Note: Descriptions are shown in the official language in which they were submitted.
CA 02233593 1998-06-02
NOVEL PROTEIN MOLECULES AND USES THEREFOR
FIELD OF THE INVENTION
The present invention relates generally to protein molecules and to
derivatives,
homologues, analogues and mimetics thereof capable of inducing or facilitating
inhibition
of blood clot formation and more particularly platelet aggregation. The
present invention
also contemplates genetic sequences encoding said protein molecules and
derivatives,
homologues, analogues and mimetics thereof. The molecules of the present
invention are
useful utter alia in a range of therapeutic and prophylactic applications.
BACKGROUND OF THE INVENTION
Platelets are essential elements involved in haemostatic events. Their role in
hemostasis
I S is distinguished by two distinct response: ( 1 ) adhesion - the
interaction of platelets with
subendothelial connective tissue and (2) aggregation - platelet to platelet
cohesion.
Abnormal platelet function may contribute to a variety of pathophysiological
conditions
including thrombosis, atherosclerosis, myocardial infarction, stroke and
pulmonary
embolism.
Inhibitors of platelet aggregation are important in the prevention and
treatment of, inter
alia, cardiovascular and cerebrovascular diseases. The use of such inhibitors
helps in the
prevention of unwanted clots which could have detrimental or debilitating
effects on
patients with high blood pressure, atherosclerosis and other related diseases.
These
inhibitors are either proteinaceous or non-proteinaceous in nature and inhibit
platelet
aggregation by a variety of mechanisms. For example, the enzyme inhibitors
such as
ADPase, tibrinogenase and phospholipase AZ and the nonenzymatic proteins such
as
disintergrins and mambin exert their inhibitory effect by various mechanisms:
These
inhibitors exert their antiplatelet actions by various mechanisms. In the case
of
nonenzymatic proteins the mechanism appears to by simpler. Mambin and
disintegrins that
contain Arg-Gly-Asp (RGD) sequence exert their action by competively blocking
,,.
b.... ,
CA 02233593 1998-06-02
_7_
fibrinogen binding to platelet glycoprotein IIb/IIIA. Ca-'+- dependent type
lectin-related
proteins exhibit the effecas on platelet agglutination and aggregation by
specifically binding
to platelet glycoprotein Ib. Among enzymes, some proteinases have been studied
for their
antiplatelet effects. The inhibitory activity of tibrinogenase was initially
thought to be due
to tibrinogen degradation, since tibri nogen is involved in the final stages
of platelet
aggregation. Subsequent studies have shown that tlbrinogen is involved in the
final stages
of platelet aggregation. Recent studies have indicated that some of the
metalloproteinases
inhibit platelet aggregation by cleaving glycoprotein Ib which is a receptor
for von-
Willebrand factor. The ADPase inhibit platelet aggregation by hydrolysis of
ADP to form
AMP, an inhibitor of platelet aggregation. Thus these enzymes physically
destroy either
ligand andlor receptor due to their inherent enzymatic activity. Accordingly,
different
inhibitors have distinct advantages and therefore new sources of anti-clotting
agents are
constantly sought.
The increasing demand for new pharmaceutical agents has led the pharmaceutical
industry
to consider molecules found in the natural environment. Accordingly, much
effort is being
spent on screening aquatic environments, riverbeds, coral) plants,
microorganisms and
higher animals for potentially useful molecules. The screening process is
often referred
to as "natural product screening".
In work leading up to the present invention, the inventors have studied snake
venom from.
the common Death Adder (Acantlcophis antarcticess) and have identified and
sequenced a
novel protein molecule capable of inhibiting platelet aggregation.
CA 02233593 1998-06-02
-3-
SUMMARY OF THE INVENTION
One aspect of the present invention provides a protein from snake venom or a
derivative,
homologue, analogue or mimetic thereof which protein is capable of inducing or
facilitating the inhibition of blood clotting.
Another aspect of the present invention provides a protein from Acattthophis
antarcticus
venom or a derivative, homologue, analogue or mimetic thereof, which protein
is capable
of inducing or facilitating the inhibition of blood clotting.
Yet another aspect of the present invention provides a protein comprising an
amino acid
sequence substantially as set forth in SEQ ID NO: I or a derivative, homolog,
analogue or
mimetic thereof or a sequence having at least 50% similarity to SEQ ID NO:I
which
protein is capable of inducing or facilitating blood clotting.
IS
A further aspect of the present invention provides a protein comprising an
amino acid
sequence substantially as set forth in SEQ ID N0:2 or a derivative, homologue,
analogue
or mimetic thereof or a sequence having at least 50% similarity to SEQ ID N0:2
which
protein is capable of inducing or facilitating blood clotting.
Yet another further aspect of the present invention provides a protein
comprising an amino
acid sequence substantially as set forth in SEQ ID N0:3 or a derivative,
homologue,
analogue or mimetic thereof or a sequence having at least 50% similarity to
SEQ ID N0:3
which protein is capable of inducing or facilitating blood clotting.
Still yet another aspect of, the present invention provides a protein
comprising an amino
acid sequence substantially as set forth in SEQ ID N0:4 or a derivative,
homologue,
analogue or mimetic thereof or a sequence having at least 50%o similarity to
SEQ ID N0:4
which protein is capable of inducing or facilitating blood clotting.
i
~ 1,
CA 02233593 1998-06-02
-4-
Still yet another further aspect of the present invention provides a protein
comprising an
amino acid sequence substantially as set forth in SEQ ID N0:4 or a derivative.
homologue, analogue or mimetic thereof or a sequence having at least 50%
similarity to
SEQ ID N0:4.
In yet another aspect of the present invention provides a protein from snake
venom having
the following characteristics:
(i) induces or facilitates inhibition of platelet aggregation;
(ii) its clotting ability is not due to its interaction between tibrinogen and
glycoproteinIIb/IIIA-complex;
(iii) its clotting ability is not due to enzymatic activity
or a derivative, homologue, analogue, or mimetic of said protein.
i 5 Another aspect of the present invention provides a peptide or a
derivative, homologue,
analogue or mimetic thereof which comprises an amino acid sequence which is at
least
50% similar to a sequence of amino acids in Acanthin and which peptide of
capable of
inducing or facilitating the inhibition of blood clotting.
c; 20 Still another aspect of the present invention provides a peptide
comprising a sequence of
amino acids from about 2 to about 50 residues having at least about 509o
similarity to a
sequence of amino acids from Acanthin wherein said peptide is capable of
inducing or
facilitating the inhibition of platelet aggregation or a derivative,
homologue, analogue or
mimetic of said peptide.
Yet another aspect of the present invention provides a peptide having the
following
characteristics:
(i) induces or facilitates inhibition of platelet aggregation;
(ii) comprises from about 2 to about 50 amino acid residues;
(iii) its clotting ability is not due to its enzymatic activity
CA 02233593 1998-06-02
1
_5_
s
or a derivative, homologue, analogue or mimetic of said peptide.
A further aspect of the present invention provides a peptide comprising the
amino acid
sequence:
GARSWLSYVN(SEQIDNO:I);
or derivatives, homologues) analogues or mimetics thereof.
Another further aspect of the present invention provides a peptide comprising
the amino
acid sequence
GPKMTLYSWEXANDVPV(SEQIDNO:S);
wherein X is cysteine or alanine or a derivative, homologue, analogue or
mimetic thereof.
Preferably, said peptide comprises the amino acid sequence
GPKMTLYSWEAANDVF'V(SEQIDN0:2).
Still yet another further aspect of the present invention provides a peptide
comprising the
amino acid sequence
APYNKNNIGIGSKTRXQ(SEQIDN0:6)
wherein X is cysteine or alanine or a derivative, homologue, analogue or
mimetic thereof.
Preferably, said peptide comprises the amino acid sequence
APYNKNNIGIGSKTRAQ(SEQ1DN0:3).
n
. ..
CA 02233593 1998-06-02
_(~_
The present invention also provides a nucleic acid molecule comprising a
sequence of
nucleotides encoding or complementary to a sequencing encoding Acanthin as
hereinbefore
defined or a derivative or homologue thereof.
Another aspect of the present invention provides a nucleic acid molecule which
encodes or
is complementary to a sequence which encodes an amino acid sequence comprising
SEQ ID
NO: l or a derivative, homologue) analogue or mimetic thereof or having at
least 50% or
greater similarity to SEQ ID NO: I or a derivative, homologue) analogue or
mimetic thereof.
Yet another aspect of the present invention provides a nucleic acid molecule
which encodes
or is complementary to a sequence which encodes an amino acid sequence
comprising SEQ
ID N0:2 or a derivative) homologue, analogue or mimetic thereof or having at
least 50% or
greater similarity to SEQ ID N0:2 or a derivative, homologue, analogue or
mimetic thereof.
I5 In yet another aspect the present invention provides a nucleic acid
molecule which encodes
or is complementary to a sequence which encodes an amino acid sequence
comprising SEQ
ID N0:3 or a derivative, homologue, analogue or mimetic thereof or having at
least 50% of
greater similarity to SEQ ID N0:3 or a derivative, homologue, analogue or
mimetic thereof.
Still yet another aspect of the present invention provides a nucleic acid
molecule which
encodes or is complementary to a sequence which encodes an amino acid sequence
comprising SEQ ID N0:4 or a derivative) homologue, analogue or mimetic thereof
or having
at least 50% or greater similarity to SEQ ID N0:4 or a derivative, homologue,
analogue or
mimetic thereof.
The present invention further providers a nucleic acid molecule comprising a
nucleotide
sequence encoding or complementary to a sequence encoding an amino acid
sequence
comprising SEQ ID NO: I or 2 or 3 or 4 or a derivative, homologue, analogue ow
mimetic
thereof capable of hybridising to said nucleic acid molecule under low
stringency conditions
at 42~C and which encodes an amino acid sequence corresponding to an amino
acid sequence
set forth in SEQ ID NO: I or 2 or 3 or 4 or a sequence having at least about
50% similarity
CA 02233593 1998-06-02
<<
_ 'J _
to SEQ ID NO: l or 2 or 3 or 4
The present invention also provides a nucleic acid molecule which encodes or
is
complementary to a sequence which encodes the peptide:
GARSWLSYVN(SEQlDN0:1);
or a derivative, homologue, analogue or mimetic thereof.
The present invention still further provides a nucleic acid molecule which
encodes or is
complementary to a sequence which encodes the peptide:
GPKMTLYSWEXANDVPV(SEQ1DN0:5);
wherein X is cysteine or alanine or a derivative, homologue, analogue or
mimetic thereof.
Preferably said peptide comprises the amino acid sequence
GPKMTLYSWEAANDVPV(SEQIDN0:2).
The present invention yet further provides a nucleic acid molecule which
encodes or is
complementary to a sequence which encodes the peptide:
APYNKNNIGIGSKTRXQ(SEQIDN0:6);
wherein X is cysteine or alanine or a derivative, homologue, analogue or
mimetic thereof.
Preferably said peptide comprises the amino acid sequence
APYNKNNIGIGSKTRAQ(~SEQIDN0:3).
Another aspect of the present invention provides the use of Acanthin in the
manufacture
of a medicament for inducing or facilitating inhibition of platelet
aggregation.
r.. -.~
CA 02233593 1998-06-02
,m .
-$_
Still another aspect of the present invention provides an agent useful for
inducing or
facilitating inhibition of platelet aggregation comprising Acanthin as
hereinbefore defined.
Even yet another aspect of the present invention provides a method of
inhibiting platelet
aggregation in a subject said method comprising administering to said subject
an anti-
clottting effective amount of Acanthin hereinbefore defined for a time and
under conditions
sufficient to inhibit platelet aggregation.
The present invention further provides a composition for use in inhibiting or
facilitating
i
inhibition of platelet aggregation comprising Acanthin as hereinbefore defined
and one or
more pharmaceutically acceptable carriers and/or diluents. The composition may
also
comprise two different types of proteins such as Acanthin and a known anti-
clotting
compound or molecule
Still another aspect of the present invention is directed to antibodies to
Acanthin and their
derivatives, homologues, analogues and mimetics. Such antibodies may be
monoclonal
or polyclonal.
Still yet another aspect of the present invention contemplates a method for
detecting
E 20 Acanthin in a biological sample from a subjeca or culture supernatant
flow or other source
said method comprising contacting said biological sample with an antibody
specific for
Acanthin or its -derivative, homologue, analogue or mimetic for a time and
under
conditions sufficient for an antibody-protein complex to form, and then
detecting said
complex.
Throughout this specification and claims which follow, unless the context
requires
otherwise) the word "comprise", or variations such as "comprises" or
"comprising", will
be understood to imply the inclusion of a stated integer or group of integers
but not the
exclusion of any other integer or group of integers.
CA 02233593 1998-06-02
,n
-9-
Single and three (otter abbreviations used throughout the specification are
detined in
Table 1.
TABLE 1
Single and three letter amino acid abbreviations
Amino Acid Three-letter One-letter
Abbreviation Symbol
Alanine Ala A
Arginine ~Arg
Asparagine Asn
Aspartfc acid Asp
Cysteine C ys C
I S Glutamine CJIn Q
Glutamic acid Glu E
Glycine GlY G
Histidine His
Isoleucine IIE: 1
Leucine Lf= L
Lysine L'.rs
Methionine Met M
Phenylalanine Phe
~
Proline I'ro P
Serine Ser S
Threonine Thr
Tryptophan T~~p
Tyrosine Tyr
Valine Val V
Any residue Xaa X
CA 02233593 1998-06-02
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 is a graphical representation of Capillary electrophoresis of
purified Acanthin.
Conditions: Capillary: 25 cm x 24 m) coated; O.1M phosphate buffer, pH 2.5;
injection:pressure loading 5 psilsec; 4 m/ run conditions: 12 kV, constant
voltage;
5 detection: UV 200 nm.
Figure 2 is a graphical representation of Electrospray ionization mass spectra
of
Acanthin.
Figure 3 is a graphical representation of the effect of Acanthin on coliagen-
and ADP-
induced platelet aggregation in human wholeblood. Each point represents the
average of
10 three measurements standard deviation. Square symbols show the effect of
acanthin on
whole blood aggregation measured using electrical impedance. Open square, ADP-
induced; Filled square, collagen-induced. Hexagon symbols show the effect of
collagen-
induced aggregation of human platelet-rich plasma. Ogen hexagon, measured by
electrical impedance; Filled hexagon, measured by turbodometric method. Filed
triangle, ADP-induced aggregation of platelet-rich plasma measured by
impedance
method.
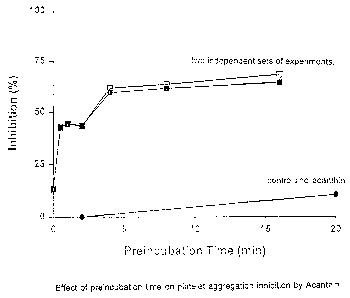
Figure 4 is a graphical representation of the effect of preincubation time on
platelet
aggregation inhibition by Acanthin. Filled circle, control (no acanthin).
Filled and open
squares show the. results of two independent sets of experiments.
Figure 5 is a schematic representation of the effect of alkylation of
histidine on the
antiplatelet effects.
CA 02233593 1998-06-02
'~,
DETAILED DESCRIPTION OF THE INVENTION
The present invention provides a protein from snake venom or a derivative,
homologue,
analogue or mimetic thereof which protein is capable of inducing or
facilitating the
inhibition of blood clotting. Said molecule is conveniently in isolated or
puritied form.
More particularly, the present invention provides a protein from Acaltthophis
antarctictts
venom or a derivative, homologue, analogue or mimetic thereof, which protein
is capable
of inducing or facilitating the inhibition of blood clotting.
In a preferred embodiment the present invention provides a protein comprising
an amino
acid sequence substantially as set forth in SEQ ID NO:I or a derivative,
homologue,
analogue or mimetic thereof or a segue~ce having at least 50% similarity to
SEQ ID NO:1
which protein is capable of inducing or facilitating blood clotting.
In another preferred embodiment the present invention provides a protein
comprising an
amino acid sequence substantially a.s set forth in SEQ ID N0:2 or a
derivative)
homologue, analogue or mimetic thereof or a sequence having at least 50%
similarity to
SEQ ID N0:2 which protein is capable of inducing or facilitating blood
clotting.
In yet another preferred embodiment the present invention provides a protein
comprising
an amino acid sequence substantially as set forth in SEQ ID N0:3 or a
derivative.
homologue, analogue or mimetic thert:of or a sequence having at least 50%
similarity to
SEQ ID N0:3 which protein is capable of inducing or facilitating blood
clotting.
Most preferably, the present invention provides a protein comprising an amino
acid
sequence substantially as set forth in SE:Q ID N0:4 or a derivative,
homologue, analogue
or mimetic thereof or a sequence having at least 50% similarity to SEQ ID N0:4
which
protein is capable of inducing or facilitating blood clotting.
CA 02233593 1998-06-02
_ 12_
The molecule according to this most preferred aspect of the present invention
is referred
to herein as "Acanthin" and is detined by the amino acid sequence set out in
SEQ ID
N0:4. Reference hereinafter to Acanthin should be read as including reference
to all other
snake venom derived proteins or peptides or encoding nucleic acid sequences
encompassed
by the present invention as well as derivatives, homologues, analogues or
mimetics
thereof.
Another aspect of the present invention provides a protein comprising an amino
acid
sequence substantially as set torch in SE;Q ID N0:4 or a derivative,
homologue, analogue
or mimetic thereof or a sequence having at least 50% similarity to SEQ ID
N0:4.
The term "similarity" as used herein includes exact identity between compared
sequences
at the nucleotide or amino acid levels. Where there is non-identity of the
nucleotide level
"similarity" includes differences between sequences which result in different
amino acids
that are nevertheless related to each other at the structural, functional,
biochemical andlor
confirmational levels. Where there is non-identity at the amino acid level,
"similarity"
includes amino acids that are nevertheless related to each other at the
structural, functional,
biochemical and/or conformational levels.
l 20 The term "blood clotting" is used in its broadest sense to include
reference to any one or
more steps of the blood clotting cascade such as, but not limited to, platelet
aggregation.
It should also be understood to refer to the aggregation of any one or more
components of
the blood such as, but not limited to, platelets. Reference to "inhibition" of
said clotting
is a reference to complete or partial prevention of blood clot formation. For
example, the
proteins of the present invention may completely prevent platelet aggregation
or they may
merely reduce the extent of platelet aggregation. Preferably said blood
clotting is platelet
aggregation. Reference hereinatter to "platelet aggregation" should be
understood as a
reference to all forms of blood clotting.
CA 02233593 1998-06-02
. " ,
- 13-
The subject of the platelet aggregation is generally an animal or bird such as
but not
limited to a human, primate. livestock animal (e.g. sheep, cow, horse, donkey,
pig),
companion animal (e.g. dog, cat), laboratory test animal (e.g. mouse, rat,
guinea pig,
rabbit, hamster), captive wild animal (e.g. deer, fox), caged bird (e.g.
parrot) and poultry
bird (e.g. chicken, duck, pheasant. goose, turkey). Preferably, the subject is
a human or
primate. Most preferably, the subjeca is a human.
Reference to a protein capable of "'inducing or facilitating" the inhibition
of platelet
aggregation should be understood as a reFerence to the inhibition of platelet
aggregation
by both direct and indirect mechanisrns. For example, said protein may
interact directly
with platelets to prevent their aggregation or, alternatively, may act
indirectly to prevent
aggregation by, for example, inhibiting one or more of the biological signals
which result
in the induction of platelet aggregation.
The term "protein" should be understood to encompass polypeptides and
proteins. The
protein may be glycosylated or unt;lycosylated and/or may contain a range of
other
molecules fused, linked, bound or otherwise associated to the protein such as
amino acids,
lipids) carbohydrates or other peptide~s, polypeptides or proteins. Reference
hereinafter
t , _ 20 to a "protein" includes a protein comprising a sequence of amino
acids as well as a protein
associated with other molecules suc:h as amino acids, lipids, carbohydrates or
other
polypeptides or proteins.
Without limiting the present invention to any one theory or mode of action,
Acanthin
exhibits the enzymatic activity of phospholipase A2. However, modifying the
active site
histidine residue to reduce by greater than 98 % the enzymatic activity of
Acanthin reveals
that phospholipid hydrolysis does not contribute to the inhibition of platelet
aggregation.
Further, unlike other known inhibitors. Acanthin does not induce platelet
aggregation via
an interaction between fibrinogen and its receptor, glycoprotein IIb-IIIA
complex, thereby
minimizing the possibility of Acanthin interfering with other adhesion
reaction.
CA 02233593 1998-06-02
- 14-
Accordingly, in another aspect the present invention provides a protein from
snake venom
having the following characteristics.
(i) induces or facilitates inhibition of platelet aggregation;
(ii) its clotting ability is not due to its interaction between tibrinogen and
glycoprotein
IIb/IIIA-complex;
(iii) its clotting ability is not due to enzymatic activity
or a derivative, homologue, analogue. or mimetic of said protein.
Prete~rably, said protein comprises an amino acid sequence substantially as
set forth in SEQ
1 D I\f O: l .
In another preferred embodiment said protein comprises an amino acid sequence
IS substantially as set forth in SEQ ID N0:2.
In yet another preferred embodiment said protein comprises an amino acid
sequence
substantially as set forth in SEQ ID N0:3.
In still yet another most preferred embodiment said protein comprises an amino
acid
sequf~nce substantially as set forth in SEQ ID N0:4.
Base~~ on the structural comparisons of Acanthin with sequences of
phospholipase A,
enzymes derived from Australian snake venoms, and using proline bracket theory
the
inventors have predicted three amino acid sequence segments which act as
interaction sites.
Accordingly, another aspect of the present invention provides a peptide or a
derivative.
homologue, analogue or mimetic thereof which comprises an amino acid sequence
which
is at least 50% similar to a sequence of amino acids in Acanthin and which
peptide is
capable of inducing or facilitating the inhibition of blood clotting.
CA 02233593 1998-06-02
-l5-
Preferably, said blood clotting is platelet aggregation.
The term "peptide" encompasses a proteinaceous molecule comprising from about
2 amino
acid residues to about 50 amino acid residues. Preferably, the peptide
comprises from
about :3 amino acid residues to about 40 amino acid residues. Even more
preferably, the
peptide comprises from about 4 amino acid residues to about 30 amino acid
residues.
Particularly preferred embodiments include peptides comprising from about 4
amino acid
residuf~s to about 20 amino acid residues such as from 4 to 10, 4 to 16, and 4
to 17 amino
acid residues.
The peptide may be gtycosylated or unglycosylated and/or may contain a range
of other
molecules fused, linked, bound or otherwise associated to the peptide such as
amino acids.
lipids, carbohydrates or other peptides, polypeptides or proteins. Reference
herein after
to a "p.~ptide" includes a peptide comprising a sequence of amino acids as
well as a peptide
I S associated with other molecules such as amino acids, lipids, carbohydrates
or other
peptidE~s) polypeptides or proteins.
In a pavrticularly preferred embodiment. there is provided a peptide
comprising a sequence
of amino acids from about 2 to about 50 residues having at least about 50%
similarity to
a sequence of amino acids from Acanthin wherein said peptide is capable of
inducing or
facilitating the inhibition of platelet aggregation or a derivative,
homologue, analogue or
mimetic of said peptide.
The percentage similarity may be greater than 60% such as at least 70% or at
least 80%
or at least 90% or higher.
Another preferred embodiment of the present invention provides a peptide
having the
followiong characteristics:
(i) induces or facilitates inhibition of platelet aggregation;
(ii) ~;:omprises from about 2 to about 50 amino acid residues;
CA 02233593 1998-06-02
._
(iii) its clotting ability is not due to its enzymatic activity
or a derivative, homologue) analogue or mimetic of said peptide.
A particularly preferred peptide of the present invention comprises the amino
acid
sequence:
GAR.SWLSYVN(SEQIDNO:1);
or derivatives, homologues, analogues or mimetics thereof.
Anoth~~r particularly preferred peptide of the present invention comprises the
amino acid
sequence
GPK.MTLYSWEXANDVPV(SEQIDNO:S);
whereiin X is cysteine or alanine or a derivative, homologue) analogue or
mimetic thereof.
Preferably, said peptide comprises the amino acid sequence
GPK:MTLYSWEAANDVPV(SEQIDN0:2).
Yet another particularly preterred peptide of the present invention comprises
the amino
acid sequence
AP~'NKNNIGIGSKTRXQ(SEQIDN0:6)
wherein X is cysteine or alanine or a derivative, homologue, analogue or
mimetic thereof.
Preferably, said peptide comprises the amino acid sequence
APB'NKNNIGIGSK'1'RAQ(SEQIDN0:3).
CA 02233593 1998-06-02
_ 17 _
The proteins and peptides of the present invention may be produced by chemical
synthetic
techniques or may be produced by recombinant DNA technology as discussed
further
below. The peptides may also be fragments of larger molecules from snake
venom. The
fragments may be naturally occurring fragments or generated by the action of
proteases.
petid;~ses) amidases, lysins or other enzymes as well as by sonic disruption,
heat, chemical
disruption and/or shearing.
Reference herein to "derivatives" includes parts, fragments and portions of
Acanthin. A
derivative also includes a single or multiple amino acid substitution,
deletion and/or
addition. Homologues include functionally, structurally or stereochemically
similar
peptides from venom from the same species of snake or from within the genus or
family
of snakes or from any other reptilian or non-reptilian species.
Preferred snakes include snakes from the family Colubridae, Elapidae,
Viperidae and
Crotalidae such as species of the genera Naja, Dertdroaspis, Bu~garrcs,
Pseccdechis,
OpJtiophagtcs and He~tachatus. Particularly preferred snakes are from the
family Elapidae
such as but not limited to King cobra (Ophiolragus hanrrah); True cobras (Naja
spp); Asian
or Indian cobra (N. naja); Egyptian cobra (N. Iraje); Spitting cobra (N.
nigeicollis); Black-
lippc~d cobra (N. melartoleuca); Cape cobra (N. rtivea); Gold's tree cobra
(Pseudohaje
r 20 gold'ii); Desert black snakes (Wnlterir~resia spp); Shield-nose snakes
(Aspidelaps spp);
Water cobras or water snakes (Bottle~gerirta spp); Black mamba (Dendroaspis
polylepis);
Mamba (D. angtcsticeps); Kraits snake (Burtgarus spp); Oriental coral snakes
(Calliophis
spp); Long-glanded coral snakes (Maticora spp); American coral snakes
(Mictcrus spp);
Southern coral snake (M. fro~talis); Eastern coral snake or Harlequin snake
(M. fulvius);
Western coral snake (Micruroides spp); Arizona coral snake (M. euryxartthus);
Death adder
(Accutthophis aatarcticus); Australian tiger snakes (Notechis spp); and
Australian
copperhead (Dertisomia spp).
CA 02233593 1998-06-02
_ 18-
Analogues and mimetics include molecules which contain non-naturally occurring
amino
acids or which do not contain amino acids but nevertheless behave functionally
the same
as the protein. Natural product screening is one useful strategy for
identifying analogues
and mimetics. Analogues of Acanthin contemplated herein include modifications
to side
chains, incorporation of unnatural amino acids and/or their derivatives during
peptide
synthesis and the use of crosslinkers and other methods which impose
conformational
constraints on the protein molecule or their analogues.
Examples of side chain modifications contemplated by the present invention
include
modifications of amino groups such as by reductive alkylation by reaction with
an aldehyde
followed by reduction with NaBH4; amidination with methylacetimidate;
acylation with
acetic anhydride; carbamoylation of amino groups with cyanate;
trinitrobenzylation of
amino groups with 2, 4, G-trinitrobenzene sulphonic acid (TNBS); acylation of
amino
I S groups with succinic anhydride and tetrahydrophthalic anhydride; and
pyridoxylation of
lysine with pyridoxal-5-phosphate followed by reduction with NaBH4.
The guanidine group of arginine residues may be modified by the formation of
heterocyclic
condensation products with reagents such as 2,3-butanedione, phenylglyoxal and
glyoxal.
The carboxyl group may be modified by carbodiimide activation via O-
acylisourea
formation followed by subsequent derivitisation, for example, to a
corresponding amide.
Sulphydryl groups may be modified by methods such as carboxymethylation with
iodoat~etic acid or iodoacetamide; performic acid oxidation to cysteic acid;
formation of
a mi Ked disulphides with other thiol compounds; reaction with maleimide,
malefic
anhydride or other substituted maleimide; formation of mercurial derivatives
using 4-
chloromercuribenzoate, 4-chloromercuriphenylsulphonic acid, phenylmercury
chloride, 2-
chloromercuri-4-nitrophenol and other mercurials; carbamoylation with cyanate
at alkaline
pH.
CA 02233593 1998-06-02
- 19-
Tryptophan residues may be moditied by, for example, oxidation with N-
bromosuccinimide or alkylation of the indole ring with 2-hydroxy-S-nitrobenzyl
bromide
or sulphenyl halides. Tyrosine residues on the other hand, may be altered by
nitration with
tetranitromethane to form a 3-nitrotyrosine derivative.
Modification of the imidazole ring of a histidine residue may be accomplished
by
alkylation with iodoacetic acid derivatives or N-carboethoxylation with
diethylpyrocarbonate.
Examples of incorporating unnatural amino acids and derivatives during protein
synthesis
include, but are not limited to, use of norleucine, 4-amino butyric acid. 4-
amino-3
hydroxy-S-phenylpentanoic acid. 6-aminohexanoic acid) t-butylglycine,
norvaline.
phenyllglycine, omithine, sarcosine. 4-amino-3-hydroxy-G-methylheptanoic acid,
2-thienyl
alanine andlor D-isomers of amino acids. A list of unnatural amino acid
contemplated
herein is shown in Table 2.
TABLE 2
Non-conventional Code Non-conventional Code
amino acid amino acid
a-aminobutyric acid Abu L-N-methylalanine Nmala
a-amino-a-methylbutyrateMgabu L-N-methylarginine Nmarg
aminocyclopropane- Cpro L-N-methylasparagine Nmasn
2S carbo;rylate L-N-methylaspartic acid Nmasp
aminoisobutyric acid Aib L-N-methylcysteine Nmcys
aminonorbornyl- Norb L-N-methylglutamine Nmgln
carbo:~ylate L-N-methylglutamic acid Nmglu
cyclohexylalanine Chexa L-N-methylhistidineNmhis
cyclopentylalanineCpen L-N-methylisolleucine Nmile
upN aumrCp(pyaal~Cweqiea-Z)-N~awwQ ayuo~y~awpCylaw-p-Q
0~
aydN aum~Cpl~zuaq-N sywQ ams~Clpnaw-p-Q
deud amuelel~Cyldeu-b nalwa auwnap~uaaw-p-Q
nqee~.ul~alel~mqlrCyaaw-~o-ourwe-NapwQ autanalosyr(ylaw-p-Q
woN aura~tl~(pdo~domwe-)-N srywa aurpilsoyl~yaaw-p-Q
~aeN aura~Cp(l~yaourwe-Z)-N upr.ua aurwelnpl~ylaw-p-Q SZ
npN aura~~~(IrC~nqomne-~)-N s~awo aura~srCyrCy;aw-~-Q
uady~ aurwelparuadl~Cy~aw-~o dsewQ aaeyedsEl~yaw-p-Q
deuey~ aurue~epuadeu-~o-lrCy~aw-pusewa auae3edse~rty~aw-~o-Q
uaday~ aurue~el~Cauadoal~Capyaaw-a~~aewo auwr~~epyaaw-p-Q
exaqay~ amuele~~CxayoprCal~Cylaw-~oelewa amuelelr~yaaw--~-Q OZ
nqe~y~ a~eir~anqomwe-~.-py~aw-~ lenQ aurlen-Q
q~eyV a~E.trCanqosroutwe-I~yaaw-pl~Cla auisoWl-Q
enN auyeniou-~ d~la ueydoad~;~~-Q
alN aumnapou-~ i4~Q amuoaay-Q
~nqawN aum~fl~lrC~nq-a-pyaaw-N-'Iaasn aur.ras-Q S t
~aamN auta~tl~lrCUaajrCy~aw-N-7oldQ aurload-4
lenwN aurlenpylaw-N-1 ayd4 autuelel~Cua4d-Q
ywN amsoirC~lrCyaaw-N-~ uioa aury~iuio-Q
dywN ueydold~~lrCylam-N-'I 7aw4 amuory~aw-4
~ylwN auruoa.rya[~C4aam-N-~ s~Cp aur:~~Cl-Q OI
~aswN auuasl~Cyaam-N-7 nap auranal-Q
o~dwN auyo~d~~Cylaw-N-~ ayQ aumnap~sr-Q
aydw N amuelepuaydl~Cyaam-N-7 svya aurptasry-Q
wowN auryliuiolrCyaaw-N-7 nI~Q pye yweanp-4
enuwN aurlenioupyaaw-N-~ ul~Q amwe~nl~-Q S
aluwN auranapoulrCy~am-N-7 s~a4 ama;srCa-Q
~awmN amuoryauyyam-N-1 dsep pre mlaedse-Q
sr(lwN amsrC~I~y~aur-N-7 .red ama'~.re-4
nalwN aumnap~y~am-N-1 leQ auiuele-Q
-OZ-
ZO-90-866l ~6S~~ZZO ~a
s~taN aura~Ct~(Irty~awoy~)-N ~nq.L auyrCt~t~Clnq-l-7 0~
i~C~qN aumrCp(~~Cuaqd~Cxo~p~t4-d)-NnqeJ pye aylnqoywe-~.
uadwN aurwett~aruadt~qaaw-N ~enwu4 auyenlrCy~aur-N-Q
deuemN auruetelrC4~deu-E~~rlaaru-Ni~C~mud aysoW~~r(yam-N-Q
~enN aura~Ct~(I~Cyapy~aw-t)-N d.nwua ue4do~d~lpq~am-N-D
iyurua auruoairhlrCyaaw-N-Q natty aum~t~(pdoidt~yau;i-Z)-N
SZ
~aswuQ auuasprhaw-N-Q apN aurarCt~(IrCdo.rdt~C~ilam-I)-N
o~dwua aurto~dpnaw-N-Q qoewN aaeW~nqosioywe~rCyuaw-N
audu~uQ aurue~Et~Cua4dt~Cqaaw-N-Qe~eN ' aum~tt~IrCr~,~aw-N
uadawN auruetep~uadota~at~ct~aw-Nuaowu4 auyau.rol~yaam-N-Q
raumrua amuorqaawt~Cyar.u-N-Q exayawN amuejetrCxayop~Cap4aaw-N
OZ
nqYSw~ a~eW~nqomwe-A,-l~Cyaw-N sr~~wuQ aursrCt~~Cylau~-N-4
d.yN aum~Cp(I~CUaa~ft~topm-~)-Nna~wuQ auranatt~r~aam-N-Q
sryN aur~~Ct~((I~ctlal~Ctozepom)-Na~iwu4 aumna~osr~rC4law-N-D
aasN aum~Ct~((t~Cya~Cxoip~y)-Nsn(wuQ aurpysrqpuaaua-N-4
mhN aumrtt~(pharCxo.rp~u-I n~~wuQ a~ewEant~~rtyaaua-N-Q
)-N S I
~aeN aura~tt~(I~Cdo~dourpiuen~-)-Nupwu4 amweant~IrC4lauu-N-Q
aqqN aumr~t~(Irtdo~dpuacldrp-~y)-Ns~C~wu4 auraas~Ca~~t4aauu-N-Q
wuqN aum~Cj~(~rCyapuarldrp-Z~Z)-NdsewuU aW iedsep4aar;u-N-4
punaN aum~Ct~paapuno~a~Ca-N usewup auae~edsepnam-N-Q
o~daN aum~Ct~~~CdoidoprCa-N ~~ewua amunlet~Cylaw-N-Q Ol
~aoaN aura~Cp~~laoo~a~Ca-N e~ewua amue~e~~C4lam-N-Q
popaN aumft~IrCaapopoatfa-N ~enwa awtent~Cylai.u-~o-4
aapaN aurarC~~~~CaapotarCa-N ~tawn aursoyprC4aam-~o-d
xayaN aum~Ct~t~xartota~Ca-N duwQ uEqdo~d~y~CUaa~w-~-Q
dayaN aura~~~pdarlop~Ca-N ylw4 amuoayt~y~a~.u-x~-Q
S
anqaN aura~t~panqota~Ca-N ~asuy auuaspiyaru-a~-D
dseN aura~t~(ptlaaw~Cxoq.rea)-No~duy auyo.rd(r(ylaur-~o-Q
n~BN aurar~t~(IrCyarCxoqiea-Z)-Nayduy aurue~epuaudpuaw-p-Q
useN aumr~p(p4lawt~weq~ea)-N uaowa auruywopuaur-~-Q
_ ~Z..
ZO-90-866l ~6S~~ZZO ~a
CA 02233593 1998-06-02
-22-
L-ethylglycine Etg penicillamine Pen
L-homophenylalanine liphe L-a-methylalanine Mala
L-a-methylarginine Marg L-a-methylasparagine Masn
L-a-methylaspartate Masp L-a-methyl-t-butylglycine Mtbug
L-a-methylcysteine Mcys L-methylethylglycine Metg
L-a-methylglutamine Mgln ~ L-a-methylglutamate Mglu
L-a-methylhistidine Mhis L-a-methylhomophenylalanineMhphe
L-a-miethylisoleucine Mile N-(2-methylthioethyl)glycineNmet
L-a-methylleucine Mleu L-a-methyllysine Mlys
L-a-rnethylmethionine Mmet L-a-methylnorleucine Mnle
L-a-n-iethylnorvaline Mnva L-a-methylornithine Morn
L-a-methylphenylalanine Mphe L-a-methylproline Mpro
L-a-methylserine Mser L-a-methylthreonine Mthr
L-a-methyltryptophan Mtrp L-a-methyltyrosine Mtyr
L-a-methylvaline Mval L-N-methylhomophenylalanineNmhphe
N-(N-(2,2-diphenylethyl) Nnbhm N-(N-(3,3-diphenylpropyl) Nnbhe
carbamylmethyl)glycine carbamylmethyl)glycine
I-cartroxy-I-(2,2-Biphenyl-Nmbc
ethylamino)cyclopropane
Crosslinkers can be used, for example, to stabilise 3 D conformations, using
homo-bifunctional
crosslinkers such as the bifunctional imido esters having (CH2)n spacer groups
with n=1 to n=6,
glutaraldehyde, N-hydroxysuccinimide esters and hetero-bifunctional reagents
which usually
contain an amino-reactive moiety such as N-hydroxysuccinimide and another
group specific-
reactive moiety such as maleimido or dithio moiety (SH) or carbodiimide
(COOH). In addition,
peptides can be conformationally constrained by, for example, incorporation of
Crc and l~[,~ -
methylamino acids, introduction of double bonds between C2 and C~ atoms of
amino acids and
the formation of cyclic peptides or analogues by introducing covalent bonds
such as forming
an amide bond between the N and C termini, between two side chains or between
a side chain
and the N or C terminus.
CA 02233593 1998-06-02
- 23 -
The present invention further contemplates chemical analogues of Acanthin
capable of acting
as antagonists or agonists of said protein. Chemical analogues may not
necessarily be derived
from Acanthin itself but may share certain conformational similarities.
Alternatively, chemical
analogues may be specifically designed to mimic certain physiochemical
properties of Acanthin.
Chemiical analogues may be chemically synthesised or may be detected
following, for example,
natural product screening.
All these types of modifications may be important to stabilise Acanthin if
administered to a
subject.
The present invention fiirther contemplates recombinant inhibitory proteins
and peptides.
Accordingly, another aspect of the present invention provides a nucleic acid
molecule
comprising a sequence of nucleotides encoding or complementary to a sequencing
encoding
Acan~rhin as hereinbefore defined or a derivative, homologue, analogue or
mimetic thereof.
A particularly useful nucleic acid molecule is one which encodes or is
complementary to a
sequence which encodes an amino acid sequence comprising SEQ ID NO:1 or a
derivative,
homologue, analogue or mimetic thereof or having at least 50% or greater
similarity to SEQ
ID NO:1 or a derivative, homologue) analogue or mimetic thereof.
Another particularly useful nucleic acid molecule is one which encodes or is
complementary to
a sequence which~encodes an amino acid sequence comprising SEQ ID N0:2 or a
derivative)
homologue, analogue or mimetic thereof or having at least 50% or greater
similarity to SEQ
ID N0:2 or a derivative, homologue. analogue or mimetic thereof.
Yet another particularly useful nucleic acid molecule is one which encodes or
is complementary
to a sequence which encodes an amino acid sequence comprising SEQ ID N0:3 or a
derivative)
homologue, analogue or mimetic thereof or having at least 50% of greater
similarity to SEQ
ID N0:3 or a derivative, homologue, analogue or mimetic thereof.
CA 02233593 1998-06-02
-24-
A most preferable nucleic acid molecule is one which encodes or is
complementary to a
sequence which encodes an amino acid sequence comprising SEQ ID N0:4 or a
derivative,
homologue, analogue or mimetic thereof or having at least 50% or greater
similarity to SEQ
ID N0:4 or a derivative, homologue) analogue or mimetic thereof.
Anotlher aspect of the present invention provides a nucleic acid molecule
comprising a
nucleotide sequence encoding or complementary to a sequence encoding an amino
acid
sequence comprising SEQ ID NO:1 or 2 or 3 or 4 or a derivative, homologue,
analogue or
mimetic thereof capable of hybridising to said nucleic acid molecule under low
stringency
conditions at 42~C and which encodes an amino acid sequence corresponding to
an amino
acid :sequence set forth in SEQ ID NO:1 or 2 or 3 or 4 or a sequence having at
least about
50% similarity to SEQ ID NO: l or 2 or 3 or 4.
A particularly useful nucleic acid molecule is one which encodes or is
complementary to a
sequE~nce which encodes the peptide:
GARSWLSYVN(SEQIDNO:1);
or a .derivative, homologue, analogue or mimetic thereof.
Another particularly useful nucleic acid molecule is one which encodes or is
complementary
to a ;sequence which encodes the peptide:
GPKMTLYSWEXANDVPV(SEQIDNO:S);
wherein X is cysteine or alanine or a derivative, homologue, analogue or
mimetic thereof.
Preferably said peptide comprises the amino acid sequence
GPKMTLYSWEAANDVI'V(SEQIDN0:2).
CA 02233593 1998-06-02
-25-
Yet another particularly usetul nucleic acid molecule is one which encodes or
is
complementary to a sequence which encodes the peptide:
APYNKNNIGIGSKTRXQ(SEQIDN0:6);
wherein X is cysteine or alanine or a derivative, homologue) analogue or
mimetic thereof.
Prefi~rably said peptide comprises the amino acid sequence
APYNKNNIGIGSKTRAQ(SEQIDN0:3).
l0
The nucleic acid molecule of the present invention is generally in isolated
form. It may also
comprise additional nucleotide sequence information fused, linked or otherwise
associated
with it either at the 3' or 5' terminal portions or at both the 3' and 5'
terminal portions. The
nucleic acid molecule may also be part of a vector) such as an expression
vector. The latter
I S embodiment facilitates production of recombinant forms of Acanthin which
forms are
encompassed by the present invention.
Reference herein to a low stringency at 42~C includes and encompasses from at
least about I%
v/v t o at least about I 5% v/v fonnamide and from at least about 1 M to at
least about 2M salt
20 for hybridisation, and at least about 1 M to at least about 2M salt for
washing conditions.
Alternative stringency conditions may be applied where necessary, such as
medium stringency,
which includes sand encompasses from at least about 16% v/v to at least about
30% vlv
fornnamide and from at least about 0.5M tv at least about 0.9M salt for
hybridisation, and at
least about 0.5M to at least about 0.9M salt for washing conditions, or high
stringency, which
25 includes and encompasses from at least about 31 % v/v to at least about 50%
v/v formamide and
frorn at least about 0.01 M to at least about 0. I 5 M salt for hybridisation,
and at least about
0.01M to at least about 0. 15M salt for washing conditions.
The present invention further encompasses host cells for the subject nucleic
acid molecules
30 and which are used to produce recombinant Acanthin. The host cells may be
prokaryotic
cells or eukaryotic cells. Examples of prokaryotic cells include E. toll,
Bacillres sp,
CA 02233593 1998-06-02
--2G-
Psertdontortns sp amongst many others. Examples of eukaryotic cells include
mammalian
cell lines (eg. CHO cells), yeast cells, fungal cells, insect cells, plant
cells and reptilian cell
lines. The ability to produce recombinant Acanthin of the present invention
permits the
large scale production of vast qualities of Acanthin for commercial uses. As
stated above,
the Acanthin may need to be produced as part of a large peptide) polypeptide
or protein
which may be used as is or may tirst need to be processed in order to remove
the extraneous
proteinaceous sequences. Such processing includes digestion with proteases,
peptidases and
amidases or a range of chemical, electrochemical, sonic or mechanical
disruption techniques.
Notwithstanding that the present invention encompasses recombinant proteins
and peptides.
chemically synthetic techniques are particularly preferred in the synthesis of
Acanthin.
Acanthin according to the present invention is conveniently synthesized based
on molecules
isolatetj from snake venom. Isolation of the venom molecules may be
accomplished by any
suitab(~~ means such as by chromatographic separation) for example using CM-
ceiluiose ion
exchange chromatography followed by Sephadex (eg. G-50 column) filtration.
Many other
technidues are available including HPLC. PAGE amongst others. Once purified)
the venom
derived molecule can be partially sequenced andlor fragments produced and used
directly
as a source of Acanthin or as a template for amino acid synthesis.
Acanthin may be synthesized by solid phase synthesis using F-moc chemistry as
described
by Carpino et al, ( 1991 ). Acanthin and fragments thereof may also be
synthesized by
alternative chemistries including, but not limited to) t-Boc chemistry as
described in Stewart
et al (1985) or by either classical methods of liquid phase peptide synthesis.
The present invention further extends to the use of Acanthin in the
manufacture of a
medicament for inducing or facilitating inhibition of platelet aggregation.
CA 02233593 1998-06-02
-27-
Yet another aspect of the present invention provides an agent useful for
inducing or
facilitating inhibition of platelet aggregation comprising Acanthin as
hereinbefore defined.
As st;3ted above the Acanthin of the present invention is useful in preventing
platelet
aggrel;ation in a subject such as a mammal (e.g. a human).
Accordingly, another aspect of the present invention contemplates a method of
inhibiting
platelet aggregation in a subject said method comprising administering to said
subject an
anti-aggregation effective amount of Acanthin hereinbefore defined for a time
and under
conditions sufticient to inhibit platelet aggregation.
In accordance with this method, more than one type of protein or peptide may
be
administered for e.g. the Acanthin may be coadministered with a known anti-
clotting
compound or molecule. By "coadministered" is meant simultaneous administration
in the
I S same formulation or in two different formulations via the same or
different routes or
sequential administration by the same or different routes. By "sequential"
administration is
meant a time difference of from seconds, minutes, hours or days between the
administration
of the two types of proteins or the protein and the known anti-clotting
compound or
molecule. The Acanthin or the Acanthin and known anti-clotting compound or
molecule
may b~e administered in any order.
Routes of administration include but are not limited to intravenously,
intraperitoneal,
subcutaneously, intracranial, intradermal, intramuscular, intraocular)
intrathecal,
intrace~rebrally, intranasally, infusion, orally, rectally, via iv drip, patch
and implant.
Intravenous routes are particularly preferred.
Another aspect of the present invention provides a composition for use in
inhibiting or
facilitating inhibition of platelet aggregation comprising Acanthin as
hereinbefore detined
and one or more pharmaceutically acceptable carriers and/or diluents. The
composition may
also comprise two different types of proteins or peptides such as Acanthin and
a known anti-
CA 02233593 1998-06-02
-28-
clotting compound or molecule.
Compositions suitable for injectable use include sterile aqueous solutions
(where water soluble)
and ,terile powders for the extemporaneous preparation of sterile injectable
solutions. They
must be stable under the conditions of manufacture and storage and must be
preserved against
the c;ontaminating action of microorganisms such as bacteria and fungi. The
carrier can be a
solvent or dispersion medium containing, for example, water, ethanol, polyol
(for example,
glycerol, propylene glycol and liquid polyethylene glycol) and the like))
suitable mixtures thereof
and vegetable oils. The preventions of the action of microorganisms can be
brought about by
'. 10 various antibacterial and antifungal agents, for example) parabens,
chlorobutanol, phenol, sorbic
acid, thirmerosal and the like. In many cases) it will be preferable to
include isotonic agents,
for example, sugars or sodium chloride. Prolonged absorption of the injectable
compositions
can he brought about by the use in the compositions of agents delaying
absorption, for example,
aluminum monostearate and gelatin.
Sterile injectable solutions are prepared by incorporating the active
compounds in the required
amount in the appropriate solvent with various of the other ingredients
enumerated above, as
required, followed by, for example, filter sterilization or sterilization by
other appropriate
means. Dispersions are also contemplated and these may be prepared by
incorporating the
various sterilized active ingredients into a sterile vehicle which contains
the basic dispersion
medium and the required other ingredients from those enumerated above. In the
case of sterile
powders for the preparation of sterile injectable solutions, a preferred
method of preparation
includes vacuum drying and the freeze-drying technique which yield a powder of
the active
ingredient plus any additional desired ingredient from a previously sterile-
filtered solution.
When the active ingredients are suitably protected, they may be orally
administered, for
example, with an inert diluent or with an assimilable edible carrier, or it
may be enclosed in hard
or ~~oft shell gelatin capsule, or it may be compressed into tablets. For oral
Therapeutic
administration, the active compound may be incorporated with excipients and
used in the form
of ingestible tablets, buccal tablets. troches) capsules, elixirs)
suspensions, syrups, wafers, and
the like. Such compositions and preparations should contain at least I % by
weight of active
CA 02233593 1998-06-02
-29-
compound. The percentage of the compositions and preparations may, of course,
be varied and
may conveniently be between about 5 to about 80% of the weight of the unit.
The amount of~
active compound in such therapeutically useful compositions in such that a
suitable dosage will
be obvtained. Preferred compositions or preparations according to the present
invention are
prepared so that an oral dosage unit form contains between about 0.1 ng and
2000 mg of active
compound.
The tablets, troches, pills) capsules and the like may also contain the
components as listed
hereafter. A binder such as gum, acacia, corn starch or gelatin; excipients
such as dicalcium
phosphate; a disintegrating agent such as corn starch, potato starch, alginic
acid and the like;
a lubricant such as magnesium stearate; and a sweetening agent such a sucrose,
lactose or
saccharin may be added or a flavouring agent such as peppermint) oil of
wintergreen, or cherry
flavouring. When the dosage unit form is a capsule, it may contain, in
addition to materials of
the above type, a liquid carrier. Various other materials may be present as
coatings or to
othen~rise modify the physical forth of the dosage unit. For instance,
tablets, pills, or capsules
may be coated with shellac, sugar or both. A syrup or elixir may contain the
active compound)
sucrose as a sweetening agent, methyl and propylparabens as preservatives, a
dye and
flavouring such as cherry or orange flavour. Any material used in preparing
any dosage unit
form should be pharmaceutically pure and substantially non-toxic in the
amounts employed.
In addition, the active compounds) may be incorporated into sustained-release
preparations
and fi~rmutations.
The present invention also extends to forms suitable for topical application
such as creams,
lotions and gels. In such forms, the anti-clotting proteins may need to be
modified to permit
penetration of the surface barrier.
Pharmaceutically acceptable carriers and/or diluents include any and a11
solvents) dispersion
media) coatings, antibacterial and antifirngal agents, isotonic and absorption
delaying agents and
the like. The use of such media and agents for pharmaceutically active
substances is well
known in the art. Except insofar as any conventional media or agent is
incompatible with the
active ingredient, use thereof in the therapeutic compositions is
contemplated. Supplementary
CA 02233593 1998-06-02
-30-
active ingredients can also be incorporated into the compositions.
It is especially advantageous to formulate parenteral compositions in dosage
unit form for ease
of administration and uniformity of dosage. Dosage unit form as used herein
refers to physically
discrete units suited as unitary dosages for the mammalian subjects to be
treated; each unit
containing a predetermined quantity of active material calculated to produce
the desired
therapeutic effect in association with the required pharmaceutical carrier.
The specification for
the novel dosage unit forms of the invention are dictated by and directly
dependent on (a) the
unique characteristics of the active material and the particular therapeutic
effect to be achieved
'. 10 and (b) the limitations inherent in the art of compounding such an
active material for inducing
or facilitating inhibition of blood clotting in living subjects.
Effective amounts of anti-clotting proteins contemplated by the present
invention will vary
depending on the severity of the pain and the health and age of the recipient.
In general terms,
1 S effective amounts may vary from 0.0l ng/kg body weight to about 100 mg/kg
body weight.
Alternative amounts include for about 0.1 ng/kg body weight about 100 mg/kg
body weight or
from 1.0 ng/kg body weight to about 80 mg/kg body weight.
Still another aspect of the present invention is directed to antibodies to
Acanthin and their
20 derivatives, homologues, analogues and mimetics. Such antibodies may be
monoclonal or
polyclonal.
In the case of small peptides) these may first need to be associated with a
carrier molecule.
25 The antibodies of the present invention are particularly useful as
therapeutic or diagnostic
agents. For example) specific antibodies can be used to screen for Acanthin
using
immunoassays or used as antagonists to inhibit Acanthin activity under certain
circumstances
such as where temporary clotting inhibition is only required. Techniques for
such
immunoassays are well known in the alt and include, for example, sandwich
assays and ELISA.
30 Knowledge of Acanthin levels may be important for monitoring certain
therapeutic protocols.
CA 02233593 1998-06-02
_31 _
Antibodies to the Acanthin (or its derivatives, homologues, analogues or
mimetics) of the
present invention may be monoclonal or polyclonal. Alternatively, fragments of
antibodies may
be used such as Fab fragments. Furthermore, the present invention extends to
recombinant and
synthetic antibodies and to antibody hybrids. A "synthetic antibody" is
considered herein to
include fragments and hybrids of antibodies.
As stated above, specific antibodies can be used to screen for the Acanthin.
The latter would
be important, for example) as a means for screening for levels of Acanthin in
a cell extract or
other biological fluid or purifying Acanthin made by recombinant means from
culture
supernatant fluid.
It is within the scope of this invention to include any second antibodies
(monoclonal, polyclonal
or fragments of antibodies or synthetic antibodies) directed to the first
mentioned antibodies
discussed above. Both the first and second antibodies may be used in detection
assays or a first
antibody may be used with a commercially available anti-immunoglobulin
antibody. An
antibody as contemplated herein includes any antibody specific to any region
of Acanthin.
Both polyclonal and monoclonal antibodies are obtainable by immunization with
Acanthin and
either type is utilizable for immunoassays. The methods of obtaining both
types of sera are well
known in the art. Polyclonal sera are less preferred but are relatively easily
prepared by
injection of a suitable laboratory animal with an effective amount of
Acanthin) or antigenic parts
thereof) collecting serum from the animal and isolating specific sera by any
of the known
immunoadsorbent~techniques. Although antibodies produced by this method are
utilizable in
virtually any type of immunoassay, they are generally less favoured because of
the potential
heterogeneity of the product.
The use of monoclonal antibodies in an immunoassay is particularly preferred
because of the
ability to produce them in large quantities and the homogeneity of the
product. The preparation
of hyt~ridoma cell lines for monoclonal antibody production derived by fusing
an immortal cell
line a~~d lymphocytes sensitized against the immunogenic preparation can be
done by techniques
which are well known to those who are skilled in the art.
CA 02233593 1998-06-02
-32-
Another aspect of the present invention contemplates a method for detecting
Acanthin in a
biological sample from a subject or culture supernatant flow or other source
said method
comprising contacting said biological sample with an antibody specific for
Acanthin or its
derivative, homologue, analogue or mimetic for a time and under conditions
sufFcient for an
antibody-protein complex to form, and then detecting said complex.
The presence of Acanthin may be accomplished in a number of ways such as by
Western
blotting and ELISA procedures. A wide range of immunoassay techniques are
available as can
be seen by reference to US Patent Nos. 4,0I6,043, 4, 424,279 and 4,018,653.
These, of
cour~~e) include both single-site and two-site or "sandwich" assays of the non-
competitive types)
as well as in the traditional competitive binding assays. These assays also
include direct binding
of a labelled antibody to a target.
Sandwich assays are among the most nsefi~l and commonly used assays and are
favoured for
use in the present invention. A number of variations of the sandwich assay
technique exist, and
all are intended to be encompassed by the present invention. Briefly, in a
typical forward assay,
an unlabelled antibody is immobilized on a solid substrate and the sample to
be tested brought
into contact with the bound molecule. After a suitable period of incubation,
for a period of time
sufficient to allow formation of an antibody-antigen complex, a second
antibody specific to the
1 - , 20 antigen, labelled with a reporter molecule capable of producing a
detectable signal is then added
and incubated, allowing time sufficient for the formation of another complex
of antibody-
antigen-labelled antibody. Any unreacted material is washed away, and the
presence of the
antigen is determined by observation of a signal produced by the reporter
molecule. The results
may either be qualitative, by simple observation of the visible signal, or may
be quantitated by
comparing with a control sample containing known amounts of hapten. Variations
on the
forward assay include a simultaneous assay, in which both sample and labelled
antibody are
added simultaneously to the bound antibody. These techniques are well known to
those skilled
in thc: art, including any minor variations as will be readily apparent. In
accordance with the
present invention the sample is one which might contain a peptide which
inhibits blood clotting
including cell extract, culture supernatant tissue biopsy, serum, saliva)
mucosal secretions,
lymph, tissue fluid and respiratory fluid. The sample is, therefore, generally
a biological sample
CA 02233593 1998-06-02
-33-
comprising biological fluid but also extends to fermentation tluid and
supernatant fluid such as
from a cell culture.
In the typical forward sandwich assay, a first antibody having specificity for
the protein or
antigenic parts thereof is either covalently or passively bound to a solid
surface. The solid
surface is typically glass or a polymer, the most commonly used polymers being
cellulose,
polyacrylamide, nylon, polystyrene) polyvinyl chloride or polypropylene. The
solid supports
may be in the form of tubes, beads, discs of microplates, or any other surface
suitable for
conducting an immunoassay. The binding processes are well-known in the art and
generally
t 0 consist of cross-linkin covalent) bindin or h sicall adsorbin the of mer-
antibod
g Y g P Y Y g, P Y Y
complex is washed in preparation for the test sample. An aliquot of the sample
to be tested is
then added to the solid phase complex and incubated for a period of time
sufficient (e.g. 2-40
minutes or overnight if more convenient) and under suitable conditions (e.g.
from room
temperature to about 37~C) to allow binding of any subunit present in the
antibody. Following
the incubation period, the antibody subunit solid phase is washed and dried
and incubated with
a second antibody specific for a portion of the hapten. The second antibody is
linked to a
reporter molecule which is used to indicate the binding of the second antibody
to the hapten.
An alternative method involves immobilizing the target molecules in the
biological sample and
there exposing the immobilized target to specific antibody which may or may
not be labelled
with a reporter molecule. Depending on the amount of target and the strength
of the reporter
molecule signal, a bound target may be detectable by direct labelling with the
antibody.
Alternatively, a second labelled antibody, specific to the first antibody is
exposed to the target-
first antibody complex to form a target-first antibody-second antibody
tertiary complex. The
complex is detected by the signal emitted by the reporter molecule.
By "reporter molecule" as used in the present specification, is meant a
molecule which, by its
chemical nature, provides an analytically identifiable signal which allows the
detection of
antigen-bound antibody. Detection may be either qualitative or quantitative.
The most
commonly used reporter molecules in this type of assay are either enzymes,
fluorophores or
radionuclide containing molecules (i.e. radioisotopes) and chemiluminescent
molecules.
CA 02233593 1998-06-02
-34-
In thc: case of an enzyme immunoassay, an enzyme is conjugated to the second
antibody,
generally by means of glutaraldehyde or periodate. As will be readily
recognized, however) a
wide variety of different conjugation techniques exist, which are readily
available to the skilled
artisan. Commonly used enzymes include horseradish peroxidase, luciferase
glucose oxidase)
beta-galactosidase and alkaline phosphatase, amongst others. The substrates to
be used with
the specific enzymes are generally chosen for the production, upon hydrolysis
by the
corre;~ponding enzyme, of a detectable colour change. Examples of suitable
enzymes include
alkaline phosphatase and peroxidase. It is also possible to employ fluorogenic
substrates which
yield a fluorescent product rather than the chromogenic substrates noted
above. In all cases,
the enzyme-labelled antibody is added to the first antibody-peptide complex)
allowed to bind)
and then the excess reagent is washed away. A solution containing the
appropriate substrate
is them added to the complex of antibody-antigen-antibody. The substrate will
react with the
enzyme linked to the second antibody. giving a qualitative visual signal,
which may be further
quantitated, usually spectrophotometrically, to give an indication of the
amount of hapten which
I S was present in the sample. "Reporter molecule" also extends to use of cell
agglutination or
inhibition of agglutination such as red blood cells on latex beads, and the
like.
Alternately) fluorescent compounds) such as fluorescein and rhodamine, may be
chemically
coupled to antibodies without altering their binding capacity. When activated
by illumination
with light of a particular wavelength, the fluorochrome-labelled antibody
adsorbs the light
enerl;y, inducing a state to excitability in the molecule, followed by
emission of the light at a
characteristic colour visually detectable with a light microscope. As in the
EIA, the fluorescent
labelled antibody is allowed to bind to the first antibody-hapten complex.
After washing off the
unbound reagent) the remaining tertiary complex is then exposed to the light
of the appropriate
wavelength the fluorescence observed indicates the presence of the hapten of
interest.
Immunofluorescene and EIA techniques are both very well established in the art
and are
particularly preferred for the present method. However, other reporter
molecules, such as
radioisotope, chemiluminescent or bioluminescent molecules, may also be
employed.
CA 02233593 1998-06-02
-35-
The present invention is further described by the following nonlimiting
examples.
EXAMPLE 1
PURIFICATION AND SEQC1ENCING OF ACANTHIN FROM VENOM
The inventors used a three-step chromatographic method to purify the
antiplatelet protein
from Acanthophis antarciterrs venom. The steps included gel filtration on Bio-
Gel P-30
column, ration exchange chromatography on a Poros HS column and reversed-phase
chromatography on a Ashahipak ODP 50 column. The protein was adjudged
homogeneous
based on capillary electrophoresis and elecarospray ionization mass
spectrometry (Fig. 1 and
2). This protein was named acanthin. The same protein can be purified by
alternative
metlhods using either different strategies or alternative columns which are
available. This
protein can also be produced by recombinant technology.
The amino acid sequence of Acanthin was determined by Edman degredation of
native and
pyridythylated protein and the peptides generated from the protein.
EXAMPLE 2
PEPTII?E IDENTIFICATION
Based on the structural comparisons with sequences of other phospholyypase A,
enzymes
from Australian snake venoms and using proline bracket theory, peptide
sequences were
predicted as potential interaction sites. These peptides were synthesized and
tested for their
anti-platelet effects.
CA 02233593 1998-06-02
-3G-
EXAMPLE 3
PEPTIDE FUNCTIONAL ASSAYS
Anti-platelet aggregation was tested in an assay which measured inhibition of
platelet
aggregation, in whole human blood. and induced by collagen. Peptide activity
was also
measured in whole human blood assays in which aggregation was induced by ~A
DP.
Binding competition between peptide and protein is examined in addition to the
structure-
activity relationships of active peptides.
EXAMPLE 4
INHIBITION OF PLATELET AGGREGATION
The protein Acanthin inhibits platelet aggregation in a dose-dependent manner
(Fig. 3). The
ICS" values (concentration of the inhibitor required to inhibit 50% of
aggregation) of ADP- and
collagen-induced platelet aggregation in human whole blood are 7 nM and 4nM,
respectively.
Thus acanthin is one of the most potent inhibitors of platelet aggregation.
E~CAMPLE 5
! 20 EFFECT OF ACANTHIN ENZYMATIC ACTIVITY ON ITS ANTI-CLOTTING
PROPERTIES
Acanthin exhibits phosphoiipase Az activity. Increasing the times of
incubation of acanthin with
the platelets does not increase the potency of inhibition (Fig. 4). Longer
incubation times
should result in increased hydrolysis of phospholipids or increased
accumulation of hydrolysis
products. If phospholipid hydrolysis is important for the antiplatelet
activity, then there will be
an increase in the antiplatelet effects when the incubation times are
increased. Results shown
in Fig. 4 indicates that phospholipid hydrolysis does not contribute to the
inhibition of platelet
aggregation. To further support this. the inventors modified the active site
Histidine residue
by p-bromophenacyibromide and tested the modified acanthin for its enzymatic
and antiplatelet
effects. Histidine-alkylated protein lost more than 98% of its enzymatic
activity, but it retains
CA 02233593 1998-06-02
_37_
significant amounts of its inhibitory ef~'ect on platelet aggregation (Fig.
5).
Those skilled in the art will appreciate that the invention described herein
is susceptible to
variations and moditications other than those specifically described. It is to
be understood
that the invention includes all such variations and moditications. The
invention also includes
all of the steps, features, compositions and compounds referred to or
indicated in this
spec:itication, individually or collectively, and any and a11 combinations of
any two or more
of said steps or features.
CA 02233593 1998-07-14
- 38 -
SEQUENCE LISTING
(1) GENERAL INFORMATION:
(i) APPLICANT: NATIONAL UNIVERSITY OF SINGAPORE
(11) TITLE OF INVENTION: NOVEL PROTEIN MOLECULES AND USES
THEREFOR
(iii) NUMBER OF SEQUENCES: 6
(iv) CORRESPONDENCE ADDRESS:
(A) ADDRESSEE: Fetherstonhaugh & Co
(B) STREET: P.O.Box 2999, Station D
(C) CITY: Ottawa
(D) PROVINCE . Ontario
(E) COUNTRY: Canada
(F) POSTAL CODE K1P 5Y6
(v) COMPUTER READABLE FORM:
(A) MEDIUM TYPE: Floppy disk
(B) COMPUTER: IBM PC compatible
(C) OPERATING SYSTEM: PC-DOS/MS-DOS
(D) SOFTWARE: PatentIn Release #1.0, Version #1.25
(vi) CURRENT APPLICATION DATA:
(A) APPLICATION NUMBER:
(B) FILING DATE: 2 June 1998
(C) CLASSIFICATION:
(vii) PRIOR APPLICATION DATA:
(A) APPLICATION NUMBER: Singapore 9800055-7
(B) FILING DATE: 6 January 1998
(viii) ATTORNEY/AGENT INFORMATION:
(A) NAME: Fetherstonhaugh & Co
(C) REFERENCE/DOCKET NUMBER: 29588-1
(ix) TELECOMMUNICATION INFORMATION:
(A) TELEPHONE: 613-235-4373
(B) TELEFAX: 613-232-8440
CA 02233593 1998-06-02
-39-
(Z) INFORMATION FOR SEQ ID NO:1:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 10 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID N0:1:
Gly Ala Arg Ser Trp Leu Ser Tyr Val Asn
10
(2) INFORMATION FOR SEQ ID N0:2:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 17 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID N0:2:
Gly Pro Lys Met Thr Leu Tyr Ser Trp Glu Ala AIa Asn Asp Val
5 10 15
Pro Val
c (2) INFORMATION FOR SEQ ID N0:3:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 17 amino acids
(BT TYPE: amino acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID N0:3:
Ala Pro Tyr Asn Lys Asn Asn Ile Gly Ile Gly Ser Lys Thr Arg
5 10 15
Ala Gln
CA 02233593 1998-06-02
-40-
(2) INFORMATION FOR SEQ ID N0:4:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 118 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID N0:4:
Asn Leu Tyr Gln Phe Gly Gly Met Ile Gln Cys Ala Asn Lys Gly
10 15
Ala Arg Ser Trp Leu Ser Tyr Val Asn Tyr GLy Cys Tyr Cys Gly
' 20 25 30
Trp Gly Gly Ser Gly Lys Pro Va:l Asp Glu Leu Asp Arg Cys Cys
35 40 45
Gln Ile His Asp Asn Cys Tyr Gly Glu Ala Glu Lys Lys Arg Cys
50 55 60
Gly Pro Lys Met Thr Leu Tyr Ser Trp Glu Cys AIa Asn Asp Val
65 70 75
Pro Val Cys Asn Ser Lys Ser AIa Cys Glu Gly Phe Val Cys Asp
80 85 90
Cys Asp AIa Ala Ala Ala Lys Cys Phe A1a Lys Ala Pro Tyr Asn
95 100 10S
Lys Asn Asn Ile Gly Ile Gly Ser Lys Thr Arg Cys Gln
110 11S
(2) INFORMATION FOR SEQ ID N0:5:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 17 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID N0:5:
Gly Pro Lys Met Thr Leu Tyr Ser Trp Glu Xaa Ala Asn Asp Val
S 10 15
Pro Val
CA 02233593 1998-06-02
l_
(2) INFORMATION FOR SEQ ID N0:6:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 17 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID N0:6:
Ala Pro Tyr Asn Lys Asn Asn Ile Gly Ile Gly Ser Lys Thr Arg
10 15
Xaa Gln
i