Note: Descriptions are shown in the official language in which they were submitted.
CA 02234~04 1998-04-07
IMPL~N~ABL~ TUBULAR PROST~E~IS
BACRGROUND OF T~B 1L~V~1ON
1. Fie1d Of the InVentiOn
The present invention relates to an implantable
tubular prosthesis having a textile substrate with a
fluid-tight microporous lining.
2. De~cription Of the Prior Art
Tubular prostheses are commonly used as vascular
grafts to replace damaged or diseased veins and arteries.
To maximize the effectiveness of any prosthesis, it is
desirable that the prosthesis have characteristics which
closely resemble that of the natural body lumen which it
is replacing.
Presently, conventional tubular prostheses and,
more specifically, vascular grafts are formed by either
weaving, knitting or braiding synthetic fibers into a
tubular structure or using a polymer such as polytetra-
fluoroethylene to create a tubular structure for use as
a prosthesis. Tubular textile structures have the
advantage of being naturally porous, which allows desired
tissue ingrowth and assimilation into the body. Porosity
must be balanced to allow for ingrowth of surrounding
tissue, yet minimize leakage during the initial
implantation. Attempts to control porosity and provide a
sufficient fluid barrier have focused on tighter stitch
construction such as knitted or woven double-velours and
biodegradable natural coatings such as collagen or
gelatin. While these grafts sought to overcome the
difficulties in achieving the porosity/fluid-tight
balance, they failed to adequately address the natural
tendency of tubular structures to kink or collapse when
the graft is twisted or bent during or subse~uent to
implantation. Thus, the prior art solutions to the
CA 02234~04 1998-04-07
.. . .
porosity/fluid-tight balance left ùnanswered the problems
of kinking and overall handling.
One conventional solution to the kinking and
collapsing problems has focused on the reinforcement of
the prosthesis walls using reinforcing fibers, rings, or
bands circumferentially placed on the tubular structure.
Additional reinforcement of this kind, however, has the
disadvantage of reducing the radial and/or longitudinal
compliance of the graft due to the increased stiffness of
the reinforcing member. A reduction in compliance
reduces the area through which blood can flow, thereby
compromising the ability of the prosthesis to adjust to
body conditions and perform naturally. Additionally,
reinforcing members are generally made from solid
structural materials which cannot be penetrated by
cellular ingrowth from surrounding tissue and may even
cause the erosion of the surrounding tissue during
contraction.
Another method of increasing the kink and crush
resistance of textile grafts is to crimp the graft, i.e.,
longitudinally compress the tubular structure. Crimping
is generally described in U.S. Patent No. 3,142,067.
While crimping serves to add a dimension of kink and
crush resistance to the graft, the intraluminal surface
formed by crimping includes peaks and valleys which
create hemodynamic turbulence within the graft as blood
passes therethrough. This turbulence affects the rate of
flow and the peaks and valleys formed on the intraluminal
surface contribute to excessive thrombus formation and
deposition of plaque.
Another disadvantage of presently available tubular
textile prostheses, in particular woven and braided
grafts, is that sutures tend to pull out or tear the
fabric thereby making it difficult to attach the
CA 02234~04 1998-04-07
. . . ~
prosthesis to the existing body lumen and to prevent
leakage at this junction. Furthermore, textile tubular
prostheses formed from a synthetic yarn tend to have ends
of the tube which easily ravel. Once the ends ravel or
fray, suturing to the existing body lumen becomes
extremely difficult.
Microporous tubing formed by stretching polytetra-
fluoroethylene (PTFE) has also been used as implantable
prostheses and especially as vascular grafts. PTFE
porous tubes are considered by some to be superior in
certain respects to conventional prostheses made of
knitted or woven fabrics. The stretched or expanded PTFE
tube has a microfibrous structure defined by the presence
of nodes inter-connected by fibrils. While PTFE grafts
have the advantage of being generally fluid-tight without
the use of pre-clotting or specialized coatings, these
grafts have limitations in their tear and tensile
strength and compliance properties. PTFE grafts often
require wrapping with a reinforcing support film to
improve undesirable dilation. Reinforcement materials
tend to impede the ingrowth of tissue necessary for rapid
healing. In addition, PTFE grafts tend to be non-
compliant as compared to textile grafts and natural
vessels, thereby lacking many of the mechanical
properties advantageous to textile grafts.
From the previous discussion it is apparent that
both conventional textile prostheses and PTFE prostheses
have respective benefits and disadvantages, but neither
offers properties which solve all of the aforementioned
problems.
Accordingly, it would be advantageous to provide a
new and improved implantable tubular prosthesis which
combines the best attributes and properties of each of
the conventional grafts. More specifically, it would be
CA 02234~04 1998-04-07
particularly desirable to form a prosthesis which has the
following characteristics: an outer surface porosity
wh~ch encourages tissue ingrowth into the prosthesis;
ravel and fray resistance for better suture retention and
tailoring; longitudinal compliance for ease of
implantation, sizing and natural vessel simulation; and a
fluid-tight lumen without the need for pretreating,
coating or pre-clotting.
~UMMARY OF THB INVENTION
The present invention addresses aforementioned the
problems associated with the prior art and provides a
soft-tissue implantable prosthesis in the form of a
composite structure including a textile substrate and an
integrated polymeric liner. The textile substrate
includes an intraluminal surface having the liner affixed
thereto, thereby rendering the tubular prosthesis fluid-
tight. Accordingly, the outer surface formed by the
textile substrate has the advantage of sufficient pore
size to enhance tissue ingrowth and promote healing as
well as other advantages associated with textile
prostheses, such as flexibility and kink resistance. The
liner provides a smooth, fluid-tight lumen to enhance
fluid flow and which is made from a polymeric material
which is naturally antithrombogenic.
The liner formed in accordance with the present
invention is preferably formed from a polymeric material.
Typical polymers for use in making the liner include, but
are not limited to polytetrafluoroethylene, urethanes,
silicones and polyesters. Preferably, expanded
polytetrafluoroethylene is used to form the liner thus
creating a microporous structure. The liner wall
thickness need only be thick enough to provide a fluid-
tight barrier to the intraluminal surface of the textile
substrate. Thus, liner wall thicknesses are preferably
CA 02234~04 1998-04-07
.
thin and on the order of about 10 to about 50 microns.
The textile substrate formed in accordance with the
present invention may be made by weaving, knitting or
braiding yarns to form a tubular structure. In the
preferred emho~iment~ the composite prosthesis including
the textile substrate and the liner is heat conditioned
to fuse the liner to the textile substrate. Thus, in one
embodiment the textile substrate is formed from fibers
having a melting temperature and bonding compatibility
substantially similar to a material forming the liner.
In an alternative embodiment, the textile substrate
may include a fusible fiber having a low melting
temperature, the fusible fiber flowing onto the liner
when melted for securing the liner to the textile
substrate. In the case where eYr~ed PTFE is used to
form the liner, it is preferable that the fusible fiber
have melt flow properties which allow the melted fiber to
flow into the pores of the liner to secure the liner to
the textile substrate.
In yet another embodiment, the textile substrate may
be formed from any known fiber and the liner may be
affixed to the substrate using an adhesive, by sewing the
components together or by any other mechanical coupling
means.
The textile substrate formed in accordance with the
present invention may be formed by warp knitting to
create a velour surface. The loops forming the velour
surface are preferably on a the exterior surface to
create a single-velour fabric. Single-velour fabrics
have many favorable properties with respect to porosity,
compliance, and suture retention.
Furthermore, the textile substrate may be formed
CA 02234504 1998-04-07
from yarns, rovings, tapes or other stranded materials.
Some of the yarns may be bioabsorbable while other yarns
are merely biocompatible. 8ioabsorbable yarns are
preferably used to create an initial porosity different
from the porosity once the bioabsorbable material has
been absorbed into the body. For example, once a
bioabsorbable yarn is absorbed into the body, a void or
pore remains in its place. Additionally, the yarns used
to form the textile substrate may be flat, twisted,
textured or preshrunk.
The invention is also directed to a process for
preparing a soft-tissue prosthesis. The process includes
the steps of winding a polymer over a smooth mandrel to
form a cylindrically-shaped liner, positioning a tubular
textile substrate over an outer surface of the liner,
heating the liner and textile substrate to a temperature
sufficient to melt a portion of one of either the textile
substrate or liner and cooling the textile substrate and
liner thereby fusing the liner to the textile substrate.
In one mhodiment, the textile substrate may be
formed from a material which has a similar melting
temperature and bonding compatibility to that of the
liner. In an alternative embodiment, the liner is formed
from expanded PTFE and the textile substrate includes a
meltable yarn such that the molten yarn flows into the
pores of the liner and, when cooled, fuses the liner to
the textile substrate.
The present invention is also directed to a method
of repairing a diseased blood vessel of a patient. The
method includes removing a diseased portion of a blood
vessel from the patient leaving a first and second open
end of the blood vessel, inserting a tubular prosthesis
between the first and second end of the blood vessel, the
tubular prosthesis being formed from a textile substrate
CA 02234~04 1998-04-07
having an intraluminal surface and a liner affixed to the
intraluminal surface of the textile substrate such that
the liner renders the tubular prosthesis blood-tight, and
securing the tubular prosthesis to the first and second
open ends of the blood vessel to form a continuous lumen
through which blood may flow.
Thus, the present invention overcomes many of the
shortcomings associated with prior art soft-tissue
prostheses. The soft-tissue prosthesis formed in
accordance with the present invention utilizes the
advantages of both a textile prosthesis and a polymer
prosthesis to create a composite structure having a
smooth, fluid-tight intraluminal surface yet providing a
porous outer structure to encourage ingrowth of
connective tissue and promote healing.
A preferred form of the textile substrate having a
fluid-tight liner, as well as other embodiments, features
and advantages of this invention will be apparent from
the following detailed description of illustrative
embodiments thereof, which is to be read in connection
with the accompanying drawings.
BRIEF DE8CRIPTION OF THE DRAWING8
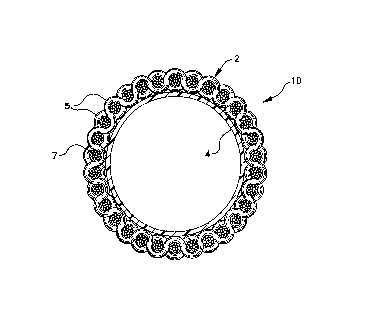
Fig. 1 is a cross-sectional view of a tubular
prosthesis formed in accordance with the preferred
embodiment of the present invention; and
Fig. 2 is a longitudinal cross-sectional view of a
tubular prosthesis formed in accordance with the
preferred embodiment of the present invention.
Fig. 3 is an illustration of a single-velour fabric
used in accordance with an embodiment of the present
invention.
CA 02234~04 1998-04-07
,
DETAILED DE8CRIP~ION OF T~E lNv~ lON
The present invention addresses the problems
associated with prior art textile prostheses and tubular
prostheses formed from polymers and provides a tubular
prosthesis in the form of a composite structure including
a textile substrate and a polymer lining affixed to an
internal lumen of the substrate. The prosthesis formed
in accordance with the present invention overcomes many
of the disadvantages of presently available conventional
tubular prostheses including controlling the porosity at
the intraluminal and extraluminal surfaces of the
prosthesis, while also providing a prosthesis which has
enhanced ravel and fray resistance and better suture
retention capabilities. Furthermore, the present
invention provides a tubular prosthesis which can be
designed to have characteristics closely resembling the
properties of a natural body lumen.
For purposes of this application, a tubular, soft-
tissue prosthesis is defined as any artificial substitute
for a natural body lumen such as a vein, artery,
esophagus or a bile duct. Although some of the
discussion in the preferred embodiment of this invention
describes a vascular graft, it is envisioned that the
composite structure including a textile substrate having
a polymer lining formed in accordance with the present
invention can be useful as a prosthesis for any soft-
tissue body lumen. Naturally, the composite structure
would be designed to meet the specific requirements of
the body lumen it is replacing.
Referring to Fig. 1, the soft-tissue prosthesis
of the preferred embodiment of the present invention
comprises a textile supporting sleeve 2 formed in the
shape of a tube and a tubular polymer lining 4 which is
affixed to an internal lumen of the textile supporting
CA 02234~04 1998-04-07
sleeve 2. The polymer lining 4 may be affixed to the
textile supporting sleeve by adhesively laminating,
separately sewing, meltably fusing or otherwise
connecting the two components. The composite structure
S of the present invention 10 thus provides a soft-tissue
prosthesis having the advantages of a textile prosthesis
with respect to outer surface porosity, compliance and
flexibility, yet the thin polymer lining 4 provides a
smooth, fluid-tight internal lumen for the composite
structure 10.
Fig. 1 is a cross-sectional view of a polymer lined
textile soft-tissue prosthesis formed in accordance with
the present invention. Fig. 1 illustrates a woven
textile supporting sleeve 2 which is woven from a
plurality of warp ends 5 and filling yarn 7. The textile
supporting sleeve 2 may be formed by knitting, weaving or
braiding a tubular structure having a desired outer
diameter to correspond to the body lumen for which it is
replacing. The tubular textile sleeve 2 provides the
soft-tissue prosthesis with mechanical strength, suture
retention, perigraft tissue attachment, radial support
and kink resistance.
As illustrated in Fig. 1, the tubular textile
substrate 2 is lined with a polymer to create a fluid-
tight barrier through the lumen of the composite
structure 10. The liner 4 formed in accordance with the
preferred embodiment is preferably fluid-tight yet
microporous. Furthermore, the liner 4 provides a very
smooth inner surface which enhances fluid flow through
small caliber soft-tissue prostheses. In the preferred
embodiment, the lining 4 is formed from expanded
polytetrafluoroethylene (ePTFE) although a variety of
polymers may be used. Furthermore, it would not be
necessary for the liner to have a large wall thickness
since it is acting as a fluid barrier and not as a
CA 02234~04 1998-04-07
support structure. Accordingly, a thin liner of about 10 to about 50
microns may be used. As previously described, the polymer liner may be
affixed to the textile substrate by any mechanical means such as stitching
or use of an adhesive. Preferably, the liner is affixed to the textile
5 substrate by heat conditioning the composite structure 10 to fuse the liner
4 to the intraluminal surface of the tubular textile substrate 2.
The composite soft-tissue prosthesis formed in accordance
with the present invention provides an implantable body lumen having
10 characteristics which more closely resemble that of a natural body lumen
in comparison to conventional polymer prostheses and textile prostheses.
For example, a vascular graft formed in accordance with the present
invention includes a liner 4 which forms the intraluminal surface of the
structure to be a smooth surface having a low porosity to prevent leakage
15 of blood and the formation of excessive thrombus on the intraluminal
surface. Conversely, the textile sleeve 2 can be woven, knitted or braided
to have a greater porosity or even a textured surface to enhance the
ingrowth of connective tissue into the vascular graft. Additionally, the
vascular graft may be formed having a relatively small diameter (less than
20 about 6mm) yet provide enhanced fluid flow due to the smooth liner 4 and
tissue ingrowth due to the porosity of the textile sleeve 2.
In a preferred embodiment, the textile substrate 2 may be
formed by weaving to create a velour surface. A single-velour surface is
25 created by a weaving technique described in commonly-owned U.S.
Patent No. 5,178,630, entitled, "Ravel-Resistant, Self-Supporting Woven
Graft".
CA 02234~04 1998-04-07
Referring to Fig. 3, the textile substrate 2 may be
woven having a yarn 6 which passes bac~ and forth through
a wall or trellis of the fabric forming a loop. These
loops constitute the velour or pile. The loops are
S formed on a single surface i.e. the exterior surface, to
create a single-velour fabric. The yarn 6 is pulled
tight on the inner surface of the textile substrate to
create a somewhat smooth surface. The liner 4 of the
preferred embodiment may be adhesively laminated,
separately sewn, meltably fused or otherwise connected to
the intraluminal surface of the single-velour substrate
of the preferred embo~i~?nt of the present invention.
The woven single-velour textile substrate includes the
advantages of being inherently kink resistant, strong,
lS longitll~;n~lly flexible, non-crimped and high in suture
retention. The single-velour substrate may be woven from
any type or combinations of fibers, including but not
limited to polyesters, polypropylenes and
polytetrafluoroethylenes.
In an alternative embodiment, the tubular textile
substrate 2 may also include a fiber comprising a
meltable fusible material. The fusible fiber may be
added to the textile substrate to aid in preventing
ravelling or fraying which may occur at the ends of the
textile tube. In such an embodiment, the textile tube
-including the fusible fiber, is heated to melt the
fusible fiber onto the surrounding yarns thereby further
enhancing the ravel and fray resistance of the textile
structure and providing a more suitable structure for
suturing to a natural body lumen.
The tubular textile substrate 2 formed in accordance
with the present invention may be woven, knitted or
braided from yarns, rovings, tapes or other stranded
material. Some of the yarns may be bioabsorbable while
other yarns are merely biocompatible. By utilizing non-
CA 02234~04 1998-04-07
12
woven tapes, such as spunbonded fabric slit into, for
example, 1/16" widths, a structure having excellent
suture retention may be formed. In this regard, the
spunbonded tape is readily pierced by a suture needle yet
possesses high tear strength and positive anchoring.
As mentioned above, the textile substrate 2 of the
composite soft-tissue prosthesis 10 formed in accordance
with the present invention may include one or more yarns
formed from bioabsorbable materials. Suitable
bioabsorbable materials include but are not limited to
poly (glycolic acid), poly (lactic acid), polydioxanoes,
polyoxalates, poly (~-esters), polycarbonates,
polyanhydrides, polyacetals, polycaprolactones, poly
(orthoesters), polyamino acids, polyurethanes,
polyaminocarbonates, polyamindes, poly (alkyl
cyanoacrylates), sebacic acid, polyethylene glycol,
polyphosphazene, bis (p-carboxy-phenoxy) propane, bis
(p-carboxyphenoxy) methane and copolymers and mixtures
thereof, provided that these materials can be formed into
a fiber suitable for use with the knitting, weaving or
braiding apparatus being used.
A single or multiple bioabsorbable yarn may be used
in the textile portion of the composite soft-tissue
prosthesis. Thus, the initial porosity will increase
once the bioabsorbable material has been absorbed into
the body.
In the preferred embodiment of the present
invention, synthetic yarns are used to form the textile
portion of the composite soft-tissue prosthesis. The
yarns may be flat, twisted, textured or pre-shrunk.
Preferably, the yarns are made from thermoplastic
materials including, but not limited to, polyesters,
polypropylenes, polyethylenes, polyurethanes and
polytetrafluoroethylenes and the like. The yarns may be
CA 02234~04 1998-04-07
of the multifilament, monofilament or spun type.
Multifilaments are preferred to increase flexibility.
Where enhanced crushed resistance is desired, the use of
monofilaments has been found to be effective.
Additionally, the yarn type and yarn denier for the
textile portion of the composite soft-tissue prosthesis
may be chosen to meet the design requirements (porosity,
flexibility and compliance) of the prosthesis, e.g.
vascular graft, being formed. Yarn denier denotes the
linear density of the yarn (number of grams mass divided
by 9,000 meters of length). Thus a yarn having a small
denier, e.g 20, would correspond with a very fine yarn,
whereas a yarn having a large denier, e.g. 1000, would
correspond to a heavy yarn. The yarns used to form the
textile portion of the present invention may have a
denier from about 20 to about 1000, and preferably from
about 40 to about 300.
The type of yarn chosen and the denier of the yarn
are important in order to form a soft-tissue prosthesis
and, more specifically, a vascular graft having proper
pore size. As previously emphasized, porosity is
important when designing a vascular graft because the
intraluminal surface must have pores small enough to
prevent the graft from leaking blood, while the outer
surface must have pores large enough to permit ingrowth
of connective tissue and promote healing. The composite
soft-tissue prosthesis of the present invention is
particularly well suited to have proper pore sizing on
both the intraluminal surface and outer surface since the
composite structure utilizes the benefits of both textile
and polymeric extrusion type prostheses. Thus, the
polymer lining 4 provides a smooth, microporous
intraluminal surface which is substantially fluid or
blood-tight. Additionally, the smooth intraluminal
surface reduces excessive formation of thrombus and
CA 02234~04 1998-04-07
.
promotes fluid flow therethrough. The outer surface is
formed from a textile substrate 2 having pores large
enough to permit connective tissue ingrowth into the
soft-tissue prosthesis to promote healing. Since the
textile substrate is lined with a thin polymer, there is
no further need to treat, coat or impregnate the textile
substrate to make it leak-resistant.
In an alternative embodiment of the present
invention, axial yarns may be dyed and inserted into
the textile portion of the soft-tissue prosthesis.
The colored axial yarn positioned on the outer surface
of the prosthesis aids the surgeon during implantation
to indicate whether the prosthesis is accurately aligned
during the procedure. Preferably, the dyed axial yarn is
black in color, formed from yarns of 40-300 denier.
A composite soft-tissue prosthesis formed in
accordance with the present invention may be made by
first choosing a mandrel with an outside diameter
corresponding to an inside diameter of a natural body
lumen which is to be replaced. The mandrel preferably
has a smooth outer surface. The liner may be produced
from expanded PTFE film or other suitable polymer, which
has been slit into a narrow tape (3-10 mm). The expanded
PTFE tape is wound onto the smooth mandrel to form the
liner. The textile substrate is made having an inner
diameter close to the outer diameter of the expanded PTFE
liner and is positioned over the liner while the liner is
still on the mandrel. The entire assembly may be placed
into an oven at a sufficiently high temperature to fuse
the textile substrate to the polymeric liner. Generally,
this heat-conditioning causes the prosthesis to shrink
slightly and densify. The heat-conditioning parameters
are chosen based upon the properties of the synthetic
materials being used to form the textile substrate and
the polymer lining. Typically, heat-conditioning is
CA 02234~04 1998-04-07
carried out at a temperature range from about 125-C to
about 225-C using a convection oven for a time of about
twenty minutes. Other means of fusing the liner to the
fabric substrate may also be used, such as the use of an
adhesive.
An alternative method of making the composite soft-
tissue prosthesis formed in accordance with the present
invention includes forming a thin wall tubular liner by
extruding a polymer. A textile substrate is made having
an inner diameter close to the outer diameter of the
polymeric liner. The textile sleeve is passed over the
liner and heat conditioned to fuse the liner within the
textile substrate.
Yet another method of forming the composite soft-
tissue prosthesis formed in accordance with the present
invention includes dip-casting a polyurethane resin onto
a mandrel to form the liner. The textile substrate is
dimensioned to be passed over the dip-casted polyurethane
liner. The composite structure is preferably heat
conditioned to fuse the textile substrate to the
polyurethane liner. The methods for making the composite
soft-tissue prosthesis described herein are merely
illustrative of several methods of manufacturing a
prosthesis formed in accordance with the present
invention. It will be obvious to those skilled in the
art that alternative methods of making the composite
soft-tissue prosthesis of the present invention may be
used without departing from the scope or spirit of the
invention.
In order for the textile substrate and polymer liner
to meltably fuse together, the textile substrate may be
formed from fibers which are similar in melting
temperature and bonding compatibility to that of the
polymer liner. For example, the textile substrate may be
. CA 02234~04 1998-04-07
16
made from fibers such as PTFE, ethylene
chlorotetrafluoroeth~lene, fluorinated ethylene-propylene
(FEP) or polyvinyl flouride. Alternatively, the textile
substrate may also incorporate a bi-component fiber
having a core formed from polyethylene terephthalate
polyester and a sheath formed from a resin of co-
polyester, polyethylene or co-polyethylene. The sheath
of the bi-component fiber melts when heated and provides
enough adhesive properties to bond the textile substrate
securely to the polymer liner. If an ~xrAn~ed PTFE liner
is utilized, it has a microporous structure formed during
stretching. Thus, the melted textile core of the bi-
component fiber may flow into the pores of the liner
mechanically bonding the textile substrate to the PTFE
liner. Fusible fibers are chosen for their melt flow
properties which allow for interstitial adhesion and
formation of an integral composite.
The present invention is also directed to a method
of repairing a diseased body lumen of a patient. The
method includes the steps of removing a diseased portion
of the body lumen from the patient thus leaving a first
and second open end of the body lumen. A composite
tubular soft-tissue prosthesis formed in accordance with
the present invention is inserted between the first and
second end of the body lumen. The composite tubular
prosthesis is formed from a textile substrate having an
intraluminal surface and a liner affixed thereto to
render the tubular prosthesis fluid-tight. The inserted
tubular prosthesis is secured to the first and second
open ends of the body lumen to allow fluid to flow
therethrough.
Although the illustrative embodiments of the present
invention have been described herein with reference to
the accompanying drawings, it is to be understood that
the invention is not :Limited to those precise
CA 02234504 1998-04-07
17
embodiments, and that various other changes and
modifications may be effected therein by one skilled in
the art without departing from the scope or spirit of the
invention.