Note: Descriptions are shown in the official language in which they were submitted.
CA 02232998 1998-03-2~
W O 97/13887 PCT~US96/16132
Description - - -
COMPOSITION AND PROCESS FOR SURFACE TREATMENT OF ALUMINUM
AND 11 S ALLOYS
Technical Field
The present invention relates to a novel composition and method for the
surface l,t:~l",ent of aluminum and its alloys, such as aluminum-manganese al-
loys, aluminum-magnesium alloys,, and aluminum-silicon alloys, that contain at
least 45 % by weight of aluminurn, all of these being jointly hereinafter briefly
designated as "aluminiferous metals", in which the surface of an aluminiferous
metal is provided with better corrosion resistance and adhesion to paint or syn-thetic resin film before said surface is painted or is laminated to a synthetic resin
film. One field in which the present invention may be particularly effectively ap-
~0 plied is the surface treatment of aluminiferous coils.Background Art
ChromdLe treatments are presently being used for the most part in the in-
dustrial surface treatment of alurninum coils. Typical examples of chromate
treatments include chrul "ic acid chromate conversion treatments and phosphoric
acid chromate conversion ll~:al,llenl~. Chromic acid chromate conversion treat-
ment solutions were put to practical use around 1950 and are still widely used
for fin members and the like in heat exchangers. This chemical conversion treat-ment solution consists primarily of chromic acid (CrO3) and hydrofluoric acid
(HF), with a promoter added, and forms a film containing some hexavalent
20 chromium. Phosphoric acid chromate conversion treatment solutions are based
on the invention in US Patent 2,438,877 from 1945. This chemical conversion
treatment solution contains chromic acid (CrO3), phosphoric acid (H3PO4), and
hydrofluoric acid (HF). The film that is formed consists primarily of hydrated
chromium phosphate (CrPO4-4H2O). Since the film does not contain hexavalent
25 chromium, it is widely used at present for the paint undercoating treatment of
beverage can bodies, particularly drawn and ironed aluminum cans and lids.
However, in the interests of environmental protection, a need has arisen for sur-
face ll~:dllnent solutions which contain no chromium. In recent years, the paint-
CA 02232998 l998-03-2~
W O 97/13887 PCT~US96/16132
ing or lamination following such surface treatment has been followed by an in-
creasingly wide range of shaping processes with the need for increasingly strin-gent levels of processing but films made from inorganic systems such as chro-
mate suffer from problems; e.g.~ the film is broken when bent sharply prevent-
s ing adequate pe~ rur" ,ance from being re~li7ed and the like. There is thus strong
demand for the development of a technique for forming a flexible film with better
corrosion resi:jldl ~ce and/or adl1esion in articles of manufacture that are shaped
after the conversion coating is formed.
Treatment solutions or methods intended to provide the surface of alumin-
iferous metals with cor,usion resistance and paint adhesion using a water-solu-
ble resin have been proposed in Japanese Laid-~)pen Patent Applications
61-91369 1-172406 1-177379 1-177380 2-608 2-609 and others. In these
conventional l, ~aL" ,ent methods the metal surface is treated with a solution con-
taining a derivative of a polyhydric phenol compound. Problems in these con-
ventional methods however are that it is difficult to form a sufficiently stable film
in a short period of time on the surface of aluminiferous metal ",aterials and
adequate corrosion resistance cannot be obtained.
n;SclQsure of the Invention
Problems to Be Solved by the Invention
The present invention is intended to remedy the aforementioned draw-
backs of the conventional technology and more specifically is intended to offer
a novel CGI I Iposilion and method for surface treating aluminiferous rnetals which
allow the surface of an aluminiferous metal to be provided in a short period of
time with better corrosion resistance and paint or laminated film adhesion
without the use of chromium and which also allow a film with better workability
to be formed.
~ummary of the Invention
It has been found that an aqueous liquid surface treatment composition
containing phosphoric acid ions condensed phosphoric acid ions an oxidizer
and a water-soluble polymer with a specific structure and that has a pH within
a specific range forms a film with better corrosion It::SiSldl ~ce and paint or lamin-
CA 02232998 l998-03-2~
- W O 97/13887 PCTrUS96/16132
ated film adhesion, as well as better workability, when a surface of an alun~inifer-~ ~
ous metal is brought into contact with such a surface treatment composition.
More particularly, a composition according to the invention for the surface
treatment of an aluminiferous metal comprises, preferably consists essentially
5 of, or more preferably consists of, water and:
(A) a component of ions of orthophosphoric acid;
(B) a component of ions of condensed phosphoric acid(s);
(C) a component of oxidizing agent; and
(D) a component of molecules of water-soluble polymer and/or oligomers
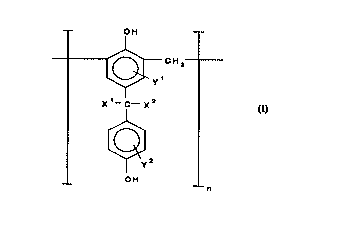
conforming, except for end groups, to the following general formula (I):
-- OH
~ CH2
X~--C---X2
( I )
~y2
OH
in which each of X' and x2 independently of each other and independently from
one unit of the polymer, said unit being defned as repr~senled by a ll lodiricdliol~
25 of formula (I) above in which the square brackets and the subscript n are omit-
ted, to another unit of the polymer represents a hydrogen atom, a C1 to Cs alkylgroup, or a C, to C5 hydroxyalkyl group; each of Y' and y2 independently of one
~ another and independently for each unit of the polymer represents a hydrogen
atom or a moiety "Z" which co,)rOr",s to one of the fcll~wi"g general formulas (Il)
30 and (Ill):
CA 02232998 l998-03-2~
W O 97/13887 PCT~US96/16132
R R 3
- C H 2--N ( ~ C H 2--N--R ( I I I
in which each of R', R2, R3, R4, and R5 in each of general formulas (Il) and
(Ill) independently represents a C, to C,0 alkyl group or a C1 to C10
hydroxyalkyl group; one moiety "Z" may be identical to or may differ from
any other moiety "Z" in the polymer molecule, so long as each "Z" con-
forms to one of the general formulas (Il) and (Ill); and n repr~:sellls a posi-
tive integer, which may be the same as or different from the value of n for
any other polymer molecule; in addition, in component (D) as a whole:
the average value for the number of Z moieties sl ~hstitl ~3d on each phen-
yl ring in the polymer molecule', which may be referred to hereinafter as
"the average value for Z moiety substitution", is from 0.2 to 1.0; the av-
~s erage value of n, which may be referred to hereinafter as "the average
degree of polymerization", is from 2 to 50, and, bec~ e it is an average,
need not be an integer;
and, optionally, one or both of the following cornponents:
(E) a component of aluminum sequestering agent that is not part of any of
the previously recited components; and
(F) a component of antifoam agent that is not part of any of the previously re-
cited components,
and in the composition as a whole, the aforementioned phosphoric acid ions (A),
condensed phosphoric acid ions (B), oxidizing agent (C), and water-soluble poly-mer (D) are present in a weight ratio (A):(B):(C):(D) of 0.1 to 30: 0.1 to 10: 0.1
1For example, if a polymer with an average degree of poly"leri~dlion of 10, which
COI lldil IS 20 benzene rings, has only 10 of these 20 benzene rings each s~ Ihstihlted with one
group Z, the mean group Z sllhstitlltion rate of the polymer is:
~(1 x 10) + (0 x 10')}/20 = 0.5
CA 02232998 l998-03-2~
- W O 97/13887 PCTrUS96/16132
to 10: 0.1 to 20. A composition according to the invention may be immediately
suitable for use as such in treating aluminiferous metal, in which instance it is
called a working composition, or it may be suitable for diluting with additional- water to form a working cornrosition, in which instance it is called a concentrate
composition. Some compositions are suitable for both purposes.
A process for the surface treatment of an aluminiferous metal according
to the present invention is charac:terized by the fact that an aqueous surface
treatment solution which contains the dror~ e~ llioned surface ll ~dll I ~el ,L compo-
sition pe, l~i";"g to the present invention and which has a pH value no more than
10 6.5 is brought into contact with the surface of an aluminiferous metal, prer~rdbly
for a total of 1 to 60 seconds, and lthe surface which has thus contacted prefer-
ably is then rinsed with water, andl then is dried and preferably heated.
Detailed Description of the Invention. Including Preferred Fmbodiments
Orthophosphoric acid, having the chemical formula H3PO4 and herein
usually desiyl Idl~3d simply as "phosphoric acid" unless the co, ll~xL requires differ-
entiating it from other phosphoric acids, and any water soluble salt or acid salt
of orthophosphoric acid that does not act adversely to the objects of the in-
vention may be used as a source of component (A) of a composition according
to the invention as defined above, and any such salt is to be under~luod, for the
20 purpose of the preferences i"dicz~lt d below, as contributing its full 6h -hiolll~llic
equivalent as orthophosphate ions (i.e., PO4~3) to the conce~ dlion thereof in any
composition according to the invention, irrespective of the actual degree of
ionization that may prevail in the composition. The phosphoric acid ions contentranges from 0.1 to 30 weight parts, and preferably 0.5 to 5 weight parts, per 0.1
25 to 20 weight parts of water-soluble polymer (D) in the aforementioned ratio. A
phosphoric acid ions content of less than 0.1 weight part in the aforementioned
blend ratio often results in inadequate reactivity between the surface treatmentsolution and the surface of the metal substrate being coated, as well as in an
inadequately formed film. More than 30 weight parts in this ratio does not harm
30 rc""~dLion of a favorable conversion coating but is uneconomical bec~llse of the
higher cost of the treatment solution and lack of any ofFsetting benefit compared
CA 02232998 l998-03-2~
W O 97/13887 PCT~US96/16132
with compositions containing somewhat less of this component. ~
Similarly, one or two or more types selected from among pyrophosphoric
acid, tripolyphosphoric acid and tetrapolyphosphoric acid, and the salts of all of
these acids, can be used to provide the condensed phosphoric acid ions in a
water-based composition of this invention, but the invention is not lirnited to the
use of these materials. Any water soluble source of any phos,uhale anions that
CGI lldill at least two atoms of phosphorus each may be used, and is to be under-
stood for the purposes of the preferences below as supplying its full stoichio-
metric equivalent as condensed phosphate anions to the composition used ac-
0 cording to the invention, irrespective of the actual degree of ionization that exists
in the composition. For example, pyrophosphoric acid ~H4P2~17), sodium pyro-
phosphate (Na4P2O7) and like compounds can be used to provide pyrophosphate
ions. In a surface treatment composition according to the present invention, thecontent of the condensed phosphoric acid ions component (B) ranges from 0.1
to 10 weight parts, and preferably 0.5 to 3.0 weight parts, per 0.1 to 20 weightparts of water-soluble polymer (D). A condensed phosphoric acid ions content
of less than 0.1 weight part in the aforementioned ratio normally results in a sur-
face treatment solution with weak etching action, preventing a film from being
adequateiy forrned. A content of more than 10 weight parts usually results in a
surface treatment solution with etching action that is too strong, which inhibits
the film-forming reaction.
In a surface treatment composition acco~ Ig to the pr~sel ll invention, the
oxidizing agent preferably comprises, more preferably consists essentially of, or
still more preferably consists of one or more sl ~hst~nces selecttsd from the group
consi~ g of hydrogen peroxide, chlorates, and nitrites; the use of hydrogen per-oxide is most preferred. The content of oxidizing agent component (C) in the
aforementioned ratio for the surface treatment composition according to the
present invention ranges from 0.1 to 10 weight parts, and preferably 2 to 5
weight parts, per 0.1 to 20 weight parts of water-soluble polymer COI "pone"l (D).
so An oxidizing agent co, ll~lll of less than 0.1 weight part usually results in a treat-
ment solution with weak etching action, preventing an adequate film from being
CA 02232998 l998-03-2~
W O 97/13887 PCTrUS96/16132
formed. More than 10 weight parts usually results in a treatment solution with
etching action that is too strong, which inhibits the film-forming reaction.
The water-soluble polymem~sed in the present invention is a water-sol-
uble polymer (a term intended herein to include oligomers) that has an average
5 degree of polymeri~lion of 2 to 5al, or preferably of 2 to 20, and that contains,
~ preferably consists essentially of, Ol more pr~r~l dbly consists of (except for end
groups) polymer units as described above in connection with general formula (I).When the alkyl or hydroxyalkyl groups represented by X1 and x2 in
general formula (I) have 6 or more carbon atoms, the resulting polymer becon ,esbulky, usually causing steric hindrance and preventing a compact film with good
corrosion reslstance from being obtained.
When the average value for Z substitutions is less than 0.2, the resulting
polymer normally has poor water solubility, and the resulting surface treatment
composition stability is usually inadequate. When the average value for Z moietysl Ihstitlltion is more than 1.0, and thus contains a substantial fraction of benzene
rings s~ Ihstitl Ited by two or more Z ç roups, the polymer usually has such a high
water solubility that a composition containing it as component (D) has great
difficulty in forming a satisfactorily protective surface film.
The alkyl or hydroxyalkyl groups expressed by R', R2, R3, R4, and R5 in
general formulas (Il) and (Ill) have 1 to 10 carbon atoms. A number of carbon
atoms of 11 or more usually results in a polymer molecule that is too bulky,
leading to a film with poor density and insuffcient corrosion resistance.
In a surface treatment composition according to the present invention, the
content of the water-soluble polymer component (D), in terms of the aforemen-
2s tioned ratios to other components, is 0.1 to 20 weight parts, and preferably 0.5
to 5 weight parts, per 0.1 to 30 weight parts of phosphoric acid ions (A). When
this ratio is less than 0.1 weight parlt, it is diffcult to form a film on the surface of
aluminiferous metals with the surface treatment composition, whereas more than
20 weight parts in this ratio is uneconomical bec~use of the higher cost of the re-
sulting surface treatment and laclc of any s~hst~ntial improvement over the
results achieved with a composition according to the invention containing 20 or
CA 02232998 l998-03-2~
W O 97/13887 PCTrUS96/16132
fewer parts in this ratio. - -
Although not narrowly limited, the pH of a working surface llt:dllllel)l com-
position according to the present invention is preferably no more than 6.5, and
even more preferably is between 2.0 and 6.5. When the pH of the surface treat-
5 ment composition is higher than 6.5, the polymer of forrnula (I) in the resultingsurface treatment composition tends to precipitate, impairing the treatment com-
position stability and its use life. When the pH is lower than 2.0, tl-e etching ac-
tion of the surFace l~t:almenl composition on the surface of the metal material is
very strong, making it difficult to form a surFace film. The pH of the surface treat-
10 ment composition can be ~justed using an acid such as phosphoric acid, nitricacid, and hydrochloric acid, or an alkali such as sodium hydroxide, sodium car-
bonate, and ammonium h~/-lr~xide. Hydrofluoric acid may be used to adjust the
pH when wastewater disposal is not a problem.
In a surface treatment method according to the present invention, the
15 aforementioned surface treatment solution preferably has a pH of 2.0 to 6.5 and
contains from 1 to 30 grams per liter (hereinafter usually abbreviated as "g/l") of
phosphoric acid ions, from 0.1 to 10 9/l of condensed phos,c I ,oric acid ions, from
0.1 to 10 g/l of oxidizing agent, and from 0.1 to 20 gA of water-soluble polymercomponent (D) as described above. When the conce"l,dlion of phosphoric acid
20 ions in a surface treatment composition according to the invention is lower than
0.1 g/l, a surface film is usually inadequately formed, whereas more than 30 g/lis uneconomical bec~se of higher costs. When the conce"l,dliG" of the con-
densed phosphoric acid ions is lower than 0.1 g/l, the etching action of the result-
ing surface treatment composition is usually too weak, and a suRace film is in-
25 adequately formed, whereas more than 10 9/l results in a surface L~ ~l" ,el ,t com-
position with etcl~ ;a properties that are so strong that the film-forming reaction
is hindered. When the oxidizing agent concentration is lower than 0.1 g/l, the
etching action of the resulting surface treatment composition is weak, usually
preventing the formation of an adequate film, whereas more than 10 g/l results
in a surface treatment composition with etching action that is too high, which
hinders the film-forming reaction. When the conce"lldlion of the water-soluble
-
CA 02232998 1998-03-2~
W O 97/13887 PCT~US96/16132
polymer component (D) is lower than 0.1 g/l, the resulting surface treatment~
composition usually has inadequal:e film formability, whereas more than 20 g/l
is economically disadvantageous because of higher costs.
When aluminum ions that have eluted from the aluminiferous metal be-
5 come mixed with the surface treatment composition, the water-soluble polymer
(D) and the metal ions sometimes fomm a conl,~lex and produce precipitation. In
such instances, an aluminum sequestering agent should be added to the surface
treatment composition. Examples of useful aluminum sequestering agents in-
clude, but are not limited to, ethylene dian ,i"e tetra-acetic acid, 1 ,2-cyclohexane-
.0 diamine tetra-acetic acid, triethanolamine, gluconic acid, heptogluconic acid, ox-
alic acid, tartaric acid, malic acid, and organophosphonic acids. When the use
of hydrofluoric acid poses no problems for wastewater treatment, it may be used
as a sequestering agent.
In a ll l~:lhod of the present invention, a working surface Ll~::dl",ent compo-
15 sition as described above is brought into conl~;l, preferably for a total of 1 to 60seconds and independently prert:r~bly at a temperature within a range from 30
to 65 oc, with a surface of an aluminiferous metal. The film formed on the sur-
face of the metal material is then preferably rinsed with water, and thereafter
preferably is heated and dried. Contact between the aluminiferous metal and a
20 working composition may be established by any convenient method, among
which immersion of the substrate in a bath of the working composition and
spraying the working composition on the metal are most cG",mon. A contact
time of less than 1 second usually results in inadequate formation of a cor,usion-
resislanl film, whereas a contact time of more than 60 seconds yields no greater25 benefits and thus leads to less operational efficiency.
When a spray treatment is used, the surface treatment composition some-
times foams and thus causes problems in the film that is formed. Although the
presence or absence of foaming and the extent of such foaming depends largely
on the spraying equipment and conditions, a defoaming agent should be added
30 to the surface l,~ "ent composition when foaming is not suitably avoided by
modification of the spraying equipmlent and conditions. The type of defoaming
CA 02232998 l998-03-2~
W O 97/13887 PCTAUS96/16132
agent, the amount used, and so forth are not narrowly limited, but the adhesion-between the resulting film and the paint or laminated film should not be compro-mised.
The film formed on the surface of the aluminiferous metal in a process ac-
cording to the invention is believed to be a film of an organic-inorganic composite
consisting primarily of phosphates and water-soluble polymer (resin) (D). The
metal substrate is etched by the ions of the phosphoric acid and condensed
phosphoric acid, at which time the pH becomes elevated in some areas at the
interface, resulting in the prec;~.iLdLion of phosphates on the surface. The amino
groups (included in the groups Z) of the water-soluble polymer (D) have chelat-
ing action, and are believed to form a type of coordination con~poun~ with the re-
generated surface of the metal sul.~ le produced by the etching. Although the
organic-inorganic composite film is h~sic~lly formed as a result of the two afore-
mentioned actions, the additional presence of the condensed phosphoric acid
ions in the surface treatment composition appears to allow some water-soluble
polymer-metal coordination compound(s) to be more readily formed, thereby al-
lowing a stable organic-inorganic complex film to be formed on the metal surfacewithin a wide pH range.
After a coating film has been formed in a process according to the inven-
tion, the film can be and preferably is heated to allow the polymer from compon-ent (D) of the working composition according to the invention that was used, andthat was incorporated into the coating film formed on the surface, to undergo fur-
ther polymeri~dlion on the surface. At least one minute at 200 ~C (in the normalambient natural atmosphere) is sufficient for the heating conditions.
A preferred expanded treatment process step sequence, using a surface
treatment composition according to the present invention, is outlined below.
(1) Surface cleaning/degreasing (any acidic, alkaline, or solvent type);
(2) Rinsing with water;
(3) Surface treatment (by method of the present invention);
(4) Riosing with water;
(5) Deionized water rinsing;
CA 02232998 l998-03-2~
W O 97/13887 PCT~US96/16132
(6) Heating and drylng; - -
(7) Painting or film lamination.
Before the method of the present invention is implemented, the surface
of the metal ",dlt :l ial is preferably c;leaned so that it is degreased of oil such as
5 rolling oil or the like remaining on the surface of the aluminiferous metal that is
being treated. No particular restrictions are imposed on the type of degreasing
agent or the degreasing method used in this step. The degreased material is
then preferably rinsed with water. The rinsing is intended to remove the de-
greasing composition from the surface; therefore, any rinsing method may be
.0 used, provided that the degreasing composition is removed from the surface.
The sur~ace l,t:aLment of the methud according to the present invention is then
implemented. The surface of the aluminiferous metal being treated is the prefer-ably further rinsed with water. This rinsing is intended to remove the surface
treatment composition, so any rins;ing method may be used, provided that the
15 unreacted surface treatment composition is removed from the surface. The
heating and drying are intended to dry offthe rinsing water, with no narrow re-
strictions imposed on the method, drying temperature, drying time, or the like,
although hot air drying or the like is generally useful for industrial purposes. The
surface of the aluminiferous metal that has been surFace treated is then paintedor la" ,i"~led with film. The coali"g ~ormed by a method according to the present
invention has good adhesion to paint or laminated films. Since the coating
formed by means of the present invention is also flexible, it also has excellentperformance after processing such as folding or drawing.
The aluminiferous metals used in the method according to the present in-
26 vention include forms such as sheets, bars, tubes, wires, and the like. No re-
sL, i~;tiGns whatever are imposed on the dimensions and configuration of the met-
al, although the method according to the present invention is especially effective
when used on aluminiferous metal coils.
The present invention is described in further detail below with reference
30 to specific working and comparison examples.
CA 02232998 l998-03-2~
- W O 97/13887 PCTAUS96/16132
Examples and Comparison Examples
Methods of Evaluation
(1 ) Corrosion Resist~nce
The cor,osiol1 resistance of aluminum materials (resistance to darkening
5 from boiling water) was evaluated by the following test: Treated aluminum ob-
jects were bent into the shape of a U around a round bar with a diameter of 1
millimeter (hereinafter usually abbreviated as "mm"); these were immersed for
30 minutes in boiling tap water; and the degree of discoloration (darkening) in
the bent parts was visually ~ssessed. No darkening was rated as "O", partial
.0 darkening was rated as "A", and total darkening was rated as "x".
(2) Paint Adhesion
Paint adhesion was tested in the following manner: Polyvinyl chloride
paint for cans was painted to a thickness of between 5 and 10 micrometres
(hereinafter usually abbreviated as "~um") on the surface of treated aluminum, fol-
lowed by 1 minute of baking at 260 oC; the samples were cut into rectangles 5
x 150 mm; the painted surfaces were thermally bonded via a polyamide film to
form test pieces; the bonded surfaces were separated by the 180 degree peel
test method; and the peel strength was evaluated at that time. The greater the
peel strength, the better the paint adhesion. In general, a peel strength of 4.020 kilograms-force (hereil1~rler usually abbreviated as "kgf') or more per 5 mm of
width is considered excellent for practical purposes.
(3) Wastewater Disposability
Used surface treatment composition waste was diluted twenty-fold with
water, and the concentration of hexavalent chromium in the thus formed compo-
25 sition was measured. For the purposes of environmental protection, no chromi- um should be detected.
F~rnple 1
An aluminum-magnesium alloy sheet (alloy according to Japanese Indus-
trial Standard, hereinafter usually abbreviated as "JIS", A5182) was degreased
so by spraying with a 2 % aqueous solution of an alkaline degreaser (trade name:
CA 02232998 l998-03-2~
W O 97/13887 PCT~US96/16132
FlNECLEANER~g) 4377K, by Nihon Parkerizing) for 5 seconds at 60 oc, and was
then rinsed clean with water. The sheet was then sprayed for 3 seconds at 60
oc with Surface Treatment Composition 1 having the ingredients noted below,
with the balance being water; it was then rinsed with tap water, then sprayed and
5 washed for 10 seconds with deionized water having a specific resistivity of atleast 3,000,000 ohm-centimeters, and was then dried for 2 minutes in a hot air
drying furnace at 80 oc.
Surface Treatment Composition 1
75 % Phosphoric acid (i.e., H3P04) : 10.0 g/l (PO43~: 7.2 g/l)
Sodium pyrophosphate (i.e., Na4P~!O7 1 0H2O) : 3.0 g/l (P2O74-: 1.2 g/l)
31 % Hydrogen peroxide in water : 10.0 g/l (H2O2: 3.1 9/l)
Polymer (1) - solids part : 2.0 g/l
pH 4.0 (Adjusted with sodium hydroxide)
Water Soluble Polymer (1 ) was according to general formula (I) when: the
average value of n = 5; each of X1 and x2 represents a hydrogen atom; each of
y1 and y2 represents a -CH2N(CH3)~ moiety or hydrogen atom; and the average
Z moiety substitution number = 0.50
Fxample 2
Aluminum alloy materials were degreased and rinsed clean in the same
20 manner as in Example 1, and they were then treated by immersion for 10 sec-
onds at 40 oC using Surface Trealment Composition 2 having the ingredients
shown below, with the balance be!ing water. This treatment was followed by
rinsing and drying under the same conditions as in Example 1.
Surface Treatment Composition 2
2!i 75 % Phosphoric acid (i.e., H3PO4) : 10.0 g/l (PO43~: 7.2 g/l)
Sodium pyrophosphate (i.e., Na4P,!O7-10H2O) : 3.0 g/l (P2O74-: 1.2 g/l)
31 % Hydrogen peroxide in water : 15.0 g/l (H2O2: 4.6 g/l)
Polymer (2) - solids part : 0.4 g/l
pH 3.0 (Adjusted with sodiurn carbonate)
30 Water soluble polymer (2) was according to general formula (I) when: the
CA 02232998 1998-03-2~
W O 97/13887 PCTrUS96116132
average value of n = 5; each of X' and x2 = a -C2H5 moiety; each of y1 and y2- -= a -CH2N(CH2CH2OH)2 moiety or a hydrogen atom; and the average value for
Z moiety substitution = 0.25.
FY~PIe 3
Aluminum alloy sheets were degreased and rinsed clean in the same
manner as in Example 1, were then spray treated for 1 second at 65 oc using
Surface Treatment Composition 3 containing the ingredients shown below, with
the balance being water, then rinsed and dried under the same conditions as in
Example 1.
~o Surface Treatment Composition 3
75 % Phosphoric acid (i.e., H3PO4) : 20.0 9/l (PO43~: 14.4 g/l)
Sodium pyrophosphate (i.e., Na4P2O7-1 OH2O) : 6.0 g/l (P2O74-: 2.4 g/l)
31 % Hydrogen Peroxide in water : 15.0 9/l (H2O2: 4-6 g/l)
Polymer (3) - solids part : 8.0 9/
~s pH 4.0 (Adjusted with sodium hydroxide)
Water soluble polymer (3) was according to general formula (I) when: the aver-
age value of n = 15; each of X' and X2 = a -C2H5 moiety; each of Y' and y2 = a
-CH2N(CH2CH2OH)2 moiety or a hydrogen atom; and the average value for Z
moiety substitution = 1Ø
20 Fy:3rnple 4
Aluminum alloy sheets were degreased and rinsed clean in the same
manner as in Exalnple 1, were then spray treated for 30 seconds at 40 oc using
Surface Treatment Composition 4 conldil ~ing the ingredients shown below, with
the balance being water, then rinsed and dried under the same conditions as in
Example 1.
Surface Treatment Composition 4
75 % Phosphoricacid (i.e., H3PO4) : 20.0g/l (PO43-: 14.4g/l)
Sodium tripolyphosphate (i.e., Na~jP30,0) : 1.2 g/l (P3O,04~: 0.8 g/l)
43 % Sodium chlorate in water : 10.0 g/l (NaClO3: 4.3 9/l)
Polymer(4) -solids part : 1.0 g/l
14
CA 02232998 l998-03-2~
W O 97/13887 PCT~US96/16132
pH 4.0 (Adjusted with aqueous ammonia~
Water soluble polymer (4) was according to general formula (I) when: the
average value of n = 15; each of X' and X2 represents a hydrogen atom; each
of Y' and y2 represents a -CH2N(CIH2OH)2 moiety or a hydrogen atom; and the
5 average value for Z moiety substihltion = 0.50
Fxample 5
Aluminum alloy sheets wer~ degreased and rinsed clean in the same
manner as in Example 1, were then spray treated for 5 seconds at 50 oc using
Surface Treatment Composition 5 containing the ingredients shown below, with
10 the balance being water, then rinsed and dried under the same conditions as in
Example 1.
Surface Treatment Composition 5
75 % Phosphoric acid (i.e., H3PO4) : 20.0 g/l (PO43- 14.4 g/l)
Pyrophosphoric acid (i-e-, H4P2O~) : 1.0 9/l (P2O74-: 0.98 g/l)
31 % Hydrogen Peroxide in water : 5.0 g/l (H2O2: 1-6 g/l)
Polymer (5) - solids part : 1.0 g/l
pH 3.5 (Adjusted with aqueous ammonia)
Water soluble polymer (5) was according to general formula (I) when: the
average value of n = 20; each of Xl and x2 = a hydrogen atom; each of Y' and
y2 = a -CH2N(CH2CH2CH2OH)2 moiety or a hydrogen atom; and the average
value for Z moiety substitution = 0. 75.
Comparative Example 1
Aluminum alloy sheets were degreased and rinsed clean in the same
manner as in Example 1, and they were then spray treated for 5 seconds at 50
~C using Surface Treatment Composition C1 having the ingredients set forth be-
low, with the balance being water. This treatment was followed by rinsing and
drying under the same conditions as in Example 1.
Surface Treatment Composition C1--with no condensed phosphoric
acid ions or oxidizing agent
75 % Phosphoric acid (i.e., H3PO4) : 20.0 g/l (PO43~: 14.4 g/l)
CA 02232998 l998-03-2~
W O 97/13887 PCT~US96/16132
Polymer(6) - solids part : 1.0 g/l
pH 3.5 (Adjusted with aqueous ammonia)
Water soluble polymer (6) was according to general formula (I) when: the
average value of n = 10; each of X1 and X2= a hydrogen atom; each of Y' and
y2 = a -CH2N(CH2CH2CH2OH)2 moiety or a hydrogen atom; and the average
value for Z moiety substitution = 0.75.
~omparative Example 2
Aluminum alloy sheets were degreased and rinsed clean in the same
manner as in Example 1, were then spray treated for 2 seconds at 50 oc using
~0 a 5 % aqueous solution of a commercially available phosphoric acid chromatetype of chemical conversion composition (tradename: ALCHROME~) K 702, by
Nihon Parkerizing). The treatment was followed by rinsing and drying under the
same conditions as in Example 1.
Comparative Example 3
Aluminum alloy sheets were degreased and rinsed clean in the same
manner as in Example 1, were then spray treated for 10 seconds at 50 oc using
a 6 % aqueous solution of a commercially available zirconium phosphate type
of chemical conversion composition (tradename. AEROSILTM 404, by Nihon Par-
kerizing). The treatment was followed by rinsing and drying under the same con-
ditions as in Example 1.
Table 1 shows the results obtained in the evaluations of the above noted
Examples 1 through 5 and Comparative Examples 1 through 3.
CA 02232998 l998-03-2~
W O 97/13887 PCTrUS96/16132
Table 1
CorrosionPaint AdhesionWast~ater
ResistanceaPeel Strength,Disros~bilibr
kgf/5 mm) (crt6 g/l)
Example 1 0 4.0 none detected
Example 2 O 4.0 none detected
Example3 0 4.0 nonedetected
Example4 0 4.0 nonedetected
Example 5 0 4.0 none detected
Comparative x 1.5 none detected
Example 1
Comparative x 4.0 0.2
~x~,3le 2
Comparative x 1.5 none detected
Example 3
It is appa,~nL from the results in Table 1 that Examples 1 through 5 which
used a method according to the present invention had better corrosion resist-
ance, adhesion, and wastewater alisposability.
In Comparative Example 1, the surface treatment composition contained
5. no condensed phosphoric acid ions or oxidizing agent, resulting in a film with in-
adequate corrosion resistance and paint adhesion. In Comparative Examples 2
and 3, convel lliGnal surface treatment compositions were used, resulting in films
with low corrosion resistance. In Comparative Example 2, the wastewater con-
tained hexavalent chromium, while in Comparative Example 3, the paint adhe-
~o sion was poor.Benefits of the Invention
It is apparent from the aforelmentioned description that the surface treat-
ment composition and method according to the present invention allow a chem-
ical conversion film with better corrosion resistance and paint adhesion to be
15 formed on the surfaces of aluminiferous metals before they are painted. The
surface treatment composition and method according to the present invention
are non-chromium and non-fluorine types, and have the exceptional merit of al-
leviating the burden of wastewater disposal.
17