F2Pmp)"
/note= "L-F2Pmp stands for 4-(phosphnno-~l;f~ ~Lhyl)-L-phe~
(ix) FEATURE:
(A) N~E/REY: Mnf~;f;~--site
(B) WC~TION:4
(D) OTHER INFORMATION: /~L~I,l~,; L-- " Gln--NH2"
( Xi ) ~;~;QU~'N(-:~ DESCRIPTION: SEQ ID NO: 65:
Xaa Ile Asn Xaa
(2) INF ~RMA~T)N FOR SEQ ID NO: 66:
;.2U ~:N~ ; CHARACTERISTICS:
(A) LENGTH: 4 amino acids
(B) TYPE: amino acid
(C) srRAN~ )N~:-~,S: single
" (D) TOPOLOGY: linear
(ii) Mt)T.F'.ClJT.F~ TYPE: peptide
CA 022275l6 l998-0l-2l
WO 97/08193 PCT/EP96/03473
- 166 -
(ix) FEATURE:
(A) NAME/~Y: ~if;.o~7-site
(B) LOCATION:l
(D) OTEIER INFORMA~T~N: /~L~lUC: L--
" N--~ 4_~mi n~h~n~oyl ]--( O--P--TSrr ) "
/note= "O--P--Ty-r stands for O--Phnsphnnn--L--Tyr"
(ix) FEATURE:
(A) NAME/~Y: M~; fi~l--site
(B) LOCATION:4
(D) OTHER ~7FORMA~T~N:/~. ~lu~L-- "Gln--NH2"
(xi) ~;yu~;N~:~ n~'RTT~TION: SEQ ID NO: 66:
Xaa Phe Asn Xaa
(2) INFORMATION FOR SEQ ID NO: 67:
( i ) ~;(JU~!;N~; CE~Ar~RT.~TICS:
(A) LENGT~I: 4 amino acids
(B) TYPE: amino acid
(C) s~rRz~N~ )N~s: single
(D) TOPOLOGY: linear
( ii ) M ~T~T~crlT~ 'Y~E: peptide
(ix) FEATt~RE:
(A) NAME/~Y: M~if;~l-site
(B) LOCATION:l
(D) OTHER INFORM~TION:/product=
"trans-3~4-dil-y~ Ly-~-inn~mnyl-(o-p-~ryr)~
/note= "O--P--Tyr stands for O-phnsFht~nn-L-rryr~
CA 022275l6 l99X-0l-2l
W O 97/08193 PCT~EP96/03473
-167-
- (ix) FEATURE:
(A) NAME/KEY: M~i f;~-site
(B) LOCATION:4
(D) OTHER INFORMATION:/product= "Gln-NH2"
(Xi) ~yU~N~ n~-~RTPTION: SEQ ID NO: 67:
Xaa Ile Asn Xaa
(2) INFnRMA~T~N FOR SEQ ID NO: 68:
( i ) ~U~N~ CEE~RACTERISTICS:
(A) T.~N~,T~: 4 arnino acids
(B) TYPE: arnino acid
(C) S~RAN~ ~: single
(D) TOPOIOGY: linear
(ii) M~T~crlT~T~ TYPE: peptide
(ix) FEATURE:
(A) NAME/REY: M~;f;e~-site
(B) LOCATION:l
(D) OTHER INFORMATION: /~l~U~ ~ -
"[N-(trans-3-1-ydl~y-~; nn~mnyl ) - ( O-P-Tyr)]"
/note= "O-P-Tyr stands for O-phnsrhnno-L-Tyyr~
(ix) FEATURE:
(A) NAME/KEY: Modified-site
(B) T~Z~TTt )N 4
(D) OTHER INFORMATION:/product= "Gln-NH2"
.
(xi) ~Qu~ DESCRIPTION: SEQ ID NO: 68:
CA 022275l6 l998-0l-2l
W O 97/08193 PCT/EP96/03473
-168-
Xaa Ile Asn Xaa
(2) INFnRMA~ToN FOR SEQ ID NO: 69:
(i) !-;IC(,~NI':N('I~: ~P-RA~"I~RT-~TICS:
(A) LENGTH: 4 amino acids
(B) TYPE: amino acid
(C) STR~Nl)~:l)N~:~S: single
(D) TOPOLCGY: line ~
( ii ) M~T~cr~Tl~ TYPE: peptide
(ix) FEATURE:
(A) NAME/KEY: M~A; f;~d-site
(B) LOCATION:l
(D) OT~ER INFORMATION~ o~ùcL-
"[N-(t-3-h-4-m-~;nn~m~yl)-(O-P-Tyr)]"
/note= "t-3-h-4-h-m-c;nn~m~yl stands for
trans-3-hydl~y-4-methoxy-~;nn~m~yl); O-P-Tyr stands for
O-Ph~l~h~ o-L-Tyr"
(ix) FEATURE:
(A) NAME/~ Y: M~;f;P~-site
(B) T~A'I'T( N 4
(D) OTHER INFnRMA~Tn~ u~ L- "Gln-NH2"
(Xi) ~U~N~ DESCRIPTION: SEQ ID NO: 69:
Xaa Ile Asn Xaa
(2) INFORMATION FOR SEQ ID NO: 70:
( i ) ~QU~N~ CHARACTERISTICS:
CA 02227516 1998-01-21
W O 97/08193 PCTA~P96/03473
-169-
_ (A) LENGTH: 2 amino acids
(B) TYPE: amino acid
(C) sTRANn~nN~ss: single
(D) TOPOLOGY: linear
(ii) M~T~crlT~ TYPE: peptide
(ix) FEATURE:
(A) NAME/REY: M~if;e~-site
(B) LOCATION:l
(D) OTHER INFORMATION: /~l~U~ L-
"N--trans--3,4-dihy~J~y-r;nnAm~yl-(O-P-Ty-r)"
(ix) FEATURE:
(A) NAME/KEY: Mn~;f;~d-site
(B) LOCATION:2
(D) OTHER INFORMATION:/~'~L- "Ile-NH2"
(Xi) S~U~N~ DESCRIPTION: SEQ ID NO: 70:
Xaa Xaa
(2) INF~RMATTON FOR SEQ ID NO: 7l:
( i ) S ~:QU~'N~ CHARACTERISTICS:
(A) T.~N~,T~ 2 amino acids
(B) TYPE: amino acid
(C) STR~Nn~nN~S: single
(D) TOPOLOGY: linear
( ii ) ~T~cr~ TYPE: peptide
(ix) FEATURE:
CA 02227516 1998-01-21
WO 97/08193 PCT/EP96/03473
- 170 - =
(A) NAME/KEY: ~; f i ~.1--site
(B) LOCATION:l
(D) OTHER INFORMATION:/product=
"N--trans--3,4-dihyd~ky-cinnamoyl-(o-p-Tyyr)"
(ix) FEATt~RE:
(A) NAME/KEY: Mn~;f;~rl--5ite
(B) LOCATION:2
(D) OTHER rNF~Rr~A~rTo~:/fJl~~ ;L-
"l-aminocy~ h~ nF~-carbonyl"
/note= "R;~ l of l-aminocy~ h~ n~ car}~oxylic acid"
( Xi ) ~ihQUhN~:h DESCRIPTION: SEQ ID NO: 7l:
Xaa Xaa
(2) l:NFORMATION FOR SEQ ID NO: 72:
(i) Sh~2Uh'N~:h' ~T~ARAt'~RT~sTIcs:
(A) LENGTH: 3 amino acids
(B) TYPE: amino acid
(C) STRAN~ N~l:.~s: single
(D) TOPOLOGY: linear
( ii ) M~)T~T~cuTlF~ TYPE: peptide
(ix) FEATURE:
(A) NAME/KEY: M~; f; e~--site
(B) LOCATION:l
(D) OTHER INFOR~TION:/yl~lu~L--
"N-trans--3,4-dihy~ ,~y-c;nn~ yl-(O-P--Tyr)"
/note= "O--P-Tyr stands for O--Phosphono-L--Tyr"
(ix) FEATuRE
CA 022275l6 l998-0l-2l
W O 97/08193 PCT~EP96/03473
-171 -
(A) NAME/KEY: Modified-site
(B) LOCATION:2
(D) OTHER INFORMATION:/product=
"l-aminocy~-1~h~n~_carbonyl"
/note= "R~ A1 of l-aminocy~1~h~n~ carboxylic acid"
(ix) FEATURE:
(A) NAME/KEY: Mn~;f;e~-site
(B) LOCATION:3
(D) OTHER INFORMATION:/product= "Asn-NH2"
(Xi) ~ U~N~ DESCRIPTION: SEQ ID NO: 72:
Xaa Xaa Xaa
(2) INFORMATION FOR SEQ ID NO: 73:
(i) SEQUENOE CHARACTERISTICS:
(A) LENGTH: 3 amino acids
(B) TYPE: amino acid
(C) STR~NI~ N~ S: single
(D) TOPOLCGY: linear
(ii) ~T-RCrlTR TYPE: peptide
(ix) FEATURE:
(A) NAME/KEY: Modified-site
(B) LOCATION:l
(D) OTHER INFORMATION:/pl~u.L-
"N-7,8-di-OH-2-0-2H-benzopyran-CO-(O-P-Tyr)"
/note= "7,8-di-OH-2-0-2H-benz~y~dn-CO stands for
- 7 ~ 8-dil~y~ )~y-2-oxo-2H~ enzopyran ) -3-carboxyl; O-P-Ty-r
stands
for O-Phosphono-L-Tyr"
CA 022275l6 l998-0l-2l
WO 97/08193 PCT~EP96/03473
-172-
(ix) FEATURE:
(A) NAME/ ~ Y: M~if;~-site
(B) LOCATION:3
(D) CT~ER INFORMATION:/product= "Asn-NH2"
(Xi) .';~ N('t': I-T'-~'RTT'TION: SEQ ID NO: 73:
Xaa Ile Xaa
(2) INFORMATION FOR SEQ ID NO: 74:
(i) ~U~N~ CHAR~RT~TICS:
(A) LENGTH: 3 amino acids
(B) TYPE: a77.~no acid
(C) S~RAN~ )N~ S: single
(D) TOPOLOGY: linear
(ii) M~T.~cr7T~ TYPE: peptide
(ix) FEATURE:
(A) NAME/ ~ Y: Mr~;f;F~-site
(B) LOCATION:l
(D) OTHER rNFORMATION: /pl~U~ L-
''N-trans-3~4-dil-ydk~y-~;nnAm~yl-(o-p-Tyr)"
/note= "O-P-Tyr stands for O-Ello~h~"o-L-Tyr"
(ix) FEATURE:
(A) NAME/ ~ Y: M~; f;~-site
(B) LOCATION:3
(D) OTHER rNFORMATION:/~lo~u~L- "Asn-NH2"
(xi) ~:QU~N~ n~RTpTIoN: SEQ ID NO: 74:
CA 02227516 1998-01-21
W 097/08193 PCTtEP96tO3473
-173-
Xaa Met Xaa
(2) INFORMATION FOR SEQ ID NO: 75:
( i ) ~QU~N~ CHARACTERISTICS:
(A) LENGTH: 3 amino acids
(B) TYPE: amino acid
( C ) S~TlR A N ~ 1 )N ~ single
(D) TOPOLOGY: linear
( ii ) ~T~T~cTTT~T~ TYPE: peptide
(ix) FEATURE:
(A) NAME/KEY: Mn~;f;~-site
(B) LOCATION:l
(D) OTHER INFORMATION: /~lO~U~ L -
"N-trans-3,4-dillydl~y-ci nn~mnyl - (O-P-Tyr)"
/note= "O-P-Tyr stands for O-phnsph~nn-L-Tyr"
(ix) FEATURE:
(A) NAME/KEY: Modified-site
(B) LOCATION:3
(D) OTHER INFORMATION:/product= "Asn-NH2"
(Xi) ~ U~N~ n~RTpTIoN: SEQ ID NO: 75:
Xaa Pro Xaa
.
(2) INFORMATION FOR SEQ ID NO: 76:
( i ) ~QU~N~ CHARACTERISTICS:
(A) LENGTH: 3 aTnino acids
CA 02227516 1998-01-21
W O 97/08193 PCT~EP96/03473
-174-
(B) TYPE: amino acid
(C) STRAN~ l )N~xs: single
(D) TOPOLOGY: linear
(ii) M~T.~.~lT.~ TYPE: peptide
(ix) FEATURE:
(A) NAME/REY: M~;fi~-site
(B) T~TT~N:l
(D) OTHER INFORMATION:/~u~L-
"3-(3~4-dihydl~y~henyl)propionyl-(O-P-Ty~)"
/note= "O-P-Tyr stands for O-Ph~sph~n~-L-Ty-r~
(ix) FEATURE:
(A) NAME/ ~ Y: M~;f;~-site
(B) T~TtYN 3
(D) OT~ER INFnRMA~TnN:/product= "Asn-NH2"
(Xi) ~U~N~ n~.~RTpTIoN SEQ ID NO: 76:
Xaa Ile Xaa
(2) INFORMA~Tt~N FOR SEQ ID NO: 77:
( i ) ~U~N~ CHARAt-~.RT~TICS:
(A) T.~Nr.~: 3 amino acids
(B) TYPE: amino acid
(C) ST~ANI)~:l)N~:~S: single
(D) TOPOLOGY: linear
( ii ) M~r.RCUT.~ TYPE: peptide
(ix) FEATURE:
CA 02227516 1998-01-21
WO 97/08193 PCTrEP96/03473
-175-
(A) NAME/KEY~ ;f;~d-site
(B) LOCATION:l
(D) OTHER INFORMATION:/product=
"N-2-(3~4-dihydlu~y~henyl)acetyl-(O-P-Tyr)"
/note= "O-P-Tyr stands for O-Phosphono-L-Tyr"
(ix) FEATURE:
(A) NAME/KEY: ~;f;~-site
(B) LOCATION:3
(D) OTHER INFORMATION:/product= "Asn-NH2"
(Xi) ~U~N~ n~RTpTIoN: SEQ ID NO: 77:
Xaa Ile Xaa
(2) INFORMATION FOR SEQ ID NO: 78:
( i ) ~U~N~ ~R~T~RIsTIcs:
(A) LENGTH: 2 amino acids
(B) TYPE: amino acid
(C) S~NI)I~ NI~ .S: single
(D) TOPOLOGY: linear
( ii ) ~T-~lT.~ TYPE: peptide
(ix) FEATURE:
(A) NAME/KEY: ~ifie~-site
(B) LOCATION:l
(D) OTHER INFORMATION:/product=
'' "N--trans--3,4--dil-y~u~y--~~inn~mnyl--(O--P--Tyr)"
/note= "O-P-Tyr stands for O-Ph~ph~n~-L-Tyr"
(ix) FEATURE:
(A) NAME/KEY: Modified-site
CA 022275l6 l998-0l-2l
W O97/08193 PCTAEP96/03473
-176-
(B) LOCATION:2
(D) OTHER INFORMATION:/product= "Gly-Nh2"
(xi) ~yu~:N~ DESCRIPTION: SEQ ID NO: 78:
Xaa Xaa
(2) INFORMATION FOR SEQ ID NO: 79:
( i ) ~U~N~ C~AR~RISTICS:
(A) LENGTH: 2 amino acids
(B) TYPE: amino acid
(C) STRAN~ ,)N~:~S: single
(D) TOPOLOGY: linear
(ii) M~T.~.~T.~ TYPE: peptide
(ix) FEATURE:
(A) NAME/KEY: M~; fii ~-site
(B) LOCATION:l
(D) OTHER INFORMATION:/~u~L-
"N--trans--3,4--d;lly~lL.J~y,inn~ yl--(O--P--Tyr)"
/note= "O-P-Ty~ stands for O-Phosphono-L-Ty-r
(ix) FEATURE:
(A) NAME/KEY: Mr~; f; ~ - site
(B) LOCATION:2
(D) OTHER INFORMATION:/product- "beta-alanyl"
/note= "~eta-alanyl stands for -NH-CH2-CH2-C(=O)-"
(Xi) ~VU~N~: DESCRIPTION: SEQ ID NO: 79:
Xaa Xaa
CA 02227516 1998-01-21
W O97/08193 PCT/EP96/03473
-177-
(2) INFORMATION FOR SEQ ID NO: 80:
(i) ~:~U~N~ CHARACTERISTICS:
(A) LENGTH: 3 amino acids
(B) TYPE: amino acid
(C) S~R;~NI~:I)NI~ : single
(D) TOPOLOGY: linear
( ii) M~T.~Cr~T.~ TYPE: peptide
(ix) FEATURE:
(A) NAME/REY: Mn~if;~A-site
(B) LOCATION:l
(D) OTHER INFORMATION: /~l~U~ ~ -
"N-trans-3,4-dihydl~y-c; nn~yl_ (O - P - Ty~ ) "
/note= "O-P-Tyr stands for O-ph~srh~n~-L-Tyr~
(ix) FEATURE:
(A) NAME/KEY: M~A; f; eA -site
(B) LOCATION:2
(D) OTHER INFORMATION:/product=
"l-amino-cycloheptane-carbonyl"
/note= "R~;c~l of l-Aminocycloheptane car~oxylic acid"
(ix) FEATURE:
(A) NAME/KEY: M~; f; ~-site
(B) LOCATION:3
(D) OTHER INFORMATION:/product= "Asn-NH2"
(Xi) ~ U~N~ DESCRIPTION: SEQ ID NO: 80:
-
Xaa Xaa Xaa
CA 02227516 1998-01-21
W O 97108193 PCT/EP96/03473
-178-
(2) INFO_~MATION FOR SEQ ID NO: 8l: A
( i ) ~U~'N~ ~T-7AR~rrT~RT~TIcs
(A) LENGTH: 3 amino acids
(B) TYPE: amino acid
(C) S'lTRZ~N1)~:1)N~ : single
(D) TOPOLOGY: linear
(ii) M~T.7~CT7T.~ TYPE: peptide
(ix) FEATURE:
(A) NAME/REY: M~;f;e~-site
(B) LOCATION:l
(D) OTHER INF~RM~rrInN /~1UdUCL -
"N--trans-3,4-d;lly~Lo~sy--cinnAm~yl--(O-P--Tyr)"
/note= "O-P-Ty-r stands for O-P1lo~yhollo-L-Tyr"
(ix) ~EATURE:
(A) NAME/KEY: M~ifif~-site
(B) LOCATION:2
(D) O'rHER rNF~RMA'rT~N:/product=
"-2-amino-2-no~ ,..,An~-car~onyl-"
/note= "R~ l of 2-amino-2-,~n,lA~,"An~ cArh~ni~ acid"
(ix) FEATURE:
(A) NAME/~ Y: M~ifi~-site
(B) LOCATION:3
(D) OTHER INFORMATION:/~r~ucL- "Asn-NH2"
(Xi) ~ :N( ~: DESCRIPTION: SEQ ID NO: 81:
Xaa Xaa Xaa
CA 02227516 1998-01-21
WO 97/08193 PCT~P96/03473
-179-
(2) INFORMATION FOR SEQ ID NO: 82:
( i ) ~QU~N~' CHARACTERISTICS:
(A) LENGTH: 2 amino acids
(B) TYPE: amino acid
(C) STRA~ :l)N~:.5.5: single
(D) TOPOLOGY: linear
(ii) M~T~crlT~ TYPE: peptide
(ix) FEATURE:
(A) NAME/REY: Mn~; f;e~-site
(B) LOCATION:l
(D) OTHER INFORMATION: /~L~U~ L-
"N-trans-3,4-dil-ydL~y-cinn~m~yl-(o-p-Tyr)"
/note= "O-P-Tyr stands for O-Phnsrhnnn-L-Ty-r"
(ix) FEATURE:
(A) NAME/REY: ~n~;f;~ -site
(B) LOCA~ION: 2
(D) OTHER INFORM~TT~N: /product=
"--2--amino--2--n~-~hnl-n~n~--carbonyl--"
/note= "R~ l of 2-amino-2-nnrhn~n~n~ carboxylic acid;
epimer
1"
(Xi) ~U~N~h' DESCRIPTION: SEQ ID NO: 82:
Xaa Xaa
(2) INFORMATION FOR SEQ ID NO: 83:
U~N~h' CEl~RACTERISTICS:
(A) LENGTH: 2 amino acids
CA 022275l6 l998-0l-2l
WO 97/08193 PCTAEP96/03473
-180-
(B) TYPE: amino acid
(C) STRAN~N~:SS: single
(D) TOPOLOGY: linear
( ii ) M~T-~lT.~ TYPE: peptide
(ix) FEATURE:
(A) NAME/REY: M~; f; ~-site
(B) LOCATION:l
(D) OTHER INFORMATION: /~lVdU~ L-
"N-trans-3,4-dillydlv~y-c;nn-~yl-(o-p-Tyr)~
/note= "O-P-Ty-r stands for O-phnsphnnn-L-Tyr~
(ix) FEATURE:
(A) NAME/REY: Mn~; f;~-site
(B) LOCATION:2
(D) OTHER INF~RM~T~N:/~lvdu~--
"-2-amino-2-"~..l~,..~ne-carbonyl"
/note= "Bivalent radical of 2-amino-2-norbornane
carboxy-lic acid
(epimer 2)"
(xi) ~U~N~ DESCRIPTION: SEQ ID NO: 83:
Xaa Xaa
(2) INFORMATION FOR SEQ ID NO: 84:
(i) ~U~N~ CHARACTERISTICS:
(A) LENGTH: 3 amino acids
(B) TYPE: amino acid
(C) ST~ANn~nN~-~S: single
(D) TOPOLOGY: linear
-
CA 022275l6 l998-0l-2l
WO 97/08193 PCT/EP96/03473
- 181 -
~, .
(ii) MOLECULE TYPE: peptide
(ix) FEATURE:
(A) NAME/KEY: Mn~;fiF~--site
(B) LOCATION:l
(D) OTHER INFORMATION:/product=
"N--trans--4--hy~lL~y--~;nn. ~-~yl--(O--P--Ty-r)~'
/note= "O--P--Tyr stands for O--Phnsphnnn--L--Tyr"
(ix) FEATt~RE:
(A) NAME/KEY: Mntl;f;~l_site
(B) LOCATION:3
(D) OTEiER INFORMATION:/product= "Asn-NH2"
(xi) ~;OU~;N~ T~.C;~'RTPTION SEQ ID NO: 84:
Xaa Ile Xaa
(2) rNFORMATION FOR SEQ ID NO: 85:
U~;N~; t~r~R~r~csTIcs:
(A) T.F~-~r-r: 2 amino acids
(B) TYPE: amino acid
(C) SIrRANI~:I)N~:.C;S: single
(D) TOPOLOGY: linear
(ii) ~T ~crlT~ TYPE: peptide
(ix) FEATURE:
(A) NA~qE/XEY: ~In~l;f;~--site
~ (B) LOCATION:l
(D) OTHER rNFORMATION: /~L ~xlu~; L--
"N--6-llyclLo~y-2-naphthoyl-(0-P-Tyr) "
CA 022275l6 l998-0l-2l
WO 97/08193 PCTAEP96/03473
-182-
/note= ''O-P-Ty-r stands for O-Phosphono-L-Ty-r
(ix) FEATURE:
(A) NAME/KEY: M~;f;~-site
( B ) T~;~TT~N: 2
(D) OTHER INFORMATION: /~l~U~ L- "Ile-NH2"
(Xi) ~ 2UlSN~ T~ 'RTT->TION: SEQ ID NO: 85:
Xaa Xaa
(2) INFORMATION FOR SEQ ID NO: 86:
(i) ,~:QII~:N~:~: ~AR~T~RISTICS:
(A) LENGTH: 2 amino acids
(B) TYPE: amino acid
(C) S~RAN~ .S: single
(D) TOPOLOGY: linear
(ii) MwT.~CTlT.~ TYPE: peptide
(ix) FEATURE:
(A) NAME/REY: M~;f;f~-site
(B) LOCATION:l
(D) OTHER INFORMATION:/~.v~u~L-
"N-trans-3,4-d;hydl~y-~;nn~m~yl-(o-p-Tyr)"
/note= "O-P-Ty-r stands for O-Ph~srhnn~-L-Tyr"
(ix) FEATURE:
(A) NAME/~ Y: M~;f;~A-site
(B) LOCATION:2
(D) OTHER INFORMATION:/product= "Gln-NH2"
CA 02227516 1998-01-21
W O 97/08193 PCTAEP96/03473
-183-
.,
~ (xi) SEQUENCE DESCRIPTION: SEQ ID NO: 86:
.
Xaa Xaa
(2) INFORMATION FOR SEQ ID NO: 87:
( i ) ~;5.~U~;N(~ '~ARAr~ RIsTIcs:
(A) IENGTH: 2 amino acids
(B) TYPE: amino acid
( C ) STRAN~ l )N~:-C S: single
(D) TOPOLOGY: linear
( ii ) M~Tl~crlT~ TYPE: peptide
(ix) FEATURE:
(A) NAME/KEY: Mn~;f;f~-site
(B) LOCATION:l
(D) OTHER INFORMATION: /~l~U~ L-
''N-trans-3,4-dilly~l~u~y-~-inn~ yl-(O-P-Ty~r)"
/note= "O-P-'~yr stands for O-ph~ph~n~-L-Tyr
(ix) FEATURE:
(A) NAME/KEY: M~;fi~-site
( B ) T~A'T'TON 2
(D) OTHER INFORMATION:/~I~u~L- "Glu-NH2"
(Xi) ~U~N~ DESCRIPTION: SEQ ID NO: 87:
Xaa Xaa
s
(2) INFORMATION FOR SEQ ID NO: 88:
( i ) ~yU~N~ CHARACTERISTICS:
CA 022275l6 l998-0l-2l
W O 97/08193 PCTrEP96/03473
-184-
(A) LENGTH: 3 amino acids
(B) TYPE: arnino acid
(C) S~R~N~ N~ : single
(D) TOPOLOGY: line ~
(ii) ~T.~crlT.T~ TYPE: peptide
(ix) FEAIURE:
(A) NAME/REY: M~ifi e~-site
(B) LOCATION:l
(D) OTHER INF~RMATT~N: /product=
"N-trans-3,4-dillydl~y-~inn~m~yl-(O-P-Tyr)"
/note= "O-P-Tyr stands for O-Ph~sphnn~-L-Tyr"
(ix) FEATURE:
(A) NAME/REY: M~ifi~ - site
(B) LOCATION:3
(D) OTHER rNF~RM~TT~: /product= "D-h~m~ph~nylalanyl"
/note= RA~;~A1 of D r~ L~h~nylAl An i n~
(Xi) ~QU~N~ DESCRIPTION: SEQ ID NO: 88:
Xaa Glu Xaa
(2) INF~RM~TO~ FOR SEQ ID NO: 89:
u~ ~ARA~T~RT~TIcs:
(A) LENGTH: 2 amino acids
(B) TYPE: amino acid
(C) STRANI)~:l)N~ : single
(D) TOPOLOGY: linear
(ii) M~T~T~cuT~ TYPE: peptide
CA 022275l6 l998-0l-2l
W O 97/08193 PCT/EP96/03473
-185-
(ix) FEATURE:
(A) NAME/KEY: MnA; f; ~ -site
(B) LOCATION:l
(D) OTHER INFORMATION:/product=
"N-trans-3,4-di1-ydl~y-~; nn -~yl-(O-P-Tyr)"
/note= "O-P-Tyr stands for O-phnq~hnnn-L-Ty-r~
(ix) FEATURE:
(A) NAME/KEY: Mn~;fi~ -site
(B) LOCATION:Z
(D) OTHER INFO~MATT~N:/product= "D-homophenylalanyl-NH2"
/note= "RA~;~A1 of D-homophenylAlAn;nr ,~
(xi) ~Q~N~ DESCRIPTION: SEQ ID NO: 89:
Xaa Xaa
(2) INFORMATION FOR SEQ ID NO: 90:
( i ) ~U~N~ CH~R~RT-~TICS:
(A) LENGTH: 2 amino acids
(B) TYPE: amino acid
(C) STRAN~ N~:~S: single
(D) TOPOLOGY: linear
( ii ) M~T.~CTlT-~ TYPE: peptide
(ix) FEATURE:
(A) NAME/KEY: M~; f; ~-site
(B) LOCATION:l
(D) OTHER INFORMATION:/product=
"N--trans--3,4-dil-yd~ y-c;nnA ~ yl--(O--P--Tyr)"
/note= "O-P-Tyr stands for O-Ph~-qph~n~-L-Tyr"
CA 02227516 1998-01-21
W O 97/08193 PCT~EP96/03473
-186-
(ix) FEATURE:
(A) NAME/~ Y: Mn~;fi~-site
(B) LOCATION:2
(D) OTHER rNFORMATION:/product=
"l-aminocyclopentane-carbonyl"
/note= "R~ l of l-aminocyclop~nt~n~ calL~ylic acid"
(xi) ~UhN~ nT~ RTPTION: SEQ ID NO: 90:
Xaa Xaa
(2) INFnRMA~ToN FOR SEQ ID NO: 9l:
(i) ~U~:N~ CHARAL~ CS:
(A) LENGTH: 2 amLnO acids
(B) TYPE: amino acid
(C) STRA~~ S: single
(D) TOPOLOGY: linear
(ii) M~T~cT~ TYPE: peptide
(ix) FEATURE:
(A) NAME/~ Y: M~ifi~-site
(B) T~A~TO~:l
(D) OTHER INFORMATION:/~l~u~-
"N-trans-3,4-dilly.l-~,~y-~~;nn~ yl-(0-P-Ty~) "
/note= "O-P-Tyr stands for O-Phosphono-L-Ty~
(ix) FEATURE:
(A) NAME/~ Y: M~;f;~-site
(B) LOCATION:2
(D) OTHER INFORMA~TO~:/~lo~u~L- "Pro-NH2"
CA 02227516 1998-01-21
WO 97/08193 PCTAEP96/03473
-187-
(Xi) ~:~Uh'N~ DESCRIPTION: SEQ ID NO: 9l:
Xaa Xaa
(2) INFORMATION FOR SEQ ID NO: 92:
(i) ~f~U~Nf_~ CHARACTERISTICS:
(A) LENGTH: 3 amino acids
(B) 'l~Y~E: amino acid
(C) STR~N~ l)N~ s: single
(D) TOPOLOGY: linear
(ii) MfT~T~cTlT~ TYPE: peptide
(ix) FEATURE:
(A) NAME/REY: ~f~;f;f-~-site
(B) LOCATION:l
(D) OTHER INFORMATION:/product= "N-2-naphthoyl-(O-P-Tyr)"
/note= "O-P-Tyr stands for O-P11o~hono-L-Tyr"
(ix) FEATURE:
(A) NAME/KEY: M~;f;~-site
(B) LOCATION:3
(D) OTHER INFORMATION:/yl~u~L- "Asn-NH2"
(xi) ~fU~Nf_~ ~.~f K T ~llfN: SEQ ID NO: 92:
Xaa Ile Xaa
..
(2) INFORMATION FOR SEQ ID NO: 93:
~ QU~:Nf_~ f-~AR~f-.T~RlSTICS:
CA 022275l6 l998-0l-2l
W O 97/08193 PCT~EP96/03473
-188-
(A) LENGTH: 3 amino acids
(B) TYPE: amino acid
(C) S~RAN~ NI~ s single
(D) TOPOLOGY: linear
(ii) M~T.~IT.~ TYPE: peptide
(ix) FEATURE:
(A) NAME/REY: M~;f;~-site
(B) LCCATION:l
(D) OTHER INFORMATION: /yl~u~L -
"N-(l-~m~ntoyl)-(O-P-Tyr)"
/note= "O-P-Tyr stands for O-Phosphono-L-Tyr"
(ix) FEATURE:
(A) NAME/REY: M~;f;~-site
(B) LCCATION:3
(D) OTHER INFORMATION:/~ ~u~L- "Asn-NH2"
(Xi) .~:~IJ~:N('~: n~RTpTIoN SEQ ID NO: 93:
Xaa Ile Xaa
(2) INFORMATION FOR SEQ ID NO: 94:
( i ) ~QU~N~ CHARACTERISTICS:
(A) LENGTH: 3 am.ino acids
(B) TYPE: arnino acid
(C) S~RANI)~ N~:~S: single
(D) TOPOIOGY: linear
(ii) M~T-~CrlT~ TYPE: peptide
CA 022275l6 l998-0l-2l
W O 97/08193 PCT/EP96/03473
-189-
(ix) FEATURE:
(A) NAME/KEY: Mn~;fie~-site
(B) LOCATION:l
(D) OTHER nNFoR~M~ATIoN:/product=
"N-cyclnh~nnyl-(O-P-Tyr)"
/note= "O-P-Tyr stands for O-Phosphono-L-Tyr"
(ix) FEATURE
(A) NAM~/KEY: M~if;~-site
(B) LOCATION:3
(D) OTHER INF~RM~TT~:/~.o~u~L- "Asn-NH2"
(xi) ~U~N~ ~T'.s~RTPTION: SEQ ID NO: 94:
Xaa Ile Xaa
(2) INFORMATION FOR SEQ ID NO: 95:
(i) ~U~ ~R~T~RT~TICS
(A) LENGTH: 2 amino acids
(B) TYPE: amino acid
(C) sT-R~NnTmN~s: single
(D) TOPOLOGY: linear
(ii) ~T~T~c~T~. TYPE: peptide
(ix) FEATURE:
(A) NAME/KEY: ~;f;~-site
(B) LOCATION:l
(D) OTHER INFORMATION: /~lV~U~ ~ -
"N-(3-cyclohexyl-propionyl)-(O-P-Tyr)"
/note= "O-P-Tyr stands for O-phns~hnnn-L-Tyr
..
(ix) FEATURE:
CA 02227516 1998-01-21
W O 97/08193 PCTAEP96/03473
-190-
(A) NAME/KEY: M~;f;e~-site
(B) LCCATION:2
(D) OTHER INFORMATION: /~l~U~ L- "Ile-NH2"
(Xi) ~U~N~ DESCRIPTION: SEQ ID NO: 95:
Xaa Xaa
(2) INF~RMATT~N FOR SEQ ID NO: 96:
( i ) ~ U~N~ CHARA~T~RT~TICS:
(A) LENGTH: 2 amino acids
(B) TYPE: amino acid
(C) STRAN~ )N~:~: single
(D) TOPOLOGY: line ~
(ii) M~r~crlr~ TYPE: peptide
(ix) FEATURE:
(A) NAME/REY: M~;f;~-site
(B) LOCATION:l
(D) OTHER INF~RMA~TON:/product=
"N-l,2,3,4-tetrahydro-2-n~ph~hnyl-(O-P-Tyr)"
/note= "O-P-Tyr stands for O-Ph~sph~n~-L-Tyr"
(ix) FEATURE:
(A) NAME/KEY: Modified-site
(B) LOCATION:2
(D) OT~ER INFORMATION:/~u~L- "Ile-NH2"
(Xi) ~:Ou~N~ DESCRIPTION: SEQ ID NO: 96:
Xaa Xaa
CA 022275l6 l998-0l-2l
WO 97/08193 PCT~EP96/03473
-191 -
(2) INFORMATION FOR SEQ ID NO: 97:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 2 amino acids
(B) TYPE: amino acid
(C) STR~Nn~n~S: single
(D) TOPOLOGY: linear
( ii ) M~T~T~crlT~ TYPE: peptide
(ix) FEAIURE:
(A) NAME/KEY: M~; f;~A-site
(B) LOCATION:l
(D) OTHER INFORMATION~ u~L-
"N--cy~l oh~rAn~yl--( O--P--Tyr ) "
/note= "O-P-Tyr stands for O-ph~sph~n~-L-Tyr
(ix) FEATURE:
(A) NAME/KEY: M~;f;~-site
(B) LOCATION:2
(D) OTHER INFORWATION:/~.~u~L- "Ile-NH2"
(Xi) ~U~N~ D~PTPTION: SEQ ID NO: 97:
Xaa Xaa
(2) INFORMATION FOR SEQ ID NO: 98:
U~N~ CHARACTERISTICS:
(A) LENGTH: 2 amino acids
(B) TYPE: amino acid
(C) sTRANn~nN~-s-~: single
CA 02227516 1998-01-21
W O 97/08193 PCT/EP96/03473
-192- v
(D) TOPOLOGY: linear
(ii) M~T~cr~ TYPE: peptide
(ix) FEATURE:
(A) NAME/~ Y: M~ifie~-site
(B) LOCATION:l
(D) OT~ER INFORMATION:/~udu~L-
"N-(2-naphthoyl)-(O-P-Tyr)"
/note= "O-P-Tyr stands for O-Ph~sph~n~-L-Tyr"
(ix) FEATURE:
(A) NAME/~ Y: M~; f;~-site
(B) LOCATION:2
(D) OT~hR INFORMATION:/p.ulu~- "Ile-NH2"
(Xi) ~yu~N~ n~rRTrJTIoN: SEQ ID ~O: 98:
Xaa Xaa
(2) rNFoRM~Tn~ FOR SEQ ID NO: 99:
( i ) ~U~N~ rr~RAr~RISTICS:
(A) LENGTH: 2 arnino acids
(B) TYPE: amino acid
(C) STR~N~ N~s~S: single
(D) TOPOLOGY: linear
(ii) ~r~cr~ TYPE: peptide
(ix) FEATURE:
(A) NAME/KEY: ~;f;~d-site
(B) LOCATION:l
CA 02227516 1998-01-21
W O 97/08193 PCT~P96/03473
-193-
(D) OTHER INFORMATION:/product=
"N-(l-adamantoyl)-(O-P-Tyr)"
/note= "O-P-Tyr stands for O-Phnsrh~n~-L-Tyr"
(ix) FEATURE:
(A) NAME/KEY: Mn~;f;~-site
(B) LOCA~ION:2
(D) ~l~LK INFORMATION: /~L~U~- L- "Ile-NH2"
(Xi) ~QU~N~ DESCRIPTION: SEQ ID NO: 99:
Xaa Xaa
(2) INFORMATION FOR SEQ ID NO: lO0:
( i ) ~QU~N~ CHARACTERISTICS:
(A) LENGTH: 2 amino acids
(B) TYPE: amino acid
(C) sT~ANn~N~s: single
(D) TOPOLOGY: linear
(ii) M~T.T~ T.T~ TYPE: peptide
(ix) FEATURE:
(A) NAME/KEY: M~;f;f~-site
(B) LCCATION:l
(D) OTHER INFORMATION:/product=
"N-(4-acetylamino-benzoyl)-(O-P-Tyr)"
/note= "O-P-Tyr stands for O-ph~srhnn~-L-Tyr
(ix) FEATURE:
(A) NAME/KEY: ~;fi~-site
(B) LOCATION:2
(D) OTHER INFORMATION:/product= "Ile-NH2"
CA 02227516 1998-01-21
W O 97/08193 PCTAEP96/03473
-194-
(Xi) ~QU~N~ DESCRIPTION: SEQ ID NO: l00:
Xaa Xaa
(2) INF~RMATT~ FOR SEQ ID NO: l0l:
( i ) ~U~N~ ~R~T~RT~TIcs:
(A) LENGTH: 3 amino acids
(B) TYPE: amino acid
(C) STR~N~ I)N~:~S: single
(D) TOPOLCGY: linear
( ii ) M~T~cuT~ TYPE: peptide
(ix) FEATURE:
(A) NAME/KEY: Mn~i fi~d-site
(B) LOCATION:l
(D) OTHER INFORMATION:/~u~-
"N--( Sll~C; n~lT~yl )--( O--P--Tyr ) "
/note= "O-P-Tyr stands for O-ph~sph~n~-L-Tyr~
(ix) FEATURE:
(A) NAME/KEY: M~;f;~-site
(B) LOCATION:3
(D) aT~R INFORMATION: /~l~U~ - "Asn-NH2"
(Xi) ~U~N~ D~.~RTPTION: SEQ ID NO: l0l:
Xaa Ile Xaa
( 2 ) INFORMATT~N FOR SEQ ID NO: l02:
CA 022275l6 l998-0l-2l
WO97/08193 PCTAEP96/03473
-195-
U~N~ CH~RACTERISTICS:
(A) LENGTH: 3 amino acids
(B) TYPE: amino acid
(C) S~R~N~ l)N~:~: single
(D) TOPOLOGY: linear
(ii) M~T.~CrlTl~ lY~E: peptide
(ix) FEATURE:
(A) NAME/KEY: M~; f; ~ - site
(B) LOCATION:l
(D) OTHER INF~RMA~TON:/~l~L-
"N-1,2,3,4--tetrahydro-2-nAph~h~yl--(O-P--Tyr)"
/note= "O-P-Tyr stands for O-ph~sph~n~-L-Tyr~
(ix) FEATURE:
(A) NAME/KEY: ~n~; f; ~ - site
(B) LOCATION:3
(D) OTHER rNFnRMA~T~N:/product= "Asn-NH2"
(Xi) ~U~N~ n~RTT~TIoN SEQ ID NO: 102:
Xaa Ile Xaa
(2) INFORMATION FOR SEQ ID NO: 103:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGT~: 4 ~m; no acids
(B) TYPE: amino acid
(C) STRANn~nN~-s~: single
(D) TOPOLOGY: linear
(ii) ~T-~Cr~.~ TYPE: peptide
CA 022275l6 l998-0l-2l
W O 97/08193 PCT~EP96/03473
-196-
(ix) FEATURE:
(A) NAM~/REY: ~ifie~-site
(B) LOCATION:l
(D) OTHER INFORMATION:/product=
"N-l,2,3,4-tetrahydro-2-naphthoyl-(O-P-Tyr)"
/note= "O-P-Tyr stands for O-E~ h~ ~-L-Tyr~
(ix) FEATURE:
(A) NAME/KEY: ~;f;~-site
(B) T~TT~N 4
(D) OTHER INFORMATION:/product= "Pro-NH2"
(Xi) ~yU~N~ n~.~Rl~TIoN: SEQ ID NO: 103:
Xaa Ile Ile Xaa
(2) INFORMATION FOR SEQ ID NO: 104:
( i ) ~U~N~ CHARACTERISTICS:
(A) LENGTH: 2 aTnino acids
(B) TYPE: amino acid
(C) S'rRZ~NI~ NI~:~S: single
(D) TOPOLOGY: linear
(ii) ~wTFcTlT~F TYPE: peptide
(ix) FEATURE:
(A) NAME/REY: ~Aifi~-site
(B) LOCATION:l
(D) OTHER INFORMATION: /P1~U~ L -
"N-trans-3,4-dihydlo~y-~innAm~yl-(o-p-Tyr)l~ -
Jnote= "O-P-Tyr stands for O-Ph~hv--o-L-Tyr"
CA 02227516 1998-01- 21
WO 97/08193 PCT~P96/03473
197
(ix) FEATURE:
(A) NAME/KEY: M~ifi~-site
(B) T~TT~ 2
(D) OTHER INFORMATION: /~l~U~ L~ hnm~rh~nylalanyl-NH2~l
/note= "~ 1 of the arnide of L-h~h~nylAl~n;n~"
( Xi ) ~OU~N~ D~RTPTION: SEQ ID NO: 104:
Xaa Xaa
(2) INFORMATION FOR SEQ ID NO: 105:
( i ) ~QU~-N~ RA~T~RISTICS:
(A) LENGTH: 3 amino acids
(B) TYPE: amino acid
( C ) S'I'RANI ~ 1 )Nl~:~ S: single
(D) TOPOLOGY: linear
(ii) M~T~crlT~ TYPE: peptide
(ix) FEATURE:
(A) NAME/KEY: M~ifi~-site
(B) LOCATION:l
(D) OTHER INFORMATION:/~I~u~L-
"N-trans-3,4-dil-ydlv~y-cinn~m~yl-(O-P-Tyr)"
/note= ''O-P-Ty-r stands for O-Phosphono-L-Ty-r"
(ix) FEATURE:
(A) NAME/KEY: ~ifie~-site
(B) L~CATION:3
(D) OTHER INFORMATION:/product= "Asn-NH2"
CA 02227516 1998-01-21
W O 97/08193 PCT~EP96/03473
-198-
( Xi ) ~QU~N~' DESCRIPTION: SEQ ID NO: 105:
Xaa Ile Xaa
(2) INFORMATION FOR SEQ ID NO: 106:
( i ) ~U~N~ r~ARAr.T~RT.~TICS:
(A) ~ENGTH: 3 amino acids
(B) lY~E: amino acid
(C) STRAN~ N~:~S: single
(D) TOPOLOGY: linear
( ii ) M~T.T~'~lT.~ TYPE: peptide
(ix) FEATURE:
(A) NAME/REY: M~ i f ~ site
(B) T~rATTnN l
(D) OTHER INFORMATION: /~l~U~ L-
''N-trans-3~4-d~ydl~y-~;nn~m~yl-(o-p-Ty-r)~
/note= "O-P-Ty-r stands for O-Phospho-l-Ty-r''
(ix) FEATURE:
(A) NAME/REY: Mn~if;~ - site
(B) T~rATT~ 3
(D) OT~ER INFORMATION:/~ludu~L- "beta-alanyl-NH2"
/note= "Beta-alanyl-NH2 stands for -NH-CH2-CH2-C(=O)-NH2"
(Xi) ~U~N~ n~rRTpTIoN: SEQ ID NO: 106:
Xaa Ile Xaa
(2) INFORMATION FOR SEQ ID NO: 107:
CA 02227516 1998-01-21
WO 97/08193 PCT~EP96/03473
-199-
QU~N~ CHARACTERISTICS:
(A) LENGTH: 3 aTnino acids
(B) TYPE: aT~nino acid
(C) STE~AN I )~:I)N~ S: siTlgle
(D) TOPOLOGY: linear
(ii) ~T~T~CTlT~ lYPE: peptide
(ix) FEATURE:
(A) NAME/KEY: ~n~if;~-site
(B) LOCATION:l
(D) OTHER INFORM~TO~ U~ L--
"N-trans-3,4-dillydl~y-cinnamoyl-(o-p-Tyr)"
/note= "O-P-Tyr stands _or O-ph~phnn~-L-Tyr
(ix) FEATURE:
(A) NAME/KEY: ~;f;~-site
(B) LOCATION:3
(D) OTHER INFORMATION:/product= "Gln-NH2"
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 107:
Xaa Ile Xaa
(2) INFORMATION FOR SEQ ID NO: 108:
:QU~N~ CHARACTERISTICS:
(A) LENGTH: 3 amino acids
(B) TYPE: aTnino acid
(C) S~R~N~ N~ S: single
(D) TOPOLOGY: linear
- ( ii ) ~TFcTlT~T~' TYPE: peptide
CA 02227516 1998-01-21
W O 97/08193 PCT~EP96/03473
- ~00 -
(ix) FEATURE:
(A) NAME/ ~ Y: Mn~;fiF~-site
(B) LCCATION:l
(D) OTHER INFORMATION:/~l~u~L-
"N--trans--3~4--dil~y~J~y--~innAmnyl--(o--P--Tyr) "
/note= "O-P-Tyr stands for O-Ehnsrhnnn-L-Tyr"
(ix) FEATURE:
(A) NAME/ ~ Y: Mn~ifi~-site
(B) LOCATION:3
(D) OTHER INFORMATION: /~l~U~ ~ - "Gly-NH2"
(xi) ~U~N~ n~rRTPTION: SEQ ID NO: 108:
Xaa Ile Xaa
(2) INFORMATION FOR SEQ ID NO: 109:
(i) ~u~N~ ~R~T~.RT~TICS
(A) LENGTH: 3 amino acids
(B) TYPE: amino acid
( C ) STR~N~ 1 )Nl'cls single
(D) TOPOLOGY: line ~
( ii ) M~T.T'CrlT.T'. TYPE: peptide
(ix) FEATURE:
(A) NAME/ ~ Y: Mn~ifi~A-site
(B) LOCATION:l
(D) OTHER rNFORMATION:/product=
"N--trans--3~4--dil~yd~y~inni~mnyl--(L--CF2--PmE))"
/note= "L-CF2-Pmp stands for 4-PhosrhnnnAifl~lnrm~thyl-L-
Phe"
CA 02227516 1998-01-21
WO 97/08193 PCTAEP96/03473
-201-
(ix) FEATURE
(A) NAME/KEY: M~; f;~-site
(B) LOCATION:3
(D) ~TR~R rN~RM~TI~N /~I~u~L- "Asn-NH2"
(Xi) ~'QU~N~ DESCRIPTION: SEQ ID NO: l09:
Xaa Ile Xaa
-
(2) rNFORMATION FOR SEQ ID NO: ll0:
( i ) ~U~N~ CE~RACTERISTICS:
(A) LENGTH: 2 amino acids
(B) TYPE: amino acid
(C) STR~N~ l)N~:~S single
(D) TOPOLO~Y: linear
( ii ) M~T.~C~lT.~ TYPE: peptide
(ix) FEATURE:
(A) NAME/KEY: ~n~;f;~-site
(B) LOCATION:l
(D) OTk~R INFORMATION:/~l~u~-
"N--trans--3,4--dil-y~o~y--r-;nn~qlTK~yl--(L--CF2Pmp)"
/note= "L-CF2Pmp stands for 4-ph~sph~n~;f11lorm~thyl-L-
Phe"
(ix) FEATURE:
(A) NAME/KEY: Modified-site
(B) LOCATION:2
(D) OTHER INFORMATION:/~.~u~- "Ile-NH2"
CA 02227516 1998-01-21
W O 97/08193 PCT~EP96/03473
-202-
( Xi ) ~U~N~ DESCRIPTION: SEQ ID NO: 110:
Xaa Xaa
(2) INFORMATION FOR SEQ ID NO: 111:
( i ) ~;S2U~;-N~ 'r~RAt'~F~RT.c~TICs
(A) T.~N~,Tr~: 3 amino acids
(B) TYPE: amino acid
(C) S~R;~NI)I~:I)N~ S: single
(D) TOPOLOGY: linear
( ii ) ~Tl~cr~T~ TYPE: peptide
(ix) FEATURE:
(A) NAME/KEY: Mn~i f; ~ - site
(B) LOCATION:l
(D) OT~ER INFORMATION:/product=
"N-( 3 _~m; nnh~n 7yloxycarbonyl)-(O-P-Tyr)"
/note= "O-P-Tyr stands for O-phnsrhnnn-L-Tyr~
(ix) FEATURE:
(A) NAME/REY: Mn~; f; e~-site
(B) LocaTIoN:3
(D) OTHER INFORMATION:/~L~L- "Asn-NH2"
(Xi) ~U~N~ n~RTpTIoN: SEQ ID NO: 111:
Xaa Met Xaa
(2) INFORMATION FOR SEQ ID NO: 112:
(i) SEQUENCE ~AR~RT~TICS:
CA 022275l6 l998-0l-2l
W O 97/08193 PCTAEP96/03473
-203-
(A) LENGTH: 4 arnino acids
(B) TYPE: amino acid
(C) STR~Nn~nN~S: single
(D) TOPOLOGY: linear
(ii) M~T.~CTIT.~ TYPE: peptide
(ix) FEATURE:
(A) NAME/KEY: Mn~;f;~-site
(B) LOCATION:1
(D) OTHER INFORMATION: /pl~U~ L -
"N-(indole-5-carbonyl)-Tyr"
(ix) FEATURE:
(A) NAME/KEY: M~;f;~-site
(B) LOCATION:4
(D) OTHER INFORMATION:/product= "Asn-NH2"
(Xi) ~:~U~N~ DESCRIPTION: SEQ ID NO: 112:
Xaa Ile Asn Xaa
(2) INFORMATION FOR SEQ ID NO: 113:
'52U~N~:~; CE~ARA~"I'F~RT~TICS:
(A) LENGTH: 3 amino acids
(B) TYPE: amino acid
(C) sTRANn~nN~.~s: single
(D) TOPOLOGY: linear
(ii) MwT~crJT~r~ TYPE: peptide
(ix) FEATURE:
CA 02227516 1998-01-21
W O 97/08193 PCTAEP96/03473
-204-
(A) NAME/REY: M~;f;~-site
(B) LOCATION:l
(D) OTHER rNFORMATION:/product=
"N-cyclohexylc~rhnnyl-(O-P-Tyr)"
/note= "O-P-Tyr stands for O-phnsphnnn-L-Tyr~
(ix) FEATURE:
(A) NAME/REY: M~ifi~-site
(B) LOCATION:3
(D) OTHER INFORMATION: /plV~U~ L- "Asn-NH2"
(xi) ~U~N~ DESCRIPTION: SEQ ID NO: 113:
Xaa Ile Xaa
(2) INFORMA~T~N FOR SEQ ID NO: 114:
~U ~:N~ ~R~.T~RISTICS:
(A) LENGTH: 3 amino acids
(B) TYPE: amino acid
(C) STRAN~ ,)N~:~S: single
(D) TOPOLOGY: linear
(ii) M~T.~.cr~.~ TYPE: peptide
(ix) FEATURE:
(A) NAME/REY: M~ifi~-site
(B) LOCATION:l
(D) OTHER rNFORMATION:/product=
"N-(2-naphthylc~rh~nyl)-(O-P-Tyr)"
/note= "O-P-Tyr stands for O-phnsph~nn-L-Tyrl'
(ix) FEATURE:
(A) NAME/KEY: Modified-site
CA 022275l6 l998-0l-2l
W O 97/08193 PCT/EP96/03473
-205-
_ (B) LOCATION:3
(D) OTHER INFORMATION:/product= "Asn-NH2"
(xi) S~r)~:N(_~: DESCRTTYrToNs SEQ ID NO: 114:
Xaa Ile Xaa
(2) INFORMATION FOR SEQ ID NO: 115:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 3 amino acids
(B) TYPE: amino acid
( C ) S'iTRZ~NI )~':1 )N~:~SS single
(D) TOPOLOGY: linear
( ii ) M~T.~CIJT.~ ~l~Y~E: peptide
(ix) FEATURE:
(A) NAME/KEY: M~if;~-site
(B) LOCATION:l
(D) OT~ER INFORMATION:/product=
"N-(indole-5-yl-carbonyl)-(L-CF2Pmp)"
/note= "L-CF2Pm~ stands for 4 - Phnsphnnn~; fl uorm~thyl-L-
Phe"
(ix) FEATURE:
(A) NAME/KEY: Mn~;f;~-site
(B) LOCATION:3
(D) OTHER INFORMATION:/product= "Asn-NH2"
-
(Xi) ~U~N~ DESCRIPTION: SEQ ID NO: 115:
Xaa Ile Xaa
CA 022275l6 l998-0l-2l
W O 97/08193 PCTAEP96/03473
-206-
(2) INFORMATION FOR SEQ ID NO: 116:
( i ) ~U~N~ CH~RA~RT.~TICS:
(A) LENGTH: 3 amino acids
(B) TYPE: amino acid
(C) STR AN I )~: I )N ~:~ S single
(D) TOPOLOGY: linear
( ii ) MnT~CrlT~ TYPE: peptide
(ix) FEATURE:
(A) NAME/REY: M~;f;~-site
(B) T~TTON:1
(D) OTHER INFORMATION: /~l~dUC L-
"N-(9-fluorenyl-carbonyl)-(O-P-Tyr)"
/note= "O-P-Tyr stands for O-ph~sph~n~-L-Tyr
(ix) FEATURE:
(A) NAME/KEY: M~;f;~-site
(B) T~T~ 3
(D) OTHER INFnRMA~TnN:/product= "Asn-NH2"
(Xi) ~U~N~ n~.~RTT'TION: SEQ ID NO: 116:
Xaa Ile Xaa
(2) INFORMATION.FOR SEQ ID N-O: 117:
:N('~: ~RA~RT~TIcs:
(A) LENGTH: 3 amino acids
(B) TYPE: amino acid
(C) STRANn~nN~S: single
CA 022275l6 l998-0l-2l
W O 97/08193 PCT~EP96/03473
-207-
s (D) TOPOLOGY: linear
( ii ) M~T.~CrJT.T~ TYPE: peptide
(ix) FEATURE:
(A) NAME/KEY: Mn~;f;~-site
(B) Tl~'l'T~N
(D) OTHER INFORMATION: /~lU~U~ L-
"N-(3-~m;n~h~n~yloxycarbonyl)-(O-P-Tyr)"
/note= "O-P-Tyr stands for O-Phosph~n~-L-Tyr"
(ix) FEATURE:
~A) NAME/KEY: ~M~; f;~-site
(B) T~N 3
(D) OTHER INFORMATION:/product= "Asn-NH2"
(Xi) ~U~N~ DESCRIPTION: SEQ ID NO: 117:
Xaa Gln Xaa
(2) INFORMATTO~ FOR SEQ ID NO: 118:
U~;N~ r~p~Rz~ i;~sTIcs
(A) LENGTH: 3 amino acids
(B) TYPE: am~no acid
( C ) S~RANI )~:I )N~:~S: single
(D) TOPOLOGY: linear
( ii ) M~T~T~crJT~ TYPE: peptide
(ix) FEATURE:
- (A) NAME/KEY: Mr~;f;~-site
(B) LOCATION:l
CA 02227516 1998-01-21
W O 97/0~193 PCT~EP96/03473
-208-
(D) OTHER INFORMATION:/product=
"N-(3-Am;nnh~n~yloxycarbonyl)-(o-p-Tyr)"
/note= "O-P-Tyr stands for O-Phnsrhnnn-L-Tyr"
(ix) FEATURE:
(A) NAME/KEY: M~;f;~-site
(B) LOCATION:2
(D) uln~R INFORMATION: /~L~U~ - "L-alpha-tert-butyl-Gly"
/note= "R~ l of L-alpha-tert-~utyl-glycine"
(ix) FEATURE:
(A) NAME/KEY: Mn~ifi~-site
(B) LOCATION:3
(D) OTHER INFORMATION:/product= "Asn-NH2"
(Xi) ~U~N~' DESCRIPTION: SEQ ID NO: 118:
Xaa Xaa Xaa
(2) rNFORMATION FOR SEQ ID NO: 119:
( i ) ~QU~N~ CHARACTERISTICS:
(A) LENGTH: 3 amino acids
(B) TYPE: amino acid
(C) S~RA~ )N~:~S: single
(D) TOPOLOGY: line ~
(ii) M~T~c~ TYPE: peptide
(ix) FEATURE:
(A) NAME/KEY: Modified-site
(B) LOCATION:l
(D) OTHER INFORMATION:/product=
"N-(3-amino-benzyloxycarbonyl)-(Pmp)"
CA 022275l6 l998-0l-2l
W O 97/08193 PCTAEP96/03473
-209-
7 /note= "Pmp stands for the radical -(4-phnsphnnnm~thyl-
Phe)-"
(ix) FEATURE:
(A) NAM~/KEY: M~;f;~-site
(B) LCCATION:3
(D) OTHER INFORMATION:/product= "Asn-NH2"
(xi) ~yU~N~ DESCRIPTION: SEQ ID NO: ll9:
Xaa Ile Xaa
(2) rNFORMATION FOR SEQ ID NO: 120:
( i ) ~U~N~ CHaRACTERISTICS:
(A) LENGTH: 3 aTnino acids
(B) TYPE: amino acid
single
(D) TOPOLOGY: linear
(ii) M~T.~CT~.~ TYPE: peptide
(ix) FEATURE:
(A) NAME/KEY: Modified-site
(B) LOCATION:l
(D) OTHER INFORMATION:/product=
"N-( 3_~m; nnh~n~yloxycarbonyl)-(O-P-Tyr)"
/note= "O-P-Tyr stands for O-Phosphono-L-Tyrosine"
(ix) FEATURE:
(A) NAME/KEY: Mn~;f;~-site
(B) LOCATION:2
(D) OTHER INFORMATION: /~l~U~ ~ -
" l-aTninocycl nh.-~n~_carbonyl"
CA 02227516 1998-01-21
W O 97/08193 PCTrEP96/03473
-210-
/note= "RAA;~Al of l-aminocycl~h~An~ carboxylic acid"
(ix) FEATURE:
(A) NAMEJ~ Y: M~A;f;~-5ite
(B) LOCATION:3
(D) OT~ER rNFORMA~T~N: /~lV~U~ L- "Asn-NH2"
(xi) ~yu~N~ DESCRIPTION: SEQ ID NO: 120:
Xaa Xaa Xaa
(2) INFO~MATION FOR SEQ ID NO: 121:
( i ) ~;I~U~;N~ ; CEI~At~'iTF~RT~TIcs:
(A) LENGTH: 3 amino acids
(B) TYPE: amino acid
(C) S'7TRAN~ 1)N~:~iS single
(D) TOPOLOGY: linear
(ii) M~T.~C~IT.~ TYPE: peptide
(lX) FEATURE:
(A) NAME/REY: M~A;f;eA-site
(B) T~ATT~
(D) OTHER INFORMATION:/product= "N-acetyl-(O-P-Tyr)"
/note= "O-P-Tyr stands for O-Phosphono-L-Tyr"
(ix) FEATURE:
(A) NAME/KEY: M~A;fi~-site
(B) LOCATION:3
(D) OTHER INFORMATION:/~ ~L-
"Asn-NH-(3-naphthalen-1-ylpropyl)"
/note= llalfa-(3-Naphthalen-l-yl-propyl)amide of Asn"
CA 02227516 1998-01-21
W O 97/08193 PCTAEP96/03473
-211 -
( Xi ) ~U~N~ DESCRIPTION: SEQ ID NO: 121:
Xaa Ile Xaa
(2) INFORM~TT~N FOR SEQ ID NO: 122:
N('-': CHA~A~T~RT-'~TICS:
(A) LENGTH: 3 amino acids
(B) TYPE: amino acid
(C) sT~N~nN~s: single
(D) TOPOLOGY: linear
(ii) M~T~crlT~ TYPE: peptide
(ix) FEATURE:
(A) NAME/REY: M~; f;~-site
(B) LOCATION:l
(D) OTHER rNFORMATION:/product= "N-acetyl-(O-P-Tyr)"
/note= "O-P-Tyr stands for O-Phosphono-L-Tyr"
(ix) FEATURE:
(A) NA~ME/KEY: ~r~;f;~-site
(B) LOCATION 3
(D) OTHER INFORMATION:/~.~u~L-
"Asn--NH-(3--(2-hy~ D~y-.aphthalen--l-yl)propyl~
/note= "radical of
alfa-(N-(3-(2-hy~lo~yllaphth~l~n 1 -yl )propylamide of Asn"
( Xi ) ~U~N~ DESCRIPTION: SEQ ID NO: 122:
-
Xaa Ile Xaa
CA 022275l6 l998-0l-2l
WO 97/08193 PCTtEP96tO3473
-212-
(2) INFORMATION FOR SEQ ID NO: 123:
~: CH~R~T~RT~TICS:
(A) LENGTH: 3 amino acids
(B) TYPE: amino acid
(C) S'1'R~NI)I~ NI~ S: single
(D) TOPOLOGY: linear
( ii ) M~T-T~lT-~ TYPE: peptide
(ix) FEATURE:
(A) NAME/REY: Mn~;f;~-site
(B) LOCaTION:l
(D) OTHER INFnRM~T~M:/~l~ù~L- "N-acetyl-(O-P-Tyr)"
/note= "O-P-Tyr stands for o-ph~sph~n~-L-Tyr"
(ix) FEATURE:
(A) NAME/KEY: M~;f;~-site
(B) LOCATION:3
(D) OTHER INFORMATION:/~I~u~L-
"Asn-HN-(3-naphthalen-2-yl-propyl)"
/note= "RA~;~A1 of al_a-N-(3-n~phthAl~n-l-yl-propyl)amide
of
Asn"
(Xi) ~U~N~ n~RTpTIoN: SEQ ID NO: 123:
Xaa Ile Xaa
(2) INFORMATION FOR SEQ ID NO: 124:
( i ) ~U~N~ CHARACTERISTICS:
(A) LENGTH: 3 amino acids
(B) TYPE: amino acid
CA 02227516 1998-01-21
W O 97/08193 PCTAEP96/03473
r ~ 213 -
(C) STR~N~ )N~:~S: single
(D) TOPOLOGY: linear
(ii) M~T~F~cuT~ TYPE: peptide
(ix) FEATURE:
(A) NAME/KEY: M~;f;~-site
(B) LOCATION:1
(D) OTHER INFORM~TTnN:/product= "N-acetyl-(O-P-Tyr)"
/note= "O-P-Tyr stands for O-pllns~h~-L-Tyr~
(ix) FEATURE:
(A) NAME/REY: M~;fi~-site
(B) LOCATION:3
(D) OTHER I~FORM~T~:/product=
"Asn-HN-(3-phenanthren-9-yl-propyl)"
/note= "RA~;~Al of alfa-N-(3-phenanthren-9-yl-propyl)amide
of
Asn"
(Xi) ~U~N~ D~.s~RTT~TIoN: SEQ ID NO: 124:
Xaa Ile Xaa
(2) INFORMATION FOR SEQ ID NO: 125:
( i ) ~U~N~ CHARACTERISTICS:
(A) LENGTH: 3 amino acids
(B) TYPE: amino acid
(C) sTRANl~l)N~s~s: single
(D) TOPOLOGY: linear
(ii) M~T.F.CUT.~ TYPE: peptide
CA 02227516 1998-01-21
WO 97/08193 PCT/EP96/03473
- 214 -
(ix) FEATURE:
(A) NA~$E/KEY: Mn~;f;~fl--site
(B) LOCATION:l
(D) ~;~ INFORMATION:/product= "N--acetyl--(O--P--Tyr)"
/note= "O--P--Tyr stands for O-phn~:phr~nn-l-Tyr~
(ix) FEATURE:
(A) NA~D3/REY: Mn~if;~l--site
(B) LOCATION:3
(D) OTE~13R rwFORMATION:/~. ~lu~
"Asn--ElN--(2--(l-br~nnArhthalen-2-yloxy)ethyl"
/note= "~ 1 of alfa-N-(2-(l-brnmt~n~rhthalen-2
yloxy)ethyl
arnide of Asn"
(xi) ~hQU~;N~; n~C:t'RTT~TION SEQ ID NO: 125:
Xaa Ile Xaa
(2) INFOR~TION FOR SEQ ID NO: 126:
(i) ~h~U~;N~ '~A~Al~ll~RT~TIcs
(A) T.~Nr.TT~ 3 arnino acids
(B) TYPE: arnino acid
(C) Sq~RAh~ l)N~ S: single
(D) TOPOLOGY: linear
(ii) M~T.~crlT.~ TYPE: peptide
(ix) EEATURE:
(A) NAME/KEY: M~l;f;~ --site
(B) IOCATION:l
(D) OTE~ER INFORMATION:/~ ~lu~-- "N-acetyl-(O-P-Tyr)"
CA 02227516 1998-01-21
WO 97/08193 PCT/EP96/03473
r -215-
/note= "O-P-Tyr stands for O-Phosphono-L-Tyr"
(ix) FEATURE:
(A) NAME/KEY: Mn~;f;~-site
(B) T~TTON: 3
(D) OTHER rNF~RMATTON:/~u~L-
"Asn-NH-(3,3-diphenyl-propyl)"
/note= "R~ of alfa-N-(3,3-diphenyl)propyl amide of
Asn"
(Xi) ~ N~:N~ DESCRIPTION: SEQ ID NO: 126:
Xaa Ile Xaa
(2) INF~RMA~T~N FOR SEQ ID NO: 127:
(i) ~U~N~ CHARACTERISTICS:
(A) T-~T~: 3 amino acids
(B) TYPE: amino acid
(C) S'1TR~NI)P:l)N~ S: single
(D) TOPOLOGY: linear
(ii) M~T.~.~.~ TYPE: peptide
(ix) FEATURE:
(A) NAME/REY: M~;fi~-site
(B) LOCATION:1
(D) OTHER INFORMATION:/product= "N-acetyl-(O-P-Tyr)"
/note= "O-P-Tyr stands for O-ph~phono-L-Tyr~
(ix) FEATURE:
(A) NAME/KEY: ~;f;~-site
(B) LCCATION:3
(D) OTHER INFORMATION:/product= "Asn-NH-3-phenylpropyl"
CA 022275l6 l998-0l-2l
W O 97/08193 PCTrEP96/03473
-~16-
/note= '~RA~;C~1 of alfa-N-(3-phenylpropyl) amide of Asn"
(Xi) ~U~'N~ n~RTr~TION: SEQ ID NO: 127:
Xaa Ile Xaa
(2) INFORMATT~ FOR SEQ ID NO: 128:
( i ) ~U~N~' CHE~R~T~RT~TICS:
(A) LENGTH: 3 arnino acids
(B) TYPE: arnino acid
~C) STRAN~ N~:.SS: single
(D) TOPOLOGY: linear
(ii) ~r.~.cr~.r~ TYPE: peptide
(ix) FEATURE:
(A) NAME/ ~ Y: M~if;~-site
(B) IOCATION:1
(D) OTHER INFORMATION:/product= "N-acetyl-(O-P-Tyr)"
/note= "O-P-Tyr stands for O-Ph~sph~nn-L-Tyr"
(ix) FEATURE:
(A) NAME/KEY: M~;f;~-site
(B) LOCATION:3
(D) OTHER INFORMATION: /plUdU~ L-
"Asn-HN-(3-(2,4-~;chlorophenyl)propyl"
/note= "RA~ 1 of alfa-N-(3-(2,4-~ hl~rophenyl-
propyl)amide of.
Asn"
( Xi ) ~Ou~N~ DESCRIPTION: SEQ ID NO: 128:
CA 02227~16 1998-01-21
WO 97/08193 PCTAEP96/03473
, -217-
_ Xaa Ile Xaa
(2) INFORMATION FOR SEQ ID NO: 129:
( i ) ~U~N~ CH~RACTERISTICS:
(A) T.T~'.N~.T~: 3 amino acids
(B) TYPE: amino acid
( C ) ST~ANI )1':1 ~NI':~ S single
(D) TOPOIOGY: linear
( ii ) M~T.~CrlT.T~' TYPE: peptide
(ix) FEATURE:
(A) NAME/REY: ~;f;F~-site
(B) LOCATION:l
(D) aTHEK INFoR~Tn~:/~lodu~L- "N-acetyl-(O-P-Tyr)"
/note= "O-P-Tyr stands for O-Ph~sph~n~-L-Tyr"
(ix) FEATURE:
(A) NAME/ ~ Y~ ;f;~-site
(B) T~T~ 3
(D) uIHER INFORMATION:/product=
"Asn-NH-(2,2-diphenyl-ethyl)"
/note= "~ 1 of alfa-N-(2,2-diphenyl-ethyl)amide of
Asn"
(xi) ~yu~N~: DESCRIPTION: SEQ ID NO: 129:
Xaa Ile Xaa
-
(2) INFORMATION FOR SEQ ID NO: 130:
(i) s~u~ CHARACTERISTICS:
CA 02227516 1998-01-21
WO 97/08193 PCT/EP96/03473
- 218 -
(A) LENGTH: 3 amino acids
(B) TYPE: amino acid
( C ) S'l'RA~nN~S: single
(D) TOPOLOGY: linear
( ii ) ~ )T~F~CIlT~T~' TYPE: peptide
(ix) FEATURE:
(A) NAME/REY: M~l;f;F~ site
(B) LOCATION:1
(D) OTHER INF~RMA~'T~: /product= "N--acetyl-(O-P-Tyr)"
/note= "O--P--Tyr stands for O--Ph~sph~n~-L--Tyr"
(ix) FEATURE:
(A) NAME/REY: M~;f; ~l-site
(B) I~ATION:3
(D) OTEIER INEnRMA'r'T~N: /~~ L--
"Asn-NH--(2-(4-chlorophenyl)ethyl)"
/note= '~RF3~ 1 of alfa-N-(2-(4-chlorophenyl)ethyl)amide
of Asn"
(xi) ~ U~;N~:~; nT~ RTTJTIoN: SEQ ID NO: 130:
Xaa Ile Xaa
(2) rNFoRM~Ic)r~ FOR SEQ ID NO: 131:
(i) SEQUENOE ~ ARAt'~RT~c:TIcs:
(A) LENGTE~: 3 amino acids
(B) TYPE: amino acid
(C) STRAN~ )N~ S: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: peptide
CA 02227516 1998-01-21
W O 97/08193 PCTAEP96/03473
-219-
A
(ix) FEATURE:
(A) NAME/KEY: M~if;~-site
(B) LOCATION:l
(D) OTHER INFORMATION: /~l~U~ L- "N-acetyl-(O-P-Tyr)"
/note= "O-P-Tyr stands for O-phnsphnnn-L-Tyr~
(ix) FEATURE:
(A) NAME/KEY: Mn~;f;~-site
(B) T~'T~')~ 3
(D) OTHER INFORMATION:/~luducL- "Asn-NH-benzyl"
/note= "RA~ 1 of alfa-N-benzylamide of Asn"
( Xi ) ~yU~N~ DESCRIPTION: SEQ ID NO: 131:
Xaa Ile Xaa
(2) INFORMATION FOR SEQ ID NO: 132:
N( ~: t'~7~R A~RISTICS:
(A) LENGTH: 3 amino acids
(B) TYPE: amino acid
(C) S'rR~NI)~:I)N~:~CS: single
(D) TOPOLOGY: linear
( ii ) M~T.~)T.~ TYPE: peptide
(ix) FEATURE:
(A) NAME/KEY: M~;f;~-site
(B) LOCATION:l
..
(D) ~THER INFORMATION:/~l~u~L- "N-acetyl-(O-P-Tyr)"
/note= "O-P-Tyr stands for O-Phosphono-L-Tyr"
CA 022275l6 l998-0l-2l
W O 97/08193 PCT~EP96/03473
-220-
(ix) FEATURB:
(A) NAMB/KBY: M~ifi~-site
(B) LCCATION:3
(D) OTHBR nNFORMATION:/yl~u~L- "Asn-NH-isobutyl"
/note= "R~ l of Alfa-N-isobutylarnide of Asn"
( Xi ) ~U~N~ DESCRIPTION: SBQ ID NO: 132:
Xaa Ile Xaa
(2) rNFORMATION FOR SEQ ID NO: 133:
(i) ~;QU~!;NCI~; CH~RA~'~ ~T-STICS:
(A) LENGTH: 3 amino acids
(B) TYPE: arnino acid
( C ) S~N~ )N~:S~S: single
(D) TOPOLCGY: linear
(ii) M~TT'CrlT~ TYPE: peptide
(ix) FEATURB:
(A) NAMB/RBY: M~ifi~-site
(B) LOCATION:l
(D) OT~BR rNFoRMATIoN:/~lulu~L- "N-acetyl-(O-P-Tyr)"
/note= "O-P-Tyr stands for O-ph~cph~no-L-Tyr
(ix) FBATURB:
(A) NAMB/RBY: ~ifi~-site
(B) LCCATION:3
(D) OT~BR INFORMATION: /~l~UC L- "Asn-NH-(3-methylbutyl)"
/note= "R~ic~l of alfa-N-(3-methylbutyl)amide of Asn"
(xi) ~U~N~ DESCRIPTION: SEQ ID NO: 133:
CA 022275l6 l998-0l-2l
WO 97/08193 PCTAEP96/03473
-221-
Xaa Ile Xaa
(2) INFORMATION FOR SEQ ID NO: 134:
( i ) ~U~N~ CHA~FRT~TICS:
(A) T-~N~: 3 amino acids
(B) TYPE: amino acid
( C ) ST~2ANI )1':1 ~N~:C s single
(D) TOPOLOGY: linear
( ii ) M~T.~CrrT.~ TYPE: peptide
(ix) FEATURE:
(A) NAME/REY: Mn~;f;~-site
(B) T~T~N l
(D) OTHER INFORMATION:/product= "N-acetyl-(O-P-Tyr)"
/note= "O-P-Tyr stands for O-ph~srh~n~-L-Tyr
(ix) FEATURE:
(A) NAME/REY: M~;f;~-site
(B) LOCATION:3
(D) ~T~K INFORMATION:/~u~L- "Asn-NH-(2-ethyl-hexyl)"
/note= "~ l of alfa-N-(2-ethyl-hexyl)amide of Asn"
(xi) ~U~N~ D~RTPTION: SEQ ID NO: 134:
Xaa Ile Xaa
(2) INFORMATION FOR SEQ ID NO: 135:
(i) ~ ut~:N(-:~; CEI~A~ RT-~TIcs
(A) LENGTH: 3 amino acids
CA 02227516 1998-01-21
W O 97/08193 PCT~EP96/03473
-222-
(B) TYPE: amino acid
(C) STR~N~ N~:SS: single
(D) TOPOLOGY: linear
( ii ) ~T-~r~.~ TYPE: peptide
(ix) FEATURE:
(A) NAME/KEY: M~if;f~-site
(B) LOCATION:l
(D) uT~R lNFnRMA~ToN:/~ v~u~ L- "N-acetyl-(O-P-Tyr)
/note= "O-P-Tyr stands for O-ph~sphnn~-L-Tyr
(ix) FEATURE:
(A) NAME/REY: M~;f;~-site
(B) T~ ~T~N 3
(D) OTHER INFORMArION:/product= "Asn-NH-(cyclohexyl)"
/note= ~'RA~;~A1 of alfa-N-(cyclohexyl)amide of Asn"
(Xi) ~U~N~'~ DESCRIPTION: SEQ ID NO: 135:
Xaa Ile Xaa
(2) INF~RMA~T~N FOR SEQ ID NO: 136:
( i ) ~QU~N~'~ CHu~R~ RT-sTIcs:
(A) LENGTH: 3 amino acids
(B) TYPE: amino acid
(C) S~R~Nl)~ N~:~S: single
(D~ TOPOLOGY: linear
( ii ) M~T~cr~ TYPE: peptide
(ix) FEATURE:
CA 022275l6 l998-0l-2l
W O 97/08193 PCT~EP96/03473
r ~ 223 ~
(A) NAME/KEY: Mn~if;~-site
(B) LOCATION:1
(D) OTHER INFORMATION:/product= "N-acetyl-(O-P-Tyr)"
/note= "O-P-Tyr stands for O-ph~sph~n~-L-Tyr~
(ix) FEATURE:
(A) NAM~/REY: M~A;f;~-site
(B) LOCATION:3
(D) OTHER INFORMATION: /~lU~U~ L-
"Asn-NH-(cyclohexylmethyl)"
/note= "R~ic~l of alfa-N-cyclohexylamide of Asn"
( Xi ) ~U~N~ DESCRIPTION: SEQ ID NO: 136:
Xaa Ile Xaa
(2) INFORMATION FOR SEQ ID NO: 137:
( i ) ~QU~N~' ~R ~T~R T.~TICS:
(A) LENGTH: 3 amino acids
(B) TYPE: amino acid
(C) S'l'R;~N~ l)Nl~ s: single
(D) TOPOLOGY: linear
( ii ) MnT.~CrlT.T' TYPE: peptide
(ix) FEATURE:
(A) NAME/KEY: M~;f;G~-site
(B) LOCATION:l
(D) OTHER INFORMATION:/product= "N-acetyl-(O-P-Tyr)"
/note= "O-P-Tyr stands for O-Phosphono-L-Tyr"
(ix) FEATURE:
(A) NAME/KEY: M~;f;~d-site
CA 02227516 1998-01-21
WO 97/08193 PCT/EP96/03473
-224-
(B) LOCATION:2
(D) OT~ER INFORMATION:/product=
"l-aminocycloh~ n~-carbonyl"
/note= ~'R~;~l of l-Aminncy~lnh~n~ carboxylic acid"
(ix) Fh-ATURE:
(A) NAME/REY: M~; f; ~-site
(B) IOCATION:3
(D) OTHER INFORMATION: /~lUdU~ L-
"Asn-NH-(3-n~phth~l~n-l-yl-propyl)~
/note= "RA~ l of alfa-N-(3-n~ph~h~l~n-l-yl-propyl)amide
o:~
Asn"
(xi) ~hQuhN.~h n~RTPTION: SEQ ID NO: 137:
Xaa Xaa Xaa
(2) INFORMATION FOR SEQ ID NO: 138:
(i) ~UhN~ ~AR~ RT~TIcs:
(A) r.~,~: 3 amino acids
(B) TYPE: amino acid
(C) S~RAN~ I)NK~.~: single
(D) TOPOLOGY: linear
( ii ) M~T.T'~TT.~ TYPE: peptide
(ix) FEATURE:
(A) NAME/KEY: Mr~; f i~ - site
(B) LOCATION:l
(D) OTHER INFORMATION:/k~u~L- "N-acetyl-(O-P-Tyr)"
/note= "O-P-Tyr stands for O-Ph~sphnn~-L-Tyr"
CA 02227516 1998-01-21
W O 97/08193 PCT/EP96/03473
-225-
(ix) FEATURE:
(A) NAME/KEY: Modified-site
(B) LOCATION:Z
(D) OTHER INFnRMATTnN~ cL-
"l-amino-cyclohexylcArh~nyl"
/note= "R~A;~1 of l-amino-cycl~h~n~ carboxylic acid"
(ix) FEATURE:
(A) NAME/KEY: Mn~ifie~-site
(B) LOCATION:3
(D) OTHER INFORMATION:/product=
"Asn-NH-(3-n~phth~l~n-2-yl-propyl)"
/note= "R~ l of alfa-N-(3-n~rhth~l~n-2-yl-propyl)amide
of
Asn"
(Xi) ~U~N~ n~.~RTT~TION: SEQ ID NO: 138:
Xaa Xaa Xaa
(2) INFORMATION FOR SEQ ID NO: 139:
(i) ~U~:N~ C~ARA~T~IsTIcs:
(A) LENGTH: 3 amino acids
(B) lY~E: amino acid
(C) S~RAN~ N~:~S: single
(D) TOPOLOGY: linear
( ii ) M~T~cuT~ TYPE: peptide
.~
J ( iX ) FEATURE:
(A) NAME/KEY: Mn~i fie~-site
(B) LOCATION:l
(D) OTHER INFORMATION:/~L~Iu~- "N-acetyl-(O-P-Tyr)"
CA 02227516 1998-01-21
W O 97/08193 PCT/EP96/03473
-226-
/note= "O-P-Tyr stands for O-Phosphono-L-Tyr"
(ix) FEATURE:
(A) NAME/KEY: M~;fi~-site
(B) LOCATION:2
(D) OTHER INFORMATION:/pl~L-
"l-amino-cyclohexyl~Arhnnyl"
/note= "RAA;~l of l-amino-cy~l~h~An~ carboxylic acid"
(ix) FEATURE:
(A) NAME/REY: M~;f;~-site
(B) LOCATION:3
(D) OTHER INFORMATION:/~v~u~L-
"Asn--NH-(3--(2--hy~lL~y~Aphth~l~n--lyl)propyl)"
/note= RA~;~A1 of
alfa-N-(3-(2-1.yd~yllAphthAlen-l-yl)propyl)amide of Asn"
(Xi) ~U~'N~ DT<'-~C-RTPTION SEQ ID NO: 139:
Xaa Xaa Xaa
(2) INFORMATION FOR SEQ ID NO: 140:
( i ) ~QU~N~ CH~R~ KT~TICS:
(A) LENGTH: 3 amino acids
(B) TYPE: amino acid
(C) STR;~NI)I~ S: single
(D) TOPOLOGY: linear
( ii ) ~T,~CrlT.~ TYPE: peptide
(ix) FEATURE:
(A) NAME/KEY: M~;f;~-5ite
(B) LOCATION:l
CA 022275l6 l998-0l-2l
W O 97/08193 PCTAEP96/03473
-227-
(D) OTHER INFORMATION: /~L~U~ L -
"N-(3-amLnobenzyloxycarbonyl)-(O-P-Tyr)"
/note= "O-P-Tyr stands for O-ph~sphnnn- L-Tyr
(ix) FEATURE:
(A) NAME/KEY: ~n~ifi~-site
(B) LOCATION:3
(D) OTHER INFORMATION: /~l~U~ L-
"Asn-NH-(3-naphthalen-1-yl-propyl)"
/note= "~ l od alfa-N-(3-n~rhth~l~n-l-yl-propyl)a-mide
of
Asn"
(Xi) ~yU~N~ n~.~rRTpTIoN: SEQ ID NO: 140:
Xaa Ile Xaa
(2) INFORMATION FOR SEQ ID NO: 141:
(i) SEQUEN OE CHARaCTERlSTICS:
(A) LENGTH: 3 am~ino acids
(B) TYPE: amino acid
(C) STR~N~ N~S single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: peptide
(ix) FEATURE:
(A) NAME/KEY: Mn~;fie~-site
(B) LCCATION:l
(D) OT~ER INFORMATION:/product=
"N--(3--;~m;nn}~n7.yloxycarbonyl)--(O--P-Tyr)"
/note= "O-P-Tyr stands for O-phns~hnnn-L-T
CA 022275l6 l998-0l-2l
W O97/08193 PCTAEP96/03473
-228-
(ix) FEATURE:
(A) NAME/KEY: Modified-site
(B) LOCATION:3
(D) OTHER INFORMATION: /plVdU~ L-
"Asn-NH-(3-(2-hydlv~yllaphthalen-lyl)propyl)"
/note= "RA~ l of
alfa-N-(3-(2-hydl~y-naphthalen-l-yl)propyl)a-m-ide of Asn"
( Xi ) ~U~N~ DESCRIPTION: SEQ ID NO: 141:
Xaa Ile Xaa
(2) INFORM~T~N FOR SEQ ID NO: 142:
(i) ::jl~;(,~U~;N~ ~'~ARAr~.RT~TICS:
(A) LENGTH: 3 amino acids
(B) lY~E: amino acid
(C) STRAN~ )N~:~S: single
(D) TOPOLOGY: linear
(ii) M~T.~T.~ TYPE: peptide
(ix) FEAIURE:
(A) NAME/REY: M~;fi~-site
(B) LOCATION:l
(D) OTHER INFORMATION: /~l~U~ L-
"N-(3-Am;n~h~n7yloxycarbonyl)-(o-p-Tyr)"
/note= "O-P-Tyr stands for O-ph~phon~-L-Tyr
(ix) FEaTURE:
(A) NAME/REY: Modified-site
(B) LOCATION:3
(D) OTHER lN ~'U~ ~TION: /~l~U~ L-
"Asn-N-(3-naphthalen-2-yl-propyl)"
CA 02227516 1998-01-21
W O 97/08193 PCTIEP96/03473
-229-
~'
~ /note= " RA~; CA 1 of alfa-N-(3-naphthalen-2-yl-propyl)amide_ of
Asn"
(Xi) ~ U~N~ n~.~rRTPTION: SEQ ID NO: 142:
Xaa Ile Xaa
(2) INFORMATION FOR SEQ ID NO: 143:
(i) ~:QU~;N~; r~ARAr'i'~RT-';TICS:
(A) LENGTH: 3 amino acids
(B) TYPE: amino acid
(C) STRAN~ N~ single
(D) TOPOLOGY: linear
( ii ) M~T~T~cr~T~T~ TYPE: peptide
(ix) FEATURE:
(A) NAME/KEY: ~;f;~-site
(B) T~rATT()N :1
(D) uTHER INFORMATION:/~Iu~L-
"N_(3_Am;n~h~n~yloxycarbonyl)-(O-P-Tyr)"
/note= "O-P-Tyr stands for O-Phnsph~no-L-Tyr"
(ix) FEATURE:
(A) NAME/KEY: ~;f;e~-site
(B) LOCATION:3
(D) OTHER rNFORMATION:/product=
"Asn-NH-(3-phenanthren-9-yl-propyl)"
/note= " ~A~ 1 ofalfa-N-(3-phenanthren-9-yl-propyl)amide
of
Asn"
CA 02227516 1998-01-21
WO 97/08193 PCT~P96/03473
-230-
(Xi) ~ :N(-~: D~RTPTION: SEQ ID NO: 143:
Xaa Ile Xaa
(2) INFO~MATT~N FOR SEQ ID NO: 144:
( i ) ~U~N~ ~ARA~RT~TIcs:
(A) T.~N~,~: 3 amino acids
(B) TYPE: amino acid
(C) STRAN~ l)N~:~S: single
(D) TOPOLOGY: linear
(ii) M~T.~.~TT.~ TYPE: peptide
(ix) FEAIURE:
(A) NAME/KEY: M~if;P~-site
(B) LOCATION: 3
(D) OTHER INFORMATION:/product=
"Asn-NH-2-(1-brnmnn~rhthalen-2-yl-oxy)ethyl"
/note= "~; ~A 1 of
alfa-N-(2-(2-brnmnn~rhthalen-2-yl-oxy)ethyl)amide of Asn"
(ix) FEATURE:
(A) NAME/KEY: M~;f;i~-site
(B) LOCATION:l
(D) OTHER INFORMATION:/product=
"N-( 3 - ~m; nnh~n~yloxycarbonyl)-(O-P-Tyr)"
/note= "O-P-Tyr stands for O-Phnsph~nn-L-Tyr"
(Xi) ~ U~N~ n~RTpTIoN: SEQ ID NO: 144:
Xaa Ile Xaa
CA 02227~16 1998-01-21
W O 97/08193
PCT/EP96/03473
t - 231 -
(2) INFORMATION FOR SEQ ID NO: 145:
( i ) ~U~N~ CH~RA~RT~TICS:
(A) LENGTH: 3 amino acids
(B) TYPE: amino acid
( C ) STR~NI)~ N~: single
(D) TOPOLOGY: linear
(ii) M~T.~.~. TYPE: peptide
(ix) FEATURE:
(A) NAME/KEY: M~;f;~-site
(B) T~Z~'T'TOl~ 1
(D) OTHER INFORM~TT~N:/~ulucL-
"N--(3--~m;nnh~n7yloxycar~onyl)--(O--P--Tyr)"
/note= "O-P-Tyr stands for O-Phnsrhn~n-L-Tyr"
(ix~ FEATURE:
(A) NAME/KEY: M~;f;~-site
(B) LOCATION:3
(D) OTHER INF~RMA~roN:/~l~lu~L- "Asn-NH-(3-methyl-butyl)"
/note= "R~ l o~ alfa-N-(3-methyl-butyl)amide of Asn"
( Xi ) ~U~N~ D~.~RTT~TION: SEQ ID NO: 145:
Xaa Ile Xaa
(~) INFORMATION FOR SEQ ID NO: 146:
(i) ~il':(;~lJl~:N~ ~AR~ 'FRlSTICS:
(A) LENGTH: 3 amino acids
(B) TYPE: amino acid
(C) S~R~N~nNF~S: single
CA 02227516 1998-01-21
W O 97/08193
PCT~EP96/03473
-232-
(D) TOPOLOGY: linear
( ii ) M~T.~CrlT.F. TYPE: peptide
(ix) FEATURE:
(A) NAME/KEY: Mn~; f;~-site
(B) T~Z~TION 1
(D) OTHER INFORMA~TO~: /~ U~ L--
"N--(3_;~m;.ln~ 7yloxycarbonyl)--(O--P--Tyr)"
/note= ''O-P-Ty-r stands for O-Pho~h~o-L-Ty-r
(ix) FEATURE:
(A) NAME/KEY: M~; f;~-site
(B) T~Z~'T'TON 3
(D) OTHER I~FORM~TION:/~1u~- "Asn-NH-cyclohexyl"
/note= "R~ l of alfa-N-(cyclohexyl)amide of Asn"
(xi) ~yu~N~ DESCRIPTION: SEQ ID NO: 146:
Xaa Ile Xaa
(2) rNFORMATION FOR SEQ ID NO: 147:
(i) ~U~N~ ~R~r.TFRT.STICS:
(A) LENGTH: 3 amino acids
( B ) TYPE: a~ino acid
(C) STRA~ N~:~S: single
(D) TOPOLOGY: linear
( ii ) M~T~cr~T~ TYPE: peptide
(ix) FEATURE:
(A) NAME/KEY: Modified-site
CA 02227516 1998-01-21
W O 97/08193
PCT~EP96/03473
-233-
- (B) LOCATION:l
(D) OTHER INFORMATION:/product=
"N-(3_~m;nnh~n~yloxycarbonyl)-(O-P-Tyr)"
/note= "O-P-Tyr stands for O-Phosphono-L-Tyr"
(ix) FEATURE:
(A) NAME/KEY: MnA;f;~A-8ite
(B) LOCATION:2
(D) OTHER rNFORMATION: /~1UdU~ L-
"-l-amino-cyclohexyl~rh~nyl-"
/note= "~A;~l of 2-aminocycl~h~n~ carboxylic acid"
(ix) FEATURE:
(A) NAME/KEY: Mn~;f;~A-site
(B) LOCATION:3
(D) OTHER I~FORMATION:/~ludu~L-
"Asn-NH-(3-naphthalen-l-yl-carbonyl)"
/note= "~A;CA1 of alfa-N-(3-n~phth~l~n-l-ylc~rhnnyl)a-mide
oi~
Asn"
(xi) S~U~N~ n~RTT~TIoN: SEQ ID NO: 147:
Xaa Xaa Xaa
(2) INFORMATION FOR SEQ ID NO: 148:
(i) ~U~N~: ~r~AR~T~RT~TIcs:
(A) LENGTH: 3 amino acids
(B) TYPE: amino acid
(C) S~R1~NI)I-:I)NI~ S: single
(D) TOPOLOGY: linear
( ii ) ~T.~CrJT.T~, TYPE: peptide
CA 022275l6 l998-0l-2l
W O 97/08193 PCT~EP96/03473
-234-
(ix) FEATURE:
(A) NAME/REY: M~;fi~-site
(B) LOCATION:l
(D) OTHER INFORMATION:/~u~L-
"N-(3-A~;nnh~n~yloxycarbonyl)-(O-P-Tyr)"
/note= "O-P-Tyr stands for O-Phnsphnno-L-Tyr"
(ix) FEATURE:
(A) NAME/KEY: M~;f;~-site
(B) LOCATION:2
(D) OT~ER INF~RMA~T~N: /~l~iU~ L -
"-l-amino-cy~l~h~YAl~Arhonyl-
~
/note= "RA~;~A1 of l-amino-cy~lnh~n~ carboxylica acid"
(ix) FEATURE:
(A) NAME/KEY: Mn~;f;e~-site
(B) LOCATION:3
(D) OTHER INF~RMA~T~:/~u~L-
"Asn-NH-(3-naphthalen-2-ylpropyl)"
/note= "R~;~A1 of alfa-N-(3-naphthalen-2-yl-propyl)amide
of
Asn"
(xi) ~U~N~ DESCRIPTION: SEQ ID NO: 148:
Xaa Xaa Xaa
(2) INFORMATION FOR SEQ ID NO: 149:
(i) ~yu~N~ CHARACTERISTICS:
(A) LENGTH: 3 amino acids
(B) TYPE: amino acid
(C) STRAN~ l)N~ S: single
(D) TOPOLOGY: linear
CA 02227516 1998-01-21
W O 97/08193
PCTrEP96/03473
-235-
( ii ) M~T~r~T T~ TYPE: peptide
(ix) FEATURE:
(A) NAME/KEY: Mn~;f;~-site
(B) T~TTnN.
(D) ul~;~ INF RM~TTC~N:t~l~luc~L--
"N--( 3 ~~; nn~n~yloxycarbon:yl )--( O-P-Tyr ) "
/note= "O-P-Tyr stands for O-Phnsphnnn-L-Tyr"
(ix) FEATURE:
(A) NAME/KEY: Mn~;fi~-site
(B) LOC~TION:2
(D) OTHER INFORM~TION:/~.v~ L -
"-l-amino-cyclohexylr~rhonyl-"
/note= '~R~ l of l-aminocycloh~An~ carboxylic acid"
(ix) FEATURE:
(A) NAME/REY: Mn~ifi~-site
(B) T~TnN 3
(D) OTHER INFORMATION:/product=
"Asn-NH-( 3 - (2-hyd~o~y~laphthalen-lyl)propyl)"
/note= "~ l of
alfa-N-(3-(2-hydloxy-naphthalen-l-yl)propyl)amide of Asn"
(Xi) ~U~N~ nT~s~RTTyrIoN: SEQ ID NO: 149:
Xaa Xaa Xaa
-