Note: Descriptions are shown in the official language in which they were submitted.
CA 02230827 1998-03-19
0050/41;229
Dispersions comprising polyurethanes having carbonyl groups in
keto function
The present invention relates to aqueous dispersions comprising a
polyurethane (A) including structural units derived from
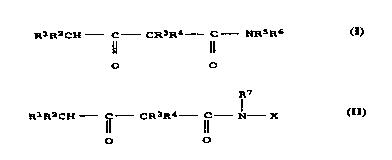
compoùnds of the formula (I)
R1R2CH - C CR3R4 - C NRsR6
Il 11
O O
where
R1, R2 ,and R3 are each hyclrogen, C1-C24-alkyl or
C6-C24-alkenyl,
R4 is hydrogen,
R5 and R6 a) together ar~e C4-C10-a1k~ne~iyl,
~) are each C2--C10-alkyl, C5-C8-cycloalkyl or
C7-C20-aralk:yl,
c) are each a :hydroxyl-terminated poly(C2-C4-alkylene
oxide), or
d) one radical, R5 or R6, is as defined under (a) to
~c) and the other radical is hydrogen or is of the
formula II
RlR2CH C CR3R4 - C 1 X II
11 ll
O O
where
45 X is C2-C6-alkanediyl and
CA 02230827 1998-03-19
0050/46229
R7 is as defined for Rs or R6 but is not of the formula II,
and
- Rs and R6 carry a tota~l of 2-5 hydroxyls attached to an
aliphatic carbons [sic~, and
- R5 and/or R6 may carry 1 or 2 hydroxyls attached to aromatic
structures or may carry one nitrile, tertiary amino, carboxyl
or sulfo group in free or salt form.
Aqueous dispersions compri~ing polyurethanes are widely known
~cf. D. G. Oertel ~Kunststoff Handbuch 7", 2nd Edition, 1983,
15 Carl Hanser Verlag, Municlh, Vienna, pp. 24-25 and 571-574). Also
known is the use of the polyurethane dispersions as coating
compositions, for example as paints or printing inks.
Processing, economics and the desired properties of the
20 subsequent coatings have combined to impose on the polyurethane
dispersions a range of re~quirements which have not yet been fully
met.
25 The service properties of surface coatings are frequently subject
to the following requirem,ents:
- The coating materials should be able to be stored for a
prolonged period without any change in their properties (eg.
rheological properties) or in the properties of the coatings
produced using them.
- The coating materials should feature minimized amounts of
so]vents, leveling agents or other volatile organic
constituents, so as to in; ize the emissions of organic
compounds when the cc,ating materials are applied and dried.
- Fo]lowing application, to the workpiece the coating material
should rapidly dry or cure so that it can be used or
processed further after just a short time.
- The coating materials should show little or no foaming
tendency when being processed.
CA 02230827 1998-03-19
' 0050/41;229
In the case of high-quality coatings and surfaces of polyurethane
coating materials, a combination of the following requirements
applies:
5 - smooth surface and high gloss
- resistance to moisture, water vapor and chemicals, such a~
di;Lute alkalis and ac:ids, and to organic solvents and
surfactants
- stability toward mechanical stresses such as impact or
abrasion
15 - freedom from inherent: color and defects such as bubbles or
cracks
- in the case of wood substrates, the coating materials should
alLow the visible structure of the wood to emerge more
st:rongly (bringing out the grain).
The development of coatings having such a combination of
propert:ies is made all the more difficult since the individual
25 perfornnance properties appear to be based on divergent structural
propert;ies. Whereas a pre!requisite of abrasion resistance is a
certain level of hardness, impact strength necessitates a certain
elastic:ity. Moreover, a glossy surface requires the coating
material to level well, which generally implies the use of
30 volati]Le organic compouncls as leveling agents.
In part;icular, the coating materials should be able to be
proces-;ed by as wide a range as possible of customary techniques.
The dil-ferent techniques, of varying complexity (where the
35 complexity correlates wit:h the quality of the resulting
coatinqs), are required so as to be able with maximum economic
efficiency to produce coatings meeting a defined level of
quality; indeed, increased complexity and effort often appear to
be jus1;ified only if matc:hed by corresponding quality. On the
40 other hand, it is logistically complex for the coatings processor
to stock a different raw material for each processing technique.
Among ~;uch techniques, the following are particularly important:
The te<hnique of cold CUI. ing (curing of the coating at room
45 temperature) with a one-component coating system is the least
complex and should satisi-y average quality requirements.
CA 02230827 1998-03-19
- ' 0050/4~229
The technique of cold curing with a two-component coating system
which, since it requires the processor to mix the system and then
gives a mixture of only limited pot life, is associated with
greater effort on his part, is intended to satisfy heightened
5 quality requirements.
The stoving technique (curing at usually 100-160~C) is suitable
for the production of coatings of the highest quality.
Normally, the surface properties of printing inks are subject to
the same requirements as already stated for the other coating
materials. In addition, it is important that they fulfill
further, specific requirements:
- high proportion of solids, especially pigments, in order to
minimize drying times, and
- good leveling even on nonpolar substrates such as
poLyethylene and polypropylene.
Requirements which should be met by articles printed with the
inks, especially those articles made of nonpolar plastics, are:
good ink adhesion to the substrate, especially under the
inEluence of water
- resistance of the inks to customary solvents, fats, oils,
su:rfactants, aqueous solutions, acids and alkalis
- good fastness properlies.
This set of requirements also gives rise in some cases to
35 conflic:ting aims, and the prior art printing inks fail to resolve
this conflict fully. For instance, it is known that the leveling
of printing inks can be improved by adding surfactant, but that
the applied inks are of cleficient water resistance. By adding
solvent: it is likewise possible to improve the wettability, but
40 this limits the ecological advantages of the water-based inks.
Polyurethane dispersions which can be used as one-component
system~; for coating various substrates are described in
45 EP-B-0 332 326. In addition to a water-dispersible polyurethane
with a molecular weight of more than 2000 which carries carbonyl
groups in keto or aldehycle function, they include a further
component which carries hydrazine or hydrazone groups, or else
CA 02230827 1998-03-19
0050/46229
s
the po]yurethane carries not only the keto or aldehyde carbonyl
groups but hydrazine and/or hydrazone groups as well. To
introduce the carbonyl ~t:ructural unit into the polyurethane it
is recommended that the latter is prepared using monomers such as
5 dihydroxyacetone, the Mic:hael adduct of diacetoneacrylamide with
diamine or alkanolamine, or the Michael adduct of 2 mol of
diacetoneacrylamide with 2 mol of diamine.
A disadvantage of the dicipersions prepared using
10 dihydroxyacetone, however, is that they produce brown films.
Said document also proposes incorporating keto groups into the
polyurethane by using monomers such as the Michael adduct of
15 diacetoneacrylamide and diethanolamine. Although such monomers
can indeed be processed 21S one-component systems which lead by
cold curing to coatings having satisfactory properties, they are
not suitable as a component of two-component systems for
producing coatings havinq a superior level of properties. Nor
20 does processing by stoving produce coatings which meet exacting
requir~sments .
In addition, aqueous dispersions comprising a water-disp~rsible,
carbonyl-containing polyurethane and a polyhydrazide are known
25 from DE-A-3 837 519, in which the carbonyl function enters the
polyurethane through the use, during preparation, of
carbonyl-containing mono- or polyalcohols, examples being
hydroxyacetone, hydroxybenzaldehyde, acetoin, benzoin, adducts of
diepoxides and ketocarboxylic acids, and ketocarboxylic esters
30 having at least one hydroxyl. Said document also recommends the
use of these dispersions as a coating material or printing ink.
The dispersions which comprise polyurethanes prepared from
polyhydroxycarbonyl compounds do not give full satisfaction when
35 shelf life is at a premium.
The mechanical properties and solvent resistance of coatings
produced from dispersions comprising a polyurethane which has
been prepared from the abovementioned monohydroxycarbonyl
40 compounds are as yet not entirely satisfactory. Moreover, these
dispersions have a tendency to form coagulum.
EP-A-0 646 609 likewise recommends the use of polyurethane
45 dispersions as printing inks. Its polyurethanes have terminal
hydrazine functional groups and can be dispersed in water owing
to the presence of ionic and polyalkylene oxide groups.
0050/46229 CA 02230827 1998-03-19
JP-A-7'i-98913 discloses polyurethane rubbers which are prepared
using amides formed from an alkanolamine and acetic acid
(H3CCOC'H2CON(CH2CH2OH)2 or H3CCOCH2CONHC(CH2CH2OH)2C2H5) and an
aluminum or iron acetoacetate complex as chain extender.
It is an object of the pr-esent invention, therefore, to provide
aqueou~; coating materials with all-round high performance which
do not have the deficienc:ies of the prior art and whose
proces-;ing to give strong, ~lossy coatings requires the use of
lO little or no volatile leveling assistants. In particular, the
coatin(~ materials should be able to be employed with maximum
flexibility; in other words, the level of properties of the
coatin~3s, which can be at:tained using them by various processing
techniques, should not be inferior to that of the prior art.
A further object is to provide printing inks which are free from
the de~Eiciencies of the prior art and can be used to produce
printed substrates, espec:ially nonpolar printed substrates, on
20 which 1:he ink adheres firmly.
We have found that these objects are achieved by the dispersions
described above.
25 The dispersions comprise a polyurethane (A) which includes
structural units derived from compounds of the formula (I)
RlR2CH C CR3R4 --C NRsR6
O O
35 where
R1, R2 and R3 are each hydrogen, C1-C24-alkyl or
C6-C24-alkenyl,
40 R4 is hydrogen,
R5 and R6 generally:
a) together are C4-C10-alkanediyl, preferably
butane-1,4-diyl or pentane-1,5-diyl,
0050/46229 CA 02230827 1998-03-19
b) are each C2-C10-alkyl, preferably C2- or C3-alkyl, or
are C5-C8-cycloalkyl, preferably cyclopentyl or
cyclohexyl, or are C7-C20-aralkyl, preferably
benzyl, or are of the formula II
R7
RlR2CH C CR3R4 - C - 1 - X II
Il 11
0 0
where
15 X is C2-C6-alkanediyl a.nd
R7 i8 as defined for RS or R6 but i8 not of the formula II,
c) ar~e each a hydroxyl-terminated poly(C2-C4-alkylene oxide),
preferably of the fo:rmula III,
I
-ICHz- CH - 0 - H III
\ / n
30 where
R8 is hydrogen, methyl ,and/or ethyl and
n is 1 to 10,
where
- Rs and R6 together carry 2 to 5 hydroxyl~ attached to an
aliphatic carbon, an,d
- R5 and/or R6 may carry 1 or 2 hydroxyls attached to aromatic
structures or may carry 1 nitrile, tertiary amino, carboxyl
or sulfo group in fr,ee or salt form.
4S
~ 0050/4~229 CA 02230827 1998-03-19
In view of the desired crosslinking density and the mechanical
propert:ies dependent thereon, the carbonyl content of the
structural elements in the polyurethane which are derived from
the compounds of the formula (I) is chosen such that there are in
5 genera]. from 3 to 140 mmol, preferably from 6 to 100 mmol,
particularly preferably from 10 to 90 mmol of these groups per
100 g of polyurethane.
The novel aqueous disper~iions are usually prepared by
I. preparing a polyurethane by reacting
a) polyfunctional isocyanates of 4 to 30 carbons,
b) polyols of which
bl) 10-100 mol%, based on the overall amount of polyols
(b), have a molecular weight of 500-5000, and
b2) 0-90 mol%, based on the overall amount of polyol~
~b), are diiunctional and have a molecular weight of
62-500 g/mol,
c) compounds of the formula I and/or condensation products
which carry alcc,holic hydroxyls and include structural
elements derived~ from compounds of the formula (I)
(condensates I),
d) if desired, furt.her polyfunctional compounds which are
different from t.he monomers (b) and (c) and have reactive
groups selected from alcoholic hydroxyl, primary amino
and secondary amino, and
~5
e) monomers which a~re different from (a), (b), (c) and (d),
have at least one isocyanate group or at least one
isocyanato-react:ive group and, moreover, carry at least
one hydrophilic or potentially hydrophilic group which
renders the polyurethane dispersible in water,
II. di.spersing the polyurethane resulting from step I in water.
45 Suitable monomers (a) are the polisocyanates customarily employed
in polyurethane chemistry.
CA 02230827 1998-03-19
~ 0050/4b229
Particular mention may be made of diisocyanates X(NC0)2 in which X
is a C4-Cl2 aliphatic, a (6-Cl5 cycloaliphatic or aromatic or a
C7-Cl5 araliphatic hydrocarbon radical. Examples of such
diisocyanates are tetramethylene, hexamethylene and
5 dodecamethylene diisocyan,ate, 1,4-diisocyanatocyclohexane,
l-isocyanato-3,5,5-trimethyl-5-isocyanatomethylcyclohexane
(IPDI), 2,2-bis(4-isocyanatocyclohexyl)propane, trimethylhexane
diisocyanate, 1,4-diisocy~anatobenzene, 2,4-diisocyanatotoluene,
2,6-diisocyanatotoluene, 4,4~-diisocyanatodiphenylmethane,
10 tetramethylxylylene diisocyanate (TMXDI),
2,4~-diisocyanatodiphenylmethane, p-xylylene diisocyanate, the
isomers of bis(4-isocyanatocyclohexyl)methane, such as the
trans/t:rans, the cis/cis and the cis/trans isomers, and mixtures
consist:ing of these compounds, especially the mixtures of the
15 respect:ive structural isomers of diisocyanatotoluene and
diisocyanatodiphenylmethclne, and in particular the mixture
consist:ing of 80 mol% 2~4 diisocyanatotoluene [sicl and 20 mol%
2,6-di:Lsocyanatotoluene. Particular advantage is also possessed
by mixlures of aromatic isocyanates, such as 2,4 diisocyanato-
20 toluene [sic] and/or 2,6--diisocyanatotoluene, with aliphatic or
cycloa:Liphatic isocyanates, such as hexamethylene diisocyanate or
IPDI, I?referably in an aliphatic to aromatic ratio of from 4:1 to
1:4.
25 Isocyanates suitable for use as compounds (a) include those which
in add:ition to the free isocyanate groups carry further, masked
isocyanate groups, for example uretdione or carbodiimide groups.
30 If desired, it is also possible at the same time to use
isocyanates having only one isocyanate group, generally in a
proporl~ion of not more than 10 mol~ based on the overall molar
amount of monomers. The rnonoisocyanates will normally carry
furthe:r functional group-;, such as olefinic or carbonyl groups,
35 and wiLl serve to introduce into the polyurethane functional
groups which make it possible for the polyurethane to undergo
dispersion, crosslinking or further, polymer-analogous reactions.
Monome:rs suitable for th:is purpose are those such as
isopropenyl-a,~-dimethylbenzyl isocyanate (TMI).
To prepare polyurethanes with a certain degree of branching or
crosslinking, it is poss:ible, for example, to use trifunctional
and tetrafunctional isocyanates, which are obtained, for example,
by brimging together difunctional isocyanates in a reaction in
45 which some of their isocyanate groups are derivatized to form
allophanate, biuret or isocyanurate groups. Examples of
CA 02230827 1998-03-19
~ ' 0050/46229
commerc:ial compounds are the isocyanurate or the biuret of
hexamet:hylene diisocyanat:e.
Examples of other suitable polyisocyanates, of higher
5 functionality, are polyisocyanates which contain urethane groups
and are based on 2,4- ancl/or 2,6-diisocyanatotoluene, isophorone
diisocyanate or tetramethylene diisocyanate on the one hand and
on low molecular mass polyhydroxy compounds, such as
trimethylolpropane, on the other.
~or good film formation and elasticity, polyols Ib) of ideal
suitab.ility are high molecular mass polyols, preferably diols
(bl), ,having a molecular weight of about 500-5000 g/mol,
15 preferably about 1000-3000 g/mol.
The polyols (bl) are especially polyesterpolyols as known, for
example, from Ull ~nnfi Encyklopadie der technischen Chemie, 4th
Edition, Volume 19, pp. l52-65, preferably those obtained by
20 reacting dihydric alcoho.ls with dibasic carboxylic acids. Instead
of the free polycarboxyl.ic acids it is also possible to employ
their anhydrides or este:rs with lower alcohols, or mixtures
thereof, in order to pre]pare the polyesterpolyols. The
polycarboxylic acids may be aliphatic, cycloaliphatic,
25 araliphatic, aromatic or heterocyclic and may be unsaturated
and/or substituted, for ~example by halogens. Examples of such
compounds are suberic, azelaic, phthalic and isophthalic acids,
phthalic, tetrahydrophthalic, hexahydrophthalic,
tetrachlorophthalic, en~ sthylenetetrahydrophthalic and glutaric
30 anhydrides, maleic acid, maleic anhydride, fumaric acid and
dimeric fatty acids. Preferred dicarboxylic acids are those of
the formula HOOC-(CH2)y~COOH in which y is 1-20, preferably an
even n.umber from 2 to 20, examples being succinic, adipic,
dodeca.nedicarboxylic and sebacic acids.
Exampl.es of polyhydric alcohols are ethylene glycol,
propane-1,2-diol, propane-1,3-diol, butane-1,3-diol,
butene-1,4-diol, butyne-1,4-diol, pentane-1,5-diol,
neopen,tylglycol, bis(hydroxymethyl)cyclohexanes such as
40 1,4-bi.s(hydroxymethyl)cyclohexane, 2-methylpropane-1,3-diol,
methyl.pentanediols, and also diethylene, triethylene,
tetraethylene, polyethylene, dipropylene, polypropylene,
dibutylene and polybutylene glycols. Preference is given to
neopentylglycol and to a.lcohols of the formula HO-(CH2)X-OH in
45 which x is 1-20, prefera.bly an even number from 2 to 20, examples
CA 02230827 1998-03-19
0050/46229
being e!thylene glycol, butane-1,4-diol, hexane-1,6-diol,
octane-1,8-diol and dodecane-1,12-diol.
Also suitable are polycarbonatediols as can be obtained, for
5 example, by reacting phoEgene with an excess of the low molecular
mass a]cohols mentioned as synthesis components for the
polyest:erpolyols.
10 Suitability extends to lactone-based polyesterdiols, which are
homo- or copolymers of lactones, preferably hydroxyl-terminated
adduct-; of lactones with suitable difunctional starter molecules.
SuitabLe lactones are preferably those derived from compounds of
the formula H0-(CH2)z-COOH in which z is 1-20, examples being
15 ~-caprc,lactone, ~-propiolactone, y-butyrolactone andtor
methyl--~-caprolactone and mixtures thereof. Examples of suitable
starter components are the low molecular mass diols mentioned as
synthe~3is components for the polyesterpolyols. The corresponding
polymers of E-caprolactone are particularly preferred. Lower
20 polyeslterdiols or polyetherdiols can also be used a~ starters for
preparing the lactone po:Lymers. Instead of the polymers of
lactones it is also poss:ible to employ the corresponding,
chemically equivalent po:Lycondensation products of the
hydroxycarboxylic acids corresponding to the lactones.
The polyesterols may also be prepared from minor amounts of
monofunctional and/or poLyfunctional monomers.
Further suitable monomer~3 (bl) are polyetherdiols. They are
30 obtainable, in particula:r, by polymerizing ethylene oxide,
propyl,ene oxide, butylene oxide, tetrahydrofuran, styrene oxide
or epichlorohydrin with itself, for example, in the presence of
BF3, or by subjecting these compounds, alone or in a mixture or in
succession, to addition reaction with starting components
35 containing reactive hydrogens, such as alcohols or amines, for
example water, ethylene glycol, propane-1,2-diol,
propane-1,3-diol, 1,2-bis(4-hydroxydiphenyl)propane or aniline.
Particular preference is given to polytetrahydrofuran with a
molecular weight ranging from 240 to 5000 and especially from 500
40 to 4500.
Likewise suitable are polyhydroxy olefins, preferably those
having 2 terminal hydroxyls, for example
a-w-dihydroxypolybutadiene [sic], a-w-dihydroxypolymethacrylic
45 [sic] esters or a-w-dihydroxypolyacrylic [sic] esters, as monomers
(bl). Such compounds are known, for example, from EP-A-0 622 378.
~ CA 02230827 1998-03-19
~ 0050/46229
12
Other ~;uitable polyols are polyacetals, polysiloxanes and alkyd
resins.
The po:lyols may also be employed as mixtures in any desired
5 proportions.
The ha:rdness and modulus of elasticity of the polyurethanes can
be increased if the polyols (b) employed include not only polyols
lO (bl) but also low molecu:Lar mass diols (b2) having a molecular
weight of about 62-500 g,~mol, preferably 62-200 g~mol.
The compounds used as monomers (b2) are in particular the
synthesis components of the short-chain alkanediols mentioned for
15 the preparation of polyesterpolyols, with preference being given
to neopentylglycol and to the unbranched diols having 2, 4, 6, 8,
10 or 12 carbons.
Based on the overall amount of polyols (b) the proportion of
20 polyols ~bl) is preferably 10-100 mol% and that of monomers (b2)
is preferably 0-90 mol%. The ratio of the polyols (bl) to the
monomers (b2) is preferably from 0.2:1 to 5:1, particularly
preferably from 0.5:1 to 2:1.
25 Compounds particularly suitable as components (c) are those of
the formula (I), which are obtainable by subjecting a diketene of
the formula ~IV)
O
l l IV
R1 ~ ~ ~ R3
R4
R2
to an addition reaction with an alkanolamine of the formula (V)
R5
H - N V
R6
CA 02230827 1998-03-19
0050/4~229
In the diketenes of the formula ~IV) and the amines of the
formula, (V), Rl, R2, R3 and R4 and R5, R6 and R7, respectively, are
as defi.ned for the formula (I).
5 It is preferred to employ a diketene in which Rl, R2 and R3 are
hydrogen or in which one of Rl and R2 is hydrogen and the other
is, li~;e R3, linear, saturated and unsubstituted hexadecyl.
lO Partic-llarly preferred amines are monoaminopolyols with two
aliphat:ically attached hydroxyls, such as
l-amino-2,3-propanediol, 2-amino-l,3-propanediol,
2-amino-2-methyl-1,3-propanediol,
2-amino-2-ethyl-1,3-propclnediol,
15 2-amino-l-phenyl-l,3-propanediol, diethanolamine,
diisopropanolamine, 3-(2--hydroxyethylamino)propanol and
N-(3-hydroxypropyl)-3-hydroxy-2,2-dimethyl-l-aminopropane.
Also suitable are monoaminopolyols having more than 2
20 alipha1:ically attached hydroxyl~, such as
tris(hydroxymethyl)methylamine) [sic],
2-[tri:i(hydroxymethyl)me1:hylamino]ethanesulfonic acid,
3-[tris(hydroxymethyl)methylamino]propanesulfonic acid,
N-[tri:3[hydroxymethyl)methyl]glycine [sicl,
25 tris(3-hydroxypropyl)methylamine, glucamine and
N-(2-hydroxyethyl)glucam:ine, or diaminodiols, such as
N,N'-b:is~2-hydroxyethyl)ethylenediamine, reaction products of a
diprimary polyetherdiamine and, per mole of polyetherdiamine,
2 mol of ethylene, propy:Lene and/or butylene oxide, the
30 condit:ions for reaction of the polyetherdiamine with the alkylene
oxide being selected so ilS to give with high selectivity the
N,N'-b.is~hydrooxyalkylam:ine) [sicl derivative havin~ two
secondi~ry aminos. Examples of the polyetherdiamines are
4,7-dioxadecane-l,lO-diamine, 4,ll-dioxatetradecane-l,l4-~; ~ m ine,
35 a-~2-~ ;n~ ~thylethyl)-~-~2-aminomethylethoxy)polyloxy(methyl-
l,2-eth~neAiyl)] having an MW of 200-3000, and
~-(3-aminopropyl)-~-~3-aminopropoxy)poly[oxy~l,4-butanediyl)]
having an MW of 300-3000.
40 Use may likewise be made of monoaminopolyols having only one
aliphatically attached hydroxyl, such as ethanolamine,
N-methylethanolamine, N-~ethylethanolamine, N-butylethanolamine,
N-cyclohexylethanolamine, N-tert-butylethanolamine, leucinol,
isoleu,-inol, valinol, prolinol, hydroxyethylaniline,
45 2-(hyd:roxymethyl)-piperildine, 3-~hydroxymethyl)piperidine,
2-(2-hydroxyethyl)piperi,dine, 2-amino-2-phenylethanol,
2-amino-l-phenylethanol, ephedrine, p-hydroxyephedrine,
CA 02230827 1998-03-19
OOSO/46229
norephedrine, adrenaline, noradrenaline, serine, isoserine,
phenylserine, 1,2-dipheny:1-2-aminoethanol, 3-amino-1-propanol,
2-amino-1-propanol, 2-amino-2-methyl-1-propanol,
isopropanolamine, N-ethyl.isopropanolamine,
5 2-amino-3-phenylpropanol, 4-amino-1-butanol, 2-amino-1-butanol,
2-aminoisobutanol, neopentanolamine, 2-amino-1-pentanol,
5-amino-1-pentanol, 2-ethy1-2-butyl-5-aminopentanol,
6-amino-1-hexanol, 2-amino-1-hexanol, 2-(2-aminoethoxy)ethanol,
3-(aminomethyl)-3,5,5-trimethylcyclohexanol,
10 2-aminobenzylalcohol, 3-aminobenzylalcohol,
2-amino-5-methylbenzylalcohol, 2-amino-3-methylbenzylalcohol,
3-amino-2-methylbenzylalcohol, 3-amino-4-methylbenzylalcohol,
3-A i n - ?thylbenzylalcohol, 1-aminoethyl-4-hydroxybenzylalcohol,
2-(4-am,inophenyl)ethanol, 2-(2-aminophenyl)ethanol,
15 1-(3-am~inophenyl)ethanol, serine [sicl, homoserine, threonine,
ethanolamineacetic acid, 4-amino-3-hydroxybutyric acid,
N-(2-hydroxyethyl)glycinenitrile, 4-(2-hydroxyethyl)piperazine
and l-amino-4-(2-hydroxyethyl)piperazine, 2-hydrazinoethanol or
diaminc,monools, such as N-(2-aminoethyl)ethanolamine,
20 1-[2-(2-hydroxyethoxy)ethyl]piperazine, and
1,3-dia~ino-2-propanol.
Preference is given to the use of compounds of the formula (I)
prepared from monoamino monoalcohols or polyamino monoalcohols,
25 based on the amounts of all monomers (c), in amounts of not more
than SCI mol%, particularly preferably not more than 20 mol%.
Those monomers (c) comprising mono- or polyamino monoalcohols,
30 employed in minor amounts, serve, for example, to control the
viscosi.ty during polyurethane synthesis.
Preferred adducts of the formula I are those of the diketene in
which ~1, R2 and R3 are hydrogen with the compounds (Ia).
Compounds of the formula (I) can, for example, be prepared-in the
fashion described for the acetoacetamide derivatives in
DE 11 4L2 859 or GB 715,896.
g~ Also suitable as components (c) are condensation products
comprising structural elements derived from compounds of the
formula (I) (condensates I), such as carbonyl-containing
polyest:erpolyols having 2L molecular weight of 300-5000, and which
are obt:ainable, for examE~le, by polycondensation of
CA 02230827 1998-03-19
0050/46229
x) compounds of the formula I in which the sum of the hydroxyls
which are attached to aliphatic carbons and are carried by
su~bstituents Rs and R6 and, if appropriate, R7 together is 2
(compounds Ia) and
y) if desired, dioils other than the compounds (Ia), having a
molecular weight of 62-500 g/mol (diols y)
10 Z) with dicarboxylic acids,
the moLar ratio of the sum of the compounds (Ia) and the diols
(y) to the dicarboxylic acids being from 2:1 to 1.05:1.
15 Preferred diols (y) and preferred dicarboxylic acids (z) are
those compoundfi also used to synthesize the polyesterdiols (bl).
To prepare the condensates (I) it is likewise possible,
preferably in minor amounts, to employ monofunctional and~or more
20 than difunctional alcohoLs or carboxylic acids. The preparation
of such condensates (I) is known, for example, from US 5,321,118.
The monomers (d), which are different from components (b) and
25 diols ~c), serve generally for crosslinking or chain extension.
In general they are tri- or higher-functional nonaromatic
alcohols, amines with 2 or more primary and/or secondary aminos,
and compounds which carry not only one or more alcoholic
hydroxyls but also one or more primary and/or secondary aminos.
Examples of trihydric and higher-functional alcohols which can be
used to establish a certain degree of crosslinking or branching
are trimethylolpropane, glycerol and sucrose.
35 Others which come into consideration are monoalcohols which as
well as the hydroxyl carry a further isocyanate-reactive group,
such as one or more primary and/or secondary aminos; one example
is monoethanolamine.
40 Polyamines, with 2 or more primary and/or secondary aminos, are
used in particular when chain extension or crosslinking is to
take place in the presence of water, since amines generally react
faster with isocyanates than do alcohols or water. This is
frequently necessary when the desire is for aqueous dispersions
45 of crosslinked polyurethanes or of polyurethanes of high
molecular weight. In such cases a procedure is followed in which
isocyanato-containing prepolymers are prepared, are dispersed
S0/46Z29 CA 02230827 1998-03-19
16
rapidly in water and then crosslinked or chain-extended by adding
compounds having two or more isocyanate-reactive amino groups.
Amines suitable for this purpose are generally polyfunctional and
5 from the molecular weight: range from 32 to 500 g/mol, preferably
from 6() to 300 g/mol, ancl contain at least two primary, two
secondary or one primary and one secondary amino group. Examples
are diamines, such as diaminoethane, diaminopropanes,
~;A inohutane5~ diaminohexanes, piperazine,
10 2,5-dimethylpiperazine, amino-3-aminomethyl-3,5,5-
trimethylcyclohexane (isophoronediamine, IPDA),
4,4'-d:iaminodicyclohexylMethane, 1,4-diaminocyclohexane,
aminoelhylethanolamine, hydrazine or hydrazine hydrate, or
triamines, such as diethylenetriamine or
15 1,8-diamino-4-aminomethyloctane.
The amines may also be employed in blocked form, for example in
the fo:rm of the corresponding ketimines (see eg. CA-l 129 128),
20 ketazimes (cf. eg. US-A ~1,269,748) or amine salts (see
US-A 4,292,226). In addition, oxazolidines as used, for example,
in US-,~ 4,192,937 constitute masked polyamines, which for
preparing the novel polyurethanes can be employed for chain
extending the prepolymer<;. When such masked polyamines are used,
25 they are generally mixed with the prepolymers in the absence of
water to form a mixture which is subsequently combined with the
dispersion water or with part of the dispersion water, such that
the ap~propriate polyamines are released by hydrolysis.
30 The polyurethanes preferably contain no polyamine or l-10 mol%,
particularly preferably 4-8 mol%, based on the overall amount of
compon,ents (b), (c) and (d), of a polyamine having at least 2
isocyanate-reactive amino groups, as monomers (d).
35 Furthermore, for chain termination, use may also be made, in
minor amounts, ie. preferably in amounts of less than lO mol%,
based ,on components (b) ,and (d), of monoalcohols. Their function
is generally similar to that of the monoisocyanates, ie.
principally to functionalize the polyurethane with free-radically
40 polymerizable C=C double bonds.
Furthermore, for chain termination, use may also be made, in
minor amounts, ie. preferably in amounts of less than 10 mol~,
based on components (b) and (d), of monoalcohols. Their function
45 is generally similar to that of the monoisocyanates, ie.
0050/46229 CA 02230827 1998-03-19
principally to functionalize the polyurethane with free-radically
polymerizable C=C double bonds lsic].
To give the polyurethanes dispersibility in water, they are
5 genera.lly composed not only of components (a) - (d) but also of
monome:rs le), different from components (a) - (d), which carry at
least one isocyanate group or at least one isocyanato-reactive
group and, in addition, at least one hydrophilic group or a group
which ,can be converted to hydrophilic groups. In the text below
lO the te:rm "hydrophilic groups or potentially hydrophilic groups"
is abb.reviated to "(potentially) hydrophilic groups". The
(potentially) hydrophilic groups react with isocyanates
substantially more slowly than the functional groups of the
monomers used to synthes.ize the polymer main chain.
The proportion of components having (potentially) hydrophilic
groups among the total amount of components (a) - (e) is
generally such that the molar amount of (potentially) hydrophilic
20 groups, based on the amount by weight of all monomers (a) to (e),
is from 30 to 1000 mmol/kg, preferably from 50 to 500 mmol/kg
and, with particular preference, from 80 to 400 mmol/kg.
The (potentially) hydrophilic groups may comprise nonionic or,
25 preferably, (potentially) ionic hydrophilic groups.
Suitable nonionic hydrophilic groups are, in particular,
polyethylene glycol ethers comprising preferably 5-100,
particularly preferably 10-80, ethylene oxide repeating units.
30 The content of polyethylene oxide units is generally from 0 to
10% by weight, preferably from 0 to 6% by weight, based on the
amount by weight of all monomers (a) to (e).
Preferred monomers containing nonionic hydrophilic groups are the
35 reaction products of a polyethylene glycol and a diisocyanate
~ which carry a terminally etherified polyethylene glycol radical.
Diisocyanates of this kind and methods of preparing them are
indicated in US 3,905,929 and 3,920,598.
Ionic hydrophilic groups are, in particular, anionic groups such
as sulfonato, carboxylato and phosphato, in the form of their
alkali. metal or ammonium salts, and also cationic groups, such as
ammoni.um groups, especially protonated tertiary amino groups or
45 quaternary ammonium groups.
CA 02230827 1998-03-19
0050/46229
Potentially ionic hydroph.ilic groups are, in particular, those
which by simple neutralization, hydrolysis or quaternization
reactions can be converted into the abovementioned ionic
hydrophilic groups, examples therefore being carboxyl, tertiary
5 amino or anhydride groups.
(Potentially) ionic monomers (e) are described at length, for
example, in Ull ?nn~ Encyklopadie der technischen Chemie, 4th
Edition, Volume 19, pp. 311-313 and DE-A-14 95 745.
Of particular significance in practice as ~potentially) cationic
monomers (e) are especially monomers containing tertiary amino
groups, examples being tris(hydroxyalkyl)amines,
15 N,N'-bis(hydroxyalkyl)alkylamines, N-hydroxyalkyldialkylamines,
tris(amlinoalkyl)amines, N,N~-bis(aminoalkyl)alkylamines and
N-aminoalkyldialkylamines, in which each alkyl and alkanediyl
indepen.dently is a C2-C6 moiety. Others which come into
consideration are polyethers which have tertiary nitrogens and
20 prefera.bly two terminal hydroxyls, as can be obtained, for
example, by alkoxylating amines which have two hydrogens attached
to the amine nitrogen, for example methylamine, aniline or
N,N'-di.methylhydrazine, in a manner known per se. Polyethers of
this ki.nd generally have a molecular weight of 500-6000 g/mol.
These t.ertiary amines are converted into the ammonium salts
either with acids, preferably strong mineral acids such as
phosphoric, sulfuric or hydrohalic acids or strong organic acids,
or by reaction with suitable quaternizing agents, such as
30 Cl-C6-alkyl halides, for example bromides or chlorides.
Suitable monomers containing (potentially) anionic groups are,
customarily, aliphatic, cycloaliphatic, araliphatic or aromatic
carboxylic and sulfonic acids which carry at least one alcoholic
35 hydroxyl or at least one primary or secondary amino group.
Preference is given to dihydroxyalkylcarboxylic acids, especially
of 3 to 10 carbons, as are described inter alia in
US-A 3,412,054. Particula.r preference is given to compounds of
the formula
0050/4~229 CA 02230827 1998-03-19
COOH
I
HO - Ra - C - Rb - OH
RC
10 in which Ra and Rb are each C1-C4-alkanediyl, and Rc i8
Cl-C4-alkyl, and especially to dimethylolpropionic acid ~DMPA).
Appropriate dihydroxysulfonic acids and dihydroxyphosphonic acids
15 such as 2,3-dihydroxypropanephosphonic acid are also suitable.
Suitab:ility extends to dihydroxy compounds having a molecular
weight of 500-10,000 g/mol and at least 2 carboxylate groups,
which are known from DE-A 39 11 827. They can be obtained by
20 reacting dihydroxy compounds with tetracarboxylic dianhydrides,
such a3 pyromellitic dianhydride or cyclopentanetetracarboxylic
dianhydride, in a molar ratio of 2:1 to 1.05:1 in a polyaddition
reaction. Particularly suitable dihydroxy compounds are the
monomers (b2) mentioned as chain extenders and also the polyols
25 (bl).
Suitable monomers (e) containing isocyanate-reactive amino groups
are aminocarboxylic acids such as lysine, ~-alanine, the adducts
of aliphatic diprimary diamines with a,~-unsaturated carboxylic
30 acids that are indicated in DE-A 20 34 479, such as
N-(2-aminoethyl)-2-aminoethanecarboxylic acid, and also the
corres]ponding N-aminoalkylaminoalkylcarboxylic acids where the
alkanediyls are of 2 to 6 carbons.
35 Where monomers having potentially ionic groups are employed, they
can be converted into the ionic form prior to, during or,
preferably, after the isocyanate polyaddition, since the ionic
monomers are frequently difficult to dissolve in the reaction
mixture. With particular preference, the carboxylate groups are
40 in the form of their salts, with an alkali metal ion or ammonium
ion as counterion.
In the field of polyurethane chemistry it i~ generally known how
45 the molecular weight of the polyurethanes can be adjusted by
choosing the proportions of mutually reactive monomers and the
CA 02230827 1998-03-19
0050/46229
arithmetic mean of the number of reactive functional groups per
molecu.le.
Normally, components (a)~ (b), (c), (d) and (e) and their
5 respective molar quantities are chosen such that the ratio A:B
between
A) th.e molar quantity of isocyanate groups and
B) th,e sum of the molar quantities of hydroxyl and of functional
groups able to react with isocyanates in an addition reaction
is from 0.5:1 to 2:1, preferably from 0.8:1 to 1.5:1,
15 particularly preferably from 0.9:1 to 1.2:1 and, with very
particular preference, as close as possible to 1:1.
In addition to components (a), (b), ~c), (d) and (e) use is made
of monomers having only one reactive group, in general in amounts
20 up to 15 mol~, preferably up to 8 mol%, based on the overall
amount of components (a) - (e).
The polyaddition reaction of components (a) - (e) is generally
25 carried out at from 20 to 180~C, preferably from 50 to 150~C,
under atmospheric or autogenous pressure.
The reaction time necessary may extend from a few minutes to
several hours. It is known in the field of polyurethane chemistry
30 how the reaction time can be influenced by a variety of
parameters such as temperature, monomer concentration and monomer
reactivity.
The reaction of the diisocyanates can be accelerated using the
35 customary catalysts, such as dibutyltin dilaurate, tin(II)
octoat.e and diazabicyclo[2.2.2]octane.
Suitable polymerization apparatus comprises stirred vessels,
especi.ally if solvents are used to provide for low viscosity and
40 good h,eat dissipation. For reaction in the absence of solvents,
the usually high viscosities and usually short reaction times
render the use of extrud.ers, especially self-cleaning multiscrew
extrud~ers, particularly suitable.
The di.spersions are usua.lly prepared by one of the following
techni.ques:
CA 02230827 1998-03-19
- ' 0050/46229
In accordance with the acetone technique, an anionic polyurethane
is prepared from components (a) - (e) in a water-miscible solvent
which boils below 100~C at atmospheric pressure. A sufficient
amount of water is added to form a dispersion in which water is
5 the coherent phase.
The prepolymer mixing tec:hnique differs from the acetone
technique in that the initial product prepared is not a fully
reacted (potentially) anionic polyurethane but a prepolymer which
10 carries isocyanate groups. In this case components (a) - (d) are
chosen so that the above--defined ratio A:B is more than from 1.0
to 3, preferably from 1.()5 to 1.5. The prepolymer is first
dispersed in water and then either crosslinked by reacting the
isocyanate groups with amines having more than 2
15 isocyanate-reactive amino groups or chain-extended using amines
having 2 isocyanate-reaclive amino groups. Chain extension also
takes place if no amine :is added. In this case, isocyanate groups
are hydrolyzed to amino groups which react, extending the chain,
with remaining isocyanate groups of the prepolymers.
In a particularly preferred variant of the acetone and of the
prepolymer mixing technique, the prepolymer is prepared in 2
steps, in the first of wllich components ~c) and, if used, (b2)
25 and some of component (a~ are first reacted with one another
until virtually all of the isocyanate groups of component (a)
have b,een reacted. The progress of this reaction can be found by
measuring the NCO value, ie. by detel ining the number of
remainin~ NCO groups. In the subsequent step, the residual
30 compon,ents may be reacted with one another to form a prepolymer
and, whether this is done or not, are added to the reaction
mixtur,e formed from (a), (c) and, if used, (b2), and the reaction
is continued.
35 Where a solvent was used in preparing the polyurethane, the
majority of the solvent is usually removed from the dispersion
by, for example, carrying out distillation under reduced
pressure. The dispersion3 preferably have a solvent content of
less than 10% by weight, and with particular preference are free
40 from solvents.
The dispersions generally have a solids content of from lO to 75%
by weight, preferably from 20 to 65% by weight, and a viscosity
of from lO to 500 mPas (measured at 20~C at a shear rate of
45 250 s~
' 0050/46229 CA 02230827 1998-03-19
22
Normal:ly, the novel aqueous dispersions are virtually free from
polyvalent metal ions.
Hydrophobic auxiliaries, which may be difficult to disperse
5 homogeneously in the finished dispersion, such as, for example,
phenol condensation resins of aldehydes and phenol and/or phenol
deriva-tives, or epoxy resins and other polymers mentioned, for
example, in DE-A-39 03 5:38, 43 09 079 and 40 24 567, which are
used im polyurethane dispersions as adhesion promoters, for
10 example, can be added to the polyurethane or to the prepolymer
even before dispersion in accordance with the methods described
in the two abovementioned documents. Examples of suitable
hydrophobic auxiliaries are specified in DE-A-39 03 538,
40 24 567 and 43 09 079.
In a variant of the present invention, the novel polyurethane
dispersions are modified with free-radically polymerizable
monomers having a C=C do~ble bond but being devoid of isocyanate
20 groups or isocyanato-reactive groups (monomers f). These monomers
comprise, in particular, the monomers normally employed in the
preparation of emulsion polymerizations [sic].
Examples of suitable monomers (f) are Cl-C6-alkyl (meth)acrylates
25 and also lauryl acrylate and ~utanediol diacrylate, or
carbonyl-cont~ining compounds, for example methyl vinyl ketone,
(meth)acrolein, crotonaldehyde, diacetone(meth)acrylamide or
diacetone (meth)acrylate.
30 Examples of other monomers are vinyl esters of C2-C20 carboxylic
acids, such as vinyl laurate, stearate, acetate and propionate,
vinyl-aromatic compounds of up to 20 carbons, such as styrene and
vinyltoluene, ethylenically unsaturated nitriles, such as
acrylonitrile and methacrylonitrile, ethylenically unsaturated
35 amides, such as acrylamide and methacrylamide, vinyl halides such
as vinyl chloride, and vinylidene chloride, and C2-C~ aliphatic
hydrocarbons with 1 or 2 C=C double bonds, such as butadiene and
ethylene.
40 The monomer (f) can be added during the synthesis of the
polyurethane (A), prior to its dispersion or to the aqueous
dispersion containing the polyurethane (A), and can be
free-radically polymerized by conventional methods, adding
free-radical initiators to the mixture of polyurethane dispersion
45 and monomer (f). It can also be metered as a feed stream into an
initiator-containing polyurethane dispersion.
CA 02230827 1998-03-19
0050/46229
If it is desired to graft the polymer formed from monomer (f)
onto the polyurethane, it is advisable to employ monomers
containing a free-radically polymerizable C=C double bond when
synthesizing the polyurethane.
To crosslink the polyurethane (A), the aqueous dispersion
normally has added to it a crosslinker (B) containing functional
substituents which react in addition or condensation with the
structu~ral units derived from compounds of the formula (I).
10 Example~s of such crosslinkers (B) are compounds having at least
one alclehyde group or at least two functional substituents
selected from the group consisting of primary amino, secondary
amino, hydrazine group, hydrazide group, aminooxy group,
isocyanate group, N-methylol group and blocked isocyanate group.
Examples of suitable polyamines are nonpolymeric amines, such as
ethylenediamine, diethylenetriamine, triethylenetetramine,
propylenediamine, butylenediamine, 1,6-hexanediamine,
20 1,12-dodecanediamine, cyclohexylenediamine, piperazine,
2-methylpiperazine, isophoronediamine, phenylenediamine,
tolylenediamine, xylylenediamine, 4,4'-diaminodiphenylmethane,
menthanediamine and m-xylenediamine. The reactive amino compound
can also be a polymer, for example an amino-containing acrylic,
25 polyest:er or polyurethaneiresin, an amino-containing
polypropylene oxide (Jeffamines), or a polyethyleneimine.
These amines can also be employed in blocked form, ie. in the
form oi their aldimines or ketimines. These blocked amines are
30 known and are described, for example, by K.J. Kim and
R.C. Williams in "Proceedings of the annual Water-Borne and
Higher Solids Symposium, New Orleans, 57, (1993)" and by
B. Vogt:-Birnbrich in ~Prc,ceedings of the 21st International
Conference in Organic Coatings, Athens, 55, (1995)" and in
35 EP-A-5'i2 469 and EP-A-584 818. Preference is given to the use of
amines blocked with aromatic aldehydes such as benzaldehyde.
Examples of suitable polyhydrazides are dicarboxylic acid
dihydrazides, as are described, for example, in EP-A-442 652 on
40 page 1], line 52 to page 12, line 1. These are derived preferably
from dicarboxylic acid~ which also form the basis for the
polyest:erdiols which can be employed as component (bl).
Furthermore, the corresponding polyhydrazone derivatives can also
be usecl, for example those derived from acetone or butanone.
CA 02230827 1998-03-19
~ ' 0050~46229
Further suitable polyhydrazides of heightened water-solubility
are described, for example, in EP-A-629 657.
Furthe:r suitable crosslinkers (B) are polyisocyanates which have
5 a crosslinking effect through transimination. Such compounds are
described, for example, in DE-A-41 21 946.
Crosslinkers containing aminooxy groups, which may also be used
10 in the form of their salts, are known, for example, from
EP-A-516 074 and from DE--A-42 19 384.
Another crosslinking option is to add mono- or polyfunctional
aldehydes, which may, if desired, also be protected, to the novel
15 dispersion.
Examples of suitable monoaldehydes are compounds of the formula
X-R9-C]~O where R9 is Cl-C6-alkanediyl and X is hydrogen or
hydroxycarbonyl. Preferred aldehydes are formaldehyde,
20 acetaldehyde and benzaldehyde.
Suitable polyfunctional aldehydes are low molecular mass
compounds, especially aliphatic aldehydes of the formula
OCH-(CH2)n-CHO where n is an integer from 0 to 8, preferably 0 to
25 4, such as glyoxal or glutaraldehyde.
Oligomers, polymers and copolymers of ethylenically unsaturated,
free-radically polymerizable aldehydes can also be used as
30 crosslinking component. Suitable examples are acrolein,
methacrolein, formylstyrene and hydroxymethylfurfuryl
(meth)acrylate. If not sufficiently soluble, such crosslinking
components can be dispersed in the aqueous phase of the
dispersion and participate in film formation when the dispersion
35 is used as a binder. Preference is given to oligomeric or
polymeric crosslinking components of this kind, with a
weight-average molecular weight of 1000-500,000.
Derivatives with protected aldehyde groups are understood as
40 being those whose reactivity is comparable with that of the free
aldehyde groups themselves. Suitable examples are acetals,
mercapltals and mercaptols, dioxolanes and dithiolanes. Preference
is given to acetal and dioxolane groups formed from the reaction
of aldlehyde groups with C1-C4-alkanols or with C2-C3-alkanediols.
OOSO/46229 CA 02230827 1998-03-19
Examples of unsaturated monomers with protected aldehyde
functions are diethoxypropyl acrylate and methacrylate, and
acryloyl- or methacryloyloxypropyl-1,3-dioxolane.
5 Further suitable aldehyde derivatives are aldimine compounds
which are obtained by reacting a substituted or unsubstituted
aromat:ic or heteroaromatic aldehyde with a mono- or
polyfunctional primary amine. Such compounds are part of general
knowledge and are described, for example, in EP 552 469 A3 and in
10 US-A-5,451,653.
Crossl.inking may also take place by way of Michael acceptorR,
suitab.1e compounds of thiR type being generally known and
15 descri~bed in DE-A-42 37 492.
Crosslinking by Michael addition is generally carried out in the
presen,ce of a catalyst which is suitably a Lewis or Bronstedt
base as described in DE-A-42 37 492.
The amounts of components (A) and (~) are preferably chosen such
that the molar ratio of the carbonyl groups of structural units
derived from compounds o~ the formula (I) to the functional
substituents of compounds ~B) is from 0.1:1 to 10:1, preferably
25 from 1.5:1 to 0.5:1.
Further suitable crosslinkers ~B) are amino resins, for example
melamine-formaldehyde condensation products as described in
D.H. Solomon, The Chemistry of Organic Film polymers, p. 235 ff.,
30 John Wiley & Sons, New York, 1967. These are preferably
melamine-formaldehyde condensation resins having a molecular
weight of preferably 250-1000, particularly preferably their
partially or completely etherified derivatives. The degree of
etherification is preferably at least 45% based on the maximum
35 possible. The melamine-formaldehyde condensation products are
etherified with C1-C4 monoalcohols, for example with methanol,
ethanol, propanol or preferably butanol, and/or with monoethers
of diols having a total of 2 to 7 carbons.
However, the melamine-formaldehyde condensation products can also
be replaced in part by other crosslinking amino resins, as are
described, for example, in Methoden der organischen Chemie
(Houben-Weyl), vol. 14/2, part 2, 4th edition, Georg Thieme
45 Verlag, Stuttgart, 1963, p. 319 ff.
CA 02230827 1998-03-19
' 0050/46229
26
Further crosslinking options arise with polyisocyanates.
Particularly suitable isocyanate compounds are the generally
known and commercially available high-solids isocyanates,
hydrophilicized and/or blocked isocyanates (cf. DE-A-42 16 536).
Suitable isocyanates are those listed as monomers ~a) which are
used to synthesize the polyurethane. Among these, particular
preference is given to the polyfunctional isocyanates having more
than 2 isocyanate groups.
Examples of suitable blocking agents for the isocyanates are
alcohols and oximes, for example acetone oxime or methyl ethyl
ketoxime.
other possible crosslinkers (B) are polymeric resins which carry
oxime-blocked isocyanate groups, as are described in
DE-A-42 37 030, DE-A-33 45 448, W0 93/01245 and US-A-5,358,997.
20 The crosslinking of polyurethanes (A) present in the novel
aqueous dispersion with a polyisocyanate takes place usually in
the presence of a basic catalyst such as tertiary alkylamine.
With the exception of the nonblocked isocyanates and the
25 aldi~ines, the novel dispersions are generally mixed with the
crosslinker at any desired moment prior to processing. It is
likewise possible to add the crosslinker to the polyurethane (A)
even prior to its dispersion in water.
The novel dispersions may additionally comprise further
water-emulsifiable or water-dispersible resins, such as polymer,
polyurethane, polyester, epoxy or alkyd resins, and commercially
customary auxiliaries and additives, such as blowing agents,
35 antifoams, emulsifiers, thickeners, leveling agents and
thixotropic agents, and colorants such as dyes and pigments.
Novel dispersions where the crosslinker (B) i8 a compound
containing aldehyde-, primary or secondary amino-, hydrazine-,
40 aminoxy-, hydrazide- or ketoxime-blocked isocyanate groups or is
an amino resin constitute systems referred to as one-component
systems, since they can be processed within any desired period of
time following their preparation.
45 Novel dispersions to which a compound with nonblocked isocyanate
groups has been added as crosslinker (B) are referred to as
two-component systems, since mixing is customarily carried out by
CA 02230827 1998-03-19
0050/46229
the dispersion user owing to the limited period of time within
which the corresponding mixture is to be processed (about 8
hours).
5 The coating compositions prepared in this way are generally
applied to the substrate workpiece by the techniques customary in
the paint industry, ie. for example by rolling, spraying,
spreading, pouring and dipping.
Subsequent drying or curing of the coating material can be
carried out either by cold curing (ie. at 0-80~C, preferably room
temperature) or by stoving (ie. normally at 80-280~C).
15 Crosslinkers particularly suitable for cold curing are those
containing aldehyde, aldimine, primary or secondary amino,
hydrazine, aminoxy or hydrazide groups.
It is supposed that the polyaddition or polycondensation reaction
20 Isic] w~hich bring about crosslinking in these systems take place
only wh.en a large proportion of the water has evaporated. The
coating compositions therefore constitute a one-component system
comprising binder and crosslinker.
25 Cold cu.ring can also be carried out in the presence of
crossli.nkers (B) containing free isocyanate groups. In the case
of this kind of processing, application of the novel dispersion
to the workpiece should take place within a period of no more
than ak,out 8 hours after the time of mixing with the crosslinker.
Cold cu.ring can likewise be carried out when the crosslinkers (B)
used contain (hetero)aromatic aldimine groups. In this case,
dependi.ng on composition, the shelf life of the novel dispersions
35 ranges from one hour to several weeks.
Where t.he coating is to be processed by stoving, particularly
suitabl.e crosslinkers are the abovementioned amino resins,
blockedl and unblocked polyisocyanates and Michael acceptors.
Even wh~en a crosslinker is absent, a certain degree of
crossli.nking of the polyu.rethane does take place under stoving
conditi.ons. This is especially the case when crosslinking takes
place i.n the presence of the Lewis or Bronstedt bases described
45 in DE~ 42 37 492, such as tertiary amines, for example
l~8-diazabicyclo[5.4.o]undec-7-ene ~DBU).
' 0050/46229 CA 02230827 1998-03-19
28
The novel coating compositions are particularly suitable for
coating wood, metal, plastics, paper, leather and textiles, for
producing moldings and printing inks, and as adhesives.
5 A feature of the novel dispersions is that even those comprising
relatively little or no leveling agent can be processed to give
high-quality coating finishes.
10 Furthe:rmore, the novel dispersions can be processed both as one-
and as two-component syslems by the techniques of both cold
curing and stoving. This is advantageous for processors who
employ 2 or more of this total of 4 processing variants, since
for different techniques it is necessary to stock only a small
15 number of polyurethane dispersions.
Moreover, aqueous dispersions comprising the polyurethane (A) are
outstandingly suitable for the production of printing inks.
20 These ]printing inks are preferably composed as follows:
(I) 15 - 30% by weight of a binder consisting essentially of
the polyurethane (A) and the crosslinker (B)
(II) ? - 15% by weight of a pigment
(III) 2 - 5% by weight of an alcohol suitable as solvent
30 (IV) 4.5 - 10% by weight of customary additives
(V) 45 - 70% by weight of water.
35 As crosslinkers (B) use is preferably made of the polyhydrazides,
described in more detail above, in the abovementioned
proportions.
The customary additives are auxiliaries and adjuvants generally
40 employed in printing inks, ie. waxes, antifoams, dispersants,
wettin,~ agents and microcides [sic], for example.
Otherwise, the components (ii) [sic] to (v) [sic] employed in the
printing inks are those generally employed in such inks, which
are known, for example, from Ullmann~s Encyclopedia of Industrial
CA 02230827 1998-03-19
~ OOSO/46229
29
Chemistry, 5th edition, Volume A22; 1993 VCH Publishers, Inc.;
pp. 14:3-155.
These printing inks are particularly suitable for printing
5 polymer filma, such as polyethylene or polypropylene films,
having a surface tension of from 30 to 50, preferably from 35 to
40, particularly preferably from 37 to 39 (in mN/m, measured with
water at 23~C). Printing can be done by the customary techniques
(cf. loc. cit. pp. 145 and 146).
The polymer films with the abovementioned surface tensions are
commercially available films which have been corona treated.
15 In combination with the recl ?n~ed substrates, these printing
inks exhibit favorable wetting properties. The printed films are
resistant to customary mechanical stresses and solvents.
Abbreviations and trade names:
ADDH: Adipic dihydrazide
Basonat~ PLR 8878: Water-emulsifiable isocyanate crosslinker from
BASF
Basophob~ WE: Polyethylene wax dispersion from BASF
Basoplast~ 20 conc.: Diketene of stearic acid from BASF
BD-1,4: 1,4-Butanediol from BASF
BHAA: Adduct of 1 mol of diethanolamine and 1 mol of
diketene
DAAM: Diacetoneacrylamide
DBU: 1,8-Diazabicyclo[5.4.0]undec-7-ene
DETA: N-(2-Aminoethyl)-1,2-ethanediamine from BASF
DMEA: 2-Dimethylaminoethanol from BASF
DMPA: Dimethylolpropionic acid from Angu~ Chemie
CA 02230827 1998-03-19
0050/46229
SC: Solids content in % by weight, measured after
distillation
IPDI: Vestanat IPDI from Huls/Isophorone diisocyanate
Luhydr~n(19 A 848 S: Aqueous, autocrosslinking polymer dispersion
from BASF
10 MEK: Methyl ethyl ketone
MW: Molecular weight
Neutral.l: Neutralizing agent for the ionic groups of
polyurethane
Neutra.1.2: As Neutral.l
20 NMP: N-Methylpyrrolidone
NCD: Theoretical crosslinking density from the reaction
of ADDH with incorporated BHAA ~in mmol/kg solids)
25 PD-1,3: 1,3-Propanediol
Pluriol~ P 600: Polypropylene glycol from BASF
30 P-THF 2000: Polytetrahydrofuran 2000 from BASF
PUD: Polyurethane dispersion
TEA: Triethylamine
TMP: Trimethylolpropane
VCD: Theoretical crosslinking density from the reaction
of DETA with isocyanate groups (in mmol/kg solids)
Wacobl.au~ 9A 918 018: Blue pigment paste from BASF ~+E.
CA 02230827 1998-03-19
~ 0050/46229
Examples
Synthesis examples for compounds of the formula I
s
Compound Ia
168.14 g of diketene (2 mol ) were added over the course of one
hour at 25~C to an initial charge of 231.3 g (2.22 mol) of
10 diethanolamine, 1000 ml of tetrahydrofuran and 25 g of pyridine.
The excess diethanolamine and the pyridine were separated off by
the ad~dition of ion exchanger (Lewatit) [sic] lOOGl, strongly
acidic) and filtration. Following the addition of 2.7 g of
triphenylphosphine to the degassed clear solution, the solvent
15 was removed on a rotary evaporator. 341.09 g of a pale yellow
liquid (90.14% of theory) were isolated. The analytical data ( 13C-
and 1H-NMR) indicate a purity of more than 95%. No starting
compounds were detected in the product, but triphenylphosphine
oxide is present.
Compound Ib
599.4 g (7.129 mol) of diketene were added over the course of
90 minutes at 25~C to an initial charge of 749.5 g (7.129 mol) of
25 diethanolamine and 1250 ml of tetrahydrofuran. The
tetrahydrofuran was subsequently removed by distillation. A
viscous, pale yellow oil was isolated, which weighed 1354.4 g
(theory 1348.9 g). The 13C- and 1H-NMR spectra confirm the
structure of the product and indicate a purity of at least 95%.
30 No starting material was detected.
Compound Ic
35 Using the procedure described for compound Ib, 261 g (3.105 mol)
of diketene were reacted with 326.4 g (3.104 mol) of
diethano~ ine in 1000 ml of acetone; however, the reaction was
carried out at 15~C and the diketene was added over the course of
15 minutes. After removal of the acetone, 596.9 g (theory
40 587.4 g) of a pale yellow-orange oil of low viscosity were
isolated. The NMR spectra confirmed the structure of the product.
No starting material was detected.
0050/46229 CA 02230827 1998-03-19
Compound Id
565.4 g (6.726 mol) of diketene were added over the course of 2
hours at 15~C to an initial charge of 707.2 g ~6.726 mol) of
5 diethanola,mine and 848.4 g of methyl ethyl ketone. Subsequently,
the solution was warmed to 40~C, 996.79 g (4.484 mol) of
isophorone diisocyanate and 0.5 ml of dibutyltin dilaurate, as a
50% strength solution in xylene, were added, and the mixture was
reacted at B5~C for 4 hours. The decrease in the isocyanate
lO content was monitored by infrared spectroscopy (isocyanate signal
at about 2270 cm~
Compound Ie
Using the procedure described for compound Ib, 1614 g (19.2 mol)
of diketene were reacted with 2018.6 g (19.2 mol) of
diethanolamine in 2422 g of THF. In this case, the initial charge
of TH~ and diethanolamine was cooled to 0~C and the addition of
20 diketene was made over about 2 h 30 min. Subsequently, the
majori.ty of the solvent was removed by distillation under reduced
pressure. The desired product, which still contained solvent
residu.es, was isolated in the form of a pale yellow oil with a
viscosity of 2400 mPa.s.
Synthesis Examples for polyurethane dispersions with carbonyl
groups
Compou.nd II
In a first stage, Pluriol P 600 was aminated: 150 ml/h of Pluriol
P 600 were reacted continuously with 450 ml/h of ammonia in a
1.2 1 tubular reactor containing 500 ml of catalyst consisting of
35 50% Ni.O, 20% Cuo and 30% ZrO2. The reaction t~ ~rature in the
reactc,r was 20~-215~C, the pressure was 200 bar and the amount of
hydrogen was 50 l/h. The highly volatile constituents (water,
ammoni.a) were distilled off at 1-3 mbar at a liquid-phase
temperatùre of up to 100~C. The product is characterized by the
40 following parameters: total amine number: 174.1 mg of KOH/G
[sic]; tertiary amine number: 1.3 mg of KOH/G [sic]; secondary
amine number: 6.3 mg of KOH/g; hydroxyl number: 30.2 mg of KOH/g,
and wa,ter content 0.06% by weight.
45 In a Piecond stage, 2040 g of the above polyetheramine were
charged to a 10 1 stirred vessel. The contents of the reactor
were rendered inert by multiple evacuation and flushing with
0050/46229 CA 02230827 1998-03-19
nitrogen. Then 500 g of propylene oxide were metered in at 105~C.
Following an after-reaction phase (to constant pressure), the
vessel was evacuated for about 20 minutes in order to remove any
readily volatile constituents. There was no need for further
5 working up. The resulting polyetheraminopolyol had the following
charac1eristics: hydroxyl number: 291 mg of KOH/g; amine number:
147 mg of KOH/g; tertiary amine number: 36.3 mg of KOH/g;
secondary amine number: 102.9 mg of KOH/g; water content 0.12% by
weight;; density 1000 g/cm3; pH 11.4, and viscosity 326 mPa.s.
In the last stage, 2640 g of the propoxylated polyetheramine and
300 g of tetrahydrofuran were combined and cooled, and 523.7 g
(6.23 mol) of diketene were added with vigorous stirring over the
course of 4 hours at 0-15~C. After the end of this addition, the
15 product was stirred at room t~ erature for 1 h and then the
solven1t was removed under reduced pressure to give a pale orange
oil wh:ich still contained solvent residues.
20 Compound III
105.14 g (1 mol) of diethanolamine were added to an initial
charge at 40~C of 512 g (1 mol) of Basoplast 20 conc. and 500 g of
toluene. At the end of the addition, the toluene was removed by
25 distilLation at 90-95~C, to give a pale brownish solid. IR
analysis confirmed the disappearance of the diketene structure.
Dispersion 1
30 110.4 g of isophorone diisocyanate (0.497 mol) and 0.07 g of
dibutyLtin dilaurate as a 50% strength solution in xylene were
added to an initial charge of 133.3 g of polytetrahydrofuran (MW
2000; 0.067 mol), 13,9 g (0.073 mol) of the compound Ia, 10.7 g
of dimethylolpropionic acid (0.08 mol), 21 g of 1,4-butanediol
35 (0.23 mol) and 43.3 g of methyl ethyl ketone, and the mixture was
reacted at 90~C for 2.5 hours. The resulting prepolymer was then
diluted with 200 g of acetone and neutralized with 6.5 g of
triethylamine (0.063 mol~. Before neutralization the isocyanate
contenlt was 0.92 g/100 g (theoretically 0.68%). By adding 500 g
40 of fully deionized water and a solution of 3 g of
diethy:Lenetriamine (0.029 mol) in 16.7 g of water followed by
removaL of the acetone, an opalescent dispersion with a solids
content of 37.5% and a p~I of 7.8 was obtained. The theoretical
conten1t of keto groups is 245, and the theoretical salt content
45 is 214 mmol~kg solids.
CA 02230827 1998-03-19
' 0050/46229
Dispersion la: Polyurethane dispersion with carbonyl groups and
adipic dihydrazide as crosslinker
0.82 g of adipic dihydrazide was added to 100 g of dispersion 1
5 (molar ratio of hydrazide to carbonyl groups of 1:1).
Dispersion lb: Polyurethane dispersion with carbonyl groups and
polyet:hyleneimine as crosslinker
0.44 g of polyethyleneimine Polymin~ G10 was added as a 20%
strength aqueous solution to 100 g of dispersion 1 (ratio of
amino to keto groups of about 1:1).
15 Dispersion 2: As dispersion 1 with direct addition of adipic acid
dihydrazide
Following the preparation procedure of dispersion 1, the
dispersion was prepared from 400 g of polytetrahydrofuran 2000,
20 41.8 g of the compound Ia, 32.19 g of dimethylolpropionic acid,
63.08 g 1,4-butanediol, 331.23 g of isophorone diisocyanate,
19.43 g of triethylamine and 8.94 g of diethylenetriamine.
24.38 g of adipic dihydrazide (78.5% pure) were added prior to
the distillative removal of the acetone. An opalescent dispersion
25 with a solids content of 36.6 and a pH of 7.8 was obtained.
Dispersion 3: As dispersion 2 with addition of adipic dihydrazide
after distillation
Dispersion 3 was prepared as for dispersion 2 but using compound
Ib instead of compound Ia and adding the adipic dihydrazide prior
to the distillative removal of the acetone.
35 An opalescent dispersion with a solids content of 36.4% and a pH
of 7.~ was produced.
Dispersion 4: polyurethane dispersion with carbonyl groups
317.8'l g of isophorone diisocyanate (1.43 mol) and 0.2 g of
dibutyltin dilaurate as a 50% strength solution in xylene were
added at 59~C to an initial charge of 400 g of polytetrahyrofuran
(0.2 mol), 81.7 g of the compound Ia (0.43 mol), 32.19 g of
45 dimethylpropionic acid (0.24 mol), 38.75 g of 1,4-butanediol
(0.43 mol) and 130 g of methyl ethyl ketone. The mixture wa~
reacted at 92~C for 5 hours. It was then diluted with 600 g of
CA 02230827 1998-03-19
0050/46229
acetone and neutralized with 19.43 g of triethylamine (0.19 mol).
The isocyanate content prior to neutralization was 0.87 g/100 g
(theore.tically 0.68%). Addition of 1350 g of fully deionized
water, 8.94 g of a solution of diethylenetrialnine (0.086 mol) in
5 50 g of water, subsequent removal of the acetone and addition,
after t:he end of distillation, of 44.16 g of adipic dihydrazide
(84.7% pure, 0.215 mol) gave an opalescent dispersion with a
solids content of 40% and a pH of 8.1. The theoretical content of
keto groups is 478, and the theoretical salt content is
10 214 mmol/kg solids. The molar ratio of hydrazide groups to keto
groups is 1:1.
Dispersion 4a
Dispersion 4a was prepared as for dispersion 4 but with no ADDH
being added. In addition, it was mixed with 0.5% by weight, based
on solids, of DBU.
20 Dispersion 5: Polyurethane dispersion with carbonyl groups
517.96 g of isophorone diisocyanate (2.33 mol) and 0.2 g of
dibutyLtin dilaurate as a 50% strength solution in xylene were
added at 65~C to an initial charge of 400 g of polytetrahydrofuran
25 (0.2 mol), 10.2 g of the compound Ib (0.58 mol), 46.95 g of
dimethylolpropionic acid (0.35 mol), 90.12 g of 1,4-but~neA;ol
(1 mol~ and 250 g of methyl ethyl ketone. The mixture was reacted
at 91~C for 6 hours. It was then diluted with 700 g of acetone and
neutra:Lized with 28.33 g of triethylcunine (0.28 mol). The
30 isocyanate content prior to neutralization was 0.90 g/100 g
(theoretically 0.79%). Addition of 1800 g of fully deionized
water, 13.76 g of diethylenetriamine (0.13 mol) as a solution in
50 g o:E water, subsequent removal of the acetone and addition,
following the end of distillation, of 59.57 g of adipic acid
35 dihydrazide (84.7% pure, 0.29 mol) gave an opalescent dispersion
having a solids content of 41% and a pH of 7.8. The theoretical
content of keto groups is 480, while the theoretical salt content
is 232 mmolJkg solids.
40 Comparison Example 1 (V): Polyurethane dispersion without
carbonyl groups
Following the procedure for dispersion 1, 400 g of
45 polytetrahydrofuran (0.2 mol), 32.1 g of dimethylolpropionic acid
(0.24 mol), 90.1 g of butanediol (1 mol) and 348.2 g of
isophoxone diisocyanate (1.565 mol) were reacted with 0.2 g of
dibutyltin dilaurate solution in 160 [lacuna] of methyl ethyl
CA 02230827 1998-03-19
0050/46229
ketone. After dilution with 600 g of acetone, an isocyanate
content of 0.8 g/100 g was measured (theoretically 0.64%). The
product was neutralized with 19.4 g of triethylamine (0.192 mol),
dispersed with 1500 g of fully deionized water and crosslinked
5 with 8.6 g of triethylenediamine (0.083 g 1 8iC]) dissolved in
50 g of water, to give a polyurethane dispersion having an
opalescent appearance, solids content of 37.5% and a pH of 7.9.
The th,eoretical salt content is 214 mmol~kg solids.
10 Comparison Example 2 (V): Example 8 of EP-A-332 326 was repeated
without addition of adipic dihydrazide
Instead of NHP, methyl ethyl ketone and acetone were used as
15 solvents, since for comparison purposes the dispersion is to be
solvent-free.
The reactive keto compound was first of all prepared from 21.75 g
of diethanolamine ( a . 207 mol) and 35.05 g of diacetoneacrylamide
20 (0.207 mol) in 35.05 g of methyl ethyl ketone.
In the subsequent synthesis of the prepolymer, the product Capa
210 was used to replace Oxyflex S 1063-120, since no closer
specification of the composition of this polyester was given.
25 Capa 210 is a polycaprolactone from Interox having a molecular
weight of 1000 g/mol (example 910 g/mol). The prepolymer was
prepared without catalyst from 400 g of Capa 210 (0.4 mol), the
adduct solution (0.207 mol), 51.18 g of DMPA (0.389 mol) and
437.93 g of isophorone diisocyanate (1.97 mol) in 201 g of methyl
30 ethyl ketone and after 4!; minutes at 90~C and cooling to 35~C had
an iso,-yanate content of 6.88 g/100 g (theoretically 6.83~).
Neutralization was carried out with 40 g of triethylamine
(0.395 mol) and dispersion with 1480 g of fully deionized water,
the prepolymer being chain-extended with 45.3 g of hydrazine
35 hydrate (0.906 mol) dissolved in 92 g of water. Distillation of
the solvents gave a milky white, slightly opalescent dispersion
with a solids content of 38.3% and a pH of 8.1. The theoretical
content of salt groups is 377, while the theoretical content of
keto groups is 201 mmol/kg solids.
The dispersion had a strong odor of its own and, in the course of
distillation and subsequent treatment, showed an increased
tenden,-y to foam.
Comparison Example 2a (V): Treatment of the dispersion from
Comparison Example 2 (V) with adipic dihydrazide
CA 02230827 1998-03-19
0050/46229
0.67 g of adipic dihydrazide was added to 100 g of the dispersion
from C~ -rison Example ~ (molar ratio of hydrazide groups to
keto groups of 1:1).
5 C.I~Prison Example 3 (V): Preparation of a PUD from a
diethanolamine/diacetoneacrylamide adduct
23.13 g of diethanolamine (0.22 mol) and 37.23 g of
10 diacetoneacrylamide (0.22 mol) were reacted under nitrogen at 85~C
for 7 h in 40 g of methyl ethyl ketone. This solution was used
without: further treatment.
Following the procedure for preparing dispersion 1, a prepolymer
15 was prepared from 400 g of polytetrahydrofuran (0.2 mol), 60.36 g
of the adduct of diethanolamine and diacetoneacrylamide
(0.22 mol), 32.19 g of dimethylolpropionic acid (0.24 mol),
57.68 g of butanediol (0.64 mol) and 317.89 g of isophorone
diisocyanate (1.43 mol) in 130 g of methyl ethyl ketone using
20 0.2 g of dibutyltin dilaurate solution. Following dilution with
600 g of acetone, the isocyanate content was 0.88 g/100 g
(theoretically 0.68%). The product was subsequently neutralized
with 1'3.43 g of triethylamine and dispersed by adding 1500 g of
fully deionized water. For crosslinking, 8.94 g of
25 diethylenetriamine were added as well. Removal of the solvents
left a white dispersion with many inhomogeneities. Some of the
polyurethane settled out overnight. The theoretical content of
keto groups is 245, while the theoretical salt content is
214 mmol/kg solids.
Comparison Example 4 (V): Preparation of a PUD from
dihydroxyacetone
Following the procedure for preparing dispersion 1, a prepolymer
35 was prepared from 400 g of polytetrahydrofuran ~0.2 mol), 19.82 g
of dihydroxyacetone (0.22 mol), 32.19 g of dimethylolpropionic
acid (().24 mol), 69.39 g of butanediol (0.77 mol) and 346.79 g of
isophorone diisocyanate ~1.56 mol) in 130 g of methyl ethyl
ketone using 0.2 g of dibutyltin dilaurate solution (reaction
40 time: 4 h). The prepolymer solution was brown in color. Following
dilution with 600 g of ac:etone, the isocyanate content was
0.79 g~'100 g (theoretically 0.68%). The product was neutralized
with 1'1.43 g of triethylamine (0.19 mol) and dispersed with
1500 g of fully deionized water. Subsecluently, 8.94 g of
45 diethylenetri~ine dissolved in 50 g of water were added. Removal
of the solvents gave a yellowish white dispersion with a solids
conten1: of 37.1~ and a pE~ of 8.1. The theoretical content of keto
CA 02230827 1998-03-19
0050/46229
groups is 245, while the theoretical salt content is 214 mmol/kg
solids. Application of the composition by knife coating to a
glass plate in a dry-film thickness of about 50 ~m gave a
yellowish film with an orange peel structure.
Comparison Example 5 (v):
An attempt was made to prepare, from an adduct of diethanolamine
lO and diacetoneacrylamide, a dispersion with a theoretically
calculated keto group content of 335 mmol/kg solids from the
following components for prepolymer synthesis: a preadduct of
34.7 g of diethanolamine and 55.85 g of diacetoneacrylamide was
reacted at 85~C for 7 h in 60 g of N-methylpyrrolidone. 400 g of a
15 polyesterdiol of adipic acid, isophthalic acid and 1,6-hexanediol
having a MW of 2000 ~Lupraphen~ VP 9206 from BASF AG), 40.24 g of
dimethylolpropionic acid, 57.68 g of 1,4-butanediol, 90 g of
methyl ethyl ketone, 362.35 g of isophorone diisocyanate and
0.2 g of dibutyltin dilaurate solution were added. After 2 h at
20 91~C, the prepolymer gelled. For this reason the r. ~-ning
components (600 g of acetone, 24.3 g of triethylamine, 1550 g of
fully deionized water and 8.8 g of diethylenetriamine) were not
added.
25 Comparison Example 6 (V): Preparation of a PUD from diacetone
alcohol
400 g of polytetrahydrofuran (0.2 mol), 32.19 g of
dimethylolpropionic acid (0.24 mol), 81.11 g of butanediol
30 (o.9 mol), 13.42 g of TMP and 393.47 g of isophorone diisocyanate
(1.77 mol) in 160 g of acetone with 0.2 g of dibutyltin dilaurate
solution were first of all reacted in a 4 1 pressure vessel under
autogenous pressure at 86~C. Then 34.35 parts of diacetone alcohol
(0.3 mol) were added and the reaction was continued at 118~C for 3
35 hours. Following dilution with 600 g of acetone the isocyanate
value was 0.81 g/100 g (theoretically 0.64%). For neutralization,
19.43 g of triethylamine (0.192 mol) were added. The dispersion
was formed by adding 1650 parts of fully deionized water, after
which crosslinking was carried out with 8.94 g of
40 diethylenetriamine (0.09 mol) dissolved in 50 g of water. Removal
of the acetone gave a slightly opalescent, milky white dispersion
with a solids content of 37.4% and a pH of 7.9. The theoretical
content of keto groups is 308, while the theoretical salt content
is 197 mmol/kg solids. Application of the composition by knife
45 coating to a glass plate in a dry-film thickness of about 50 ~m
without film-forming auxiliaries gave a film with numerous stress
cracks directed from the side toward the center. Butylglycol of
CA 02230827 1998-03-19
0050/46229
39
neutral pH was slowly applied dropwise; coagulum formed after
only the first few drops.
The dispersions of Examples 6 to 10 and of Comparison Examples
5 7(V) to lO~V) were prepared using the monomers indicated in
Table 1 and by the method indicated in Example 3.
CA 02230827 1998-03-19
0050/46229
~ O t~ t~ '~
-- O ~,~ O I I ~ r~ O O
t
- o ,~ o I I ~ I ~ ~ ~ ~ ~ ~ ~ ~ ~ Q
'~ o
.~ td --
~' ~ ~ ~ É~
o rt IIn I ~ I''I I I O O ' O
~, ~r
n J
_. I a) - ~ r
u 1~ ~ 0 r~ ~
o ~ ~:1 0
'~ O ~ --
E~ o o ~ t~ I ~ t~~ ~ I a~ r~ Z ~~
~ o ~
O
"~ O ~ r ~ t~ ~ t~ V
.~ ~ ~
~,g td
t~ o ~ ~ I t~ ~ o I ~ r~ r-
d o ~,
D ~ ~ ~ I ~ ~o O I ~ O ~ r- ~ ~ ~ = _
g
, ~ R. O
r~~ I ~ ~ I ~ ~ I ~ ~ t~ o ~ ~ 0
. m
Zo 2 -'
' I ~ ~ I I ~ ~ r~ ~ z ~ O rn
. rn ~ t.~ ~ ~ ~ m H ~ ~ ~ ~ td
_I
0050/46229 CA 02230827 1998-03-19
Dispersion 11
Dispersion 11 was prepared in the same way as dispersion 6 but
5 without adding ADDH.
Solids content: 39.6
ph [sic:~: 7.9
10 Dispersion lla
Disper~ion lla was prepared by mixing dispersion 11 with 0.5% by
weight, based on solid resin, of DBU.
15 Dispersion llb
Dispersion llb was prepared by mixing 100 parts of dispersion 11
with 2 45 parts of adipic acid dihydrazide (molar ratio of
hydrazide groups to carbonyl groups of 1:1).
Dispersion 12
Dispersion 12 was prepared like dispersion 11
25 Solids content: 40.8%
pH: 7.9.
Dispersion 12a
30 Dispersion 12a was prepared by mixing 100 parts of dispersion 12
with 2.52 parts of adipic acid dihydrazide (molar ratio of
hydrazide groups to carbonyl groups of 1:1).
35 Dispersion 13
Using the procedure indicated in the case of Example 3, a
disper~ion was prepared from the following componentR: 678.4 g of
compound II, 53.65 g of DMPA, 87.42 g of 1,4-butanediol, 537.97 g
40 of IPDI, 40.48 g of TEA and 17.2 g of DETA. The product was an
opalescent dispersion having a solids content of 23.9% and a pH
of 8.4. The theoretical content of keto groups is 952, while the
theoretical salt content is 228 mmol/kg solids.
CA 02230827 1998-03-19
~ 0050/46229
- 42
Dispersion 13a
To 100 parts of dispersion 13 there were added 1.96 parts of
adipic acid dihydrazide (molar ratio of hydrazide groups to
5 carbonyl groups of 1:1).
Dispersion 14
Using the procedure indicated in the case of Example 3, a
dispersion was prepared from the following components: 400 g of
polytetrahydrofuran 2000, 368.33 g of compound III, 83.16 g of
DMPA, 36 05 g of 1,4-butanediol, 455.72 g of IPDI, 50.19 g of TEA
and 17.2 g of DETA. The product was an opalescent dispersion
15 having a solids content of 24.9% and a pH of 8Ø The theoretical
content of keto groups is 400, while the theoretical salt content
is 352 mmol/kg solids.
A. Performance tests as coating materials
The results of the tests of performance as coating materials are
reproduced in Tables 2a, 2b, 2c, 3, 4 and 5.
B. Preparation and performance testing of printing inks
The dispersions of Table 6 were mixed with the amounts stated
therein of pigments and other auxiliaries, by stirring the
following components into the dispersion in the following
30 sequence:
1. a solution of ammonia and water
2. a solution of Basophob and the re!~;ning water
3. isopropanol
4. p:igment paste
After optional storage the printing inks were applied to
Corona-treated polyethylene with a surface tension of 38 mN/m by
knife coating, then subjected to forced drying at 90~C or at 60~C
for 2 minutes. The dry-film thickness was s6 ~m.
The results of the tests of performance for printing inks are
given in Table 7.
CA 02230827 1998-03-19
0050/46229
43
bq
a~ ~
D ~ ~ ~ --I ~ ~ ,r~ t~
-
U~
C S ~ ~ ~ O U~ o ~~ O _~
_
_
P~ S
,~ _ 3
r ~a S 4 "~ ~
4 ~
~n _ J
,, 0 ~ h
., ~ o ~ ~
U ~ "
S ~,
~ a~
U ~ ~ ~ ~ l~ ~ ~ ~ ~ ~ ~ er t~ ~ ~ ~ U
'' u~
~ u~
E~ C
N g~ ~ ~ r1 r l a
CA 02230827 1998-03-19
~ 0050/46229
44
Table 2b
Test results of the films prepared in accordance with e)
Disper- Swelling THFAV THF Swelling AV ethanol
~ion ethanol (50%)
(50%)
1 881 % 18.6 % 767 % 14.2 %
la 401 % 4.0 % 222 % 0.4 %
lb 610 % 6.7 % 166 % 1.3 %
l(V) dissolved / dissolved
2(V) highly / 952% 15.9%
swollen*
152a(V) highly / 1011% 20.0%
swollen*
4 488 % 6.0 % 196 % 1.9 %
295 % 0.0 % 179 % 0.0 %
*very tacky
Table 2c
Steam test f)
25DigpersionVisual - Nail Visual - 1 h* Nail
immed.* hardness - hardness - 1 h
immed.* *
1 3 3 3 2
1~ 2 3 2 2
l(V) 3 5 3 2
2(V) 5 5 5 2
2atV) 5 5 5 2
~ 3 5 1 2
35 .; 3 5 2
~i 4 5 4 3
6a 2 2 1 2
6b 4 5 2 2
40 12 2 2 2 2
l;!a 1 2
13 2 4 1 2
13a 2 4
* 0 = best score, 5 = worst score
CA 02230827 1998-03-19
~ ' 0050/46229
These results show that even without crosslinkers the novel
carbonyl-containing dispersion has improved resistance
properties. Moreover, the addition of crosslinker in the case of
the comparison product does not improve the resistance.
Table 3
Test results of the surfaces prepared in accordance with b)
10Dispersion 112 lla llb
Acetone testl)
l number ] ( j )
60~C 50 50 > 100
70~C 60 > 100
80~C 75
15 90~C > 100
Pendu:Lum hardne~sl)
[number] (c)
60~C 126 123
70~C 126 131 124
20 80~C 124 128 135
90~C 114 122 137
122
Erichsen
indentationl )
25[mm] (i)
60~C > 10 > lO > 10
70~C > 10 > 10 > 10
80~C > 10 > 10 > 10
90~C > 10 > 10 > 10
30Crosshatchl)
lscore*] (h)
60~C 1 0 - 1 1
70~C 1 0 - 1 l
80~C 0 - 1 0 - l 0 - 1
90 C O O - 1 0 - 1
Abrasion [mg] (g) 23.9 / 23.6 25.8 / 24.5 26 / 24.7
* 0 = best score, 5 = worst score
0 l) Films drawn out on gradient oven panels; 2) flash rusting
The results from Tab. 3 show that, using a catalytic amount of a
basic catalyst, the results obtained are c. p~rable with those
obtained with a stoichiometric amount of crosslinker. The
45 addition of both DBU ~dispersion lla) and ADDH (dispersion llb)
prevents flash rusting.
CA 02230827 1998-03-19
0050/46229
46
r~ ~ O ,~ N C o o o
a ~
D o rO~ O
O A O
C~ U~ ~ o r~l It~ ~ O O t~
~1 ~1 A A A
._ ~
:0
~ A ~ ~ u
.' a,
~ ~'~ e~r O ,~1 U~ O _~ o ~ E3
U~
S~ ~ Ir~ ~ O ~ N --I ~
.~ ,4
0 ~7U'l O r1 N ~ r1 0 11~ ~
tS ~r
~r N ~,, o~ o~ ~J
-1 A A
IU
C ~ ~ O ~ o ~1 0 u) a~ rt
~rl --
u r~ ~ ~ ~ ~ ,~ ~ ~
~ G a~
ul ~
J C~ CD ~ O ~~~ ~ a~~ ~ ~ ~ ~ S
u
~ ~ h
In ~ u~
U~
r_ IU
~D ~j ~ r r1 r l U
o UQ ~ ~ k ~ ~ ~ ~ 'I
UJ _ ~C ~ Ul r5 ~ ; n5
U r~l ~ ~ O C .C
I ~u ~ C r ~ ~
r- Cr_~ O r-- _
10E~ ' ~t ltr l ~ UQ ~ C C U~ 3
Q, 1 r_ ~ D ~ ~ ~D Q ~U a~ O -I
r_ ~ ~ n5 ~ n- IQ ~ h h ~~
~I C C) ~ r5 ~ O dP r_ ,~ r_ ,~ r_ 1) UQ
U O U~ C ~ ID1-1 CO ~ UQ Ic UQ ~
Q ~ a~ IQ ~ ~~.~ D D U ~ ~Q O
U~ O ~D ~ ~ ~ UQ ~l dP -I ~ I CJ
~D 1~ C ~ I r5 '~ r l
n5 _ - O ~ ~D~ .) ~ )
U~ Q ~ U~ ~ ~Q ,~ r
r r~ E~ r_ 5~r~ ~ UQ ~ U~-- IQ C~ I
r5 ~ J r ~ rr5 ~ ~D IU IU ~I r
- I ~ ri ~ D O E-l ~ E-~
U 1~ U~ r5 , ,Ci r~ ~ ~ ~ IU ~r
U~ ~ ~ E3 o ~ 11 UQ R
.a ~ ~/ c c ~ ~ ~ r5 ~ O S O ~C w E~
n5 Q~ ~rl r- w -I ~ C 1~ 'rl N ~r n5 ~ n5 ~ O ~C O
E~ E-l h ~ ~ ~l~i ~ -- X ~ 3: NZ ~1 ~ ~ ~ E~ o
CA 02230827 1998-03-19
0050/46229
47
Table 6: Preparation of printing ink formulations ~parts by
weight):
Dispersion Parts Ammonia Isopro- Basophob Water Wacoblau
25% panol WE
strength
2 49.2 2.0 5.0 5.0 38.8 25
~l 45.0 2.0 5.0 5.0 43.0 25
li 46.3 2.0 5.0 5.0 41.7 25
7 47 2.0 5.0 5.0 41 25
~3 46.5 2.0 5.0 5.0 41.5 25
~3 q9.2 2.0 5.0 5.0 38.8 25
46.2 2.0 5.0 5.0 41.8 25
7 (v) 39.1 2.0 5.0 5.0 38.9 25
25 8 (V) 41.6 2.0 5.0 5.0 46.4 25
9 (V) 42.7 2.0 5.0 5.0 45.3 25
10 (V) 52.6 2.() 5.0 5.0 35.4 25
CA 02230827 1998-03-19
0050/46229
48
-~n ~ 8 8 8 8 8 8 8
r ~
~ u tr 8 o 8 8 ~ 8 8 ~ 8 8 ~ 8 8
~u
o î~ ~ ~ ~ c ~ 8~ o o o ~ o o o
w
0
.
'
~ ~ ~ O ~
~ .
~ '~
-
x ~ ~ w _. ~ o o o o o --~ o o o ~ ~ ~ ~
A~ A,l W ~P C ~
~,1 ~;~ O ---- O O O O O O O O --I O O O O
o' o~ o~ ~ o~ 'o~ y o~ o~ ou o~ o~ ou ~o)
o o o o o o o o o o o o o
'~ ~O O~ O ~O ~O ~O O) ~D ~O ~D ~O ~O
h
~ ' ~ '~ ' N
~ C~ El g ~ ~ ~ ~g U') o u
Co~ ~o~ o~ ~ oU o~ ~o~
O O ~ O ~ O O ~ O ~ O
E~
a c~ a a Q a a a a a a
E~
CA 02230827 1998-03-19
~ 0050/46229
49
n~ ~
-- 0 o o o o o o o o o o o o o o o
o o o o o o O o o o O o o o o
~. ~
~ ~o ~ 8 ~
- ~a , . . o
h ~ O o o o h ~a
. ~
0
O ,o ,~ 0 o o O O ~ ~
~1 , _
J 0 ~ ~ ~ ~ ~ ~ ~ ~ ~
a~ ~u 0 ~ ~
,~ O ~
~, ~ - O
,
o ~_I ~ o ~ o o ,~ ,~,~ r~ ~ o o o
.__ o I I a o I ~ I I O ~ I I ~ ~ ~
o
o~ ou ~o,ou o. o, o~ ou o~ ~o,ou y ou Co~~o~~o~U r~
o o o o O o o o o o o o o o o
''I ~O ~ o o~ o o~
P. I I I I I I I I I I I I I I IC~ ~
O p, ,~ O ~ ~ ~ r.~ Or,~ I'O~ O~ In In ~ Id
rl 0 0 r l r1r l _~r1_~r ~ _Ir1r I r l ~ r~l ll
U OOU O~OU OU0~ ~ ~,
o ~ ~ o o ~ ~ o ~ ~ ~ ~ h ~ ~ '
o ~o ~ n
~D r,~ r
tn ~c
D~ O p p p p p p p p p p p p p ~ U
~~ r,~ ~ O O 0 5~ 0
~ a -a a a a a a 3 a a a a 3 a a r~ ~
0050/46229 CA 02230827 1998-03-19
Test methods
5 a) Film formation on glass
The dispersions without additives were knife-coated onto
glass plates in a dry-film thickness of about 50 ~m and were
dried under standard conditions. After drying, the films were
assessed visually. If defects were present in the film
(inhomogeneities~ stress cracks, clouding, orange peel
structure, craters, etc.) then the amount of butylglycol
added to the dispersion~ wa~ just that which gave a film
surface which after drying was clear, glossy and free from
defects. The amount of butylglycol required is indicated as
the solvent de an~ in % by weight.
b) Film formation on panels
The dispersions were drawn using a film-drawing frame onto
standard metal panels or onto gradient oven panels with a
wet-film thickness of generally 150-200 ~m, dried at room
temperature for 10 minutes, and then stoved.
c) The surface hardness (Pd hardness) was determined in
accordance with DIN 53157 using a Ronig apparatu~. Table 1
indicates the number of strokes. The measurements were made
at various times, indicated in Table 2a, following the
application of the dispersions by knife coating.
d) Film thickness: determined in accordance with DIN 50 982
e) Swelling experiments
So as to obtain comparable results, 5% by weight of
butylglycol was added to all of the dispersions in order to
improve film formation. ~ilms about 2 mm thick were cast.
After 10 days, film pieces were subjected to swelling in
tetrahydrofuran (THF) or 50% strength ethanol for 24 h. After
redrying, the leaching lo~s (AV) and swelling value were
determined.
CA 02230827 l998-03-l9
- 0050/46229
51
f) Steam test
The steam test was carried out in accordance with DIN 68860B
on two-coat systems on wood. Visual assessment and testing of
the nail hardness were carried out immediately (imm.) and
again after one hour.
g) Abrasion testing:
50 g of dispersion with a determined quantity of butylglycol
added were stirred for 5 to 10 min using a Dispermat, left to
stand for 1 day, and applied with a 200 ~m wire doctor to
Abraser glass plates. After conditioning for 1 day at room
temperature, for 16 hours at 60~C and for at least 48 hours
in a controlled-climate chamber, abrasion testing was carried
out as follows using the Taber Abraser instrument model 503:
grindstone CS 10; load 2 xl kg; 1000 revolutions with 80%
suction.
h) Crosshatch: testing was in accordance with DIN 53 151
i) Erichsen indentation: testing was in accordance with ISO 1520
j) Acetone/MEK test: an iron panel of grade St 1405 was coated
with the formulation or dispersion to be tested. After
drying, a plug of cotton soaked in acetone was rubbed
backward and forward over a selected site of the coated panel
under slight pressure (1 xforward, 1 xbackward comprises 1
double stroke DS). The test is carried out for 50 - 100 Dss.
Where the film has not been worn away after that, it is
regarded as being crosslinked or fully cured.
35 k) Sulfuric acid test 28% strength: this was carried out as
described by Dr. Kurt Herberts (DKH), Wuppertal, as follows:
a small plug of cotton which had been soaked in the above
acid was placed on the test specimen. After 4 hours at 60~C
in a convection oven, the sample was then assessed in
accordance with DIN 53 230 Tab. 1 with a score from 0 to 5
(0 = very good, 5 = poor).
1) Sulfuric acid test 38% strength and sodium hydroxide solution
test 1 and 5% strength: the test was carried out as for
method i), with storage being at room temperature for
24 hours.
OOSO/46229 CA 02230827 1998-03-19
52
m) Viscosity:
The flow time was determined in accordance with DIN 53 211
using a DIN 4 cup.
n) Adhesive strength:
The adhesive strength was assessed via the Tesa tear-off
method, in %. In this test a strip of adhesive tape of
20 - 25 mm in width (Tesafilm 104 - Beiersdorf AG) was stuck
to the print to be tested, pressed down uniformly and pulled
off sharply. Testing was carried out firstly after drying and
secondly after storage in water.
o) Wet-wiping strength:
The print was placed while still wet on a smooth, Rolid
substrate. Under slight pressure, it was wiped with a soft
moist paper cloth 50 times in the same direction. An
assessment was made visually of whether and to what extent
the paper had become colored and/or the print had been wiped
off from the substrate.
p) Wet-rub creasing resistance:
Testing was carried out following the testing of wet adhesion
by subjecting a knife-coated film to circular rubbing 20
times "under water" against an identical film.
q) Wet adhesion:
The dried, knife-coated film was placed in a bucket of water.
~y gentle rubbing of the coating "in water" using the thumb,
an assessment was made of whether the wet coating could be
rubbed off from the substrate or not. Testing was carried out
after 30 minutes and 24 hours of storage in water.
40 r) Gloss: the gloss was assessed visually.
s) Leveling: the leveling was assessed visually.