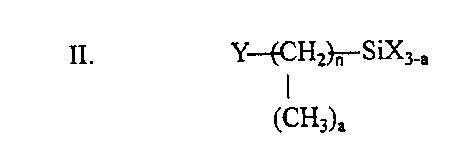Note: Descriptions are shown in the official language in which they were submitted.
CA 02233744 2001-06-07
1VIETHOD AND COMPOSITIONS FOR DECORATING VITREOUS ARTICLES WITH
RADIATLON CURABLE INKS HAVING IMPROVED ADHESION AND
DURABILITY
The invention is in the field of applying decorative indicia to substrates
such as glass and
other vitreous articles, and in particular, for pre-treating articles to be
decorated with radiation
~~urable inks to cause the inks to exhibit improved adhesion to the vitreous
substrate.
Back' ground of the Invention
Commercial ceramic and glassware is often decorating by applying a pattern in
colored
1 S ink on the surface of the substrate with screen printing, offset printing,
or any other direct
application technique. The glass is then baked at high temperatures to bond
the indicia to the
glass surface. This process, sometimes referred to as applied ceramic labeling
(ACL), exhibits
certain drawbacks. Often the ink compositions contain heavy metals and
volatile organic
solvents (VOC's). Both VOC's and heavy metals are undesirable from the
environmental point
of view. Second, ACL requires high temperature ovens for the baking step,
which results in
considerably energy Usage and an increased potential for worker injury due to
the high
temperatures at which the process operates. Moreover, the high temperature
ovens are
expensive, cumbersome, pieces of equipment that require considerable floor
space in factories.
On the other hand, use of radiation curable, particularly ultraviolet (UV)
radiation
curable, organic pigmented inks for decoration of glass and other vitreous
materials is very
1
~ . ~.._ CA 02233744 1998-03-31
f~ .
desirable. Organic inks generally can be cured by exposure to radiation, such
as UV radiation,
thus obviating the need for high temperature baking. In addition, UV curable
organic inks can be
formulated to contain little or no VOC's or other nonaqueous solvents. One
problem with
organic inks is that they often do not exhibit adhesion equivalent to that
obtained with ACL.
Glass beverage and cosmetic containers must pass very stringent quality
control tests, depending
on the material which is sold in the container. For example, the decorative
indicia on certain
types of beverage bottles must be able to withstand pasteurization (exposure
to water having a
temperature of 70° C. for one hour), or exposure to solutions of
caustic base for extended periods
of time at elevated temperatures.
Thus, there is a continuing effort to formulate radiation curable inks and
utilize
decorating methods which provide decorative indicia having durability and
adhesion equivalent
to that of ACL.
An object of the invention is to provide a composition to be used for pre-
treating vitreous
articles prior to application of radiation curable inks to cause the inks to
exhibit improved
durability and adhesion.
An object of the invention is to provide a method for pre-treating vitreous
articles with a
composition which will cause the radiation curable inks subsequently applied
to to the article, to
exhibit improved adhesion and durability.
An object of the invention is to provide a method for decorating glass and
other vitreous
articles with radiation curable ink compositions which exhibit improved
adhesion and durability.
~ ,mm ~f t_he Invention
sssa3-~ .aoo 2
CA 02233744 2001-06-07
The invention comprises a method for applying decorative indicia to the
surface of a
vitreous article comprising the steps of:
(a) applying to the surface of the article a primer composition containing a
solvent and a silane
coupling agent capable of forming a chemical bond between the surface of the
vitreous article
and an organic, radiation curable composition, wherein said solvent comprises
water,
(b) after the applied primer composition is substantially dry, applying an
organic
radiation curable ink composition over the primer composition in a desired
design,
(c) curing the organic ink composition on the article by exposing it to the
radiation by
which it is curable, thereby causing the coupling agent to form a chemical
bond between the
surface of the vitreous article and the cured organic ink composition.
The invention also comprises a method for pre-treating a vitreous article
prior to
application of organic radiation curable decorative indicia, comprising
applying to said article a
primer composition comprising, by weight of the total composition:
75-99.99% water,
0.01-20% of a silane coupling agent having the general formula:
II. Y-(CHz}~-SiX3_,
(CH3)a
wherein n = 0-3
a = 0-2
3
CA 02233744 1998-03-31
r.
Y = -NH2, CH2=CH-, CH2=C-COO-, CH2=CH-COO-, CH3-NH-, NH2-CO-NH-, HS-,
CH3
-Cl, NH2(CH2~NH-,
H2C- N-
I
H2C CH
or CHI-- CH-(CH2)y0-;
N
X is each independently -CH3, -Cl, -OCOR', -OC2H40CH3, -(OC2H4)2OCH3, or -OR,
where R is a C ,_2a straight or branched chain alkyl, and R' is a C,-3 alkyl
or alkenyl, wherein R
and R' are preferably methyl or ethyl, and y is 1-3.
0.001-20% of a nonionic surfactant which is a polyethylene glycol glyceryl
fatty acid
ester.
The invention also comprises a primer composition for radiation curable inks
comprising,
by weight of the total composition:
75-99.99% water,
0.01-20%of a silane coupling agent having the general formula:
B, Y-(CH~SiX3_a
(CH3)a
wherein n = 0-3
a = 0-2
Y = -NH2, CH2~H-, CH2=C-COO-, CH2=CH-COO-, CH3-NH-, NHrCO-NH-, HS-,
CH3
sasaa-~ .dx 4
--.. CA 02233744 1998-03-31
-Cl, NH2(CH2)2NH-,
H2C- N-
H2C CH
or CHI-- CH-(CH2)YO-;
N
X is each independently -CH3, -Cl, -OCOR', -OC2H40CH3,-(OC2H4~OCH3, or -OR,
where R is a C 1_2o str~ght or branched chain alkyl, and R' is a C1_3 alkyl or
alkenyl, wherein R
and R' are preferably methyl or ethyl, and y is 1-3.
0.001-20% of a nonionic surfactant which is a polyethylene glycol glyceryl
fatty acid
ester.
All percentages mentioned herein are percentages by weight unless otherwise
indicated.
The term "vitreous article" when used in accordance with the invention shall
mean glass,
ceramic, tile, and similar vitreous materials. The articles which may be
decorated or printed
according to the method of the invention may be in any shape or form, such as
a container,
sheet, tile, figurine, or the like. In the preferred embodiment of the
invention the article is made
of glass or ceramic and is a container, such as a cosmetic or beverage
container. The method of
the invention and the compositions used in the method are further described
below.
Thr ~'Oh'~OSITIONS USED IN THE METHOD OF THE INVENTION
sssaa-~.doo 5
CA 02233744 1998-03-31 ,=
The primer compositions which are used to pre-treat the vitreous article prior
to
decoration with the radiation curable ink contain a solvent and a coupling
agent capable of
forming a chemical bond between the surface of a vitreous article and an
organic radiation
curable composition. The primer composition may also contain other ingredients
such as
surfactants, and other materials. Surfactants will improve the wettability and
levelling capability
of the primer composition, i.e. the primer composition is better able to wet
the surface to which it
is applied, and the radiation curable ink compositions will apply more evenly.
~~) The Solvent
The solvent may be aqueous or non-aqueous or a mixture of both types of
solvents,
provided the coupling agent is soluble in it. Suitable non-aqueous solvents
include aliphatic or
aromatic ketones such as acetone, diacetone alcohol, dihydroxyacetone, ethyl
butyl
valerolactone, methyl ethyl ketone, and the like; aliphatic or aromatic
alcohols such as methanol,
propanol, benzyl alcohol, butoxyethanol, butoxypropanol, butyl alcohol, 3-
methyl-3-methoxy-
butanol, t-butyl alcohol, butylene glycol, diethylene glycol, abietyl alcohol,
propylene carbonate,
hexyl alcohol, isopropanol, and the like; glycol ethers; esters such as butyl
acetate, ethyl acetate,
etc. Also suitable as non-aqueous solvents are volatile linear or cyclic
silicones such as
cyclomethicone or dimethicone, or volatile paraffinic hydrocarbons. The primer
composition
comprises 75-99.99%, preferably 85-99.5%, more preferably 90-99% of the
solvent, which is
preferably water.
(b1 The Coupling Agent
The coupling agent is a compound capable of forming a chemical bond between
the
surface of a vitreous article and the organic, radiation curable composition.
The vitreous article
sas4s-~.aoc 6
fw CA 02233744 1998-03-31
is generally comprised largely of inorganic material which has free functional
groups such as
hydroxyl groups. Preferably, the bonding that occurs between the coupling
agent and the
inorganic surface is via the formation of hydrogen bonds mainly between the
free hydroxyl
groups of the inorganic substrate and the functional groups of the coupling
agent. In some
instances, the hydrophilic functional groups of the coupling agent are formed
when the coupling
agent comes into contact with water. For example, in the case where the
coupling agent is an
alkoxy silane, the alkoxy groups present in the silane tend to hydrolyze upon
exposure to water
to form hydroxyl groups, and thus the silane becomes a silanol. Preferably the
functional groups
of the coupling agent are hydrophilic groups such as hydroxyl, hydroxy-
polyethyleneoxy,
carboxylate, sulfonate, sulfate, phosphate, amine, or mixtures thereof. The
coupling agent/
solvent composition generally comprises 0.01-25%, preferably 0.05-15%, more
preferably 0.1-
10% of the coupling agent. While a variety of coupling agents will provide
this type of bonding,
the preferred coupling agents are silanes. In this case, the bonding that
occurs between the silane
coupling agent and the inorganic surface of the vitreous material is via the
formation of hydrogen
bonds between the hydroxyl groups of the inorganic material and the
hydrophilic, preferably
hydroxyl, groups of the silane which are formed by hydrolysis of the silane
functional groups
once it is exposed to water. An example of this reaction is set forth below:
O OCH3
II
Silane CH3-C-C-O-(CH~3-Si-OCH3
II I
CH2 OCH3
sss4a-~ .dog 7
CA 02233744 1998-03-31
~~ t:_
Hydrolysis in aqueous solution
O OH
II I
Silanol CH3-C-C-O-(CH2)3-Si-OH
II I
CH2 OH
Thus, it should be understood that when the silane in an aqueous solution it
may be present in the
"silanol" form. Suitable silanes include those having the general formula:
I. Y-(CH2)n SiX3, wherein
n = 0-3,
Y = H2N-, CH2=CH-, CH2=C-COO-, HS-, -Cl,
(
CH3
O
or CIA- CH-CH20-;
X = -OR, -Cl, -O-C-CH3
II
O
R = C ,-2o straight or branched chain alkyl, preferably methyl or ethyl.
Also suitable are silanes having the general formula:
II. Y-{CH~ SiX3_a
I
(CH3)a
wherein n = 0-3
a = 0-2
98943-1.doc
CA 02233744 2001-06-07
Y = -NH2, CH;2=CH-, CH2=C-COO-, CH2=CH-COO-, CH3-NH-, NH2-CO-NH-, HS-,
C.H3
-Cl, NH2(CH2)2NH-,
HZC- N-
I I O
HZC CH
or CHI-- CH-(CH2)y0-;
N
1 S X is each independently -CH3, -Cl, -OCOR', -OC2H40CH3, -(OCZH4)20CH3, or -
OR,
where R is a C i_20 straight or branched chain alkyl, and R' is a C1-3 alkyl
or alkenyl, wherein R
and R' are preferably methyl or ethyl, and y is 1-3.
Examples of ;>ilanes having the above formulas are those sold by Huls America
(now
SIVENTO Ine.) under the DynasylanTM and HydrosilTM tradenames, namely AMMOTM,
1110,
AMEO-PTM, AMEO-TTM, 1151, 1211, 1302, 1505, 1506, DAMOTM, DAMO-TTM, 1411,
TRIAMOTM, 2201, IiVIEOTM, MEMO-ETM, GLYMOTM, MTMOTM, 3201, 3403, CPTMOTM,
VTCTM, VTMOTM, VTEOTM, VTMOEOTM, SILFINTM, HS 2629TM, HS 2759TM, HS 2781TM,
HS 2775TM, and HS 2776TM, and the like.
Other organofunctional silanes such as those disclosed in U.S. patent no.
5,221,560 are
also suitable. Such organosilanes are
acryloxyfunctional silanes including 3-methacryloxypropyltrimethoxysilane,
3-acryloxypropyltrirnethoxysilane, 2-methacryloxyethyltrimethyoxysilane,
2-acryloxyethyltrimethyoxysilane, 3-methacryloxypropyltriethoxysilane,
3-acryloxypropyltriethoxysilane, 2-methacryloxyethyltriethoxysilane,
2-acryloxyethyltriefhoxysilane, etc.
9
~, CA 02233744 1998-03-31 ;-
,.
Particularly preferred are GLYMO and MEMO-E. GLYMO is
3-glycidoxypropyltrimethoxysilane and MEMO is 3-trimethoxysilylpropyl
methacrylate.
The primer composition may also contain various other additives such as
surfactants,
S leveling agents, viscosity modifiers, and the like.
Preferably, the primer composition contains 0.001-20%, preferably 0.01-15%,
more
preferably 0.05-10% of a surfactant which may be cationic, anionic,
amphoteric, zwitterionic, or
nonionic. Suitable amphoteric surfactants are generally derivatives of
aliphatic secondary or
tertiary amines wherein one aliphatic radical is a straight or branched chain
alkyl of 8 to 18
carbon atoms and the other aliphatic radical contains an anionic group such as
carboxy,
sulfonate, sulfate, phosphate, or phosphonate. Suitable zwitterionic
surfactants include betaines,
as well as those of the general formula:
l"3lx
RAY-CI-~R~Z
wherein R2 contains an alkyl, alkenyl or hydroxy alkyl radical of from about 8
to about 18 carbon
atoms, from 0 to about 10 ethylene oxide moieties and 0 or 1 glyceryl moiety;
Y is selected from
the group consisting of nitrogen, phosphorus, and sulfur atoms; R3 is an alkyl
or
monohydroxyalkyl group containing about 1 to 3 carbon atoms; x is 1 when Y is
a sulfur atom,
and 2 when Y is a nitrogen or phosphorus atom; R4 is an alkylene or
hydroxyalkylene of from
about 1 to about 4 carbon atoms, and Z is a radical selected from the group
consisting of
carboxylate, sulfonate, sulfate, phosphonate, and phosphate groups. Suitable
anionic surfactants
sssas-~ .dog 10
CA 02233744 1998-03-31
';.
include alkyl ether sulfates and sulfonates, as well as succinates,
succinimate, and olefin
sulfonates.
Preferably, the surfactant is a nonionic surfactant. Nonionic surfactants are
generally
compounds produced by the condensation of alkylene oxide groups with a
hydrophobic
compound. Classes of nonionic surfactants are:
(a) Long chain dialkyl sulfoxides containing one short chain alkyl or hydroxy
alkyl radical of
from about 1 to 3 carbon atoms and one long hydrophobic chain which may be an
alkyl, alkenyl,
hydroxyalkyl, or ketoalkyl radical containing from about 8 to 20 carbon atoms,
from 0 to 10
ethylene oxide moieties, and 0 or 1 glyceryl moiety.
(b) Polysorbates, such as sucrose esters of fatty acids. Examples of such
materials include
sucrose cocoate, sucrose behenate, and so on.
(c) Polyethylene oxide condensates of alkyl phenols, for example the
condensation products of
alkyl phenols having an alkyl group of 6 to 20 carbon atoms with ethylene
oxide being present
in amounts of about 10 to 60 moles of ethylene oxide per mole of allcyl
phenol.
(d) Condensation products of ethylene oxide with the reaction product of
propylene oxide and
ethylene diamine.
(e) Condensation products of aliphatic alcohols having 8 to 18 carbon atoms
with ethylene
oxide, for example a coconut alcohoUethylene oxide condensate having 10 to 30
moles of
ethylene oxide per mole of coconut alcohol, the coconut alcohol fraction
having 10 to 14 carbon
atoms.
(f) Long chain tertiary amine oxides such as those corresponding to the
general formula:
R~R2R3N0
98943-1.dx 11
CA 02233744 2001-06-07
wherein R, contains an alkyl, alkenyl or monohydroxyalkyl radical ranging from
about 8 to 18
carbon atoms in length, from 0 to about 10 ethylene oxide moieties, and from 0
to about 1
glyceryl moiety and FCz and R3 are each alkyl or monohydroxyalkyl groups
containing from
about 1 to about 3 carbon atoms.
(g) Long chain tertiary phosphine oxides corresponding to the general formula:
RR,R2P0
wherein R contains an alkyl, alkenyl, or monohydroxyalkyl radical having 8 to
18 carbon atoms,
from 0-10 ethylene oxide moieties and 0 or 1 glyceryl moiety, and RZ and R3
are each alkyl or
monohydroxyalkyl group containing from about 1 to 3 carbon atoms.
(h) Alkyl polysaccharides having a hydrophobic group of 6 to 30, preferably
10, carbon atoms
and a polysaccharide: group such as glucose, galactose, etc. Suitable alkyl
polysaccharides are
octyl, nonydecyl, undecyldodecyl, tridecyl, tetradecyl, pentadecyl, hexadecyl,
heptadecyl, and
octadecyl, di-, tri-, tetra-, penta-, and hexaglucosides, galactosides,
lactosides, glucoses,
fructosides, fructoses, and so on.
(i) Polyethylene glycol (PEG) glyceryl fatty esters, which are the reaction
product of
polyethylene glycol, glycerin, and fatty acids. Suitable PEG glyceryl fatty
esters may be
monoesters, diesters, or triesters. Such compounds are manufactured by
Goldschmidt
Corporation under the TAGAT tradename. Suitable monoesters include, for
example, those
having the general i:ormula:
RC(O)OCHZCH(OH)CH2(OCHZCH2)"OH
wherein n is 2-200 and RC(O)- is a hydrocarbylcarbonyl group wherein R is an
aliphatic radical
having 7 to 30, preferably 8-20 carbon atoms.
12
CA 02233744 2001-06-07
(j) Other nonionic surfactants that may be used include 00.18
alkyl(C,.~)polyhydroxy fatty acid
amides such as C~2.~8 methylglucamides, N-alkoxy polyhydroxy fatty acid
amides, N-propyl
through N-hexyl C~2_,;3 glucamides and so on.
Other suitable nonionic surfactants include fluorinated nonionic surfactants.
The term
"fluorinated nonionic surfactant" means a fluorine containing compound having
at least one
liphophilic group or portion and at least one hydrophilic group or portion.
Examples of such
surfactants are set forth in U.S. patent no. 4,961,976 . Also suitable are
fluorocarbon
surfactants, such as those marketed under the Fluorad trademark by 3M Company.
These
fluorochemical surfactants include fluorinated alkyl esters, fluorinated alkyl
polyoxyethylene
ethanols, and the like. Fluorinated alkyl alkoxylates are marketed by 3M under
the trademark
FC-171.
The preferred nonionic surfactants for use in the present invention are
polyethylene
glycol glyceryl fatty esters of the formula
RC(O)OCH2CH(OH)CH2(OCH2CH2)"OH
1 S wherein n is 2-200 and RC(O)- is a hydrocarbylcarbonyl group wherein R is
an aliphatic radical
having 8-20 carbon atoms. Particularly preferred is a polyethylene glycol
glyceryl fatty acid
monoester sold by Goldschmidt Corporation under the tradename Tagat SSTM,
which is PEG-5
glycerylmonostearate. In the preferred embodiment of the invention TegoglasTM
TS is used which
is an aqueous solution of Tagat SST~z, sold by Goldschmidt Corporation.
The preferred primer compositions in accordance with the invention comprises:
(a) 90-99.9f% water,
(b) 0.1-10% of a silane coupling agent having the general formula:
13
'-._CA 02233744 1998-03-31
II. Y-(CH2}~ SiX3_e
(CH3)a
wherein n = 0-3
a = 0-2
Y = -NH2, CH2=CH-, CH2~-COO-, CH2=CH-COO-, CH3-NH-, NH2-CO-NH-, HS-,
CH3
-C1, NH2(CH2)2NH-,
H2C- N_
H2C CH
or CHI- CH-(CH2)y0-;
N
X is each independently -CH3, -C1, -OCOR', -OC2H4OCH3, -(OC2H4)2OCH3, or -OR,
where R is a C i.2o straight or branched chain alkyl, and R' is a C,_3 alkyl
or alkenyl, wherein R
and R' are preferably methyl or ethyl, and y is 1-3; and
(c) 0.001-10% of a nonionic surfactant which is a polyethylene glycol glyceryl
fatty
ester, having the general formula
RC(O)OCH2CH(OH)CH2(OCH2CH~"OH
wherein n is 5-200 and RC(O)- is a hydrocarbylcarbonyl group wherein R is
preferably an
aliphatic chain having 8-20 carbon atoms.
More preferably, the primer agent composition comprises:
3 0 (a) 90-99.99% water,
(b) 0.1-10% of a silane selected from the group consisting of
sss.a~-~ .dx 14
CA 02233744 2001-06-07
3-glycidoxypropyltrimethoxysilane, 3-trimethoxysilylpropyl methacrylate, and
mixtures thereof;
and
(c) 0.001-10°ro of a nonionic surfactant which is a polyethylene glycol
glyceryl fatty acid
monoester, where the fatty acid is stearic acid.
Preferably, the primer composition has a pH of less than 7, preferably a pH of
1-6, more
preferably a pH of 3.:5 to 4.5 with a range of 3.8 to 4.3 being most suitable.
The pH of the
composition can be adjusted with acids or bases which are usually used for
this purpose, i.e.
amines, acetic acid, ~u~d so on.
The pre-treatment method of the invention may be used with a wide variety of
radiation
curable ink compositions provided that such compositions contain a monomer,
oligomer, or low
molecular weight polymer that is radiation curable.
Suggested ranges of the monomer, oligomer, or polymer in the radiation curable
composition are 0.1-99%, preferably 0.5-95%, more preferably 1-90% by weight
of the total
composition.
The bis-phenol-A epoxy resins as described in U.S. Patent No. 5,656,336
are suitable. Such i.nk compositions comprise:
bis phenol-A epoxy resin having the formula:
CA 02233744 2001-06-07
CH3
I. '~
CH2 H-CH O- O ' O -CH2CHCH
CHI OH n
O
CH3
_ ~ ~ O-CH2Cu~---aH2_
CH3
wherein n = 0-20,
Also suitable for use as the ink are compositions containing acid functional
monomers or
oligomers, such as those disclosed in U.S. Patent Application Serial No.
868,409, filed June 3,
1997, entitled Method and Compositions For Decorating Glass, by inventors
Melvin Kamen and
Ming Hu. These ink compositions preferably contain about 5-95%, more
preferably about
10-85%, most preferably about 15-75% of a monomer, oligomer, or low molecular
weight homo-
or copolymer having at least one free acid group. A variety of such materials
are suitable,
provided they have at least one free acid group, such as a carboxylic acid,
sulfonic acid, or
phosphoric acid group. The phrase "having at least one free acid group" means
that the
monomer, if used, hays at least one free acid group, or the oligomer, if used,
contains at least one
monomer unit containing a free acid group, or if a homo- or copolymer is used,
at least one
monomer unit thereof contains at least one free acid group. Preferably the ink
composition
contains a monomer or oligomer, in particular an ethylenically unsaturated
monomer or oligomer
16
CA 02233744 2001-06-07
having at least one free acid group. Examples of preferred monomers or
oligomers include those
having carboxylic acid functional groups, e.g. either the monomer:
I.
R1
CH2=C
COOH
wherein R, is H, a C~,.3o straight or branched chain, substituted or
unsubstituted, saturated or
unsaturated alkyl, anal, aralkyl, a pyrrolidone, or a substituted or
unsubstituted aromatic,
alicyclic, or bicyclic ring where the substitutents are C1_3o straight or
branched chain alkyl, or
halogen, or the monomer:
II.
Ri
I
CHZ==C
COOR2
wherein R~ is as defined above, and R2 is X-COOH wherein X is a C1_3o str~ght
or branched
chain alkyl, aryl, arylalkyl, or -{-EH2CH2-O~Y-COOH or -~CHZCH2CH2-O~-POOH
wherein Y is a C~_"~ straight or branched chain alkyl and n is 1-10,000, or an
oligomer formed
from one of those monomers, as well as mixtures of the foregoing.
Preferably the monomer is of Formula II wherein R~ is H or CH3, and RZ is X-
COOH
wherein X is a C1_,o straight or branched chain alkyl, more preferably ethyl.
More preferably R2
is B-carboxyethyl, e.g. as in B-carboxyethyl acrylate, which is sold in the
form of a mixture with
acrylic acid and oligomers of acrylic acid, under the tradename 13-CEATM by
UCB Radcure, Inc.
17
CA 02233744 2001-06-07
13-CEATM contains both ethylenic unsaturation and carboxylic acid
functionality. Much of the
carboxylic acid functionality comes from acrylic acid dimer. 13-CEATM is a
mixture of about 40%
by weight B-carboxyethylacrylate, about 40% by weight of oligomers of acrylic
acid, and about
20% by weight of monomeric acrylic acid. The B-carboxyethylacrylate component
of this
mixture has the following formula:
O
II
H02C:- CHi- CH~-~ -CH=CH2
In addition, carboxylic acid functional oligomers, such as aromatic acid
methacrylate half
esters and aromatic acid acrylate half esters, are also suitable acid
functional oligomers for use in
the method of the invention. Examples of such oligomers are partial esters of
low molecular
weight copolymers of ethylenically unsaturated dicarboxylic acid anhydrides
such as those
disclosed in U.S. patent no. 4,722,947. These partially esterified copolymers
correspond to the
following formula:
R3 R4 R3 Ra R3 R4
I I I I I
--~ '-'C
I I I I l
C C-0 C=O C=O C=O
x 0~~~~0 y I I Z I I P
OH OR.6 OH OB
18
CA 02233744 2001-06-07
wherein Rl and RZ are each independently hydrogen, C,.2o alkyl, aryl, alkaryl,
cycloalkyl, or
halogen; R3, R-0, and :EtS (see below) are each independently hydrogen, C1_ZO
alkyl, or aryl; and
R6 is the same or different and is alkyl, aralkyl, or an alkyl substituted
aralkyl radical containing
about 1 to 20 carbon atoms as well as oxyalkylated derivatives thereof; and
the subscripts x, y, z,
and p are each whole numbers such that the surn of x, y, z, and p may range
from about 3 to 20;
and x, p, and y are each equal to or greater than 1, and z may be 0; and B is
OAOCOCRSCHz
wherein A is a linear or branched divalent alkylene of from about 1 to 20
carbon atoms, or an
oxyalkylated derivative thereof as described for R6.
Particularly preferred aromatic partial esters of anhydride containing
copolymers are
those sold by Sartomer, Inc. under the SarboxTM tradename, such as Sarbox SB-
400TM,
SB-SOOTM, and SB-600TM. Particularly preferred is aromatic acid methacrylate
half ester in
ethoxylated trimethylolpropane triacrylate, which is sold by Sartomer, Inc.
under the tradename
Sarbox SBSOOESOTN'.
Other suitable carboxylic acid functional monomers include acrylic acid,
bisacrylamidoacetic acid, 4,4-bis(4-hydroxphenyl)pentanoic acid, 3-butene-
1,2,3-tricarboxylic
acid, 2-carboxyethyl acrylate, itaconic acid, methacrylic acid, 4-vinylbenzoic
acid, and mixtures
of these materials.
Examples o;f monomers containing sulfonic acid groups include 2-acrylamido-2-
methyl-
1-propanesulfonic acid; 2-methyl-2-propene-1-sulfonic acid, 2-propene-1-
sulfonic acid, 4-
styrenesulfonic acid, 2-sulfoethyl methacrylate, 3-sulfopropyldimethyl-3-
methacrylamidopropyl
ammonium inner s~~lt, 3-sulfopropyl methacrylate, vinysulfonic acid, and so
on.
19
CA 02233744 2001-06-07
Examples of monomers containing phosphoric acid functional groups include
bis(2-
methacryloxyethyl)phosphate, monoacryloxyethyl phosphate, and so on.
Also suitable for use as the ink. compositions such as disclosed in U.S.
Patent No.
5,487,927. These radiation curable compositions contain a cationically curable
cycloaliphatic
epoxide, preferably one having at least two epoxy groups per molecule.
Polymeric
cycloaliphatic epoxides are suitable also, such as those formed by the
reaction products of
epichlorohydrin and phenol or a phenolformaldehyde resin, diepoxy resin,
epoxidized oils, and
epoxidized polyolefins. Such epoxides include novolac epoxides, glycidyl
ethers of various
types including diglycidyl ethers of bisphenol, diglycidyl ethers of
butanediol, and the like. Also
suitable are homopolymers or copolymers that contain pendant epoxide groups
such as those
made from glycidyl methacrylate or acrylate with or without other
ethylenically unsaturated
monomers. Examples of such cycloaliphatic epoxides are 3,4-
epoxycyclohexylmethyl-3,4-
epoxycyclohexane carboxylate, bis-(3,4-epoxy-cyclohexylmethyl)-adipate,
vinylcyclohexene
diepoxide, bis(2,3-epoxycyclophenyl)ether, epoxidized butadiene, 2,3-epoxy-2-
methylcyclohexylmethyl-3,4-epoxy-2-methylcyclohexane carboxylate, or mixtures
thereof.
These cycloaliphatic; epoxides are sold by Union Carbide Chemicals and
Plastics Company
under the tradename~ CyracureTM
Other radiation curable inks are as set forth in U.S. Patent Nos. 5,571,359
and 5,562,951.
These compositions comprise monomers, oligomers, or other low molecular weight
polymers
made from monomer units such as epoxide, cycloaliphatic epoxide, vinyl
chloride, styrene, ethyl
acrylate, vinyl acetate, difunctional acrylic monomers such as hydroxy alkyl
acrylates or
hydroxy alkyl meth,acrylates, vinyl butyrate, vinul methyl ether, methyl
methacrylate, isobornyl
acrylate, acrylonitrile and mixtures thereof.
CA 02233744 2001-08-O1
The radiation curable ink compositions may also contain other ingredients such
as
pigments, photointiators, photosensitizers, coupling agents, surfactants and
the like.
The radiation curable ink compositions used in the method of the invention may
be clear
or pigmented. If pigmented, ranges of 0.01-SO%, preferably 0.05-40%, more
preferably 0.1-35%
by weight of the total composition, of pigment is suggested. Suitable pigments
include organic
and inorganic pigments. Examples of such pigments are set forth in U.S. patent
no. 5,178,952,
Inorganic pigments include extender pigments such as baryites , barium
sulfate, calcium
carbonate, talc, clay, alumina, titanium dioxide, white carbon, Chinese white,
zinc sulfide,
lithopone, ultramarine, Prussian blue, cobalt, chrome oxide, viridian chrome
green yellows,
oranges, and reds, cadmium, chromium, iron oxides, carbon black, metallic
pigments, aluminum
powder, bronze powder, zinc chromate, strontium chromate, zinc dust, copper,
and so on.
Examples of suitable organic pigments include azo pigments, indolinones,
isoindolinones, vat
pigments, the Lakes, pthalocyanine pigments and so on. The preferred pigrnent
to impart white
color to the ink composition is titanium dioxide. Preferred red and yellow
pigments are
isoindolinones and pyrrolopyrrols as disclosed in U.S. patent nos. 4,415,685;
4,579,949;
4,791,204; 4,666,455; 5,074,918; 4,783,540; 4,914,211; 4,585,878; as well as
U.S. Patent No.
5,571,59, of Kamen, et al.
These pyrrolopyrrols are generally of the formula:
21
CA 02233744 2001-06-07
R, X
R~-- ~ -R3
:X RZ
wherein R, and R2 are each independently alkyl, arylalkyl, aryl, substituted
or unsubstituted
isocyclic or heterocyclic aromatic radicals; R3 and R4 are each independently
H, substituted or
unsubstituted alkyl, ;alkoxycarbonyl, amyl, phenyl, benzoyl, benzyl,
arylalkyl, aryl, alkanoyl,
C5~ cycloalkyl, alkenyl, alkynyl, carbamoyl, alkylcarbamoyl, arylcarbamoyl, or
alkoxycarbonyl;
and X is O or S. Preferred is a compound wherein R, and R2 are each
independently phenyl or
naphthyl, R3 and R4 are hydrogen, and X is O. Particularly preferred is
pyrrolo 3,4-C pyrrol-1,4-
dione, 2,5-dihydro-3,6-di-4-chlorophenyl which has a CAS number 84632-65-5 and
is known by
the common name C.I. pigment red 254. This pigment is commercially available
from Ciba-
Geigy .Pigments Division, Newport, DE, under the tradename Irgazin DPP Red 80.
Other Ciba-
Geigy red pigments sold under the tradename IrgazinTM are also suitable.
Suitable isoindolinones are as set forth in U.S. patent nos. 3,884,955,
3,867,404,
4,978,768, 4,400,507, 3,897,439, 4, 262,120 and 5,194,088. Preferred
isoindolinones are tetrachloro-
cyanobenzoic acid alkyl esters, particularly benzoic acid, 2,3,4,5-tetrachloro-
6-cyano-methyl ester
which is reacted wivth 2-methyl-1,3=benzenediamine and sodium methoxide. This
pigment composition
has the common name C.I. Pigment Yellow 109 and is available commercially from
Ciba-Geigy
22
CA 02233744 2001-06-07
Pigments Division, rdewport DE under the tradename Irgazin yellow 2GLTE. Other
pigments in
the Irgazin Yellow sE:ries as manufactured by Ciba-Geigy are also suitable.
With certain ink colors it may also be desired to include in the ink
composition a
photosensitizer, which is generally defined as a molecule which absorbs
radiant energy which it
then passes on to the; photoinitiator. The photosensitizer then returns to its
energetic ground state
while the photoinitiator is activated and undergoes chemical changes as if it
had itself absorbed
the energy. As the photosensitizer often absorbs energy in a different part of
the spectrum then
does the photointiatior, thus a more effective use of the light source can be
achieved. If a
photosensitizer is used, generally a 1:10 to 1:200, more preferably 1:50 to
1:100 ratio of
photosensitizer to photoinitiator respectively, is suggested. Typical examples
of photosensitizers
which assist in cationic curing are thioxanthone compounds, in particular
isopropyl thioxanthone
which is marketed ~.uider the Escacure ITX trademark by Sartomer. Suggested
ranges of
photosensitizers are; 0.01-20%, preferably 0.05-15%, more preferably 0.1-10%
by weight of the
total ink composition.
The compositions of the invention comprise 0.1-25%, preferably 0.5-20%, more
preferably 1-15% by weight of the total composition of a photoinitiator which
catalyzes the
polymerization of the monomers upon exposure to the radiation by which the
monomers are
curable. There are generally two types of photoinitiators: free radical and
cationic. Free radical
initiators are more commonly used with ethylenically unsaturated monomers and
oligomers,
while cationic photoinitiators are used with epoxy or vinyl ether functional
resins. Suitable free
23
CA 02233744 2001-06-07
radical-type photoiniators include carbonyl compounds such as ketones,
acetophenones,
benzophenones, and derivatives thereof. Examples of such materials include,
for example,
methyl ethyl ketone; benzophenone; benzyl dimethyl ketal; 1-
hydroxycyclohexylphenylketone;
diethyoxyacetophenone; 2-methyl-1-(methylethiophenyl)-2-(4-morpholinyl)-1-
propanone; 2-
benzyl-2-N,N-dimeth~ylamino-1,4(4-morpholinophenyl)-1-butanone; 2,2-dimethoxy-
2-phenyl
acetophenone; 2-methyl-1-[4-(methylthio)phenyl]-2-morpholino propan-1-one; 2-
hydroxy-2-
methyl-1-phenyl-propan-1-one; 4-(2-hydroxyethoxy)phenyl-(2-hydroxy-2-
methylpropyl)ketone;
and a mixture of bis(2,6-dimethyoxybenzoyl)-2-4-4-trimethylpentyl phosphine
oxide and 2-
hydroxy-2-methyl-1-phenyl-propan-1-one.
If the monomers in the ink compositions used in the method of the invention
cure by
cationic mechanism:., it is desirable to add a cationic photoinitiator which
catalyzes cross-linking
of the resin upon exposure to the radiation to which the resin is sensitive.
Various types of
cationic photoinitiators are suitable. Both ionic cationic photoinitiators
such as opium salts or
organometallic salts are suitable as well as non-ionic cationic
photoinitiators such as
organosilanes, latent sulphonic acids and the like. Preferred are
photosensitive opium salts, in
particular, opium salts such as those disclosed in U.S. patent pos. 4,058,401,
4,138,255,
4,161,478, and 4,17:5,972. Triaryl svlphonium salts are most preferred, in
particular triaryl
sulphonium salts such as those sold by Union Carbide under the tradename
Cyracure UVI
6990TM and 6974TM. Also suitable are ferrocenium salts such as those sold
under the IrgacureTM
tradename by Ciba-~Geigy, in particular Irgacure 261TM. Sulphonyloxy ketones
and silyl benzyl
ethers are also good cationic photoinitiators. A detailed analysis of the
mechanism of cationic
24
CA 02233744 2001-06-07
curing is disclosed in "Photosensitized Epoxides as a Basis for Light-Curable
Coatings" by
William R. Watt, American Chemical Society Symposium, Ser. 114, Epoxy Resin
Chemistry,
Chapter 2, 1979, and in "Chemistry and Technology of UV and EB Formulation for
Coatings,
Inks, and Paints," Volume 3, entitled "Photoinitiators for Free Radical and
Cationic
Polymerization, K.K. Dietliker, pages 332-374 (1991). Photosensitive onium
salts are used as
photoinitiators in cationic curing, in particular, onium salts such as those
disclosed in U.S. patent
nos. 4,058,401, 4,13f>,255, 4,161,478, and 4,175,972. Triaryl sulphonium salts
are most
preferred, in particular triaryl sulphoniurn salts such as those sold by Union
Carbide under the
tradename Cyracure UVI 6990 and 6974.
~l Defoa_m__i_n_g Agen
Suitable defoaming agents will cause the ink to apply smoothly on the glass
substrate
without bubbles or cmevenness. If defoaming agents are used, ranges of 0.01-
20%, preferably
0.05-15%, more preferably 0.1-10% by weight of the total composition is
suggested. A wide
variety of defoamers are suitable, for example, those sold by BYK Chemie under
the BYK
tradename or by Tego Chemie Services, USA under the FOAMEXTM tradename.
Particularly
suitable is FOAMEX NTM, a silicone based defoaming agent, which comprises
dimethylpolysiloxane dispersed in silicic acid. Also preferred is BYK-033
which is a mixture of
about 5% by weight ethoxylated alkylphenol, and 92% by weight of heavy
petroleum distillates
(or paraffin). Other defoaming agents are also suitable, for example,
polyvinyl ethers such as
BYK-052 and BYh:-053. BYK-052 is polyvinylbutyl ether in Stoddard solvent. BYK-
354, a
polyacrylate solution, and BYK-022, a mixture of hydrophobic solids and foam
destroying
polysiloxanes in polyglycol, may also be used.
i-. CA 02233744 1998-03-31
(~ Other In~~edients
Suitable photoinitators, coupling agents, and surfactants may be added to the
ink
compositions. Examples of such materials and ranges for addition are as
mentioned above. In
the preferred embodiment of the invention both the primer composition and the
radiation curable
ink composition contain a coupling agent so that the coupling agent is, in
essence, applied to the
vitreous article twice.
In the method of the invention, the primer composition is applied to the
vitreous article,
which is preferably glass. The composition may be applied in a variety of
ways, such as by
spraying, dipping, roller coating, painting, screening, and the like.
Preferably, the primer
composition is applied to the article by spraying, either manually or online
in a factory setup
where an appropriate container integrated into the line will spray the
composition on the glass
container before it passes on to the printing workstation. In most factory
setups the line setup
will be such that the glass article will emerge from the molding oven and pass
through the
spraying station prior to entering the printing workstation. The article may
be sprayed with the
primer composition while the article is still warm, or the article can be
sprayed while it is at room
temperature. Preferably, the primer composition is applied to the article
while it is an elevated
temperatures, for example from 100 to 400° F., just after it emerges
from the molding oven.
After the primer composition is applied to the article it may enter the
printing workstation
immediately, or it may be printed at some later time. In another preferred
embodiment, the
article is sprayed while at room temperature, and then heated to an elevated
temperature of about
150 to 200, preferably 160-170° F. In the alternative, the article is
sprayed while at room
sss4~-~ .ax 26
~'CA 02233744 1998-03-31
i_
temperature, then the composition is dried with an air gun or dryer at room
temperature. The
article then enters the printing station. Alternatively, the article may be
heated first to an
elevated temperature, then sprayed, before entering the printing station. The
solvent portion of
the sprayed composition rapidly evaporates leaving the coupling agent and
other ingredients on
the surface of the article. It has been found that if the article is heated to
an elevated temperature
after it is sprayed, the coupling agent more readily reacts with the glass
surface to form a
chemical bond.
After the primer composition is sprayed onto the article and the solvent has
substantially
evaporated, it is decorated with the radiation curable composition. Obviously,
the primer
composition is applied to the article in the area where the radiation curable
indicia is applied.
The ink composition is applied to the article to be decorated in a
predetermined design
using a variety of printing methods including screen printing, offset
printing, gravure, hand
painting, curtain or roller coating, and the like. After the ink is applied
the substrate or article is
irradiated with UV or actinic radiation using a conventional light source. The
term "UV" means
ultraviolet light which generally has a wavelength of 190 to 500, preferably
200 to 450
nanometers. The term "actinic" means radiation having a wavelength of 200 to
600 nanometers.
Electron beam may be used instead of a UV light source. If a tJV conveyer is
used, it is set up so
that the substrate passes through the beam of radiation for an amount of time
appropriate to
completely cure the ink composition and cause it to adhere to the substrate.
If desired, the
substrate may be moved through the conveyer in one or more passes to achieve
the required
curing. The appropriate time varies depending on the ink formula, but
generally curing is
complete in a period of time ranging from fractions of a second to several
minutes. It is
sss43-~.doc 27
CA 02233744 2001-06-07
preferred, that by the Mime the decorated substrate or article is removed from
the conveyer, the
ink is completely cured and fused to the substrate surface, providing a bright
true color. While,
the newly screened glass container may be exposed to slightly elevated
temperature prior to UV
curing the applied ink; on the substrate, or to an additional post-UV cure
application of heat to
S finally polymerize the ink on the substrate, this is not really necessary.
This ink is well suited for
use in automated systems such as the multiple color printing apparatus
disclosed in
U.S. Patent No. 5,98:1,326, by Kamen, et al., entitled "Apparatus and Method
for Screen Printing
Radiation Curable Compositions", or with the methods disclosed in U.S. Patent
No. 5,562,951,
by Kamen et al., entitled "Method for Printing Articles with Multiple
Radiation Curable
Compositions".
In the method of the invention, multiple colors may be applied to the glass
substrate by
spraying the article, then applying and curing one color, and repeating the
process until as many
successive colors as desired have been applied to the glass in complete or
partial registration.
In another embodiment of the invention, it is possible to apply an unpigmented
ink
composition on the glass substrate in predetermined design after spraying the
substrate with the
primer composition. For example, a substrate such as a container may be
decorated in a pre-
determined design by first spraying the article with the primer composition,
then silk screening
the unpigmented irik composition on the substrate and curing with the
appropriate radiation. A
layer of hot stampizig foil is then compressed against the substrate with a
press which is heated to
a temperature su~cient to cause the hot stamping foil to adhere to the printed
ink design but not
to the ink-free areas of the glass. Hot stamping foil is generally a laminate
comprised of a carrier
28
~CA 02233744 1998-03-31 i'
material (often polyester or a similar material capable of release), a release
film between the
carrier and a subsequent decorative coat which is usually color or a
metallized coat, most often
aluminum or colored aluminum. The foil may contain other optional layers such
as one or more
protective layers, hot melt adhesive layers, etc. between the metallized layer
or layers and the
carrier material. More specifically, hot stamping foil can be defined as a
multilayer web
comprised of a backing film carrier, a release coating, one or more protective
top coatings, one or
more color coatings, and a hat melt adhesive in that order. The hot stamping
foil is then
compressed against the container with the hot melt adhesive layer being
compressed against the
substrate. The compress, which may be a standard hot stamping press or a hand
held press, is
heated to a temperature sufficient to cause the hot melt adhesive layer of the
hot stamping foil to
adhere to the ink decorated portion of the substrate. Generally this
temperature ranges from
about 250 to 400° F. Temperatures higher than this may cause
deterioration of the hat stamping
foil. The application of heat causes the adhesive side of the hot stamping
foil to become
adhesively adhered to the ink design but not to the ink-free areas of the
substrate. When the
compress is removed, a portion of the foil laminate adheres to the ink
decoration but not to the
ink free areas of the glass. In particular, adhered to the ink design on the
substrate is the hot melt
adhesive layer, the color coatings, and the protective top coatings, in that
order, of the hot
stamping foil. Portions of the release coating may or may not be adhered to
the protective top
coating because the release coating is designed to melt upon application of
heat and cause the
polyester carrier backing layer to release from the protective top coat layer
and some remnants
may remain.
sss4~-~.dx 29
' ~CA 02233744 1998-03-31
The resulting hot stamped substrate exhibits a metallic gold, silver, or
colored appearance
depending on the color of the hot stamping foil.
The compositions and method of the invention appreciably improve adherence of
radiation cured decorative indicia to vitreous articles.
The invention will be further described in connection with the following
examples which
are set forth for the purpose of illustration only.
A primer composition in accordance with the invention was made according to
the
following formula:
w w°
MEMO-E* 0.5
Tegoglas TS * * 0.5
Water 99.0
*A silane sold by Huts America, Somerset, New Jersey, which has the formula
CH2=C(CH3)-COO-(CH~3-Si(OCH3)3
** an aqueous solution of PEG-5 glycerylmonostearate, Goldschmidt Corporation,
McDonald,
PA.
The water was poured into a separate container, and a few drops of acetic acid
added to
adjust the pH of the water to a range of 3.5 to 4.5, preferably 3.8 to 4Ø
The Tegoglas TS was
added with gentle stirring until completely dissolved and the solution was
clear. The MEMO
was added with stirring until dissolved and the solution was clear. The pH of
the composition
was 3.8. The composition was poured into a container equipped with a spray
nozzle.
sss4~.~.dx 30
I
CA 02233744 2001-06-07
A red ink composition having the following formula was prepared as follows:
~L~Le
SB500 E50~ 15.0
PhotomerTM 61732 35.0
CN 1043 15.0
SR 25644 2.0
SR 5025 13.0
Irgazin DDP Red BO~' 12.0
I-9071 3.0
I-18008 3.0
Foamex N9 1.0
MEMO1 0.8
A-1310" 0.8
Aromatic acid methacrylate half ester in ethoxylated trimethylolpropane
triacrylate (50:50,
w/w), Sartomer Company, Inc;., Exton, PA.
2 UV/EB curable acrylate oligomer, Henkel Corp. Kankakee, IL
3 Epoxy acrylate + tripropylene glycol acrylate
4 2(2-ethoxyethoxy)ethylacrylate, Sartomer Company, Exton, PA.
5 Ethoxylated trimethylolpropane triacrylate, Sartomer Company
6 Pigment red 254, I;diketopyrrolopyrrole), Ciba Geigy Corp., Hawthorne, New
York
2-methyl-1-[4-me~thylthio)phenyl]-(4-morpholine)-1-propanone, Ciba Geigy Corp
A mixture of 25% by weight bis(2,6-dimethyoxybenzoyl)(2,4,4-
trimethylpentyl)phosphine
oxide and 75'% by weight 1-hydroxycyclohexylphenyl ketone, Ciba Geigy
Polydimethylsilo~;ane dispersed in silicic acid, Goldschmidt Corporation,
Hopewell, VA.
'° 3-methacryloxypropyltrimethoxysilane, Huls America.
11 Gamma isocyanotopropyltriethoxysilane, Osi Specialties, Inc., Danbury, CT.
The composition of Example 1 was sprayed on brown glass alcoholic beverage
bottles
heated to a temperature of 160 to 1?0° F. After the bottles cooled to
room temperature, at which
point they were substantially dry, the composition of Example 2 was applied to
the glass bottles
by silk screening indicia onto the glass through a 255 mesh screen. The ink
was cured on the
glass by exposure to a 9 mm. ultraviolet D bulb having a power of 600 watts
per inch,
31
~
~-~.CA 02233744 1998-03-31
manufactured by Fusion Inc., for about half a second. The decorated bottles
were allowed to
cool.
Adhesion was tested on several of the cooled bottles by immersion in water
heated to a
temperature of 70° C. (158° F) for 1 hour. The ink remained
adherent to the glass, although in
some areas the ink was thin and spotty.
The remaining bottles were divided into three groups and subjected to a post-
cure heat
treatment as follows:
(a) 250° F. (121.1° C.) for 2 minutes
(b) 290° F. (143.3° C.) for 1 minute
(c) 300° F. (148.9° C.) for 1 minute
Bottles (a), (b), and (c) were immersed in water heated to 70° C. for
one hour. Adhesion
of the ink to (a), (b), and (c) was excellent. The ink appeared to be
untouched. No evidence of
peeling or chipping was present.
Bottles (a), (b), and (c) were then immersed in a solutions of concentrated
NaOH heated
to a temperatures of 150° and 160° F. to evaluate resistance of
ink to removal by hot caustic.
A number of (a) bottles were immersed in 4.5% NaOH heated to a temperature of
about 150° F.
The ink composition on one of the (a) bottles washed off after 4 minutes.
Three more of the (a)
bottles were immersed in 4.5% NaOH heated to a temperature of 160° F.
The ink was removed
on the bottles after 2 minutes, 53 seconds; 2 minutes 45 seconds; and 2
minutes 40 seconds;
respectively.
sas4~-~.doo 32
i
~;,CA 02233744 1998-03-31
The composition of Example 1 was sprayed onto glass containers having a
temperature of
170° F. After the bottles cooled to room temperature, the composition
of Example 2 was applied
to the glass bottles by silk screening indicia onto the glass through a 255
mesh screen. The ink
was cured on the glass by exposure to a 9 mm. ultraviolet D bulb having a
power of 600 watts
per inch, manufactured by Fusion Inc., for about half a second. The bottles
were divided into
four groups and heat treated as follows:
('noun Tem~(E1 Time
1 165 2 min.
2 1 g0 1 min. 1 S sec.
3 200 2 min.
4 220 1 min. 30 sec.
The following results were obtained after immersing the treated bottles in
water having a
temperature of 70° C. for one hour:
1 S ~i2~p Temueraihme F A esion
1 165 good
2 1 g0 small parts not good,
majority excellent
3 200 small parts not good,
majority excellent
220 excellent
The composition of Example 1 was sprayed onto glass containers having a
temperature of
170° F. After the bottles cooled to room temperature, the composition
of Example 2 was applied
to the glass bottles by silk screening indicia onto the glass through a 255
mesh screen. The ink
sasa~.~ .do° 33
-_
CA 02233744 1998-03-31 '
was cured on the glass by exposure to a 9 mm. ultraviolet D bulb having a
power of 600 watts
per inch, manufactured by Fusion Inc., for about half a second.
The composition of Example 1 was sprayed on glass alcoholic beverage bottles
at room
temperature using a standard spray dispenser. The bottles were then exposed to
a Varitemp Heat
Gun from Master Appliance Corporation, Racine WI, for less than 60 seconds
until they reached
a surface temperature of 160 to 170° F. The ink composition of Example
2 was applied to the
glass bottles by silk screening indicia onto the glass through a 255 mesh
screen. The ink was
cured on the glass by exposure to a 9 mm. ultraviolet D bulb having a power of
600 watts per
inch, manufactured by Fusion Inc., for about half a second. The decorated
bottles were allowed
to cool to room temperature. The adhesion of the decorative indicia to the
bottles was tested as
follows, with the ratings excellent, good, fair, poor, unacceptable being
correlated with grades SB
to 1B respectively, on the cross-cut tape test as defined by the ASTM,
Designation No. 3359-87:
pasteu_ri ation - 70g ~. one hour
Two bottles were immersed in water heated to a temperature of 70° C.
for one hour. The
adhesion on both bottles was excellent, i.e. corresponded to rating SB on the
cross-cut tape test.
While the invention has been described in connection with the preferred
embodiment, it is
not intended to limit the scope of the invention to the particular form set
forth but, on the
contrary, it is intended to cover such alternatives, modifications, and
equivalents as may be
included within the spirit and scope of the invention as defined by the
appended claims.
sssa~~.aoc 34