Note: Descriptions are shown in the official language in which they were submitted.
CA 02232788 1998-03-23
WO 97/13823 PCT/US96/12549
FIRE BARRIER FELT
Field of the Invention
- This invention relates to a fire barrier felt
that deters the spread of flames, smoke, vapors, and/or
heat, during a fire.
Background of the Invention
Fire barrier materials (often referred to as
firestop materials or fire retardant materials) are
used to reduce or eliminate the passage of smoke and
flames through openings between walls and floors and
the openings caused by through-penetrations in
buildings, such as the voids left by burning or melting
cable insulation resulting from a fire in modern office
buildings. Characteristics of fire barrier materials
suitable for typical commercial use include flexibility
prior to exposure to heat, the ability to insulate
and/or expand, and the ability to harden in place upon
heating (i.e., to char) sufficiently to deter the
passage of heat, smoke, flames, and/or vapors.
Although many such materials are available, the
industry has long sought better and more effective
materials. For example, many commercially available
materials protect for only limited periods of time
because of poor stability at elevated temperatures or
poor mechanical strength under high-pressure water
sprays .
Foams, caulks, putty-like materials are known
for use in various fire retardant applications. For
example, urea formaldehyde resin foams are known to be
used for filling gaps between concrete floor slabs and
upright curtain walls. Such foams typically require
1
CA 02232788 1998-03-23
WO 97/13823 PCT/LTS96/12549
some form of support (e. g., a thin sheet of metal)
because the mechanical strength of foams is typically
relatively low, and that of a charred foam is generally
even lower. Intumescent compounds (e. g., expandable
graphite and hydrated metal silicate granules
incoporating an oxy boron compound) have been used with
polymeric binders to form caulks for use in filling
narrow joints (e.g., less than about one inch), or
small holes (less than about one inch in diameter).
Such materials can also include crosslinking and/or
fire retardent compounds (e. g., phosphates), thickeners
(e. g., cellulose), and fillers (e. g., cellulosic fibers
or inorganic fibers). These compositions, however, are
typically flowable and therefore generally not capable
of maintaining their shape prior to being charred
without some type of support. That is, most of these
compositions are generally not self-supporting.
Nonflowable (i.e., self-supporting), fire
retardent compositions are known. For example,
elastomeric sheets containing intumescent compounds are
known for use in pipe wraps or cable tray wraps. Also,
rigid boards containing polymeric foams in combination
with alkali metal silicates are known for use as
thermal insulating covers on surfaces such as walls,
ceilings, doors, and the like. Such rigid foam boards,
however, are typically coated with a protective layer
of material to render them moisture resistant. More
flexible, water resistant sheet materials are also
known. For example, expandable ceramic insulating
fiber felts are known for use in furnaces; however, if
such felts are used in a nonenclosed space, such as a
curtain wall or a wall penetration, heated and
expanded, they will often crumble and fall out. Other
conventional felts are used as fire barriers, but some
2
CA 02232788 1998-03-23
WO 97!13823 PCT/US96/12549
of these are not very flexible, and most are not self-
supporting at high temperatures.
Thus, what is needed are additional fire
barrier materials that can be used in a wide variety of
- 5 applications, particularly applications that require a
flexible self-supporting material that when exposed to
~ heat, either expands and hardens or simply hardens into
a more rigid self-supporting material that provides
insulation properties.
Summary of the Invention
The present invention provides a flexible
fire barrier felt and a method of producing the fire
barrier felt. The felt includes: at least about 10
weight percent (wt-$) of an organic polymeric binder;
at least about 5 wt-$ of organic fibers having pendant
hydroxyl groups (preferably cellulosic fibers); and at
least about 10 wt-$ of a heat absorbing compound;
wherein the felt contains at least about 0.3 wt-$ of
phosphorus, as provided by a phosphorus-containing
compound. This phosphorus-containing compound can be
the heat absorbing compound if the heat absorbing
compound also contains phosphorus. Typically, however,
the heat-absorbing compound does not contain
phosphorus; that is, it is a non-phosphorus-containing
compound. Thus, a phosphorus-containing compound that
is distinct from the heat absorbing compound is used as
the source of the phosphorus. All weight percentages
used herein are on a dry weight basis and are based on
3o the total weight of the felt.
The felt of the present invention is self-
supporting, and when subjected to a temperature of at
least about 350°C, the self-supporting felt forms a
self-supporting char. This char will typically occupy
3
CA 02232788 1998-03-23
WO 97/13823 PCT/LTS96/12549
the same volume as the original felt, or it will occupy
a larger volume, depending on the type of heat
absorbing compound the felt contains. If the char
needs to occupy a larger volume than that of the
original felt to be effective, the heat absorbing -
compound is an intumescent compound (i.e., a compound
that expands upon exposure to heat). Preferred
intumescent compounds are those selected from the group
consisting of intercalated graphite, mica, perlite,
to vermiculite, hydrated sodium silicate, and combinations
thereof. If the char will be effective if it occupies
substantially the same volume as that of the original
felt, the heat absorbing compound is an endothermic
compound. Preferred endothermic compounds are those
selected from the group consisting of alumina
trihydrate, magnesium ammonium phosphate, zinc borate,
magnesium hydroxide, gypsum, and combinations thereof.
In another aspect, the method of making the
fire barrier felt comprises: preparing an aqueous
suspension of an organic polymeric binder, organic
fibers having pendant hydroxyl groups, a heat absorbing
compound, and an optional phosphorus-containing
compound: precipitating the binder, heat absorbing
compound, and optional phosphorus-containing compound
onto the organic fibers; casting the precipitated
suspension onto a screen to form a felt; and drying the
felt; wherein the dried felt comprises at least about
10 wt-$ organic polymeric binder, at least about 5 wt-~
organic fibers having pendant hydroxyl groups, and at
least about 10 wt-$ heat absorbing compound; wherein
the felt contains at least about 0.3 wt-~ phosphorus,
as provided by a phosphorus-containing compound (which
can be the heat absorbing compound, the optional
phosphorus-containing compound, or both). Preferably,
4
CA 02232788 2006-04-06
60557-5774
the step of preparing an aqueous suspension includes the
steps of preparing a first aqueous suspension of the fibers;
preparing a second aqueous suspension of the organic
polymeric binder, heat absorbing compound, and optional
phosphorus-containing compound; and combining the first and
second aqueous suspensions.
In another aspect, the invention provides a
flexible fire barrier felt comprising: (a) at least 10 wt-o
of an organic polymeric binder; (b) at least 5 wt-o of
organic fibers having pendant hydroxyl groups; and (c) at
least 10 wt-o of a heat absorbing compound; wherein the felt
contains at least 0.3 wt-o phosphorus, as provided by a
phosphorus-containing compound; and wherein all weight
percents are based on the total dry weight of the felt.
In a further aspect, the invention provides a
flexible fire barrier felt comprising: (a) about 10-50 wt-
of an organic polymeric binder; (b) about 5-75 wt-o of
cellulosic fibers; and (c) about 10-70 wt-o of a heat
absorbing compound; wherein the felt contains about 0.3-3
wt-% phosphorus, as provided by a phosphorus-containing
compound; and wherein all weight percents are based on the
total dry weight of the felt.
In a still further aspect, the invention provides
a method of making a flexible fire barrier material
comprising: (a) preparing an aqueous suspension of an
organic polymeric binder, a heat absorbing compound, an
optional phosphorus-containing compound, and organic fibers
having pendant hydroxyl groups; (b) precipitating the
polymeric binder, heat absorbing compound, and optional
phosphorus-containing compound onto the fibers; (c) casting
the precipitated suspension onto a screen to form a felt;
and (d) drying the felt; wherein the dried felt comprises:
5
CA 02232788 2006-04-06
60557-5774
(i) at least 10 wt-% organic polymeric binder; (ii) at least
wt-o organic fibers having pendant hydroxyl groups; and
(iii) at least 10 wt-% heat absorbing compound; wherein the
felt contains at least 0.3 wt-% phosphorus, as provided by a
5 phosphorus-containing compound; and wherein all weight
percents are based on the total dry weight of the felt.
The following terms are used herein:
"binder" refers to an organic polymeric material;
"cellulosic" refers to materials which are
carbohydrate polymers typically derived from wood or cotton;
"char" is a carbonaceous residue formed upon
heating the felt at a temperature of at least about 350°C,
such as would be experienced when exposed to flames;
"char strength" is a measure of the ability of the
carbonaceous residue ("char") to remain intact, i.e., the
strength of the residue;
"felt" refers to a compressed, porous nonwoven
material;
"flexible" refers to the drapability of the felt;
a flexible felt is one that can be fitted into a
construction joint and subjected to building movement
(seismic, thermal, wind sway, etc.) without breaking or
significantly cracking;
"heat absorbing compound" refers to a compound
that reacts to create an insulating barrier;
"intumescent" refers to a material that expands to
at least about two times its original volume upon heating at
a temperature, typically above about 100°C;
5a
CA 02232788 1998-03-23
WO 97/13823 PCT/US96/12549
"inorganic fibers" refer to mineral wool,
glass, glass-ceramic, or ceramic materials in the form
of fibers;
"organic fibers" refer to natural or
synthetic polymeric materials, such as cellulosic -
materials, in the form of fibers;
"rayon fiber" refers to an extruded '
cellulosic material in the form of fibers typically at
least 0.63 cm long; and
"self-supporting" means that the felt and the
char formed from it has sufficient cohesive strength to
support its own weight.
Brief Description of the Drawings
FIG. 1 is a test set-up for determining the
hotside/coldside test performance of a fire barrier
felt of the present invention.
FIG. 2 is a test set-up for determining the
performance of a fire barrier felt of the present
invention as a heat barrier in a joint.
Detailed Description
The present invention provides a flexible
fire barrier felt and a method of producing this felt.
The felt can be in a variety of shapes, such as a mat
or a sheet, or a complex shape (e.g., a cup or a
clamshell), which can be formed through vacuum forming
processes. The flexible felt is also self-supporting.
During a fire, the fire barrier felt forms a self-
supporting char that has sufficient integrity to
effectively seal against the passage of heat, smoke,
flames, and/or vapors. The felt also effectively
6
CA 02232788 1998-03-23
WO 97/13823 PCT/US96/12549
insulates against the transfer of heat. When used as a
fire barrier, the felt prevents a rapid rise in the
temperature on the cold side of the felt. The
insulating characteristics of the felt are comparable
t 5 to similar thicknesses of conventional insulating
materials such as mineral wool and intumescent mats.
~ In addition to the amount and type of
components chosen to prepare the felt, the flexibility
of the felt depends on its thickness. For example, a
l0 0.63 cm thick felt in the form of a mat can be easily
wrapped around a pipe 5.08 cm in diameter without
visible cracking or spelling. A thicker felt, such as
that in the form of a 1.25 cm thick mat, however, will
typically exhibit some visible cracking or spelling at
15 the surface when wrapped around a small diameter
object, such as a 2.5 cm diameter pipe. A thinner
felt, in the form of a 0.16 cm thick mat will generally
exhibit greater flexibility, and can be wrapped around
a 0.63 cm diameter pipe without showing evidence of
20 cracking or spelling. Thus, a flexible felt will
retain its structural integrity after being bent or
curved.
Once the felt is exposed to a temperature of
at least about 350°C, such as would result from exposure
25 to flames in a fire, it forms a carbonaceous residue
known as char. This char is also self-supporting,
although it is not flexible. That is, when the felt is
positioned in a construction joint and subjected to
fire, the resultant char will remain in position and
30 can support its own weight. It will generally not
crumble or disintegrate under the conditions typically
experienced in a fire. This does not mean that it will
not crumble under heavy pressure; rather, to
demonstrate its rigidity, the char will typically
7
CA 02232788 2006-04-06
60557-5774
resist penetration resulting from a pencil under light
hand pressure. Thus, the char serves to protect against
the transfer of heat.
The flexible fire barrier felt of the present
invention is generally useful in applications in which
endothermic or intumescent fire retardant mats are
commonly used, such as in window and door edges, in
dynamic joints, and as insulation for pipe wraps and
electrical cable trays. It is particularly useful when
to used as a fire retardant barrier in a dynamic joint.
Dynamic joints are generally linear openings in a
building, such as joints within floors and walls or
between floors and walls, which are designed to allow
for building movement. Dynamic joints are often
referred to in the trade as "construction joints,"
"soft joints," "expansion joints," and "seismic
joints." A common type of a dynamic joint, known as an
"exterior wall gap," is present between exterior walls
or curtain walls and the structural elements of a
2o building. Typically, the felt, which can be in the
form of a mat is draped across an opening between, for
example, a wall and a floor, with sufficient slack to
allow for slight movement in the joint. It may be used
in combination with mineral wool insulation or other
insulation materials, and may be held in place with
pins, clamps, or adhesives. A preferred method of
installing a fire retardant mat in a curtain wall using
adhesive is described in U.S. Patent No. 5,765,332, "Fire
Barrier Protected Dynamic Joint" filed on February 21,
1995 .
The fire barrier felt includes a heat
absorbing compound, an optional phosphorus-containing
compound, organic fibers having pendant hydroxyl
groups, and an organic polymeric binder. For each of
8
CA 02232788 1998-03-23
WO 97/13823 PCT/LTS96/12549
these components, one or more materials can be used.
That is, a felt can include one or more heat absorbing
compounds, one or more different types of organic
fibers, etc. The heat absorbing compound provides
insulating characteristics to the material. That is,
the material is a thermal insulator, both before and
after exposure to the effects of a fire. Preferably,
the heat absorbing compound is selected from the group
consisting of an intumescent compound, an endothermic
to compound, and mixtures thereof.
As used herein, an endothermic compound is
one that absorbs heat by releasing water of hydration.
These compounds contribute to the insulating
characteristics of the fire barrier felt by absorbing
thermal energy and releasing gases (such as water
vapor) at a temperature above room temperature (i.e.,
25-30°C). Preferably, the gases are released at a
temperature below about 500°C. Thus, compounds
containing water molecules (i.e., water of hydration)
2o are suitable for use in the practice of this invention.
Preferably, these compounds are in a form that is
insoluble in water or only slightly soluble in water.
That is, preferably they exhibit no more than about 5~
solubility in water at about 25°C and, more preferably,
no more than about 10~ solubility in water at about
50°C. As used herein, all solubility percentages are
weight percentages based on the weight of the material
(fully hydrated if the molecules contain water of
hydration) and the total weight of the solution.
Suitable endothermic compounds include, but are not
limited to, alumina trihydrate (A1Z03~3H20), hydrated
zinc borate (ZnB20q ~ 6H20) , calcium sulfate (CaS04 ~ 2H20;
~ also known as gypsum), magnesium ammonium phosphate
(MgNH9P04 ~ 6H20) , magnesium hydroxide (Mg (OH) 2) . The
9
CA 02232788 1998-03-23
WO 97/13823 PCT/US96/12549
magnesium ammonium phosphate is preferred because it
can serve a dual purpose of acting as an endothermic
compound and providing a source of phosphorus. Another
preferred endothermic material is alumina trihydrate,
such as that commercially available under the trade
designation "SOLEM SB-36" from J.M. Huber Corp., Solem
Div., Norcross, GA. This latter material is available
as a powder with 90~ of the particles having a diameter
of about 6-60 micrometers.
As stated above, an intumescent compound is
one that expands to at least about two times its
original volume upon heating. During heating, the
intumescent compound expands the felt and generates
gas. Typically, this occurs at a temperature above
about 100°C. An intumescent compound contributes to the
insulating ability of the fire barrier felt by
increasing the total volume, absorbing some thermal
energy during a fire, and creating a generally tight
seal around construction elements. When the fire
barrier felt of the present invention includes an
intumescent compound, it is particularly suitable for
use with a firestop clamping assembly which surrounds
pipes and other types of through-penetrations in walls
and ceilings. Such a firestop assembly is described,
for example, in U.S. Pat. No. 5,103,609 (Thoreson et
al.), and describes one or more pieces of a fire
barrier felt wrapped around a pipe and held in place by
a metal clamping assembly. When heated, such as when
exposed to the heat and flames of a fire, the fire
barrier felt expands to fill gaps, such as the gap
created if the pipe collapses. Typically, when an
intumescent material is used, the fire barrier felt of
the present invention expands to at least about three
CA 02232788 1998-03-23
WO 97/13823 PCT/US96/12549
times its original volume, and preferably, to at least
about nine times its original volume.
Suitable intumescent compounds are
substantially insoluble in water. That is, preferably,
they do not exhibit more than about 5 wt-~ solubility
at about 25°C, and, more preferably, not more than about
wt-$ solubility at about 50°C. Examples of such
compounds include intercalated graphite, hydrated
alkali metal silicates, vermiculite, perlite, and mica.
10 A preferred intumescent graphite material is an acid
intercalated graphite having an acid-neutralized
surface commercially available under the trade
designation "GRAPHITE IG-338-50" from UCAR Carbon of
Cleveland, OH. A preferred intumescent compound is a
granular hydrated alkali metal silicate intumescent
composition commercially available under the trade
designation "EXPANTROL 4BW" from the 3M Company of St.
Paul, MN.
Typically, the heat absorbing compound (or a
mixture of heat absorbing compounds) is present in the
flexible fire barrier felt in an amount of at least
about 10 wt-~, based on the total dry weight of the
felt. Felts with less than this amount are generally
not effective for this use as they either do not expand
sufficiently or do not absorb enough heat to act as an
effective thermal barrier. Preferably, the heat
absorbing compound is present in an amount of at least
about 20 wt-$, and more preferably, at least about 25
wt-~. Typically, the heat absorbing compound is
present in the flexible fire barrier felt in an amount
. of no greater than about 70 wt-$. Felts with more than
this amount typically are not sufficiently flexible for
use in dynamic joint applications.
11
CA 02232788 1998-03-23
WO 97/13823 PCT/US96/12549
The optional phosphorus-containing compound
(or a mixture of such compounds) is present in the
flexible fire barrier felt of the present invention to
provide flame retarding characteristics to the
material. Preferably, the flame retarding phosphorus-
containing compound is substantially water insoluble.
That is, preferred materials are not more than about 5~ '
soluble at about 25°C, and, more preferably, not more
than about 10~ soluble at about 50°C. Suitable flame
to retarding phosphorus-containing compounds include
phosphates, such as magnesium ammonium phosphate,
polymer-encapsulated ammonium polyphosphate, and
organic phosphate oils. Red phosphorus may also be
suitable for use in this invention. Phosphate
compounds are commercially available or can be readily
synthesized by techniques known in the art. For
example, magnesium ammonium phosphate can be prepared
by the reaction of magnesium chloride, dihydrogen
ammonium phosphate, and magnesium hydroxide in an
aqueous solution. Magnesium ammonium phosphate also is
commercially available under the trade designation
"BUDIT 370" from Cometals Inc. of New York, NY. A
preferred phosphate-containing compound is melamine
formaldehyde encapsulated ammonium polyphosphate, which
is commercially available under the trade designation
"HOSTAFLAM 422" from Hoechst Celanese of Summit, NJ
(containing 31 wt-~ phosphorus + 5~). A preferred
organic phosphate oil (e.g., a phosphate ester) is
commercially available under the trade designation
"SANITIZER 141" from Monsanto of St. Louis, MO
(containing 2.7-3.0 wt-~ phosphorus).
Typically, phosphorus is present in the fire
barrier felt in an amount of at least about 0.3 wt-~
phosphorus in the felt (calculated as the weight
12
CA 02232788 1998-03-23
WO 97/13823 PCT/US96/12549
percent of phosphorus provided by a phosphorus-
containing compound), based on the total dry weight of
the felt. Felts with less than this amount tend to
decompose too rapidly when exposed to a flame.
- 5 Preferably, phosphorus is present in the felt in an
amount of at least about 0.5 wt-~, and more preferably,
- at least about 0.7 wt-~. Typically, the phosphorus is
present in the fire barrier felt in an amount of no
greater than about 3 wt-~. Felts with more than this
to amount are generally not cost effective. As stated
previously, this amount of phosphorus is provided by a
phosphorus-containing compound, which can be the heat
absorbing compound, which is present in the felt in an
amount sufficient to provide the desired level of
15 phosphorus.
Organic fibers having pendant hydroxyl groups
are present in the flexible fire barrier felt of the
present invention to strengthen the felt and to improve
its flexibility. The fibers also contribute to the
20 structural integrity of the charred material after
exposure to heat. The organic fibers having pendant
hydroxyl groups are preferably cellulosic fibers.
Suitable cellulosic fibers include cotton, linen, hemp,
wood pulp, and rayon fibers. Preferably, the
25 cellulosic fibers are at least about 0.63 cm long, and,
more preferably, they are at least about 1.25 cm long.
Preferred cellulosic fibers are rayon fibers
commercially available under the trade designations
"RAYON 3D 1/2" and "RAYON 3D 1/4" from MiniFiber, Inc.
30 of Johnson City, TN. These fibers are provided as
chopped fibers in 1.25 cm and 0.63 cm lengths. The "D"
designation refers to the denier of the fiber.
Typically, the organic fibers having pendant
- hydroxyl groups, preferably cellulosic fibers, (or
13
CA 02232788 1998-03-23
WO 97/13823 PCTlUS96/12549
mixtures of different types of such fibers) are present
in the fire barrier felt in an amount of at least about
wt-~, based on the total dry weight of the felt.
Felts with less than this amount tend to lack
5 sufficient tensile strength and flexibility, and are -
less self-supporting before and after exposure to fire.
Preferably, they are present in an amount of at least
about 8 wt-$. Typically, the cellulosic fibers are
present in the fire barrier material in an amount of no
l0 greater than about 75 wt-~ . Felts with more than this
amount generally tend to be too weak, lacking
cohesiveness and flexible strength. Preferably, they
are present in an amount of no greater than about 30
wt-~.
Although not being bound by theory, it is
believed that the organic fibers having pendant
hydroxyl groups, and particularly cellulosic fibers, in
conjunction with the phosphorus-containing compound,
assist in stabilizing char formation when the material
is subjected to heat. Again, although not being bound
by theory, it is believed that this is because
phosphorus interacts with the pendant hydroxyl groups
on the surface of the fibers and, in the presence of
the polymeric binder, creates a firm carbonaceous
network upon exposure to heat. The felt of the present
invention can include other organic fibers that do not
have pendant hydroxyl groups, such as polyethylene and
polypropylene fibers. These may be included to modify
the strength of the felt or modify the hand of the felt
3o and are further discussed below with respect to
fillers.
Although other fire protection materials '
include phosphorus-containing compounds and cellulose
(see, e.g., U.S. Pat. Nos. 5,232,976 (Horacek et al.)
14
CA 02232788 1998-03-23
WO 97/13823 PCT/US96/12549
and 5,175,197 (Gestner et al.)), the cellulose is in
the form of powder used to thicken a caulk composition.
In the felt of this invention, it is important that the
cellulose be in the form of fibers because fibers are
capable of forming internal structures (e. g., networks)
that contribute to the strength and resiliency of the
felt even after exposure to heat.
Inorganic fibers may be desirable to include
in the felt because they contribute to the high
to temperature durability of the felt, but are by their
nature brittle, which tends to reduce the flexibility
of-the felt. However, inorganic fibers (such as glass
and ceramic fibers) tend to add fire resistance to the
felt and strength to the char. Thus, it is preferable
is to add at least about 5 wt-~, based on the total dry
weight of the felts, of inorganic fibers to the felt.
Felts with less than this amount are generally less
useful at temperatures exceeding 1000°C. More
preferably, they are present in an amount of at least
20 about 10 wt-~ and may be present in amounts up to about
75 wt-~. As with all other components of the felt, one
or more different types of inorganic fibers can be
used.
The binder is an organic polymeric material
25 which preferably has elastomeric properties. That is,
the polymer has rubber-like properties, such as
conformability and stretch. The binder can be either a
thermoplastic polymer or a thermoset polymer.
Preferably, the binder is a latex, i.e., a polymer that
3o is dispersed or dispersible in water. Such polymeric
binders are commercially available either as an aqueous
dispersion or as powders or liquids, which can then be
dispersed in water before use. Suitable polymers
include acrylates, natural rubbers, styrene butadiene
CA 02232788 1998-03-23
WO 97/13823 PCT/US96/12549
copolymers, butadiene acrylonitrile copolymers,
urethane elastomers, polyvinylidene fluoride,
polyamide, polyisoprene, polychloroprene, and
polybutadiene. Preferred latex binders include an
acrylate polymer, which is commercially available under
the trade designation "RHOPLEX HA-8" from Rohm and Haas
Co. of Philadelphia, PA, and an ethylene/vinyl '
acetate/acrylate terpolymer, which is commercially
available under the trade designation "AIRFLEX 600BP"
l0 from Air Products and Chemicals, Inc. of Allentown, PA.
Typically, the organic polymeric binder is
present in the fire barrier felt in an amount of at
least about 10 wt-~, based on the total dry weight of
the felt. Felts with less than this amount are
generally not sufficiently flexible. Preferably, the
organic polymeric binder is present in an amount of at
least about 20 wt-$. Typically, the organic polymeric
binder is present in the fire barrier material in an
amount of no greater than about 50 wt-$. Preferably,
it is present in an amount of no greater than about 25
wt-~.
Fillers can be used to add reinforcement,
adjust the stiffness, or alter the handleability of the
flexible fire barrier felt of the present invention.
Fillers include, but are not limited to, fumed silica,
clay, fly ash, perlite, vermiculite, glass powders
(also known as glass frits), sodium aluminates, zinc
borate, boric oxide, inorganic fibers (e. g., glass
fibers, glass ceramic fibers, ceramic fibers, mineral
fibers, and carbon fibers), and organic fibers (e. g.,
nylon fibers, thermoplastic polyethylene fibers, and
polyester fibers). Some of the refractory materials,
such as ceramic fibers, glass powders, as well as
sodium aluminates, zinc borate, boric acid, and the
16
CA 02232788 1998-03-23
WO 97/13823 PCT/US96/12549
like, may also .serve an additional fire retardant
purpose. Preferred fillers include glass fibers such
as those commercially available under the trade
designation "MICROFIBER 106/475," which is available
from Schuller International, Defiance, OH, or ceramic
fibers commercially available under the trade
designation "FIBERFRAX 7000M," which is available from
Carborundum of Niagara Falls, NY. Other suitable
ceramic fibers are commercially available as "NEXTE1,"
to ceramic fibers from the 3M Company of St. Paul, MN.
Preferably, like the cellulosic fibers, the inorganic
fibers range in length from about 0.3 cm to about 2.5
cm, and more preferably, from about 0.63 cm to about
1.25 cm.
Other additives, such as colorants (e. g.,
FeZ03), which may aid in product identification,
fungicides, and bactericides, may be added to the fire
barrier felt. Also defoamers, which are typically
petroleum derivatives, may be added during the process
of making the felt. Defoamers are used to minimize
foaming and facilitate processing. Surfactants may
also be used in the felt making process and typically
are used to assist in incorporating all of the
materials into the felt.
The fire barrier felt of the present
invention is typically formed into a flexible mat.
This mat can be made by using conventional wet-forming
techniques typically used in the paper-making industry.
These can include hand laid or machine laid techniques.
3o For example, a handsheet mold, a Fourdrinier paper
machine, or a rotoformer paper machine can be used to
make a flexible mat. In addition to a wet-laid or
paper-making method, various vacuum forming methods can
be used to make three-dimensional, complex shapes, such
17
CA 02232788 1998-03-23
WO 97/13823 PCT/US96/12549
as honeycombs or shells, as is known to those skilled
in the art of vacuum forming.
Typically, a polymeric binder (as a latex) is
mixed with the heat absorbing compound, an optional
phosphorus-containing compound, and a surfactant to
form a homogeneous suspension, referred to herein as a
"premix." This premix can also contain other desired
additives, but does not typically contain fibers, such
as ' cellulosic, glass, ceramic, or mineral fibers.
Because fibers are typically received from a supplier
in the form of bundles, the fibers are not free-
flowing. Therefore, it is desirable to individualize
the fibers by subjecting them to high shear forces
before adding all the fibers and the premix containing
the polymer together. This is done by mixing the
fibers in, for example, a blender with a large volume
of water to form a slush. Sodium aluminate is
typically added to the fiber slush to produce a high pH
solution (typically ranging from about 8 to about 10).
The sodium aluminate is washed away during processing
and typically does not contribute to the final weight
of the felt. The premix is then pumped into a
container holding a "slush" of the fibers. Preferably,
the mixing of the premix with the fiber slush is done
at a controlled temperature, e.g., at about 45-55°C.
When the fiber slush and premix containing the polymer
are mixed together, the mixture is at a basic pH,
typically within a range of about 8-10. A pH change
is preferably used to precipitate the suspension;
however, other methods are available to precipitate the
suspension. Such methods are known and used in the
paper making industry. For acidifying the suspension,
aluminum sulfate is typically used. It is believed
that this causes precipitation of the polymer, heat
18
CA 02232788 1998-03-23
WO 97/13823 PCT/US96/12549
absorbing compound, and optional phosphorus-containing
compound onto the fibers, which aids in the felt making
process. The aluminum sulfate washes away during
processing and typically does not contribute to the
final weight of the felt. A defoamer can be added at
any point during the mixing process when it appears
necessary to reduce the amount of foaming. Suitable
defoamers include petroleum derivatives such as
"FOAMMASTER II" from Henkel of Ambler, PA.
To make a felt, the mixture is cast onto a
paper-making screen, such as a Fourdrinier screen, to
remove excess water, pressed or blotted to remove as
much water as possible, and then dried using a steam
drum drier or conventional oven. It may be desirable
during the casting process to add more defoaming agent
this is typically done by spraying some of the agent
onto the felt. The felt can be made over a large range
of thicknesses, depending upon the equipment used to
make the felt. A typical felt ranges in thickness from
about 0.15 cm to about 1.25 cm, and preferably about
0.32 cm to about 0.63 cm.
It may be desirable to laminate the fire
barrier material to a restraining layer such as a metal
foil (e. g., aluminum or steel foil), graphite foil,
insulating blanket, or other fire barrier sheets.
Lamination can be done, for example, by pressing two
materials together at room temperature or by running
them through laminating rollers (which typically use
pressure and heat). Also, an adhesive can be used to
~ laminate two layers together. Lamination is
particularly desirable when the fire barrier material
of the present invention contains an intumescent
compound, because the laminated layer acts to control
the direction of expansion of the material. Other
19
CA 02232788 1998-03-23
WO 97/13823 PCT/US96/12549
materials useful as restraining layers are described in
U.S. Pat. No. 4,467,577 (hicht), and include metal
screen, paper, cardboard, and rubber or plastic sheets.
The fire barrier felt of the present
invention can be used for sealing or isolating openings
in building components, such as gaps between walls,
cavities, interspaces, wall breaks, cable ducts, and '
the like. The felt may also be useful as heat
insulation for roofs, walls, and floors, lining for
metal panels and doors, and backing for fire retarding
walls.
Objects and advantages of this invention are
further illustrated by the following examples, but the
particular materials and amounts thereof recited in
these examples, as well as other conditions and
details, should not be construed to unduly limit this
invention. All parts and percentages are by weight
unless otherwise indicated.
Examples
Hotside/Coldside Test
For fire testing, a dynamic joint assembly
was built to simulate that used in a building.
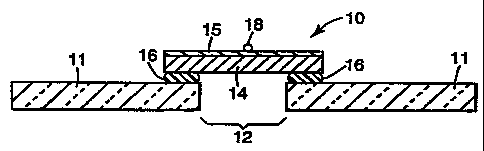
Referring to FIG. 1, dynamic joint assembly 10 included
a 0.19 m3 gas-fired furnace commercially available as a
kiln from Olympic Kilns of Atlanta, GA (not shown),
which was covered with 5 cm thick ceramic slab 11
having a 10.2 cm by 10.2 cm square opening 12. A 17.8
cm by 17.8 cm square felt test specimen 14 was centered
over the top of the opening 12. One side of the felt
test specimen 14 was covered with 0.05 mm thick
aluminum foil tape 15. The foil side faced upward to
the coldside. The edges of the test specimen 14 were
CA 02232788 1998-03-23
WO 97/13823 PCT/US96/12549
held in place by a fire retardant caulk 16 commercially
available under the trade designation "2000+
FIREBARRIER CAULK" from the 3M Company of St. Paul, MN.
This caulk also acted to keep the heat and flame on the
- 5 hotside of felt test specimen 14. The temperature on
the coldside was monitored using a 15.2 cm thermocouple
pad (not shown) over thermocouple 18. The temperature
on the hotside was monitored by the permanent furnace
thermocouples as described in ASTM (American Society
l0 for Testing Materials) Test Method E119-88.
The test was run according to ASTM Test
Method E119-88, entitled "Standard Test Methods for
Fire Tests of Building Construction and Materials."
This test was used to determine the difference in
15 temperature between the hot and cold sides and to
evaluate the duration for which these fire barrier
materials contained a fire or retained structural
integrity. The assembly was subjected to the
temperature and time conditions shown in Figure 1 of
20 ASTM E119-88. Temperatures were recorded every minute.
Joint Fire Test
A construction joint was formed that
simulated a 2 hour fire rated floor, according to ASTM
25 Test Method E119-88. Referring to FIG. 2, to form
construction joint test assembly 20, two concrete slabs
21 and 22 (198 cm long x 73.7 cm wide x 11.4 cm deep)
were poured and cured. The concrete slabs were
positioned on top of a 2.72 cubic meter floor furnace
30 (not shown) built to ASTM Test Method E119-88
specifications. The joint 23 formed between the two
slabs was 20.5 cm wide and 198 cm long. A 0.56 cm
thick test specimen (felt) 24 was covered on one side
with aluminum foil tape 25. For this test, the
21
CA 02232788 1998-03-23
WO 97/13823 PCT/LTS96/12549
aluminum foil 25 was positioned to face the fire, or
hotside. A ceramic cloth cover 29 was draped across
the top of the opening to support the thermocouple 30
and positioned by weighting down the ends on top of the
slabs 21 and 22 using weights 26 and 27. The felt 24
was adhered to the faces of the concrete slab by
applying a silicone adhesive 28 commercially available '
as "3M FIRE BARRIER SEAL & BOND SILICONE" from the 3M
Company of St. Paul, MN. The adhesive was troweled
to onto the concrete faces at a thickness of about 0.16
cm, allowed to cure to a tacky consistency for about 15
minutes, and the felt was then pressed into place.
This was allowed to cure for 24 hours before running
the fire test.
m,~~+-
To conduct flex testing, a commercially
available flex tester was obtained from Arcon
International of Lawrenceville, GA. A sample of a fire
barrier felt was tested according to ASTM Test Method
E1399-91, entitled "Cyclic Movement and Measuring the
Minimum and Maximum Joint Widths of Architectural Joint
Systems," in a 15 cm wide joint. That is, the joint is
flexed from 100 open to 100 closed, leaving a space
such that the closed position is twice the thickness of
the felt. This avoids compressing the felt. ASTM Test
Method E1399-91 is used to evaluate compression and
deflection characteristics of architectural joint
systems, including fire barriers used in such joints.
3o The results are reported in the number of flex cycles
it takes to damage or break the felt, so it will no
longer perform as an effective fire barrier.
22
CA 02232788 1998-03-23
WO 97/13823 PCT/US96/12549
Volume Expansion Test
A one inch diameter disk of the felt was
punched out using a premade die. A simple expansion
test was done to measure the expansion in only one
direction for felts constrained to expand in one
direction, since 95~ of the expansion of these felts
occurs in a direction normal to their surface, wherein
thickness of charred disk
to Expansion Ratio = ---------___________
_________ ( 1 )
thickness of initial disk
In the following examples, the component amounts are
expressed by their weight in grams or kg; their dry
weight percent, based on the total dry weight of the
felt, follows in parentheses.
Example 1
This example describes the preparation of a
felt containing an intumescent material. A premix was
made by mixing together in a low shear blender (total
volume: 151.4 liters) 68.0 kg of an acrylate latex
(55g solilds, a terpolymer of ethylene-vinyl acetate-
acrylate commercially available under the trade
designation "AIRFLEX 600BP" from Air Products and
Chemicals, Inc. of Allentown, PA), 18.1 kg (which
provides 0.3 wt-$ of phosphorus to the felt)) an
30, organic phosphate (a phosphate ester (oil) commercially
available under the trade designation "SANITIZER 141"
from Monsanto Chemical Co. of St. Louis, MO), 0.5 kg of
a surfactant (a sodium salt of polymeric carboxylic
acid (30~ active in solution) commercially available
under the trade designation "TAMOL 850" from Rohm &
Haas of Philadelphia, PA), and 15.1 kg of alumina
23
CA 02232788 1998-03-23
WO 97/13823 PCTlLTS96/12549
trihydrate (commercially available under the trade
designation "SOLEM SB 36" from Solem Manufacturing,
J.J. Huber Corp.of Fairmount, GA). After stirring for
about 10 minutes, 3.0 kg (which provides 0.6 wt-$
phosphorus in the felt) of a second phosphorus compound
(a melamine-coated organic phosphate commercially
available under the trade designation "HOSTAFLAM 422" '
from Hoechst Celanese of Summit, NJ), 531 kg of
graphite (a sulfuric acid treated graphite flake with a
pH neutralized surface commercially available under the
trade designation "GRAPHITE IG-338-50" from UCAR Carbon
Co. of Danbury, CT) and 37.8 liters of water were added
to the initial mixture. This premix was stirred until
ready to pump into another container holding a slush of
fibers.
A high shear, large capacity blender was
charged with about 3634 liters of water, 1.4 kg of a
32~ sodium aluminate (Na2A1204) aqueous solution
(commercially available under the trade designation
"NALCO 2372" from Nalco Chemical Co. of Naperville,
IL), and 3.0 kg of fiberglass (a low melting glass
fiber commercially available under the trade
designation "MICROFIBER 106/475" from Schuller
International of Defiance, OH). The fibers were mixed
in the blender for about 30 seconds. Following this,
22.7 kg of ceramic fiber (a high temperature ceramic
fiber commercially available under the trade
designation "FIBERFRAX 7000M" from Carborundum of
Niagara Falls, NY) were added and mixed for 2 minutes.
While this was mixing, 2.3 kg of 1.25 cm rayon fiber
and 7.6 kg of 0.63 cm rayon fiber were sprinkled into
the mixer. An additional 757 liters of water was used
to rinse this mixture out of the blender while
transferring to a larger container.
24
CA 02232788 2006-04-06
60557-5774
The fiber slush was pumped into a large vat
and the premix containing the binder was pumped into
the vat. The mixture was continually mixed to prevent
settling. The temperature of the stirring mixture was
held at about 50°C (+ 5°C) . About 18 kg of a 25 wt-~
aluminum sulfate (Alz (S04) 3~ 14H20) solution (commercially
available under the trade designation "NALCO 7530" from
Nalco Chemical Co, of Naperville, IL) was added to the
diluted mixture with stirring.
l0 The mixture was cast into felts using a
conventional Fourdrinier paper-making machine employing
a steam drum drier. To minimize foaming during casting
into felts, about 25 mL of a defoaming agent (a
petroleum derivative commercially available under the
trade designation "FOAI~IASTER II" from Henkel of
.Ambler, Pa.) was added to the mixture over the course
of mixing and during casting by adding as necessary
from a spray bottle. The cast felt had a weight per
unit area of about 40-80 grams per 154.8 square centimeters
2o and a thickness ranging from about 0.28 cm to about
0.56 cm.
The resulting felt with a thickness of 0.29
cm was used in the "Hotside/Coldside Fire Test,"
described above and depicted in FIG. 1. There was a
difference of about 426°C between the hot and cold
sides, indicating that the felt was a very effective
barrier to heat. The temperature gradient reached
equilibrium after about 15 minutes.
The felt, at a thickness of about 0.56 cm,
was used in the "Joint Fire Test," described above and
depicted in FIG. 2. This sample achieved a 1 hour fire
rating per ASTM E119-88. The fire test ran for a total
of 2 hours. The fire was stopped and the sample
allowed to cool until it could be examined. The char
CA 02232788 1998-03-23
WO 97/13823 PCT/US96/12549
remained in place and was still solid (i.e., it had not
crumbled and remained an integral piece) at the end of
the 2 hours.
Another sample of the felt was subjected to
5000 flex cycles according to the "Flex Test" described
above. This sample passed the test. By this it is
meant that the felt did not fall apart (e.g., crack) '
after 5000 flex cycles. Expansion tests were done
using 2.5 cm diameter disks which were die cut from a
to felt. Thirty disks were tested by placing in an oven
at 350°C for 5 minutes. The volume of expansion was
calculated using equation (1) in the "Volume Expansion
Test" described above. The volume of expansion was
about 8-13 times the original (unexpanded) volume.
The felt of Example 1 was also used in a PVC
pipe firestop apparatus, such as that described in U.S.
Pat. No. 5,103,609 (Thoreson et al.). Sufficient
layers of the felt were wrapped around the pipe to
create a 1.89 cm thick layer. After about 8 minutes of
exposure to fire, the felt expanded and closed off the
pipe and produced a hard char which was difficult to
dislodge after a two hour E119 test.
Example 2
This example describes the preparation of a
felt containing an endothermic material. A premix was
made as described in Example 1 by mixing together in a
low shear blender 90.7 kg of "AIRFLEX 600BP" acrylate
latex, 18.1 kg (which provided 0.2 wt-~ phosphorus to
the felt) of "SANITIZER 141" organic phosphate, and 0.5
kg of "TAMOL 850" surfactant. While this was mixing,
3.6 kg (which provided 0.5 wt-$ phosphorus to the felt)
of "HOSTAFLAM 422" organic phosphate and 120.6 kg of
"SOLEM SB 36" alumina trihydrate were added with
26
CA 02232788 1998-03-23
WO 97/13823 PCTJLTS96/12549
stirring. This mixture formed a smooth paste. About
38 liters of water were added to reach a pourable
consistency. This premix was allowed to stir and held
until ready to pump into another container holding a
slush of fibers.
A high shear, large capacity blender was
. charged with about 3634 liters of water, 1.8 kg of
"NALCO 2372" sodium aluminate solution, and 2.5 kg of
"MICROFIBER 106/475" glass fiber. The fibers were
l0 mixed in the blender for about 30 seconds . Then, 22 . 7
kg of ceramic fiber (high temperature ceramic fiber
commercially available under the trade designation
"FIBERFRAX 7000M") were added and mixed for 2 minutes.
While this was mixing, 2.5 kg of 1.25 cm rayon fiber
(commercially available under the trade designation
"RAYON 3D 1/2" from Mini Fiber, Inc. of Johnson City,
TN) and 10.7 kg of 0.63 cm rayon fiber (commercially
available under the trade designation "RAYON 3D 1/4"
from Mini Fiber, Inc. of Johnson City, TN) were
sprinkled into the mixer.
The fiber slush was pumped into a large vat
and the premix containing the binder was pumped into
the vat. The mixture was continually mixed to prevent
settling. The temperature of the stirring mixture was
held to 50°C (+5°C) . About 20.84 kg of "NALCO 7530" 25
wt-$ aluminum sul fate (Ale ( S04 ) 3 ~ 14H20) solution was
added to the diluted mixture with stirring. An
additional 757 liters of water was used to rinse this
mixture out of the blender while transferring to a
larger container.
The mixture was cast into felts using a
conventional Fourdrinier paper-making machine employing
a steam drum drier. To minimize foaming during casting
' into felts, about 25 mL of "FOAMMASTER II" defoaming
27
CA 02232788 2006-04-06
60557-5774
agent was added to the mixture as necessary during
mixing and during casting. The cast felt had a weight
per unit area of about 15-25 grams per 154.8 square
centimeters and a thickness ranging from about 0.13 cm to
about 0.38 cm.
The resulting felt, at a 0.25 cm thickness,
was used in the "Hotside/Coldside Fire Test," described
above and depicted in FIG. 1. There was a difference
of about 426°C between the hot and cold sides, after
to reaching equilibrium, indicating that the felt was a
very effective barrier to heat. After 15 minutes, the
coldside temperature was 340°C and at 1 hour the
coldside temperature was 591°C.
A similar test was run using one layer of
the felt, which was 0.29 cm thick, from Example 1 on
the hotside with 2 layers of the felt, which was 0.25
cm thick, from Example 2 on the coldside. This layered
system reached 96°C on the coldside in 30 minutes and
255°C in 60 minutes.
Example 3
This example describes the preparation of a
sample of another intumescent fire barrier felt. A
premix was prepared by mixing 4S grams of "AIRFLEX
600BP" acrylate latex, 12 grams (which provided 0.3 wt-
$ phosphorus to the felt) of "SANITIZER 141" organic
phosphate, 6 drops of "TAMOL 850" surfactant, 2 grams
(which provided 0.6 wt-$ of phosphorus to the felt) of
an organic phosphate (a melamine-coated organic
phosphate commercially available under the trade
designation "HOSTAFLAM 422" from Hoechst Celanese of
Summit, NJ), and 35 grams (35.8 wt-~) of "GRAPHITE IG-
338-50" graphite by hand in a beaker until homogeneous.
28
CA 02232788 1998-03-23
WO 97/13823 PCT/US96/12549
About 50 mL of deionized water were worked in until the
mixture was smooth and pourable.
A fiber mixture was prepared by combining
23.5 grams (24.5 wt-~) of "RAYON 3D 1/4" rayon fiber
(0.63 cm long) and 2 liters of deionized water in a
blender. Ten drops of "NALCO 2372" sodium aluminate
solution were added. The temperature was held at 50°C
and the material was mixed at high speed for 6 seconds
to individualize the fibers. The fiber slush was
poured into a 5 liter beaker. Agitation was provided
by a stirring rod powered by a pneumatic mixer to
prevent settling. The mixture containing the latex was
poured into this fiber slush and 3 drops of "FOAMMASTER
II" defoamer was added. Over a period of about 2
minutes, 25 grams of "NALCO 7530" 25 wt-$ aluminum
sulfate was poured into the mixture. The agitation was
continued for a few seconds until the latex was visibly
precipitated onto the fibers. That is, the cloudiness
of the suspension disappeared and the fibers could be
seen to flocculate. The mixture was transferred to a
20.3 cm x 20.3 cm papermaker (commercially available as
a Handsheet Maker from Williams Apparatus Co. of
Watertown, NY) and drained to remove the water. The
resultant soft felt was then pressed with blotter paper
at 420 Pascals for 5 minutes to remove as much water
as possible. The felt was dried for 60 minutes in a
lab oven at 128°C. The felt was 0.3 cm thick.
Three samples were cut into 2.5 cm diameter
coupons and tested for expansion at 350°C, according to
equation (1) in the "Volume Expansion Test." The
expansion for the three samples was 9-10 times the
original volume. This felt passed the "Flex Test" as
described above at 5000 flex cycles.
29
CA 02232788 1998-03-23
WO 97/13823 PCT/US96/12549
Example 4
This example describes the preparation of a
felt. A premix was prepared by mixing 67.5 grams of
"AIRFLEX 600BP" acrylate latex, 5 grams of "SOLEM SB36"
alumina trihydrate, 5 grams (which provided 1.6 wt-$ '
phosphorus to the felt) of "HOSTAFLAM 422" organic
phosphate, 6 drops of "TAMOL 850" surfactant, and 35 -
grams of "GRAPHITE IG-338-50" graphite by hand in a
beaker until homogeneous. About 50 mL of deionized
water were then worked into the mixture until it was
smooth and pourable.
A fiber slush was prepared by combining 2.5
grams of "RAYON 3D 1/4" rayon fiber (0.63 cm long),
0.75 gram of "RAYON 3D 1/2" rayon fiber (1.25 cm
long), 1.0 gram of glass fibers (commercially available
under the trade designation "MICROFIBER 106/475" and
7.5 grams of "FIBERFRAX 7000M" ceramic fiber with 2
liters of deionized water in a blender. Ten drops of
"NALCO 2372" sodium aluminate solution were added. The
temperature was held to 50°C and the material was mixed
at high speed for 6 seconds to individualize the
fibers. The fiber slush was poured into a 5 liter
beaker. Agitation was provided by a stirring rod
powered by a pnematic mixer to prevent settling.
The mixture containing the latex was poured
into the fiber slush and 6 drops of "FOAMMASTER II"
defoamer was added. Over a two minute period, 25 grams
of "NALCO 7530" 25 wt-$ aluminum sulfate was poured
into the mixture. The agitation was continued for a
few seconds until the latex was visibly precipitated
onto the fibers. That is, the cloudiness of the
suspension disappeared and the fibers could be seen to
flocculate. The mixture was transferred to a 20.3 cm x
20.3 cm papermaker (commercially available as a
CA 02232788 1998-03-23
WO 97/13823 PCT/US96/12549
Handsheet Maker from Williams Apparatus Co. of
Watertown, NY) and drained to remove the water. The
resultant soft felt was then pressed with blotter paper
at 420 Pascals for 5 minutes to remove as much water as
- 5 possible. The felt was dried for 60 minutes in a lab
oven at 128°C. The felt was 0.28 cm thick.
Three 2.5 cm diameter coupons were cut from
the dried felt and tested for expansion at 350°C
according to equation (1) in the "Volume Expansion
Test." The average expansion of the three samples was
9.4 times the original volume. The felt passed 2000
flex cycles of the "Flex Test."
Example 5
A premix was prepared by combining 30 grams
(16.5 wt-$) of "AIRFLEX 600BP" acrylate latex, 9 grams
(which provided 0.3 wt-$ phosphorus to the felt) of
"SANITIZER 141" organic phosphate, 6 drops of "TAMOL
850" surfactant, 2 grams (which provided 0.8 wt-$
phosphorus to the felt) of "HOSTAFLAM 422" organic
phosphate, 30 grams (38.8 wt-$) of magnesium ammonium
phosphate (a low temperature endothermic powder of the
formula MgNH4P03- 8H20 commercially available under the
trade designation "BUDIT 370" from Budenheim
Chemicals/Cometals, Inc. of New York, NY) and mixing by
hand in a beaker until homogeneous. About 175 mL of
deionized water were worked in until the mixture was
smooth and pourable.
A fiber slush was prepared by combining 1.25
grams of "MICROFIBER 106/475" glass fiber, 11.25 grams
of "FIBERFRAX 7000M" ceramic fiber, 5.25 grams of
"RAYON 3D 1/4" rayon fiber (0.63 cm long) and 1.25
grams of "RAYON 3D 1/2" rayon fiber (1.25 cm) with 2
liters of deionized water in a blender. Ten drops of
31
CA 02232788 1998-03-23
WO 97/13823 PCT/iTS96/12549
"NALCO 2372" sodium aluminate solution were added.
The temperature was held at 50°C and the material was
mixed at high speed for 6 seconds to individualize the
fibers. The fiber slush was poured into a 5 liter
beaker. Agitation was provided by stirring rod powered '
by a pneumatic mixer to prevent settling.
The premix containing the latex was poured
into the fiber slush and 3 drops of "FOAMMASTER"
defoamer was added. Over a two minute period, 50 grams
of "NALCO 7530" 25 wt-~ aluminum sulfate was poured
into the mixture. The agitation was continued for a
few seconds until the latex was visibly precipitated
onto the fibers. The mixture was transferred to the
papermaker used in Example 3 and drained to remove the
water. The resultant soft felt was then pressed with
blotter paper at 420 Pascals for 5 minutes to remove
water. The felt was dried for 60 minutes in a lab oven
at 128°C.
Two layers of the resulting 0.32 cm thick
felt were used in a "Hotside/Coldside Fire Test," as
described above and depicted in FIG. 1. These two
layers were placed over a 0.28 cm thick felt made
according to Example 1. A piece of 0.05 mm thick
aluminum foil tape was placed over the top layer. All
layers were sealed together at the edges with a fire
retardant caulk (commercially available as "2000+
FIREBARRIER CAULK" from the 3M Company of St. Paul,
MN). The thermocouple on the coldside showed a
significant leveling off of the temperature rise above
about 149°C corresponding to the endothermic release of
water associated with the magnesium ammonium phosphate. ,
After 30 minutes the temperature on the coldside of the
felt was 224°C and at 60 minutes it was 342°C.
32
CA 02232788 1998-03-23
WO 97/13823 PCT/US96/12549
The felt was subjected to a 5000 flex cycles
according to the "Flex Test" described above and
passed. Thermal gravimetric analysis of the felt
showed weight loss peaks of about 100°C and about 300°C
~ 5 corresponding to loss of water from magnesium ammonium
phosphate.
various modifications and alterations of this
invention will become apparent to those skilled in the
art without departing from the scope and spirit of this
invention, and it should be understood that this
invention is not to be unduly limited to the
illustrative embodiments set forth herein.
33