Note: Descriptions are shown in the official language in which they were submitted.
CA 02232340 1998-03-17
Bifunctional Nicotinamide-Chelating Agents
Such a8 N2S2 for RAdioactive Isotopes
The invention relates to new chelating agents that contain
nicotinamides, pharmaceutical agents that contain these
compounds, their use in radiodiagnosis and radiotherapy, a
process for the production of t:hese compounds and agents, and
conjugates of these compounds with substances that selectively
accumulate in diseased tissue, especially peptides.
The use of radiopharmaceut:ical agents for diagnostic and
therapeutic purposes has been known for a long time in the field
of biological and medical research. In particular,
radiopharmaceutical agents are used to visualize certain
structures, such as, for example, the skeleton, organs, or
tissue. Diagnostic application requires the use of radioactive
agents which, after administrat:ion, accumulate specifically in
the structures in patients that: are to be examined. These
radioactive agents that accumu]ate locally can then be traced,
plotted, or scintigraphed usiny suitable detectors, such as, for
example, scintillation cameras or other suitable recording
processes. The dispersion and relative intensity of the detected
radioactive agent characterize the site of a structure where the
radioactive agent is located and can show the presence o
anomalies in structure and func:tion, pathological changes, etc.
Similarly, radiopharmaceutical agents can be used as therapeutic
agents to irradiate specific pathological tissues or regions.
Such treatment requires the production of radioactive therapeutic
CA 02232340 1998-03-17
agents that accumulate in specific structures, tissues, or
organs. By concentrating these agents, therapeutic radiation is
brought to bear directly on the pathological tissue.
The use of both diagnostic: and therapeutic
radiopharmaceutical agents requires compounds that can be
radiolabeled. In the case of metallic radionuclides, the metal
can be present in free form as an ion or in the form of a metal
complex with one or more ligands. Examples of metallic
radionuclides that can form complexes are technetium-99m and the
various rhenium isotopes. The former is used in diagnosis, and
the latter is employed in therapy. The radiopharmaceutical
agents contain suitable vehicles and additives that allow
injection, inhalation, or ingestion by patients, just like
physiological buffers, salts, etc.
The radionuclide that is used most often for tasks in
nuclear medicine is technetium-99m, which, owing to its
advantageous physical properties (no corpuscular radiation, 6
hours of physical half-life, 140 KeV of gamma-radiation) and the
low radiation exposure that results from it, is especially well
suited as a radioisotope for in vivo diagnosis. Technetium-99m
can easily be obtained from nuc:lide generators as pertechnetate
and can be used in this form directly for the production of kits
for routine clinical needs.
The production of radiopharmaceutical agents first requires
the synthesis of a suitable ligand. Then, the complex with the
radionuclide is visualized separately (labeling). To do this,
the ligand that is produced, invariably in the form of a freeze-
CA 02232340 1998-03-17
dried kit, is reacted under complexing conditions with a solution
that contains the radionuclide. If, f,or example, the production
of a technetium-99m radiopharmaceutical agent is desired, the
ligand that is produced is mixed with a pertechnetate solution
with the addition of a suitable reducing agent, and the
corresponding technetium complex is produced under suitable
reaction conditions. These complexes are then administered to
the patient in a suitable way by injection, inhalation, or
ingestion.
The solutions that contain the radionuclide can, as in the
case of technetium-99m, be obtained from an available Mo-99/Tc-
99m nuclide generator, or may be ordered from a manufacturer, as
in the case of rhenium-186. The complexing reaction is carried
out at suitable temperatures (e.g. 20~-100~C) within periods
ranging from a few minutes to several hours. To ensure complete
complexing, a large excess (more than a 100-fold excess in the
metal-radionuclide) of the ligand that is produced and enough
reducing agent to ensure complete reduction of the radionuclide
that is used are necessary.
Radiopharmaceutical agents are produced by combining the
radionuclide complex, in an amount that is sufficient for
diagnostic or therapeutic application, with pharmacologically
acceptable radiological vehicles. This radiological vehicle
should have advantageous properties for the administration of the
radiopharmaceutical agent in the form of an injection,
inhalation, or ingestion. Examples of such vehicles are HSA,
aqueous buffer solutions, e.g., tris-(hydroxymethyl)aminoethanes
CA 02232340 1998-03-17
(or their salts), phosphate, citrate, bicarbonate, etc., sterile
water, physiological common sa]t solution, isotonic chloride or
dicarbonate-ionic solutions or normal plasma ions, such as Ca2',
Nat, ~ and Mg2'.
Since technetium can be present in a number of oxidation
stages (+7 to -1), it is often necessary for radiopharmaceutical
agents to contain additional agents, which are known as
stabilizers. The latter keep the radionuclide in a stable form
until it has reacted with the ligand. These stabilizers can
contain agents that are known as transfer or auxiliary ligands,
which are especially useful for stabilizing and complexing the
metal in a well-defined oxidation stage until the target ligand
complexes the metal via a ligand exchange. Examples of this type
of auxiliary ligands are (including their salts) gluconoheptoic
acid, tartaric acid, citric acid, or other common ligands, as is
explained in more detail later.
In a standard fashion, radionuclide-containing
radiopharmaceutical agents are produced by the ligand first being
synthesized and then being reacted with the metal-radionuclide in
a suitable way to form a corresponding complex, in which the
ligand necessarily must be present unchanged after complexing,
with the exception of the cleavage of optionally present
protective groups or hydrogen ions. The removal of these groups
facilitates the coordination of the ligand on the metal ion and
thus results in quick complexing.
To form technetium-99m complexes, pertechnetate is first
obtained from a nuclide generator and shifted, with the aid of
CA 02232340 1998-03-17
suitable reducing agents (e.g., SnCl2, S20~2~, etc.), into a lower
oxidation stage, which then is stabilized by a suitable chelating
agent. Since technetium can be present in a number of oxidation
stages (+7 to -1), which can greatly alter the pharmacological
properties by altering the charge of a complex, it is necessary
to provide chelating agents or complex ligands for technetium-ggm
that can bind technetium securely, tightly, and in a stable
manner to a defined oxidation stage to keep undesirable
biodistribution, which impedes reliable diagnosis of
corresponding diseases, from occurring due to in vivo redox
processes or technetium releases from the corresponding
radiodiagnostic agents.
The efficiency of radionuclides in in vivo diagnosis and in
therapy depends on the specificity and selectivity of the labeled
chelates with respect to the target cell. These properties are
enhanced by coupling the chelat:es to biomolecules according to
the "drug-targeting" principle. Offered as biomolecules are
antibodies, their fragments, hormones, growth factors, and
substrates of receptors and enzymes. Thus, in British Patent
Application GB 2,109,407, the use of radiolabeled monoclonal
antibodies against tumor-associated antigens is described for in
vivo tumor diagnosis. Direct protein labelings via donor groups
(amino, amide, thiol, etc.) of the protein (Rhodes, B. A. et al.,
J. Nukl. Med. 1986, 27, 685-693) or by introducing complexing
agents (US Patent 4,479,930 and Fritzberg, A. R. et al., Nucl.
Med. 1986, 27, 957) with technetium-99m have also been described.
These experimental methods are not available for clinical use,
CA 02232340 1998-03-17
however, since, on the one hand, their selectivity is too low
and, on the other hand, the background activity in the organism
is too high to make in vivo imaging possible.
Regarded as suitable complexing agents for technetium and
rhenium isotopes are, e.g., cyclic amines as they are described
by Volkert et al. (Appl. Radiol. Isot. 1982, 33; 891) and
Troutner et al. (J. Nucl. Med. 1980, 21; 443), which have the
drawback, however, that they frequently are able to bind
technetium-99m in good yields only starting from a pH > 9. N202
systems (Pillai, M. R. A., Troutner, D. E. et al.; Inorg. Chem.
1990, 29; 1850) are in clinical use. Non-cyclic N4 systems, such
as, e.g., the HMPA0, suffer from low complex stability as a major
disadvantage. Because of its instability (Ballinger, J. R. et
al., Appl. Radiat. Isot. 1991, 42; 315; Billinghurst, M. W. et
al., Appl. Radiat. Isot. 1991, 42; 607), Tc-99m-HMPA0 must be
administered within 30 minutes after it is labeled, so that the
portion of decomposition products that have a different
pharmacokinetics and separation can be kept small. Such
radiochemical contaminants hamper the detection of diseases that
are to be diagnosed. Coupling these chelates or chelating agents
to other substances that accumulate selectively in foci of
disease cannot be accomplished by simple means, so that the
latter are dispersed in general in an unspecific manner in the
organism.
N2S2 chelating agents (Bormans, G. et al.; Nucl. Med. Biol.
1990, 17; 499), such as, e.g., ethylenedicysteine (EC;
Verbruggen, A.M. et al.; J. Nucl. Med. 1992, 33; 551)
CA 02232340 1998-03-17
specifically meet the requirement for sufficient stability of the
corresponding technetium-99m complex, but form radiodiagnostic
agents with a purity of greater than 69% starting only from a pH
of the complexing medium > 9. N3S systems (Fritzburg, A.; EP-
0173424 and EP-0250013) form stable technetium-99m complexes but
must be heated to temperatures of about 100~C to form a uniform
radiopharmaceutical agent.
In recent years, the demand for radiodiagnostic agents that
accumulate specifically in diseased tissue has increased. This
can be accomplished if complexing agents can be readily coupled
to selectively accumulating substances and, in so doing, do not
lose their advantageous complexing properties. Since it very
frequently happens, however, that after a complexing agent is
coupled to such a molecule with the aid of its functional groups,
a weakening of complex stability is observed, previous attempts
to couple chelating agents to selectively accumulating substances
do not appear to have been very satisfactory since a
diagnostically non-tolerable portion of the isotope from the
conjugate is released in vivo (Brechbiel, M. W. et al.; Inorg.
Chem. 1986, 25, 2772). It is t:herefore necessary to produce
bifunctional complexing agents that carry both functional groups
for binding the desired metal ion and a (another, several)
functional group(s) for binding the selectively accumulating
molecule. Such bifunctional ligands make possible a specific,
chemically defined binding of technetium or rhenium isotopes to a
wide variety of biological materials even if a so-called
prelabeling is carried out. Several chelating agents, coupled to
CA 02232340 1998-03-17
monoclonal antibodies (e.g., EP-0247866 and EP-0188256) or fatty
acids (EP-0200492), have been described. As chelating agents,
however, the already mentioned N2S2 systems are used, which are
not very suitable owing to their low stability. Since both the
selectively accumulating substances are very different in terms
of their properties and also in terms of the mechanisms according
to which they are concentrated, it is further necessary to vary
the couplable chelating agent and to be able to adapt the
physiological requirements of the coupling partner with respect
to its lipophilia, membrane permeability, etc.
The object of the invention is therefore to make available
stable complex compounds that are or can be coupled to various
selectively accumulating compounds, without their specificity and
selectivity being fundamentally affected. In addition, the
object exists of preparing such couplable chelating agents or
complexes that have a greater chemical variation range of the
substituents, in order to be able to match the latter to the
above-referenced requirements. In this case, the requirements
for the application of these compounds to humans must be met in
terms of the radiation dose taken up and the stability and
solubility of the compounds.
This object is achieved according to the invention in that
new chelating agents that contain bifunctional thiol-substituted
nicotinamides and their coupling products with specifically
accumulating compounds are made available.
CA 02232340 l998-03-l7
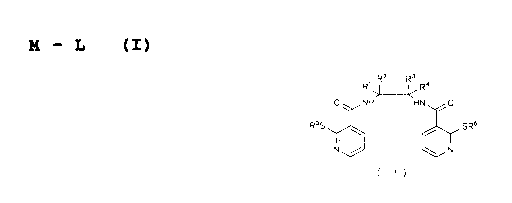
The subject of the invention is compounds of general formula
(I)
M - L (I)
in which
M means a radioisotope of Tc or Re and L means a ligand
of general formula (II)
, R~ R~ R4
0~ NH HN ~ O
R 55 ~~ SR6
N~ N
( ! I )
in which
Rl and R3 are the same or different and in each case stand
for a hydrogen atom and/or for a branched or unbranched
C1-6 alkyl radical or together an optionally
substituted, saturated, or unsaturated, aliphatic or
aromatic C36 cycle,
R2 and R4 are the same or different and in each case stand
for a hydrogen atom, for a branched or unbranched C1-6
alkyl radical or a radical -Co-R7,
in which
R7 represents a hydroxyl group, a branched or
straight-chain, cyclic or polycyclic C130 alkoxy,
alkenyloxy, polyalkenyloxy, alkinyloxy,
polyalkinyloxy, aryloxy, alkylaryloxy or
arylalkyloxy group, which optionally is
CA 02232340 l998-03-l7
substituted with hydroxy, oxy, oxo, carboxy,
aminocarbonyl, alkoxycarbonyl, amino, aldehyde or
alkoxy groups with up to 20 carbon atoms and/or
optionally is interrupted and/or substituted by
one or more heteroatoms from the series O, N, S,
P, As and Se, and optionally together form an
anhydride or represents an N(RaRb) group,
whereby
R~ and Rb are the same or different and/or
represent a hydrogen atom, a branched or
straight-chain, cyclic or polycyclic C130
alkyl, alkenyl, polyalkenyl, alkinyl,
polyalkiny]., aryl, alkylaryl or arylalkyl
radical, which optionally is substituted with
hydroxy, oxy, oxo, carboxy, aminocarbonyl,
alkoxycarbonyl, amino, aldehyde or alkoxy
groups with up to 20 carbon atoms and/or
optionally is interrupted and/or substituted
by one or more heteroatoms from the series O,
N, S, P, As and Se,
R5 and R6 are the same or different and in each case stand
for a hydrogen atom, for a branched or unbranched C1-6
alkyl radical or for a sulfur protective group.
Preferred compounds of general formula (I) are distinguished
in that in each case R1 and R3 are hydrogen atoms.
CA 02232340 1998-03-17
Especially preferred compounds of general formula (I) are
distinguished in that R1, RZ and R3 are hydrogen atoms, and R4
stands for a radical -Co-R7,
in which
R7 represents a hydroxyl group, a branched or straight-
chain, cyclic or polycyclic C130 alkoxy, alkenyloxy,
polyalkenyloxy, alkinyloxy, polyalkinyloxy, aryloxy,
alkylaryloxy or arylalkyloxy group, which optionally is
substituted with hydroxy, oxy, oxo, carboxy,
aminocarbonyl, alkoxycarbonyl, amino, aldehyde or
alkoxy groups with up to 20 carbon atoms and/or
optionally is interrupted andtor substituted by one or
more heteroatoms from the series 0, N, S, P, As and Se
or is an N(RaRb) group,
whereby
Ra and Rb are the same or different and/or
represent a hydrogen atom, a branched or straight-
chain, cyclic or polycyclic C130 alkyl, alkenyl,
polyalkenyl, alkinyl, polyalkinyl, aryl, alkylaryl
or arylalkyl radical, which optionally is
substituted with hydroxy, oxy, oxo, carboxy,
aminocarbonyl, alkoxycarbonyl, amino, aldehyde or
alkoxy groups with up to 20 carbon atoms and/or
optionally is interrupted and/or substituted by
one or more heteroatoms from the series 0, N, S,
P, As and Se.
CA 02232340 1998-03-17
Another subject of the invention relates to new bifunctional
thiol-substituted nicotinamide ligands of general formula (II)
Rl J ~ R~
0~NH HN~, O
R5S ~ " SR6
N N
( I I )
in which
R1, R2, R3, R4, R5 and R6 have the meaning that is indicated
above.
Preferred are ligands of general formula (II) according to
the invention in which R1 and R3 are hydrogen atoms.
Especially preferred are ligands according to the invention
in which R1, R2 and R3 are hydrogen atoms, and R4 stands for a
radical -Co-R7
in which
R7 represents a hydroxyl group, a branched or straight-
chain, cyclic or polycyclic C130 alkoxy, alkenyloxy,
polyalkenyloxy, alkinyloxy, polyalkinyloxy, aryloxy,
alkylaryloxy or arylalkyloxy group, which optionally is
substituted with hydroxy, oxy, oxo, carboxy,
aminocarbonyl, alkoxycarbonyl, amino, aldehyde or
alkoxy groups with up to 20 carbon atoms and/or
optionally is interrupted and/or substituted by one or
more heteroatoms from the series O, N, S, P, As and Se
or is an N(R~Rb) group,
CA 02232340 l998-03-l7
13
whereby
Ra and Rb are the same or different and/or
represent a hydrogen atom, a branched or straight-
chain, cyclic or polycyclic C130 alkyl, alkenyl,
polyalkenyl, alkinyl, polyalkinyl, aryl, alkylaryl
or arylalkyl radical, which optionally is
substituted with hydroxy, oxy, oxo, carboxy,
aminocarbonyl, alkoxycarbonyl, amino, a~dehyde or
alkoxy groups with up to 20 carbon atoms and/or
optionally is interrupted and/or substituted by
one or more heteroatoms from the series O, N, S,
P, As and Se.
Another subject of the invention is conjugates that contain
a compound of general formula (I and/or II) and substances that
selectively accumulate in diseased tissue, whereby between the
latter, a covalent bond exists and this is present in amide form
in the case of substances that contain carboxyl or amino groups,
such as naturally occurring or modified oligonucleotides, in
which degradation is prevented or hampered by naturally occurring
nucleases, peptides, proteins, antibodies or their fragments, or
is present in imide form in the case of substances that contain
hydroxyl groups, such as fatty alcohols that are in ester form or
in the case of substances that contain aldehyde groups.
Especially preferred conjugates are distinguished in that
the substances that accumulate in diseased tissue mean peptides
such as endothelins, partial sequences of endothelins, endothelin
analogs, endothelin derivatives, endothelin antagonists or
CA 02232340 l998-03-l7
14
angiotensins, partial sequences of angiotensins, angiotensin
analogs, angiotensin derivatives and angiotensin antagonists.
In other preferred conjugates according to the invention,
the peptides have the following sequences
Cys-Ser-Cys-Ser-Ser-Leu-Met-Asp-Lys-Glu-Cys-Val-Tyr
Phe-Cys-His-Leu-Asp-Ile-Ile-Trp,
Cys-Ser-Cys-Ser-Ser-Trp-Leu-Asp-Lys-Glu-Cys-Val-Tyr-
L
Phe-Cys-His-Leu-Asp-Ile-Ile-Trp,
Cys-Thr-Cys-Phe-Thr-Tyr-Lys-Asp-Lys-Glu-Cys-Val-Tyr-
I
Phe-Cys-His-Leu-Asp-Ile-Ile-Trp,
F=
Cys-Ser-Ala-Ser-Ser-Leu-Met-Asp-Lys-Glu-Ala-Val-Tyr-
Phe-Cys-His-Leu-Asp-Ile-Ile-Trp,
Cys-Ser-Cys-Lys-Asp-Met-Thr-Asp-Lys-Glu-Cys-Leu-Asn-
Phe-Cys-His-Gln-Asp-Val-Ile-Trp,
CA 02232340 1998-03-17
Ala-Ser-Cys-Ser-Ser-Leu-Met-Asp-Lys-Glu-Cys-Val-Tyr-
Phe-Ala-His-Leu-Asp-Ile-Ile-Trp,
Ala-Ser-Ala-Ser-Ser-Leu-Met--Asp-Lys-Glu-Ala-Val-Tyr-
Phe-Ala-His-Leu-Asp-Ile-Ile-Trp,
Cys-Ser-Cys-Ser-Ser-Leu-Met-Asp-Lys-Glu-Cys-Val-Tyr-
Phe-Cys-His-Leu-Asp-Ile-Ile-Trp,
Cys-Thr-Cys-Phe-Thr-Tyr-Lys-Asp-Lys-Glu-Ala-Val-Tyr-
Phe-Ala-His-Leu-Asp-Ile-Ile-Trp,
J
Cys-Val-Tyr-Phe-Cys-His-Gln-Asp-Val-Ile-Trp,
N-Acetyl-Leu-Met-Asp-Lys-Glu-Ala-Val-Tyr-Phe-Ala-His-Gln-
Asp-Val-Ile-Trp,
Asp-Arg-Val-Tyr-Ile-His-Pro-Phe-His-Leu,
Ac-Asp-Arg-Val-Tyr-Ile-His-Pro-Phe-His-Leu,
Asp-Arg-Val-Tyr-Ile-His-Pro-Phe,
Arg-Val-Tyr-Ile-His-Pro-Phe,
CA 02232340 1998-03-17
16
Arg-Val-Tyr-Ile-His-Pro-Phe-His-Leu,
Sar-Arg-Val-Tyr-Val-His-Pro-Ala,
For-Met-Leu-Phe,
For-Met-Leu-Phe-Lys,
die Teilsequenzen
[Key:]
die Teilsequenzen = the partial sequences
His-Leu-Asp-Ile-Ile-Trp,
D-Trp-Leu-Asp-Ile-Ile-Trp,
Phe-D-Trp-Leu-Asp-Ile-Ile-Trp,
Val-Tyr-Ile-His-Pro-Phe,
Val-Tyr-Ile-His-Pro,
or the cyclic amino acid sequences
cyclo-(DTrp-DAsp-Pro-DVal-Leu),
cyclo-(DGlu-Ala-alloDIle-Leu-DTrp).
CA 02232340 l998-03-l7
Another subject of this invention is also compounds of
general formula (II)
R? R~
G ~ NH HN ~ O
R55~ SR6
N ~J N
( I I )
in which
R1 and R3 are the same or different and in each case stand
for a hydrogen atom and/or for a branched or unbranched
C1-6 alkyl radical, or together for an optionally
substituted, saturated or unsaturated, aliphatic or
aromatic C36 cycle,
R2 and R4 are the same or different and in each case stand
for a hydrogen atom, for a branched or unbranched C1-6
alkyl radical or a radical -Co-R7,
in which
R7 represents a hydroxyl group, a branched or
straight-chain, cyclic or polycyclic C130 alkoxy,
alkenyloxy, polyalkenyloxy, alkinyloxy,
polyalkinyloxy, aryloxy, alkylaryloxy or
arylalkyloxy group, which optionally is
substituted with hydroxy, oxy, oxo, carboxy,
aminocarbonyl, alkoxycarbonyl, amino, aldehyde or
alkoxy groups with up to 20 carbon atoms and/or
optionally is interrupted and/or substituted by
CA 02232340 l998-03-l7
18
one or more heteroatoms from the series 0, N, S,
P, As and Se and optionally together form a
carboxylic acid anhydride, or an N(RaRb) group,
whereby Ra and Rb are the same or different
and/or represent a hydrogen atom, a branched
or straight-chain, cyclic or polycyclic C130
alkyl, alkenyl, polyalkenyl, alkinyl,
polyalkinyl, aryl, alkylaryl or arylalkyl
radical, which optionally is substituted with
hydroxy, oxy, oxo, carboxy, aminocarbonyl,
alkoxycarbonyl, amino, aldehyde or alkoxy
groups with up to 20 carbon atoms and/or
optionally is interrupted and/or substituted
by one or more heteroatoms from the series 0,
N, S, P, As and Se,
R5 and R6 are the same or different and in each case stand
for a hydrogen atom, for a branched or unbranched C1-6
alkyl radical, or for a sulfur protective group,
their conjugates with substances that selectively accumulate in
diseased tissue, whereby between the latter, a covalent bond
exists and this is present in amide form in the case of
substances that contain carboxyl or amino groups, such as
naturally occurring or modified oligonucleotides, in which
degradation is prevented or hampered by naturally occurring
nucleases, peptides, proteins, antibodies or their fragments, or
is present in imide form in the case of substances that contain
hydroxyl groups, such as fatty alcohols that are in ester form or
CA 02232340 1998-03-17
19
in the case of substances that contain aldehyde groups, as well
as their complexes with radioisotopes of Tc or Re.
The production of the compounds of general formula (II)
according to the invention is carried out in that the free thiol
group of the 2-mercaptonicotinic acid is protected in a way that
is known in the art and then the carboxyl group is activated in a
way that is known in the art, and reacted in an aprotic solvent
with the addition of a suitable base with compounds of general
formula (III)
H2N_CR1R2_CR3R4_NH2
(III)
in which R1, RZ, R3 and R4 have the meaning that is indicated
above,
at temperatures of -20~C to 180~C to compounds of general
formula (II)
, R2 R~ R4
O;~Nh HN~O
~5S ~ ~, SR6
N N
I )
in which R1, R2, R3, R4, R5 and R6 have the meaning that is
indicated in claim 1,
and optionally present protective groups are cleaved off in
a way that is known in the art.
Another subject of the invention is kits, which are used for
the production of radiopharmaceutical agents and which consist of
CA 02232340 1998-03-17
a compound of general formula (II) or compounds of general
formula (I andtor II) that contain a conjugate according to the
invention and substances that accumulate selectively in tissues,
a reducing agent and optionally an auxiliary ligand, which are
present in the dry state or in solution, as well as directions
for use with a set of reaction instructions for the reaction of
the described compounds with technetium-99m or Re in the form of
a pertechnetate solution or perrhenate solution.
The subject of the invention is also a radiopharmaceutical
composition for noninvasive in-vivo visualization of organs,
receptors and receptor-containing tissue and/or arteriosclerotic
plaque, which contains a compound of general formula (I) or
compounds of general formula (I and/or II) that contain a
conjugate according to the invention and substances that
accumulate selectively in tissues, optionally with the additives
that are commonly used in galenicals, whereby the compound is
prepared in a kit with technetium-99m or Re in the form of a
pertechnetate or perrhenate solution.
In a method for implementing a radiodiagnostic
investigation, the radiopharmaceutical composition is
administered to a patient in an amount of 0.1 to 30 mCi,
preferably 0.5 to 10 mCi per 70 kg of body weight, and the
radiation that is given off by the patient is recorded.
Surprisingly enough, many of the chelates that are
synthesized and labeled with technetium-99m or Re show higher
stability than comparable N2S2 and N3S systems, which are
described in the literature. Thus, e.g., in the case of a
CA 02232340 l998-03-l7
21
substance according to the invention (Example 2), which was
coupled to an alkylamine, no decomposition product could be
observed after 24 hours. It was also found by competitive tests
that the Tc-99m or Re chelating agents described in this
invention complex better than the comparable N2S2, N3S and
propylenaminoxime systems. The chelates and chelating agents
that are described in this invention are thus clearly better
suited for diagnostic and therapeutic purposes than the
previously known systems. Special advantage lies in the
restrained labeling conditions. Thus, after the protective
groups are cleaved off, the labeling of the ligands according to
the invention as well as their coupling products on substances
that accumulate selectively in diseased tissue is possible at
room temperature and at physiological pH. By selecting suitable
protective groups, which can be cleaved off under different
reaction conditions depending on the coupling product, it is
always ensured that undesirable secondary reactions cannot occur
in the purification of the coupling products. This carries the
danger that no undesirable cross-linking reactions or oxidations
of free sulfhydryl groups to disulfides occur under purification
conditions. Such alterations often affect the labeling yield and
radiochemical purity and thus also the background by
unspecifically bound technetium in a disadvantageous manner. The
establishment of sulfur protective groups or their cleavage is
carried out according to methods that are known to one skilled in
the art. Another basic advantage of the compounds according to
the invention lies in the high stability of the free aromatic
CA 02232340 l998-03-l7
22
thiols, which makes special protective measures (e.g., protective
gas atmosphere) superfluous in the handling of coupling products.
The coupling to substances that selectively accumulate in
diseased tissue is also carried out according to methods that are
known to one skilled in the art (e.g., Fritzberg et al.; J. Nucl.
Med. 26, 7 (1987)), for example by reaction of electrophilic
groups of the complex ligand with nucleophilic centers of the
substances that accumulate selectively in diseased tissue or by
reaction of nucleophilic groups of the chelating agent with
electrophilic groups of the substances that selectively
accumulate in diseased tissue.
As coupling participants, i.a., various biomolecules are
used. Thus, e.g., ligands that bind to specific receptors and
can thus detect alterations of the receptor thickness include,
i.a., peptides, steroid hormones, growth factors and
neurotransmitters. Coupling products with steroid hormone-
receptor-affine substances make possible an improved diagnosis of
breast and prostate cancer (S. J. Brandes and J. A.
Katzenellenbogen, Nucl. Med. Biol. 15, 53, 1988). On various
occasions, tumor cells exhibit an altered density of receptors
for peptide hormones or growth factors, such as, e.g., the
"epidermal growth factor" (EgF). The concentration differences
can be used for selective concentration of cytostatic agents in
tumor cells (E. Abound-Pirak et al.; Proc. Natl. Acad. Sci. USA
86, 3778, 1989). Other biomolecules are metabolites that can be
incorporated into the metabolism of cells, which show an altered
metabolism; these include, e.g., fatty acids, saccharides,
CA 02232340 1998-03-17
peptides and amino acids. Fatty acids that are coupled to the
less stable N2S2 systems were described in EP-0200492. Other
metabolic products, such as saccharides, deoxyglucose, lactate
and amino acids (leucine, methyl methionine, glycine) were used
with the aid of PET technology for graphic visualization of
altered metabolic processes (R. Weinreich, Swiss Med. 8, 10,
1986). Also, nonbiological substances such as misonidazole and
its derivatives, which bind irreversibly to cell components in
tissues or tissue parts at reduced oxygen concentration, can be
used for specific concentration of radioactive isotopes and thus
for graphic visualization of tumors or ischemic regions (M. E.
Shelton, J. Nucl. Med. 30, 351, 1989). Finally, the coupling of
new chelating agents to monoclonal antibodies or their fragments,
polysaccharides such as dextrans or starches, bleomycins,
hormones, enzymes, polypeptides such as polylysine and
nucleotides such as DNA or RNA is also possible. Coupling
products of the chelates according to the invention or their
complexes with technetium-99m or Re with fatty alcohols, fatty
alcohol derivatives or with fatty alcohol amines or their
derivatives have proven advantageous for the detection of
arteriosclerotic vascular diseases. These derivatives were
administered to WHHL rabbits, which show high LDL concentrations
in the blood by a genetic defect of the LDL receptor and thus
have arteriosclerotic lesions. About 1 to 6 hours after the
derivatives are administered to WHHL rabbits, a large degree of
concentration in arteriosclerotic plaque was detected. Up until
now, only very late stages of artherogenesis could be diagnosed
CA 02232340 1998-03-17
24
with an invasive process. The compounds according to the
invention therefore offer the decisive advantage of diagnosing
many earlier stages of arteriosclerosis with a noninvasive
process.
It is unimportant whether a labeling of the described
chelating agent with technetium-99m is carried out before or
after coupling to the selectively accumulating molecule. For
coupling to the selectively accumulating molecule after
complexing, however, there is a precondition that the reaction of
the radioactive complex with the accumulating compound proceeds
quickly under conservative conditions and almost quantitatively,
so that subsequent purification is not necessary.
The production of the pharmaceutical agents according to the
invention is carried out in a way that is known in the art,
whereby the complexing agents according to the invention are
dissolved in aqueous medium with the addition of a reducing
agent, preferably tin(II) salts, such as -chloride,
-pyrophosphate or -tartrate -- and optionally with the addition
of the additives that are commonly used in galenicals -- and then
sterilized by filtration. Suitable additives are, for example,
physiologically harmless buffers (e.g., tromethamine), small
additions of electrolytes (e.g., sodium chloride), stabilizers
(e.g., gluconate, phosphates or phosphonates). The
pharmaceutical agent according to the invention is present in the
form of a solution or in freeze-dried form and is mixed shortly
before administration with a Tc-99m-pertechnetate solution,
CA 02232340 l998-03-l7
eluted from commercially available MoTc generators or a
perrhenate solution.
In the case of nuclear-medicine in vivo use, the
pharmaceutical agents according to the invention are dosed in
amounts of lx10-5 to 5X104 nmol/kg of body weight, preferably in
amounts of between lx10-3 to 5X102 nmol/kg of body weight.
Starting from an average body weight of 70 kg, the amount of
radioactivity for diagnostic applications is between 0. 05 to 50
mCi, preferably 5 to 30 mCi per 70 kg of application. For
therapeutic uses, between 5 and 500 mCi, preferably 10 to 350
mCi, is administered. The administration is carried out normally
by intravenous, intraarterial, peritoneal or intertumoral
injection of 0.1 to 2 ml of a solution of the agent according to
the invention. Intravenous administration is preferred.
The following examples are used for a more detailed
explanation of the subject of the invention.
CA 02232340 1998-03-17
26
Example 1
2-(S-Piperonyl)mercaptonicotinic acid
1.55 g of anhydrous 2-mercaptonicotinic acid (10 mmol) is
suspended in 10 ml of glacial acetic acid, and about 2.28 g of
piperonyl alcohol (15 mmol) and 2.1 ml of BF3-diethyletherate (15
mmol) are added to it. It is stirred for 1-2 hours at room
temperature, whereby all is dissolved in clear form. Then, it is
concentrated by evaporation in a rotary evaporator at a bath
temperature of 40~C. The oily residue is dissolved in ethyl
acetate. By trituration with diethyl ether, the protected
nicotinic acid derivative crystallizes out.
Yield: 72%
Analysis:
Cld: C 58.12 H 3.83 N 4.84 0 22.12 S 11.08
Fnd: C 57.77 H 3.92 N 4.65 S 11.01
2-(8-Piperonyl)mercaptonicotinic acid-N-hydroxys~ccinimido-ester
2.27 g of DCC (11 mmol) in 50 ml of anhydrous
dichloromethane is added in drops to a solution of 2.89 g of acid
1 (10 mmol), 2.80 ml of triethylamine and 1.15 g of N-
hydroxysuccinimide (10 mmol) in 50 ml of anhydrous
dichloromethane while being stirred at -10~C, and it is stirred
for 2 hours at 0~C and for 4 hours at room temperature. Then, it
is cooled to -20~C, and precipitated urea is filtered out. After
the solvent is drawn off, the residue is chromatographed (silica
gel, dichloromethane).
CA 02232340 1998-03-17
27
Yield: 74%
Analysis:
Cld: C 55.96 H 3.65 N 7.25 0 24.85 S 8.30
Fnd: C 55.65 H 3.74 N 7.41 S 8.20
N,N'-Bis[2-(8-piperonyl)mercaptonicotinecarbamoyl]-
ethylenediamine 3
5.78 of activated ester 2 (200 mmol) in a little anhydrous
dichloromethane and 20. 2 g of triethylamine (200 mmol) are added
to a stirred solution of 6.01 g of ethylenediamine (100 mmol) in
a little anhydrous dichloromethane at 0~C. It is stirred for 2
hours at 0~C and for another 24 hours at room temperature. Then,
it is concentrated by evaporation in a vacuum and taken up in
dichloromethane. It is washed twice with 0. 5N HCl and saturated
sodium bicarbonate solution, dried on magnesium sulfate, and the
solvent is drawn off. The residue is crystallized by trituration
with diethyl ether.
Yield: 39%
Analysis:
C 59.79 H 4.35 N 9. 30 0 15.93 S 10.64
C 59.61 H 4.45 N 9. 24 S 10.52
N,N'-Bis r 2-mercaptonicotinecarbamoyl]ethylenediamine 4
603 mg of protected nicotinic acid derivative 3 (1 mmol) and
a trace of anisole are added to 10 ml of trifluoroacetic acid in
an oxygen-free environment at room temperature, and it is
refluxed for 1 hour. Then, the trifluoroacetic acid is drawn off
CA 02232340 1998-03-17
in a vacuum, and the residue is taken up in dichloromethane.
After washing with saturated sodium bicarbonate solution and
water, it is dried with sodium sulfate and concentrated by
evaporation. The oily residue is crystallized by trituration
with diethyl ether.
Yield: 89%
Analysis:
Cld: C 50.28 H 4.22 N 16.75 0 9.57 S 19.81
Fnd: C 50.20 H 4.35 N 16.56 S 19.08
N,N'-Bis[2-mercaptonicotinecarb~moyl]ethylenediamine, technetium-
99m complex
10 mg of compound 5 is dissolved in 1.0 ml of ethanol. 50
~l of this ligand solution is mixed with 250 ~l of ethanol, 50 ~l
of a deoxygenated aqueous citrate solution (50 mg/ml), 2.5 ~l of
a deoxygenated tin(II) chloride solution (5 mg/ml of O.lN HCl)
and 100 ~l of a pertechnetate solution (400-1000 ~Ci). After an
incubation time of 10 minutes, the reaction mixture is examined
by HPLC to determine the purity of the Tc complex formed:
LiChrospher RP-18 column, 5 ~, 125 x 4 mm; gradient elution of
100% A after 100% B within 15 minutes (eluant A:
acetonitrile/Na-phosphate 5 mmol, pH 2.0 (10/90); eluant B:
acetonitrile/Na-phosphate 5 mmol, pH 2.0 (75/25); 1 ml/min. The
radiochemical purity is > 99%.
CA 02232340 1998-03-17
29
Example 2
2-~8-TriphenYlmethyl)mercaptonicotinic acid 5
1.55 g of anhydrous 2-mercaptonicotinic acid (10 mmol) is
suspended in 10 ml of glacial acetic acid, and about 2.6 g of
triphenylmethylcarbinol (10 mmol) and 2.1 ml of BF3-
diethyletherate (15 mmol) are added to it. It is stirred for 1-2
hours at room temperature, whereby almost all is dissolved in
clear form. Then, it is concentrated by evaporation in a rotary
evaporator at a bath temperature of 40~C. The oily residue is
dissolved in ethyl acetate. By trituration with diethyl ether,
the protected nicotinic acid derivative crystallizes out.
Yield: 90%
Analysis:
Cld: C 75.54 H 4.82 N 3.52 O 8.05 S 8.07
Fnd: C 75.06 H 4.93 N 3.64 S 8.18
2-(~-TriphenYlmethyl~mercaPtonicotinic acid-N-hydroxy-
succinimidoester 6
2.16 g of DCC (11 mmol) in 50 ml of anhydrous
dichloromethane is added in drops to a solution of 3.97 g of acid
1 (10 mmol), 2.80 ml of triethylamine and 1.15 g of N-
hydroxysuccinimide (10 mmol) in 50 ml of anhydrous
dichloromethane while being stirred at -10~C, and it is stirred
for 2 hours at o~C and for 4 hours at room temperature. Then, it
is cooled to -20~C, and precipitated urea is filtered out. After
the solvent is drawn off, the residue is chromatographed (silica
gel, dichloromethane).
CA 02232340 1998-03-17
Yield: 64%
Analysis:
Cld: C 70.43 H 4.48 N 5.66 O 12.94 S 6.48
Fnd: C 70.22 H 4.68 N 5.46 S 6.44
N,N'-Bis[2-(8-TriphenYlmetbYl~mercaptonicotinecarbamoyl~-
diaminoPropionic acid ethyl ester 7
First, 9. 89 g of activated ester 6 (20 mmol) in a little
anhydrous dimethylformamide and then 5.05 g of triethylamine (50
mmol) are added while being cooled with ice to a suspension of
2. 05 diaminopropionic acid-ethyl ester dihydrochloride (10 mmol)
in a little anhydrous dimethylformamide at 0~C. It is stirred
for 2 hours at 0~C and for another 24 hours at room temperature.
Then, it is concentrated by evaporation in a vacuum and taken up
in dichloromethane. It is washed twice with 0.SN HCl and
saturated sodium bicarbonate solution, dried on magnesium
sulfate, and the solvent is drawn off. The residue is
chromatographed (silica gel, dichloromethane).
Yield: 29%
Analysis-:
C 74.13 H 5.20 N 6.29 O 7.18 S 7.20
C 73.83 H 5.45 N 6.34 S 7.28
N,N'-Bisr2-(S-TriphenYlmethyl)mercaPtonicotinecarbamoyl-
diaminoPropionic acid 8
8.91 g of the ester is stirred in aqueous/ethanolic
potassium hydroxide solution (4.0 g = 72 mmol of KOH, 20 ml of
CA 02232340 1998-03-17
water, 40 ml of ethanol) for 6 hours at room temperature. Then,
it is diluted with water and acidified with semiconcentrated HCl.
The precipitate is suctioned off, washed and dried.
Yield: 90%
Analysis:
Cld: C 73.76 H 4.91 N 6.49 O 7.42 S 7.43
Fnd: C 73.41 H 5.03 N 6.54 S 7.56
N,N'-Bis[2-mercaptonicotinec~rbamoyl~di~minopropionic acid 9
863 mg of acid 8 (1 mmol) is treated for 45 minutes at 0~C
with 10 ml of anhydrous HF in the presence of 5 ml of anisole and
3.5 ml of diethyl sulfide. After the acid is evaporated, the
remaining residue is taken up in ethyl acetate and washed with
sodium bicarbonate solution and water and dried on sodium
sulfate. After the solvent is drawn off, a slowly crystallizing
oil remains.
Yield: 43%
Analysis:
Cld: C 47.61 H 3.73 N 14.81 O 16.91 S 16.95
Fnd: C 47.48 H 3.87 N 15.03 S 16.46
N,N'-Bis r 2-mercaptonicotinec~rbamoYlldiaminopropionic ~cid,
technetium-99m complex
10 mg of compound 5 is dissolved in 1.0 ml of ethanol. 50
~l of this ligand solution is mixed with 100 ~l of ethanol, 150
~l of phosphate buffer with a pH of 8.5, 50 ~l of a deoxygenated
aqueous citrate solution (50 mg/ml), 2.5 ~l of a deoxygenated
CA 02232340 1998-03-17
32
tin(II)-chloride solution (5 mg/ml of O.lN HCl) and 100 ~1 of a
pertechnetate solution (400-1000 ~Ci). After an incubation time
of 10 minutes, the reaction mixture is examined by HPLC to
determine the purity of the Tc complex formed: LiChrospher RP-18
column, 5 ~, 125 x 4.6 mm; gradient elution of 100% A after 100%
B within 15 minutes (eluant A: acetonitrile/Na-phosphate 5 mmol,
pH 2.0 (10/90); eluant B: acetonitrile/Na-phosphate 5 mmol, pH
2.0 (75/25); 1 ml/min. The radiochemical purity is > 97%.
Example 3
N,N'-Bis[2-(~-triphenYlmethyl)mercaptonicotinecarbamoYll-
di~minopropionic acid hexylamide 10
1.13 g of DCC (5.5 mmol) in 25 ml of anhydrous
dichloromethane is added in drops to a solution of 4.32 g of acid
8 (5 mmol), 1.5 ml of triethylamine and 575 mg of N-
hydroxysuccinimide (5 mmol) in 100 ml of anhydrous
dichloromethane while being stirred at -10~C, and it is stirred
for 2 hours at 0~C. Then, a solution of 506 mg of hexylamine (5
mmol) in dichloromethane is added in drops within 30 minutes. It
is first stirred for another 2 hours at 0~C, and then stirred for
12 hours at room temperature. The product is filtered off from
urea, and the filtrate is concentrated by evaporation in a vacuum
and taken up in dichloromethane. After filtration was again
performed, it is washed twice with 0.5N HCl and saturated sodium
bicarbonate solution, dried on magnesium sulfate, and the solvent
is drawn off. The residue is crystallized by trituration with
diethyl ether.
CA 02232340 l998-03-l7
33
Yield: 81%
Analysis:
Cld: C 74.89 H 5.86 N 7.40 O 5.07 S 6.78
Fnd: C 74.71 H 5.98 N 7.31 S 6.91
N,N'-Bis r 2-mercaptonicotinecarbamoylldiamino-propionic acid hexY
amide 11
946 mg of amide 10 (1 mmol) is treated for 45 minutes at 0~C
with 10 ml of anhydrous HF in the presence of 5 ml of anisole and
3.5 ml of diethyl sulfide. After the acid is evaporated, the
remaining residue is taken up in dichloromethane and washed
several times with water and dried. Chromatographic purification
on silica gel with dichloromethane yields 272 mg of an oil.
Yield: 59%
Analysis:
Cld: C 54.64 H 5.90 N 15.17 O 10.40 S 13.89
Fnd: C 55.04 H 6.03 N 15.43 S 13.66
N,N'-Bi 8 r 2-mercaptonicotinecarbamoyl~diamino-propionic acid
hexylamide 11, technetium-99m complex
10 mg of compound 11 is dissolved in 1.0 ml of ethanol. 50
,ul of this ligand solution is mixed with 250 ~Ll of ethanol, 50 ,ul
of a deoxygenated, aqueous citrate solution (50 mg/ml), 2.5 ,lLl of
a deoxygenated tin(II)-chloride solution (5 mg/ml of O.lN HCl)
and 100 ,ul of a pertechnetate solution (400-1000 ,UCi). After an
incubation time of 10 minutes, the reaction mixture is examined
by HPLC to determine the purity of the Tc complex formed:
CA 02232340 1998-03-17
LiChrospher RP-18 column, 5 ~, 125 x 4.6 mm; gradient elution of
100% A after 100% B within 15 minutes (eluant A:
acetonitrile/Na-phosphate 5 mmol, pH 2.0 (10/90); eluant B:
acetonitrile/Na-phosphate 5 mmol, pH 2.0 (75/25); 1 ml/min. The
radiochemical purity is > 97%.
Example 4
N,N'-Bi~r2-(~-triphenylmethyl)mercaptonicotinecarbamoyl]-
ethylenediaminocArbonyl-D-Trp-Leu-Asp-Ile-Ile-Trp 12
211 mg of DCC (1.1 mmol) in 5 ml of anhydrous
dichloromethane is added in drops to a solution of 863 mg of acid
8 (1 mmol), 280 ~1 of triethylamine and 115 mg of N-
hydroxysuccinimide (1.0 mmol) in 10 ml of anhydrous
dichloromethane while being stirred at -10~C, and it is stirred
for 2 hours at 0~C. Then, a solution of 845 mg of H2N-D-Trp-Leu-
Asp-Ile-Ile-Trp-COOH (1 mmol) and DMF are added in drops within
30 minutes. It is first stirred for another 2 hours at 0~C and
then stirred for 12 hours at room temperature. The product is
filtered off from urea, and the filtrate is concentrated by
evaporation in a vacuum and taken up in dichloromethane. After
filtration was again performed, it is washed twice with 0.5N HCl
and saturated sodium bicarbonate solution, dried on magnesium
sulfate, and the solvent is drawn off. The residue is
crystallized by trituration with diethyl ether.
Yield: 36%
CA 02232340 l998-03-l7
Analysis:
Cld: C 68.94 H 5.96 N 9. 95 O 11.36 S 3.80
Fnd: C 70.02 H 6.08 N 9.78 S 3.52
N,N'-Bis r2 -mercAPtonicotinecarbamoyl] ethylene-diaminocarbonyl-D-
Trp-Leu-Asp-Ile-Ile-Trp 13
1.69 g of peptide 12 (1 mmol) is treated for 45 minutes at
0~C with 20 ml of anhydrous HF in the presence of 5 ml of anisole
and 3. 5 ml of diethyl sulfide. After the acid is evaporated, the
remaining residue is taken up in 5% acetic acid, washed several
times with diethyl ether and freeze-dried. Chromatographic
purification on Sephadex G-10 with 0. 2 M acetic acid yields 579
mg of an oil.
Yield: 48%
Analysis:
Cld: C 58.79 H 6.02 N 13.94 O 15.93 S 5.32
Fnd: C 58.39 H 6.31 N 13.88 S 5.22
N,N'-Bisr2-mercaptonicotinecarbamoyl]ethylene-diaminocarbonyl-D-
Trp-Leu-Asp-Ile-Ile-Trp, technetium-99m complex
10 mg of compound 13 is dissolved in 1.0 ml of ethanol. 50
~1 of this ligand solution is mixed with 100 ~1 of ethanol, 150
,ul of phosphate buffer with a pH of 8.5, 50 ,ul of a deoxygenated
aqueous citrate solution (50 mg/ml), 2.5 ,ul of a deoxygenated
tin(II) chloride solution (5 mg/ml of O.lN HCl) and 100 ,ul of a
pertechnetate solution (400-1000 ,UCi). After an incubation time
of 10 minutes, the reaction mixture is examined by HPLC to
CA 02232340 1998-03-17
36
determine the purity of the Tc complex formed: LiChrospher RP-18
column, 5 ~, 125 x 4.6 mm; gradient elution of 100% A after 100%
B within 15 minutes (eluant A: acetonitrile/Na-phosphate 5 mmol,
pH 2.0 (10/90); eluant B: acetonitrile/Na-phosphate 5 mmol, pH
2.0 (75/25); 1 ml/min. The radiochemical purity is > 96%.
Example 5
Diaminosuccinic ~cid ethyl ester 14
Dry HCl gas is introduced into the mixture of 5 g of
diaminosuccinic acid (34 mmol) and 100 ml of ethanol while being
stirred for 1.5 hours, and it is heated to boiling for 6 hours.
After cooling to room temperature, the solvent is drawn off.
6.97 g of white crystals remains.
Yield: 74%
Analysis:
Cld: C 34.67 H 6.55 N 10.11 0 23.09
Fnd: C 34.82 H 6.71 N 9.96
N,N'-Bi~r2-(S-triphenylmethyl)mercaPtonicotinecarbamoyll-diamino-
succinic acid ethyl ester 15
744 mg of the activated ester (20 mmol) in a little
anhydrous THF and 2.02 g of triethylamine (20 mmol) are added to
a stirred solution of 2.77 g of 14 (10 mmol) in a little
anhydrous THF at 0~C. It is stirred for 2 hours at 0~C and for
another 24 hours at room temperature. Then, it is concentrated
by evaporation in a vacuum and taken up in dichloromethane. It
is washed twice with 0.5N HCl of saturated sodium bicarbonate
CA 02232340 1998-03-17
37
solution, dried on magnesium sulfate, and the solvent is drawn
off. The residue is crystallized by trituration with diethyl
ether.
Yield: 41%
Analysis:
Cld: C 72.33 H 5.23 N 5.82 O 9.97 S 6.66
Fnd: C 72.09 H 5.43 N 5.76 S 6.46
N,N'-Bis[2-(8-triPhenYlmethyl)mercaptonicotinecarbamoyll-di~mino-
succinic acid 16
5.87 of the ester is stirred in aqueous/ethanolic potassium
hydroxide solution (4.0 g = 72 mmol of KOH, 20 ml of water, 40 ml
of ethanol) for 6 hours at room temperature. Then, it is diluted
with water and acidified with semiconcentrated HCl. The
precipitate is suctioned off, washed and dried.
Yield: 87%
Analysis:
Cld: C 71.50 H 4. 67 N 6.18 O 10.58 S 7.07
Fnd: C 70.94 H 4.85 N 6.16 S 7.11
N,N'-Bis r 2-l8-triphenYlmethyl)mercaptonicotinecarbamoyl]-diamino-
succinic acid ~nhYdride 17
3.32 g (5 mmol) of the succinic acid derivative and 1.17 g
(15 mmol) of acetyl chloride are refluxed until the succinic acid
derivative has gone completely into solution. The excess acetyl
chloride is drawn off in a vacuum, and the residue is dried in a
CA 02232340 l998-03-l7
38
vacuum with phosphorus pentaoxide and recrystallized from
dichloromethane/petroleum ether.
Yield: 81%
Analysis:
Cld: C 72.95 H 4.54 N 6.30 O 9.O0 S 7.21
Fnd: C 72.65 H 4.67 N 6.11 S 7.44
N~N~-Bisl2-~8-triphenylmethyl)mercaptonicotinecarbamoyl]-2~3-
diamino-2-rcarbonyl~Val-Tyr-Ile-His-Pro-Phe)-propionic acid 18
The solution of 775 mg of the peptide H2N-Val-Tyr-Ile-His-
Pro-Phe-COOH in a little dimethylformamide is slowly added to a
solution of 889 mg of the acid anhydride and 505 mg of
triethylamine in anhydrous dimethylformamide, and it is stirred
for 24 hours at room temperature. Then, the solvent is drawn off
in a vacuum, and the residue is crystallized by trituration with
diethyl ether.
Yield: 31%
Analysis:
Cld: C 67.85 H 5.69 N 10.10 O 12.50 S 3.85
Fnd: C 67.54 H 5.78 N 10.01 S 3.47
N,N'-Bisr2-mercaptonicotinecarb_moyll-2,3-diamino-2-
rcarbonyl~Val-TYr-Ile-His-Pro-Phe)lpropionic acid 19
1.66 g of peptide 18 (1 mmol) is treated for 45 minutes at
0~C with 20 ml of anhydrous HF in the presence of 5 ml of anisole
and 3. 5 ml of diethyl sulfide. After the acid is evaporated, the
remaining residue is taken up in 5% acetic acid and washed
CA 02232340 1998-03-17
several times with diethyl ether and freeze-dried.
Chromatographic purification on Sephadex G-10 with 0.2 M acetic
acid yields 636 mg of an oil.
Yield: 54%
Analysis:
Cld: C 57.03 H 5.64 N 14.25 O 17.64 S 5.44
Fnd: C 57.23 H 5.71 N 14.18 S 5.23
N,N'-Bisr2-mercaptonicotinecarbamoyllethylene-aiaminocarbonyl-D-
Trp-Leu-Asp-Ile-Ile-Trp, technetium-99m comPlex
10 mg of compound 19 is dissolved in 1.0 ml of ethanol. 50
~l of this ligand solution is mixed with 100 ~l of ethanol, 150
~l of phosphate buffer with a pH of 8.5, 50 ~l of a deoxygenated
aqueous citrate solution (50 mg/ml), 2.5 ~l of a deoxygenated
tin(II) chloride solution (5 mg/ml of O.lN HCl) and 100 ~l of a
pertechnetate solution (400-1000 ~Ci). After an incubation time
of 10 minutes, the reaction mixture is examined by HPLC to
determine the purity of the Tc complex formed: LiChrospher RP-18
column, 5 ~, 125 x 4.6 mm; gradient elution of 100% A after 100%
B within 15 minutes (eluant A: acetonitrile/Na-phosphate 5 mmol,
pH 2.0 (10/90); eluant B: acetonitrile/Na-phosphate 5 mmol, pH
2.0 (75/25); 1 ml/min. The radiochemical purity is > 94%.