Note: Descriptions are shown in the official language in which they were submitted.
CA 02229896 1999-05-18
D-SHO-98F
METHOD FOR FORMING A DENTAL RESTORATION
This invention relates to a method for forming and/or repairing dental
restorations.
BACKGROUND OF THE INVENTION
In crown and bridge prosthodontics, metal copings are conventionally
used to provide the essential structural strength and rigidity necessary for a
dental restoration to resist the forces of mastication. In a ceramic-to-metal
dental restoration, the metal coping forms the understructure, over which is
applied a fired-on coating of porcelain or acrylic. A coating of porcelain is
used over the coping for aesthetics and to simulate natural teeth. To the
dental patient, color and the overall appearance of the dental restoration are
critical factors in the satisfaction of the restoration. Accordingly, the
color of
the metal coping is important and should enhance the aesthetics of the
restoration. For a ceramic-to-metal dental restoration, the metal coping
should enhance the porcelain by providing a background color contrast.
In Applicant's US Patent No. 5,234,343 entitled Moldable Dental
Material and Method, a dental material composition is taught which can be
readily shaped or molded into any desired shape for repairing and/or forming
a dental restoration. The composition of the material and method of
application is taught in Applicant's earlier U.S. Patent No's. 4,742,861 and
4,990,394. In general, the dental material is preferably composed of both
high fusing temperature metal particles and low fusing temperature metal
particles combined in a matrix with a volatile binder for forming a dental
restoration directly on a die or model of the tooth or teeth to be restored.
The
1
CA 02229896 1999-05-18
D-SHO-98F
material is shaped on the die into a desired configuration and heat-treated
at a temperature to vaporize the binder and to melt, or substantially melt,
the
low-fusing temperature metal particles resulting in a porous, sponge-like
structure having the shape it was given prior to heat treatment. A low-melting
temperature filler material, preferably of gold, is then melted into the
sponge-
like structure to form a solid metal coping, with a configuration identical to
the
configuration of the shaped material on the die before heat treatment and
without experiencing distortion and/or shrinkage.
The solidified metal should possess a desirable color, which is
reproducible with high accuracy, for use in forming a dental restoration.
Heretofore, the process was sensitive to temperature variations in the
furnace during heat treatment and even minor variations in temperature
during the heat-treatment procedure would permit some oxidation of the
metals to occur, which could deleteriously affect its color, and even more
seriously, could inhibit the flow of filler material into the porous sponge,
which
would affect the size of the solidified sponge. In fact, even the type of
furnace
used or its condition was able to affect the ability to accurately control the
temperature during the heat-treatment procedures. Although sophisticated
furnace temperature control equipment is commercially available, the
implementation of such equipment is costly and would be unacceptable to
the dental practitioner. The sensitivity to temperature variation also limited
the process to the fabrication within the furnace of one restoration at a time
which made the process very costly.
2
CA 02229896 1999-05-18
D-SHO-98F
In US. Patent No. 5,332,622 the above identified temperature
sensitivity problem was solved by the addition of activated carbon to the
dental material composition. However the process was limited in a practical
sense to the use of a refractory die. Although dental laboratories
conventionally use refractory dies this is not cost effective or practical in
the
fabrication of only a limited number of restorations or custom restorations,
of
for example 1-3, at one time. In this situation it is preferred for the dental
laboratory to use a conventional stone working die or any other type of
conventional non-refractory working die which is not to be heat treated. A
method has been discovered in accordance with the present invention for
forming and/or repairing dental restorations utilizing the principles and
methodology taught in Applicant's earlier U.S. Patent No's. 5, 234, 343,
4,742,861 and 4,990,394 which overcomes the above identified temperature
sensitivity problems during heat treatment for use with a non-refractory
working die. This method is particularly suited to the fabrication of a
limited
number of restorations at one time where a non-refractory stone, metal or
polymer die is preferred by the laboratory technician or dentist.
SUMMARY OF THE INVENTION
The method of the present invention for forming, repairing or restoring
a dental restoration can be readily practiced either at the dental laboratory
or
by the dentist in the dental office assuming a dental furnace is available.
The method of the present invention is characterized by the steps of:
3
CA 02229896 1999-05-18
D-SHO-98F
forming a base material comprising high-fusing temperature metal
particles and a volatile binder substantially or entirely of wax;
applying the base material to a non-refractory die;
shaping the base material upon said die into a desired configuration;
removing the shaped base material from the die;
combining particles of activated carbon to a dental investment material
to form a combined dental investment material having activated carbon in a
concentration above at least 0.005 wt. %;
investing the shaped base material with the combined dental
investment material;
heat treating the shaped base material in the investment at
temperatures up to 1200 C to vaporize the volatile binder from the base
material for forming a porous structure with a void volume of at least 20%;
and
filling the porous structure with a filler material to form a finished dental
coping.
BRIEF DESCRIPTION OF THE DRAWINGS
Additional advantages of the present invention will become apparent
from the following detailed description of the invention when read in
conjunction with the accompanying drawings of which:
Figure 1 is a perspective view of a non-refractory working die shown
in a conventional stone working model for practicing the present invention;
4
CA 02229896 1999-05-18
D-SHO-98F
Figure 2 is a perspective of the non-refractory working die of Figure 1
removed from the model for illustrating an initial preparatory step in the
process of the present invention;
Figure 3 is an illustration in perspective of the preferred method of
applying base material to the non-refractory working die of Figure 2;
Figure 4 is a further illustration in perspective of the procedure for
shaping the base material after application upon the non-refractory working
die of Figure 3;
Figure 5 is yet another illustration in perspective of the shaped base
material having the form of a finished coping upon its removal from the
working die of Figure 4;
Figure 6 is a perspective view of the shaped base material invested
in a dental investment material prior to heat treatment;
Figure 7 is an illustration in perspective of the preferred method for
adding filler material to the invested coping after heat treatment;
Figure 8 is a perspective view of the finished coping upon completion
of heat treatment and after removal of the investment with the finished coping
shown sitting upon a die for convenience and having a section of the coping
removed for illustrative purposes; and
Figure 9 is an exploded view of a section of the finished coping of
Figure 8 shown in cross section.
DETAILED DESCRIPTION OF THE INVENTION
The method of the present invention permits any conventional stone,
metal or polymer non-refractory die to be used for forming, repairing or
5
CA 02229896 1999-05-18
D-SHO-98F
restoring dental restorations. In accordance with the present invention
particles of activated carbon are added to a conventional investment
material and the working die is a conventional non-refractory stone, metal or
polymer die which is invested with the investment material as will hereafter
be explained in greater detail.
The method of the present invention is practiced by first forming
a base material of a high-fusing temperature metal component, a low
fusing dental metal component and a volatile binder substantially or
entirely of wax. The high-fusing temperature metal component is critical
to the composition of the base material and may be a single metal or
metal alloy, preferably of precious metals such as platinum and palladium
in any desired proportion to one another from zero to one hundred
percent and may include gold in any desired concentration. Additional
constituents may be added such as Ag, Cu, Mg, Al, Zn, and other metals
of the platinum group of elements of the third and fourth group of
elements. The total weight percent of the elements other than gold,
silver, and the platinum group metals should not exceed ten percent. Gold
may be added in any proportion to the high-fusing temperature metal
component to increase the affinity of the high-fusing temperature metal
component to the low-fusing temperature metal component or to itself in
the absence of the low fusing component. In the latter instance gold may
represent the major constituent of the high fusing metal composition and
depending upon its concentration will lower the melting temperature of
the high fusing component to as low as 900-950 C.
6
CA 02229896 1999-05-18
D-SHO-98F
The high fusing particles should also be of an irregular shape
preferably in the form of flakes, that is, platelets and should be very thin.
The
size and dimensions of the irregular, flake-like particles play an important
function. The very thin platelets of high-fusing particles interleave one
another to provide sufficient mechanical integrity to form a porous structure
during heat treatment and even without the presence of low-fusing particles
will surprisingly retain its structure after heat treatment with minimal
shrinkage. It is postulated that even without low-fusing particles, the heat-
treatment operation forms localized, autogenous joints which maintain the
structural integrity of the porous structure after heat treatment. However,
the
porous structure formed with the use of low-fusing particles as part of the
composition is still preferred. Moreover it is preferred but not critical to
the
invention that at least fifty percent (50%) of the high-fusing metal particles
have a thin, cross-sectional, average thickness of less than about 1.5
microns. The following test, in combination with the examples given below,
should be employed to determine if fifty percent (50%) of the high-fusing
particles meet this 1.5 micron thickness limitation: (a) the surface area of
the
largest two-dimensional surface (or its "projected image") for each of the
high-fusing particles should be measured, (b) the total surface area of all of
the high-fusing particles should be calculated, and (c) the cumulative surface
area of the high-fusing particles below 1.5 microns in average thickness
should then be divided by the computed total surface area. The surface area
calculation is a simple two-dimensional measurement of the area
circumscribing the flat, planar surface containing the largest two-dimensional
7
CA 02229896 1999-05-18
D-SHO-98F
image of each particle. If the planar geometry of the particle were rectangu-
lar, the surface area would simply be the length times the width. As an
illustration, assume a high-fusing particle flake geometry of 5p(long) x
10p(wide) x 3 microns thick. The largest two-dimensional surface area is 5p
x 10N. For a second illustration, assume a geometry of 20p(long) x 5p(wide)
x 1 p(thick). Again, the largest two-dimensional surface area is 20p x 5p. As
a third example, assume a flake geometry of a ball having a diameter of 20
microns. A two-dimensional projected image would be a circle having a
surface area of rrrz or n100. The taking of a "projected image" of the largest
two-dimensional surface maybe necessary based on undulations and
irregularities in the flake surfaces which would otherwise complicate the
surface area calculation. The cumulative total of the surface area for all of
the
particles is preferably determined by statistical analysis. There are
commercial analytical instruments and techniques available which may be
used for computing the surface area of the particles. Preferably, most of the
particles will have a very thin cross-sectional thickness of less than about
1.5
microns. However, since it is possible to break larger particles into many
smaller particles, it is necessary to make a surface area measurement to
determine if at least fifty percent (50%) of the total population of the high-
fusing particles in the composition are of proper thickness.
As stated above although the base material may be limited to only
high-fusing metal particles it is preferred to include low-fusing metal
particles
to enhance the wetting of the high-fusing particles during heat treatment. The
particles of low-fusing temperature metal are composed preferably of gold or
8
CA 02229896 1999-05-18
D-SHO-98F
a gold alloy, with gold as the major constituent. The preference for gold as
the major constituent of the low-fusing component is based on its known
characteristics of workability, biocompatibility, nonoxidizing properties, and
color. The low-fusing metal particles must, of course, have a melting
temperature below that of the high-fusing metal particles. The preference for
gold as the major constituent of the low-fusing component is based on
its known characteristics of workability, biocompatibility, non-oxidizing
properties and color. The low fusing metal particles must, of course, have
a melting temperature below that of the high fusing metal particles. When
the high-fusing metal particles possess a flake-like geometry and are very
thin, they overlap to form a lattice network of particles. This assures
adequate strength even when the composition is thinned down near the
dental margin without flaking. The thin flakes also assure a compact,
open-pore structure of uniform porosity, which also provides a greater
reliability of dimensional control over the voids in the heat-treated
structure.
Upon heat treatment of the base material, the binder should vaporize
to leave a porous, sponge-like structure having a capillary network of
multiple
voids uniformly distributed throughout the structure, with a void volume
preferably of at least twenty percent (20%), and up to eighty percent (80%).
Although the binder may be any suitable vehicle which will vaporize
upon heat treatment to facilitate the formation of a porous structure dental
wax is preferred. The binder may include organic or inorganic components
to control the malleability of the dental material. The term "wax," for
purposes
of the present invention, means any natural wax, mineral wax, or organic
9
CA 02229896 1999-05-18
D-SHO-98F
wax, or combination thereof. The wax composition is not critical as long as
it melts relatively cleanly without leaving a residue. The viscosity of the
wax
is also not critical although for purposes of the present invention a
relatively
viscose or "hard" wax should be used. The concentration of the wax binder
is preferably high enough to assure a void volume of at least twenty percent
(20%). When the concentration of binder is at least twenty percent (20%) by
volume, the relationship between void volume and binder is substantially one-
to-one.
The base material of the present invention is shaped upon a working
die of non-refractory material. A conventional stone working model of dental
teeth is shown in Figure 1 with a removable stone working die 22 . The die
22 may be composed of any non-refractory composition. Initially it is
preferred to apply any conventional isolation material 15 over the stone
working die 22 using a brush 16 so as to facilitate the separation of the base
material from the working die 22 after shaping as shown in step 5. The use
of a liquid or semi-liquid isolation material is very conventional in
dentistry
and typically used with standard investing and casting procedures. The base
material 23 is preferably applied to the working die 22 from a conventional
wax spatula 20. The wax spatula 20 is preferably a conventional electrically
heated wax spatula so that the base material 23 may be fed from the spatula
20 at an elevated temperature to control the ease of application of the base
material when higher viscosity waxes are used in the base material. After
applying the base material 23 to the working die 22 it is shaped to form a
desired configuration 25, preferably in the form of a dental coping, as shown
CA 02229896 1999-05-18
D-SHO-98F
in Figure 4 using a hand wax carver or other conventional implement. The
shaped base material 25 is then removed from the working die 22 as shown
in Figure 5 in preparation for investing.
Activated carbon particles are then added to a conventional dental
investment material or to any conventional industrial investment material
preferably for high temperature application. Activated carbon is a well-known,
porous, carbonaceous material formed by heat-treating carbon or subjecting
it to reaction with gases, sometimes adding chemicals, for example, zinc
chloride, during or after carbonization, in order to increase its porosity.
Its
high porosity results in a very high surface area of many orders of magnitude
larger than its untreated surface area. Activated carbon has a large
absorption capacity to different gases. The carbonaceous particles from
which activated carbon is formed may be of any conventional carbon
material, including carbon black, coke flour, calcined lamp black flour, and
the like. Suitable amounts of the activated carbon particles to be added to
the dental investment material may range from five-thousands of one percent
(0.005%) of the weight of the investment material used in investing the
shaped base material 25 to about five percent (5%) of the weight of the
investment material. Finely divided particles of activated carbon is preferred
in particle sizes of less than 250 microns average. The activated carbon
particles functions both as a reducing agent and to assist the filler in
filling in
the capillary network formed by the base material during the heat-treatment
procedures. The activated carbon substantially burns during heat treatment
leaving little or no residue. Carbonaceous materials other than activated
11
CA 02229896 1999-05-18
D-SHO-98F
carbon are not suitable for satisfying the above objectives in the present
invention and therefore even if present are of no avail.
The shaped base material 25 is filled with investment material
containing activated carbon 27 as shown in Figure 6. A supporting pin 28
may be used to facilitate placement of the invested base material 25 on a
firing tray (not shown) for insertion into a furnace (not shown). The invested
base material 25 is then heat treated to convert the base material into a
porous structure 29 of identical shape and without distortion. The porous
structure 29 has a high void volume of above at least 20% and preferably
over 30% void volume although the porosity may be as high as 80%. The
usual heat-treatment for the base material is done in stages with an
intermediate heat treatment stage of between 500 C and 800 C and a final
heat treatment stage between 800 C and 1200 C. The total heat treatment
for all the heat treatment stages of the base material totals generally less
than 30 minutes and preferably less than 15 minutes.
A filler material is melted into the voids of the heat-treated porous
structure 29 to solidify the structure for forming the final dental
restoration.
The filler material may be any suitable ceramic or metal composition,
preferably a precious metal composition. The filler material may also be
formed of a matrix of particles mixed with a wax binder having a similar
composition and concentration similar to the composition and concentration
of the binder used to form the porous structure. A minimum binder
concentration of at least about twenty percent (20%) by volume is preferred,
and up to eighty-five percent (85%) by volume. Fifty percent (50%) or more
12
CA 02229896 1999-05-18
D-SHO-98F
of the overall weight of the filler composition is preferably of individual or
alloyed particles, of any size, containing between 90% to 98.5% gold and
between 1.5% to 8.5% silver, preferably 2% to 5%, with the remainder
selected from the group of metals such as copper, zinc, aluminum,
magnesium, gallium, indium, tin, or any of the platinum group metals and/or
elements from the third or fourth groups of elements of the periodic table.
The weight of the remainder should not exceed seven percent (7%) of the
total weight. The other fifty percent (50%) of the filler composition may be
composed entirely of gold, although other metals may be included, provided
the silver content of the total filter composition is limited to no more than
ten
percent (10%) by weight, and the total of the other metals is also limited to
ten percent (10%) by weight. The addition of metals, other than gold and
silver, may be added to provide a melting gradient during melting of the
filler
material.
If wax is used as the binder in the filler, its composition is not critical,
and any natural wax, mineral wax, organic wax, or synthetic wax composition
may be used. The preferred wax for the filler is relatively soft and tacky,
and
should melt relatively cleanly, as should any other binder constituent,
without
leaving a significant residue. By using a relatively soft and tacky wax for
the
filler it may be readily compressed into a compacted strip 30 of any desired
geometrical shape. The strip 30 may have any thickness generally between
twenty-five (25) microns and ten (10) millimicrons. The strip 30 is placed
over
the porous structure 29 which is still filled with the investment material 27
before being returned to the furnace for heat treatment. The strip 30 may be
13
CA 02229896 1999-05-18
D-SHO-98F
affixed to the porous structure 39 using any conventional tweezers or holder
40. Heat treatment occurs at a temperature sufficient to melt the filler into
the voids of the porous structure 29 to densify the structure thereby forming
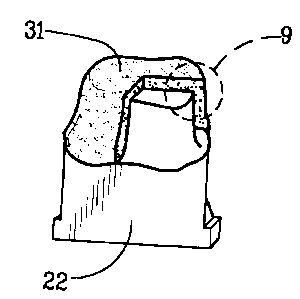
upon removal of the investment 27 a finished dental coping 31. In figure 8
the finished dental coping 31 is shown seated for convenience upon the
working die 22 and has a section removed therefrom to more clearly identify
the final finished coping 31 as having a central body region 33 with
symmetrical inner and outer layers 32 as shown in figure 9. The central
body region 33 is composed substantially of the base material metals and
less than 50% of the filler metals whereas the inner and outer layers are
substantially or entirely of gold.
14