Note: Descriptions are shown in the official language in which they were submitted.
CA 02233724 1998-03-31
-1-
The present invention relates to a method for the prediction and diagnosis of
cardiac allograft rejection.
BACKGROUND OF THE INVENTION
The cardiac natriuretic peptides (NP) atrial natriuretic factor (ANF) and
brain
natriuretic peptide (BNP) are polypeptide hormones synthesized, stored and
released by
cardiac muscle cells (cardiocytes). In many ways, the endocrine heart is a
modulator of
systems such as the sympathetic nervous system, the renin-angiotensin-
aldosterone
system and other determinants of vascular tone, extracellular fluid volume and
renal
function.
ANF and BNP are synthesized by cardiocytes as preprohormones that are
enzymatically processed to yield prohormones and, ultimately, hormones that
are
released into the circulation. In humans, the prohormone proANF is a
polypeptide that
contains 126 amino acids (ANF1_126) fat is processed to ANF ,_98and ANF 99-126
The latter
being the biologically active portion. Human proBNP, on the other hand, is 108
amino
acids long and it is processed to BNPI_,6 and BNP.,.,_~08. The latter being
the biologically
active peptide. Both the C-terminal and N-terminal portions of NPs circulate
in blood.
For example, Hunt et al (1995, Biochem Biophys Res Comm 214:1175-1183) report
the
detection of hBNP and N-terminal ProBNP within human plasma, and note an
increase
in the plasma levels of both peptides with congestive heart failure.
Under certain pathophysiological conditions affecting the cardiovascular
system,
synthesis and release of both ANF and BNP are significantly augmented in both
atrial
and ventricular cardiocytes. Increased production of ANF and BNP by the
mammalian
ventricle is a hallmark of cardiac hypertrophy and failure (Sadoshima J. et al
Am J
Hypertens 1995;8:301-310). Further, it is now known that measurement of the
circulating levels of different fragments of these hormones in plasma is a
powerful means
to identify elderly subjects at risk of heart failure (Davis KM. et al. JAMA
1992;267:2625-2629), establish long term prognosis after (myocardial
infarction) MI
CA 02233724 2003-02-13
-2-
(Hall C. et al J Am Coll Cardiol 1995;26(6):1452-1456), stratify patients in
terms of
response to angiotensin-converting enzyme inhibition post MI (Motwani JG. et
al Lancet
1993;341:1109-1113) and to demonstrate asymptomatic left ventricular
dysfunction
(Lerman A, Jr. et al Lancet 1993;341:1105-1109; Arad M et al Cardiology
1996;87:12-17).
Myocarditis is also characterized by an increase in synthesis and release of
NP
from the heart (Takemura G. et al. IntJCardiol 1995;52:213-22:). While the
biological
basis of increased production of NP during cardiac hypertrophy and failure is
conceptually placed within the re-expression of the cardiac fetal phenotype
seen with
chronic hemodynamic overload and heart failure, the basis for increased
production of
NP in myocarditis is not understood. Increased ventricular gene expression in
intact
cardiocytes surrounding foci of degenerative changes or necrosis has been
observed in
both animals models and human myocarditis. Mice inoculated with
encephalomyocarditis virus, a model of myocarditis with heart failure, showed
significantly increased ANF plasma levels and ventricular ANF mRNA 10 and 30
days
after infection when compared to non-infected controls (Kanda T. et al.
JPharmacol &
Exptl Ther 1995;274:494-498). Treatment of the mice 24 h after inoculation
with a
combination of the immunomodulators OK432 and human interferon-a A/D prevented
the development of cardiomyopathy and hypertrophy and down regulated the
expression
of ANF mRNA in the ventricles to near normal levels.
W097/32900 (Mischak RP. et al, published September 12, 1997 discloses
monospecific antibodies to hBNP and their use as diagnostic reagents for the
detection
of BNP levels in plasma of patients with congestive heart disease. The
specific epitopes
ofthe MAb's include fragments ofthe mature BNP (BNP77_~og) peptide. These
fragments
include: 5-13 hBNP, 1-10 hBNP, 15-25 hBNP, and 27-32 hBNP.
EP542255 (Tsuji T. et al. published May 19, 1993 discloses monoclonal
antibodies that recognize the C-terminus of hBNP, and
CA 02233724 1998-03-31
-3-
the use of these MAb's within RIA's. The assay involves the determination of
hBNP in
plasma and can be used for the diagnosis of diseases such as hypertension and
altered
states of the heart and kidney.
Ationu et al (1993a, Cardiovas Res. 27:2135-2139) disclose the monitoring of
circulating BNP and ANF levels in paediatric cardiac transplant recipients. In
this study
increased plasma BNP and ANF levels were noted within patients during the
first year
after transplant. When BNP levels were re-assayed at 2.5 or 3 years following
transplantation, the levels were reduced. Another study (Geny B. et al 1988, J
Thorac
Cardiovas Surgery 115:473-475) considered the relationship between BNP levels
before
and immediately following heart transplantation, or coronary artery bypass
grafting, and
concluded that no meaningful relationship was present. Rather it was observed
that
following transplantation, plasma BNP levels, which are typically elevated,
decreased
and returned to earlier levels. Ationu et al (1993b, Cardiovas Res. 27:188-
191) disclose
the assessment of plasma BNP levels following heart transplantation. Levels of
BNP
were noted to increase following transplantation, however, there no
relationship was
observed within plasma or ventricular BNP levels, and rejection episodes.
These authors
report the desire to derive a non-invasive marker in order to monitor such
situations,
however, no such relationship was noted with either of these NP's. A similar
lack of
correlation has been noted in several studies examining circulating ANF levels
following
cardiac transplantation (e.g. Masters RG. et al Can J Cardiol. 1993,9:609-
617).
In all of these studies, there is no disclosure of a relationship between
circulating
BNP levels and transplant rejection, nor is there any demonstration that
circulating BNP
levels can be used to monitor cardiac allograph rejection. Rather, several of
these
references demonstrate the lack of such a correlation, and lack of utility of
BNP levels
as an indicator of rejection. Even though the prior art has failed to report
any meaningful
relationship between levels of BNP in biological fluids and transplant
rejection, the
present invention demonstrates such a relationship, and provides a method for
the
detection of myocardial allograph rejection by determining BNP levels within
biological
fluids.
CA 02233724 1998-03-31
-4-
SUNiNIARY OF THE INVENTION
The present invention relates to a method for the prediction and diagnosis of
cardiac allograft rejection involving the determination of brain natriuretic
peptide.
According to the present invention there is provided a method of diagnosing
cardiac transplant rejection within a patient comprising, obtaining a sample
of a
biological fluid from said patient, and determining the level of a brain
natriuretic
peptide (BNP) or a fragment thereof, within said sample of body fluid. This
invention
also relates to the method as described above wherein said step of determining
the
concentration of BNP involves an assay comprising at least one antibody
exhibiting
affinity for said BNP or a fragment thereof. Furthermore, this invention is
directed
to the method as described above wherein said at least one antibody comprises
a
polyclonal antibody, a monoclonal antibody, or a combination thereof. This
invention
is also directed to the above method wherein said biological fluid comprises
plasma,
urine or cerebrospinal fluid. The present invention also embraces the above
method,
wherein the BNP is BNP.,~_,os, and wherein a level of about or greater than
300 pg/mL of
said BNP.,.,_,og within said biological fluid is an indication of a rejection
episode.
This invention also relates to a method as defined above, wherein at least two
said
samples of body fluid from said patient are obtained over a period of time,
and the levels
within these two samples are compared to determine a change in said BNP levels
within
said biological fluid.
Furthermore, this invention is directed to the method as defined above,
wherein
an increase in said level of BNP or fragment thereof is a predictor of
transplant rejection.
This invention also embraces the method as described above, wherein said BNP
is
selected from the group consisting of mature BNP or a fragment thereof, and
ProBNP or
a fragment thereof, or a combination of mature BNP and ProBNP, and wherein
said
ProBNP comprises BNP,_,6, BNP,_ZS> , or BN~Z_~6 , and said mature BNP
comprises
CA 02233724 1998-03-31
-5-
BNP~,-,og, or a combination of two or more of BNP,_z6, BNP1_zs,, or BNPsz_zb,
and BNP,.,_
~os~
This invention also embraces the method as described above wherein said assay
comprising at least one antibody exhibiting affinity for said BNP or a
fragment thereof
is selected from the group consisting of RIA, ELISA, fluoroimmunoassay,
immunofluorometric assay, and immunoradiometric assay, and wherein the assay
is
performed in the liquid, or solid phase.
The present invention provides for a method for diagnosing cardiac transplant
rejection within a patient by assaying the levels of BNP within biological
fluids.
Existing methods for determining cardiac transplant rejection require the
determination
of a range of clinically significant criteria including those based upon
tissue biopsy.
Even though prior art studies demonstrate a relationship between BNP levels
and heart
failure, the prior art failed to note any correlation between BNP levels and
cardiac
rejection. As a result of characterizing the correlation between BNP levels
and cardiac
rejection, the method of the present invention provides for a sensitive,
simple, reliable
and effective method for predicting rejection episodes.
CA 02233724 1998-03-31
-6-
BRIEF DESCRIPTION OF THE DRAWINGS
These and other features of the invention will become more apparent from the
following description in which reference is made to the appended drawings
wherein:
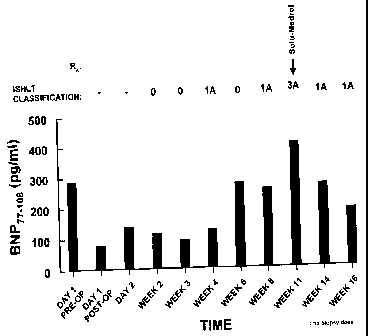
FIGURE 1 shows the relationship between plasma BNP"_lo8 levels before and up
to
16 weeks following a transplant, and the ISHLT classification. A rejection
episode occurred at week 11 (ISHLT classification 3A). Therapeutic treatment
for acute rejection was administered during week 11
FIGURE 2 shows the relationship between plasma BNP~~_log levels for 16 weeks
following a transplant. An acute rejection episode occurred at weeks 2 (ISHLT
classification 3A), 3 (ISHLT 4) and 10 (ISHLT 4). Therapeutic treatment was
administered during weeks 2, 3 and 10.
FIGURE 3 shows the correlation between plasma BNPI_zs, BNP"_lo$ and BNPz_~6
levels in transplant patients. Also shown is the frequency histogram for each
variable.
FIGURE 4 shows the correlation between plasma ANFI_3o, ANFz~9g , ANF~9_126
BNPI_~, BNPsz-76, BNP~~-lob levels in patients diagnosed with heart failure.
Histograms
DESCRIPTION OF PREFERRED EMBODIIVVIENT
The present invention relates to a method for the prediction and diagnosis of
cardiac allograft rejection involving the determination of natriuretic
peptides.
As used herein, ProBNP refers to the entire 1-108 amino acid sequence of the
brain natriuretic peptide, and it is also referred to as BNPi_los~ Specific
fragments of
CA 02233724 1998-03-31
_7_
BNP are referred to based on the numbering of the ProBNP molecule. For
example,
the mature, 32 amino acid, active BNP portion is referred to as BNP~.,_,os,
other
fragments used within this invention include the N-terminal portion of ProBNP,
for
example, which is not to be considered limiting in any manner, BNPi_u, and the
C-
terminal region of the cleaved portion of BNP, for example but not limited to,
BNP52_,6
(note: BNPSZab is derived from the N-terminal region of ProBNP). However,
other
portions of the ProBNP molecule may also be suitable for the method of this
invention.
A similar nomenclature is used with regards to ANF. The entire ProANF is
ANF1_126, while specific portions of this peptide are numbered with reference
to the
ProANF molecule. These fragments include, but are not limited to, ANF,_3o,
ANF.,ø9s~
ANF~_1z6~
Both the BNP and ANF peptides, or fragments thereof, are detectable within
biological fluids, for example plasma, urine and cerebrospinal fluid. The
occurrence
of these peptides or associated fragments can readily be determined within
these
biological fluids following the method of this invention as well as using
methods
known to one of skill in the art.
The term "heart failure", refers to a clinical syndrome in which impaired
cardiac pumping decreases ejection and impedes venous return. This is a
hemodynamic determination of heart performance. Typically, a heart that
exhibits an
ejection fraction of less than about 45 % (compared to the blood received), is
considered to be in heart failure.
By "rejection" or "rejection episode" it is meant a clinically significant
determination, based upon a biopsy, that results in an ISHLT (International
Society of
Heart and Lung Transplantation) Standardized Grading System; Billingham ME. et
al
J Heart Transplant 1990, 9:587-593) grade of 1 or higher. Such a grade,
together with
clinical criteria, evident to one of skill in the art, indicates the
requirement for boosting
the patients immune suppression following a transplantation.
CA 02233724 2003-02-13
An aspect of the present invention as disclosed herein relates to the
determination of plasma BNP levels in diagnosing and predicting rejection in
cardiac
transplant patients. In addition, the data obtained on NP and gene expression
and its
relationships to clinical, histopathological and hemodynamic variables help
elucidate
the pathophysiological basis of rejection and the clinical and molecular
determinants
of increased NP plasma levels observed after cardiac transplantation and, in
general,
in cardiac inflammatory disease.
In order to assess the impact of transplantation on BNP circulating levels
plasma BNP levels were determined in adult patients undergoing cardiac
transplantation. Other studies also assessed the relationship between
different portions
of BNP in plasma in patients following either transplantation or those
exhibiting
symptoms of heart failure. ANF plasma levels were also determined in order to
establish any relationship between the levels of these peptides in response to
rejection
episodes or heart failure.
In the context of this invention, methods for the detection of BNP or ANF may
include a variety of known techniques that would be evident to one of skill
within the
art. Such methods include but are not limited to radioimmunoassay (RIA),
ELISA, and
other immunological-based assays, including fluoroimmunoassay,
immunofluorometric
assay, and immunoradiometric assay, involving radioactive tracers,
colourometric,
fluorogenic or enzymatic markers, or chemical luminescence, or
immunoturbimetry
(e.g. W097/32900; Mischak RP. et al, published September 12, 1997; EP542255;
Tsuji T. et al, published May 19, 1993. These immunological assays may involve
the
use of either polyclonal or monoclonal antibodies, or a combination thereof,
that
recognize specified portions of either BNP or ANF.
Based upon the results presented below, there is no specific portion of the
BNP
that is preferred in order to generate these polyclonal or monoclonal
antibodies for the
purposes of this invention. Rather, any fragment of ProBNP (i.e. BNP,_,o8 ) or
ProANF
CA 02233724 1998-03-31
-9-
(ANF1_126), may be used for the preparation of antibodies and be suitable for
detecting NP
levels in order to determine pending rejection episodes. Several of these
peptides are also
available commercially (e.g. Advanced ChemTech). The antibodies so prepared
may be
used for the detection of ANF or fragments thereof, or BNP or fragments
thereof, or a
combination of both ANF and BNP, or a combination of the fragments of ANF or
BNP,
using the methods as described herein.
Initially, two groups of patients were studied. For those undergoing
transplantation during the study period, right heart catheterization and
hormonal assays
in blood were performed simultaneously immediately pre-operatively and
post-operatively at 24 hours and during each endomyocardial biopsy. For those
whose
transplantation occurred prior to the study period right heart catheterization
and hormonal
assays in blood were performed during each endomyocardial biopsy. Grading of
the
degree of cellular rejection was done using the working formulation of the
ISHLT.
Figure 1 shows individual data from a transplant performed during the study
period and therefore, pre-transplant plasma BNP~,_lo8 are shown.
Transplantation resulted
in a decrease of plasma BNP.,.,_,08 levels immediately after transplantation
suggesting that
the source of this peptide in the pre-transplant period was the diseased
ventricle. Plasma
2O ANF99_126 levels although decreasing after transplantation, continued to be
elevated (data
not shown) suggesting that the major contributors of ANF99_lzb to pre-
transplant and
post-transplant plasma levels were the atria. Steadily increasing ANF99_~z6
and BNP,.,_los
plasma level preceded an overt rejection episode as assessed by clinical and
histological
criteria. Further, successful treatment of the rejection episode caused plasma
level of
both peptides to decrease, although the most noticeable trend was for BNP. It
is of
interest to note that increased BNP levels are observed several weeks prior
(e.g. weeks
6 and 8) to the rejection episode (week 11), yet biopsy analysis produced an
ISHLT
classification of 0 or 1A.
Figure 2 shows a patient who was transplanted prior to the study period and,
therefore, pre-transplant ANF99-,z6 and BNP,.,_,o$ plasma levels could not be
determined.
CA 02233724 1998-03-31
-10-
This patient suffered from more serious rejection episodes and, in this and in
other cases,
the BNP plasma level is correspondingly higher than in patients with milder
rejection
(Compare y-axis scales between figures).
The temporal pattern of change in plasma levels of BNP,.,_,og in terms of
association with rejection episodes was similar to that shown in Figure 1.
However,
plasma levels of ANF99-m6 (data not shown) and BNP.,.,_~og (in Figure 2) were
independent
of each other during rejection episodes that required additional
immunosuppressive
treatment.
Some patients were characterized by maintaining consistently higher levels of
~F99-126 t~'oughout the study period while others maintained higher BNP~~_lo$
plasma
levels. Any patient requiring therapeutic intervention due to a rejection
episode,
however, invariably had relatively higher BNP"_lo8 plasma levels. Furthermore,
rejection
episodes were preceded by increasing BNP.,.,_,08 plasma levels.
The relative proportion of mean plasma levels of ANF99_,26 to BN$.,_,08 in
individual patients showed widely varying values. a patient with prevalence of
BNP,~_,og
had mean plasma levels of ANF99_,zb of 295.0 ~ 19.17 pg/mL and BNP,.,_,08 of
677.31 ~
133.69 pg/mL (n=7) while a patient with prevalence of ANF99_,z6 had mean
plasma levels
Of ANF99-126 of 192.0 ~ 28.9 pg/mL and BNP"_,o8 of 109 ~ 17.38 pg/mL (n=13).
Overall,
plasma ANF9g_,26 levels were slightly lower than those for BNP"_,o8 (239.07 ~
11.54
pg/mL (n = 84) and 278.66 ~ 34.6 pg/mL (n= 84), respectively, p>0.05).
Without wishing to be bound by theory in any manner, it is possible that the
ANF
plasma levels may be a measure of extracellular volume status while that of
BNP
indicates cardiac hypertrophy. These underlying events, superimposed to the
specific
effects) of the inflammatory process together with varying degrees of
preservation of
renal function in individual patients, may explain the variation in NP ratios
found.
CA 02233724 1998-03-31
-11-
Only three of the patients included in this pilot study had pulmonary wedge
pressure (PWP) and right atrial pressure (RAP) consistently determined at the
time of
blood sampling for NP determination. Simple correlation analysis of the
untransformed
data and Bonferroni probability showed that a significant correlation existed
between
PWP and RAP in the three patients but not between these and ANF or BNP plasma
levels
or between NP levels themselves. Lack of correlation between cardiac filling
pressures
and ANF plasma levels has been previously reported for transplant patients
(Hare J.M et
al Am J Cardiol 1991, 67:391-397) although others (Magovern J.A. et al, J.
Heart
Trasnpl. 1987, 6:193-198) report a good correlation between these parameters.
It is
likely that the differences in volume status and cardiac factors and degrees
of rejection
as outlined in above may also explain these differences. Indeed, simple
inspection of the
correlation curves reveals that outliers leading to declare non-significant
correlations
between NP or between NP and cardiac filling pressures are often those NP
values that
are associated with clinically significant rejection episodes requiring
aggressive
treatment. These findings clearly suggest that in those cases rejection, and
not filling
pressures, is a major determinant of NP, particularly BNP"_,og, plasma levels.
These data indicate that rejection episodes needing therapeutic intervention
are
associated with BNP,~_,08 plasma levels greater than 300-400 pg/mL. The values
shown
in Figures l and 2 also suggest that pooling of cardiac hormone levels data in
the general
population of cardiac transplant patients could lead to declare non-
significant associations
between increasing plasma BNP."_,o$ and clinically significant rejection
episodes because
each patient evolves from its own baseline.
In no instance were BNP~,_,o8 plasma levels of over 300 pg/mL observed without
association with a rejection episode. This observation indicates that the
determination
of plasma BNP."_,o8 has few false positives. Conversely, all rejection
episodes that
required additional immunosuppressive therapy were always associated with, and
preceded by, an increase of BNP,~_,o8 plasma levels. In an instance of
difficult
histopathological diagnosis, a tentative grading of ISHLT = 3A (multifocal
moderate
rejection) that would be expected to be associated with additional
immunosuppressive
CA 02233724 1998-03-31
-12-
therapy, BNP levels were in a downward trend and at 40 pg/mL on the day of the
biopsy.
These levels were well below an average of 1,150 pg/mL (range 300-2480 pg/mL)
seen
in association with additional immunosuppressive therapy and clinical
indications of
rejection in other patients. On clinical basis, no additional treatment was
given to this
patient despite a possible 3A grade and none was required 3 weeks later when a
biopsy
was graded "0" and BNP levels were 25 pg/mL. This incident further emphasizes
the
occasional problem encountered with biopsy sampling and the value of
investigating
BNP as a marker of rejection.
Finally, in all instances studied, mild rejection (lymphocytic infiltrate
without
cardiocyte necrosis) leading to a more serious rejection episode was
accompanied by an
increase in plasma BNP levels. The studies as described herein therefore
indicate that
BNP plasma levels are useful in predicting the outcome of rejection episode,
and can be
used as an means for determining the need for administering immunosuppressive
therapy.
Even the simplest of the methods developed to date for the measurement of ANF
or BNP require facilities that are only found in tertiary care facilities.
Even then,
collection of blood samples is often subjected to variations that are not
acceptable in the
quantitation of hormones that circulate at concentrations of pg per mL. In
addition, most
procedures require the handling of radioactive materials and take long
processing time
when compared to biopsies. For these reasons, the feasibility of replacing the
measurement of BNP»_,og with assays to quantitate NP fragments that are known
to
circulate at much higher concentrations. Higher concentration of peptide could
be
detected with faster, simpler and inexpensive technology. In order to enhance
the
sensitivity for the detection of circulating levels of BNP, a comparison
between
different portions of ProBNP and BNP~~_log were examined.
Plasma N-terminal portion of BNP was compared to BNP~~_,o$ by
radioimmunoassay the following values were obtained: N-terminal portion =
2,895513
pg/mL (n=31) and for BNP"-,o8 = 279134 (n=84). Pairwise comparison of samples
for
which both values were available in the same sample (n=19) gave a correlation
CA 02233724 1998-03-31
-13-
coefficient (Pearson's) = 0.969 (Bonferroni p<0.0001 ). Furthermore, the
average levels
of BNPI_u are up to 10 fold higher than the levels of BNB l08 (see Tables 1
and 2
"Means") . An assessment of plasma BNP.,.,_los, BNP,_u, and BNPSZa6 levels in
patients
following transplantation (Figure 3) and heart failure (Figure 4) indicated
that the
circulating levels of the N-terminal, and C-terminal region of the cleaved
portion of
ProBNP are highly correlated with levels of BNP,.,_log. The detection of
BNPI_zs and
BNPsz-76 also includes detection of BNPI_loa ~d possible other BNP species
within the
plasma.
As a result of the high degree of correlation between the cleaved N-terminal
and
C-terminal portions of the cleaved portion of the peptide and the mature
peptide in
plasma obtained from transplant and heart failure patients, and as a result of
the high
levels of these peptides within plasma, the detection of any portion of the
BNP peptide
may be used as an indicator of rejection episodes, heart failure or other
heart disease.
These results indicate that plasma BNP levels may be determined with
techniques that
are less sensitive, less exacting, less expensive and faster as would be
achieved by
replacing the radioimmunoassay by a colorimetric technique. Such technique
would also
be of general applicability for other diagnostic and prognostic purposes in
cardiology.
While this invention is described in detail with particular reference to
preferred
embodiments thereof, said embodiments are offered to illustrate but not limit
the
invention.
Examples
Patients undergoing orthotopic cardiac transplantation at the University of
Ottawa Heart Institute were studied. Parallel, hemodynamic, neuroendocrine and
histopathological measurements were made.
It is the standard of care that patients undergoing transplantation
immediately
prior to surgery have the right internal vein cannulated for measurement of
CA 02233724 1998-03-31
-14-
intra-cardiac pressures including the right atrial, pulmonary arterial and
pulmonary
capillary wedge pressures. Blood samples are withdrawn from the right atrium
for
assays of ANF and BNP. On day 1 following transplantation prior to removal of
the
cannula from the jugular vein the pressures are measured and blood taken for
the
hormonal assays. Subsequently it is routine for these patients to undergo
right heart
catheterization and endomyocardial biopsy at a decreasing frequency and as
guided by
the clinical circumstances. This is performed on an out-patient basis. During
this
procedure the internal jugular vein is cannulated, the intra-cardiac pressures
are
measured and a samples of tissue from the right ventricular endomyocardium is
removed for histopathological assessment. At this time blood is collected
again for
hormonal assays. Patients are followed for a period of time following their
transplant
during which time each patient will have catheterizations and biopsies.
Hormonal
assays are performed during each such procedure.
Typically, a histological grading of the biopsy of ISHLT grade 3A or higher
is considered clinically significant rejection necessitating a boost in the
patient's
immune suppression. Typical maintenance immune suppression for patients
consists
of oral cyclosporine, azathioprine and prednisone. In the case of rejection
this is
supplemented by three days of pulse high dose intravenous steroids.
Recalcitrant
rejection requires the use of such drugs as OKT3 (monoclonal antibody against
T3
lymphocytes) .
Biopsy And Blood Processing
Four to six biopsies obtained from routine percutaneous transvenous right
ventricular endomyocardial biopsies are fixed in 10% neutral buffered
formalin, paraffin
embedded and sectioned as to obtain five micron step sections through the
entire block.
An average four to seven glass slides with three to four sections at each
levels are stained
with Haematoxylon-phloxine-saffron and interpreted by a cardiovascular
pathologist.
Grading of the degree of cellular rejection is done using the working
formulation of the
CA 02233724 1998-03-31
-15-
International Society for Heart and Lung Transplantation Standardized Grading
System
(Billigham ME. et al J Heart Transpl. 1990, 9:587-593).
Two 15 mL blood samples are collected per patient prior to and within 24 of
cardiac transplantation and each time right heart catheterization is
performed, in
pre-chilled VacutainerTM tubes with EDTA anticoagulant. The blood is
centrifuged
immediately at 4°C and the plasma kept at -80 °C until one tube
is extracted for
determination of ANF99-,26 and BNP~.,_,08. The plasma of the second tube is
used for
analysis using assays for proBNP fragments (BNP,_zs ~d BNPSZ-~6)
Extraction Of Plasma And Tissue For Radioimmunoassay
Plasma samples are acidified by adding 100 ~cl/mL of I M HCI and passed
through Sep-Pak C1g cartridges (Millipore, Milford, MA) pre-wetted with 5 mL
of 80%
acetonitrile (ACN) in 0.1 % trifluoroacetic acid (TFA) and 10 mL of 0.1 % TFA.
The
cartridges with the absorbed peptides will be washed with 20 mL of 0.1 % TFA,
and then
eluted with 3 mL of 60% ACN in 0. 1% TFA. The elates are freeze-dried and
processed
for radio immunoassay as previously described (Sarda IR. et al. Clin Biochem.
1989
22:11-15).
Assay for N terminal (NT ) BNP
For the purposes of developing a simpler method to measure NT-BNP, an ELISA
protocol is pursued. Two approaches are possible for measuring soluble
antigens with
ELISAs: 1. Technology based on a direct competitive protocol, or, 2.
Technology based
on a "sandwich" technique involving a competitive reaction plus a reaction
with a second
antibody directed to a second epitope in the antigen. a capture and secondary
polyclonal
and monoclonal antibodies against the N-terminal portion of BNP52_.,6 used is:
Lys-Ser-Arg-Glu-Val-Ala-Thr-Glu-Gly-Ile-Arg-Gly-His-His-Arg-Lys-Met-Val-Leu-
Tyr-Thr-Leu-Arg-
Ala-Pro-Arg
CA 02233724 1998-03-31
-16-
Production Of Monoclonal Antibodies
Synthetic NP52_,6 are coupled to bovine thyroglobulin using the carbodiimide
method (Skowsky WR et al. 1972, J Lab Clin Med 80:134-144). Female BALBoC mice
are immunized with the BNPSZ-,6- thyroglobulin conjugates mixed with 100 ,ug
of
murarnyl peptide, dissolved in phosphate buffered saline. Protocols for
immunization
and for the fusion of spleen cells from the immunized mice with cells of the
non-
secreting mouse SP2-O plasmacytoma line are described in detail, elsewhere
(Mime
RW. et al 1992, in Immunological Methods for Studying and Quantifying
Lipoproteins
and Apolipoproteins, Converse CA and Skinner ER eds, Oxford U Press, pp 61-
84). Two
methods of screening are used to identify specific antibodies in the culture
supernatants
of hybridomas. In the first protocol, BNPsa-~6 is adsorbed to polystyrene
Removal Wells
(Dynatech) and after washing and saturation of the wells, they are
successively exposed
to hybridoma culture supernatant and IZSI_anti-mouse IgG. In the second
protocol, wells
are coated with affinity-purified, anti-mouse IgG and then successively
exposed to
hybridoma culture supernatant and to 'ZSI-BNP52_.,6, as appropriate. Details
of the two
screening protocols have been reported along with a discussion of their
relative merits
(Mime RW. et al 1992, in Immunological Methods for Studying and Quantifying
Lipoproteins and Apolipoproteins, Converse CA and Skinner ER eds, Oxford U
Press,
pp 61-84).
Production of Polyclonal Antibodies
Polyclonal antibodies to ANF and BNP are commercially available (e.g.
Advanced ChemTech). However, antibodies to specific portion of the BNP
molecule are
prepared as follows (Sarda IR. et al. Clin Biochem. 1989 22:11-15): 3 mg of
peptide in
0.3 mL of 20 mM HCI is mixed with 3 mg of keyhole limpet haemocyanin (Sigma)
dissolved in 0.3 mL of 20 mM HCL. Thirty mg of 1-ethyl 3- (3-
dimethylaminopropyl)
carbodiimide in 0.2 mL of water is added and the reaction allowed to proceed
at room
temperature for 1 h. After I h, 0.6 mL of 20 mM NaOH is added to bring the pH
to
CA 02233724 1998-03-31
-17-
about 6.5 and left to stand for about 1 h at room temperature and then diluted
to 5 mL
with 0.9 % NaCI. The peptide conjugate is emulsified in twice its volume with
complete
Freund's adjuvant (Difco Laboratories, Detroit, Michigan). Five 3-month-old
New
Zealand white rabbits are immunized by injecting the emulsion intramuscularly
into the
biceps femoralis, so that each animal receives a total of 3 mL of the
emulsion. Animals
are boosted with the conjugate emulsified with Freund's incomplete adjuvant at
4-week
intervals and bled 10-14 days after each booster injection by ear vein
puncture. Serum
is aliquoted and stored at - 70°C. Titres are determined by standard
binding curves
using a previously published protocol (Sarda IR. et al. Clin Biochem. 1989
22:11-15).
Tracer peptides are iodinated using the chloramine T method or the
Bolton-Hunter reagent as appropriate and previously published (Sarda IR. et
al. Clin
Biochem. 1989 22:11-15). Purification is carried out by RP-HPLC using a Vydac
C1g
column and a 60-min gradient of 8-35 % acetonitrile containing 0.1 % TFA at a
flow
rate of 1.5 mL/ min. The specific activity of labelled peptides is determined
using the
self displacement method described by Morris (1976, Clin Chem Acta 73:213-
216).
Alkaline phosphatase-antibody conjugates for ELISAs are prepared from
affinity purified polyclonal or monoclonal antibodies and alkaline phosphatase
(enzyme
immunoassay grade, Boehringer-Mannheim) according to standard methodology (van
Vunakis H, Langone JJ. 1980 Immunological Techniques, pp. 1-525)
Statistical Analysis
Correlation analysis with posthoc test for statistical significance are
carried out
between continuous variables using standard procedures. Multivariate analysis
(SYSTAT, an SPSS statistical package) is used to examine the independent
predictive
value of BNP values and rejection versus other risk factors including age,
disease, sex,
etc.. Receiver-operating analysis (reference) is carried out to determine the
relationship between sensitivity and specificity of BNP plasma values at
different
CA 02233724 1998-03-31
-18-
diagnostic cutoff values. The data of figure 3 and 4 were generated using the
SYSTAT
Graph Module.
Results
Data obtained from a transplant performed during the study period including
pre-transplant plasma ANF99_,z6 and BNP~~_lo8 are shown in Figure 1. The study
period
covered a 16 week period. Following transplantation, a decrease of plasma
BNP~,_,o8
levels is observed. Plasma ANF99_126 levels also decreased after
transplantation, but
continued to be elevated throughout the monitoring period. Plasma levels of
both
ANF99_lz6 and BNP~~_los increased with the onset of a rejection episode (ISHLT
3A).
Following treatment of the rejection episode plasma levels of both peptides
decreased,
most noticeably for BNP."_,os~
Results from a patient, transplanted prior to the study period are shown in
Figure
2. Two serious rejection episodes were noted during this study (weeks 2, 3 and
10), and
both corresponded with increased levels of plasma BNP,,_,o$ (see weeks 2, 3
and 7). a
corresponding increase in the levels of ANF were not detected This and other
analysis
have also indicated that the BNP,~_~o8 plasma level is higher in patients with
more sever
rejection episodes. Furthermore, if therapeutic intervention due to a
rejection episode
was required, plasma levels of BNP~,_~og were also noted to be comparatively
higher. In
all cases however, rejection episodes were preceded by increasing BNP~~_,o8
plasma
levels.
The relative proportion of mean plasma levels of ANF99_lzb to BNP~~-,o$ in
individual patients showed widely varying values. a patient with prevalence of
BNP.,.,_,og
had mean plasma levels of ANF99-i26 of 295.0 t 19.17 pg/mL and BNP.,.,_,08 of
677.31 ~
133.69 pg/mL (n=7) while a patient with prevalence of ANF99-,z6 had mean
plasma levels
of ANF99-,zb of 192.0 ~ 28.9 pg/mL and BNP.,~_,og of 109 ~ 17.38 pg/mL (n=13).
Overall,
plasma ANF99-126 levels were slightly lower than those for BN~,?,og (239.07 ~
11.54
pg/mL (n = 84) and 278.66 ~ 34.6 pg/mL (n= 84), respectively, p>0.05).
CA 02233724 1998-03-31
-19-
An assessment of the use of other portions of the BNP molecule for determining
the onset of transplant rejection in 19 patients is shown in Figure 3. The
correlation
in plasma levels between each of the portion of BNP tested and BNP77-108 is
shown
in Table 1.
Table 1: Pearson Correlation Matrix and Matrix of Bonferroni Probabilities for
the
data presented in Figure 3 (n=19)
Means: BNPI_zs BNP77-ioa BNPsz-~6
3.263 ng 490.640 pg 486.016 pg
Pearson Correlation Matrix
BNPI_ZS BNP~~_los BNPs2_~6
BNPI_~ 1.000 - -
BNP~~_lo$ 0.969 1.000 -
BNPsza6 0.866 0.920 1.000
Bartlett Chi-Square statistic: 76.539 df=3; Prob=0.000
Matrix of Bonferroni Probabilities
BNPi_ZS BNP77-ios BNPsz-~6
BNPI_~ 0.0 - _
BNP~~_log 0.000 0.0 -
BNPSZa6 0.000 0.000 0.0
These results indicate that fragments, in addition to BNP."_log, of the ProBNP
molecule may be effectively used for the determination of plasma BNP levels.
Due to
CA 02233724 1998-03-31
-20-
the higher levels of BNP55_.,6 and BNPI-~ Present within plasma, assays based
on these
fragments provide a more sensitive monitoring of plasma BNP levels.
The levels of BNP and ANF were also determined in 27 patients diagnosed as
exhibiting heart failure. the results of such a study are shown in Figure 4
and Table
2.
CA 02233724 1998-03-31
-21-
Table 2: Pearson Correlation Matrix and Matrix of Bonferroni Probabilities for
the
data presented in Figure 4 (n=27)
S Means: ANFI_3o ANF~4_9g ANF~_126 BNPi_u BNP~~_log BNPsz-~6
6.536 ng 2.903 ng 119.84pg 1.369 ng 154.239 pg 503.749 pg
Pearson Correlation Matrix
1 O ANF , ", ANF~. .,o ANF"., ..,~ RNP, .,~ RNP.... ."~ RNP__ _.
ANF1-30 1.000 - - - - -
ANF74-98 0.806 1.000- - - -
ANF99-126 0.627 0.5591.000 - - -
BNPI_zs 0.773 0.7760.525 1.000 - -
15 BNPT,_,os 0.925 0.8150.655 0.825 1.000 -
BNPsz-~6 0.856 0.7410.716 0.833 0.936 1.000
Bartlett Chi-Square ; Prob=0.000
statistic: 170.933 df=15;
Matrix of Bonferroni
Probabilities
20 BNP1_zsBNI'n-Los BNPsz-~6
ANFi_3o 0.0 - - -
ANF~96 0.000 0.0 - -
ANF~_,26 0.007 0.0360.0 -
BNPI_zs 0.000 0.0000.074 0.0 - -
25 BNPr,_,os 0.000 0.0000.003 0.000 0.0 -
BNPszab 0.000 0.0000.000 0.000 0.000 0.0
30 These results again indicate the high degree of correlation between
different
portions of the BNP or ANF molecules with the biologically active BNP or ANF
CA 02233724 1998-03-31
-22-
peptides. Furthermore, this results demonstrates the utility of BNP, or a
fragment
thereof, for the diagnosis of heart failure and other heart related diseases.
The present invention has been described with regard to preferred embodiments.
However, it will be obvious to persons skilled in the art that a number of
variations
and modifications can be made without departing from the scope of the
invention as
described herein.