Note: Descriptions are shown in the official language in which they were submitted.
CA 0222916~ 1998-02-10
W O 97/09388 PCT~US96/lZ286
ARTICLES COATED WIT~ ELECTROP~IOTOGRAPEIIC
TONER RE(~ E RELEASE COATINGS
Field of the Invention
The present invention relates to release coatings and articles coated with
such release co~tings In particular, the present invention relates to release coatings
which anchor printed indicia, even when cont~cting a repositionable pressure-
sensitive adhesive.
Background of the Invention
A well known note pad comprises a stack of paper sheets, each having a
narrow strip of low-tack pressure-sensitive adhesive ~ cent one edge on its rearside by which the sheets can be temporarily adhered to substrates such as
doc~-m~ntc or other articles (often for message-bearing purposes), by which strip of
adhesive the sheets are adhered together in the pad. In one pad configuration, all of
the strips of adhesive are along one side of the note pad, whereas in another pad
configuration the adhesive strip on each s-lcceccive sheet in the pad is along the
opposite side ofthe pad as is illustrated in U.S. Patent No. 4,416,392. Note pads in
both of these configurations are currently being marketed under the trademark
"Post-it" by Minnesota Mining and M~nllf~ctllring Company, St. Paul, Minnesota.
A number of conventional electrophotoconductive devices such as p~ el ~
and copiers employ dry toner. These printers and/or copiers employ toner to form a
latent image ~ n~rel~ble from an im~in~ device to a substrate such as paper, tag or
label stock. The toned latent image is then subjected to heat fusing in order toobtain the best print quality and toned image density.
Although the reslllting images are often of good quality, there are problems
associated with the im~ging processes employed by printers and/or copiers. For
example, there is the problem of backgrounding which is the ~ccumlll~tiQn of
various unwanted materials such as toner particles on the substrate.
Other conventional devices for office and commercial printing employ inks
co.l~;..;..g pigm~ntc or inks along with a carrier vehicle. Examples of such printing
CA 0222916~ 1998-02-lO
W O 97/09388 PCTAUS96/12286
devices are ink jet, offset, flexographic, duplicators, and electrophotographic
printers. The images are applied to the substrate, and the vehicle is absorbed into
the substrate and evaporates. Occasionally, the substrate may be subjected to heat
to ~onh~nce evaporation of the ink vehicle.
Problems with such printers include "ghosting", in which ink transfer to the
substrate is inc~mplete. Typically, the residual ink is transferred to another roller in
the printer and subsequently transferred to the substrate out of register. This results
in a lightly colored image. Additional problems with ink jet printing is the tendancy
of ink to dewet from the substrate, and failure to absorb into the substrate or dry.
In either case, the ink is smeared when a subsequent sheet contacts the image.
When printed sheets are formed into note pads, a release coating is typically
used to provide facile removal of the sheets from pads. Such a release coating must
be receptive to common writing instruments and printing inks (see U.W. Patent No.
5,154,962 columns 1 and 2 related background). The release coating is s~lected to
permit high quality images with specific printing technologies and ready separation
from the adhesive.
It has long been industry practice for commercial printing to employ offset
printing devices located in centralized production facilities. However, desktop
publishing and electronic commerce is rapidly çh~nging the printing industry away
from centralized production with a single printing technology to decentralized
production using various and multiple printing technologies. For in~t~nce~ a
printing requirement may require both offset printing (e.g., a co~ )a,ly logo)
followed by xerographic ~;u~,lo~ alion (e.g., a person's name).
In any event, the release-coated substrate must accept and firmly anchor
2~ indicia. The coated substrate must first accept the inidicia without "backg,.,unding"
in electrophotographic devices, "ghosting" in some printers, or smearing in liquid
ink printers. Additionally, when formed into pads, the printed indicia must firmly
anchor to the substrate without transferring to the adhesive of the preceding note in
the pad.
Coatings have been prepared to ~nh~nce ink or toner particle receptivity.
World Patent Application No. US/90/03286 (Josephy et al.) describes a toner
,
CA 0222916~ 1998-02-10
W O 97/09388 PCTrUS96/12286
receptive coating which can be applied to paper. U.S. Patent No. 5,154,962
describes indicia receptive release co~tingC which are particularly useful for writing
with pens co~ g waterbased inks. However no known refernces teach the use
of a release coating which accepts and firmly anchors indicia from a wide array of
S devices in~ rling offset, ink jet, and xerographic printers. Frequently,
customization is performed using sheetfed devices such as laser printers, ink jet
printers, and xerographic copiers. This printing is useful when pl epaling printed
note pads. Such pads may be prepared using the structures and methods described
in U.S. Patent No. 5,382,055. Thus, there currently exists a need for a release
10 coating which will accept and anchor toner particles, offset inks, and other liquid
inks and toners.
Summary of the Invention
The present invention comprises articles coated release coatings that are
15 capable of accepting and anchoring printed indicia and yet, .n~ c good release
plOpelLies. The release coating ofthe present invention is coated onto a substrate
such as paper. The articles are of the form described in U.S. Patent No. 5,382,055
and are useful to prepare a small amount of notes or note pads. The release coating
COll~ lises a polymer having at least one vinyl polymeric segm~nt having a T~
20 bt;lwt;en -10~C and 65~C and at least one siloxane polymeric segm~nt Plerel~bly,
the Tg should be between 200C and 450C of the vinyl polymer se~n~nt
In one embodiment of the present invention, the coating is a copolymer
which comprises the formula:
(Rl)3 x IGs ~(R3)3 q
(G SR2~,~Si (~~Y OSi\(R4SG4)q
wherein
Rl are monovalent moieties which can independently be the same or
di~el ell~ and are selected from the group con.cicting of alkyl, aryl,
alkaryl, alkoxy, alkylamino, hydroxyl, hydrogen, and fluoroalkyl;
CA 0222916~ 1998-02-10
W O 97/09388 PCTAUS96/12286
R2 can independently be the same or dirrelen~ and are divalent linking
groups;
R3 are monovalent moieties which can independently be the same or
di~el elll and are s.olected from the group con~;sL~.Ig of alkyl, aryl,
alkylaryl, alkoxy, alkylamino, hydroxyl, hydrogen, and fiuoroalkyl;
R~ can independently be the same or different and are divalent linking
groups;
x is an integer of 0 to 3;
y is an integer of 10 or greater;
q is an integer of 0 to 3;
G5 and G6 are monovalent moieties which can independently be the same or
di~rt;l GIlL selected from the group coll~;sL"Ig of alkyl, aryl, alkaryl, alkoxy,
alkylamino, fluoralkyl, hydrogen, and -WSA wherein W is a divalent linking groupand A is defined below; and
G2 and G4 are A whe~ein A is a vinyl polymeric se~m~nt or block concictin~
csf-nti~lly of a polymerized free radically polymerized monomer.
In another embodiment of the present invention, the coating comprises a
copolymer of D and E monomers copolymerized to form a polymeric backbone
with F monomer graf'~ed thereto wherein:
D is at least one free radically polymerizable vinyl monomer;
E is at least one polar monomer copolymerizable with D, the amount of E
being up to 30% of the total weight of all monomers, and
F is a monomer having tbe general formula
X-(Y)nSiR~, n)Zm wherein
X is a vinyl group copolymerizable with the D and E monomers,
Y is a divalent linking group where n is zero or l;
m is an integer of 1 to 3;
R is hydrogen, lower alkyl (e.g., methyl, ethyl, or propyl), aryl (e.g., phenyl
or substituted phenyl), or alkoxy groups; and
CA 0222916~ 1998-02-lO
W O 97/09388 PCT~US96/12286
z is a monovalent siloxane polymeric moiety having a number average
molecular weight above about 1,000 and is ess~nti~lly unreactive
under copolymerization conditions.
The present invention also in~ des a toner receptive article comprising a
5 liner, a release coating and a substrate such as a label mounted over the liner.
Brief Description of the Drawin~s
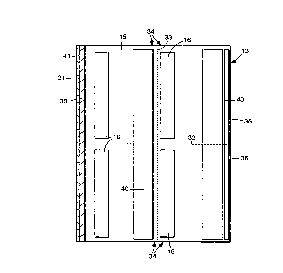
Figure 1 is a rep. ~:se ~~aLion of an embodiment of Applicants' invention
tested in accordance with the procedures described below.
Figure 2 is a representation of Avery 30-up stock #5160 that has been tested
in accordance with the procedures described below.
Figure 3 is an edge view of a sheet assembly according to the present
invention.
Figure 4 is a reduced sectional view taken approximately along line 4-4 of
15 Figure 3.
Description of the Preferred Embodiment(s)
The present invention incl~ldes an article coated with a release coating that iscapable of anchoring toner. The release coating has at least one siloxane polymeric
20 segmPnt and at least one vinyl polymeric se mlont having a Tg between -10~C and
65~C. By virtue of its çhpnnic~l composition and structure and the res llt~nt
p~.,pe.~ies, the release coating is well-suited to control toner anchorage
applications. In particular, it is thought that the silicone segm~nt pl esenL~ a low
energy, "siliconized" release surface and the higher energy vinyl polymeric Se m~nt
25 provides the adhesion for the toner particles.
In one embodiment of the present invention, the coating is a copolymer
which comprises the formula:
(G S /Si--(O~y--OSi \(R 3 q
CA 0222916~ 1998-02-10
W O 97/09388 PCT~US96/12286
Rl are monovalent moieties which can independently be the same or
di~rt;. t;lll which are selected from the group consisting of alkyl, aryl, alkylaryl,
alkoxy, alkylamino, hydroxyl, fluoroalkyl, and hydrogen. Preferably, Rl are
monovalent moieties which can independently be the same or dirrele;ll~ sPIected
S from the group consisting of Cl~ alkyl and llydlo~yl. Most preferably, R, is
sPlected from the group con~ietins~ of methyl and butyl.
R2 are divalent linking groups which can independently be the same or
dirr~r~llL~ Suitable divalent linking groups include but are not limited to the
following: Cl to C10 alkylene, arylene, alkylarylene and alkoxyalkylene. Preferably,
10 R2 is sPIected from the group consisting of Cl 3 alkylene and C,-C10 alkylarylene due
to ease of ~yllLlle~is of the compound. Most preferably, R2 is selected from thegroup consisting of-CH2-; 1,3-propylene; and
CH2~CH2CH2--
R3 are monovalent moieties which can indepPn~lPntly be the same or
1~ dirrt;rell~ which are selected from the group consisting of allcyl, aryl, alkaryl, alkoxy,
alkylamino, hydroxyl and fluoroalkyl, and hydrogen. Preferably, R3 are monovalent
moieties which can in~lepenrlPntly be the same or di~e:~ellL sPlected from the group
cc n.~ ~l;..g of C~ alkyl and hydroxyl. Most plere ably, R3 is s~lectecl from the
group consisting of methyl and butyl.
R4 are divalent linking groups which can independently be the same or
dirrt;re,~. Suitable divalent linking groups include but are not limited to the
following: C~ to C~0 alkylene, arylene, alkylarylene and alkoxyalkylene. Preferably,
R4 is sPIected from the group consisting of Cl3 alkylene and C7-C10 alkylarylene for
reasons of ease of synthesis. Most preferably, R4 is selected from the group
25 co~ ;g of-CH2-; 1,3-propylene; and
CH2~H2CH2
G5 and G6 are monovalent moieties which can independently be the same or
diLrele.l~ selçcted from the group consisting of alkyl, aryl, alkylaryl, alkoxy,
CA 0222916~ 1998-02-10
W O 97/09388 PCTAJS96/12286
alkylamino, fluoralkyl, hydrogen, and -WSA wherein W is a divalent linking groupand A is defined below.
W are divalent linking groups. Suitable divalent linking groups inr.l~lde, but
are not limited to, Cl to ClO alkylene, alkarylene, arylene, and alkoxyalkylene.5 Preferably, W is selected from the group consi~Li,lg of methylene and propylene.
G2 and G4 are the same or dirrelt;"L and colllplise A. A is a vinyl polymeric
seg...~ consisting ess~nti~lly of polymerized free radically polymerizable
monom~r. A can comprise either a homopolymer segment or block or a copolymer
segm~nt or block. The toner anchorage properties of the coating are determined by
the vinyl polymeric segment content. The chemical nature or composition of the
vinyl polymeric seg- I~ri " ~ can be modified independent of the release aspect to
improve toner anchorage and adhesion to the substrate. Thus, the release coatingcan be chemically tailored to provide a specific level of toner anchorage. The
selection of the composition of A is typically based upon the int~.ncled use of the
release coating and the properties the release coating must possess in order to
accomplish its int~nrled purpose.
A can in~ d~, but is not limited to, those monomers wherein the free
radically polymerizable monomer or monomers are chosen such that a vinyl segm~nthas a T,~ or Tl" above about -20~C. The plere-led free radically polymerizable
monolllc;l ~ are selected from the group consisting of styrene, methyl meth~r.rylate,
methyl acrylate, acrylic acid, meth~t~.rylic acid, acrylonitrile, isobornyl acrylate,
isobornyl meth~rylate, N-vinyl pyrrolidone, butyl meth~.rylate, isopropyl
m~th~- rylate, vinyl acetate, hydroxy propylacrylate, hydroxy ethyl acrylate andmixtures thereo~
The amount and composition of the vinylic segm~nt to silicone should range
from about 98 to about 2 parts preferably, from about 40 to about 60 parts by
weight. It is p~c;rel~t;d that the vinyl polymeric segm~nte should have a molecular
weight in the range of 2,000 to 80,000, more preferably 5,000 to 50,000.
The release p. o~el Lies of the coating are determined by both the silicone
~ 30 content (weight pelcellLage) present in the copolymer and the molecular weight of
the silicone segm~nt, with higher silicone content and/or molecular weight
CA 0222916~ 1998-02-10
WO 97/09388 PCTAUS96/12286
providing easier release. A copolymer or copolymer blend can, therefore, be
I-.hPmic~lly tailored to provide a specific level of release which can be reproduced
with con~ictency, thus making possible the variation of the release properties of a
liner over a range of values in a controlled fashion.
The silicone polymeric segment must have an average molecular weight
above about 1000 in order for the release coating to function properly. Preferably,
the silicone polymeric se~mPnt has a number average molecular weight of about
1000 to about 20,000. Most p~trel~ly, the silicone polymeric se~mçnt has a
number average molecular weight ranging from about 2,000 to about 15,000. The
silicone polymeric segmPnt can comprise about 2 to 60 wt% of the release coatingin order to allow for a wide range of release pe,rc,lnlallce.
In another embodiment of the present invention, the release coating
comprises a copolymer of D and E monomers copolymerized to form a polymeric
backbone. Gra~ed to the backbone is an F monnm~r. The D and E monomers
provide the toner anchorage properties of the coating and the F monomer providesthe release properties.
The D monomer or monomers (there may be more than one) are chosen
such that the backbone Tg or Tm is above about -20~C. Representative examples ofD monomers include styrene, vinyl acetate, vinyl chloride, vinylidene chloride,
acrylonitrile and acrylic or meth~rylic acid esters of nontertiary alcohols or tertiary
alcohols such as mPth~nnl, ethanol, propanol, iso,olopallol, butanol, isobutanol,
cyslohPY~nol, benzyl alcohol, dodecanol, heY~-iec~nol, and oct~dec~nol, the
alcohols having from 1 to 18 carbon atoms.
Especially ~ rt;l-~d D monomers include methyl meth~çrylate~ butyl
meth~rylate~ vinyl acetate, partially hydrolyzed vinyl acetate, methyl acrylate and
octadecyl acrylate.
Repl t;~e.lLa~ e E monomers useful in practicing the invention, and which
may be used either individually or in co---binalion, include carboxylic acids such as
acrylic acid, mPth~rylic acid, itaconic acid, maleic acid, fumaric acid, and 2-
carbo~yelllyl acrylate and their ammonium or metal salts; sulfonic or phosphonicacids such as 2-sulfoethyl mpth~rylate~ 3-sulr~,prol yl acrylate, 3-sulropropyl
CA 0222916~ 1998-02-10
W O 97/09388 PCTAUS96/12286
meth~rylate, styrene sulfonic acid, and vinyl benzyl phosphonic acid and their
~mmnnil-m or metal salts; amides such as acrylamide, meth~rylamide, N,N-
dimethyl acrylamide, and N-vinyl pyrrolidone; and monomers having hyd~ yl
functionality (e.g., 2-hydloxycl}lyl acrylate, 2-hydroxyethyl meth~-~rylate,
S llydl~xy~JIopyl acrylate, and dihydroxypropyl acrylate), ammonium functionality
derived from reaction of amine-cG..~ monomers (e.g., N,N,-
dimethylaminoethyl methacrylate and vinyl pyridine) with alkylating agents or protic
acids, or zwitterionic functionality such as that derived by reaction of amine
monomers with hydrogen peroxide or propane sulfone.
The F monomer has the general formula:
X~(Y)nSiR(3 m)Zm
X is a vinyl group copolymerizable with the D and E monomers.
Y is a divalent linking group.
R comprises hydrogen, lower alkyl groups such as methyl, ethyl, or propyl,
lS aryl groups such as phenyl or substituted phenyl and alkoxy groups such as
methoxy and ethoxy groups.
Z is a monovalent siloxane polymeric moiety having a number average
molecular weight above about 1,000 and is ees~-nti~lly unreactive under
copolymerization conditions.
The plcrcl-cd F monomer may be further defined as having an X group
which has the general formula
IRl R2
CH=C
wherein Rl is a hydrogen atom or a COOH group and R2 is a hydrogen atom, a
methyl group, or a CH2COOH group.
The Z group of the F monomer has the general formula
- R3
R4~ r
R5
CA 0222916~ 1998-02-10
W O 97/09388 PCT~US96/12286
where R3 and R5 are independently lower alkyl, aryl, or fluoroalkyl, where loweralkyl and fluoroalkyl both refer to alkyl groups having from one to three carbonatoms and where aryl refers to phenyl or substituted phenyl. R4 may be alkyl,
alkoxy, alkylamino, aryl, hydroxyl, or fluoroalkyl, and r is an integer from about 5
5 to about 700. Preferably, the F monomer has a general formula sPIected from the
group consisting ofthe following, where m is 1, 2 or 3, p is zero or 1, R" may be
alkyl or hydrogen, and X R, and Z are as defined above:
1~l
X--C--O--(cH2)q--(O)p--Si(R)3--mZm
wherein q is an integer from 2 to 6;
1~l
X--C--O--Si(R)3--mZm
1~l ~}~(CH2)q~(0)p--Si(R)3-mZm
wherein q is an integer from zero to 2;
Ol H O R"
X--C--O--CH2--CH2--N--C--N--(cH2)q Si(R)3--mZm
wl-c-cin q is an integer from 2 to 6;
X--C--O--CH2--CH2--N--C--~Si(R)3-mZm
and
Ol OH R"
X--C--O--CH2--CH--CH2--N--(cH2)q--Si(R)3--mZm
wherein q is an integer from 2 to 6.
The release coating of the present invention may comprise the copolymers
of the two embodiments alone, or may comprise copolymers blended with other
CA 0222916~ 1998-02-lO
W O 97/09388 11 PCTAJS96/12286
co"l~ible homopolymers and/or copolymers. The low percentage of silicone
cont~ined in the copolymers makes the copolymers readily conlpa~ible with
polymers of similar composition to the vinyl Polymeric blocks or segm~nt~ In
addition, there are several pairs of rli~imil~r polymers that yield compatible blends
S due to specific interaction as described by S. Krause in Polymer Blends, ~c~dçmic
Press, New York~ 1978. Introduction of a low level of silicone block onto one ofthese polymers will not infl~lçn~ e compatibility.
In addition, additives, fillers or pigment~ such as ~lllmin~, silica, tit~n~te~ or
calcium carbonate may, of course, be added to the copolymer compositions.
The release coating ofthe present invention should provide sl-ffi~i~nt
anchorage to anchor at least 50% ofthe toner. It is understood that "subst~ntis-lly"
means at levels of at least 50%. More preferably, it should anchor at least 70% of
the toner.
In addition, the release coating should have a surface release value not
greater than about 10 oz./in (11 N/dm). It should be understood that this upper
limit applies to use with highly aggressive pressure-sensitive adhesives (PSAs)
which have peel adhesion values of 45 N/dm or higher. PSAs as a group fall into
three broad categories (1) low (5-15 N/dm), (2) intern ~ te (25-50 N/dm), and (3)
high (60-100 plus N/dm) peel adhesion ranges. It is appale-,~ that the degree ofrelease can be s~lected to match the aggressiveness of the PSA with which it will be
in contact and it is only for the most aggressive PSAs that a release value as high as
10 oz/m. (11 N/dm) would be sÇlecte~l Release co~ting.c for less aggressive PSAswould be selected to be correspondingly lower.
The release compositions do not require curing or cros~linking; however, if
solvent ,e~ nce is desired for a particular application, cros~linking can be effected
by standard methods well-known in the art, such as radiation curing (electron beam
or ultraviolet light) or ~h~mic~l cros~linking
The release coating compositions may be applied to any suitable backing or
liner by means of conventional coating te~hniquçs such as wire-wound rod, direct- 30 gravure, offset gravure, reverse roll, air-k~ife and trailing blade co~tinp Suitable
CA 02229l6~ l998-02-lO
W O 97/09388 12 PCT~US96/12286
liners include paper, non-woven fabrics and films of thermoplastic resins such as
polyesters, polyamides, polyolefins, polycarbonates and polyvinyl chloride.
In addition, any substrate which can be applied to a liner by a p.es~u~e
.sensitive adhesive can be ut~ ecl For example, paper is a suitable substrate.
In a prefe.. ed embodiment, the release coating ofthe present invention is
utilized as a co...ponent of label stock. In this p~ t;re. I ~d embodiment, one or more
non-contimlQus substrates such as labels are adhered to protective liner by a suitable
adhesive. To permit the substrate to be removed from the liner, the release coating
of the present invention is coated the liner.
A particularly useful configuration is a sheet assembly wherein the assembly
can be described as follows and in reference to Figures 3 and 4. The sheet assembly
10 is illustrated with release coatings 40 on at least one nonadhesive bearing area of
the rear surfaces 15 ofthe sheets 12 and 13. The release coatings 40 can be applied
strips and are slightly wider than strips 16 of adhesive, and extend transversely of
the sheets 12 and 13 parallel to the edges 30, 36 and 37 with each strip releasecoating 40 being a~ cçnt and eYten~ing along an edge of a portion 34 of the sheet
12 and 13 that will be formed by sep~Li~g the sheets 12 and 13 along the paths of
weakness 31 and 33, and in a position such that when the rear surfaces 15 of thesheets are placed face to face as illustrated in Figure 3, the strip layers 16 of the
20 adhesive will contact and will be generally centered on the strip release co5~ting~ 40.
Also, as illustrated, the entire front surface 14 of each of the sheets 12 and 13 is
coated with a release coating 41, althought such a coating is not necessarily
required. For example, the illustrated release coatings 40 on the rear surfaces 15
and the release co~ting~ 41 on the front surface 14 ofthe sheets 12 and 13 are not
25 needed when the layers 16 of the adhesive are of low-tack or repositionable
adhesive (e.g., the low-tack adhesives based on tacky, ~l~ctQm~ric copolymer
microspheres t~iccl~sed in U.S. Patent Nos. 3,961,140 and 3,857,731). The use ofthe release co~tin~s 40 and 41 allows the use of layers 16 of pressure-sensitiveadhesive that are more aggressive or permanent than repositionable adhesive.
30 Suitable release co~ting~ 40 and 41 are materials and tre~tm~nts those ofthe present
invention. Even with layers 16 of aggressive p. ~;s~u. ~;-sensitive adhesive the release
CA 0222916~ 1998-02-10
W O 97/09388 13 PCTAJS96/12286
co~ting.C 40 and 41 might not be required if the sheets 12 and 13 are of a material,
or are illlpl ~A ~ ed with the release material, from which the layer 16 of adhesive
readily releases.
While Figures 3 and 4 I c;preselll a particular configuration of a sheet
5 assembly, such illustrations and descriptions are merely illustrative of the present
invention and other embodiments could be contemplated to be within the scope of
this invention.
The following examples are illustrative in nature and are not int~nded to
limit the invention in any way.
TEST SAMPLES
The polymer solutions of Examples 1 to 19 ~1iccllcced below were diluted to
15% solids in distilled water. The solutions were then coated onto commercially
available roll base paper with a gravure roll having a pyramidal pattern of 200 cells
15 per inch. A two roll direct gravure coating was applied to each sample. Two
di~elenL roll base papers were ~ltili7ed Supercalendered CIS paper supp1ied by the
Simpson Paper Colll,~)a,ly was coated with the diluted polymer solutions of
Examples 1 to 4 and m~çhine glazed base paper sold by Akrosil were coated with
the diluted polymer solutions of Examples 4 to 19. After co~ting, the base papers
20 were dried at 77~C.
CA 0222916~ 1998-02-10
W O 97/09388 14 PCT~US96/12286
Test Methods
Release Properties
The release property of an adhesive refers to ease that an adhesive separates
from another surface. It is the force required to remove a ~exible adhesive tapefrom a test sample at a specific angle and rate of removal. It is measured in
Newtons per clecimeter (N/dm). Two test methods are used to evaluate the releaseand indicia anchorage properties of coated flexible sheet materials. Both tests are
modified versions of the industry standard peel adhesion test ASTM D3330-78
PSTC 1 and 3 used to evaluate PSA coated materials. The two modified release
property tests are described below.
1. Release Value
Each test sample was conditioned overnight at conct~nt temperature (22~C)
and humidity (50% RH). Thereafter, a 5 .08 cm by 25 .4 cm strip of the test sample
is l~min~ted to a consLa-~l 90~ angle jig commercially available as the Deltron Ball
Slide from the J.R. Brass Co. of Eden Prairie, MN with double coated tape. Then a
2.54 cm strip of a PSA coated (#810 tape, co"~".e--;ially available from the ~.c.ci n~e
ofthis application) test tape was rolled down onto the l~min~te with a 1.82 kg
rubber roller. The force required to remove this tape at 90~ and 30.5 crn/minutewas then measured was measured by a Sintech/Instron Tensile Tester System
conllll~l.,;ally available from Sintech Corporation, a division of MTS Systems
Co~ol~lion, Research Triangle Park, North Carolina.
2. Toner Receptivity Testin~
The toner receptivity ofthe test sarnples was aeses.sed by printing on a 21.6
cm x 27.9 cm test sarnple an asterisk pattern, i.e., (****), in an Hewlett Packard
LaserJet II printer. The imaged coated sheets then sat overnight in a controllede.~virolllllent of 21~C and 50% RH. Thereafter, a 2.54 cm x 25.4 cm strip of
ScotchTM Brand 810TM tape m~nllf~ctllred by the Minnesota Mining and
M~mlf~ rin~ COlll~ was rolled down over the imaged test samples using two
passes of a 1.82 kg rubber roller. After the two passes, the test samples were
CA 0222916~ 1998-02-lO
W O 97/09388 PCT~US96/12286
allowed to sit in a controlled environment of 21 ~C and 50% RH for 24 hours. Then
the ScotchTM Brand 810TM tape was l~min~ted image side up to the stage of in
TIMI Release and Adhesion Tester sold by Testing ~hines Inc. of Mityville, New
York with double coated tape. The 810~ tape was then removed at a peel angle of
180~C at 3048 cm/minute. Image analysis was used to determine the amount of
indicia which ~ ed anchored to the coated sheet.
Abbreviations
AA - acrylic acid
10 AIBN- 2-21-azobisbutyronitrile
BMA - butyl m~th~rrylate
EMA - ethyl meth~çrylate
IPA - isopropyl alcohol
KF2001 - a ~llel-,a~Lofunctional dimethyl siloxane with 4-5 mole % mercapto
functionality co,.. --el cially available from Shin-Etsu.
MA - meth~crylic acid
MAA - methyl acrylate
MEK - methyl ethyl ketone
MMA - methyl meth~crylate
20 ODA- octadecyl acrylate
Example 1
The composition of Example 1 was prepared in the following manner:
First, a solvent borne sample was plepa-ed by charging a 32 oz. reaction
25 bottle with 45 grams of mercaptofunctional dimethyl siloxane with 4-5 mole
l..elcapto functionality commercially available as KF-2001 from Shin-Etsu, 169
grams of methyl acrylate, 11 grams acrylic acid, 335 grams of methyl ethyl ketone
(MEK) and 0.56 grams 2-2'-azobisisobuLy~ul iL.ile (AIBN). The solution mixture
was then purged with nitrogen for 2 mim-tes at a rate of 1 L/min, after which the
- 30 bottle was sealed. The sealed bottle co.. l;.il.;.. g the clear solution was tumbled in a
CA 0222916~ l99X-02-10
W O 97/09388 16 PCTAUS96/12286
collsL~ temperature bath for 20 hours at 55~C re~ tin~ in a viscous cloudy whitesolution.
Thereafter, a waterborne solution was pl ~pal ed by filling a gallon jar with
860 grams deionized (DI) water and 9 grams NH4OH. Next, 53 7 grams of the
5 solvent borne solution (40.0 % solids) was added to the solution in the gallon jar.
The res~llting solution was placed on a shaker and shaken for one-half hour in order
tG con'lplete the neutra~ation. The i~ was then stripped from the resllitin~
viscous solution on a rotary evaporator at 40~C using an aspirator vacuum to yield
19.0 % solids aqueous solution. An additional amount of DI water was added to
10 obtain 15.0 % solids solution. The ingredients utilized in forming a solvent borne
solution and water borne solution and the amount of the ingredients utilized arereported in Tables 1 and 2 respectively. The ingredients of the release coating, the
weight percentage of the ingredients and the test results are reported in Table 3 .
Examples 2-20
The copolymers of Examples 2-20 were prepared in accordance with the
procedure oullh~ed in Example 1. The ingredients utilized in forming a solvent
borne solution and water borne solution and the amount of the ingredients utilized
are reported in Tables 1 and 2 respectively. The ingredients of the release coating,
20 the weight percentage of the ingredients and the test results are reported in Table 3 .
CA 02229165 1998-02-10
W O 97/09388 PCT~US96/12286
17
TABLE 1
F.Y~mrle Ingredients Utilized in Polymerization Amount of Ingredients
Utilized (~ms.)
1 RUF2001~IAIA~ EK~ ~IBN 45/169/11/335/0.56
2 RUF200L~AIA~ EK~ AIBN 25/70/10/157.5/0.52
3 ~F2001~IAJAI~EK~AIBN 50/140/20/315/0.53
4 ~F2001AhL~VL~AJ~DEK~AIBN 50/140/10/300/0.5
~UF2001~VL~VAA/~DEK~AIBN 52/182/26/390/0.65
6 ~F2001AVL~AvL~A~DEK~AlBN 62/173/25/390/0.65
7 RUF2001rhL~VL~A~DEK~AlBN 60/140/20/330/0.55
8 ~F20011YL~ML~A/~DEK~AIBN 60/140/10/315/0.53
9 ~UF2001~YL~VL~A/~DEK~AlBN 50/120/10/300/0.5
~UF2001~U~VU~A/O{hLAl~CK~AlBN 65/130/13/52/390/0.65
11 EUF2001D\lA/~LA,VOlbLAV~EK~AlBN 30/75/7.5/37.5/225/0.38
12 ~UF2001/~L~/~L~AVOlhlAv~EK~AlBN 37.5/60/7.5/45/225/0.38
13 K~F20011hL~AhLA~VOlhLAv~EK~AlBN 37.5/45/7.5/60/225/0.38
14 EUF2001~ XU~AVO~bLA~EK~AlBN 37.5/30/7.5/75/225/0.38
~UF200 _ K~AIBN 39.5/71/7.9/31.6/225/0.38
16 ~F2001/~AhU~A/~ A~rE 1AlBN 35.7/78.6/7.1/28.6/225/0.38
17 ~UF2001~kLA~YLAA~Qf~lAl~EK~AlBN 34.1/81.8/6.8/27.3/225/0.38
18 EUF2001~ U~A/OD~A~EK~AIBN 32.6/84.8/6.5/26.1/225/0.38
19 ~F2001/~AhU~A~ AI~E 1AlBN 31.2/87.5/6.2/25/225/0.38
K F2001_K;/AIBN 50/100/10/40/300/1.0
CA 02229165 1998-02-10
W O 97/09388 18 PCTnJS96/12286
TABLE 2
FY~mrle Ingredients Utilized In Amount of Ingredients Utilized
Pl~a,~Lion of Water-borne (gms.)
Solution
Polymerl/NH40H/H20 537/9/860
2 Polymer/NH40H/H20 537/9.0/645
3 Polymer/NH4oH/H2o 520/8.8/650
4 Polymer/NH4oEvH2o 326/5/540
Polymer/NH4oHlH2o 320/9/500
6 Polymer/NEI40H/H20 320/8/500
7 Polymer/NH4oH/H2o 321/540/9
8 Polymer/NH40EIlH20 325/5/540
g PolymerlNH4oHtH2o 317/5/540
Polymer/NH4oHlH2o 320/4.3/500
11 Polymer/NH4oH/H2o 360/7.5/576
12 Polymer/NH4oH~H2o 345/4.6/530
13 Polymer/NH4oHlH2o 345/4-7/530
14 Polymer/NH4o~vH2o 345/4.6/520
Polymer/NH4oH/H2o 340/5/530
16 Polymer/NH4oHtH2o 340/4 4 /530
17 Polymer/NH4oH/H2o 340/4.2/530
18 Polymer/NH4oEvH2o 340/4/530
19 Polymer/NH4oHlEI2o 340/4/530
PolymertNH4oHlH2o 480/5/760
1 The polymer is the reaction product of KF2001, MA and AA and/or
MAA or MI~LA
CA 02229165 1998-02-10
W O 97/09388 PCT~US96/12286
19
Table 3
Ex.Ingredients Wt % Tg~ C Tape Release Pe~ ge
Ingredients g/inch Indicia
rece~Livily
Initial
MA/KF2001/AA 70/20/5 11 A 51 8
2MA/KF2001/AA 70/25/10 16 A 10 75
3MA/KF2001/AA 70/25/5 20 A 15 68
4MA/KF2001/MAA 70/25/5 18 A 8 88
SMA/KF2001/MAA 70/20/10 26 A 10 56
6MA/KF2001/MAA 70/25/10 26 A 9 38
7MA/KF2001/MAA 70/30/10 26 A - 25
8MA/KF2001/MAA 70/30/5 ? A - 22
9MA/KF2001/MAA/ 60/25/10/5 ? A - 58
MAA
10MA/KF2001/MAAt 50/25/20/5 40 A 14 92
MAA
11MAlKF2001/MAAt 50/20/25/5 44 A 23 78
MAA
12MA/KF2001/MAA/ 40/25/30/5 18 A 34 75
MAA
13MAIKF2001/MAA/ 30/25/40/5 22 A 88 64
MAA
14MA/K~2001/MAA/ 20/25/50/5 30 A 157 50
MAA
15MA/KF2001/MAA/ 45/25/20/5 42 A 23 84
MAA
16MA/KF2001/MAA/ 55/25/20/5 38 A 15 88
MAA
17MA/KF2001/MAA/ 60/25/20/5 36 A 37 88
MAA
18MA/KF2001/MAA/ 70/25/20/5 34 A 31 90
MAA
19MA/KF2001/MAA/ 70/25/20/5 33 A 58 85
MAA
20MAIKF2001/MAA/ 50/25/20/5 40 B 27 92
MAA
A = Acrylic
B = Rubber
s
CA 0222916~ 1998-02-10
W O 97/09388 PCTAUS96/12286
Fx~mrle21
The composition of Example 21 was pl ~pal ed in the following manner:
First, a solvent borne sample was prepared by charging a 4 ounce glass
bottle with 4 grams of 15K silicone macromer (SiMac), the pl~palaLion of which is
described in U.S. Patent No. 7,728,571, 16 grams ethyl meth~rrylate ~EMA), 30
grams methyl ethyl ketone ~MEK) and 0 06 g 2-2'-aGobi~l,u~lu~ ile (AIBN).
Thereafter, the contents of the bottle was purged with nitrogen gas for two ~ esThen the bottle was sealed and t~lmhled in a 55~C water bath for 48 hours.
The sample was then diluted to 10% solids for co~tin~ 40 grams of 40%
solids polymer solution, 96 grams oftoluene and 24 grams of isoplopanol (~A)
were added to a 16 ounce wide mouth jar. The mixture was then shaken to form a
homogenous rnixture. The ingredients utilized in forming a solvent borne solution
and the amount of the ingredients utilized are reported in Tables 4 and 5
re~ c~,Li~ely. The ingredients of the release coating, the weight percentage of the
ingredients and the test results are reported in Table 6.
FY~mples 22-27
The copolymers of Examples 22-27 were prepared in accordance with the
procedure outlined in Example 21. The ingredients utilized in forming a solvent
borne solution and the amount of the ingredients utilized are reported in Tables 4
and 5 respectively.
TABL;E 4
FYz-mple Ingredients Utilized in Polylll~ Lion Amount of Ingredients
Utilized (~ms.)
21 15KSIMAC/EM~K/AIBN 4/16/30/0.06
22 15KSIMACt~M~K/AIBN 5/7.5/7.5/30/0.06
23 15KSIMAC/EMA/BMA/MEK/AIBN 5/7.5/7.5/30/0.06
24 15KSlMAC/ EMA/MA/~K/AIBN 5/7.5/7.5/30/0.06
15KSIMAC/ M~L9JBMA/MEK/AIBN 5/7.5/7.5/30/0.06
26 15KSIMAC/EMA/ODA/MEK/AIBN 5/11/4/30/0.06
27 15KSIMAC/EMA/ MEK/AIBN 5/7.5M.5/30/0.06
CA 0222916~ 1998-02-lO
W O 97/09388 21 PCT/US96/12286
TABLE 5
Example Ingredients Utilized In Preparation of Amount of Ingredients
Solvent-borne dilution Utilized (~ms.)
21 polymer2/toluene/IPA 40/96/24
22 polymer/toluene/IPA 40/96/24
23 polymer/toluene/IPA 40/96/24
24 polymer/toluene/IPA 40/96/24
polymer/toluene/IPA 40/96/24
26 polymer/toluene/IPA 40/96/24
27 polymer/toluene/IPA 40/96/24
2 The polymer is the reaction product of the components listed in Table 5 for each
5 example.
Table 6
Ex. Ingredients Wt% Tg ~ Tape Release Indicia% Toner
Ingredients C g/ nch RecepLivily
In tial
21 EMA/15KSiMac 80/20 47 A 3~ 88
22 EMA/MMA/15K 37.5/37.5/25 84 A 70 88
SiMAC
23 EMA/BMA/15K 37 5/37.5/25 41 A 61 90
SiMac
24 EMA/MA/15KSi 37.5/37.5/25 35 A 23 87
Mac
MMA/BMA/15K 37.5/37.5/25 57 A 32 88
SiMac
26 EMAIODA/15K 55/20/25 -- A 91 96
SiMac
27 EMA/AA/15KSi 37.5/37.5/25 85 A 34 99
Mac
A=acrylic
Example 28
A sheet assembly particularly adapted to be printed on both sides in the
10 types of printers described above was made which had the structure illustrated in
Figure 1 except that four instead of two layers 16 of pressure sensitive adhesive
were placed in spaced relationship on each sheet 12 and 13. The sheets 12 and 13
CA 0222916~ 1998-02-10
WO 97/09388 22 PCTAUS96/12286
were each 20 pound bond paper and were each about eight and one half by 11
inches in size, with the sheet 13 being slightly longer so that it projected about 0.1
inch past the edge 37. The layers 16 of pressure sensitive adhesive were
microsphere structured copolymer adhesive described in U.S. Patent 3,691,140
(Silver) dispersed in n-heptane at 8 percent solids. The layers of release coating 40
were cro~link~cl Syl Off7676 coated at 20 percent solids in 2-butanone. The paper
was first primed with a vinyl solution co.l~ g zinc oxide. The release coating 41
was a polymer described in U.S.S.N. 08/040876 Example 1, except the composition
was KF2001/M~ 25/50/20/5 dispersed in 2-butanone ~Example 20) to
1.5 percent solids. The adhesive was coated to provide an adhesion comparable toPost-it(~ notes (i.e., apl)loxil-lated 0.6 N/dm adhesion to glass) in 0.75-inch (1.9
cm) wide stripes 16 e~t~n~ling the full width ofthe sheets 12 and 13. The opposing
strip of release coating 40 was coated with a gravure cylinder to provide 1.25-inch
(3.2 cm.) wide stripes ~ n~ling the full width ofthe sheets 12 and 13. The
materials were coated on a wide web and subsequently converted and folded to
form the sheet assemblies 10.
The initial release ofthe sheet was 166 g/m. Subsequently, the sheets were
printed using ink jet, a xerographic, and offset printers. In each case, the test
pattern was a solid block of black ink or toner. The ink jet printer was an HP
1200C color ink jet printer commercially available from Hewlett-Packard, Palo
Alto, CA. The xerographic printer was a Lanier 6540 copier cornmercially available
from Lanier Worldwide, Inc., Atlanta, GA.
The percent of inidicia receptivity were as follows:
~ 1200 C 100 percent
Lanier 6540 92 percent
The test results indicate that the release coating of the present invention
anchors indicia much more effectively than the release coatings utilized in the
Co.llpal~ e F.~mples Visually this is seen in a comparison of Figs. 1 and 2
wher~,;ll the samples were tested in accordance with the Toner Receptivity Test
described above. The asterisk pattern in Fig. 2 was not subst~nti~lly anchored
col..pal~d to Applicants' release coating shown in Fig. 1. Applicant's composition
CA 02229l6~ l998-02-lO
W O 97/09388 23 PCTAUS96/12286
utilized in Fig. 1 comprises ~2001 in the following amounts
50/5/20/25.
Thus, the coating of the present invention ~ignifis~ntly decreases the
att~n-l~nt problems associated with indicia when printed and subsequently used as a
5 stack of pressure-sensitive notes.
In sl-mm~ry, a novel article coated with a unique toner receptive release
coating is described. Although specific embodiments and examples of the present
invention have been described herein, it should be born in mind that these are by
way of explanation and illustration and the present invention is not limited thereby.
10 Certainly, modifications which are within the o,di,la,y skill in the art are considered
to lie within the scope of this invention as defined in the following claims inC ~ ing
all equivalents.