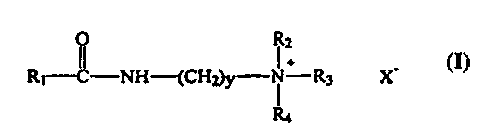Note: Descriptions are shown in the official language in which they were submitted.
CA 02233332 1998-03-27
WO 97/I2020 PCT/US96115578
1
LIQUID LAUNDRY DETERGENTS CONTAINING SELECTED
ALKYL AMIDOALKOYL QUATERNARY AMMONIUM COMPOUNDS
Field of the Invention
The present invention relates to detergent compositions containing
surfactants selected from quaternary ammonium surfactants. More particularly,
the
invention is directed to detergent compositions containing a nil-LAS
surfactant
system comprising anionic surfactants selected from the group consisting of
alkyl
alkoxylated sulfates and alkyl sulfates, said composition further containing
specific
quaternary ammonium surfactants.
Background of the Invention
The present invention relates to liquid detergents containing a surfactant
~ 5 system which is free of linear alkyl benzene sulfonate surfactants (LAS),
said liquid
detergent having optimum greasy stain removal performance.
The recent trend towards partial or total replacement of linear alkyl benzene
sulfonate surfactants (LAS) has urged the detergent formulators to rebalance
their
formulations with different surfactants.
2o There is thus a standing desire for performance and flexibility reasons to
make available a surfactant system capable of providing optimum detergency
performance which is equivalent to that of LAS-containing detergents.
The above objective has been met by a surfactant system comprising anionic
surfactants selected from the group consisting of alkyl alkoxylated sulfates
and alkyl
25 sulfates, said surfactant system fiurther comprising a cosurfactant
selected from the
group of quaternary ammonium surfactants which are stable in the detergent
product.
It has been surprisingly found that detergent compositions containing said
surfactant system exhibit detergency performance equivalent to that of LAS
containing detergents.
30 In addition, it was found that the liquid detergent compositions containing
the selected quaternary ammonium surfactants of the present invention, provide
excellent greasy stain removal performance without detriment to the suds
' characteristics of the compositions. This finding allows the formulator to
reduce the
level of suds suppressing agents, thereby facilitating the formulation of
concentrated
35 liquid detergents.
Quaternary ammonium surfactants are described in the art. The properties of
these surfactants are very strongly influenced by the type of substituent.
Chain
CA 02233332 1998-03-27
WO 97/12020 PCT/LTS96115578
2
length, degree of saturation, branching or the presence and number of
hydroxylic or
ethoxy groups mainly determine the properties of the surfactant. Whereas
typical
textile-conditioning actions are performed by cationic surfactants with two
long
alkyl chains. cationic surfactants with only one long alkyl chain have been
reported
to improve the detergency performance in laundry detergents. EP-A-224
describes
liquid built laundry detergent compositions comprising a general class of
quaternary
ammonium surfactants. Decyltrimethyl ammonium chloride is described. EP 8142
describes a liquid builder-free heavy duty detergent comprising a quaternary
ammonium compound of a general formula. Octyldihydroxyethylmethyl ammonium
1 O halides are described.
For optimum grease detergency performance, however, these compositions
of the prior art require high level of cationic surfactant. These high levels
of cationic
surfactants in turn, generate excessive foaming, thereby raising problems of
automatic washing machine compatibility. If, on the other hand, the cationic
surfactant is reduced at a level at which foam regulation is no longer a
problem, the
beneficial grease detergency characteristics of quaternary compounds are
diminished.
In contrast, the surfactant system of the present invention provides optimum
grease and oil removal performance, thereby not adversely affecting the suds
characteristic of the detergent compositions formulated therewith.
In addition, it has been found that liquid detergent compositions formulated
with said surfactant system are extremely useful when the liquid detergent
compositions are in direct contact with the fabrics such as during
pretreatment.
Summary of the Invention
The present invention relates to liquid detergent compositions comprising a
nil-LAS surfactant system (less than 5%, preferably substantially free of LAS,
e.g.,
less than 0.1 % by weight of detergent composition). Said surfactant system
comprising anionic surfactants selected from the group of alkyl alkoxy
sulfates and
alkyl sulfates and selected quaternary ammonium surfactants. The detergent
compositions comprise at least 5%, more preferably from about 10% to about
65%,
even more preferably from about 15% to about 40%, by weight, of the anionic
surfactant as described herein. The quaternary ammonium surfactant is present
in an
amount ranging from about 0.5% to about 10%, preferably from about 1% to about
'
4%, by weight, of the total detergent composition.
Detailed description of the Invention
CA 02233332 1998-03-27
WO 97/12020 PCT/LTS96/15578
3
The surfactant system of the detergent compositions according
to the present
invention comprise anionic surfactants selected from the
group of alkylalkoxy
sulfates and alkyl sulfates.
Alkyl alkoxylated sulfates and/or alkyl sulfates - The
alkyl alkoxylated
sulfate surfactants hereof are water soluble salts or acids
of the formula
RO(A)mS03M wherein R is an unsubstituted C l0-C24 alkyl
or hydroxyalkyl group
having a C l 0-C24 alkyl component, preferably a C 12-C
1 g alkyl or hydroxyalkyl,
more preferably C 12-C 15 alkyl or hydroxyalkyl, A is an
ethoxy or propoxy unit, m
is greater than zero, typically between about 0.5 and about
6, more preferably
1 O between about 0.5 and about 3, and M is H or a cation which
can be, for example, a
metal cation (e.g., sodium, potassium, lithium, calcium,
magnesium, etc.),
ammonium or substituted-ammonium cation. Alkyl ethoxylated
sulfates as well as
alkyl propoxylated sulfates are contemplated herein. Specific
examples of substituted
ammonium cations include ethanol-, triethanol-, methyl-,
dimethyl, trimethyl-
ammonium cations and quaternary ammonium cations such as
tetramethyl-
ammonium and dimethyl piperidinium cations and those derived
from alkylamines
such as ethylamine, diethylamine, triethylamine, mixtures
thereof, and the like.
Exemplary surfactants are C 12-C 15 alkyl polyethoxylate
( 1.0) sulfate (C 12-
C 15E( 1.0)M), C 12-C 15 alkyl polyethoxylate (2.25) sulfate
(C 12-C 15E(2-25)M),
2o C 1 ~-C 1 ~ alkyl polyethoxylate (3.0) sulfate (C 12-C
1 SE(3.0)M), and C 1 ~-C 1 ~ alkyl
polyethoxylate (4.0) sulfate (C 12-C 15E(4.0)M), wherein
M is conveniently selected
from sodium and potassium.
The alkyl sulfate surfactants hereof are water soluble
salts or acids of the
formula ROS03M wherein R preferably is a Cl0-C24 hydrocarbyl,
preferably an
alkyl or hydroxyalkyl having a C l 0-C 1 g alkyl component,
more preferably a C 12-
C 15 alkyl or hydroxyalkyl, and M is H or a cation, e.g.,
an alkali metal cation (e.g.
sodium, potassium, lithium), or ammonium or substituted
ammonium (e.g. methyl-,
dimethyl-, and trimethyl ammonium cations and quaternary
ammonium cations such
as tetramethyl-ammonium and dimethyl piperidinium cations
and quaternary
ammonium cations derived from alkylamines such as ethylamine,
diethylamine,
triethylamine, and mixtures thereof, and the like).
The quaternary ammonium compound - The quaternary ammonium
surfactant according to the present invention has the formula:
CA 02233332 1998-03-27
WO 97/12020 PCT/US96/15578
4
R2
R i-CI -NH-(CH~y-N ~ R3 X-
R4
whereby R 1 is a C6-C 1 g alkyl, preferably a C 12-14 alkyl; y is from about 1
to about
6, preferably from about 1 to about 3, more preferably 3; R2, R3 and R4 are
either
the same or different and can be H, a Cl-Cg alkyl, preferably a Cl-C3 alkyl,
Cl-Cg
alkoxy, aryl, alkaryl or alkoxyaryl; X- is a counterion, preferably a halide,
e.g.,
chloride, acetate, sulfate, OH-, or methylsulfate.
Preferred quaternary ammonium surfactants are those whereby Rl is C12-
C 14, R2, R3, and R4 are CH3.
1 O Preferred quaternary ammonium surfactants have the above formula, and are
present in the detergent compositions herein in an amount ranging from about
0.5%
to about 10%, by weight, of the total detergent composition.
Detergent ingredients - In another embodiment of the present invention, a
liquid detergent composition is provided comprising the surfactant system of
the
present invention mixed with detergent ingredients. A wide range of secondary
surfactants can be used in the detergent composition of the present invention.
The
detergent compositions according to the present invention comprise a
surfactant
system which is substantially free of linear alkylbenzene sulfonate
surfactant. A
typical listing of anionic, nonionic, ampholytic and zwitterionic classes, and
species
of these surfactants, is given in US Patent 3,664,961 issued to Norris on May
23,
1972.
When included therein, the laundry detergent compositions of the present
invention typically comprise from about 1% to about 35%, preferably from about
5%
to about 20%, by weight of secondary surfactants.
Other suitable anionic surfactants that can be used are alkyl ester sulfonate
surfactants including linear esters of Cg-C2p carboxylic acids (i.e., fatty
acids) which
are sulfonated with gaseous S03 according to "The Journal of the American Oil
Chemists Society", 52 (1975), pp. 323-329. Suitable starting materials would
include
natural fatty substances as derived from tallow, palm oil, etc.
3o The preferred alkyl ester sulfonate surfactant, especially for laundry
applications, comprise alkyl ester sulfonate surfactants of th;e structural
formula
R3 - CH(S03M) - C(O) - OR4
wherein R3 is a Cg-C20 hydrocarbyl, preferably an alkyl, o:r combination
thereof, R4
is a C1-C6 hydrocarbyl, preferably an alkyl, or combination thereof, and M is
a
CA 02233332 2001-02-07
i. 5
canon which forms a water soluble salt with the alkyl ester sulfonate.
Suitable salt-
forming canons include metals such as sodium, potassium, and lithium, and
substituted or unsubstituted ammonium canons, such as monoethanolamine,
diethanolamine, and triethanolamine. Preferably, R3 is Cl0-CI6 alkyl, and R4
is
methyl, ethyl or isopropyl. Especially preferred are the methyl ester
sulfonates
wherein R3 is C 1 p-C I 6 alkyl.
Other anionic surfactants useful for detersive purposes can also be included
in the laundry detergent compositions of the present invention. These can
include
salts (including, for example, sodium, potassium, ammonium, and substituted
ammonium salts such as mono-, di- and triethanolamine salts) of soap, Cg-C22
primary of secondary alkanesulfonates, Cg-C24 olefinsulfonates, sulfonated
polycarboxylic acids prepared by sulfonation of the pyrolyzed product of
alkaline
earth metal citrates, e.g., as described in British patent specification No.
1,082,179,
Cg-C24 alkylpolyglycolethersulfates (containing up to IO moles of ethylene
oxide);
~ 5 alkyl glycerol sulfonates, fatty acyl glycerol sulfonates, fatty oleoyl
glycerol sulfates,
alkyl phenol ethylene oxide ether sulfates, paraffin sulfonates, alkyl
phosphates,
isethionates such as the acyl isethionates, N-acyl taurates, alkyl
succinamates and
sulfosuccinates, monoesters of sulfosuccinates (especially saturated and
unsaturated
C 1 ~-C 1 g monoesters) and diesters of sulfosuccinates (especially saturated
and
20 unsaturated C6-C12 diesters), sulfates of alkylpolysaccharides such as the
sulfates of
alkylpolyglucoside (the nonionic nonsulfated compounds being described below),
and alkyl polyethoxy carboxylates such as those of the formula RO(CH2CH20)k-
CH2C00-M+ wherein R is a Cg-C22 alkyl, k is an integer from 0 to 10, and M is
a
soluble salt-forming cation. Resin acids and hydrogenated resin acids are also
25 suitable, such as rosin, hydrogenated rosin, and resin acids and
hydrogenated resin
acids present in or derived from tall oil. Further examples are described in
"Surface
Active Agents and Detergents" (Vol. I and II by Schwartz, Perry and Berch). A
variety of such surfactants are also generally disclosed in U.S. Patent
3,929,678,
issued December 30, 1975 to Laughlin, et al. at Coltunn 23, line 58 through
Column
30 29, line 23.
When included therein, the laundry detergent compositions of the present
invention typically comprise from about 1 % to about 40%, preferably from
about 5%
to about 25% by weight of such anionic surfactants.
One class of nonionic surfactants useful in the present invention are
35 condensates of ethylene oxide with a hydrophobic moiety to provide a
surfactant
having an average hydrophilic-lipophilic balance (HLB) in the range from 8 to
17,
preferably from 9.5 to 14, more preferably from 12 to I4. The hydrophobic
CA 02233332 1998-03-27
WO 97/12020 PCT/US96/15578
6
(lipophilic) moiety may be aliphatic or aromatic in nature and the length of
the
polyoxyethylene group which is condensed with any particular hydrophobic group
can be readily adjusted to yield a water-soluble compound having the desired
degree
of balance between hydrophilic and hydrophobic elements.
Especially preferred nonionic surfactants of this type are the Cg-C 15 primary
alcohol ethoxylates containing 3-12 moles of ethylene oxide per mole of
alcohol,
particularly the C 12-C 15 primary alcohols containing 5-8 moles of ethylene
oxide
per mole of alcohol.
Another class of nonionic surfactants comprises alkyl polyglucoside compounds
1 o of general formula
RO (CnH2nO)tZx
wherein Z is a moiety derived from glucose; R is a saturated hydrophobic alkyl
group that contains from 12 to 18 carbon atoms; t is from 0 to 10 and n is 2
or 3; x is
from 1.3 to 4, the compounds including less than 10% unreacted fatty alcohol
and
less than 50% short chain alkyl polyglucosides. Compounds of this type and
their
use in detergent are disclosed in EP-B 0 070 077, 0 075 995 and 0 094 118.
Very suitable as nonionic surfactants are poly hydroxy fatty acid amide
surfactants of the formula
R2 - C(O) - N(R1) - Z,
wherein R1 is H, or R1 is C1-4 hydrocarbyl, 2-hydroxy ethyl, 2-hydroxy propyl
or a
mixture thereof, R2 is CS_31 hydrocarbyl, and Z is a polyhydroxyhydrocarbyl
having
a linear hydrocarbyl chain with at least 3 hydroxyls directly connected to the
chain,
or an alkoxylated derivative thereof. Preferably, R1 is methyl, R2 is a
straight C11-
15 alkyl or alkenyl chain such as coconut alkyl or mixtures thereof, and Z is
derived
from a reducing sugar such as glucose, fructose, maltose, lactose, in a
reductive
amination reaction.
Highly preferred nonionics are amine oxide surfactants. The compositions of
the
present invention may comprise amine oxide in accordance with the general
formula I:
R1(EO)x(PO)y(BO)zN(O)(CH2R)2.qH2O (I).
In general, it can be seen that the structure (I) provides one long-chain
moiety
R1(EO)x(PO)y(BO)z and two short chain moieties, CH2R'. R' is preferably
selected
from hydrogen, methyl and -CH20H. In general R1 is a primary or branched
hydrocarbyl moiety which can be saturated or unsaturated, preferably, R1 is a
primary alkyl moiety. When x+y+z = 0, R1 is a hydrocarbyl moiety having
chainlength of from about 8 to about 18. When x+y+z is different from 0, Rl
may
CA 02233332 2001-02-07
7
be somewhat longer, having a chainlength in the ranee C l 2-C~.1. The general
formula also encompasses amine oxides wherein x+y+z = 0, R 1 = C g-C l g, R' =
H
and q = 0-2, preferably 2. These amine oxides are illustrated by C 1 ~_ 14
alkyldimethyl amine oxide, hexadecyl dimethylamine oxide. octadecylamine oxide
and their hydrates, especially the dihydrates as disclosed in U.S. Patents
5,07,501
and 5,071,594.
The invention also encompasses amine oxides wherein x+y+z is different from
zero, specifically x+y+z is from about 1 to about 10, Rl is a primary alkyl
group
containing 8 to about 24 carbons, preferably from about 12 to about 16 carbon
t o atoms; in these embodiments y + z is preferably 0 and x is preferably from
about 1
to about 6, more preferably from about 2 to about 4; EO represents
ethyleneoxy; PO
represents propyleneoxy; and BO represents butyleneoxy. Such amine oxides can
be
prepared by conventional synthetic methods, e.g., by the reaction of
alkylethoxysulfates with dimethylamine followed by oxidation of the
ethoxylated
~ 5 amine with hydrogen peroxide.
Highly preferred amine oxides herein are solids at ambient temperature, more
preferably they have melting-points in the range 30°C to 90°C.
Amine oxides
suitable for use herein are made commercially by a number of suppliers,
including
Akzo Chemie, Ethyl Corp., and Procter & Gamble. See McCutcheon's compilation
2o and Kirk-Othmer review article for alternate amine oxide manufacturers.
Preferred
commercially available amine oxides are the solid, dihydrate ADMOX~ 16 and
ADMOX 18, ADMOX 12 and especially ADMOX 14 from Ethyl Corp.
Preferred embodiments include hexadecyldimethylamine oxide dihydrate,
dodecyldimethylamine oxide dihydrate, octadecyldimethylamine oxide dihydrate,
25 hexadecyltris (ethyleneoxy)dimethyl-amine oxide, and tetradecyldimethyl-
amine
oxide dehydrate.
Whereas in certain of the preferred embodiments R' is H, there is some
latitude with respect to having R' slightly larger than H. Specifically, the
invention
further encompasses embodiments wherein R' is CH20H, such as hexadecylbis(2-
30 hydroxyethyl)amine oxide, tallowbis(2-hydroxyethyl)arnine oxide,
stearylbis(2-
hydroxyethyl)amine oxide and oleylbis(2-hydroxyethyl)amine oxide,
dodecyldimethylamine oxide dehydrate.
The compositions according to the present invention may further comprise a
builder system. Any conventional builder system is suitable for use herein
including
35 aluminosilicate materials,, silicates, polycarboxylates and fatty acids,
materials such
as ethylenediamine tetra-acetate, metal ion sequestrants such as aminopoly-
phosphonates, particularly ethylenediamine tetramethylene phosphoric acid and
CA 02233332 1998-03-27
WO 97/12020 PCT/CTS96/15578
8
diethylene triamine pentamethylene phosphonic acid. Though less preferred for
obvious environmental reasons, phosphate builders can also be used herein.
Suitable polycarboxylates builders for use herein include citric acid,
preferably in the form of a water-soluble salt, derivatives of succinic acid
of the
formula R-CH(COOH)CH2(COOH) wherein R is C 10-20 alkyl or alkenyl,
preferably C12_16~ or wherein R can be substituted with hydroxyl, sulfo
sulfoxyl or '
sulfone substituents. Specific examples include lauryl succinate, myristyl
succinate,
palmityl succinate 2-dodecenylsuccinate, 2-tetradecenyl succinate. Succinate
builders are preferably used in the form of their water-soluble salts,
including
sodium, potassium, ammonium and alkanolammonium salts.
Other suitable polycarboxylates are oxodisuccinates and mixtures of tartrate
monosuccinic and tartrate disuccinic acid such as described in US 4,663,071.
Especially for the liquid execution herein, suitable fatty acid builders for
use herein
are saturated or unsaturated C 10-18 fatty acids, as well as the corresponding
soaps.
Preferred saturated species have from 12 to 16 carbon atoms in the alkyl
chain. The
preferred unsaturated fatty acid is oleic acid. Other preferred builder system
for
liquid compositions is based on dodecenyl succinic acid and citric acid.
Detergency builders are normally included in amo~.mts of from 3% to 50% by
weight of the composition preferably from 5% to 30% and most usually from 5%
to
25% by weight.
Optional detergent ingredients - Preferred detergent compositions of the
present
invention may further comprise one or more enzymes which provide cleaning
performance and/or fabric care benefits. Said enzymes include enzymes selected
from cellulases, hemicellulases, peroxidases, proteases, gluco-amylases,
amylases,
lipases, cutinases, pectinases, xylanases, reductases, oxidases,
phenoloxidases,
lipoxygenases, ligninases, pullulanases, tannases, pentosanases, malanases, J3-
glucanases, arabinosidases or mixtures thereof.
A preferred combination is a detergent composition having a cocktail of
conventional applicable enzymes like protease, amylase, lipase, cutinase
and/or
cellulase in conjunction with the lipolytic enzyme variant D96L at a level of
from SO
LU to 8500 LU per liter wash solution.
The cellulases usable in the present invention include both bacterial or
fungal
cellulase. Preferably, they will have a pH optimum of between 5 and 9.5.
Suitable '
cellulases are disclosed in U.S. Patent 4,435,307, Barbesgoard et al, which
discloses
fungal cellulase produced from Humicola insolens. Suitable cellulases are also
'
disclosed in GB-A-2.075.028; GB-A-2.095.275 and DE-OS-2.247.832.
CA 02233332 2001-02-07
9
Examples of such cellulases are cellulases produced by a strain of Humicola
insolens (Humicola grisea var. thermoidea), particularly the Humicola strain
DSM
1800.
Other suitable cellulases are cellulases originated from Humicola insolens
having a molecular weight of about SOKDa, an isoelectric point of 5.5 and
containing
415 amino acids. Especially suitable cellulases are the cellulases having
color care
benefits. Examples of such cellulases are cellulases described in United
States patent
No. 5,520,838 issued May 26, 1996 (Novo).
Peroxidase enzymes are used in combination with oxygen sources, e.g.
1 o percarbonate, perborate, persulfate, hydrogen peroxide, etc. They are used
for
"solution bleaching", i.e. to prevent transfer of dyes or pigments removed
from
substrates during wash operations to other substrates in the wash solution.
Peroxidase enzymes are known in the art, and include, for example, horseradish
peroxidase, ligninase, and haloperoxidase such as chloro- and bromo-
peroxidase.
Peroxidase-containing detergent compositions are disclosed, for example, in
published PCT International Application WO 89/099813 and in Canadian Patent
application No. 2,122,987, filed on October 28, 1992.
Said cellulases and/or peroxidases are normally incorporated in the detergent
composition at levels from 0.0001 % to 2% of active enzyme by weight of the
2 o detergent composition.
Preferred commercially available protease enzymes include those sold under
the trademarks Alcalase, Savinase, Primase, Durazym, and Esperase by Novo
Nordisk A/S (Denmark), those sold under the trademarks Maxatase, Maxacal and
Maxapem by Gist-Brocades, those sold by Genencor International, and those sold
2 5 under the trademarks Opticlean and Optimase by Solvay Enzymes. Also
proteases
described in our co-pending application CA 2,173,105 can be included in the
detergent composition of the invention. Protease enzyme may be incorporated
into
the compositions in accordance with the invention at a level of from 0.0001%
to 2%
active enzyme by weight of the composition.
3 o A preferred protease herein referred to as "Protease D" is a carbonyl
hydrolase variant having an amino acid sequence not found in nature, which is
derived from a precursor carbonyl hydrolase by substituting a different amino
acid
for the amino acid residue at a position in said carbonyl hydrolase equivalent
to
position +76, preferably also in combination with one or more amino acid
residue
35 positions equivalent to those selected from the group consisting of +99,
+101, +103,
+104, +107, +123, +27, +105, +109, +126, +128, +135, +156, +166, +195, +197,
+204, +206, +210, +216, +217, +218, +222, +260, +265, and/or +274 according to
CA 02233332 2001-02-07
.,
the numbering of Bacillus amyloliquefaciens subtilisin, as described in the
patent of
A. Baeck et al. entitled "Protease-Containing Cleaning Compositions" U.S.
Patent
No. 5,679,630 issued October 21, 1997.
Highly preferred enzymes that can be included in the detergent compositions
5 of the present invention include lipases. It has been found that the
cleaning
performance on greasy soils is synergistically improved by using lipases.
Suitable
lipase enzymes include those produced by microorganisms of the Pseudomonas
group, such as Pseudomonas stutzeri ATCC 19.154, as disclosed in British
Patent
1,372,034. Suitable lipases include those which show a positive immunological
1 o cross-reaction with the antibody of the lipase, produced by the
microorganism
Pseudomonas fluorescens IAM 1057. This lipase is available from Amano
Pharmaceutical Co. Ltd., Nagoya, Japan, under the trade mark Lipase P "Amano,"
hereinafter referred to as "Amano-P". Further suitable lipases are lipases
such as M 1
Lipase~ and Lipomax~ (Gist-Brocades). Highly preferred lipases are the D96L
lipolytic enzyme variant of the native lipase derived from Humicola lanuginosa
as
described in US Patent No. 5,837,010. Preferably the Humicola lanuginosa
strain
DSM 4106 is used. This enzyme is incorporated into the composition in
accordance
with the invention at a level of from 50 LU to 8500 LU per liter wash
solution.
Preferably the variant D96L is present at a level of from 100 LU to 7500 LU
per liter
2 0 of wash solution. More preferably at a level of from 150 LU to 5000 LU per
liter of
wash solution.
By D96L lipolytic enzyme variant is meant the lipase variant as described in
patent application WO 92/05249 viz. wherein the native lipase ex Humicola
lanuginosa aspartic acid (D) residue at position 96 is changed to Leucine (L).
2 5 According to this nomenclature said substitution of aspartic acid to
Leucine in
position 96 is shown as : D96L.
Also suitable are cutinases [EC 3.1.1.50] which can be considered as a special
kind of lipase, namely lipases which do not require interfacial activation.
Addition
of cutinases to detergent compositions have been described in e.g. WO-A-
88/09367
3 0 (Genencor).
The lipases and/or cutinases are normally incorporated in the detergent
composition at levels from 0.0001 % to 2% of active enzyme by weight of the
detergent composition.
Amylases (& and/or (3) can be included for removal of carbohydrate-based
3 5 stains. Suitable amylases are TermamylR (Novo Nordisk), FungamylR and BANK
(Novo Nordisk).
CA 02233332 2001-02-07
'' 11
The above-mentioned enzymes may be of any suitable origin, such as
vegetable, animal, bacterial. fungal and yeast origin.
Said enzymes are normally incorporated in the detergent composition at
levels from 0.0001% to 2% of active enzyme by weight of the detergent
composition.
Other suitable detergent ingredients that can be added are enzyme oxidation
scavengers which are described in European Patent application 553607 published
August 4, 1993. Examples of such enzyme oxidation scavengers are ethoxylated
tetraethylene polyamines.
Other components used in detergent compositions may be employed, such as
soil-suspending agents, soil-release polymers, abrasives, bactericides,
tarnish
inhibitors, coloring agents, foam control agents, corrosion inhibitors and
perfumes.
Preferably, the liquid compositions according to the present invention are in
"concentrated form"; in such case, the liquid detergent compositions according
to the
~ 5 present invention will contain a lower amount of water, compared to
conventional
liquid detergents. The level of water is Iess than ~0%, preferably less than
30% by
weight of the detergent compositons.
- Said concentrated products provide advantages to the consumer, who has a
product which can be used in lower amounts and to the producer, who has lower
20 shipping costs.
The liquid compositions are especially effective when applied directly to
soils and stains in a pretreatment step before washing the fabrics.
' The detergent compositions of the present invention can also be used as
detergent additive products. Such additive products are intended to supplement
or
25 boost the performance of conventional detergent compositions.
The detergent compositions according to the present invention include
compositions which are to be used for cleaning of substrates, such as fabrics,
fibers,
hard surfaces, skin etc., for example hard surface cleaning compositions (with
or
without abrasives), laundry detergent compositions, automatic and non-
automatic
30 dishwashing compositions.
The following examples are meant to exemplify compositions of the present
inventions, but are not necessarily meant to i~mn the scope of the invention.
CA 02233332 1998-03-27
WO 97/12020 PCT/LTS96/15578
12
O
R'~OH O CH3C1 O
~N~NH~ toluene - I + ~
I (p~ ~N~N R CH3CN ~N~N~R
R=Ctt/~s 0 ~ H a ~ H
-Hz0 1 2
(Cocoylamidopropyl)dimethylamine (1}. Coconut fatty acid (150.00 g,
0.749 mol), N,N dimethyl-1,3-propanediamine (86.00 g, 0.933 mol), and p-
toluenesulfonic acid (7.12 g, 0.037 mol) are combined with toluene (300 mL) in
a I
L flask fitted with a Dean-Stark trap, condenser, and argon inlet. The mixture
is
heated to reflux for 18 h at which time the theoretical amount of water is
collected.
The mixture is concentrated by rotary evaporation, taken-up in 1500 mL of
dichloromethane and washed twice with 200 mL of 50°ro K2C03 solution.
The
organic layer is dried over MgS04, filtered, and concentrated by rotary
evaporation
to give 196.38 g (92.2%) of 1 as an off white solid. The structure is
confirmed by
1 H and 13 C NMR.
(Cocoylamidopropyl)trimethylammonium chloride (2).
GCocoamidopropyl)di-methylamine (100.00 g, 0.343 mol) and acetonitrile (100
mL)
are combined in a glass autoclave liner. The liner is placed in an autoclave
and its
contents are treated with methyl chloride gas (65 psig, 6~-85 °C) for
18 h. The
cooled mixture is removed with the addition of dichloromethane (500 mL). The
mixture is concentrated by rotary evaporation to give 109.63 g (93.5%) of 2 as
an
off white solid. The structure is confirmed by 1H and 13C NMR.
Example II
0
R'~OH O (CH30)zsOz O
~ i ~NHZ tol~ ~N~N~R CH3CN ~N~N~R
(pTSA) I H ~~ I H
R~~ ~ O (CH3S04)
-H20
3 4
(Lauroylamidopropyl)dimethylamine (3}. Lauric acid ( 140.28 g, 0.711
mol), N,N dimethyl-1,3-propanediamine (72.65 g, 0.711 mol), and p-
toluenesulfonic
acid (3.47 g, 0.018 mol) are combined with toluene (250 mL) in a 1 L flask
fitted
with a Dean-Stark trap, condenser, and argon inlet. The mixture is heated to
reflux
for 18 h at which time the theoretical amount of water is collected. The
mixture is -
washed twice with 200 mL of 50% K2C03 solution. The organic layer is dried
over
CA 02233332 1998-03-27
WO 97/12020 PCT/LTS96/I5578
13
MgS04, filtered, and concentrated by rotary evaporation to give 184.80 g
(92.4%) of
3 as a white solid. The structure is confirmed by 1H and 13C NMR.
(Lauroylamidopropyl)trimethylammonium methylsulfate (4).
(Lauroylamido-propyl)dimethylamine (184.00 g, 0.654 mol) and acetonitrile (500
mL) are combined in a round bottomed flask fitted with a condenser and argon
inlet.
The mixture is magnetically stirred until it is homogeneous. At this point,
dimethylsulfate (82.46 g, 0.654 mol) is added to the reaction mixture. The
mixture
is heated to reflux for 2 h. The cooled mixture is concentrated by rotary
evaporation
to give 259.39 g (97.3%) of 4 as a white solid. The structure is confirmed by
1 H and
13 C NMR.
CA 02233332 2001-02-07
14
EXAMPLE III
Liquid detergent compositions are made according to the following.
by weight Of the detergent cnmnncitinr,~
B C D
C 1 ~-C 1 ~ alkyl ethoxylated sulfate2 8 11 5
Quaternary Surfactant* 2 2 2 1
C 1 ~-C 14 alkyIdimethyl amine oxide- _
C 1 ~-C 15 alkyl sulfate 17 12 7 8
C I 2-C 14 N-methyl glucamide 5 4 4 3
C I ~-C I 4 fatty alcohol ethoxylate6 1 1 I
C12-Clg fatty acid I 1 4 4 3
Citric acid anhydrous l 3 3 2
Diethylenetriamine pentamethylene 1 1 1 0.5
phosphoric
acid
Monoethanolamine g
-Sodium hydroxide 1 2.5 1 1.5
Propanediol 14.~ 13.1 10.0 8
Ethanol 1.8 4.7 s.-1 1
I
Amylase (300KNU/g) 0.1 0.1 0.1 O.I
Lipase D96/L ( 100KNU/g) 0.1 0.15 0.1 0.15
S ~
Protease (35g/1) 0.5 0.5 0.5 0.5)
Endo-A (5000 CEVLJ/g) 0.05 0.05 0.05 0.5
Carezyme~ (5000 CEW/g) 0.09 0.09 0.09 0.9
Terephthalate-based polymer 0.5 - 0.3 0.3
Boric acid ?:4- 2.8 2.8 2.4
Sodium xylene sulfonate - 3 - _
DC 3225C 0.04 0.04 0.03 0.03
2-butyl-octanol I 1 1 1
Branched silicone 0.3 0.3 0.3 0.3
,
~
Water & minors ~ Up
to
100%
The quaternary surfactant are chosen from those of E.rample I or II.
The above liquid detergent compositions i A-Dl are found to be very efficient
in the removal of greasy/oily soils under various usage conditions while
having a
controlled suds profile.