Note: Descriptions are shown in the official language in which they were submitted.
CA 02228290 1998 - 01 - 29
PCT/US96112482
WO 97105287
ChemiluminesCent Method of Monitoring Products A~ter
Heat Treatment
Reference to Pr~or Applications
This application claims the benefit of the priority
date of U.S. Provisional Patent Application S.N.
60/001,706 filed July 31, 1995, titled ''Chemiluminescent
Heat Monitoring Process and System for Biological
lC ACtiVity''-
3ac~ground of the Invention
It is desirable to validate rapidly and effectively
the heat treatment of products in the preparation of
pharmaceutical biologicals and food products with a rapid,
sensitive and accurate method to verify that the product
has been heat-treated to an adequate or certified
temperature within the time in which the product has been
so heat-treated. The determination of acid phosphatase
(AP) in products as an indicator for cooking to a
temperature has been reported in associative literature.
Enzymatic tests have been used for monitoring milk
pasteurization, such as the Scharer Test (the "Standard
Method for Examination of Dairy Products", 16th Ed., 1992,
R. T. Marshall, Ed., ch. ll, p.418), which is used
worldwide to monitor standard pasteurization, however, it
takes about thirty minutes to detect one unit of AP in the
Scharer Test. New pasteurization and ultrapasteurization
procedures are at a level of o.01U, and thus cannot be
monitored with the Scharer Test. A test known as the
Fluorophos acid test (the "Standard Method for E~Am;n~tion
of Dairy Products", 16th Ed., 1992, R. T. Marshall, Ed.,
ch. 11, p.418) using a fluorescent substrate is also used;
however, this test has a limit of detection of 0.05U, and
takes about three minutes to perform.
,
CA 02228290 1998-01-29
W O 97/05287 PCT~US96/12482
It is desirable to provide for a new and improved,
effective, rapid, chemiluminescent method and test with
low limits of detection for the monitoring of heat
processes in various products.
5S~ ~ry of the Invention
The invention relates to a chemiluminescent method of
monitoring of products after heat treatment. More
particularly, the invention concerns a rapid, sensitive
and accurate method to examine products which are heat
processed to reduce or eliminate microbiological or
biological activity, so as to validate any heat treatment,
and/or processing of the product and to verify that the
product has been subjected to an adequate temperature
within the time of the heat treatment process.
15The invention concerns a chemiluminescent method for
monitoring or validating the processing of the heat-
treatable product, by monitoring the concentration of a
receptor or enzyme in the product by chemiluminescent
means. The method comprises obtaining a sample of the
heat-treated product directly from the product itself or
from a surface contaminated with the product; and a~mix;ng
this sample, typically and optionally, with a buffer-type
solution such as saline buffer solution of pH 6-1~ or
water. The method includes combining the sample, either
before or after incubation, with a room temperature (20-
25~C), stable, chemiluminescent compound, usually in the
presence of one or more chemiluminescent enhancers, which
chemiluminescent compound is susceptible to the enzyme in
the sample, which triggers the decomposition of the
chemiluminescent compound to provide for chemiluminescence
from the admixed sample and compound. In the preferred
embodiment, the enzyme present in the compound would
comprise phosphatase or lactoperoxidase, and in one
preferred embodiment, the chemiluminescent compound would
comprise a 1,2-dioxetane compound, with an enhancer to
render the chemiluminescent test method more sensitive.
The test method includes incubating the sample with
the chemiluminescent compound and/or enhancer for a
CA 02228290 1998-01-29
W O 97/05287 PCTAUS96/12482
selected incubation time period and incubation
temperature, typically, for example, about 1-15 minutes
and for example at 25-65~C, or more typically 1 to about
3 minutes at 30-45~C, to provide for the enzymatically
- 5 cleavable labile substituent of the chemiluminescent
compounds to be cleaved. The chemiluminescent compound
may be added to the sample before or after the incubation
perlod .
The method also includes, preferentially, bringing
the incubation to the selected incubation time period and
employing a stopper solution, typically a buffer solution
having a pH, for example, of about 3-12. The method then
includes observing and counting the chemiluminescent light
emission over time (seconds/minutes) for an indication of
the temperature and time profile of the sample that was
subjected to heat processing.
The invention relates to a method of monitoring of
pharmaceutical, clinical, and food products, for instance
and including dairy products such as milk, cheese and
meat, or nonbiological products, to monitor the heat-
treating and to validate the heat treatment of the heat-
treatable product or a sample taken therefrom. The
present invention provides a rapid and sensitive
chemiluminescent method to examine products and heat
processes to reduce or eliminate microbiological or
biological activity, and particularly to prolong the
shelf-life of a biological product.
The present chemiluminescent methods have been
demonstrated to be over 100 times more sensitive than
current prior art methods and allow monitoring over a 12
log microbial reduction in pasteurization,
ultrapasteurization and ultra short time, high temperature
processes (UHT) of treating products. The present methods
avoid many of the limitations of prior art test processes
by incorporating chemiluminescent compounds with or
without chemiluminescent enhancers to make the test more
sensitive, and can detect enzymes, for example acid
CA 02228290 1998-01-29
W O 97/05287 PCT~US96/12482
phosphatase (AP) or other enzymes or receptors, down to
about O.OOlU of AP in about 3 minutes.
In the past, lactoperoxidase has been suggested for
monitoring UHT, but, due to sensitivity, only
ultrapasteurization can be monitored, while the
chemiluminescent test method enhances the sensitivity over
times and makes it possible to monitor UHT.
Traditional color assay sensitivity is not adequate,
however, using the chemiluminescent test method of the
invention, up to 12 log reduction of bacteria can be
guaranteed. The chemiluminescent test method may be used
for the monitoring of the pasteurization of serums, and,
for example, acid phosphatase could be demonstrated as a
marker for proper pasteurization, with 12 log reductions
of various microorganisms.
The chemiluminescent test method may also be
profitably employed in pasteurization and sterilization of
liposomes, and liposome containing products, including a
receptor antibody or enzyme which has the chemiluminescent
activity heat treatment and can thus be monitored in short
time periods such as seconds or minutes. More
particularly, heat processed food products, such as meats,
vegetables and fruit (canned or juices), can be monitored
by monitoring reduction and peroxidase activity, for
instance, for example, horseradish peroxidase,
glucoronidase phosphatase (for example potato acid
phosphatase) or epirase, at very low levels, down to lo-lS
mols or lower. Thus, the chemiluminescent method enhances
greatly the sensitivity and provides for rapid, accurate
real time monitoring of bacterial reduction in the heat-
treated sample being tested.
The chemiluminescent test method is carried out by
obtaining a sample of the product to be tested, either
directly from the surface or interior of the product, or
from any surface contaminated by the product, and
typically in one case may be employed by using a swab on
a stick, with the swab dry or premoistened with water or
a sterilizable saline or buffer solution, to retain the
-
CA 02228290 1998-01-29
W ~ 97/05287 PCT~US96/12482
sample of the product. The sample thus obtained is
admixed, either before or after the incubation step with
a chemiluminescent compound which is susceptible to the
enzyme or receptor present in the sample, or inherently or
- 5 integrally which is added to the sample as an enhancer.
The chemiluminescent compound may be selected through
a wide variety of compounds, but more particularly, for
example, may be a 1,2-dioxetane compound by Tropix, Inc.
o~ Bedford, Massachusetts. Generally, chemiluminescent
compounds are also employed in combination with a
sufficient amount of enhancers to provide for increased
amplification o~ sensitivity in connection with the
enzymatic cleavage, which enhancers are usually selected
based upon the particular enzyme, such as phosphatase,
which is to be cleaved.
The sample, which contains a receptor or more
particularly a cleavable enzyme, which is either integral
with the sample or which is added, for example, may be
phosphatase. Chemiluminescent compounds and the enhancers
may be either added before or after incubation; however,
incubation is carried out with the sample for a selected
time period and temperature, usually with a
chemiluminescent compound present. Incubation is
terminated employing a stopper solution, and immediately
thereafter, the sample, typically in a translucent or
transparent glass or container, is placed within a
luminometer such as portable luminometer well, and the
luminescence of the sample, in relative light units (RLU)
are determined relative to time in order to provide for a
determination of the amount of receptor or enzyme
remaining in the heat-treated sample. The
chemiluminescent test method thus permits the
determination as to whether the treated product was
subject to a proper heat-treating process, for example, as
required by the U.S. Code of Federal Regulations which
specifies cooking temperatures in connection with food
products, with the acid phosphatase as an indicator for
the cooking time and temperature.
CA 02228290 1998-01-29
96~12482
~ 2 7 5~ 1991
The chemiluminescent substrate reagents used in the
test may vary; however, some substituted l,2-dioxetanes
and enhancers suitable for use therewlth are described in
U.S. Patent 5,330,900, issued July 19, 1994, and U.S.
Patent 5,338,847, issued July 23, 1996, both hereby
incorporated by reference. The reagents may be employed
in solution or in tablet form. For example, the reagent
may comprise a compressed tablet of a bulking agent (like
microcellulose or other inert filler material), and
optionally with other additives like stabilizers, anti-
oxidants, binder agents, dyes, etc. For example, a 30
- milligram cellulose tablet having about 1-20 micrograms of
the reagent and enhancer may be used to detect alkaline
phosphatase or acid phosphatase in the test sample.
. ~ .
The test method will be described for the purposes of
illustration only in connection with certain preferred
illustrated embodiments, however, it is recognized that
various changes, modifications, additions and improvements
may be made to the illustrated test method embodiment by
those persons skilled in the art, all falling within the
spirit and scope of the invention.
Brief Description of the R~hodiments
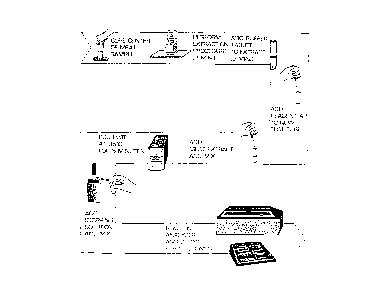
~ Fig. 1 shows a flow diagram of the meat patty test
procedure;
Fig. 2 is a graph depicting the 4 log reduction of
phosphatase activity from ground chicken meat when heated
at cooking temperatures ranging from 630C to 830c;
Fig. 3 is a graph illustrating the detection limit of
residual uncooked meat (raw chicken meat) portion in a
sample;
Figs. 4-7 are graphs illustrating the reduction of
phosphatase activity under treatments at four different
temperatures and cooklng times.
Description of the F~hodiments
The Food Safety and Inspection Service, the USDA's
agency responsible for safety of meat and poultry product,
has established new requirements for cooked uncured meat
AMENDED. S~IEET
CA 02228290 1998-01-29
W O 97/05287 PCT~US96/12482
products designed to "protect the public health by
reducing the.risk of foodborne illness from certain types
of meat products prepared in USDA inspected plants". The
rules, as set forth in the "Requirements for Cooked
Uncured Meat Products", published in the FSIS
R~ckgrol~n~er, August 1993, pages 1-4, and the "Code of
Federal Regulations", published in the Fe~er~l Register,
August 2, 1993, 9 CFR Parts 318 and 320, set heating,
cooking, handling and storage requirements for meat
processing facilities (including supermarkets), plants,
restaurants, hotels and other institutional kitchens. The
program~ 8 goal is to reduce pathogens throughout the food
chain "f~om the farm to the consumer's fork".
Requirements include:
1. Cook;n~ Tem~er~tllre vs. T;me:
Operators are instructed to measure the temperature,
every hour, of at least one product, in the center using
a device accurate within 10F, to ensure control of heat
processing (see Table 1, for permitted heat processing
time and temperature, from the Code of Federal Regulations
9 CFR Parts 318 and 320.
Table 1
Jable 1: Tei"~,erdlura requ~l ~",eli~s for cooked uncured
meatj ~roduct
~F ~CTime(Seconds)Product Type
140 60.0 -- partially cooked
145 63.0180 fully cooked
150 66.060 fully cooked
155 68.316 fully cooked
157 69.4,lO fully cooked
Source: Code of Federal Regulations (9 CFR Parts
318 and 320)
CA 02228290 1998-01-29
W O 97/05287 PCTAUS96/12482
2. Prevent;on of Cross-co~t~;n~t-on:
Prevent bacteria from raw products from cross-
contamination of fully cooked products. Handling areas
for raw and cooked meat must be either physically
separated or products should be processed at different
times after thorough cleaning and sanitizing of the area.
Detailed sanitation instructions include:
-Surfaces, equipment - germicidal sanitizer
equivalent to 50 ppm chlorine
-Employee '6 hands - similar to above
-Garments, aprons and gloves identified and
designated only for either fully cooked or uncooked
processing
-Fully cooked product to be stored with other product
shall first be packaged or covered.
The phosphatase assay is a rapid tool for quality
assessment of these requirements. The use of phosphatase
as an indicator for heating process has been recognized by
the milk industry worldwide for more than two decades.
Phosphatase activity has been previously reported as
a possible indicator for the presence of uncooked meat in
a cooked or pre-cooked poultry product. The use of acid
phosphatase measurement as a monitor of end-point
temperature in poultry tissue has been presented at the
1993 IAMFES (International Assoication of Milk, Food and
Environmental Sanitarians) meeting by a group headed by
Carl E. Davis from USDA's Russell Research Center in
Georgia. They found a similar time/temperature dependent
decrease in mean chicken breast acid phosphatase activity.
This research was published in an article titled "Rapid
Fluorometric Analysis of Acid Phosphatase Activity in
Cooked Poultry Meat", by Carl E. Davis and William E.
Townsend, in the Jollrn~l of Foo~ Protect,on, Vol. 57, No.
12, pp. 1094-1097.
The Applicants have found that beef phosphatase
thermal stability coincides with the time/temperature
requirement established by regulatory agencies and
accepted by the Industry (see Table 1). Phosphatase
CA 02228290 1998-01-29
W O 97/05287 PCTrUS96/12482
thermal stability correlates well with log reduction of
bacteria. The sensitivity of the assay (linear over 4
logs of activity) makes the chemiluminescent test method
useful for determining if temperature requirements for
- 5 either partially or fully cooked meat products have been
met at the processing plant or other locations.
The test method is a valuable tool in quality
control, as it is rapid, simple and easy to interpret.
The simplicity and min~m~l preparation of samples make the
test applicable to on-line monitoring, post cooking
sampling and on-site testing using a portable unit.
Multiple samples can be prepared simultaneously, which
makes the procedure even more desirable. Such meat
samples can be extracted, buffered (Buffer Table provided)
and tested in under 15 minutes. The kit uses a stable,
ready-made chemiluminescent reagent substrate (e.g., AP
reagent) and a stopping solution to stabilize the results.
The precooked meat patty rule also establishes
requirements for prevention of cross-contamination in
common areas used for handling raw meat as well as fully
cooked patties. Efficiency of cleaning and sanitizing of
the handling areas can be monitored by swabbing areas and
testing. Any residual raw product can be easily detected
and results can be recorded for HACCP documentation. So
far, test performance has not been affected by the
presence of sanitizers routinely used by the processing
industry (e.g., chlorine, detergents).
A flow diagram of the meat patty test procedure is
shown in Fig. 1 of the Drawings. The test results,
obtained from a luminometer, are expressed in relative
light units (RLU). Luminometers are available in portable
or bench top laboratory models. The AP reagent comprises
1,2-dioxetane, a chemiluminescent substrate CSPD~
(Disodium 3-(4-methoxyspiro{1,2-dioxetane-3,2'-(5' chloro)
tricyclo[3.3.1.1.1~ 2] decan}-4-yl)phenyl phosphate - of
Tropix, Inc., of Bedford, Massachusetts) and a luminescent
enhancer material, LAMS, Luminescence Amplifying
Materials, e.g., TMQ, poly(vinylbenzyltrimethylammonium
CA 02228290 1998-01-29
W O 97/05287 PCT~US96/12482
chloride), and others as listed in U.S. Patent 5,330,900.
Phosphatase activity was examined in raw chicken,
beef, pork and turkey. Samples were obtained from local
supermarkets and tested up to the expiration day. All
samples exhibit high phosphatase activity with results
ranging from 1 to 3 x 106 RLU (see Table 2) When samples
were fully cooked to 750C and tested again, phosphatase
activity dropped 4 logs. Results from the fully cooked
meats establish the range for negative phosphatase.
Table 2
Table 2: Charm Phosphatase Test- Typical results forpartially cooked and
fully cooked groun ~ b~ef
Matrix TemperatureResults Reduction
~F ~C ~ULU %
Raw NA NAl,250,000 0
pa~iallycooked 60 l40500,000 >50
fullycooked 69.4 l57 200 >99
To evaluate further the applicability of the test to
the marketplace, hamburgers from various fast food
restaurants were tested. In all but one, the phosphatase
activity was net zero, indicating over 4 log reduction in
activity (over 12 log reduction of E. coll) . In one
sample, bought during rush hour as medium rare, low
phosphatase activity was detected (3 log reduction). This
indicates the potential of undercooking when rare to
medium meat patties are served at peak hours.
Enzyme activity was measured versus the regulatory
requirement of temperature and time of cooking. The
results indicate that response curves of phosphatase
activity are correlated with cooking time of 0-3 minutes,
at temperature ranges of 63-69OC. Fig. 2 of the drawings
shows the 4 log reduction of phosphatase activity from
ground chicken meat when heated at temperatures ranging
from 630 C to 830C.
CA 02228290 .998-0.-29pCT~s 9 6 / 1 2 4 8 2
1~ R~ 'PT~ 2 7 FEB 1997
The sensitivity level of the assay was established in
regard to the detection o~ residual uncooked portions of
the sample. Fig. 3 shows the detection limit determined
to be as little as 0.005~ of residual raw meat in a cooked
chicken meat sample.
Bee~ phosphatase activity was measured at four
di~ferent temperatures and compared to log E. coli
reduction over three minutes. The temperature o~ beef
muscle extract was raised (measured by thermocouple probe)
to 63, 67, 67.8 and 68.9~C and maintained at these
temperatures. Maximum of 4 log reduction in phosphatase
- activity could be measured by the test method up to three
minutes. At least 6 log reduction of E. coli was measured
at 2 log reduction of phosphatase activity under any of
these treatments, as shown in Figs. 4-7 of the drawings.
Table 3 summarizes the predicted log reduction when
USDA time and temperature requirements are met. Hold time
requirements will increase the log reduction of E. coli by
an additional 6 logs. The total log reduction of E. coli
at 63~C is 12.1, 67~C is 18.5, 67.8~C is l9.1, and 68.9~C
is 18.8.
Table 3
.~
Fi~re -- :~n~ ~ol~ R~uction
Temp.At 0 seconds During Dwell Time Total
~C (log) (sec) (log) (log)
4 63.0 6.1 180 6 12.1
67.0 8.5 60 6 1 8.5
6 67.8 1 1.1 16 6 19.1
7 68.9 10.8 10 6 18.8
The reduction in phosphatase activity, as measured by
the test method, is 2 logs or more. Table 2 shows
examples of test method results from uncooked, raw,
partially cooked (60~C), and fully cooked (69.4OC) bee~.
At 60~C (1400F), there is a 50~ or greater drop in RLU
IDED ~
CA 02228290 1998-01-29
W O 97/05287 PCTrUS96/12482
values, at 69.4OC (1570F), the difference is greater than
99~. A screening level can be set at 99% reduction (2
logs) which give a 95~ confidence level for fully cooked
beef burger.
Any food processing plant contains a potentially
hazardous situation in terms of microbial contamination
from raw meat or milk contaminating the finished product.
Similarly, in distribution centers, supermarkets and
restaurants, cross-contamination from raw meat to finished
product can occur when same surfaces or equipment is used.
Phosphatase activity has been tested as a marker for
contamination. By modifying the standard swab procedure
for microbial quality, a rapid test method has been
developed which identifies contamination by raw meat,
blood or serum at extremely low levels. 0.1 mg tissue or
0.1~1 serum spread over 10 x 10 cm area can be detected
using a sterile swab and testing for phosphatase activity
(see Table 4). After cleaning with 50 ppm chlorine
solution, phosphatase activity was removed. Chlorine at
50 ppm did not interfere with phosphatase activity.
Table 4
Tabh 4~ anltatlon r~stlnq uslng CPT on 4 by 4 In h squar~ ar~as
Square ;I~ial~ninp T~ ~ :- Re~t~
~LI~* -5~ppm-ch~olinc thcn.. RLJ. ource(mg) RLU
A ~9956rinsed with water ' 0~ bov ne serum (0.2) 34~1
B 3663erinsed with water ,80bov ne serum (0.2) 32~
C 11580norinse,airdry 348groundbeef(10) 147~ 9
D 2492norinse,airdry 396groundbeef(10) 2976~1
~raw meat ~).ocessing surface
The following examples further illustrate the
effectiveness of Applicants' invention.
~ le 1
Testing residual raw milk/meat/fish or other food
products.
This test measures the activity of phosphatase as
indicative of raw tissue, milk or serum in cooked products
(e.g., pasteurized milk, cheese, cooked meat, salami, cold
CA 0222829o l998-ol-2~ cT/u s 9 6 1 1 2 4 8 2
lG6 Rec'~Ç~TIP~ rEB 1997
cuts, smoked fish). It also can be used to detect cross-
contamination ~rom raw material on processing surfaces,
equipment or packaging intended for finishing products.
A sample is obtained using a probe with a sample-
collecting swab at one end. The swab can be dry forsampling wet surfaces, or is moistened with a water/buffer
solution, for example, for meat products and solid dairy
products, like cheese.
The test sample is added in a test container, such as
an elongated plastic tube having a transparent well at one
end,, to a buffer solution such as saline buffer, pH 6-10,
with preservatives (e.g. benzoic acid, sorbate), and a pH
indicator such as phenol red at 0.001~.
A chemiluminescent compound which is cleaved by
phosphatase is employed, such as a tablet with a 1,2-
dioxetane phosphatase substrate, (comprised of, e.g., CPD-
Star~, a product of Tropix, Inc.), freeze dried and made
into a tablet for use in the test method.
After incubating, the cleaving reaction is stopped by
adding a stopper solution, for example, a stopping
solution 0.0025-0.025M ethylene diamine tetroacetic acid
(EDTA), 0.05-0.2M Tris base or other biological buffers,
and optionally a pH indicator, 0.1-0.3 NaCl, pH 8-11).
Results in testing cooked ground beef hamburgers is
shown in Table 5A.
Table 5A: Test results in Relative Light Units (RLU) of
various ground beef samples held at various
temperatures and times
3 ~ Sample #1Sample #2Sample #3 Sample #4Sample #5 Sample #6
Temp. ~C (~F):53 (128)57 (135)59 (138) 63 (145)65 (149) 69 (156)
Hold Time ->:60 sec.60 sec.60 sec.60 sec. 60 sec.16 sec.
Repli~-te # F _U RL J RLU ~. ~ RLU R_U
12~ 0 1622 ' 10 1, 3
- ~ 22 ~0
i;~.J ' ~.5 8~ 6
6~ ~ 7 _
;~ 12 ~,2 0~ 1 6
6264 1C' ~ 1 8 2,7
Average 62, . 11541 02 ~ :s 0 64 5
+/- Range 4676 6285 4704 1542 95 96
% activity 95 68 60 21 0.3 8 0.3 4
13
~MENDED SHE~T
CA 02228290 1998-01-29
W O 97/05287 PCTAJS96/12482
Table 5B: Test of a dozen hamburgers purchased at a local
food chain restaurant
Hamburger # CHEF (RLU) Hamburger # CHEF (RLU)
0 7 37
2 0 8 0
3 0 9 0
4 0 10 0
0 11 0
6 0 12 0
~ le 2
The purpose of this study is to demonstrate the test
method's performance, precision and accuracy in predicting
completeness of cooking level of cooked ground beef.
Inadequate cooking has been the major cause of stomach
poisoning from pathogenic bacteria like E. coli and
salmonella.
The chemiluminescent test uses the presence of
phosphatase activity to determine whether cooked meats
have met CFR specified cooking temperatures. The test
method uses a chemiluminescent substrate for rapid
determination of phosphatase activity. The procedure
includes the sampling step, which includes using a
premoistened wet swab to sample the core of the meat
(after splitting the meat sample to expose the inner
core). Also, it can be used to swab an equipment surface
(e.g., a slicing machine, counter top surface), or other
surfaces to test for residual raw meat/milk. In the
incubation step, the swab is brought into contact with the
chemiluminescent substrate, for one to ten minutes at a
temperature range from room temperature to 650C, for
example, 550C for one minute. After incubation and at the
reading step, the reaction is terminated by raising the
pH, and stabilized by adding a stopping solution and
immediately counting relative light units (RLU) using a
14
CA 02228290 1998-01-29
W O 97/0~287 PCT~US96/12482
photometric luminometer and placing the well of the test
container with the sample, buffer and chemiluminescent
compound, in the luminator.
The average test for raw beef is in the range of
~ 5 15,000 to 20,000 RLU, while fully cooked beef gives
results in the range of 0-300 RLU (see Table 2).
- Results for ground beef heated to various
temperatures and hold times are listed in Table 5A.
Using the results for fully cooked meat, a cut off
for determining incompletely cooked meat can be set at the
upper range (e.g. 300 RLU). In the Applicants' field
samples (Table 5B), all the hamburgers were properly
cooked (all results below 300 RLU). In the Applicants'
own cooking experiment, (Table 5A), the Applicants
effectively screened low temperature cooked products
(Samples 1-4) from adequately processed and cooked
products (Samples 5 and 6).
The chemiluminescent test method accurately detects
raw meat and also can distinguish fully cooked meats from
incompletely cooked meats. Meat processed at a
temperature 20C below CFR specifications and for thirty
seconds too short a time (Sample 4), was identified as
positive in this study. Samples properly processed, and
hamburgers purchased from a local restaurant, were
negative for residual raw meat.
e 3
The test method has also been employed for ultra high
temperature (UHT) processed milk (e.g., milk treated in a
microwave ultra high temperature short time process) by
testing for the enzyme lactoperoxidase in the milk, as
well as for or an alkaline phosphatase test for milk.
The UHT milk test includes dispensing lml of a
buffered lactoperoxidase to 100~1 milk sample, incubate at
350C for one minute, add 100~1 of reagent LPO (tablet or
liquid) and count in analyzer. The LPO reagent comprises
a luminol (2,3 aminophthalhydrazide) and perborate as
chemiluminescent substrate for lactoperoxidase. Sodium
CA 02228290 1998-01-29
W O 97/05287 PCTrUS96/12482
carbonate pH 11 is added as an enhancer at the end o~ one
minute of incubation).
The alkaline phosphatase test for milk includes:
mixing a reagent AP (alkaline tablet with CDP-Star~
chemiluminescent substrate and enhancer from Tropix,
Inc.), and milk in a test tube; incubating for three
minutes at 350C, adding a stopping solution to the
incubated sample and analyzing or counting by a
luminometer the emitted light. The alkaline phosphatase
test covers the pasteurization range from 60~C to 75~C,
whil ethe lactoperoxidase test covers UHT treatments up to
95~C
ExamDle 4
The test method may further be employed as a
validation test for microwave heat treatment of blood,
blood derivatives, body fluids, or fermentation processes
o~ therapeutics to eliminate bacteria, viruses and
mycoplasme. This ultra-high temperature, short-time (UHT)
process is described in U.S. Patent 5,000,000,
incorporated herein by reference. Enzymes like acid and
alkaline phosphatase, galactosidase, glucoronidase, and
peroxidases can be used to determine is sufficient heat
was applied in the UHT process.