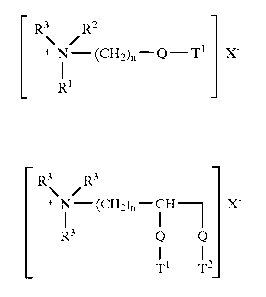Note: Descriptions are shown in the official language in which they were submitted.
CA 02232413 1998-03-16
W O 97/11142 PCTAJS96/14865
STABILISED FABRIC SOFTENING COMPOSITIONS
Field of the invention
The present invention relates to fabric softening
compositions showing excellent: stability upon storage. More
particularly, it relates t-o liquid fabric softening
compositions.
~ackqround of the invention
Liquid fabric compositions are well known to the consumer
and may be divided in two types: concentrated compositions
with 5~ to 80~ of fabric softening agents and diluted
compositions with 1~ to 5~ of fabric softening agents.
Concentrated fabric softening compositions are well
appreciated by consumer. However, concentrated compositions
may be expensive. This may be due to processing cost and/or
material cost of the formulation. Diluted'.fabric softening
compositions, are also known by consumer for providing a
cheaper alternative to concentrated compositions without
reducing the softness performance.
Consumer acceptance of such compositions is determined not
only by the performance achieved with these products but
the aesthetics associated therewith. Viscosity of the
product is therefore an important aspect of the successful
formulation of such commercial products: stable medium to
medium-high viscosities being highly preferred by consumer.
By medium-high viscosities is meant viscosities of SOcps to
CA 02232413 1998-03-16
W O 97/11142 PCTAJS96/14865
150cps when the fabric softening composition is in a
diluted form and viscosities of 30cps to 90cps when the
fabric softening composition is in a concentrated form. 7
To this end, thickeners such as compounds of the
polyacrylamide, polysacharide or polyurethanes type have
been widely used in such compositions. Although, these
compounds are effective in providing the thickening effect,
they increase the cost of the formulation without adding
any other benefit to the product.
Another aesthetic point which may be of concern to the
consumer is that of resulting odour of the product. To this
end, products which contain a high level of perfume
relative to the total amount of biodegradable fabric
softening components and fatty acid components present
within the composition are most preferred. However, a
problem encountered with such high ratios is that of
stability of the perfume, especially where the fabric
softening composition contains a low amount of such actives
(i.e sum of biodegradable fabric softening components and
fatty acid components), especially less than 10~ by weight
of the composition.
Still another important aspect of successful formulation of
such commercial product is that of the resulting storage
stability after exposure to high temperatures.
The Applicant has now found that the use o,f high levels of
fatty acids relative to the level of biodegradable fabric
softening agents in a liquid fabric softening composition
overcomes the problems.
It is therefore an object of the invention to provide a
liquid fabric softening composition which has a suitable
viscosity, provide excellent softening benefit and is
lnexpens lve .
-
CA 02232413 1998-03-16
W O 97/11142 PCTrUS96/14865
It is another object of the invention to provide a liquid
fabric softening composition with effective storage
s~ability.
r
It is another object of the invention to provide a liquid
fabric softening composition which also contain high level
of perfumes relative to the total amount of biodegradable
fabric softening components and fatty acid components.
SummarY of the invention
The present invention relates to a liquid fabric softening
composition comprising
a)- one or more biodegradable fabric softening compounds,
b)- one or more fatty acid compounds,
wherein the ratio of said fa.bric softening agents to said
~fatty acid compounds is from 25:1 to 6.5:1.
In a preferred embodiment of the invention, the liquid
fabric softening composition further comprises a perfume
composition in a ratio of said perfume to said total amount
of biodegradable fabric softenlng components and fatty acid
components of 1:40 to 1:2.
In another preferred embodlment of the invention, the
liquid fabric softening composition is used in the rinse
cycle of a laundry washing process.
Detailed description of the invention
Biodearadable fabric softeninq com~ound
An essential component of the invention is a biodegradable
fabric softening compound.
_
CA 02232413 1998-03-16
W O 97/11142 PCTAUS96/14865
Fabric softening compositions, in particular fabric
softening compositions to be used in the rinse cycle of
laundry washing processes, are well known.
Biodegradable quaternary ammonium compounds contain
long chain alk(en)yl groups interrupted by functional
groups such as carboxy groups.
Said materials and fabric softening compositions
containing them are disclosed in numerous publications such
as EPA 040 562, and EPA 239 910.
In EPA 239 910 is disclosed that a pH range of from
2.5 to 4.2 provides optimum storage stability to said
rapidly biodegradable ~mmonium compounds.
The quaternary ammonium compounds and amine precursors
herein have the formula (I) or (II), below :
R3\ R2
+ Nl--(CH2~--Q__T 1 X
Rl
(I)
or
\
+ Nl -(CH2~-CH ~ X
R3 Q Ql
Tl T2
(II)
Q is selected from -O-C(O)-, -C(O)-O-, -O-C(O)-O-,
-NR4-C(o)-, -C(o)-NR4-;
Rl is (CH2)n-Q-T2 or T3;
CA 02232413 1998-03-16
W O 97/11142 PCT~US96/14865
R2 is (CH2)m-Q-T4 or T5 or R3;
R3 is C1-C4 alkyl or Cl-C4 hydroxyalkyl or H;
R4 is H or Cl-C4 alkyl or C1-C4 hydroxyalkyl;
T1, T2, T3, T4, T5 are independently Cl1-C22 alkyl or
alkenyl;
n and m are integers from 1 to 4; and
X~ is a softener-compatible a~ion.
Non-limiting examples of softener-compatible anions include
chloride or methyl sulfate.
The alkyl, or alkenyl, chain Tl, T2, T3, T4, T5 must
contain at least 11 carbon atoms, preferably at least 16
carbon atoms. The chain may be straight or branched.
Tallow is a convenient and inexpensive source of long
chain alkyl and alkenyl material. The compounds wherein
T1, T2, T3, T4, T5 represents the mixture of long chain
materials typical for tallow are particularly preferred.
Speci~ic examples of quaternary ~mm~n ~um compounds
suitable for use in the aqueous fabric softening
compositions herein include :
1) N,N-di(tallowoyl-oxy-ethyl)-N,N-dimethyl ammonium
chloride;
2) N,N-di(tallowyl-oxy-ethyl)-N-methyl, N-~2-hydroxyethyl);
3) N,N-di(2-tallowyl-oxy-2-oxo-ethyl)-N,N-dimethyl ammonium
chloride;
4) N,N-di(2-tallowyl-oxyethylcarbonyloxyethyl)-N,N-dimethyl
ammonium chloride;
5) N-(2-tallowyl-oxy-2-ethyl)-N-(2-tallowyloxy-2-oxo-
ethyl)-N,N-dimethyl ammonium chloride;
6) N,N,N-tri(tallowyl-oxy-ethyl)-N-methyl
ammonium chloride;
7) N-(2-tallowyl-oxy-2-oxoethyl)-N-(tallowyl-N,N-dimethyl-
ammonium chloride; and
CA 02232413 1998-03-16
W O 97/11142 PCT~US96/148G5
8) 1,2-ditallowyl-oxy-3-trimethylammoniopropane chloride;
and mixtures of any of the above materials.
Of these, compounds 1-7 are examples of compounds of
Formula (I); compound 8 is a compound of Formula (II).
Particularly preferred is N,N-di(tallowoyl-oxy-ethyl)-
N,N-dimethyl ammonium chloride, where the tallow chains are
at least partially unsaturated.
The level of unsaturation of the tallow chain can be
measured by the Iodine Value (IV) of the corresponding
fatty acid, which in the present case should preferably be
in the range of from 5 to 100 with two categories of
compounds being distinguished, having a IV below or above
25,
Indeed, for compounds of Formula (I) made from tallow
fatty acids having a IV of from 5 to 25, preferably 15 to
20, it has been ~ound that a cis/trans isomer weight ratio
greater than 30/70, preferably greater than 50/50 and more
preferably greater than 70/30 provides optimal
concentrability.
For compounds of Formula (I) made from tallow fatty acids
having a IV of above 25, the ratio of cis to trans isomers
has been found to be less critical unless very high
concentrations are needed.
Other examples of suitable ~uaternary ammoniums of
Formula (I) and (II) are obtained by, e.g. :
- replacing "tallow" in the above compounds with, for
example, coco, palm, lauryl, oleyl, ricinoleyl, stearyl,
palmityl, or the like, said fatty acyl chains being
either fully saturated, or preferably at least partly
unsaturated;
- replacing "methyl" in the above compounds with ethyl,
ethoxy, propyl, propoxy, isopropyl, butyl, isobutyl or t-
butyl;
- replacing ~'chloride" ln the above compounds with bromide,
methylsulfate, formate, sulfate, nitrate, and the like.
CA 02232413 1998-03-16
W O 97/11142 PCTAJS96/14865
In fact, the anion is merely present as a counterion of
the positively charged quaternary ammonium compounds. The
nature of the counterion is not critical at all to the
practice of the present invention. The scope of this
invention is not considered limited to any particular
anion.
By "amine precursors thereof" is meant the secondary or
tertiary amines corresponding to the above quaternary
ammonium compounds, said amines being substantially
protonated in the present compositions due to the claimed
pH values.
For the preceding biodegradable fabric softening
agents, the pH of the compositions herein is an essential
parameter of the present invention. Indeed, it influences
the stability of the quaternary ammonium or amine
precursors compounds, especially in prolonged storage
conditions.
The pH, as defined in the present context, is measured
in the neat compositions at 20~C. For optimum hydrolytic
stability of these compositions, the neat pH, measured in
the above-mentioned conditions, must be in the range of
from 2.0 to 4.5. Preferably, where the liquid fabric
softening compositions of the invention are in a
concentrated form, the pH of the neat composition is in the
range of 2.0 to 3.5, while if it is in a diluted form, the
pH of the neat composition is in the range of 2.0 to 3Ø
The pH of these compositions herein can be regulated by the
addition of a Bronsted acid.
Examples of suitable acids include the inorganic mineral
acids, carboxylic acids, in particular the low molecular
weight (C1-C5) ca~boxylic acids, and alkylsulfonic acids.
Suitable inorganic acids include HCl, H2S04, HNO3 and
H3PO4. Suitable organic acids include formic, acetic,
citric, methylsulfonic and ethylsulfonic acid. Preferred
acids are citric, hydrochloric, phosphoric, formic,
methylsulfonic acid, and benzoic acids.
CA 02232413 1998-03-16
W O 97/11142 PCT~US96/14865
The quaternary ammonium or amine precursors compounds
herein are present at levels of from 1% to 80~ of
compositions herein, depending on the composition execution
which can be dilute with a preferred level of active
biodegradable fabric softening components from 1~ to 5~, or
concentrated, with a preferred level of active
biodegradable fabric softening components from 5~ to 80~,
more preferably 10~ to 50~, most preferably 15~ to 35~ by
weight.
Additional fabric softening materials may be used in
addition to the biodegradable fabric softener. Theses may
be selected from additional cationic fabric softening
material such as di-long alkyl chain ammonium chloride,
nonionic, amphoteric or anionic fabric softening material
excluding fatty acids as defined herein after. Disclosure
of such materials may be found in US 4,327,133; 4,421,792;
4,426,299; 4,460,485; 3,644,203 and 4,661,269.
Fattv acid
Another essential component of the invention is a fatty
acid compound.
Suitable fatty acids include those containing from 10 to
25, preferably from 12 to 25 total carbon atoms, with the
fatty moiety cont~;n;ng from 10 to 22, preferably from 16
to 22, carbon atoms. The shorter moiety contains from 1 to
4, preferably from 1 to 2 carbon atoms.
The level of unsaturation of the tallow chain can be
measured by the Iodine Value (IV) of the corresponding
fatty acid, which in the present case should preferably be
in the range of from 5 to 100, more preferably in the range
of from 0 to 25.
Specific examples of fatty acid compounds suitable for
use in the aqueous fabric softening compositiOnS herein
CA 02232413 1998-03-16
W O 97/11142 PCTAUS96/14865
include compounds selected from lauric acid, myristic acid,
palmitic acid, stearic acid, arachidic acid, behenic acid,
oleic acid, coconut fatty acid, tallow fatty acid,
partially hydrogenated tallow fatty acid and mixtures
thereof.
A most preferred fatty acid compound is tallow fatty acid
with an Iodine Value (IV) of 18.
Another essential element of the invention is the ratio of
said biodegradable fabric softening agents to said fatty
acid compounds. Preferred ratios of said biodegradable
fabric softening agents to said fatty acid compounds are
from 25:1 to 6.5:1, more preferably from 20:1 to lo:1 and
most preferably from 20:1 to 15:1.
Ratios below 6.5:1 would tend to provide fabric softening
compositions with a poor storage stability due to a phase
instability, while ratios above 25:1 would not produce
sufficient built-on viscosity of the fabric softening
compositior.s to be noticeable.
Compositions according to the present invention have
further been found to be beneficial to high ratios of
perfumes relative to the total amount of biodegradable
fabric softening components and fatty acid components which
allows the use of highly scented product favoured by some
consumer. By high ratios is meant ratios of perfume to said
total amount of biodegradable fabric softener components
and fatty acid components components of 1:40 to 1:2,
preferably 1:20 to 1:2 and more preferably 1:10 to 1:3.
O~tional inqredients
Fully formulated fabric softening compositions can
contain polymers having a partial or net cationic charge.
CA 022324l3 l998-03-l6
W O 97/11142 PCT~US96/148~5
Such polymers can be used at levels of from 0.001~ to
10~, pre~erably 0.01~ to 2~ by weight of the compositions.
Such polymers having a partial cationic charge can be
polyamine N-oxide containing polymers which contain units
having the following structure ~ormula (A):
(A) Ax
I
R
wherein P is a polymerisable unit, whereto the R-N~O
group can be attached to or wherein the R-N~O group forms
part of the polymerisable unit or a combination of both.
O O O
Il 11 11
A is -NC-, -CO-, -C-, -O-, -S-, -N- ; x is O or 1;
R are aliphatic, ethoxylated aliphatics, aromatic,
heterocyclic or alicyclic groups or any combination thereof
whereto the nitrogen of the N~O group can be attached or
wherein the nitrogen of the N~O group is part o~ these
groups.
The N~O group can be represented by the following
general structures :
O O
(R~x~ )y =N -~R)x
(R3)Z
wherein R1, R2, and R3 are aliphatic groups, aromatic,
heterocyclic or alicyclic groups or combinations thereof, x
or/and y or/and z is O or 1 and wherein the nitrogen o~ the
CA 02232413 1998-03-16
W O 97/11142 PCTAUS96/14865
N~O group can be attached or wherein the nitrogen of the N
~O group forms part of these groups.
The N~O group can be part o~ the polymerisable unit (P)
or can be attached to the polymeric backbone or a
combination of both.
Suitable polyamine N-oxides wherein the N~O group forms
part of the polymerisable unit comprise polyamine N-oxides
wherein R is selected from aliphatic, aromatic, alicyclic
or heterocyclic groups.
One class of said polyamine N-oxides comprises the group
of polyamine N-oxides wherein the nitrogen of the N~O
group forms part of the R-group. Preferred polyamine N-
oxides are those wherein R is a heterocyclic group such as
pyridine, pyrrole, imidazole, pyrrolidine, piperidine,
quinoline, acridine and derivatives thereof.
Another class of said polyamine N-oxides comprises the
group of polyamine N-oxides wherein the nitrogen of the N~
o group is attached to the R-group.
Other suitable polyamine N-oxides are the polyamine
oxides whereto the N~O group is attached to the
polymerisable unit.
Preferred class of these polyamine N-oxides are the
polyamine N-oxides having the general formula (A) wherein R
is an aromatic, heterocyclic or alicyclic groups wherein
the nitrogen of the N~O functional group is part of said R
group.
Examples of these classes are polyamine oxides wherein R
is a heterocyclic compound such as pyridine, pyrrole,
imidazole and derivatives thereof.
Another preferred class of polyamine N-oxides are the
polyamine oxides having the general formula (A) wherein R
are aromatic, heterocyclic or alicyclic groups wherein the
nitrogen of the N~O functional group is attached to said R
groups.
Examples of these classes are polyamine oxides wherein R
groups can be aromatic such as phenyl.
-
CA 02232413 1998-03-16
W O 97/11142 PCTAUS96/14865
12
Any polymer backbone can be used as long as the amine
oxide polymer formed is water-soluble and has dye transfer
inhibiting properties. Examples of suitable polymeric
backbones are polyvinyls, polyalkylenes, polyesters,
polyethers, polyamide, polyimides, polyacrylates and
mixtures thereof.
The amine N-oxide polymers useful herein typically have a
ratio of amine to the amine N-oxide of 10:1 to 1:1000000.
However the amount of amine oxide groups present in the
polyamine N-oxide containing polymer can be varied by
appropriate copolymerization or by appropriate degree of N-
oxidation. Preferably, the ratio of amine to amine N-oxide
is from 2:3 to 1:1000000. More preferably from 1:4 to
1:1000000, most preferably from 1:7 to 1:1000000. The
polymers of the present invention actually encompass random
or block copolymers where one monomer type is an amine N-
oxide and the other monomer type is either an amine N-oxide
or not. The amine oxide unit of the polyamine N-oxides has
a PKa < 10, preferably PRa ~ 7, more preferred PKa c 6.
The 'polyamine N-oxide containing polymer can be obtained
in almost any degree of polymerisation. The degree of
polymerisation is not critical provided the material has
the desired water-solubility and dye-suspending power.
Typically, the average molecular weight of the polyamine
N-oxide containing polymer is within the range of 500 to
1000,000; preferably from 1,000 to 50,000, more preferably
from 2,000 to 30,000, most preferably from 3,000 to 20,000.
Such polymers having a net cationic charge include
polyvinylpyrrolidone (PVP) as well as copolymers of N-
vinylimidazole N-vinyl pyrrolidone, having an average
molecular weight range in the range 5,000 to 100,000,
preferably 5,000 to 50,000; said copolymers having a molar
ratio of N-vinylimidazole to N-vinylpyrrolidone from l to
0.2, preferably from 0.8 to 0.3.
Other o~tional in~redients include :
CA 022324l3 l998-03-l6
W O 97/11142 PCT~US96/148~5
Additional softenin~ aaents : which are nonionic fabric
softener materials. Typically, such nonionlc fabric
softener materials have a HLB of from 2 to 9, more
typically from 3 to 7. Such nonionic fabric softener
materials tend to be readily dispersed either by
themselves, or when combined with other materials such as
single-long-chain alkyl cationic surfactant described in
detail hereinafter. Dispersibility can be improved by
using more single-long-chain alkyl cationic surfactant,
mixture with other materials as set forth hereinafter, use
of hotter water, and/or more agitation. In general, the
materials selected should be relatively crystalline, higher
melting, (e.g. ~40~C) and relatively water-insoluble.
The level of optional nonionic softener in the
compositions herein is typically from 0.1~ to 10~,
preferably from 1~ to 5~.
Preferred nonionic softeners are fatty acid part-.al
esters of polyhydric alcohols, or anhydrides thereof,
wherein the alcohol, or anhydride, contains from 2 to 18,
preferably from 2 to 8, carbon atoms, and each fatty acid
moiety contains from 12 to 30, preferably from 16 to 20,
carbon atoms. Typically, such softeners contain from one
to 3, preferably 2 fatty acid groups per molecule.
The polyhydric alcohol portion of the ester can be
ethylene glycol, glycerol, poly (e.g., di-, tri-, tetra,
penta-, and/or hexa-) glycerol, xylitol, sucrose,
erythritol, pentaerythritol, sorbitol or sorbitan.
Sorbitan esters and polyglycerol monostearate are
particularly preferred.
The fatty acid portion of the ester is normally derived
from fatty acids having from 12 to 30, preferably from 16
to 20, carbon atoms, typical examples of said fatty acids
being lauric acid, myristic acid, palmitic acid, stearic
acid and behenic acid.
Highly preferred optional nonionic softening agents for
use in the present invention are the sorbitan esters, which
CA 02232413 1998-03-16
W O 97/11142 PCT~US96/14865
are esterified dehydration products of sorbitol, and the
glycerol esters.
Commercial sorbitan monostearate is a suitable material.
Mixtures of sorbitan stearate and sorbitan palmitate having
stearate/palmitate weight ratios varying between 10:1 and
1:10, and 1,5-sorbitan esters are also useful.
Glycerol and polyglycerol esters, especially glycerol,
diglycerol, triglycerol, and polyglycerol mono- and/or di-
esters, preferably mono-, are preferred herein (e.g.
polyglycerol monostearate with a trade name of Radiasurf
7248).
Useful glycerol and polyglycerol esters include mono-
esters with stearic, oleic, palmitic, lauric, isostearic,
myristic, and/or behenic acids and the diesters of stearic,
oleic, palmitic, lauric, isostearic, behenic, and/or
myristic acids. It is understood that the typical mono-
ester contains some di- and tri-ester, etc.
The l~glycerol esters" also include the polyglycerol,
e.g., diglycerol through octaglycerol esters. The
polyglycerol polyols are formed by condensing glycerin or
epichlorohydrin together to link the glycerol moieties via
ether linkages. The mono- and/or diesters of the
polyglycerol polyols are preferred, the fatty acyl groups
typically being those described hereinbefore for the
sorbitan and glycerol esters.
S7~7-factant/Concentration Aids
Although as stated before, relatively concentrated
compositions of the unsaturated material of Formula (I)
and ~II) above can be prepared that are stable without the
addition of concentration aids, the concentrated
compositions of the present invention may require organic
and/or inorganic concentration aids to go to even higher
concentrations and/or to meet higher stability standards
depending on the other ingredients.
Surfactant concentration aids are typically selected from
the group consisting of single long chain alkyl cationic
CA 02232413 1998-03-16
W O 97/11142 PCTrUS96/14865
sur~actants; nonionic surfactants; amine oxides; fatty
acids; or mixtures thereof, typically used at a level of
from 0 to 15~ of the composition.
Such mono-long-chain-alkyl cationic surfactants useful in
the present invention are, preferably, quaternary ammonium
salts of the general formula :
[R2N+R3 ] X-
wherein the R2 group is C10-C22 hydrocarbon group,
pre~erably C12-Clg alkyl group of the corresponding ester
linkage interrupted group with a short alkylene (C1-C4)
group between the ester linkage and the N, and having a
similar hydrocarbon group, e.g., a fatty acid ester of
choline, preferably C~2-C14 (coco) choline ester and/or
C16-C18 tallow choline ester at from O.1~ to 20~ by weight
of the softener active. Each R is a Cl-C4 alkyl or
substituted (e.g., hydroxy) alkyl, or hydrogen, preferably
methyl, and the counterion X~ is a so~tener compatible
anion, for example, chloride, bromide, methyl sulfate, etc.
Other cationic materials with ring structures such as
alkyl imidazoline, imidazolinium, pyridine, and pyridinium
salts having a single C12-C30 alkyl chain can also be used.
Very low pH is required to stabilize, e.g., imidazoline
ring structures.
Some alkyl imidazolinium salts and their imidazoline
precursors useful in the present invention have the general
formula :
CH2 CH2
N~ ,N\ C2H4- Y-R X
R8
wherein y2 is -C(O)-O-, -O-(O)C-, -C(O)-N(R5)-, or
-N(R5)-C(o)- in which R5 is hydrogen or a Cl-C4 alkyl
radical; R6 is a C1-C4 alkyl radical or H (for imidazoline
precursors); R7 and R8 are each independently selected from
CA 02232413 1998-03-16
W O 97/11142 PCTAJS96/14865
16
R and R2 as defined hereinbefore for the single-long-chain
cationic surfactant with only one being R2.
Some alkyl pyridinium salts useful in the present
invention have the general formula :
R - N ~ X
wherein R2 and X- are as defined above. A typical
material of this type is cetyl pyridinium chloride.
Nonionic Surfactant (AlkoxYlated Materials)
Suitable nonionic surfactants for use herein include
addition products of ethylene oxide and, optionally,
propylene oxide, with fatty alcohols, fatty acids, fatty
amines, etc.
Suitable compounds are substantially water-soluble
surfactants of the general formula :
R2 _ y _ (C2H40) z - C2H40H
wherein R2 is selected from the group consisting of
primary, secondary and branched chain alkyl and/or acyl
hydrocarbyl groups; primary, secondary and branched chain
alkenyl hydrocarbyl groups; and primary; secondary and
branched chain alkyl- and alkenyl-substituted phenolic
hydrocarbyl groups; said hydrocarbyl groups having a
hydrocarbyl chain length of from 8 to 20, preferably from
10 to 18 carbon atoms.
Y is typically -O-, -C(O)O-, -C(O)N(R)-, or
C(O)N(R)R-, in which R2 and R, when present, have the
meanings given hereinbefore, and/or R can be hydrogen, and
z is at least 8, preferably at least 10-11.
CA 02232413 1998-03-16
W O 97/11142 PCTAJS96/148G5
The nonionic surfactants herein are characterized by an
HLB (hydrophilic-lipophilic balance) of from 7 to 20,
preferably from 8 to 15.
Examples of particularly suitable nonionic surfactants
include
Straight-Chain, Primary Alcohol Alkoxylates such as
tallow alcohol-EO(11), tallow alcohol-EO(18), and tallow
alcohol-EO(25);
Straight-Chain, Secondary Alcohol Alkoxylates such as 2-
C16EO(11); 2-C2oEO(ll); and 2-C16EO(14);
Alkyl Phenol Alkoxylates, such as p-tridecylphenol EO(11)
and p-pentadecylphenol EO(18), as well as
Olefinic Alkoxylates, and Branched Chain Alkoxylates such
as branched chain primary and secondary alcohols which are
available from the well-known "OXO" process.
Amine Oxides
Suitable amine oxides include those with one alkyl or
hydroxyalkyl moiety o~ 8 to 28 carbon atoms, preferably
~rom 8 to 16 carbon atoms, and two alkyl moieties selected
from the group consisting of alkyl groups and hydroxyalkyl
groups with l to 3 carbon atoms.
Examples include dimethyloctylamine oxide,
diethyldecylamine oxide, bis-(2-hydroxyethyl)dodecylamine
oxide, dimethyldodecyl-amine oxide, dipropyltetradecylamine
oxide, methylethylhexadecylamine oxide, dimethyl-2-
hydroxyoctadecylamine oxide, and coconut fatty alkyl
dimethylamine oxide.
Electrolyte Concentration Aids
Inorganic viscosity control agents which can also act
like or augment the effect of the surfactant concentration
aids, include water-soluble, lonizable salts which can also
optionally be incorporated into the compositions of the
present invention. Incorporation of these components to the
composition must be processed at a very slow rate.
CA 02232413 1998-03-16
O 97/11142 PCTAUS96/14865
Components of this type have now been found less needed in
the compositions of the invention, especially where such
compositlons are in a concentrated form. This has result in
a simplification of the process formulation (i.e process
time reduction).
A wide variety of ionizable salts can be used. Examples
of suitable salts are the halides of the Group IA and IIA
metals of the Periodic Table of the Elements, e.g., calcium
chloride, magnesium chloride, sodium chloride, potassium
bromide, and lithium chloride. The ionizable salts are
particularly useful during the process of mixing the
ingredients to make the compositions herein, and later to
obtain the desired viscosity. The amount of ionizable salts
used depends on the amount of active ingredients used in
the compositions and can be adjusted according to the
desires of the formulator. Typical levels of salts used to
control the composition viscosity are from 20 to 20,000
parts per million (ppm), preferably from 20 to 11,000 ppm,
by weight of the composition. Where the compositions of the
invention are in a concentrated form, levels of salts used
to control the composition viscosity are reduced by 2~ to
50~ of such typical levels.
Alkylene polyammonium salts can be incorporated into the
composition to give viscosity control in addition to or in
place of the water-soluble, ionizable salts above. In
addition, these agents can act as scavengers, forming ion
pairs with anionic detergent carried over from the main
wash, in the rinse, and on the fabrics, and may improve
softness performance. These agents may stabilize the
viscosity over a broader range of temperature, especially
at low temperatures, compared to the inorganic
electrolytes.
Specific examples of alkylene polyammonium salts include
l-lysine monohydrochloride and 1,5-diammonium 2-methyl
pentane dihydrochloride.
CA 02232413 1998-03-16
W O 97/11142 PCT~US96/14865
19
Another optional ingredient is a liquid carrier. The
liquid carrier employed in the instant compositions is
preferably at least primarily water due to its low cost
relative availability, safety, and environmental
compatibility. The level of water in the liquid carrier is
preferably at least 50~, most preferably at least 60~, by
weight of the carrier. Mixtures of water and low molecular
weight, e.g., c200, organic solvent, e.g., lower alcohol
such as ethanol, propanol, isopropanol or butanol are
useful as the carrier liquid. Low molecular weight alcohols
include monohydric, dihydric (glycol, etc.) trihydric
(glycerol, etc.), and higher polyhydric (polyols) alcohols.
Still other optional ingredients are stabilizers, such as
well known antioxidants and reductive agents, Soil Release
Polymers, emulsifiers, bacteriocides, colorants, per~umes,
preservatives, optical brighteners, anti ionisation agents,
antifoam agents, enzymes, chelants and builders.
CA 02232413 1998-03-16
W O 97/11142 PCT~US96/148~5
The invention is illustrated in the following non limiting
examples, in which all percentages are on a weight basis
unless otherwise stated.
Exam~le 1
The following di~uted liquid fabric softening compositions
A and B were prepared, where Composition A is in accord
with the invention and Composition B is a prior art
composition:
A B
DEQA ~1) 2.6 2.9
Fatty acid (2) 0 3
Hydrochloride acid 0.02 0.02
Perfume 1.0 1.0
Silicone antifoam 0.01 0.01
Dye lOppm lOppm
Water and minors to balance to 100
Viscosity (cps)* l 100 1 10
(1) Di-(tallowyloxyethyl) dimethyl ammonium chloride
(2) Stearic acid of IV20
* as measured with a Brookfield viscosity meter set to a
speed of 60 rpm (lHertz)
Composition A was seen to have a good phase stability and a
stable medium viscosity while Composition B was seen to
have perfume phase separation and a water-like viscosity.
Exam~le 2
The following concentrated liquid fabric softening
compositions C to E were prepared, where Composition C is
CA 02232413 1998-03-16
W O 97/11142 PCT~US96/14865
21
in accord with the invention and Compositions D and E are
prior art compositions:
C D E
DEQA (1) 18.0 ls.o 19.0
Fatty acid(2) 1.0 - -
Hydrochloride 0.02 0.02 0.02
acid
Polyethylene 0.6 0.6 0.6
Glycol 4000
Perfume 1.0 1.0 1.0
Electrolyte 60Oppm 60Oppm 120Oppm
(3)
Silicone 0.01 0.01 0.01
antifoam
Dye 5Oppm 5Oppm 5Oppm
Water and minors to balance to 100
Viscosity* 40 ~5000 40
(cps~
(1) Di-(tallowyloxyethyl) dimethyl ammonium chloride
(2) Tallow fatty acid of IV=18
(3) Calcium chloride
* as measured with a Brookfield viscosity meter set to a
speed of 60 rpm (lHertz)
On freshly made products, compositions C was seen to have a
good phase stability and a stable medium viscosity as did
Composition E which had compensated the absence of fatty
acid with an increased level of electrolyte while
Composition D was seen to have a gel-like viscosity.
Storaae stability results
Compositions C and E were then assessed for their storage
stability.
CA 02232413 1998-03-16
W O 97/11142 PCT~US96/14865
Viscosity measures were first made on freshly made product.
The products were then put in a room with constant
temperature for a specified period of time (See table
below).
After said storage period, two viscosity measures were made
on the products:
l-when the product is at the specified storage room
temperature, and
2-when the product has been left to cool to ambiant
temperature (about 22~C-24~C) after storage as specified
under point 1.
The results are as follows:
~iscosity C E
stability
on freshly made 40 cps 40 cps
product
After one month at115 cps 75 cps
50~C
After cooling from50 cps 1400 cps
50C to 23C
After one month at35 cps 53 cps
35C
After cooling from30 cps 200 cps
35C to 23C
After storage, it can be seen that composition C of the
invention has a better storage stability than Composition
E.