Note: Descriptions are shown in the official language in which they were submitted.
CA 02228308 l998-0l-30
W O 97/05473 PCTAUS96/12651
OPTICAL MICROPR08ES AND METHODS FOR SPECTRAL ANALYSIS OF MATERIALS
FIELD OF THE INVENTION
This invention relates to improved means and methods for deriving spatially
~ dirr~ t~l analytic information from a specimen by analyzing the results of the interaction of
electromagnetic radiation with the specimen, and in particular to new and useful devices and
methods of using data generated by such devices to provide in vivo diagnostic information on
said specimen. This is achieved by spatially limiting a probing electromagnetic beam to have
m~ximllm power density within a small volume element and limiting the accepted response
detected to ecsenti~lly only the same volume element.
BACKGROUND OF THE INVENTION
A significant need exists for an instrument capable of automatically providing a rapid
analysis, for example, of cancerous, ~ e~ecl, or otherwise injured tissue. In particular, there
remains a need for instruments capable of accurately diagnosing the size and stage of cancerous
growth or otherwise injured tissue. Today, the medical field generally relies on visual analysis
and tissue biopsies to analyze biological tissue for specific pathologies and abnormalities.
Various forms of biochemical im~ging are used as well, and finally, the optical response of
various pathologies are being used in attempts to characterize biological tissues. These prior art
techniques, however, contain serious drawbacks.
For example, performing a tissue biopsy and analyzing the extracted tissue in the
laboratory requires a great deal of time. In addition, tissue biopsies can only characterize the
tissue based upon representative samples taken from the tissue. This results in a large number of
resections being routinely performed to gather a selection of tissue capable of completely
representing the sample. In addition tissue biopsies are subject to sampling and inte~ ion
errors. Magnetic resonance im~gingis a successful tool, but it is expensive and has serious
limitations in detecting pathologies that are very thin or in their early stages of development.
One technique used in the medical field for tissue analysis is in~ ce~l fluorescence. Laser
induced fluorescence utilizes a laser tuned to a particular wavelength to excite tissue and to cause
the tissue to fluoresce at a set of secondary wavelengths that can then be analyzed to infer
characteristics of the tissue. Fluorescence can originate either from molecules normally found
CA 02228308 l998-0l-30
W 097/05473 PCT~US96/12651
-- 2 -
within the tissue, or from molecules that have been introduced into the body to serve as marker
molecules.
Although the mech~ni~m~ involved in the fluorescence response of biological tissue to
UV excitation have not been clearly defined, the fluorescence xign~tllre of neoplasia appears to
5 reflect both biochemical and morphological changes. For example, useful auto fluorescence
spectral markers may reflect biochemical changes in the mitochondria, e.g., in the relative
concentration of reduced nicotinamide adenine dinucleotide (NADH) and flavins. Mucosal
thickening and changes in capillary profusion are structural effects that have been hlLel~ Led as
causing some typical changes in the spectroscopic record.
The major molecules in biological tissue which contribute to a fluorescence emission
response to 337nm near-W light excitation, have been identified as tryptophan (390nm
emission), chromophores in elastin (410nm) and collagen (300nm), NADH (470nm), flavins
(520nm) and melanin (540nm). It should be noted, however, that in tissue, there is also some
peak shifting and changes in the overall shape relative to the pure compounds. Accordingly, the
15 sample can be illl-min~tçcl with a W beam of sufficiently short wavelength and responses
recorded from the above enumerated wavelengths of light in order to cletermine the presence of
each of above identified contributions to tissues types
It has been further shown that hemoglobin has an absorption peak between 400 and540nm, while both oxyhemoglobin and hemoglobin have strong light absorption above 600nm.
20 Blood distribution may also influence the observed emission spectra of elastin, collagen, NAD,
and NADH. Further compounds present in tissue which may absorb emitted light and change the
shape of the dctected emission spectra include myoglobin, porphyrins, and dinucleotide co-
enzymes.
Among other general background, we note a general belief that neoplasia has high levels
25 of NADH because its metabolic p~ w~y is prim~rily anaerobic. The inability of cells to elevate
their NAD+: NADH ratio at confluence is a characteristic of transformed cells related to their
defective growth control. The ratio of NADH-: NADH is an indicator of the metabolic capability
of the cell, for example, its capacity for glycolysis versus gluconeogenesis. Surface fluorescence
has been used to measure the relative level of NADH in both in vitro and i~ vivo tissues.
30 Emission spectra obtained from individual myocytes produces residual green fluorescence
probably origin~ting from mitochondrial oxidized flavin proteins, and blue fluorescence is
con~i~tent with NADH of a mitochondrial origin.
CA 02228308 1998-01-30
W O 97/05473 PCTrUS96/12651
-- 3 --
Collagen, NADH, and flavin adenine dinucleotide are thought to be the major
fluorophores in colonic tissue and have been used to spectrally decompose the fluorescence
spectra. Residuals between the fits and the data resemble the absorption spectra of a mix of oxy-
and deoxy-hemoglobin, thus the residuals can be attributed to the presence of blood.
S Alfano, U.S. Patent No. 4,930,516, teaches the use of lllmin~scence to distinguish
cancerous from normal tissue when the shape of the visible lllmin~scence spectra from the
normal and cancerous tissue are substantially different, and in particular when the cancerous
tissue exhibits a shift to the blue with different intensity peaks. For example, Alfano discloses
that a distinction between a known healthy tissue and a suspect tissue can be made by colllpal;llg
the spectra of the suspect tissue with the healthy tissue. According to Alfano, the spectra of the
tissue can be generated by exciting the tissue with substantially monochromatic radiation and
culll~ling the fluorescence induced at at least two wavelengths.
Alfano, in U.S. Patent No. 5,042,494, teaches a technique for distinguishing cancer from
normal tissue by varying the excitation wavelength and observing differences in the shapes of the
l S visible luminescence spectra for normal and cancerous tissue. Alfano further teaches, in U.S.
Patent No. 5,131,398, the use of lllmin~scence to distinguish cancer from normal or benign tissue
by employing (a) monochromatic or substantially monochromatic excitation wavelengths below
the visible band at about 31 Snm, and, in particular, between about 260 and 31 Snm, and,
specifically, at 300nm, and (b) comparing the resulting luminescence at two wavelengths about
340 and 440nm. While clearly defined color differences in certain dense tumor tissue are
diagnostically useful, the approach does not distinguish between normal, malignant. benign,
tumorous, dysplastic, hyperplastic, inflamed, or infected tissue. Inability to define these subtle
distinctions in diagnosis would make ~ fiate tre~tment choices nearly impossible. While the
simple ratio, difference and comparison analysis of Alfano and others offer promise for
development as useful tools in cancer research and provocative indicators of tissue status, more
effective methods are required to provide accurate and robust clinical tools.
It is quite evident from the above that the actual spectra obtained from biological tissues
are extremely complex and thus difficult to resolve by standard peak m~tt~hing programs, spectral
deconvolution or comparative spectral analysis. Furthermore, spectral shifting further
complicates such ~LL~ JL~ at spectral analysis. Last, laser fluorescence and other optical
responses from tissues typically fail to achieve depth resolution because either the optical or the
electronic instrumentation commonly available for these techniques entails integrating the signal
CA 02228308 1998-01-30
WO 97/05473 PCT~US96/12651
--4-
emitted by the excited tissue over the entire illllmin~ted tissue volume.
Rosenthal, U.S. Patent No. 4,017,192, describes a technique for automatic detection of
abnormalities, including cancer, in multi-cellular bulk bio-medical specimens, which overcomes
the problems associated with complex spectral responses of biological tissues. Rosenthal teaches
5 the ~lct~rmin~tion of optical response (tr:~n~mi~ion or reflection) data from biological tissue over
a large number of wavelengths for numerous samples and then the correlation of these optical
responses to conventional, clinical results to select a few test wavelengths and a series of
constants to form a correlation equation. The correlation equation is then used in conjunction
with optical responses at the selected wavelengths taken on an uncharacterized tissue to predict
10 the status of this tissue. However, to obtain good and solid correlations, Rosenthal excises the
tissues and obtains in essence a homogeneous sample in which the optical responses do not
include the optical ~i~;nzltnres of underlying tissues. Rosenthal's methods, therefore, cannot be
used in in vivo applications as contemplated in the present invention.
In studies carried out at the Wellman Laboratories of Photomedicine, using a single fiber
15 depth integrating probe, Schomacker has shown that the auto-fluorescence of the ~i~n~tllre of
human colon polyps in vivo can be an indicator of four different states: normality, benign
hyperplasia, pre-cancerous, and m~lign~nt neoplasia. See Schomacker et al., Lasers Surgery and
Medicine~ 1 2, 63-78 (1 992), and Gastroenterolo~y 1 02, 11 55-11 60 (1 992). Schomacker further
teaches using multi-variant linear regression analysis of the data to distinguish neoplastic from
20 non-neoplastic polyps. However, using Schomacker's techniques, the observation of mucosal
abnormalities was substantially impaired by the signal from the submucosa, since 87% of the
fluorescence observed in normal colonic tissue can be attributed to submucosal collagen. An
instrument which could discriminate the sources in depth would improve perform~nce
Accordingly, there is a need for a more effective and accurate device to characterize
25 specimens, and particularly in vivo specimens, which will obtain responses from well defined
volume elements within the specimens, and furthermore, there is a need for methods to
automatically interpret such data in terms of simple diagnostic information about the volume
elements.
It is therefore the main object of the instant invention to provide methods and means to
30 analyze a sample by inducing interaction between electromagnetic radiation and a defined,
controllable, and localized volume element within a sample and spatially restricting the response
observed to this small volume element.
CA 02228308 1998-01-30
W O 97/05473 PCTrUS96/12651
It is also an object of this invention to provide means for analyzing volume elements of
material em~n~ting weak or spatially masked signals, such as a volume element of m~teri~l
located below a proximal plane or surface of the specimen being analyzed, or above a plane that
~ emits interfering signals.
It is an additional object of the instant invention to provide means for automatically
determining the nature and/or the pathological state of biological tissues, by measuring the
response of discrete volume elements in the tissues to an exciting beam and correlating the
responses to responses of known pathologies, or to ensembles of pathology responses and
extrinsic medical data.
It is yet another object of the invention to provide in vivo diagnostic information for
tissues that are directly observable, or tissues that are accessible optically either by direct
observation or through rigid or flexible optical paths such as laparoscopes or endoscopes.
It is a further object of the invention to rapidly analyze or detect regions of cancerous,
diseased, or otherwise injured tissue with a high degree of accuracy and at low cost.
It is a further object of the invention to monitor tissue to determine whether organ or skin
grafts are viable.
It is further object of the invention to measure oxygen perfusion, hemoglobin, other
metabolites, and tissue or blood chemicals in vivo.
It is a further object of the invention to monitor temporal and spatial distribution of drugs,
especially. drugs used in photodynamic therapy.
Further objects of the invention include analyzing volume elements of a tissue whose size
is clinically significant to a physician.
These and other objects will be ~ual~llL from the description that follows.
SUMMARY OF THE INVENTION
In its broadest terms, the invention provides an instrument for eliciting and detecting a
response to radiation from delineated regions of a sample such as tissue, which is, hlLuiLiv~ly
speaking, a strong and "pure", or undiluted response. While particle beams have proven highly
effective for qualitative microanalysis of materials (e.g., chemical elements and metzlli7e~
- 30 microstructure) that can be probed by point-like high energy sc~nning, the situation is far
different for ~LL~ L)L~ to elicit responses to lower energy radiation from more complex systems in
more transparent media, such as organic materials in living systems, or chemical reactions in
CA 02228308 1998-01-30
WO 97/05473 PCTAUS96/12651
--6-
fluids. For example, acoustic (e.g. compressional wave) radiation and photonic radiation both
suffer from a number of noise-introducing drawbacks which include scattering, mode-
conversion, absorption and re-emission, and a number of related or analogous effects such that
collected responses to such radiation become diluted, impure, filtered or otherwise substantially
S altered by unrelated noise from spatially neighboring regions or from physically co-located but
substantively uninteresting intcrmixed material. The invention solves this drawback by a probe
which stimulates and collects responses from a volume element such as a layer of tissue, a
localized growth, or a reaction volume that is delin.-~t( ~l in accordance with an active process. In
general, this is achieved by arranging that the stimulation beam, such as an illumination beam,
10 has a defined irradiance distribution that falls greatly outside a first region, and that the
instrumentation for collecting responses to the stimulation has a collection efficiency that has
similar spatial discrimination about a second region, with the first and second regions
intersecting to define the probed volume element. Thus a volume element is defined by the
overlapping spatial distributions of stim~ tion and of detection sensitivity.
The invention is most readily understood using as an example the illumination of a
sample and collection of light from the sample, and accordingly in the following description the
terms "light", "illumination" or "radiation" shall be used throughout. However, it is applicant's
intention that the foregoing terms, both in the disclosure and in the claims appended hereto, shall
be understood to mean and include any form of electromagnetic radiation that may be formed
20 into beams and focused or otherwise shaped or spatially distributed using field stops, apertures
and focusing elements, and also to include acoustic radiation.
In order to more completely define and describe the invention herein, certain terms are
defined as set forth below. The invention contemplates the use of field stops which are large
compared to the resolution of the illl]min~ting and collecting path objective lenses, respectively,
25 yet which are used in configurations that together strongly define localized volume elements
from which responses are collected. Applicants have found the most useful quantitative
definitions of this aspect of the invention to involve the concepts of encircled power and
co~ e spot size. In the absence of diffraction, images could be precisely predicted by
geometrical optics, including the effects of aberrations; the concept of colll~dLi~e spot size uses
30 a comparison of the size of an image predicted by geometric optics with the image predicted
when diffraction is properly taken into account or when the image is accurately measured in the
presence of diffraction effects and residual aberrations. Encircled power is the ~raction of the
CA 02228308 l998-0l-30
W O 97/05473 PCTAUS96/12651 --7--
total optical flux in an image surface that is found within a stated boundary, for example, the
geometric image. Furthermore, resolution will vary with the position of an image with respect to
the principal points of the optical system; therefore, when accurately defined, the definition will
include the location at which the resolution is specified, which is here taken to be in the plane of
5 the field stop. For the purposes of this specification and its associated descriptions and claims,
the terms "large field stop", "field stop larger than the diffraction limited spot", and "field stop
larger than the classical resolution of its objective" mean a field stop which is large compared to
the size of the image of a point object formed by an associated well-corrected objective when
that point object is so disposed that its image falls on the location of the field stop.
A "volume-microprobe" or "volume-microprobe configuration" or "weakly confocal
configuration" means
1 ) a configuration which employs radiation to characterize a sample, and
a) which uses a pair of field stops in the illlmnin~ting, and collecting paths,
wherein the field stops are larger than the classical resolution at those field stops
of their associated objectives, and
b) in which the field stops are conjugated at least in part through a common
volume element of a sample, and~0
c) employs the observed radiation to characterize the common volume element;
or
2) a configuration in which the illllrnin~tin~ and collecting field stops are physically
the same stop.
A "volume-probe" means
1 ) a volume-microprobe configuration; or
2) any configuration which employs radiation to characterize a sample, and
a) limits the volume element from which information is extracted to a sub-
volume of the illumination and observation paths, and
b) thereby discrimin~t~ against radiation em~n~ting outside said limited volume
element, and
c) limits the volume element to less than the entire volume of the sample, and
d) permits predetçrmin~tion and at least partial control of the size and location of
said volume element to effectively discriminate between locations within the
sample.~0
CA 02228308 1998-01-30
W 097/05473 PCTrUS96tl2651 -8-
Similarly, the term "optical" and "radiation" shall include both electromagnetic and
acoustic optics and radiation, and "volume-element" shall refer generally to a localized volume
defined by the optical configuration.
In general, instruments embodying the invention operate by collecting an optical response
5 from a defined volume element, detection of this response forming a "record" which may be
either perm~nent or ephemeral. The record may, for example, be an electrical signal from a
photodetector or from a detector processing circuit, may be a visual display on a display screen,
or may be a mathematically quantified measurement or set of mea~ult:lllents stored in a memory
or hard record form.
The discussion below shall for simplicity generally assume an "ideal" medium, i.e., a
medium in which scattering or effects dependent on index of refraction of that medium do not
sensibly affect the propagation of light therein.
Turning now to a representative instrument, the objectives of the invention are achieved
by providing two optical assemblies which conjugate their field stops via the volume element
15 from which the optical response is sought. The first optical assembly is ~lesi~ne~l to image a field
stop which selectively transmits a beam from a light source, or other source of radiation, into a
volume element. The second optical assembly is designed to collect light or radiation çm~nsltin
from the target volume element, and largely only f}om the element, and transmit the light or
radiation to a detector for further analysis of the interaction of the first transmitted beam with the
20 volume element. The first optical assembly includes a field stop to achieve selective
illumination of the selected volume élement and the second optical assembly includes a second
field stop to restrict acceptance of the çnn~n:~ting radiation or light into the collection optics, to
that from the target volume element. Furthermore, a controller is provided, which in some
embocliment.~ of the instant invention, adjusts the depth of the selected volume element relative
25 to the surface of the specimen by controlling the respective image regions of the two optical
assemblies while keeping their conjugation and having the sampled volume element as a
common conjugation point for both assemblies. The general dimensions of these field stops are
always large in order to sample, or process, physiologically interesting volume elements and are
thus larger than the classical resolution of their objectives. The images of the field stops forrned
30 by the respective objectives of the two assemblies in the sampled volume element encompass
substantially all (e.g., preferably more than 95%) of the flux passing through the non diffraction
limited (geometric) images of the respective field stops, with a~plu~l;ate correction for losses in
CA 02228308 l998-0l-30
W O 97/05473 PCTAUS96/12651
_ g _
the optics and the specimen. The field stops' dimensions may be selected to define sample
volume elements that include a physiologically me~ningful size of tissue which encompasses at
least a few cells.
In some embodiments of the invention, the illnmin~ting and collection optics are the
same elements and filtering or beam splitting is used to separate the illllmin~ting beam from the
collected beam.
A configuration in which the illumination and collection field stops are conjugated is
generally considered a confocal arrangement, whereby the field stops' images formed by the
optical assemblies (the illllminz~tinp; and the collecting assemblies) overlap within the object
being viewed. Confocal arrangements in which the field stops are small "pinholes" (so that
diffraction effects govern the depth discrimination) are well known in the prior art. For example,
the confocal microscope disclosed in U.S. Patent No. 3,013,467 issued to Marvin Minsky,
provides for a double focusing, or "confocal" device having two optically conjugate pinhole field
stops, which improves the contrast of images formed by rejecting substantially all received light
ori~in~ting outside the diffraction limited focal volume, i.e., by controlling the lateral field-of-
view and by minimi7ing the effective depth of field surrounding the image surface. The data
obtained from any single such point is of little utility and in a confocal microscope only the
relative responses of a plurality of points, usually obtained by laterally sc~nning the sample,
provide the desired high contrast t~o-dimensional image.
In the present invention. t~y contrast, the field stops define a volume element with
dimensions much larger than the diffraction limited spots of the illllminz-1ing and collecting
objectives and data obtained from a single volume element contains a clean spectral signal that is
used for its diagnostic information. Indeed, while confocal microscopes obtain images of target
surfaces by the convolution of optical responses obtained from a large nurnber of closely spaced
points in a sample, the instant invention can be described as a non im~ging volume microprobe,
in that no image of the sampled specimen need be obtained, but significant analytic data are
obtained from discrete volume elements sampled. In other words, we are interested in
optimi7inp the amount of absolute optical response energy (signal) obtained from a finite volume
element, while in classical confocal microscopy the enhanced contrast in the response from a
- 30 plurality of sub-microscopic point-like sources is the desired end. As a result of these opposite
goals, confocal microscopy must use at least one field stop that is smaller than the classical
resolution of its objective, and obtain readings from a plurality of adjacent points, while we use
CA 02228308 1998-01-30
WO 97/05473 PCTrUS96/12651
- 10-
field stops that are much larger than the diffraction limited spot sizes of their objectives and
obtain useful responses from single volume elements. The use of large field stops produces
volume-limited responses many orders of magnitude stronger, and filn~ ment~lly alters the
signal-to-noise ratio of collected light, allowing detection and rapid measurement of phenomena
5 by their optical cign~tllres that often cannot be even observed by confocal microscopy. By way
of contrast, the inherent depth discrimin~tion of the confocal microscope, that is its depth of field
or depth of its diffraction limited focal volume element. depends on the wavelength of the
illllmin~ting light and on the nature of the interaction of that light with the sample; whereas the
inherent depth discrimination in the present invention depends solely on the geometric
10 parameters of the optical system.
The optical responses from the selected volume elements bear important information
about the volume elements, such as the chemical, morphological, and in general the
physiological nature of the volume elements. When the sample is spectrally simple, these optical
responses may be analyzed by classical spectral techniques of peak mz3tching, deconvolution or
15 intensity determination at one or more selected wavelengths. One such system could be for
example, a system for the ~l~termin:~tion of the degree of homogeneity of a mixture or a solution
of a plurality of compounds. However, when the samples are complex biological specimens, as
mentioned above, the spectral complexity observed with prior art instruments is often too great to
obtain meaningful diagnosis. When such biological specimens are analyzed for subtle
20 characteristics, we surprisingly found that the application of correlation transforms to spatially
filtered optical responses obtained from discrete volume elements, or the use of such transforms
in conjunction with data obtained through non imz~ging microscopy, yields diagnostically
meaningful results.
A method of practicing this aspect of the invention is as follows, we first select a training
25 set or sample of a specific target pathology; such a sample preferably has at least ten specimens.
OptiGa! responseC are first Go!!eGted ~o.m. ~uel! defined vo!ume ele,~,ents in the specilllens cLrld
recorded. The same volume elements that have been sampled with the non imz~ging volume
microprobe of the instant invention are excised and biopsied. That is, histopathological analysis
of the excised volume elements is carried out in a pathology laboratory, and the specimens are
30 scored on an all,iLIdly scale (e.g. from zero to ten) which relates to the extent of the pathology, C
(for instance a specific cancer) being characterized. These scores, Cj, where Cj is the score value
assigned to the specimen j within the training set, should be as accurate as possible, and thus an
CA 02228308 l998-0l-30
W O 97/05473 PCT~US96/12651
average of a number of pathologists' scores ((let~rmint?~l on the same volume elements, j, can be
used). We now create a set of equations ~,ajc F(Ijj) = Cj, where i (iecign~t~ a spectral window
(usually between 5 to 50 nm) and F(Ijj) is a specific function of the measured response intensity
- or other characteristics of the spectral response in the window i for volume element j . The
function F may sometimes be the response intensity itself, in that window, namely, F(Ijj) = Ijj, or
F(Ijj) = (dIjj)/d~)/Ijj, where ~ is the median wavelength in the window i, or other functions. The
factors ajc~ the correlation transforms coefficients for the pathology C, are now found from the set
of equations created above, by applying well known numerical methods, such as multivariate
linear regression analysis. In such analysis the number of wave length windows i required to
obtain faithful correlations between the optical responses and the pathological derivations of the
values Cj, is minimi7.?tl and the set of correlation coefficients a~-c for the pathology C are found.
When we now record responses (Ijk), which form a vector in the space of i optical windows,
minimi7~--1 to a limited number of discrete elements from a sample outside the said training set
and apply the transform operator (aiC) on the vector F(Ijk), namely obtain the sum ~,ajc F(Ijk) = Ck,
result is a "score" quantifying the target pathology C for the volume element sampled.
Instruments embodying the invention are deemed useful for characterizing turbid
m~t~ri~l~, such as biological tissue, water, plastics, coatings, and chemical reaction processes,
and may offer particular benefits in analysis of biological tissue, both in vitro and in vivo. To
provide internal analysis, the invention is adapted to work with existing types of rigid and
flexible endoscopes. The invention can be adapted by connecting the first and second optical
elements with optical fibers to either an endoscope, laparoscope, or arthroscope.
BRIEF DESCRIPTION OF THE DRAWINGS
For a more complete understanding of the nature and objects of the invention, reference
should be made to the following detailed description and the accompanying drawings, in which:
FIG. 1 shows a block diagram of a volume probing system according to the presentinvention;
FIG. 2 shows a schematic representation of a volume probing system according to the
present invention;
- 30 FIGS. 3A, 3B and 3C illustrate illumination and light collection portions of a system, and
their alignment to define observable volume elements, respectively.
FIG. 4 shows a schematic representation of another embodiment of a volume probing
CA 02228308 1998-01-30
W 097/05473 PCTAJS96/12651
-12-
system according to the invention,
FIG. 4A is a diagram of a practical system implementing the srllem~tic of FIG. 4.
FIGS. 4B and 4C are graphs of light collected using the system of FIG. 4A;
FIG. 5 shows a schematic representation of a multi-wavelength embodiment of a volume
5 probing system according to the invention;
FIGS. 6A and 6B illustrate an embodiment adapted to a colposcope;
FIGS. 7 illustrates an embodiment adapted for three-axis sç~nning of a sample;
FIG. 8 shows an embodiment for probing neoplasia confined to the epithelium of
muscosal tissue;
FIGS. 9A-9B illustrate an embodiment in which stationary illumination optics provide z-
axiS probe sç~nnln~;;
FIGS. 1 OA- 1 OE illustrate alternative implementations of illumination and collection
elements; and
FIG. 11 illustrates an endoprobe embodiment.
DETAILED DESCRIPTION OF THE INVENTION
FIG. 1 shows a volume probing system 10 according to the invention for illnmin~ting a
volume element in a sample l 8 and for detecting radiation em~n~ting from an illnmin~ted target
volume element in sample 18. The radiation em~n:~ting from the volume element is modified in
20 a number of aspects relative to the impinging radiation, and is herein termed the "optical
response" or simply, the "response" of the volume element sampled. These modifications carry
the unique "~ign:~tllre" of the volume element sampled and can be correlated to chemical,
morphological, physical and physiological characteristics of the volume element. The optical
response can take the form of any one or a combination of the responses of matter irradiated by
25 electromagnetic radiation, including reflection, tr~n~mi~ion, selective absorption, various forms
of ~c~ttering, various form of luminescence, and particularly fluorescence.
The system 10 has an illnmin~tor 12 for generating radiation, a first light restrictor 14,
and directing (objective) optics 16? collectively, the illllmin~tin~ optics, for illnmin~ting sample
18 with a beam formed of the radiation passed by the light restrictor 14. Probing system 10 also
30 includes collector optics 20 (collection objective), a second light restrictor 22, and a detector 24,
collectively, the collection optics, for detecting radiation collected from the illnmins~ted sample
18 which passes the second light restrictor. In addition, system 10 contains a controller 26 which
CA 02228308 l998-0l-30
W O 97/05473 PCT~US96/12651 -13-
can be coupled with the illumination and collection optics or parts thereof, for coor lin~ting their
positions so the illumination is directed to, and response is collected from a selected volume
element, e.g., for aiming the probe to provide discrete depth sections or volume element
- sampling. This is specifically achieved by having field stops in the illllrnin~ting and collecting
S restrictors 14 and 22 conjugated to each other via the sampled volume element as will be further
detailed below.
In operation, ilhlmin~tQr 12, restrictor 14, and directing optics 16 together illllmin~t~ a
volume element in sample 18. Illnminzltor 12 generates radiation and directs it towards restrictor
14. Light restrictor 14 selectively transmits portions of the radiation on to the illnmin~ting
objective 16, which focuses, or images, the light restrictor field stop onto a volume element
contained within sample 18.
Collector optics 20, restrictor 22, and detector 24 jointly operate to detect radiation
em~n~ting toward the collector optics from at least a portion of the illnmin~te~l volume element.
Collector optics 20 form an image of the illllmin~ted volume element contained in sample 18 on
the field stop of the second light restrictor 22. The second restrictor 22 then selectively passes
the radiation from the collector 20 on to detector 24. Detector 24, in turn, identifies
characteristics of the electromagnetic radiation, such as its intensity at one or more spectral lines
or regions of the spectrum.
The cooperative restrictions of the illumination and response collection provide spatial
filtering of all responses outside of a discrete volume element, thus dramatically increasing the
signal to background ratio of the detected signal from that volume element.
Controller 26 can sequentially adjust the positions of directing optics 16 and collector
optics 20 to illllmin:~te and detect a plurality of volume elements contained in sample 18, or it
can adjust the positions of the light restrictor field stops to achieve the same effect. In another
aspect of this invention, controller 26 scans the illumination beam and the detecting beam across
a two dimensional area of sample 18. This is done by redirecting the beam axes of system 10,
relative to sample 18, to various positions offset in a direction orthogonal to the axis of the beam
of radiation illllmin~ting a volume element within sample 18. Thus, controller 26 provides for
movement or aiming of system 10 relative to sample 18 along three mutually orthogonal axes or
~ 30 independent directions.
FIG. 2 illustrates representative physical elements implementinp; a system 10 according to
the invention for probing a plurality of volume elements within a sample. The illustrated system
CA 02228308 1998-01-30
WO 97/05473 PCT~US96/12651 -14-
lO includes light source or illnmin~t--r 12, an illnmin~tor coupler 32,illnminzltor coupling optics
34, first light restrictor 14, cont~ining the first field stop 36, and im~ging means, shown as an
illumination objective 16 consisting of the lenses 38 and 42, the latter being the front assembly
ofthe objective 16, and aperture stop 40 for ill~ lg a volume element 46 contained in
5 sample 18 with an input radiation beam 44 derived from the first light restrictor. FIG. 2 further
illustrates a collector 20, consisting of lenses 52 Cobjective front assembly) and 56, and aperture
stop 54, a second light restrictor 22 cont~ining a second field stop 58, detector coupling optics
60, detector coupler 68, and detector 24 for capturing and analyzing the return radiation 48
em~n~tin~ from the volume element 46.
l O Illnmin~tQr coupler 32 in conjunction with illnmin~tor coupling optics 34 provide a path
for electromagnetic radiation to travel from illnmin~tor 12 to the restrictor 14. Detector coupler
68 and detector coupling optics 60 provide a path for electromagnetic radiation to travel from
the second light restrictor 22 to detector 24. Coupler 32 and coupler 68 can be either an optical
fiber having a diameter ranging typically from 50 micrometers to 200 micrometers, or a wave
guide. Generally, the optical fibers or wave guides are multi-mode, i.e. couplers 32 and 68 can
transmit broadb~ncl electromagnetic signals. Ilh1min~tor coupling optics 34 and detector
coupling optics 60 each typically contains a lens or similar element for re~1igning or m~tchin~;
the electromagnetic waves traveling between couplers 32 and 68, respectively and the other
portions of the system. The lenses in the coupling optics 34 and 60 for~n a precisely aligned
transition minimi7ing power loss as the electrom~gn~tic radiation exits or enters couplers 32 and
68 respectively.
The illustrated first light restrictor 14 contains a field stop 36 which is intended to be
representative of any restrictor used in optical systems to limit the field of view. Generally,
reference to a "field stop" in this specification is int~n~le~l to mean any opening having any
shape, such as a circular, elliptical, square, slot-like or rectangular opening which limits the field
of view. In addition, it is understood to include openings having a static shape and size as well
as openings having an adjustable shape and size, such as a restrictable iris, and to include system
elements having an aperture which are commonly utilized to perform the function of a field stop
such as an optical fiber of defined effective cross-sectional area. In operation, field stop 36
selectively restricts light entering directing optics 16 by blocking light. Lens 38, as illustrated,
co11im~t~s the light passing through the field stop 36. In one embodiment, the collim~te~l rays of
light in the first portion of the illumination assembly enable directing optics 16, which act as an
CA 02228308 1998-01-30
W O 97/05473 PCT~US96/126~1
- 15 -
illumination objective, to be shifted axially without requiring any further adjustment of the
position of light restrictor 14. Such adjustments, which are under the control of controller 26,
allow for chzlnging the focal point of the beam 44 in the axial direction to illnmin~tt? a selected
discrete volume element along the beam axis.
Objective optics 16 can include a first aperture stop 40 to limit the aperture of the
objective lens in addition to the opening defined by the edge of the lens assembly 38 and 42 for
ims~ging the resultant beam into a defined probe volume 46 in sample 18. If provided, such an
aperture stop 40 serves to further restrict or define the cross-sectional size of the electromagnetic
radiation beam projected by lens 42 to illnmin~te sample 18. Lens 42 generally operates by
10 causing a beam of radiation 44 to converge to a narrow image of the field stop 36 at a focal
region in sample 18. The volume in sample 18 where the beam of radiation 44 comes to a focus
includes the probed volume element 46, as described in more detail in FIG. 3.
Light restrictor 14 and the focusing power of directing optics 16 cause the intensity of
input radiation 44 to be strongly peaked at the focal region of the objective optics 16 at which the
15 opening of restrictor 14 is imaged so that the intensity of radiation 44 falls off both transversely
and along the line of sight away from the aforementioned focal region in sample 18.
When input radiation 44 illnmin~tes the probed volume element 46, the radiation
interacts with materials within the sample volume limited by the field stop 36. This interaction
generally results in a secondary beam of light em~n~ting from the illnmin~ted sample in all
20 directions (47~ steradians). This secondary beam, the response of the sample to the impinging
radiation, can include reflection off material within the sample volume, scattering, and
absorption (observed as lack of response in specific parts of the spectrum), as well as unique
fluorescent emissions of light. A portion of this optical response from the sample volume, the
collected radiation 48, reaches the collector and is picked up and coupled to the detector 24.
The collector assembly 20 can include a front objective lens 52, a second a~ Lule stop
54, and a relay lens 56 which together gather the radiated energy 48 em~n~ting from the probed
volume 46 and image the largest cross section of said volume element perpendicular to the beam
onto the field stop 58. In one embodiment, lens 52 has a focal length equal to its distance from
said cross section and thus can gather the collected radiation 48 and redirect the radiation 48 in a
30 collim~tecl manner. The aperture stop 54 selectively passes a radiated beam of defined size from
the light g7ltherin~ objective lens 52, and a second lens 56 images the aforementioned cross
section of the volurne element 46 into the second light restrictor 22.
CA 02228308 1998-01-30
W O 97/05473 PCTrUS96/12651
-16-
The illustrated second light restrictor 22 contains a field stop 58. Field stop 58is
inten-1ed to be representative of any opening used in optical systems common in the art. In
operation, collecting element 20 images on field stop 58 the volume element 46, or to be more
accurate, the largest cross section of said volume element perpendicular to the collection optics'
axis. Therefore, field stops 58 and 36 are conjugated to each other via the volume element 46.
The light passing through the field stop 58 travels through coupling optics 60 and detector
coupler 68 to reach detector 24. The spatial filtering of the illumination beam to have the highest
irradiance in the sample volume element 46, coupled with the spatial filtering of light em~n~ting
from the volume element 46, combine to strongly discriminate against reception of light
ems~nsltinF from regions outside the volume element 46.
The effect of mutual conjugation of the field stops in restricting the volume element from
which em~n~ted radiation is detected will be better understood by ex~minin~ FIGS. 3A,3B, and
3C, which represent cross sections of the optical system.
FIG. 3A illustrates the illumination subsystem, where the ilhlmin~ting field stop 36is
imaged by optical element 71 (representative ofthe objective 16 of FIG. 2). Marginal rays 200
and 201 origin~ting in the illllmin~tor12 (FIG. 2) propagate from point A of the field stop 36,
and marginal rays 202 and 203 propagate from opposite point B. The marginal rays 200 and 202
pass through the margin C of the aperture stop 40, whereas marginal rays 201 and 203 pass
through the opposite margin of the aperture stop D. Rays 200 and 201 converge in the image
plane 250 at A', the image of point A. Similarly, rays 201 and 203 converge in the image plane
at point B', the image of B. A' and B' are extremities of the cross section of the image 36' of field
stop 36. If field stop 36is a circular aperture, points A' and B' lie on opposite ends of a diameter
of its circular image 36'. Marginal rays 200,201,202, and 203 then continue prop~g~ting into the
specimen 18 as shown in FIG. 3A.
In the geometric optics approximation to non diffraction limited im~ging, marginal rays
200,201,202. and 203 are geometric boundaries of the input radiation 44. In the absence of
scattering within the specimen 18, all input radiation 44is contained within an illllmin~t~-l
volume 461 bounded by points H, B',C, D, A',K.Allillllmin~ting radiation prop~g~ting through
interior points of the field stop 36, which also propagates through the aperture stop 40,will pass
through the volume element 46 whose cross section is bounded by points A', F, B', and E. When
field stop 36is circular, volume element 46is biconical and formed of two right circular cones
with apices at E and F and with common base 36'. As will be made more clear in FIG. 3B,
-
CA 02228308 1998-01-30
W 097/05473 PCT~US96/12651
-17-
volume element 46 is the only volume element in specimen 18 in which radiation 44 is
unvignetted.
The irradiance is maximum at the image 36' and falls rapidly outside the volume element
46. For clarity, in FIG. 3A, the relative size of the field stop 36 and its image 36' are shown
5 greatly exaggerated compared to the diameter of the aperture stop 40 or to the working distance
from the objective 71 to the image plane 250. Thus, the irradiance inside the illnmin~te.d volume
461 and outside the volume element 46 falls approximately as the square of the distance from the
center L of the image of the field stop 36', for example at point G.
The radiation 44 interacts with the specimen 18 which, as a result, is made to em~n:~,te
10 radiation. The nature of the em~n~te-l radiation depends on the nature of the interaction and may
include em~n:~tion into the entire sphere (4~ steradians) surrounding any source point G in the
illnmin~ted volume 461, for example, when the em~n~ted radiation is fluorescence, or may be a
more directional emztn~tion such as a forward dispersion or partially absorbed beam, or a
backscattered beam. The strength of this emanation from any source point G will be proportional
15 to the strength of the radiation 44 at that point.
For clarity of illustration first consider the collection subsystem separately from the
illumination subsystem. Because of the inherent reversibility of fundamental optical laws, any
argument properly applied to the distribution efficiency of the illumination subsystem can be
shown to apply to the collection efficiency of the conjugated collector subsystem. This
20 reversibility is illustrated in FIG. 3B showing the collection subsystem. Volume 461' contains
sources which emanate radiation which can be collected and then detected by the detector 24
(FIG. 2), and, when illumination and collection are considered together, will correspond, at least
in part, to the illnmin~te~l volume 461 of FIG. 3A.
As illustrated in FIG. 3B, radiation em~n~1ing from a point in the volume 461' will reach
25 the detector 24 only if that radiation propagates though both the collector aperture stop 54 and
the collector field stop 58. The radiation em~n~tecl from the specimen 18 which can reach the
detector is included in the radiation 48. In non diffraction limited im~ging, marginal rays 300,
301, 302. and 303 are geometric boundaries ofthe collectible radiation 48. In the absence of
sc?ttering within the specimen 18, all collectible radiation 48 em~n~tes from, and is contained
30 within, the volume 461' bounded by points H"', B"', C", D", A"', K"'. All em~n~te~l radiation 48
prop~g~tinp~ through interior points of the field stop 58, which also propagates through the
aperture stop 54, will pass, or be projectable, through the volume element 46' whose cross
CA 02228308 1998-01-30
WO 97/05473 PCT~US96/12651
-18-
section is bounded by points A"', F"', B"', and E"'. When field stop 58iS circular, volume
element 46'iS biconical and formed of two right circular cones with apices at E"' and F"' and
with common base 58'.
Any ray of radiation 48 which passes through the field stop 58 must also propagate
S through the image 58', if it originates in 18 below the image 58' (on the far side of plane 350
from objective 72), for example at point G"'. Furthermore, any ray of 48 which passes through
field stop 58 must also propagate so that it can be projected backwards through the image 58', if
it originates above image 58' (between plane 350 and the objective 72). Firstly, all radiation
which can be collected must be directed into the collection aperture of the objective 72, that is,
10 lie within the volume 461' and be directed toward the aperture stop 54; secondly, all radiation
that achieves the detector 24 must propagate through the field stop 58. This second condition
causes the collection efficiency of the collection subsystem to fall off approximately as the
square of the distance of a source point, for example, G"' from the center L"' of the image 58' of
the detector field stop.
For clarity, in FIG. 3B, the relative size ofthe field stop 58 and its image 58' are shown
greatly exaggerated compared to the diameter of the aperture stop 54 or to the working distance
from the objective 72 to the image plane 350. In actual practice, the angle C"F"'D" closely
approximates the angle C"E"'D", and, in calculations of efficiency or irradiance, either can be
replaced by the angle C"L"'D" with small loss in accuracy. The function sin(l/2/C"L"'D") is the
20 working numerical aperture (NA) of the objective 72 and defines the nominal collection angle of
the objective for light falling on the aperture stop. From FIG. 3B it can be seen that, for source
points in 461' but outside the volume element 46' and significantly below the plane 350, for
example the source point G"', the solid angle subtended by the image of the field stop 58' from
G"' is smaller than the solid angle subtended by the aperture stop from the center L"' of the image
25 58'. Thus, rays emitted from G"' may lie within the solid angle collected by the objective 72, but
will not propagate through the field stop 58, because no such rays can propagate through the field
stop without first passing through its image 58'. Therefore, the collection efficiency for such
points falls as the ratio of the solid angles so subtended, which can be expressed as falling as the
square of the distance of a source point, for example, G"', from the center of the image 58'. For
30 rays emitted into 48 from source points in the sample 18 outside volume element 46' and
between the plane 350 and the objective 72, the image 58' subtends a smaller solid angle than the
aperture stop; thus for these source points, the area of the aperture stop through which rays may
CA 02228308 1998-01-30
W O 97/05473 PCTrUS96/12651
- 19-
propagate and pass the field stop is reduced, and the collection efficiency falls in the same
manner.
For source points within the volume element 46', any ray emitted into the actual- collection angle of the objective 72 may propagate to the detector 24. Thus, volume element 46'
S is the only volume element of specimen 18 from which em~n:~ting radiation 46 is unvignetted.
Thus, vignette operates in applicant's non diffraction limited conjugated optical system to
limit the volume element from which significant signal is detected.
FIG. 3C shows the volume element 46" defined by the conjugated illumination and
collection optics when the observation angle ~ (50 in FIG. 2) is not 180~. As can be seen from
FIG. 3C, the depth discrimination is generally improved and the signal is only slightly weaker
than when a common field stop and common objective assembly are used, because of the
snhst~nti~l but incomplete overlap of volume elements 46 of FIG. 3A and 46' of FIG. 3B. The
shared volume element 46" which consists of the intersection of volume elements 46 and 46' is
the volume element through which the field stops are conjugated. In general, with the field stops
l S centered as shown and their images crossing only at a point, the peaks of the unvignetted
biconical volume elements are clipped to provide a smaller element. of lesser top-to-bottom
depth, shaped like the intersection of conic sections, from which the optical response is
selectively enhanced. The illumination and collection systems may also be shifted
translationally, so that this crossed intersection of field stop images assumes a less symmetrical
shape- e.g., a sliver, wedge or sheet-which is even more localized and may be shaped like or
aligned obliquely with a feature of the sample being observed. In that case although the total
signal may be greatly reduced, the proportion of detected signal em~n~ting from the sample may
be increased, enhancing its useful information content or its correlatability to a characteristic of
interest.
System 10 uses the conjugate stops 36 and 58 to limit the fields of view, both
transversely and in depth, within sample 18, defining probe volumes elements tailored to the
intended chara~;L~;li~Lion task of the system. A person trained in the art would note that while the
field stops 36 and 58 are conjugate to each other via volume element 46", as shown in FIG. 3C,
the two field stops are not confocal, in that the respective images of the two field stops, the cross
- 30 sections 36' and 58' respectively in FIG. 3C, do not overlap but have only a common line, the
intersection of the cross sections 36' and 58'. In general, the optical paths, the field stop shape
and position, and the objective elements may be aligned so that they define volume elements
CA 02228308 1998-01-30
W097/05473 PCT~US96/12651
-20-
which are both localized and oriented. For example, shapes such as slivers. wedges, meniscii,
conic sections and the like may be realized by the intersection of illumination and collection
regions.
A person trained in the art will also appreciate that rather than full conjugation of the two
S field stops, the invention also includes other non im~ging volume microprobe systems, wherein
the detection volume is defined by sheared conjugation, namely the partial overlap of the field
stop images, and thus the partial overlap of the two biconical structures discussed herein. One
f~ d embodiment of such systems employs the illllmin~tin~ and collection optics parallel to
each other and the objectives slightly displaced or provided with a wedge to form a common
10 image overlap region in the specimen of the two field stops, which is offset from the respective
optical axis of both assemblies.
As illustrated in FIG. 3C, in any of these configurations the interaction of the impinging
beam 44 with matter of volume element 46", which is the volume common to both light paths
cited herein, is thus spatially filtered twice and the detected light from this volume may be
expected to have an amplitude that drops off as the product of the illumination and the collection
distributions. Much lower levels of light ill-lmin~te out of focus volume elements in sample 18,
so that advantageously, the non-selected elements do not significantly mask the image of the
more brightly illllmin,.tc~l focal region, or probe volume 46. Furthermore, from the low level
light that does reach out of focus material, not inside a selected volume element, only a very
small portion reaches the collecting optics. In essence, the contribution to the detected signal of
light origin~ting outside the common volume element 46" decreases as the fourth power of the
distance from the common center of the biconical structures cited herein. This results in a many-
fold enhancement of the return signal which may be collected using apparatus of the present
invention from the selected volume element, relative to the signal collected from outside that
volume element.
As for size, by way of example, for probing a biological tissue sample having a
fundamental microstructure consisting of cells with a characteristic dimensions of one to twenty
micrometers, and a clinical macrostructure which may extend across layers or growth processes
ten to a thousand times larger, a probe instrument may have its illumination field stop defining
30 the illumination volurne selected to be tens to hundreds of micrometers in size; the collection
field stop may also be tens to hundreds of micrometers in size. In typical embo~limt?nt~,
contemplated by applicant, the illumination and collection field stops are between twenty-five
CA 02228308 l998-0l-30
W O 97/05473 PCTAUS96/12651 - 21 -
micrometers and five hundred micrometers, and the m~gnification of the objective is between
one tenth and one. The characteristic dimensions d of the field stops in typical embo~liment~ are
set by these requirements. For practical optical systems, this requires the use of large field stops.
- The image of a point-like object formed by a well-corrected objective lens is not point-
S like. It is generally complex, consisting of a brightly illnmin~tl~l central spot, which
encomp~xe~ more than one-half of the power in the image, with the rçm~ining power distributed
over the image in accordance with the shape of the aperture stop and in accordance with the
amplitude and phase distribution of the light incident on the aperture stop. Evaluation of
Maxwell's Equations in the ~ u~liate approximation show that the central spot has dimension
d' = k~/NA, where ~ is the wavelength of monochromatic incident light, NA is the image-side
working numerical aperture of the objective, and k is a coefficient of order unity. For example,
for the most common case in practical optics, a plane or spherical wave incident on a circularly
symmetric well-corrected objective, the image is the Airy pattern f~rnilizir to one skilled in the
art, and the diameter across the first nulls surrounding the central maximum is d'~ 1 .2~/NA;
approximately 84% of the power in the image is contained within that diameter.
As another example common in practical optics, in the case where the incident light is a
lowest order TEMoo laser mode, the irradiance at the image is distributed as a G~n~ n function
with the conventionally defined diffraction spot diameter d'~ 0.64~/NA; at that diameter the
image irradiance is approximately 13.5% of the maximum irradiance at the center of the
diffraction pattern and the power encomp~ecl is approximately 87% of the total power in the
image. Accordingly, the field stops contemplated for use in the present invention have diameter
d>k~/NA where k is the constant of proportionality ~plv~liate to the aperture stop and incident
light employed. For any practical combination of objective lens, aperture stop, and illumination,
it is sufficient for d > 2~/NA, and in practice usually d>>~/NA.
Neither is the image of a large object formed by a well-corrected objective the perfect
replica predicted by geometric optics. Careful ~x~rnin~tion of the edges of the geometric image
will reveal diffraction effects, but these effects are negligible except for images whose size
vx i ~ tec the diffraction spot, because substantially no optical power is found outside the
geometrical image. Thus the distribution of optical power in the image plane can be used as a
criterion of whether an object, like a field stop, is so small that diffraction effects dominate the
optical perform~nre~ of its associated objective. One such criterion is, for example, that 95% of
the optical power from an object which reaches the image surface be encompassed by the
CA 02228308 1998-01-30
W 097/05473 PCTAUS96/12651
- 22 -
geometrical image of that object.
In the embodiment of FIG. 2, of the instant invention, the selection of the volume element
46, along the optical axis is achieved by the synchronous axial movement of the objectives' front
assemblies (42 and 52 respectively). This, since we chose to have the front assembly of the
5 objectives at a distance equal their respective focal point from the selected volume element 46. It
should be understood, however, that when a new volume element need be selected, either the
sample is moved relative to the two (fixed) optical assemblies or one of the optical assemblies is
moved relative to other to keep the selected volume element as the conjugating point for the two
field stops.
Other means to obtain volume selection along the optical axis, z, of the illumination
system, ~ltili7ing other movements of elements are also contemplated, including the movement of
the field stop, or movement of other optical elements that modify the im~ging (1i~t~nce of the
objective of the illumination system. However, any movement in one of the two optical
assemblies is to be compensated by a related movement in the other, e.g., in the collection optical
assembly to assure that conjugation or sheared conjugation of the two field stops is achieved via
the selected volume element 46.
FIG. 4 shows a modified system 10' which reduces the need for coupling motive
assemblies in a non im~ging volume microprobe, wherein a common objective assembly is
employed to illllmin~te a specimen and to collect light along the same axis or path. System 10'
has an illllmin~tor 12, coupling optics 34. beam splitter 100, first light restrictor 14, objective 16,
detector 24, and a controller 26 conFIG.d for sampling volume elements at different depths by
shifting the front objective optics elements 42. The beam splitter 100 is interposed between the
front and back elements of coupling optics 34 and reflects light ent-oring from the direction of the
light restrictor 14, but passes light arriving from the direction ofthe illllmin~t--r 12. The
reflected light from beam splitter 100 is directed towards the detector 24.
In operation, illllmin~tor 12 generates a beam of radiation which travels through coupling
assembly 34 and the beam splitter 100 towards the light restrictor 14. Light restrictor 14
selectively transmits the beam of radiation which has passed through a field stop 36 to the
objective 16. Objective optics 16 form an image in the sample 18 ofthe field stop opening 36.
Light reflected or emitted from sample 18 is then collected by the objective optics 16 and is
focused back through the same field stop opening 36 in the light restrictor 14, which thus
selectively passes only a portion of the radiation from the sample 18 that reaches the objective.
CA 02228308 l998-0l-30
W O 97/05473 PCT~US96/1265]
- 23 -
The portion passed consists predo", i ~ ly or e~sçnti~lly of responses from the volume element
46. The selectively transmitted beam continues towards beam splitter 100. Beam splitter 100
redirects at least a portion of the selectively l~ beam towards the detector 24 for
recording analysis and char~teri7~tion. As in the embodiment of FIG. 2, the illumination source
S and light restrictor form a larger than-diffraction-limited illumination spot of localized illl~llsily
in the sample.
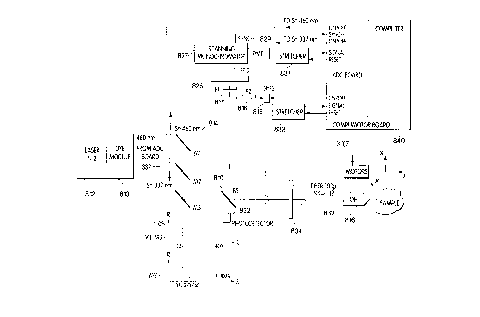
FIG. 4A shows another embodiment 10" wherein a common objective assembly is
employed to illllmin~t~ a specimen and collect light along the same path. System 10" has as
illuminator a laser, for example a Melles Griot 05-LHP-121-111 with lin-early polarized output at
10 633 nm wavelength, a beam modulator assembly 997 employed to pulse the laser output to
permit synchronous detection for signal enhancement, a polarization sensitive dichroic beam
splitter 100, for example one manufactured by the Reynard Corporation for use with 633 nm
radiation at 45 degrees incidence, disposed so that it reflects more than 90% of the incident
polarized laser radiation, a coupling lens 913, for example Melles Griot LAO011 with focal
15 length of 20 mm, which couples the laser radiation into illumination coupler 32 while m~tching
the acceptance numerical aperture of the coupler 32. This coupler consists of an optical fiber, for
example a two meter length of CeramOptic UV200/220A12 with 200 micron core and numerical
aperture (NA) of 0.12 enclosed in a nylon jacket and tPrmin~tçd with Augat SMC connectors, an
optical head comprised of coupling optics assembly 34, field stop 36, for example a National
20 Aperture stainless steel field stop with a 100 micron laser cut aperture in a PA-3 Adapter, and an
objective assembly 16~ which collectively serve to illllmin~te the sample 18. The sample 18 may
be a phantom used to calibrate the performance of the system 10", consisting for example, of
scattering elements and a fluorescent dye, Nile blue, dispersed in a transparent medium, or, for
example, it may be comprised of scattering elements dispersed in a transparent medium under the
25 surface of which is inserted a thread soaked in a dye such as Nile blue to mimic a small cancer,
or it may be comprised of natural tissue like chicken breast into which is inserted a thread soaked
in a drug normally used for cancer therapy, so as to mimic the absorbency and emission
char~tt?ri~fics of a small tumor treated with such a drug, alternatively, the sample may be such a
tumor so treated. The illllmin~1e~1 Nile blue emits fluorescence concentrated at wavelengths
30 between 670 nm and 780 nm. The optical head collects the fluorescence generated in volume
element 46 of sample 18 and couples it into illumination coupler 32 which emits into the
acceptance numerical aperture of the coupling lens 913 which, in turn collimzlt~s the collected
CA 02228308 1998-01-30
WO 97/05473 PCT~US96/12651
- 24 -
radiation and passes it to the dichroic beam splitter 100. Beam splitter 100 transmits more than
90% of the fluorescence at wavelengths greater than 670 nm. The transmitted fluorescence
passes through a set of optical filters 915, for example Schott R68 glass, which Ll~ less than
one part in 104 of scattered 633 nm illumination but transmit more than half of the fluorescence
5 to detector photomultiplier 924, which may be, for example, an H~m~m~tcu R928. The electrical
output 930 of the photomultiplier 924 is passed to a lock-in amplifier 926, for example a
Princeton Applied Research SR530 amplifier. The electrical output 931 of the pulse generator
925, (a Hewlett Packard 8003A) is adjusted to oscillate, for example, at approximately 850 Hertz
to control the beam modulator assembly and to synchronize the modulator assembly and lock-in
10 amplifier. The lock-in amplifier processes the electrical output 930 of the photomultiplier 924
and displays its average strength on a display and also passes a signal proportional to the average
strength to an oscilloscope 926, for example a Tektronix 475. The displayed signal strength is
proportional to the fluorescence emitted from the volurne element 46 being measured. A
controller 26 is employed to move the optical head 914 in three orthogonal directions so that the
15 fluorescence of dirf~lcllL volume elements may be measured.
Specifically, the modulator assembly comprises an acoustic optic deflector, for example
an Isomet 1201E with 221A-2-39 driver, which generates deflected first and higher order beams
when pulsed by the pulse generator 925 and which generates an undeflected zeroth order beam
when the pulse generator output is off. The output beam passes from the laser 912 through the
20 acousto optic deflector 991 to a turning mirror 992 which directs the beam to a mode selector
995. The mode selector 995 might be, for example approximately 800 mm distant from the
deflector 991, so that the deflected beams impinge the mode selector well separated from the
impingement point of the undeflected zeroth order beam. The mode selector contains an aperture
which is large enough, for example approximately one millim~ter in diameter, that the first order
25 beam can pass, whereas the body of the mode selector hlLc~ and blocks tr~ncmiccion of the
other modes. The first order compric.oc the laser illumination which impinges on a second
turning mirror 924 and which is directed onto the beam splitter 100 through an adjustable iris
996 which is set, for ex~mple7 to ~ploxil.~ately 3.5 mm in aperture. The adjustable iris acts to
remove any other residual deflector modes which leak through the mode selector 995 and to
30 remove any portions of the incident laser beam other than the lowest order TEMoo Gaussian
mode which predomin~fes in the output of the laser illllminzl1Or 912. This final cleaning of the
illumination beam reduces sc~ttering by the following optical elements of illumination into the
CA 02228308 1998-01-30
W O 97/05473 PCT~US96/12651
- 25 -
optical path directed toward the photomultiplier. Thus the laser illumination which enters the
optical head 914, is a cleaned up beam pulsed at the pulse frequency of the pulse generator 925.
The optical head coupler includes front and back elements 341 and 342, respectively, for
example a pair of Edmund Scientific M6387 achromats with focal length 17 mm. Sllk~emhly
34 matches the numerical aperture of the output of the illumination coupler fiber 32, and directs
the illumination through the field stop 36 which may be, for example a circular aperture of
diameter 100 microns which selectively passes the incident illumination with an efficiency of
about thirty percent to the back element 38 of the objective assembly. Element 38, for example a
Melles Griot 01LAO 028/078 with focal length of approximately 31.5 mm, is selected to
maximize efficiency of coupling through the aperture stop 40 which is typically in the range 8 to
8.5 mm in diameter and integral to front element 42. Front element 42 is one of a set of
microscope objectives chosen so that their m~gnification causes the image of the field stop to
assume a desired diameter. Front element 42 may be, for example an American Optical Corp.
44X objective with focal length of approximately 4 mm and NA of 0.66, an infinity-corrected
American Optical 20X PlanAchromat objective with focal length of approximately 8.5 mm and
NA of 0.5, or an infinity-corrected Optics for Research 10X objective with focal length of
approximately 20.3 mm and NA of 0.2 with long working distance of approximately 12 mm.
These objectives were used to perform the measurements shown in FIG. 4B which verify the
predicted performance of the system 10".
FIGS. 4B and 4C show measurements made with a practical working system of the
embodiment of FIG. 4A.
In the embodiment of FIGS. 4 and 4A, the collecting and illllmin~ling subsystems are
congruent and several elements of FIGS. 3A and 3B are physically realized by the same
elements; specifically the objectives 71 and 72, the aperture stops 40 and 54, and the field stops
36 and 58 are physically realized by the same h~.lw~. In these embo~liment~, the field stops 36
and 58 are automatically conjugated through their common images 36' and 58' (FIGS. 3A and
3B) which are also common, as are the unvignetted illnmin~ted and source volume elements 46
and 46' of FIGS. 3A and 3B. In this embodiment, the illumination efficiency and collection
efficiency are highest at the common field stop image and, relative to distant points, for example
~ 30 G in FIG. 3A or G"' in FIG. 3B, remain high in volume element 46. Similarly the collection
efficiency is high in volume element 46, which is identical to volume 46'. Overall efficiency of
illumination and collection for a source point in 460, which is identical to 461 (FIGS. 3A and
CA 02228308 1998-01-30
WO 97/05473 PCTAUS96/12651
- 26 -
3B), for example G, drops approximately as the product of the two (illumination and collection)
efficiencies times a function relating the m:~gnitll(le of emanation to that of illumination, i.e.,
approximately as the fourth power of distance from the center of the image 36' which is identical
to image 58'. For points not on the optical axis, it can be shown that the drop off is even faster.
5 Aside from losses in the specimen, a factor which does not mzlteri~lly affect the above
reasoning, were it not for the limitation imposed by the collection field stop 58 the total flux of
collected radiation 44 em~n~ting from out of focus volume elements disposed along sphere 450
(450') could contribute approximately as much background signal to the radiation detected by the
detector 24 as does the volume element 46 (46'~, because the area of sphere 450' inside the
10 illllmin~t~l and em~n~ting volume 461 (461 ') increases approximately as the square of its
distance from the image 36' (58'). However, in applicant's system, because of the collection field
stop 58, the collection efficiency drop off is fast enough to ensure that the integrated background
from all volume elements of 461 ' does not overwhelm the signal collected from the focal region,
and specifically from the unvignetted volume element and its near neighborhood.
The full width half power depth discrimin~tion of the non im~gin~ volume microprobe
system,o, is approximately o=1 .4d"/N~ where d" is the diameter of the detector field stop image
58' and NA is the image-side working numerical aperture of the objective 72, and is thus
determined solely by the geometry of the optical configuration. By contrast, in confocal
microscopy, where diffraction dominated mechz-nicm~ control the illumination and collection
efficiencies, the depth resolution /~ depends on the nature of the interaction of the incident light
with the medium. For example, T. Wilson in Handbook of Biological Confocal Microscopy, J.
B. Pawley, Ed., Chapter 11, 113-126, Plenum Press, New York, 1990 shows that forfluorescence confocal microscopy, ~~2.8~NA2, where ~ is the wavelength of monochromatic
illllmin~ting radiation, whereas ~ ~ 1 .4~/NA2, when the incident and em~n~te~l light are coherent.
FIG. 4C shows two typical two dimensional scans of artifacts similar to a small
photosensitizer-rich tumor using the d~ ldLUS of FIG. 4A. The first specimen was a 200,L~ thread
(Talon-American Sewing Bee mercerized cotton #50) saturated in Nile blue, dried, and then
coated with a thin layer of polymer (Duco cement) to prevent diffusion of the dye into the host
tissue. The suture was then inserted 500-800,u below the surface in chicken breast. Excitation
was at 633 nm and fluorescence was observed at wavelengths >670 nm. Depth resolution was
76,u and cross-scan resolution was ~25,u; the suture is clearly resolved. Minimal data
processing was used; for ease in establishing isointensity curves, the raw data was fitted with a
CA 02228308 1998-01-30
W O 97/05473 PCT~US96/12651
-27-
sixth degree polynomial and the constant background was removed, the isointensity contours are
plotted with the 50% contour bolded. Using another embodiment of FIG. 4, a second specimen
was e~mined. The second specimen was also mercerized cotton and was 130~ in diameter
- (Coats and Clark #50) ~imil~rly treated with benzoporphyrin derivative monoacid (BPD-MA), a
S drug used for photodynamic therapy of tumors. The chicken breast was partially immersed in
phosphate buffered saline and sealed with Dow Handiwrap plastic film. Here, the plot is raw
data (relative intensity vs. depth (z) and cross scan (x). These strong clear signals are possible
when a marker dye is used to enhance the signal of small tumors, and requires minim~l signal
processing to be useful to the surgeon. When the native fluorescence of tumorous tissue is
10 observed, more complex processing is generally necessary.
In FIG. 4C, the "depth" dimension lies along the optical axis of the appa~alus (the vertical
axis). The horizontal "cross" dimension was perpendicular to the thread. The functional
difference in these two axes is that the depth and transverse resolutions differ: the depth
resolution is ~76~, and the transverse (cross) resolution is ~25,u.
Although not of direct clinical interest, additional minor signal analysis will improve the
measurement even further. The contours are lengthened in the depth direction. Depth resolution
was approximately 50,u larger than the radial resolution, and, thus, we expect the 50% contour
depth to be about approximatel-~ I OO,u greater than the contour width. The actual measured
difference was about 125,u. Also. because the thread is not much larger than the depth
resolution, at the center of the thread. the observed probe-volume is filled with fluorescing target;
whereas, at the edge of the thread. it is under filled. In these circumstances a simple
deconvolution algorithm is expected to recover the cylindrical shape.
To minimi7~ the amount of laser illumination at 633 nm which is scattered from the
elements of the system 10" into the detection photomultiplier, the ~lefell~d embodiment 10" of
FIG. 4A, may position the thin reflective field stop aperture carrier tilted at an angle, for example
two degrees, so that illumination not passed is not reflected into the collection optical path,
further, the inner diameter of the optical head assembly package may be threaded and painted
with an absorbing diffuse paint so that light scattered to the walls is optimally absorbed or
blocked. A spatial filter may also be inserted in the collection path between the beam splitter
100 and the optical filter assembly 915 so that reflection from the connector at the end of the
illumination coupler 32is blocked but return fluorescence is passed.
As will be understood in part from the foregoing description and in part form the detailed
CA 02228308 1998-01-30
WO 97/05473 PCT~US96/12651 -28-
discussion below, the volume microprobe of this invention enables one to detect or monitor a
broad range of physiological effects or states. By way of example, the probe may be directed
with depth discrimination optics to monitor skin or organ grafts. Conditions such as how well
the graft is being vascularized may be readily ~letçct~--l as will be the optical ~ign~hlres of
S necrosis or of rapidly proliferating tissue (repair). Blood perfusion, hence vascnl~ri7~tion can be
measured spectrally, as can be various hemoglobins, and these measurements are resolved in
depth.
Measuring the depletion of drugs used in photodynamic cancer therapy during a
treatment is a particularly important application. One of the current drawbacks of photodynamic
10 therapy techniques is that there is no reliable way to quantify the dose actually delivered to a
tumor. Photodynamic therapy drugs are chemicals which, when flooded with light of the
opliate wavelength (usually red or near IR) are photoactivated and become toxic. They also
collect somewhat preferentially in rapidly proliferating tissue. To date, all chemicals of this type
are porphyrin derivatives related to the chemicals that cause porphyria and sun-sensitivity all
15 fluoresce readily. FIG. 4C shows the detection of one such drug, Benzoporphyrin derivative
monoacid, showing a highly selective spatial detection, when illnminz~tt?d with the device of FIG.
4A.
Another application is to determine the depth and viability of burned tissue. The hardest
decision for a trauma specialist to make in the treatment of burns is where and whether to debride
20 burned tissue. If an area is likely to recover, sç~rring, recovery time, and infection risk will be
lower if it is left alone. The best criterion for zl~e~ing such health is the depth to reasonably
healthy vasculature. This may be det( rrninto-l by looking at native optical ~ign~tllres, or
preferably by looking at a marker drug through the eschar, etc. Indocyanine green is a
fluorescent dye which has been used for this purpose; it is generally recognized as safe and is
25 used as an indicator of cardiac sufficiency. When injected intravenously, it is rapidly distributed
throughout the body and does not leak from healthy blood vessels. Thus by det~rminin~ its
depth under a burn, one could determine the depth of healthy blood vessels. Green and his
collaborators at M~.s~t~hll~etts General Hospital have patented a technique for observing its
fluorescence under excitation by first a red and then a UV probe. The W-excited fluorescence
30 is scattered more than the red-excited fluorescence, so a dirrerelllial measurement is an indicator
of burn depth. However, with the present invention, a direct and more accurate measurement is
achieved by setting volume elements at dirreLelll depths and observing the optical response to
CA 02228308 l998-0l-30
W O 97/05473 PCTAJS96/12651
-29-
precisely localize the region of intact vasculature.
While the invention has been described in very broad terms of illnmin~tin~ and detecting
light from selected volume elements in a sample where the volume elements are defined in part
- by a relatively large aperture source, the invention is understood to include instruments and
5 methods employing any known type of illumination, and indeed because of its enhanced signal
levels, their sharp spatial z-axis discrimination (namely along the axis of illumination), and their
correspondence in size or even shape to tissue layers or biological features of interest, applicants
expect that the use of the invention will reveal significant spectral response information to be
available with instruments of the present invention even using some illumination sources which
10 have previously yielded no useful analytic information of this type. Furthermore, the invention
contemplates use of a full range of detectors for receiving the collected spectral information to
detect not just amplitude or power, but entire spectra or sets of spectral features. By way of
example, FIG. 5 describes a non im~ging volume microprobe in which an illllmin:~torl2 contains
a plurality of light sources, specifically, a nitrogen laser 101 emitting light at 337 nm, a white
15 light source 102, a laser diode 104, and a light emitting diode 106. FIG. S further illustrates a
detector 24 having a sc~nning monochromator 108 and a spectrograph 110. Illuminator 12
provides multiple different light sources for probing a volume element, and detector 24 provides
multiple devices for detecting and/or plotting characteristics of the electromagnetic radiation
em~n~ting from the selected volume element. In general, illllmin~torl2 may generate
electromagnetic radiation having wavelengths ranging from below the ultraviolet to far-infrared
wavelengths, while the detector 24 and its associated data processing unit 1 12 may detect
discrete or continuous spectral inforrnation and may further include signal conditioning elements
for filtering, integrating, time-shifting and differentizlting, as well as for further processing the
collected response signal. In particular, data processor 1 12 also include means to carry on
25 analysis on a set of training specimens, for example to carry out multivariate statistical analysis,
so as to derive a correlation transform matrix, and means for applying the transform matrix
correlating responses from a specimen outside the training set to a number of pathological states
that could be present in said specimen outside said training set.
In operation, light emitting diode 106 of this system generates a red sensing or targeting
30 light that provides a visual aid for identifying the general area within sample 18 that is
illllmin~fed by the light emitted from system 10 and may be used as a targeting or steering beam
of radiation for generating steering signals to redirect the direction in which system 10 is
CA 02228308 1998-01-30
W 097/05473 PCT~US96/12651
-30-
pointing, and thus to define or stabilize the area in which the selected volume element being
examined is positioned. Suitable beam steering arrangements which operate, for example, with
galvanometer-controlled steering mirrors to aim an image or feature, and controlling one or more
steering mirrors in response to displal~ennent are known in the art. White light source 102
5 produces a broad band signal, while the nitrogen laser 101 and the laser diode 104 each generate
electromagnetic radiation having particular wave lengths. For example, nitrogen laser 101 can
generate ultraviolet light with a wave length of 337 nanometers and laser diode 104 can generate
a beam of light having a wave length of 780 nanometers, while white light source 102 can
produce a beam of light co~ a plurality of wavelengths and useful for eliciting optical
10 responses including absorption and reflection responses in a broad band of the electromagnetic
spectrum.
Nitrogen laser 101 can be used to excite the material within the volume element 46, and
cause it to fluoresce. ~mplitudes and wavelengths of fluorescence that em~n~tes from the
targeted volume element will bear important diagnostic or analytic information characteristic of
15 the volume element, and the collection system together with the data analysis system can use the
fluorescence response to provide diagnostic information on the volume element. White light
source 102 and laser diode 104 can also be used to generate beams of radiation which interact
selectively with the volume element 46 so that its response to the radiation can be collected and
diagnostic information derived from such responses. Such responses can include scattering,
20 absorption and reflection characteristics. In some embodiments of this invention, when using a
non im~ging volume microprobe such as described in FIG. 2 in conjurrction with a plurality of
light sources and detectors as described in FIG S the angular spatial distribution relative to the
min~ting bearn of the response of the targeted volume element can be used for analytic
purposes as well Such an application would involve, for instance, the continuous monitoring of
25 the growth process of bacteria in fluid or gelled growth media, or the monitoring of complex
fermentation processes
The detector portion 24 of the instrument of FIG. 5 includes a sç~nning monochromator
108 and a spectrograph 110 for analyzing radiation response emzln~ting from the targeted volume
element 46. Detector 24 analyses the radiation coming from sample 18 by first passing the
30 radiation through spectrograph 110 or monochromator 108. Spectrograph 110 disperses the
collected radiation beam into a spectrurn for further analysis, and monochromator 108 isolates
particular regions of the spectrum from the collected dispersed beam of radiation to determine
CA 02228308 l998-0l-30
W O 97/05473 PCT~US96/12651 -31-
their intensity or other properties. Once spectrograph 110 or monochromator 108 have isolated
the particular wavelengths or spectral region of interest, this further analysis can take place. In
particular, the detector 24 system can ~letçrrnine the particular wavelengths of light contained in
the collected radiation, and the characteristics of the detected wavelengths. Characteristics of
S interest at particular wavelengths include: the intensity of the radiation at the wavelength; the
polarization direction, if any, at the wavelength, and the phase shift at the wavelength. Other
characteristic of interest in the collected radiation includes its fluorescence life time, when the
line being observed is fluorescent, and wavelength shifts of the emission peaks at the
wavelengths of interest.
For control, storing and analysis of the detector data, FIG. S also illustrates a data
processor 112 coupled with detector 24, and a memory unit 114 coupled with data processor 112.
Data processor 112 can be, for example, a general purpose programmable computer, and the
memory unit 114 can be an electronic storage device such as a digital memory chip, a floppy or
hard disc, a magnetic tape, or a read/write compact disc. Data processor 112 can also contain a
15 read only memory on which resides a library of correlation transforms vectors or matrices, the
former used in conjunction with automated diagnostic of single pathologies and the latter in the
automated diagnosis of a plurality of pathologies. It may further contain a set of computer
instructions, modules and subroutines for tagging a set of samples, adding data fields provided
by keyboard input and deriving one or more correlation transforms which are then stored,
20 possibly updated or modified, and m~int~ined in a library, as is described further below.
In general, the invention is intended to operate at least partially to record and generally
also compile and analyze the responses it collects. In some low cost embodiments of the instant
invention, only diagnostic prediction of pathologies is provided, namely, the system is equipped
with a library of correlation transform vectors or matrices for specific diagnostics functions, and
25 the system only registers the signals Ijj and calculates functions F(Ijj) required to provide a
diagnostic score Cj, for a volume element j, as is further described below.
In broad terms, the output from detector 24 is fed to the data processor 112, which can
process the output from detector 24 or can store the data in memory unit 114 for processing at a
later time. Data processor 112 can also compare a first data set obtained from detector 24 with a
second data set obtained from memory unit 114. For example, data processor 112 can calculate
correlations between a first data set representative of the material being probed and a second data
set in memory unit 114. In accordance with a ~l~f~lled embodiment of this aspect of the
CA 02228308 l998-0l-30
W 097/05473 PCTAUS96/12651
-32-
invention, the second data set may amount to a library of optical response data or most preferably
incl~l(les a m~them~tical model abstracted from such a library, as described below in the section
entitled, "Methodology and Operation of the Non Tm~ginp~ Volume Microprobe."
Memory unit 114 can be used to store a large body of data about particular materials. For
S example, memory unit 1 14 can store data concerning the characteristics of light which has
interacted with a particular type of biological tissue, or memory unit 114 can store data
concerning the characteristics of light emitted by particular types of biological tissues in response
to excitation by each of a set of wavelengths of light, or can store such spectra indexed by tissue
depth, or other complex multidimensional spectral data derived from a prior set of observations.
Memory unit 1 14 can further store information associating particular characteristics of
light obtained from a biological tissue sample with a particular diagnosis. For example, the ratio
of light reflected at one wavelength to light reflected at a second reference wavelength can be
associated with a cancerous tissue growth as in certain known observations, or may be associated
with a clinically relevant condition such as a thickening of one layer of tissue, a precancerous
15 metabolic change, or a m~lign~ncy, based on correlation with the spectral library and previous
clinical characterizations. Thus, correlation with annotated or stored digitized spectra may
provide a diagnostic j~lr1gment, even without the identification of any specific individual spectral
features, such as peaks or absorbance bands, that have been required for diagnosis in the past.
Methodologv and Operation of the Non Tm~ing Volume Microprobe
In the prior art, spectral and chemical analysis of complex and heterogeneous matrices
was hindered by the inability to limit the response obtained from such matrices to regions with a
high degree of homogeneity. A large group of microprobes were developed to handle this
problem, and indeed, there exist electron microscopes and ion microprobes and various other
devices capable of providing analytic information, both morphological and to some extent
chemical (mostly elemental) on a point by point basis, or even through sections (such as with the
ion microprobe) of specimens. Unfortunately these methods all require the placement of the
sample in vacuum and the eventual destruction of the specimen, and furthermore these methods
are not conducive to the analysis of organic materials. In vivo microprobe analysis of biological
30 tissue poses requirements that are somewhat different than those of classical microprobes.
Particularly, it is not necessary to have a resolution greater the typical dimensions of
dirr~lc"~ islt~ ~1 tissues, but it is desirable to have analytic tools that can be operated by personnel
CA 02228308 l998-0l-30
W O 97/05473 PCT~US96/12651 -33-
without specific training in the analytic arts, such as physicians, process control personnel and
other professionals. With the invention of the non im~ing volume microprobe as set forth in the
present application, such microprobing of biological tissues in vivo, and other specimens in their
natural environment is achieved. There are numerous approaches by which the response data
S from such a non im~ging volume microprobe is useful, and without limiting the scope of the
instant invention, we describe herein some of these methods.
In one embodiment of the instant invention, responses from a volume element, which
represent or at least contain specific ~ign~tllres of the interaction of the material within the
volume element with the impinging radiation, are presented in terms of received light intensities
10 for a set of various discrete wavelengths, or wavelength bins or as a spectrum of the response. A
researcher trained in the specific analytic art can then use these spectra to recognize or deduce
important information about the volume element from his knowledge of the impinging radiation
and the modes of interaction of that radiation with his target material. A variety of analytic tools
such as software programs ~1e~ignecl to conduct spectral peak fitting, or spectral deconvolution
15 can be directly applied to further increase the researcher's basic understanding of the underlying
interactions and provide the researcher information on the chemical. morphological and
physiological nature of the target volume element. This in accordance with basic principles
known in the art, with the change, in accordance with the present invention, that the data
provided to the researcher are directly taken by the instrument and derived from a well defined
20 volume element, rejecting interferences and response weakening of the relevant spectrum from
light origin~ting outside the target volume element. Thus background noise no longer drowns
out the signal of interest and hinder the researcher's ability to differentiate specific features
within a largely heterogeneous sample. Because of this ability to obtain a clean spectrum from a
heterogeneous sample~ the non im~gin~ volume microprobe of the instant invention can apply its
25 collected volume response data as input to a relatively simple numerical analysis module of
conventional type to carry out classical absorption spectroscopic analysis, scattering analysis,
fluorescence analysis, Raman scattering and other parametric or characterizing analysis without
the complications that occur when applied to less well-defined or to buried signals.
In another embodiment of the instant invention, directed to users that do not possess the
30 technical skills to derive meaningful conclusions from raw responses observed, the system is
equipped with a library of correlation transforms dedicated to the users special diagnostic or
analytic needs, so the system is essentially pre-calibrated for specific analytic tasks. The method
CA 02228308 1998-01-30
W O 97/05473 PCTAJS96/12651 -34-
of calibrating the non im~ging volume microprobe is further detailed herein.
For simplicity of the following description, we will assume that the goal of the method is
to calibrate a non im~ing volume microprobe for the diagnosis of the presence or lack of a
certain condition in particular tissues that are accessible to optical visualization, either on the
S extçrn~l skin, or in the cervix, or in other cavities accessible via endoscope or laparoscope, such
as the various segments of the gastrointestinal tract (starting from the mouth, through the
esophagus and the stomach, and, by rectal çx:~min~tion, the colon), or various organs in the
peritoneal cavities that are accessible via exploratory laparoscopy. In may of these situations, a
physician who is not a trained spectroscopist, presently must view the suspected tissues, and
10 when discoloration or other morphological abnormalities are present, must excise samples from
such areas and send them to a pathology laboratory for microscopic ex~min~tion to determine the
presence or lack of cancer pathology, as well as the stage of possible cancer. The present
invention provides, during the visual exzlmin~tion, a non-invasive optically derived diagnostic
scoring to ~lçtermine the nature of the suspected pathology of the suspicious target tissue, so that
15 immediate action can be taken, if necç~ry, and in any case avoiding unnecessary excision of
tissue for biopsies. Moreover, when calibrated as described below, the non im~ginE~ volume
microprobe of the instant invention provides automated diagnosis of such viewed tissues by a
physician, without the need for a pathologist to examine such tissues under the microscope.
In order to calibrate the non im~ging volume microprobe for a specific pathology, we first
20 select a training set of specimens for the specific pathology. The term "training set" is used
herein to denote a group of tissue specimens on which very exacting determination of the state of
each specimen has been previously conclucte~l in a pathology laboratory. Furthermore, prior to
excision for such biopsies, each specimen in the training set preferably has been subjected, in
vivo, to illumination and detection with the non im~ging volume microprobe of the instant
25 invention to provide a stored spectral response record. For the purpose of this description, let us
assume that the target volume elements of this training set (those tissues that are later subjected
to a pathology laboratory ~lstçrmin~tion of their pathological state) are investigated with the
volume microprobe of FIG. 5 They are illnmin ~tecl with both a laser UV source (101) and a
broad band white light source ( 102). Let the intensities of the responses to the UV and white
light excitations of the targeted volume element within the specimen j be denoted Jui and I
respectively, where u and i are central wavelengths within spectral bands of the spectral
responses to the UV and to the white light excitations respectively. For example, the Jui may be
CA 02228308 1998-01-30
WO 97/OS473 PCTAJS96/12651
-35-
fluorescence and the Iij may be backscatter responses, respectively. These data are stored in
memory unit 1 14 for future analysis. The training set are excised after recording the responses
obtained with the non im~ing volume microprobe and pathological (let~rmin~tion of the state of
each specimen are recorded in the form of scores Cj, where j is the identity of the specimen and
S Cj is a number selected according to the specimen state on a monotonic scoring scale, for
example a single-axis scale of zero to ten, where zero denotes normal tissue and ten corresponds
to a characterization as a fully entrenched and deep cancerous tissue change. This training set is
used to calibrate non im~ging volume microprobes for future ~l~termin~tions of the presence or
lack of each of the tissue pathologies represented in the set, so it is important that great care be
taken in arriving at an objective determinz~tion of the pathological state of the training set.
Preferably, the same samples are examined microscopically by a number of independent
pathologists in a blind experiment, and only such specimens for which there is a valid threshold
of agreement between the various pathologists are included in the training set.
Once the scores Cj of the specimens in the training set have been carefully determined,
the values of Iij and Jui previously stored in memory unit 1 14, are used to set up a set of j
correlation equations:
~,aj F(Ijj) + ~bu F(Juj) = Cj (1)
The band widths around the wavelengths i and u of the collected narrow band responses
to white light and UV light respectively, may generally be set between S and 50 nm, depending
on the spectral resolution achievable or desirable in the system's detection monochromator 108 or
spectrograph 1 10.
The selection of the functions F depends to some extent on the nature of responses
received. When collecting almost featureless spectral responses (namely a spectral response
which is relatively smooth and changes slowly with the wavelength), then one may select the
response intensities, or norm~li7~l intensities, namely, F(Ijj) = Iij or F(Iij) = Iij/K, respectively,
where K is either the maximum response in the received spectrum or K the response at a
predetermined wavelength (which, for example, in biological tissues, may be a response
associated with the presence of water, or of hemoglobin). When the expected spectrum contains
a number of sharper features, one may set F(Iij) = (dIij/d~)Iij, where ~ is the wavelength.
~ 30 The data processor 112 next performs a regression analysis to minimi7~ the number of
wavelengths i and u used to obtain a valid correlation and to solve the set of minimi7P~l equations
(1) for the correlation constants. This regression analysis is carried out using the j equations
CA 02228308 1998-01-30
W097/05473 PCT~US96/12651
-36-
obtained experiment~lly, using in essence the correlation constant as unknowns, for which a
solution having the best correlation is sought. The minimi7~tion is carried out to extract a
minimllm number of wavelengths whose responses Ijj and Jui provide satisfactory correlation with
the phenomenon being measured. It should be appreciated that during the calibration process7 a
5 greater amount of data is collected than absolutely necessary, and much of these data are
interrelated. In m~them~tical terms, the minim~l set may be a basis of responses for this tissue.
Once a minim~l set of responses has been cletermineA, this allows the taking of a minim~l set of
responses during subsequent actual diagnostic use of the non im~ging volume microprobe (e.g.,
responses at a minim~l number of narrow wavelength bands), and thus accelerates the procedure.
The methods used for obtaining the minim~l set of wavelengths and the associatedcorrelation coefficients aj and bu are well known in the prior art and include multivariant linear
regression analysis and univariant linear regression analysis. Other statistical tools such as neural
networks analysis are also available and can also be applied to this task. These statistical tools
have been reduced to simple software programs such as those sold or available, for instance,
15 under the name STATISTICA by Statsoft, Inc. or PREDICT by Neural Ware, Inc.
In general, we denoted by the values Ijj and Jui~ the responses of the volume element to
white light and UV excitation respectively. Other responses might be used to characterize the
volume element 46, more generally, we denote all responses which are responses from volume
elements that correlates with certain pathologies Rjj. We find that it is sometimes advantageous
20 to include as part of the responses Rjj, i.e., to include as a data field in the memory record
representing Rjj. other information about a volume element which was not determined with the
help of the non imz~ging volume microprobe but still contributes to improvement in the
correlation between the observed responses and the pathologies diagnosed. Such information
might include general medical record information, such as classification of the subject in which
25 the volume element resides, including, but not limited to features like sex, age, race, and weight.
Such information, when its inclusion in the regression improves the confidence level of the
regression, can be included as additional "artificial" responses Rjj. The index i therefore
represents the type of response obtained whether it is obtained with the non im~ging microprobe
(one or more types of responses as well as the spectral band from which the response is
registered) or by other means such as extrinsic patient, population or source data.
The set of equations ( 1 ) from which the correlation coefficients are derived can thus be
simplified to be:
CA 02228308 1998-01-30
W O 97/05473 PCTAJS96/126~1 -37-
~,aj F(Rjj) = Cj (2)
For notational simplicity we call the ordered values ~aj}the correlation vector (a) for the
pathology C, and the ordered responses Rjj the responses vector (Rj) for volume element j in the
training set. The functional responses vector (F(Rj) is similarly defined as the ordered functions
5 of the responses elements in the responses vectors (Rj). Similarly, the ordered scores Cj can be
termed the pathology score vector (C) for said training set. The process of calibrating the non
im~ging volume microprobe for a given pathology C, consists therefore of compiling the all the
response vectors (Rj) and their corresponding pathology score vector (C) and from these data,
after generating the functional response vector (F(Rj), ~let~rmining a minim:~l correlation vector
10 (a) which is the calibration vector of the non im~ging volume microprobe. These mathematical
constructs are then stored in memory, together with basic software for applying them to a new
response.
Clinical operation of the probe proceeds as follows. When a calibrated instrument is now
used to determine the extent of a given pathology C in a volume element outside the training set,
15 (where this new volume element is denoted k) the responses vector (R,~) is registered by the
instrument on the volume element k, and to the extent that some of the responses Rik are artificial
responses (e.g., extrinsic data from medical records, such as sex or race), these are entered into
the data processing unit 1 12. and the score for the pathology C for volume element k, Ck, iS
predicted by obtaining the product of the correlation vector (a) with the functional responses
20 vector (F(Rj)), namely: C,; =~,a, F(R"~). Thus the use of the calibrated non imslging volume
microprobe on a volume element k ~hose pathological state Ck is unknown, allows for the
immediate and automatic testing and diagnosis of the pathology C in the volume element k by
application of the stored mathematical operator to the observed Response vector.It should be appreciated by persons trained in the art that the non imslgin~ volume
25 microprobe of the instant invention can be calibrated to diagnose a plurality of dirrelell~
pathologies Pm~ where each m denotes a specific pathology. When used in this fashion, the task
of calibrating the instrument for this plurality of pathologies consists as before of taking a
training set of j responses Rjj and scores Pmj~ where i denotes the bandwidth of the response or the
type of artificial response, j the volume element or the specimen in the training set, and Pmj the
score for pathology m on specimen j. As before, during calibration we obtain a number of
correlation vectors (am), each for the specific pathology m. In operation of the calibrated non
im~gin~ volume microprobe, the correlation vector (a) mentioned above is now replaced with a
CA 02228308 1998-01-30
WO 97/05473 PCT~US96/12651
-38-
correlation matrix {a} whose elements are ajm, the functional responses vector (F(Rk)) for an
uncharacterized specimen, k, is replaced with the matrix {F(Rk)} whose element are F(Rjmk), and
the diagnostic results are given as a vector (P)k whose elements are Pmk obtained by the product
of the correlation matrix {a} with the functional responses matrix {F(Rk)} . Thus, once a set of
S diagnostic conditions of interest has been identified and a suitable training set of responses
processed to generate these matrices, the diagnostic score for each condition is computed
automatically for a new sample by a simple matrix operation on the volume element response.
It should also be appreciated that in the practical embodiment of this method of analysis,
the correlation created will use the same responses, or at least a partially overlapping set of
10 responses--e.g., magnitudes of detected radiation in a specific set of defined wavelength bands--
for different pathologies. Thus only a vector of responses (Rk) (having elements Rjk) is required
which includes the minimz~l set of responses from volume element k to obtain diagnostic scores
Pmk. The matrix {a} can also be termed the correlation transform matrix, since it transforms one
set of measurable values (or observables), to another set of numbers or values, which are the
15 desired pathology scores. This is achieved by multiplying the correlation transform matrix, {a},
with the vector, the functional responses vector, (F(Rk)) to obtain a transformation of the
responses vector (Rk) to a vector of diagnostic scores (P)k.
It should be further appreciated that by probing a plurality of adjacent volume elements in
the optical axis direction (the z axis) one obtains the penetration depth of certain pathologies by
20 plotting the scores Pm(z) for the pathology m as function of depth z. Similarly, an artificial three
dimensional image of the pathological state of an area can be obtained by repeating the
procedure for a number of adjacent volume elements in the xy plane (the plane orthogonal to the
optical axis of the non im~ging volume microprobe), where the gray scale or the color for each
volume element correlates to the diagnosed scores Pm(x,y,z).
Since, as described above, the volume probe collects the response from a well localized
area, this manner of plotting "pathology gradients" within a sample may elucidate the growth
processes involved in (1i~ç~e-1 tissue, and is expected to elucidate complex relationships between
such processes and different surrounding tissue types.
A correlation transform method such as exploited herein, for predicting diagnostic or
30 analytic information on an unknown specimen by correlating optical responses of a training set
to independent detçrmin~tion of diagnostic or analytic data on the training set, has been shown
by Rosenthal to work well on artificially homogenized samples that are large enough to provide a
CA 02228308 1998-01-30
WO 97/05473 PCTrUS96/12651
-39-
set of responses pos~es~inP~ large signal to noise ratio. It is surprising that the ç~p~ncle-l but
similar method of the instant invention yields good correlation on very minuscule volume
elements in vivo. In classical spectroscopy, for instance as described by Alfano, spectra or
optical responses of ~ e~e~l tissues are compared to similar spectra or responses of healthy
5 tissues to attempt a ~ nostic reading on the target tissue. This method generally is not sensitive
and robust enough for in vivo clinical application because of the large variations encountered
between subjects and the nature of the tissue examined. When using the above described
correlation transform method, we purposefully avoid using comparison of spectral responses in a
target tissue to the responses of any existing (healthy or pathological) tissue. Since no one
10 specific tissue can represent all the variations encountered between subjects, subject to subject
variations cause spectral distortions that invariably weaken the ability of the prior art to obtain
robust diagnostic determin~tion of pathologies. Furthermore, by our inclusion of non optical
responses together with the optical responses as part of the correlation transform algorithm, we in
essence build a completely artificial model (based on the training set) of the pathology, which by
15 itself is never reproduced in any one subject or tissue. This novel approach coupled with the
spatial filtering of the optical responses to a small volume element, are believed to account for
the success of the invention in predicting diagnostic states of tissues in vivo.It will be understood that the enhanced information content of the detected optical
responses may be observed by other processing regimens known to be useful for analysing data
20 sets. Thus, for example, rather than the particular correlation regimen described above for
deriving transforms T to evaluate particular pathologies, the instrument may apply transforms
derived by any of the processes of peak m~tl~hing, spectral deconvolution, spectral ratio
m~tching, self norm~li7~1ion~ Fourier transform analysis, discrimin~nt analysis, linear univariate
and multivariate regression analysis, non-linear univariate and multivariate regression analysis,
25 partial least squares regression analysis, principal component analysis, and neural network
analysis. As with the above described embodiment, it may apply these processes to directly
compare a sample with a stored data set, or may apply a stored transform T developed by such a
derivation process.
It should be emph~i7e~1 that the enhanced localized information content of the optical
responses collected by the present invention may allow discernment or distinction of states which
need not be so extreme as to amount, for example, to an entrenched or established disease state.
Indeed, slight degrees of differentiation, transient metabolic or circulatory effects and other such
CA 02228308 l998-0l-30
WO 97/05473 PCT~US96/12651
-40-
conditions may all be revealed in the collected spectral data. Thus the ~lçtectç~l condition may be
one ore more of the set of burn, necrosis, infl~mm~tion, tissue repair, graft hez~ling, graft
rejection, lesions, cancer, cancer precursors, benign hyperplasia, benign dysplasia, tumors, the
presence and concentration of specific compounds wherein said compounds are naturally
5 occurring agents and their metabolites, the presence and concentration of therapeutic and illicit
agents and their metabolites, and other such pathologies or conditions. Furthermore, as applied
to samples of non-biological material or pure chemicals, the responses may be processed to
detect diverse physical or physico-chemical phenomena such as spectral redistribution,
polarization shift, temporal shift, spectral shift, Zeeman splitting, Stark splitting, phase ~hifting,
10 shifting of the frequency and amplitude modulation of the intensity of the em~n~tç-l light with
respect to the illumination.
Also, the nature of the collected light may vary depending on properties of the sample
and the optics involved, to selectively detect one or more of scattered illumination, tr~n~mittç~l
illumination, attenuated illumination, reflected illumination, Raman scattered ilhlmin~tion,
15 autofluorescence stimulated by the illumination, and marker and therapeutic agent fluorescence
stimulated by the illumination The basic illumination in turn may be provided by a broad band
source, a narrow band source, a substantially monochromatic source, a light emitting diode, a
laser or a frequency and/or arnplitude modulate intensity source.
20 Exemplary Instruments
Returning now to a discussion of instrumentation of the present invention for particular
applications, FIGS. 6A and 6B illustrate a colposcope embodiment of the invention.
As shown in FIG. 6A. a non im~ging volume microprobe 220 of the present invention
attaches to the patient-side dovetail of a conventional colposcope 210 and couples its
25 illumination and collecting paths into the objective path of the colposcope so that the viewing
gynecologist may see and identify an area where diagnostic scoring of specific tissue is desired,
both at the surface observed and to some depth below the surface. In this instrument, the
colposcope has an effective working distance of about 30 cm and the optical head 220 is aligned
and attached to the colposcope housing to m~int~in stable ~lignment with the colposcope, while
30 small steering mirrors inject or catch the probe beams. A joystick 201 and coupling mirror allow
the optical paths of the non im~ging volume microprobe to be steered within the colposcopic
field of view and overlap this field of view. The head design permits mounting from either side
CA 02228308 1998-01-30
W O 97/05473 PCTAUS96/12651
-41-
to suit the handedness of the gynecologist, so that the ColpoProbeTM does not intrude into the
space used by the gynecologist for manipulating instruments like biopsy forceps. The rem~in(ler
of the instrument is connected to the optical head by a set of optical fibers approximately 5 m
long This allows the bulky portion of the system to be well out of the way.
In operation, a gynecologist would use a non im~ging volume microprobe that has been
precalibrated, namely, it has in its memory a correlation matrix {a} as described above which
correlates light responses collected from target volume elements in the cervix with any of a
number of possible pathologies such as, but not limited to, infl~mm~tion, repair, high and low
grade squamous intraepithelial lesions, or neoplasia. The gynecologist points the non im:~ging
10 volume microprobe to the desired zone, where a pathology is suspected. The gynecologist may
also enter "artificial" responses, i.e., non-optical information such as age, if postmenopausal, how
long, if premenopausal, time in the menstrual cycle, or other extrinsic medical-record-type
information. These artificial responses correspond to further variables which may be values on
which the correlation transform operates. The gynecologist then starts the registration of the
15 desired responses from the subject zone with the non im~ging volume microprobe, including z
sc~nning of the depth of the suspected tissue pathology. The instrument samples and holds the
observed response when triggered, or it may be configured to automatically sample a plurality of
responses in a local pattern. Once the non im~ging volume microprobe has m~mlzllly or
automatically taken the requisite number of responses, the correlation transforrn is applied to the
20 responses vector to transform it to a vector of scores for the pathologies for which the instrument
was calibrated. This is achieved in the processor 112 by calculating the vectors of functional
responses (F(Rk)) for each volume element, k, sampled by the probe, and multiplying it by the
coITelation transforrn matrix {a} which resides in memory 114 and was derived during the
calibration of the non im~ging volume microprobe.
One prototype instrurnent for volume-limited enhanced signal collection of tissue has
been developed for better biopsy of cervical cancer and dirr~lcllLial diagnosis of cervical
abnormalities. This instrument, to be sold under the trade name ColpoProbeTM is shown
schematically in FIG. 6B as instrument 10''', and is designed for non-cont~ctin~ clinical
applications of spectrophotometric measurements to this diagnosis task. The ColpoProbeTM
30 examines spectral ~ign~tllres of tissue in dirrclcllt states of health, for example by measuring and
processing autofluorescence and spectral b~k~c~tt~r measurements from volume elements of a
size on the order of a few hundred microns on a side or bigger, interesting to the gynecologist at
CA 02228308 l998-0l-30
W 097/05473 PCTrUS96/126~1 - 42 -
colposcopic working distances, typically 300 mm. The mea~u.~ lent mode and the data
proces.~ing each figure substantially in providing clinically useful data.
FIG. 6B illu~LldLes the module 220 in greater detail. A plurality of fibers 202 couple
various source or detector elements to positions within the module, and various position switches
5 and motorized controls move the illumination and collection fiber assemblies to mzlint~in a
desired focal distance and control their mutual overlap in the specimen so as to define a selected
probe volume as shown in FIGS. 3A-3C. An assembly 221 of relay mirTors in the optical head
220 couples these elements into the optical path and m~int~in~ dir~e~ sources and paths
separate, allowing one or more illumination sources to be switched in, and collected light to be
10 conducted back to the detector assembly, without interference. A curved coupling mirror acts as
the common front objective assembly for the analyzer and viewing optical paths. Notably,
following the basic light sources and their relay or coupling fibers, all beam-forming and beam
directing elements are reflective, thus avoiding the chromatic aberrations of refractive optics.
The various fibers coupling the light sources to their respective relay lenses may be of relatively
15 large diameter, for example 100-300~m, and serve as large aperture sources. The fiber ends may
be shifted by stepper motors to laterally shift, and to advance or retract the depth of focus in the
target tissue.
FIG. 6B also shows other major components of the instrument 10' ' ' as well as the
optical head 220, spectral sources, a spectral measurement section, a control computer 240, and
20 analysis software.
The optical coupling between the ColpoProbeTM and the colposcope is mediated by a
wavelength-selective aiming milror 230 which is controlled by a joystick 201 and does not block
the field-of-view. This makes colposcope operation "transparent" to the gynecologist when the
ColpoProbeTM is not actuated, i.e., the colposcope can be used as if the ColpoProbeTM were not
25 mounted and there are no noticeable visual or mechanical effects. When Z~'.tll~t~i, the
ColpoProbeTM enters either an aiming or measuring operating mode, selected by convenient
push-buttons. The joy-stick allows the physician to select a sample point within the colposcope
field-of-view by using a marker beam projected by the ColpoProbeTM. In the aiming mode, the
gynecologist moves the pointing beam by manipulating the joystick to select a site for
30 ex~min~tion. The physician then selects the measurement mode. After a sample point is
selected, instrument pointing is held fixed and a depth scan is made from 2.5 mm above to 2.5
mm below the aiming point.
CA 02228308 1998-01-30
W O 97/05473 PCTrUS96/126~1
- 43 -
The aiming light remains on, so that, if desired, it is simple to precisely biopsy the spot
selected. Also, because multiple biopsies are frequently performed, a microphone is installed in
the optical head so that the physician can identify the location of each sample as it is taken.
The ColpoProbeTM employs four optical ch~nnel~ to interrogate the cervix. An
S illumination channel 231 merely passes light from the colposcope's internal illLullill~Lion source
to the cervix and visual data from the cervix back to the colposcope, without introducing any
further effects visually noticeable to the physician. A white-light channel 232 uses a broad-band
5,300~K light source for spectral backscatter measurements. A red, 635 nm, channel 233 is the
aiming channel which provides the marker beam. This beam provides a visually discernible
10 bright spot, which shows the aim of the instrument, and may also be used for an automated
tracker control feedback loop. Finally, the 337 nm UV channel 234 is used to excite
fluorescence and for backscatter measurements. FIG. 6B also shows a fifth channel at 780 nm to
measure the position of the volume element whose spectral signature is being measured with
respect to the cervical surface. In a ~l~f~ d embodiment, this function is assumed by the red
15 channel 233.
FIG. 6B is a schematic of the device. Because of the wide spectral range covered by the
several light sources, no refracting elements are employed in the optical train; this avoids
significant problems with secondary spectrum associated with refractive focusing elements. In
addition, these optics permit p~ ging of the instru~ent in the space availabie to a usual
20 colposcope add-on. The following description proceeds generally from left to right and top to
bottom of that diagram, FIG. 6B.
Among the principal components of the optical head 220 are mirrors Mj for focusing the
interrogating beams on the cervix, at a ~ t~nc e of approximately 300 mm from the colposcope
objective lenses, and for necessary beam manipulation; mirrors Mj are shown schem~tically as
25 oblique heavy black line segments with closely spaced short ruling behind the reflecting surface.
The optical head also contains wavelength-selective beam-combiners (dichroic mirrors) shown as
short oblique heavy line segment~ without rulings. Other elements are a depth sç~nning
meçh~ni~m for the excitation channel and a range detector.
A transmitter block 250 contains the excitation laser 251, for example a Laser Sciences
30 VSL-337ND-S, and the 5,300~K white-light source 252, for example a Welsh-Alyn M24E001.
Also shown are a monitor for measuring excitation output energy 261 and a sc~nning
monochromater 262, for example a Monospec 18 Spectrograph Model 479, which analyzes
CA 02228308 1998-01-30
WO 97/05473 PC~rUS96/12651 -44 -
b~- kcç~ttered white light. The excitation source in the prototype is a nitrogen laser. This may
be replaced with a dye laser head, if another, or more than one, excitation wavelength is needed.
The nitrogen laser is pulsed, for example at 25 times per second; during the intervals
between its 5 ns pulses, the white-light, aiming, and ranging sources are independently pulsed
5 under computer control.
The receiver block 260 contains the aiming and position monitoring channel source 253,
for example a 635 nm laser diode, the position monitoring receiver 261, a 337 nm b~qek~ç~tt~r
receiver 262, and the fluorescence spectral analyzer 264, which may be, for example an Acton
Spectropro 150 and a Princeton Instruments Spectral Multichannel Analyzer with an intensified
10 CCD detector package model CCD576MGE.
The control co~ uLel 240 integrates the operation of the various sub-assemblies, for
example through the use of National Instruments LabView(~) software. The computer controls
system operation and ~ligiti7~?c and stores raw data for further analysis. In addition, the processor
and memory of control computer 240 may also perform the functions of the processor 112 and
15 memory 114 of FIG. 5.
FIG. 7 shows another embodiment of an optical probe module 300 in accordance with the
present invention. Module 300 is implemented in a single housing 310 cont~ining the basic
optical beam ~lefining elements previously described, and coupled by input and output fiber
optics 313, 311, to the illumination and detector portions of the apparatus, respectively. The unit
20 3 l O is shown as mounted on an x, y, z stage, and a computer controls the chopping of the input
beam, spectral selection, stepping of the apparatus over a sample, and recording an analysis of
the received illumination such that many hundreds of measurements on different probe volumes
may be acquired over a short time to provide a spectral profile throughout the sample.
FIG. 8 is another embodiment of a prototype non imzlging volume probe built by the
25 applicants for probing early cancers, which in this case are cancers origin~ting in mucosal
tissues, and specifically for detecting autofluorescence signals from neoplasia confined to the
epithelium of the mucosal lining of body cavities, while specifically rejecting hlL~lrelillg signals
from collagen in underlying stromal tissue. The embodiment of FIG. 8 is int~nclecl to probe
autofluorescence excited by two wavelengths; 337 nm and 460 nm. The illumin~tc-r 812 is a
30 pulsed nitrogen laser at 337 nm with an ~tt~Ch~ dye laser module 813 for generation of 460 nm
excitation. A shutter mechanism 814 is controlled by a computer 840 to switch between the two
excitation wavelengths, and a shutter mech~ni~m 815 switches a long wavelength optical cut off
CA 02228308 1998-01-30
W O 97/05473 , PCTrUS96/12651
-45-
filter 826 into the collector optical path when the 460 nm excitation is employed. Optical filter
826 passes collected fluorescence longer than the 460 nm excitation light but blocks scattered
excitation from entering the collection spectral discriminator 827 and detector 828. Optical filter
817 blocks excitation radiation at 337 nm, but passes longer wavelength fluorescence.
Photodetector 832 monitors the output of the excitation laser to permit pulse by pulse
comparison of the collected signal by providing a correction signal indicative of the changes in
the laser illuminator output. Fluorescence intensity follows the illumination pulse intensity,
which pulse has duration of approximately 5 nanoseconds; and electrical pulse stretchers 831 and
832 lengthen the output electrical signals which are generated by these short pulses of
10 fluorescence so that the signal processor may accurately determine the energy carried in each
pulse. The wide spectral bandwidth of this implementation requires that chromatic aberrations
be closely controlled, and this is accomplished by use of all-reflective optical elements; mirrors
826 and 834 are off-axis parabolic elements used to couple light efficiently into the sc~nning
monochromator and into the optical fiber 832, respectively. The optical head 816 is an all
15 reflective microscope objective selected for its long working distance and high numerical
aperture The optical fiber 832 couples excitation illumination into the head and fluorescence
from the head into the detector optical path. Separation of the illumination and collection optical
paths is accomplished by a beam splitter 810.
In addition, this app~lus incorporates a preferred implementation of the field stop in
20 which the end of the multimode fiber 832 closest to the objective 816 is the first, as well as the
second field stop This arrangement contrasts to that of the embodiment of FIG. 6B, wherein
separate multimode fibers are employed as the first and the second field stops for the
ColpoProbeTM implementation.
FIGS. 9A and 9B illustrate a further variation of this illumination assembly. In this
25 embodiment, a plurality of input tr~n~mi~ion fibers 313a, 313b... are disposed in a helix around
a cylindrical contour of radius R with the face of each fiber offset from the preceAing one by a
short distance L and directing its output in the axial direction A. The cylinder of radius R may
for example be a large aperture collection fiber, or may be a mechanical element such as a tube
or ring. Other combinations will be evident to one skilled in the art. The output beam from each
30 of the many fibers 313 may be focused by the single objective lens 301 into a different probe
volume region in the sample 320. FIG. 9A illustrates the output beams from two fiber ends
313a, 313b with their outside edge rays denoted by 1, 2, respectively, focused into two different
CA 02228308 1998-01-30
W O 97/05473 PCT~US96/12651 - 46 -
probe volumes in the sample. FIG. 9B shows a detail of the sample in the region of the
corresponding probe volumes. Each of the corresponding probe volumes 340a,340b... lie within
a small cylindrical plug of radius R' = I m I R, where I m I is the magnitude of the m~gnification
of the objective system. Furthermore each probe volume is offset along the z axis and slightly
displaced to the side in a helical fashion around the plug-like probe volume by a z-axis offset of
m2L where L is the original tr~n~mi.~ion fiber axial step spacing. Thus, the fixed array of fibers
serve to define a sampling plug at a plurality at different depths within the sample 320. This
geometry is absolutely fixed, being determined by the fixed spacing of the fiber ends and the
power of the objective 301, so the sampling within the different layers of tissue is accurate and
substantially independent of the stability of the instrument 301. This feature is expected to
greatly enhance reproducibility of depth resolved tissue spectroscopy. In an endoscopic
embodiment, the illumination and collection assemblies are necessarily defined in a much closer-
focal configuration, and the probe tip itself may define the subject spacing or form part of the
optics, or both.
Various optical configurations may be employed to achieve non-im:~ging volume probe
configurations with effective rejection of unwanted signals. FIG.1 OA shows one such
embodiment, wherein the illumination and collection beams 44',48' are each essentially
collim~te-l In this case, a collim~ting field stop FS essentially defines the width of the collection
window, while the crossing angle affects the dimensions and aspect ratio of the volume element.
In another embodiment of the non-im~ging volume microprobe configuration, the
illumination and collection beams may cross, and may employ a common field stop FS, but still
utilize a common objective lens assembly. This is achieved as shown in FIG.10B. Here, source
12 and detector 24 are each obliquely directed to cross at the stop FS, while an objective
assembly 21 is placed so that different portions a, a' of its clear aperture are used for illumination
and collection. A baffle B assures clean separation of the two sub-regions of the shared objective
aperture. At the image of field stop FS in the sample 18, the two beams cross clt-fining a stably-
aligned sheared-conjugation volume element. Other more conventional approaches to sharing of
the aperture of an objective assembly may also be used, for example by using small mirrors to
fold-in separate optical paths, passing through two separate field stops, into different locations on
the objective lens.
Another useful embodiment employs multiple apertures or pupils in the objective
assembly, with the pupils arranged in spaced-apart pairs for illumination and detection. One
CA 02228308 1998-01-30
W O 97/05473 PCT~US96/12651
-47-
such embodiment, shown in FIGS. 1 OC and 1 OD, makes maximum use of crossed beamasymmetry while also minimi7inp crosstalk. Rather than simply dividing the objective aperture
into two parts where the adjacent partitions are less effective in enhancing depth resolution, a
mask M in or on the objective 16 divides the pupil into a set of N diametrically opposed pairs of
5 sub pupils (2N sub-pupils), Pla, P,b,...PNa, PNb each pair being coupled to one self-conjugate field
stop, such as the fiber-end field stops shown in FIGS. 9A and 9B. Here, crosstalk generated by
scattering is reduced to a minimum, while the use of the full-diameter spacing of the objective 16
mz~ximi7es the depth resolution achievable by the crossed beams. The N pairs of pupils may be
arranged in any desired distribution, and may simultaneously take N response rç~din~;~
10 FIG. 1 OD shows the orientation of a pupil mask M which may be used to define the set of pupils
on a lens. The single objective with a masked pupils can be replaced with individual objectives;
this requires somewhat more complex alignment, but reduces the effects of aberrations. More
important, the separate objective lenslets can be micro-lenses which adapt well to the spatial and
focal constraints for a small instrument like an endoscope. These are ~ d embodiments for
15 an endoscope as described in FIG. 11 below.
In yet another embodiment of the invention, a second or further field stop may be used in
combination with a dispersive element to correct for residual chromatic aberrations of a
holographic or refracting objective. Generally, the best available objectives for wide spectral
bandwidth fluorescence confocal microscopy are well corrected chromatically. They form good
20 images with constant mzlgnification over ranges from about 300 nm to over 1,000 nm. However,
even these superb lenses suffer from excessive "secondary spectrum" over a portion of that
spectral range. Secondary spectrum is a wavelength-dependent shift of the axial position of an
image with respect to the position of the lens. Even for large field stops, the shift of the image
has two adverse effects: (1) the out of focus bundle of rays emzln~tin~ from the displaced image
25 rapidly becomes larger than the field stop diameter and energy is lost, and (2) loss of confocal
conditions (conjugations of FS, and FS2) leads to reduction in discrimination against background
and depth sectioning capabilities. Partially for this reason~ the previously described systems rely
on reflective objectives in our wide-spectral-bandwidth systems. However, by using a dispersing
element as part of the beam separator, one can then place secondary field stops FS; at the correct
30 locations to be conjugate to the first field stop. As shown in FIG. lOE, an aberration-corrected
dispersive element DE may be used to disperse the collected light into separate beam locations
for each ~j. These separate beams ~j are then each coupled into a corresponding fiber end FSj,
CA 02228308 1998-01-30
W097/05473 PCT~US96/126Sl
-48-
which serves as a secondary field stop located at the correct conjugate position for each ~ j. The
dispersive element may, for example, be a holographically-formed element, a prism, or a
classical tr~n~mi~cion or reflection grating.
Yet another embodiment of a probe in accordance with the present invention may be
5 employed in endoscopes and other contact or internal remote sensing applications. Such an
embodiment is illustrated in FIG. 11. In this embodiment~ an illumination fiber and a reception
fiber each pass through an elongated body 500, which may be a conventional catheter, to an
im~ging head 510 which resides at the tip of the body or catheter. Tip 510 may include active
focusing optics, for example, one or more rod lenses which are adapted to move to adjust probe
10 volume depth. However preferably, the endoscope embodiment employs a multifiber
arrangement, for example one such as shown in FIG. 9A, to define different probe volumes by
simply applying a light input to different ones of the illumination fibers 51 3a,..., extemally of the
device without requiring motion of any optics within the im~ging head itself. Such an assembly
may be rigidly and fully encapsulated to provide a smooth curved contact face that is brought
15 into contact with the tissue surface in order to project to and collect light from dirr~ t stations
below the surface. For applications where wide spectral bandwidth is not es~çn1i~1, radially
graded index of refraction rods (GRIN lenses) advantageously may termin~te or follow the fibers
to allow very simple optical zllignment and short focal distances to be obtained in geometries
suitable for endoscopic rnanipulation against tissue in a human body. The FIG. illustrates one
~0 such lens 514 positioned for focusing the light from the plurality of helically-arranged input
fibers 513a, 513b, 513c.
In each of the above embodiments, the probe volume is defined by the intersection of
illumination and im~ging beams in a manner to provide large signal strength while effectively
discrimin~ting against stimulation, scattering, reflection and luminescence and collection of light
:25 from the shadow region of tissue. A large illumination stop effectively provides the desired level
of illumination in the probe volume enabling meaningful spectral measurements to be taken.
Among the various embodiments of the invention, several constructions are particularly
advantageous. These include embodiments wherein the illumination and collecting optics are
reflective, or catadioptic, and alleviate spectral aberrations. They also include a~zudlus in which
30 the ill--min~ting and collecting field stops are physically realized by the same element, and
embo-liment~ wherein the collected beam is physically separated from the illumination beam
path after it has passed bac~ through the common field stop. Another such construction employs
CA 02228308 1998-01-30
W O 97/05473 PCTAJS96/12651
-49-
a multimode fiber as the illumination field stop. One particularly advantageous device for
probing localized subvolumes which can be varied in position achieves this by controlled
translation of the field stops along respective beam axes of the illumination and collecting optics.
This may also be achieved by translation of the objectives along their respective optical axes.
While the invention has been shown and described with reference to specific pl~f~ d
embodiments, and several exemplary methods of use, those skilled in the art will understand that
variations in form and detail may be made without departing from the spirit and scope of the
invention. Indeed, having described the invention, further modifications or variations will occur
to those skilled in the art, and such modifications and variations are understood to be within the
10 scope of the invention, as defined by the claims appended hereto.