Note: Descriptions are shown in the official language in which they were submitted.
CA 022331~4 1998-04-22
0050/46319
Aromatic sulfoxides and sulfones, processes for their prepara-
tion, and their use as herbicides
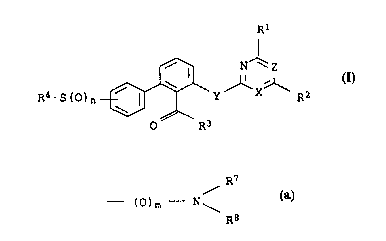
5 The present invention relates to aromatic sulfoxides and sulfones
of the formula I
N'~z
R4-S(0)n ~ Y X R2
0 R3
where the substituents and th.e index have the following meanings-:
X is nitrogen or a methine group C-H;
20 Y is oxygen or sulfur;
Z is nitrogen or a group C--R5;
n is 1 or 2;
Rl is halogen, alkyl, haloa:Lkyl, alkoxy, haloalkoxy, alkylthio,
alkylamino and/or dialky:Lamino;
R2 is alkyl, haloalkyl, alkoxy, haloalkoxy;
R3 is hydrogen;
a radical oR6;
a radical
~ R7
- (~)m - N
where R7 and R3 can be identical or different and where m can
assume the values 0 or 1;
45 R4 is alkyl, haloalkyl, benzyl;
- CA 022331~4 1998-04-22
0050/46319
R5 is hydrogen, halogen, alkyl or together with R1 a unit -
CH2-CH2-O-;
R6 is hydrogen, an alkali met;al cation, the equivalent of an al-
kaline earth metal cation" or an organic ammonium ion;
an alkyl group which can have attached to it one to five ha-
logen atoms and/or one or two of the following radicals:
alkoxy, alkylthio, cyano, phenyl, substituted phenyl, a
radical -o-N=CR9Rl0 where R9 and Rlo can be identical or dif-
ferent;
an alkyl group which can have attached to it one to five ha-
logen atoms and a 5-membe:red heteroaromatic ring contA;n;ng
~ 15 one to three nitrogen atoms, or a 5-membered heteroaromatic
ring contA;n;ng one to th:ree nitrogen atoms and additionally-
one sulfur or oxygen atom in the ring, it being possible for
them to have attached to them one to four halogen atoms and/
or one to two of the following radicals: alkyl, haloalkyl,
alkoxy, haloalkoxy and/or alkylthio;
an alkenyl or an alkynyl group, it being possible for these
groups, in turn, to have attached to them one to five halogen
atoms;
a radical -N=CR9Rl0 where R9 and R10 can be identical or
different;
R7 and R8 are hydrogen;
alkyl, alkenyl or alkynyl, it being possible for each of
these radicals to have attached to it one to five halogen
atoms and/or one to two of the following groups: alkoxy,
alkylthio, cyano, alkylca.rbonyl, alkoxycarbonyl,
bisdialkylamino, cycloalk:yl;
phenyl or substituted phe!nyl;
together are a cyclized alkylene chain or together are a
cyclized alkylene chain c:ontaining a hetero atom, which can
be oxygen, sulfur or nitr.ogen, it being possible for each of
them to have attached to them one to three alkyl substitu-
ents;
45 R9 and R10 are alkyl which can have attached to it a phenyl rad-
ical or an alkoxy and/or an alkylthio group, or are cycloal-
kyl, phenyl, or together are an alkylene chain which can have
CA 022331~4 1998-04-22
0050/46319
attached to it one to five alkyl groups and which can be
bridged via an alkylene chain;
with the proviso that, if R1 ilnd R2 are methoxy and R3 is a hy-
5 droxyl group and if Y is oxyglen and Z is a methine group CH, n is
2 and S(~)n is in the p-position of the phenyl ring, R4 is other
than methyl, where
substituted phenyl means that the phenyl ring can have attached
10 to it one to five halogen atoms, one to three alkyl or alkoxy
groups and/or one to three of the following radicals: nitro,
cyano, haloalkyl, haloalkoxy, alkylthio, alkylamino,
dialkylamino, alkylcarbonyl, alkoxycarbonyl, phenyl, or phenyl
which is substituted by one to three halogen atoms or one to
15 three methyl groups.
Patent Applications W0 91/13065 and DE-A 3919435 describe
salicylic acid derivatives having an aromatic substituent in the
6-position which has attachedi to it an alkylthio or alkylsulfonyl
20 substituent, these salicylic acid derivatives being herbicidally
active. The activity of the compounds described in these
publications is not always sa~tisfactory with a view to the
herbicidal activity or the selectivity in crop plants.
25 It was therefore an object of the present invention to provide
salicylic acid derivatives which have an improved biological ac-
tion.
Accordingly, we have found the salicylic acid derivatives I de-
30 fined at the outset. The novel compounds I have an outst~n~ing
herbicidal action and an improved selectivity in crop plants.
Furthermore, we have found processes for the preparation of the
compounds I and their use as herbicides.
The compounds of the formula I are accessible via several routes.
A route which has proved par~icularly advantageous is via the
benzo[1,3]dioxinones IV whic]h can be prepared from the aromatic
tin compounds IIa and the benzodioxinones III using a palladium
40 catalyst, by a method similar to the procedure described in EP
657441, and which are first opened with a nucleophile R3-H in the
presence or absence of a base to give the salicylic acid deriva-
tives V, which are then reacted with heterocycles of the type VI,
in the presence or absence of a base:
- CA 02233l~4 1998-04-22
0050/46319
SnR
R4 - S ( O ) n ~ Rl f~ R4 - S ( O ) n
IIaIII IV
base
lOR3 -- H ~R4-S(O)n ~ ~ ~ R3YH base
V N'~Z
R13 ~ X ~ R2
VI
N'~Z
~y X ~ R2
R4-S(O)n ~ ~ R3
The radicals have the abo~...entioned meanings, Rll is Cl-C6-alkyl
and Cl-C6-cycloalkyl, R12 is a halogen atom, preferably bromine or
30 iodine, or a trifluoromethylsulfonyloxy group, Rl3 is a
nucleofugic leaving group such as halogen, alkyl- or aryl-
sulfonyl.
Furthermore, it is possible lto react a sulfur compound IIb with a
35 tin-substituted benzoic acid of the formula VII where Rl4 is un-
substituted or substituted benzyl, Cl-C4-alkyl, dihydropyranyl,
trialkylsilyl, alkoxyalkyl and dialkoxyalkyl, using a palladium
catalyst, and to convert the resulting benzoic acid VIII into the
salicylic acids Va where R3 := OH, which can be reacted by a method
~0 similar to the above process to give the active ingredient Ia
where R3 = OH:
- ~ CA 02233l~4 l998-04-22
0050/46319
R12
R4-S (~)n {~ + ~ PdO/PdII ~ ~R14
Rll3Sn l ~ R4-S(O)n ~ O OH
IIb VII VIII
~ R4-S(O)n ~ OH base
O OH
Va N'~Z
R13 ~ X ~ R2
VI
Rl
~ N'~Z
~ o X ~ R2
R4-S(O)n ~ O ~ OH
Ia
A catalytically active palladium compound is employed in each of
the two abovementioned processes. Any palladium salts or palla-
dium complexes which are at :Least partially soluble in the reac-
30 tion mixture are suitable. The oxidation level of the palladiumcan be 0 or 2. The following counterions are suitable, inter
alia, for the palladium salts: chloride, bromide, iodide, sul-
fate, acetate, trifluoroacetate, acetylacetonate or hexafluoro-
2,4-pentadionate. A large number of different palladium complexes
35 can be used. The only prere~lisite is that the ligands on the
palladium can be displaced b~y the substrate under the reaction
conditions. Substances which are particularly suitable are phos-
phine ligands, eg. aryl-alkylphosphines such as, inter alia,
methyldiphenylphosphine, iso]propyldiphenylphosphine, triaryl-
40 phosphines such as, inter alia, triphenylphosphine, tritolyl-
phosphine, trixylylphosphine, trihetarylphosphines such as
trifurylphosphine or dimeric phosphines. Also highly suitable are
olefinic ligands such as, inter alia, dibenzylideneacetone or its
salts, cycloocta-1,5-diene or amines such as trialkylamines (eg.
45 triethylamine, tetramethylethylenediamine, N-methylmorpholine) or
pyridine.
CA 022331~4 1998-04-22
0050/46319
The complex used can be employed directly in the reaction. For
example, this procedure may be followed using tetrakistri-
phenylphosphinepalladium(0), bistriphenylphosphinepalladium
dichloride, bistriphenylphosphinepalladium diacetate, a dibenzyl_
5 ideneacetone/palladium(0) complex, tetrakismethyldiphenyl-
phosphinepalladium(0) or bis(l,2-diphenylphosphinoethane)-
palladium dichloride. It is also possible to use a palladium salt
and additionally a suitable ligand, these substances only then
forming the catalytically active complex in situ. This procedure
10 is suitable, for example, in the case of the abovementioned salts
and phosphine ligands such as trifurylphosphine or tritolyl-
phosphine. Palladium complexes such as tris(dibenzylidene-
acetone)dipalladium, bis(dibenzylideneacetone)palladium or
1,5-cyclooctadienepalladium di.chloride can also be further
15 activated by adding ligands such as trifurylphosphine or
tritolylphosphine.
0.001 to 10 mol%, in particular 0.005 to 5 mol%, of the palladium
compound (salt or complex), based on the compound II or VII, are
20 generally used. Larger amounts are possible, but rather uneconom-
ical. The amount of IIa or VII based on the reactant III or IIb
is generally at from 0.8 to 3, preferably 0.95 to 1.5, mol equiv-
alents. Suitable for the react;ion are all solvents which do not
themselves react with the substrates used. Polar solvents accel-
25 erate the reaction. Particularly suitable substances are etherssuch as diethyl ether, methyl tert-butyl ether, dimethoxyethane,
tetrahydrofuran, dioxane, amides such as dimethylformamide,
dimethylacetamide, N-methylpyrrolidone, dimethylpropyleneurea or
amines such as triethylamine. The use of mixtures, eg. of ethers
30 with amides, is frequently advantageous. Alkyl alcohols and water
are also suitable as components for mixtures, in particular when
the radical B contains a boron atom. The addition of
tetraalkylammonium halides or alkali metal halides such as
lithium chloride is frequentl~y advantageous and particularly
35 recommended when Rl2 represent:s a sulfonyloxy radical.
The reaction temperature is from -20 to 200~C, preferably from 50
to 160 C. The reaction times are usually from a few minutes to
50 hours, in most cases 0.5-10 hours. When using low-boiling sol-
40 vents, it is sometimes advantageous to carry out the reaction inan autoclave under the inherent pressure.
The organotin compounds of the formula VII are prepared by metal-
lating the benzoic acid on which they are based with a suitable
45 base at low temperatures and subsequently reacting the product
with a trialkyltin compound to give VII:
CA 022331~4 1998-04-22
0050~46319
COOH
COOH 1) base I SnRll
R14 ~ 2) R12_SnRl13 ~ 14~ ~ ~ 3
VII
Suitable bases are, primarily, cycloalkyl- or alkyl-lithium com-
10 pounds, particularly suitable are the commercially available iso-
mers of butyl- and hexyllithium. Frequently, it is expedient to
add an auxiliary to promote t!he metallation process. Substances
which are suitable for this purpose are ethers, alcoholates such
as potassium tert-butylate, or amines such as tetramethylethyl-
15 enediamine. The metallation process can be carried out at from(-130) C to 0 C, preferably from ~-100) to (-50) C. All solvents
which are usually used for metallation processes are also suit-
able for the present reaction, particularly expedient solvents
being diethyl ether, methyl tert-butyl ether, tetrahydrofuran and
20 simple hydrocarbons, and it may be advantageous to use mixtures
of these substances. The reaction times for the metallation pro-
cess can be from some minutes to a few hours. Then, the trialkyl-
tin compound is added, Rl2 be:ing the customary leaving groups,
preferably chlorine or bromine. As regards the temperature during
25 the addition and the subsequent reaction time, what has been said
above also applies here. This may be followed by aqueous or non-
aqueous work-up, and it may be advantageous in the former case to
keep the pH of the aqueous phase constant by using a buffer. The
yield may be increased substantially when a substance which is
30 suitable for destroying excess base is added prior to work-up
while the temperatures are still low. Substances which are suit-
able for this purpose are, for example, carbon dioxide, water,
alkyl halides or benzyl halides. If required, the organotin com-
pounds of the formula VII can be purified further, for example by
35 chromatography on silica gel. They are stable during work-up and
when exposed to water at various pH values and can be stored at
room temperature.
Another possibility of synthesizing active ingredients of the
40 formula VII where n is 2 is t:o convert a formyl compound IX into
the corresponding crotonaldehyde X, which is then reacted via the
cyclohexenone XI and the salLcylic acid derivative XII in accor-
dance with EP 402751 to give the compound Ib where R3 = oR6.
CA 02233l54 l998-04-22
0050/46319
H 11
O ~ So2-R4 H ~~ ~'~ ~ So2-R4
IX X
O O
B ~~~ ~~~ 3 ~ 502-R4
OH ~
R6
~ Rl
~ So2-R4
XII N '~Z
R13~X ~R2
VI
N'~Z
,~,,J~ o X~ R2
R4-S(o)n ~ ~ ~ oR6
Ib
Benzoic acid derivatives, ie. compounds Ia where R3 is an OH
group, can also be synthesized by converting a suitable precursor
Ib where R3 is oR6 into the free acid by means of hydrolysis or
hydrogenation.
CA 02233154 1998-04-22
0050/46319
hydrolysis or
O X ~ R2 hydrogenation
R4-S~o)n ~J ~ oR6
Ib
Rl
N ~ Z
15 R4-S(O)n Ia o ~ X ~ RZ
Compounds of the formula I can also be synthesized by starting
20 from the free acids, ie. substances where R3 is OH, and converting
them into an activated form ~;uch as a halide or an imidazolide,
which is then reacted with a nucleophile R3-H in the presence or
absence of a base. Alternatively, it is also possible first to
activate the salicylic acids III and then to react the resulting
25 derivatives V with heterocycles IV to give the active ingredi-
ents I.
R4~5~0)n ~ YH ~ y X ~ R2
O OH R4-S(O)nOH Ia
1. activation
2. R3-H 1. activation
2. R3-H
l ll IV
40 R4~S(O~n ~ ~ YH IRl
O R3 ~ N'~Z
V ~y X \ R2
R4-S(O)n ~ ,J>~
o R
CA 022331~4 1998-04-22
0050/46319
The various oxidation levels of the sulfur do not have to be pre-
selected in the starting materials but can be converted into each
other at any suitable intermediate level:
monooxidation ~, R
~ R ~ R-S~0
\ oxidation ~ reduction
~ I
dioxidation
R-S02 ~ R
20 R is any radical described in the above synthesis methods. Exam-
ples of oxidants are hydrogen peroxide, organic peracids such as
meta-chloroperbenzoic acid or peracetic acid, iodates, perio-
dates, potassium permanganate, iodosylbenzene, acetyl nitrate or
nitric acid. A catalyst, eg. sodium tungstate or phenylselenous
25 acid, can be added if this promotes the reaction.
In the description, the substituents mentioned preferably have
the following meanings:
30 Cl-C4-alkyl: methyl, ethyl, l-propyl, 2-propyl, l-butyl, 2-butyl,
2-methylpropyl, l,1-dimethyl0thyl;
Cl-C6-alkyl: Cl-C4-alkyl and also l-pentyl, 2-pentyl, 3-pentyl,
2-methylbutyl, 3-methylbutyl, 2-methyl-2-butyl, 3-methyl-2-butyl,
35 l,l-dimethylpropyl, 1,2-dimet:hylpropyl, 2,2-dimethylpropyl,
l-hexyl, 2-hexyl, 3-hexyl, 2--methylpentyl, 3-methylpentyl,
4-methylpentyl, 2-methyl-2-pentyl, 3-methyl-2-pentyl,
4-methyl-2-pentyl, 2-methyl-3-pentyl, 3-methyl-3-pentyl,
2,2-dimethylbutyl, 2,3-dimethylbutyl, 3,3-dimethylbutyl,
40 3,3-dimethyl-2-butyl, 2-ethy]Lbutyl, 1,1,2-trimethylpropyl,
1,2,2-trimethylpropyl, in particular methyl, ethyl, propyl,
2-propyl, butyl, 2-butyl, l,L-dimethylethyl, pentyl,
2,2-dimethylpropyl, hexyl;
45 Cl-C4-haloalkyl: chloromethyl, difluoromethyl, dichloromethyl,
trifluoromethyl, trichloromethyl, chlorodifluoromethyl,
l-fluoroethyl, 2-fluoroethyl, 2,2-difluoroethyl,
CA 02233l~4 l998-04-22
0050/46319
1,1,2,2-tetrafluoroethyl, 2,2,2-trifluoroethyl, 2-chloro-
1,1,2-trifluoroethyl and pentafluoroethyl, decafluorobutyl,
1,1-bistrifluoromethyl-2,2,2-t;rifluoroethyl, preferably
difluoromethyl, trifluoromethyl, trichloromethyl and
5 chlorodifluoromethyl;
C3-C6-cycloalkyl: cyclopropyl, cyclobutyl, cyclopentyl, cyclo-
hexyl, especially preferably cyclopropyl, cyclopentyl and cyclo-
hexyl;
Cl-C4-alkylcarbonyl: acetyl, propionyl, 1-propylcarbonyl,
2-propylcarbonyl, 1-butylcarbonyl, 2-butylcarbonyl, 2-methyl-
propylcarbonyl, 1,1-dimethyle1:hylcarbonyl;
- 15 Cl-C4-alkoxycarbonyl: ethoxycarbonyl, propoxycarbonyl, 1-propyl-
oxycarbonyl, 2-propyloxycarbonyl, 1-butyloxycarbonyl, 2-butyloxy-
carbonyl, 2-methylpropyloxyca~bonyl, 1,1-dimethylethoxycarbonyl;
C3-C6-alkenyl: propenyl, 1-but.enyl, 2-butenyl, 2-methylpropenyl,
20 pentenyl, 2-pentenyl, 2-methy:Lbutenyl, 3-methylbutenyl,
2-methyl-2-butenyl, hexenyl, :2-hexenyl, 3-hexenyl, 2-methyl-
pentenyl, 3-methylpentenyl, 4-methylpentenyl, 2-Methyl-2-pent-
enyl, 3-methyl-2-pentenyl, 4-methyl-2-pentenyl, 2,3-dimethyl-
butenyl, 2-ethylbutenyl, 3,3-dimethylbutenyl, 2,3-dimethyl-2-but-
25 enyl;C3-C6-alkynyl: propynyl, butynyl, 2-butynyl, pentynyl, 2-pentynyl,
3-methylbutynyl, hexynyl, 2-hexynyl, 3-hexynyl, 3-methylpentynyl,
4-methylpentynyl, 4-methyl-2-]pentynyl;
30 Cl-C4-alkoxy: methoxy, ethoxy, propoxy, 1-methylethoxy, butoxy,
2-butoxy, 1-methylpropoxy, 2-methylpropoxy, l,1-dimethylethoxy,
in particular methoxy, ethoxy, 1-methylethoxy;
Cl-C4 -haloalkoxy: difluoromethoxy, trifluoromethoxy,
35 chlorodifluoromethoxy, 1-fluoroethoxy, 2-fluoroethoxy,
2,2-difluoroethoxy, 1,1,2,2-tetrafluoroethoxy, 2,2,2-tri-
fluoroethoxy, 2-chloro-1,1,2-trifluoroethoxy and penta-
fluoroethoxy, 1,1,2,3,3,3-hexafluoropropoxy, heptafluoropropoxy,
decafluorobutoxy, 1,1-bistrifluoromethyl-2,2,2-trifluoroethoxy,
40 preferably difluoromethoxy, trifluoromethoxy and chlorodifluoro-
methoxy;
Cl-C4-alkylcarbonyloxy: acetoxy, propionyloxy, 1-propylcarbonyl-
oxy, 2-propylcarbonyloxy, l-blutylcarbonyloxy, 2-butylcarbonyloxy,
45 2-methylpropylcarbonyloxy, l,1-dimethylethylcarbonyloxy;
CA 022331~4 1998-04-22
0050/46319
C1-C4-alkylthio: methylthio, et:hylthio, propylthio, l-methyl-
ethylthio, butylthio, 2-butylt:hio, l-methylpropylthio, 2-methyl-
propylthio, l,l-dimethylethylthio, in particular methylthio,
ethylthio, l-methylethylthio;
C1-C4-alkylamino: methylamino, ethylamino, propylamino, 1-methyl-
ethylamino, butylamino, 2-butylamino, 1-methylpropylamino,
2-methylpropylamino, l,1-dimethylethylamino, in particular
methylamino, ethylamino, l-methylethylamino;
di-Cl-C4-alkylamino: dimethylamino, N-methyl-N-ethylamino,
diethylamino, N-methyl-N-propylamino, N-ethyl-N-propylamino,
dipropylamino, diisopropylamin.o, N-isopropyl-N-methylamino,
N-ethyl-N-isopropylamino, N-isopropyl-N-propylamino, dibutyl-
15 amino, di-2-methylpropylamino, di-l-methylpropylamino, N-butyl-
N-methylamino and its isomers, N-butyl-N-ethylamino and its
isomers, N-butyl-N-propylamino and its isomers;
C3-C6-alkylene chain: propylene, butylene, pentylene, hexylene;
Preferred compounds of the formula I are those where the
substituents and the index have the following meanings:
X is nitrogen;
25 Y is oxygen or sulfur;
Z is nitrogen or a group C-R5;
n is 1 or 2;
R1 is alkyl, haloalkyl, alkoxy, haloalkoxy;
R2 is alkoxy;
30 R3 is hydrogen;
a radical oR6;
R~ is alkyl;
R5 is hydrogen or together with Rl is a unit -C~2-CH2-0-;
R6 is hydrogen, an alka]Li metal cation, the equivalent of an
alkaline earth metal cation, or an organic ammonium ion;
an alkyl group which can have attached to it one to five
halogen atoms and/or one or two of the following
radicals: alkoxy, phenyl, substituted phenyl;
an alkenyl or an alkynyl group, it being possible for
these groups, in tur:n, to have attached to them one to
five halogen atoms;
a radical -N=CR9Rl0 where R9 and R10 can be identical or
different;
CA 02233l~4 l998-04-22
0050/46319
R9,Rl0 are alkyl which can have attached to it an alkoxy and/or
an alkylthio group, or together are an alkylene chain;
with the proviso that, if Rl a~nd R2 are methoxy and R3 is a hy-
5 droxyl group and if Y is oxygen and Z is a methine group CH, n is
2 and S(~)n is in the p-position of the phenyl ring, R4 is other
than methyl, where
substituted phenyl means that the phenyl ring can have attached
10 to it one to five halogen atoms, one to three alkyl or alkoxy
groups and/or one to three nitro groups.
Other preferred compounds of the formula I are those where the
substituents and the index have the following meanings:
X is nitrogen;
Y is oxygen or sulfur;
Z is nitrogen or a group C-R5;
n is 1 or 2;
20 Rl is Cl-C4-alkyl, Cl-C4--haloalkyl, Cl-C4-alkoxy,
Cl-C4-haloalkoxy;
R2 is Cl-C4-alkoxy;
R3 is hydrogen;
a radical oR6;
25 R4 is alkyl;
R5 is hydrogen or together with Rl is a unit -CH2-CH2-O-;
R6 is hydrogen, an alkali metal cation, the equivalent of an
alkaline earth metal cation, or an organic ammonium ion;
a Cl-C6-alkyl group which can have attached to it one to
five halogen atoms and/or one or two of the following
radicals: Cl-C4-alkoxy, phenyl, substituted phenyl;
a C3-C6-alkenyl or a C3-C6-alkynyl group, it being
possible for these groups, in turn, to have attached to
them one to five halogen atoms;
a radical -N=CR9Rl0 where R9 and Rl~ can be identical or
different;
R9,Rl0 are Cl-C4-alkyl which can have attached to it a
Cl-C4-alkoxy and/or a Cl-C4-alkylthio group, or together
are a C3-Cll-alkylen~e chain;~5
CA 02233l~4 l998-04-22
0050/46319
:14
with the proviso that, if Rl and R2 are methoxy and R3 is a hy-
droxyl group and if Y is oxygen and Z is a methine group CH, n is
2 and S(O) n is in the p-position of the phenyl ring, R4 is other
than methyl, where
substituted phenyl means that the phenyl ring can have attached
to it one to five halogen atoms, one to three Cl-C4-alkyl or
Cl-C4-alkoxy groups and/or one to three nitro groups.
- 10 Other preferred compounds of the formula I are those where the
substituents and the index have the following meanings:
X is nitrogen;
Y is oxygen or sulfur;
15 Z is nitrogen or a grollp C-H;
n is 1 or 2;
Rl is alkoxy;
R2 is alkoxy;
R3 is hydrogen;
a radical oR6;
R4 is alkyl;
R6 is hydrogen;
an alkyl group which can have attached to it one to five
halogen atoms, a phenyl ring or an alkoxy group;
an alkenyl or an alkynyl group;
a radical -N=CR9R10 where R9 and Rl~ can be identical or
different;
R9,Rl0are alkyl or together are an alkylene chain;
with the proviso that, if Rl and R2 are methoxy and R3 is a hy-
droxyl group and if Y is oxygen, n is 2 and S~O) n is in the
35 p-position of the phenyl ring, R4 is other than methyl.
With a view to the herbicida:L activity, particularly preferred
compounds of the formula I a:re those where the substituents and
the index have the following meanings:
X is nitrogen;
Y is oxygen or sulfur;
Z is nitrogen or a group C-H;
n is 1 or 2;
45 Rl is Cl-Cg-alkoxy;
R2 is Cl-C4-alkoxy;
CA 022331~4 1998-04-22
0050/46319
1!5
R3 is hydrogen;
a radical oR6;
R4 is C1-C4-alkyl;
R6 is hydrogen;
a Cl-C6-alkyl group which can have attached to it one to
five halogen atoms, a phenyl ring or a C1-C4-alkoxy
group;
a C3-C6-alkenyl or a C3-C6-alkynyl group;
a radical -N=CR9R10 where R9 and R10 can be identical or
different;
15 R9 and Rlo are C1-C4-alkyl or together are a C3-Cg-alkylene chain;
with the proviso that, if R1 and R2 are methoxy and R3 is a hy-
droxyl group and if Y is oxygen, n is 2 and S(O) n is in the
p-position of the phenyl ring, R4 is other than methyl.
Other preferred compounds of the formula I are those where the
substituents and the index have the following meanings:
X is nitrogen;
25 Y is oxygen or sulfur;
Z is nitrogen or a group C-H;
n is 1 or 2;
R1 is methoxy;
R2 is methoxy;
30 R3 is hydroxyl;
R4 is alkyl;
with the proviso if when Y is oxygen, n is 2 and S(O) n is in the
p-position of the phenyl ring, R4 is other than methyl.
With a view to the herbicidal activity and tolerance by crop
plants, especially preferred ,-ompounds of the formula I are those
below in which the substituents and the index have the following
40 meanings:
X is nitrogen;
Y is oxygen or sulfur;
Z is nitrogen or a group C-H;
45 n is 1 or 2;
R1 is methoxy;
CA 022331~4 1998-04-22
0050/46319
R2 is methoxy
R3 is hydroxyl;
R4 is Cl-C4-alkyl;
s
with the proviso that if Y is oxygen, n is 2 and S(O) n is in the
p-position of the phenyl ring, R4 is other than methyl.
Very especially preferred is t.he compound 2-(4,6-dimethoxypyrim-
10 idin-2-yloxy)-6-(3-methylsulfonylphenyl)benzoic acid.
The compounds I and their agriculturally useful salts, both as
isomer mixtures and in the form of the pure isomers, are suitable
as herbicides. The herbicidal compositions which comprise I effi-
15 ciently control vegetation on uncultivated land, in particular athigh rates of application. In crops such as wheat, rice, maize,
soya beans and cotton, they act against broad-leaved weeds and
grass weeds without causing any notable damage to the crop
plants. This effect is observed especially at low rates of ap-
20 plication.
Taking into consideration the versatility of the applicationmethods, the compounds I, or compositions comprising them, can
additionally be employed in a further number of crop plants for
25 removing undesirable plants. Suitable crops are, for example, the
following:
Allium cepa, An~n~s comosus, Arachis hypogaea, Asparagus
officinalis, Beta vulgaris ss]p. altissima, Beta vulagris ssp.
30 rapa, Brassica napus var. napus, Brassica napus var.
napobrassica, Brassica rapa var. silvestris, Camellia sinensis,
Carthamus tinctorius, Carya illinoinensis, Citrus limon, Citrus
sinensis, Coffea arabica (Coffea canephora, Coffea liberica),
Cucumis sativus, Cynodon dactylon, Daucus carota, Elaeis
35 guineensis, Fragaria vesca, Glycine max, Gossypium hirsutum,
(Gossypium arboreum, Gossypium herbaceum, Gossypium vitifolium),
Helianthus annuus, Hevea brasiliensis, Hordeum vulgare, Humulus
lupulus, Ipomoea batatas, Juglans regia, Lens culinaris, Linum
usitatissimum, Lycopersicon lycopersicum, Malus spp., Manihot
40 esculenta, Medicago sativa, Musa spp., Nicotiana tabacum (N.ru-
stica)~ Olea europaea, Oryza sativa, Phaseolus lunatus, Phaseolus
vulgaris, Picea abies, Pinus spp., Pisum sativum, Prunus avium,
Prunus persica, Pyrus commllnis, Ribes sylvestre, Ricinus
communis, Saccharum officinarum, Secale cereale, Solanum
45 tuberosum, Sorghum bicolor (c. vulgare), Theobroma cacao, Trifo-
CA 022331~4 1998-04-22
OOSO/46319
lium pratense, Triticum aesti~m, Triticum durum, Vicia faba,
Vitis vinifera, Zea mays.
In addition, the compounds I can also be used in crops which are
5 tolerant to the action of herbicides as a result of breeding, in-
cluding genetic engineering methods.
The herbicidal compositions or the active ingredients can be ap-
plied pre- or post-emergence. If the active ingredients are less
10 well tolerated by certain crop plants, application techniques may
be used where the herbicidal c:ompositions are sprayed, with the
aid of the spraying equipment, in such a way that they come into
as little contact as possible with the leaves of the sensitive
crop plants while the active iLngredients reach the leaves of un-
15 desirable plants growing underneath, or the naked soil surface(post-directed, lay-by).
For example, the compounds I or the herbicidal compositions com-
prising them can be applied in the form of directly sprayable
20 aqueous solutions, powders, suspensions, also highly concentrated
aqueous, oily or other suspen;ions or dispersions, emulsions, oil
dispersions, pastes, dusts, mt~terials for spraying or granules,
by means of spraying, atomizing, dusting, scattering or pouring.
The use forms depend on the intended purposes; in any case, they
25 should ensure the finest possible distribution of the active in-
gredients according to the invention.
Suitable inert additives are mineral oil fractions of medium to
high boiling point, such as k,erosine or diesel oil, furthermore
30 coal tar oils and oils of veg,etable or ~n;m~l origin, aliphatic,
cyclic and aromatic hydrocarbons, eg. paraffin, tetrahydronaph-
thalene, alkylated naphthalenes or their derivatives, alkylated
benzenes or their derivatives, methanol, ethanol, propanol, buta-
nol, cyclohexanol, cyclohexanone, or strongly polar solvents such
35 as n-methylpyrrolidone or water.
Aqueous use forms can be prepared from emulsion concentrates,
suspensions, pastes, wettable powders or water-dispersible gran-
ules by adding water. To prepare emulsions, pastes or oil disper-
40 sions, the substances, as such or dissolved in an oil or solvent,can be homogenized in water by means of wetting agent, adhesive,
dispersant or emulsifier. Alternatively, it is possible to pre-
pare concentrates composed of active substance, wetting agent,
adhesive, dispersant or emulsifier and, if desired, solvent or
45 oil, which are suitable for dilution with water.
. CA 022331~4 1998-04-22
0050/46319
1~3
Suitable surfactants (adjuvants) are the alkali metal salts, al-
kaline earth metal salts and ammonium salts of aromatic sulfonic
acids, eg. ligno-, phenol-, naphthalene- and dibutylnaphthalene-
sulfonic acid, and of fatty ac:ids, alkyl- and alkylarylsulfo-
5 nates, alkyl sulfates, lauryl ether sulfates and fatty alcoholsulfates, and salts of sulfated hexa-, hepta- and octadecanols,
and of fatty alcohol glycol ethers, condensates of sulfonated
naphthalene and its derivative~s with formaldehyde, condensates of
naphthalene or of the naphthalenesulfonic acids with phenol and
10 formaldehyde, polyoxyethylene octylphenol ether, ethoxylated iso-
octyl-, octyl- or nonylphenol, alkylphenyl polyglycol ethers,
tributylphenyl polyglycol ether, alkylaryl polyether alcohols,
isotridecyl alcohol, fatty alcohol/ethylene oxide condensates,
ethoxylated castor oil, polyoxyethylene alkyl ethers or polyoxy-
15 propylene alkyl ethers, lauryl alcohol polyglycol ether acetate,sorbitol esters, lignosulfite waste liquors or methylcellulose.
Powders, materials for spreading and dusts can be prepared by
mixing or grinding the active substances together with a solid
20 carrier.
Granules, eg. coated granulates, impregnated granules and homoge-
neous granules, can be prepare!d by binding the active ingredients
to solid carriers. Solid carriers are mineral earths such as sil-
25 icas, silica gels, silicates, talc, kaolin, limestone, lime,chalk, bole, loess, clay, dolomite, diatomaceous earth, calcium
sulfate, magnesium sulfate, magnesium oxide, ground synthetic ma-
terials, fertilizers such as c~monium sulfate, ammonium phos-
phate, ammonium nitrate, ureas and products of vegetable origin
30 such as cereal meal, tree bar}c meal, wood meal and nutshell meal,
cellulose powders or other so]Lid carriers.
The concentrations of the act:Lve ingredients I in the ready-to-
use preparations can be varied within wide ranges. In general,
35 the formulations comprise from 0.001 to 98 % by weight, prefer-
ably 0.01 to 95 % by weight, of active ingredient. The active in-
gredients are employed in a purity of from 90 % to 100 %, prefer-
ably 95 % to 100 % ~according to NMR spectrum).
40 The compounds I according to the invention can be formulated for
example as follows:
I 20 parts by weight of the~ compound No. 1.001 are dissolved in
a mixture composed of 80 parts by weight of alkylated ben-
zene, 10 parts by weight of the adduct of 8 to 10 mol of eth-
ylene oxide to 1 mol of oleic acid N-monoethanolamide,
5 parts by weight of calcium dodecylbenzenesulfonate and
- CA 022331~4 1998-04-22
0050/46319
5 parts by weight of the adduct of 40 mol of ethylene oxide
and 1 mol of castor oil. Pouring the solution into 100,000
parts by weight of water and finely distributing it therein
gives an aqueous dispersion which comprises 0.02 % by weight
of the active ingredient.
II 20 parts by weight of the compound No. 2.001 are dissolved in
a mixture composed of 40 I?arts by weight of cyclohexanone,
30 parts by weight of isobutanol, 20 parts by weight of the
adduct of 40 mol of isooctylphenyl and 10 parts by weight of
the adduct of 40 mol of ethylene oxide to 1 mol of castor
oil. Pouring the solution into 100,000 parts by weight of wa-
ter and finely distributing it therein gives an aqueous dis-
persion which comprises 0.02 % by weight of the active ingre-
dient.
III 20 parts by weight of the active ingredient No. 2.057 are
dissolved in a mixture composed of 25 parts by weight of cy-
clohexanone, 65 parts by weight of a mineral oil fraction of
boiling point 210 to 280 C and 10 parts by weight of the add-
uct of 40 mol of ethylene oxide and 1 mol of castor oil.
Pouring the solution into 100,000 parts by weight of water
and finely distributing it therein gives an aqueous disper-
sion which comprises 0.02 % by weight of the active ingredi-
ent.
IV 20 parts by weight of the active ingredient No. 1.001 are
mixed thoroughly with 3 plarts by weight of sodium diisobutyl-
naphthalene-a-sulfonate, 17 parts by weight of the sodium
salt of a lignosulfonic acid from a sulfite waste liquor and
60 parts by weight of a pulverulent silica gel and the mix-
ture is ground in a hammer mill. Finely distributing the mix-
ture in 20,000 parts by weight of water gives a spray mixture
which comprises 0.1 % by weigh of the active ingredient.
V 3 parts by weight of the active ingredient No. 2.001 are
mixed with 97 parts by weight of finely divided kaolin. This
gives a dust which comprises 3 % by weight of the active in-
gredient.
VI 20 parts by weight of the active ingredient No. 2.057 are
mixed intimately with 2 parts by weight of calcium dodecyl-
benzenesulfonate, 8 parts by weight of fatty alcohol polygly-
col ether, 2 parts by we:ight of the sodium salt of a phenol/
urea/formaldehyde condensate and 68 parts by weight of a par-
affinic mineral oil. This gives a stable oily dispersion.
- CA 022331~4 1998-04-22
OOSO/46319
VII 1 part by weight of the compound 1.001 is dissolved in a mix-
ture composed of 70 parts by weight of cyclohexanone,
20 parts by weight of ethoxylated isooctylphenol and 10 parts
by weight of ethoxylated castor oil. This gives a stable
emulsion concentrate.
VIII 1 part by weight of the compound 2.001 is dissolved in a
mixture composed of 80 parts by weight of cyclohexanone and
20 parts by weight of Emulphor EL. This gives a stable emul-
sion concentrate.
To widen the spectrum of action and to achieve synergistic ef-
fects, the aromatic sulfoxide-; and sulfones can be mixed, and ap-
plied jointly, with a large number of representatives of other
15 groups of herbicidal or growth-regulating active ingredients.
Suitable substances for mixtures are, for example, diazines,
4H-3,1-benzoxazine derivatives, benzothiadiazinones, 2,6-dinitro-
anilines, N-phenylcarbamates, thiocarbamates, halocarboxylic
acids, triazines, amides, urea6, diphenyl ethers, triazinones,
20 uracils, benzofuran derivatives, cyclohexane-1,3-dione
derivatives which have attached to them, in the 2-position, for
example a carboxyl or carbimino group, quinolinecarboxylic acid
derivatives, imidazolinones, sulfonamides, sulfonylureas,
aryloxy- and heteroaryloxyphenoxypropionic acids and their salts,
25 esters and amides, and others.
It may furthermore be advantageous to jointly apply the compounds
I, alone or in combination with other herbicides, in the form of
a mixture with still further crop protection agents, for example
30 with pesticides or agents for controlling phytopathogenic fungi
or bacteria. Also of interest is the miscibility with mineral
salt solutions, which can be employed for treating nutrient and
trace element deficiencies. Non-phytotoxic oils and oil concen-
trates can also be added.
Depending on the inte~e~ purpose, the season, the target plants
and the growth stage, the application rates of active ingredient
are from 0.001 to 3.0, preferably 0.01 to 1.0, kg of active in-
gredient (a.i.) per ha.
Use Examples
The herbicidal action of the aromatic sulfoxides and sulfones of
the formula I was demonstrated by greenhouse experiments:
CA 022331S4 1998-04-22
0050/46319
The culture containers used were plastic flowerpots containing
loamy sand with approximately 3.0 % of humus as the substrate.
The seeds of the test plants were sown separately for each spe-
cies.
For pre ~ ?rgence treatment, the active ingredinents which had
been suspended or emulsified in water were applied directly after
sowing by means of finely distributing nozzles. The containers
were irrigated gently to promlote germination and growth and sub-
lO sequently covered with translucent plastic hoods until the plantshad rooted. This cover causes uniform germination of the test
plants unless this has been adversely affected by the active in-
gredients.
15 For post-emergence treatment, the test plants were first grown to
a height of 3 to 15 cm, depending on the plant habit, and only -
then treated with the active ingredients which had been suspended
or emulsified in water. The t:est plants are either sown directly
and grown in the same containers or first grown separately as
20 seedlings and transplanted into the test containers a few days
prior to treatment. The rate of application for the post-emer-
gence treatment is 0.0625 or 0.0313 kg of a.i. per ha.
Depending on the species, the plants were kept at from 10 to 25 C
25 or 20 to 35~C. The test period extended over 2 to 4 weeks. During
this time, the plants were tended, and their response to the in-
dividual treatments was evaluated.
Assessment was carried out u-;ing a scale from 0 to 100. 100 means
30 no emergence of the plants o~ complete destruction of at least
the aerial parts, and 0 means no damage or normal course of
growth.
The plants used in the greenhouse experiments belonged to the
35 following species:
CA 022331~4 1998-04-22
0050/46319
2'2
Scientific name Common name
Alopecurus black grass
myosuroides
Avena fatua wild oats
5 Echinochloa barnyard grass
crus-galli
Orysa sativa rice
10 Comparison Example
The compound according to the invention and the known comparison
agent were employed post-emergence in the greenhouse by the meth-
ods described above.
The comparison agent used is:
A from WO 91-13065, Ex. 1-12
O
~ ~ = ~ C
OH N
O--
The data in Table 1 show the clearly superior action of the com-
30 pound according to the invention in comparison with Example A.
Table 1
Comparison of results from post-emergence greenhouse experiments
R2~ ~ O--
~ ~ N ~
OH \ o
CA 022331S4 1998-04-22
0050/46319
23
Ex.No. 2.057 A
R1 SO2Me H
5 R2 H SO2Me
Rate of application 0.0625 0.0313 0.0625 0.0313
(kg/ha a.i.)
damage in %
lO Test plants
ORYSA 0 0 20 10
ALOMY 95 95 0 0
AVEFA 95 95 ~ ~
15 ECHCG 95 95 70 60
Synthesis Examples:
The procedures shown in the synthesis examples below were used to
20 obtain other compounds I using suitable different starting com-
pounds. The resulting compounds and physical data are given in
the tables which follow. Compounds without these data can be syn-
thesized by similar methods from suitable starting materials. The
structures shown in the tables describe especially preferred ac-
25 tive ingredients of the formula I.
Example 1
2-(4,6-Dimethoxy-pyrimidin-2-yloxy)-6-(3-methylsulfinylphenyl)-
30 benzoic acid (Compound 2.001):
a. 5-(3-Methylthiophenyl)-2,2-dimethyl-4H-(1,3)benzodioxin-
4-one: 3.72 g (155 mmol~l of magnesium filings were introduced
into 5 ml of dry THF ancl activated with a few grains of
iodine. The mixture was refluxed, the cooling equipment was
removed, and a solution of 29.6 g (146 mmol) of 1-bromo-3-me-
thylthiobenzene in 90 m]L of dry THF was added dropwise in
such a manner that the reflux was retained. The mixture was
kept under reflux for a further 3 hours, during which time
most of the magnesium d:issolved. The mixture was allowed to
cool to room temperature, a solution of 47.5 g (146 mmol) of
tributyltin chloride in 60 ml of dry THF was added dropwise,
and the mixture was refluxed for 3 hours. After cooling, the
batch was poured into 500 ml 5 % strength ammonium chloride
solution and the aqueous phase was extracted four times using
methylene chloride. The combined organic phases were washed
using water and saturated sodium chloride solution, dried
- CA 022331S4 1998-04-22
0050/46319
24
over sodium sulfate and concentrated. The residue which
remained was purified on silica gel (deactivated using
hexamethyldisalazane) using hexane/acetone. This gave 57.4 g
of a colorless oil (GC purity: 84 %). 31.92 g (65 mmol) of
this oil, 20.6 g (63 mmol) of 2,2-dimethyl-5-trifluoro-
methylsulfonyloxy-4H-(1,3)benzodioXin-4-one, 8.1 g (190 mmol)
of lithium chloride, 1.46 g of tetrakistriphenylphosphine-
palladium(0), 60 mg of 2,6-bis-tert-butyl-4-methylphenol were
heated in 140 ml of dioxane for 6 hours at 140 C in an auto-
clave. The mixture was subsequently concentrated in vacuo,
the residue was chromatographed on silica gel using toluene/
ethyl acetate mixtures an,d the product was then stirred with
hexane. Yield: 13.4 g of a colorless solid, m.p. lll-llS C.
15 b. 5-(3-Methylsulfinylphenyl)-2,2-dimethyl-4H-(1,3)benzodio-
xin-4-one: 3.6 g (12 mmol) of 5-(3-methylthiophenyl)-
2,2-dimethyl-4H-(1,3)benzodioxin-4-one were introduced into
50 ml of dry acetonitrile, and 2.77 g (12.6 mmol) of
iodosylbenzene and 0.24 g of phenylselenous acid were then
added at 45 C. The mixture was stirred for 14 hours at 45 C,
a further 0.28 g of iodosylbenzene were then added, and stir-
ring was continued for 2.5 hours at 45 C. The reaction mix-
ture was then poured into 200 ml of water and extracted using
methylene chloride. The combined organic phases were ex-
tracted by shaking with water and saturated sodium chloride
solution, dried over sodium sulfate and concentrated. The
residue was repeatedly extracted by stirring with hexane and
dried in a vacuum drying oven at 50 C. 3.45 g of a colorless
solid of m.p. 136-146 C remained.
c. 6-(3-MethylsulfinylphenyL)salicylic acid: 3.17 g (10 mmol) of
5-(3-methylsulfinylphenyL)-2,2-dimethyl-4H-(1,3)benzodio-
xin-4-one were suspended in a solution of 1.65 g (25 mmol) of
potassium hydroxide (85%) in 30 ml of water and the mixture
was refluxed for 5 hours. After cooling, the mixture was
extracted by shaking with ether, and the aqueous phase was
acidified to a pH of 2 using orthophosphoric acid and
extracted using ether. The combined ether phases of the
second extraction were washed using water and saturated
sodium chloride solution, dried over sodium sulfate and
concentrated. The residue was extracted repeatedly by
stirring with hexane and dried in a vacuum drying oven at
50 C. 1.75 g of a colorless solid of m.p. 136-163~C remained.
45 d. 2-(4,6-Dimethoxypyrimidin-2-yloxy)-6-(3-methylsulfinyl-
phenyl)benzoic acid: The product of c) (1.49 g, 5.4 mmol) was
introduced into 20 ml of dimethyl sulfoxide, 1.21 g
CA 022331~4 1998-04-22
0050/46319
(10.8 mmol) of potassium t:ert-butylate were added at room
temperature, and stirring was continued for 0.5 hours. 1.18 g
(5.4 mmol) of 4,6-dimethoxy-2-methylsulfonylpyrimidine were
then added and the mixture was stirred overnight at room
temperature. The reaction mixture was subsequently poured
into water which had been acidified with phosphoric acid and
the solid which separated out was filtered off with suction.
The product was washed wit:h water and dried in a vacuum
drying oven at S0 C. Yield 2.07 g of a colorless solid. Melt-
ing range 161-254~C. NMR(C'DC13, 270MHz): 2.71(s, 3H),
3.81(s, 6H), 5.78(s, lH), 7.21-7.32(m, 2H), 7.44-7.72(m, SH).
Example 2
15 2-(4,6-Dimethoxypyrimidin-2-y]oxy)-6-(3-methylsulfonylphenyl)-
benzoic acid (Compound 2.057)
a. 6-(3-Methylthiophenyl)salicylic acid: 7.2 g (24 mmol) of
5-(3-methylthiophenyl)-2,2-dimethyl-4H-(1,3)benzodioxin-4-one
were suspended in a solution of 3.95 g (60 mmol) of potassium
hydroxide (85%) in 150 ml of water and the mixture was
refluxed for 10 hours. After cooling, the mixture was
extracted by shaking with ether, and the aqueous phase was
acidified to a p~ of 2 using orthophosphoric acid and
extracted using ether. The combined ether phases of the
second extraction were washed using water and saturated
sodium chloride solution, dried over sodium sulfate and
concentrated. The residue was extracted repeatedly by
stirring with hexane and dried in a vacuum drying oven at
50 C. 5.1 g of a colorless solid of m.p. 135-138~C remained.
b. 6-(3-Methylsulfonylphenyl)salicylic acid: 2.05 g (7.9 mmol)
of 6-(3-methylthiophenyl)salicylic acid were dissolved in
20 ml of glacial acetic acid and treated with 0.15 g of
sodium tungstate. 1.97 g (17.4 mmol) of hydrogen peroxide
solution (30% strength) were added dropwise at 35~C and stir-
ring was continued for 15 minutes. The batch was then treated
with three times the amount of water and extracted using
MTBE. The combined MTBE phases were washed using water and
saturated sodium chloride! solution, dried over sodium sulfate
and concentrated. The reeidue was extracted repeatedly by
stirring with hexane and dried in a vacuum drying oven at
50 C. 1.9 g of a colorless solid of m.p. 148-161~C remained.
45 c. 2-(4,6-Dimethoxypyrimidin-2-yloxy)-6-(3-methylsulfonyl-
phenyl)benzoic acid: The product of b) (1.65 g, 5.65 mmol)
was placed into 20 ml of dimethyl sulfoxide, 1.27 g
CA 022331S4 1998-04-22
0~50/46319
2'6
(11.3 mmol) of potassium 1;ert-butylate were added at room
temperature, and stirring was continued for 0.5 hour. 1.27 g
(5.65 mmol) of 4,6-dLmethoxy-2-methylsulfonylpyrimidine were
then added and the mixture was stirred overnight at room
temperature. The reaction mixture was subsequently poured
into water which had been acidified with phosphoric acid and
the solid which separated out was filtered off with Suction.
The product was washed wi'th water and dried in a vacuum
drying oven at 50 C. Yield 1.95 g of colorless solid. Melting
range 78-120~C. NMR(CDCl3, 270MHz): 3.07(s, 3H), 3.80(s, 6H),
5.78~s, lH), 7.22-7.33(m, 2H), 7.46-7.63(m, 2H), 7.71 (d,
lH), 7.90(d, lH), 8.01(s, lH).
Table l
Rl -
N~Z
~I~X~ R2
R4~S(O)n ~ oR3
2 5 No. Z Rl R2 R3 R4 Y n X Physical data
(mp.[~C],NMR)
1.001 CH OMe OMe OH methyl O 1 N 183-186
1.002 CH OMe OMe OH methyl S 1 N
1.003 CH Me OMe OH methyl O 1 N
1.004 CH CF3 OMe OH methyl O 1 N
1.005 C-CH2-CH2-O OMe OH methyl O 1 N
1.006 CH OCHF2 OCHF2 OH methyl O 1 N
1.007 CF OMe OMe OH methyl O 1 N
1.008 N OMe OMe OH methyl O 1 N
1.009 CH OMe OMe O-Li+ methyl O 1 N
1.010 CH OMe OMe O~Na+ methyl O 1 N
1.011 CH OMe OMe O~ K+ methyl O 1 N
1.012 CH OMe OMe O-(1/2 Ca2+) methyl O 1 N
1013 CH OMe OMe O-NH4+ methyl O 1 N
1.014 CH OMe OMe O-NBu4+ methyl O 1 N
1.015 CH OMe OMe O-CH3 methyl O 1 N
1.016 CH OMe OMe O-cH2-cH=cH2 methyl O 1 N
1.017 CH OMe OMe O-CHz-C_ ('H methyl O 1 N
1-018 CH OMe OMe O-CH2-CF3 methyl O 1 N
1.019 CH OMe OMe O-(CH2)2-O-CH3 methyl O 1 N
1.020 CH OMe OMe O-CH2-S-CH3 methyl O 1 N
1.021 CH OMe OMe O ,~ \ methyl O 1 N
\~\~;l
1.022 CH OMe OMe O-N=C(CH3)2 methyl O 1 N
1023 CH OMe OMe O-N=C(CH2)s methyl O 1 N
CA 022331~4 1998-04-22
0050/46319
27
1.024 CH OMe OMe O-N=C(CH2k~ methyl O 1 N
1.025 CH OMe OMe O-N=C(CH3)(0CH3) methyl O 1 N
1.026 CH OMe OMe N~ / methyl O 1 N
/ \
0 ~
1.027 CH OMe OMe O-N(CH3)2 methyl O 1 N
1.028 CH OMe OMe OH ethyl O 1 N
1.029 N OMe OMe OH ethyl O 1 N
1.030 CH OMe OMe OH ethyl S 1 N
1.031 CH Me OMe OH ethyl O 1 N
1.032 CH CF3 OMe OH ethyl O 1 N
1.033 C-CH2-CH2-O OMe OH ethyl O 1 N
1.034 CH OCHF2 OCHF2 OH ethyl O 1 N
1.035 CF OMe OMe OH ethyl O 1 N
1.036 CH OMe OMe O-Li+ ethyl O 1 N
1.037 CH OMe OMe O~Na+ ethyl O 1 N
1.038 CH OMe OMe O-NBu4+ ethyl O 1 N
1.039 CH OMe OMe O-CH3 ethyl O 1 N
1.040 CH OMe OMe O-cH2-cH=(-H2 ethyl O 1 N
1.041 CH OMe OMe O-CH2-C_ CH ethyl O 1 N
1.042 CH OMe OMe O-CH2-CF3 ethyl O 1 N
1.043 CH OMe OM 0~ \ ethyl O 1 N
1.044 CH OMe OMe O-N=C(CH3) ~ ethyl O 1 N
1.045 CH OMe OMe O-N=C(CH2)s ethyl O 1 N
1.046 CH OMe OMe O-N=C(CH2)7 ethyl O 1 N
1.047 CH OMe OMe O-N=C(CH3)~0CH~) ethyl O 1 N
1.048 CH OMe OMe N~ / ethyl O 1 N
/ \
o rs
1.049 CH OMe OMe O-N(CH3)2 ethyl O 1 N
1.050 CH OMe OMe OH n-propyl O 1 N
1.051 CH OMe OMe OH isop~ 1 O 1 N
1.052 CH OMe OMe OH n-butyl O 1 N
1.053 CH OMe OMe OH s-butyl O 1 N
1.054 CH OMe OMe OH t-butyl O 1 N
1.055 CH OMe OMe OH trifluoromethyl O 1 N
1.056 CH OMe OMe OH benzyl O 1 N
3 5 1.057 N OMe OMe OH methyl S 2 N
1.058 CH OMe OMe OH methyl S 2 N
1.059 CH Me OMe OH methyl O 2 N
1.060 CH CF3 OMe OH methyl O 2 N
1.061 C-CH2-CH2-O OMe OH methyl O 2 N
1.062 CH OCHF2 OCHF2 OH methyl O 2 N
1.063 CF OMe OMe OH methyl O 2 N
1.064 N OMe OMe OH methyl O 2 N
1.065 CH OMe OMe O-Li+ methyl O 2 N
1.066 CH OMe OMe O~Na+ methyl O 2 N
1.067 CH OMe OMe O~ K+ methyl O 2 N
1.068 CH OMe OMe O-(1/2 Ca2+) methyl O 2 N
1.069 CH OMe OMe O-NH4+ methyl O 2 N
1.070 CH OMe OMe O-NBu4+ methyl O 2 N
1.071 CH OMe OMe O-CH3 methyl O 2 N
1.072 CH OMe OMe O-cH2-cH=cH2 methyl O 2 N
1.073 CH OMe OMe O-CH2-C- ('H methyl O 2 N
- CA 02233154 1998-04-22
0050/46319
1.074 CH OMe OMe O-CH2-CF3 methyl O 2 N
1.075 CH OMe OMe O-(CH2k-O-('H3 methyl O 2 N
1.076 CH OMe OMe O-CH2-S-CH3 methyl O 2 N
5 1.077 CH OMe OMe O ~ ( < methyl O 2 N
1.078 CH OMe OMe O-N=C(CH3)2 methyl O 2 N
1.079 CH OMe OMe o-N=c(cH2)s methyl O 2 N
1.080 CH OMe OMe O-N=C(CH2h methyl O 2 N
1.081 CH OMe OMe O-N=C(CH3)(OCH3) methyl O 2 N
1.082 CH OMe OMe N=( / methyl O 2 N
/ ~';
O
1.083 CH OMe OMe O-N(CH3)2 methyl O 2 N
1.084 CH OMe OMe OH ethyl O 2 N 133-136~C
15 1.085 N OMe OMe OH ethyl O 2 N 79-82~C
1.086 CH OMe OMe OH ethyl S 2 N
1.087 CH Me OMe OH ethyl O 2 N
1.088 CH CF3 OMe OH ethyl O 2 N
1.089 C-CH2-CH2-O OMe OH ethyl O 2 N
1.090 CH OCHF2 OCHF2 OH ethyl O 2 N
1.091 CF OMe OMe OH ethyl O 2 N
1.092 CH OMe OMe O-Li+ ethyl O 2 N
1.093 CH OMe OMe O~Na+ ethyl O 2 N
1.094 CH OMe OMe O-NBu4+ ethyl O 2 N
1.095 CH OMe OMe O-CH3 ethyl O 2 N
1.096 CH OMe OMe O-CH2-CH=C:H2 ethyl O 2 N
2 5 1.097 CH OMe OMe O-CH2-C _ C]H ethyl O 2 N
1.098 CH OMe OMe O-CH2-CF3 ethyl O 2 N
1.099 CH OMe OMe O ,~ ~ ethyl O 2 N
~
1.100 CH OMe OMe O-N=C(CH3)zl ethyl O 2 N 124~C
1.101 CH OMe OMe O-N=C(CH2).i ethyl O 2 N
1.102 CH OMe OMe O-N=C(CH2),r ethyl O 2 N 133-142~C
1.103 CH OMe OMe O-N=C(CH3)(0CH3) ethyl O 2 N
1.104 CH OMe OMe N=~ / ethyl O 2 N
/ ~,S
O
1.105 CH OMe OMe O-N(CH3)2 ethyl O 2 N
1.106 CH OMe OMe OH n-propyl O 2 N
1.107 CH OMe OMe OH isopropyl O 2 N
1.108 CH OMe OMe OH n-butyl O 2 N
1.109 CH OMe OMe OH s-butyl O 2 N
1.110 CH OMe OMe OH t-butyl O 2 N
1.111 CH OMe OMe OH trifluoromethyl O 2 N
1.112 CH OMe OMe OH benzyl O 2 N
1.113 N OMe OMe OH methyl O 1 CH
1.114 N OMe OMe OH ethyl O 1 CH
1.115 N OMe OMe OH methyl O 2 CH
1.116 N OMe OMe OH ethyl O 2 CH
CA 022331S4 1998-04-22
0050/46319
Table 2
N'~Z
R4-S(O)n ~ Y X ~ R2
No. Z Rl R2 R3 R4 Y n X Physicalda~
~mp.rC],NMR)
2.001 CH OMe OMe OH methyl O 1 N 2.71(s,3H),
3.81(s,6H),
5.78(s,1H),
7.21-7.32
(m,2H),7.~-
7.72(m,SH)
2.002 CH OMe OMe OH methyl S 1 N
2.003 CH Me OMe OH methyl O 1 N
20 2 004 C~ CF3 OMe OH methyl O 1 N
2.005 C-CH2-CH2-O OMe OH methyl O 1 N
2.006 CH OCHF2 OCHF2 OH methyl O 1 N
2.007 CF OMe OMe OH methyl O 1 N
2.008 N OMe OMe OH methyl O 1 N
2.009 CH OMe OMe O-Li+ methyl O 1 N
2.010 CH OMe OMe O~Na+ methyl O 1 N
25 2.011 CH OMe OMe O~ Kt methyl O 1 N
2.012 CH OMe OMe 0-(112 Ca2+) methyl O 1 N
2.013 CH OMe OMe O-NH4+ methyl O 1 N
2.014 CH OMe OMe O-NBu4+ methyl O 1 N
2.015 CH OMe OMe O-CH3 methyl O 1 N
2.016 CH OMe OMe O-cH2-cH-cH2 methyl O 1 N
30 2.017 CH OMe OMe O-CH2-C_CH methyl O 1 N
2.018 CH OMe OMe O-CH2-CF3 methyl O 1 N
2.019 CH OMe OMe O-(CH2)2-O-CH3 methyl O 1 N
2.020 CH OMe OMe O-CH2-S-CH3 methyl O 1 N
2.021 CH OMe OMe O~.N methyl O 1 N
2.022 CH OMe OMe O-N=~CH3)2 methyl O 1 N
2.023 CH OMe OMe O-N=C~CH_)s methyl O 1 N
2.024 CH OMe OMe O-N=C(CH2)7 methyl O 1 N
40 2.025 CH OMe OMe O-N=~CH3)(0CH3) methyl O 1 N
2.026 CH OMe OMe ~ / methyl O 1 N
~- s
O
2.027 CH OMe OMe O-N(cH3)2 methyl O 1 N
2.028 CH OMe OMe OH ethyl O 1 N
45 2.029 N OMe OMe OH ethyl O 1 N
2.030 CH OMe OMe OH ethyl S 1 N
2.031 CH Me OMe OH ethyl O 1 N
2.032 CH CF3 OMe OH ethyl O 1 N
CA 022331s4 1998-04-22
0050~46319
2.033 C-CH2-CH2-O OMe OH ethyl O 1 N
2.034 CH OCHF2 OCHF2 OH ethyl O 1 N
2.035 CF OMe OMe OH ethyl O 1 N
2.036 CH OMe OMe O-Li+ ethyl O 1 N
2.037 CH OMe OMe O~Na+ ethyl O 1 N
2.038 CH OMe OMe O-NBu4+ ethyl O 1 N
2.039 CH OMe OMe O-CH3 ethyl O 1 N
2.040 CH OMe OMe O-cH2-cH=lcH2 ethyl O 1 N
2.041 CH OMe OMe O-CH2-C--C'H ethyl O 1 N
2.042 CH OMe OMe O-CH2-CF3 ethyl O 1 N
10 2.043 CH OMe OMe O ~ ~ ethyl O 1 N
~\N'
2.044 CH OMe OMe O-N=C~CH3)l2 ethyl O 1 N
2.045 CH OMe OMe O-N=C(CH2~1s ethyl O 1 N
2.046 CH OMe OMe O-N=c(cH2'J7 ethyl O 1 N
2.047 CH OMe OMe O-N=C(CH3'1(0CH3) ethyl O 1 N
2.048 CH OMe OMe ~ / ethyl O 1 N
~ S
~
2.049 CH OMe OMe O-N(CH3)2 ethyl O 1 N
20 2.050 CH OMe OMe OH n-propyl O 1 N
2.051 CH OMe OMe OH isopropyl O 1 N
2.052 CH OMe OMe OH n-butyl O 1 N
2.053 CH OMe OMe OH s-butyl O 1 N
2.054 CH OMe OMe OH t-butyl O 1 N
2.055 CH OMe OMe OH trifluoromethyl O 1 N
25 2.056 CH OMe OMe OH benzyl O 1 N
2.057 CH OMe OMe OH methyl O 2 N 3.07(s,3H),
3.80(s.6H),
5.78(s,lH),
7.22-7.33
(m, 2H),
7.46-7.63
(m,2H),
7.71d,1H),
7.90(d,1H),
8.01(s,lH)
2.058 CH OMe OMe OH methyl S 2 N
2.059 CH Me OMe OH methyl O 2 N
35 2.060 CH CF3 OMe OH methyl O 2 N
2.061 C-CH2-CH2-O OMe OH methyl O 2 N
2.062 CH OCHF2 OCHF2 OH methyl O 2 N
2.063 CF OMe OMe OH methyl O 2 N
2.064 N OMe OMe OH methyl O 2 N
2.065 CH OMe OMe O-Li+ methyl O 2 N
2.066 CH OMe OMe O~Na+ methyl O 2 N
2.067 CH OMe OMe O~ K+ methyl O 2 N
2.068 CH OMe OMe O~(1/2 Ca2+) methyl O 2 N
2.069 CH OMe OMe O-NH4+ methyl O 2 N
2.070 CH OMe OMe O-NBu4+ methyl O 2 N
2.071 CH OMe OMe O-CH3 methyl O 2 N
2.072 CH OMe OMe O-CH2-CH=CH2 methyl O 2 N
45 2.073 CH OMe OMe O-CH2-C - CH methyl O 2 N
2.074 CH OMe OMe O-CH2-CF-~ methyl O 2 N
2.075 CH OMe OMe O-(CH2)2-O-CH3 methyl O 2 N
2.076 CH OMe OMe O-CH2-S-CH3 methyl O 2 N
- CA 022331~4 1998-04-22
0050/46319
.~1
2.077 CH OMe OMe O ~( methyl O 2 N
2.078 CH OMe OMe O-N=C(CH3)2 methyl O 2 N 128-132~C
2.079 CH OMe OMe o-N=c(cH2)s methyl O 2 N 48-52~C
2.080 CH OMe OMe O-N=C(CH2)7 methyl O 2 N 98-102~C
2.081 CH OMe OMe O-N=C(CH3)(0CH3) methyl O 2 N
2.082 CH OMe OMe N=~ / methyl O 2 N
0 ~'~
2.083 CH OMe OMe O-N(CH3)2 methyl O 2 N
2.084 CH OMe OMe OH ethyl O 2 N
2.085 N OMe OMe OH ethyl O 2 N
2.086 CH OMe OMe OH ethyl S 2 N
2.087 CH Me OMe OH ethyl O 2 N
2.088 CH CF3 OMe OH ethyl O 2 N
2.089 C-CH2-CH2-O OMe OH ethyl O 2 N
2.090 CH OCHF2 OCHF2 OH ethyl O 2 N
2.091 CF OMe OMe OH ethyl O 2 N
2.092 CH OMe OMe O-Li+ ethyl O 2 N
2.093 CH OMe OMe O~Na~ ethyl O 2 N
2.094 CH OMe OMe O-NBu4~ ethyl O 2 N
2.095 CH OMe OMe O-CH3 ethyl O 2 N
2.096 CH OMe OMe O-cH2-cH=('H2 ethyl O 2 N
2.097 CH OMe OMe O-CH2-C_ CH ethyl O 2 N
2.098 CH OMe OMe O-CH2-CF3 ethyl O 2 N
2 5 2.099 CH OMe OMe O ,~/ ~ ethyl O 2 N
2.100 CH OMe OMe O-N=C(CH3)2 ethyl O 2 N 155-157~C
2.101 CH OMe OMe O-N=C(CH2)s ethyl O 2 N 93-96~C
2-102 CH OMe OMe O-N=C(CH2~7 ethyl O 2 N 157-162~C
2.103 CH OMe OMe O-N=C(CH3)(0CH3) ethyl O 2 N
2.104 CH OMe OMe N=~ / ethyl O 2 N
O ~
2.105 CH OMe OMe O-N(CH3)2 ethyl O 2 N
2.106 CH OMe OMe OH n-propyl O 2 N 65-67~C
2.107 CH OMe OMe OH isopropyl O 2 N
2.108 CH OMe OMe OH n-butyl O 2 N
2.109 CH OMe OMe OH s-butyl O 2 N
2.110 CH OMe OMe OH t-butyl O 2 N
2.111 CH OMe OMe OH trifluoromethyl O 2 N
2.112 CH OMe OMe OH benzyl O 2 N
2.113 N OMe OMe OH bethyl O 1 CH
2.114 N OMe OMe OH ethyl O 1 CH
2.115 N OMe OMe OH methyl O 2 CH
2.116 N OMe OMe OH ethyl O 2 CH
2.117 CH OCH3 OCH3 O-N=C~CH3'12 t-butyl O 2 N 173-176~C
2.118 CH OCH3 OCH3 OH t-butyl O 2 N 146-148~C
2.119 CH OCH3 OCH3 O-N=C(CH2)s n-propyl O 2 N 137-142~C
2.120 CH OCH3 OCH3 O-N=C(CH3)2 n-propyl O 2 N 144-146~C
CA 02233l~4 l998-04-22
0050/46319
/CH3
2.121 CH OCH3 OCH3 O-N=C methyl O 2 N100-103~C
OC2'H5
/CH3
2.122CH OCH3 OCH3 O-N=C ethyl O 2 N 97-108~C
OC2H5
Table 3
R4-S(O)n
rJ~x ~ R2
~ R3
No. Z Rl R2 R3 R4 Y n X Physicsldata
(mp.rC],NMR)
3.001 CH OMe OMe OH methyl O 1 N
3.002 CH OMe OMe OH methyl S 1 N
3.003 CH Me OMe OH methyl O 1 N
3.004 CH CF3 OMe OH methyl O 1 N
3.005 C-CH2-CH2-O OMe OH methyl O 1 N
3.006 CH OCHF2 OCHF2 OH methyl O 1 N
3-007 CF OMe OMe OH methyl O 1 N
3.008 N OMe OMe OH methyl O 1 N
3.009 CH OMe OMe O-Li+ methyl O 1 N
3.010 CH OMe OMe O~Na+ methyl O 1 N
3.011 CH OMe OMe O~ K+ methyl O 1 N
3.012 CH OMe OMe O-(1/2 Ca2+) methyl O 1 N
3.013 CH OMe OMe O-NH4+ methyl O 1 N
3.014 CH OMe OMe O-NBu4+ methyl O 1 N
3.015 CH OMe OMe O-CH3 methyl O 1 N
3.016 CH OMe OMe O-cH2-cH=cH2 methyl O 1 N
3.017 CH OMe OMe O-CH2-C- ('H methyl O 1 N
3.018 CH OMe OMe O-CH2-CF3 methyl O 1 N
3.019 CH OMe OMe O-(CH2)2-O-CH3 methyl O 1 N
3 5 3.020 CH OMe OMe O-CH2-S-CH3 methyl O 1 N
3.021 CH OMe OMe O ,~/~ methyl O 1 N
\_</ \~1
~ '
3.022 CH OMe OMe O-N=C(CH3)2 methyl O 1 N
3.023 CH OMe OMe O-N=C(CH2)s methyl O 1 N
3.024 CH OMe OMe O-N=C(CH2)7 methyl O 1 N
3.025 CH OMe OMe O-N=C(CH3)(0CH3) methyl O 1 N
3.026 CH OMe OMe N=~ / methyl O 1 N
O r
3.027 CH OMe OMe O-N(CH3)2 methyl O 1 N
3.028 CH OMe OMe OH ethyl O 1 N
CA 022331;i4 1998-04-22
0050/46319
313
3.029 N OMe OMe OH ethyl 0 1 N
3.030 CH OMe OMe OH ethyl S- 1 N
3.031 CH Me OMe OH ethyl 0 1 N
3.03Z CH CF3 OMe OH ethyl 0 1 N
3.033 C-CH2-CH2-O OMe OH ethyl 0 1 N
3.034 CH OCHF2 OCHF2 OH ethyl 0 1 N
3.035 CF OMe OMe OH ethyl 0 1 N
3.036 CH OMe OMe O-Li+ ethyl 0 1 N
3.037 CH OMe OMe O~Na+ ethyl 0 1 N
3.038 CH OMe OMe O-NBu4+ ethyl 0 1 N
3.039 CH OMe OMe O-CH3 ethyl 0 1 N
3-040 CH OMe OMe O-CH2-CH=C'H2 ethyl 0 1 N
3.041 CH OMe OMe O-CH2-C--C]H ethyl 0 1 N
3.042 CH OMe OMe O-CH2-CF3 ethyl 0 1 N
3.043 CH OMe OMe O ¦ \ ethyl 0 1 N
\~\N
3.044 CH OMe OMe O-N=C(CH3)~1 ethyl 0 1 N
3.045 CH OMe OMe O-N=C(CH2).; ethyl 0 1 N
3.046 CH OMe OMe O-N=C~CH2),~ ethyl 0 1 N
3.047 CH OMe OMe O-N=C(CH3)( 0CH3) ethyl 0 1 N
3.048 CH OMe OMe N==~ / ethyl O 1 N
O ~
3.049 CH OMe OMe O-N(CH3)2 ethyl 0 1 N
3.050 CH OMe OMe OH n-propyl 0 1 N
3.051 CH OMe OMe OH isopropyl O 1 N
25 3.052 CH OMe OMe OH n-butyl 0 1 N
3.053 CH OMe OMe OH s-butyl 0 1 N
3.054 CH OMe OMe OH t-butyl O 1 N
3.055 CH OMe OMe OH trifluoromethyl 0 1 N
3.056 CH OMe OMe OH benzyl 0 1 N
3.057 CH OMe OMe OH methyl 0 2 N
3.058 CH OMe OMe OH methyl S 2 N
30 3.059 CH Me OMe OH methyl 0 2 N
3.060 CH CF3 OMe OH methyl 0 2 N
3.061 C-CH2-CH2-O OMe OH methyl 0 2 N
3.062 CH OCHF2 OCHF2 OH methyl 0 2 N
3.063 CF OMe OMe OH methyl 0 2 N
3.064 N OMe OMe OH methyl 0 2 N
3.065 CH OMe OMe O-Li+ methyl 0 2 N
3.066 CH OMe OMe O~Na+ methyl 0 2 N
3.067 CH OMe OMe O~ K+ methyl 0 2 N
3.068 CH OMe OMe 0-(1/2 Ca2+) methyl 0 2 N
3.069 CH OMe OMe O-NH4+ methyl 0 2 N
3.070 CH OMe OMe O-NBu4+ methyl 0 2 N
3.071 CH OMe OMe O-CH3 methyl 0 2 N
3.072 CH OMe OMe O-cH2-cH=cH2 methyl 0 2 N
3.073 CH OMe OMe O-CH2-C- ('H methyl 0 2 N
3.074 CH OMe OMe O-CH2-CF3 methyl 0 2 N
3.075 CH OMe OMe O-(CH2)2-O-CH3 methyl 0 2 N
3.076 CH OMe OMe O-CH2-S-CH3 methyl 0 2 N
CA 02233l~4 1998-04-22
0050~463 19
3.077 CH OMe OMe O ,~ ~ methyl O 2 N
\1/ \\N
3.078 CH OMe OMe O-N=C~CH3)2 methyl O 2 N
3.079 CH OMe OMe O-N=C{CH2)s methyl O 2 N
3.080 CH OMe OMe O-N=C(CH2)7 methyl O 2 N
3.081 CH OMe OMe O-N=C~CH3)(0CH3) methyl O 2 N
3.082 CH OMe OMe N=~ / methyl O 2 N
o ~ S
3.083 CH OMe OMe O-N(CH3)2 methyl O 2 N
3.084 CH OMe OMe OH ethyl O 2 N
3.085 N OMe OMe OH ethyl O 2 N
3.086 CH OMe OMe OH ethyl S 2 N
1 5 3.087 CH Me OMe OH ethyl O 2 N
3.088 CH CF3 OMe OH ethyl O 2 N
3.089 C-CH2-CH2-O OMe OH ethyl O 2 N
3.090 CH OCHF2 OCHF2 OH ethyl O 2 N
3.091 CF OMe OMe OH ethyl O 2 N
3.092 CH OMe OMe O-Li+ ethyl O 2 N
3.093 CH OMe OMe O~Na+ ethyl O 2 N
3.094 CH OMe OMe O-NBu4+ ethyl O 2 N
3.095 CH OMe OMe O-CH3 ethyl O 2 N
3.096 CH OMe OMe O-cH2-cH=cH2 ethyl O 2 N
3.097 CH OMe OMe O-CH2-C--('H ethyl O 2 N
3.098 CH OMe OMe O-CH2-CF3 ethyl O 2 N
2 5 3.099 CH OMe OMe O ~/ \ ethyl O 2 N
3.100 CH OMe OMe O-N=C~CH3)2 ethyl O 2 N
3.101 CH OMe OMe o-N=c(cH2)s ethyl O 2 N
3-102 CH OMe OMe O-N=C(CH2h ethyl O 2 N
3.103 CH OMe OMe O-N=C~CH3)(0CH3) ethyl O 2 N
3.104 CH OMe OMe N=~ / ethyl O 2 N
/ \
O
3.105 CH OMe OMe O-N(CH3)2 ethyl O 2 N
3.106 CH OMe OMe OH n-propyl O 2 N
3.107 CH OMe OMe OH isopropyl O 2 N
3.108 CH OMe OMe OH n-butyl O 2 N
3.109 CH OMe OMe OH s-butyl O 2 N
3.110 CH OMe OMe OH t-butyl O 2 N
3.111 CH OMe OMe OH trifluoromethyl O 2 N
3.112 CH OMe OMe OH benzyl O 2 N
3.113 N OMe OMe OH methyl O 1 CH
3.114 N OMe OMe OH ethyl O 1 CH
3.115 N OMe OMe OH methyl O 2 CH
3.116 N OMe OMe OH ethyl O 2 CH