Note: Descriptions are shown in the official language in which they were submitted.
CA 02234772 1998-04-15
W O 97/17411 PCT~US96/16399
E[EAT-A CTIVATABLE ALDEIESI~E C O MPOSITION
The invention relates to a cro~clin~Pd acrylic heat-activatable adhesive with
low activation t~~ ,. alul t;, and products co~ g said adhesive.
Most emhedded-lens and ~ a~ st~Pd-lens ~~,L-o-t;ne.;li~e cheetinglc~ in
particular for the J9pZI~e~e~ traffic sign market, are heat applical)le cheetinge having a
heat activatable adhesive. However, cube corner r~l urene~ e .cl.r~ g.~ are
typically only adllercd through the use of p-~s~u-.i-se.l~ e adhesives, which are
slllsl~ ~-I;slly di~rel_.ll from heat activatable a~hes;~es. See, e.g., Adhesion and the
Formu1ation of Adhesives 2d Ed., Wake, pp. 98-99 (Elsevier Applied Science
PL~ , 1986). Heat applicable cube corner ~~,I-u-.;nective chee~ would be
adv-s-nt-s-geQl~s since they would have better hsn~lling characteristics, better quality,
and easy msintPnsnr.e.
A heat applicable cube corner ~c;l~ nective cheeting should have the
15 desired optical clarity; have the desired level of tack, so that the ~he~l;g is
p. efe. ably positionsble~ but does not slide on the substrate once its position is fixed;
have the ability to be applied at around 70~C or less by a heat lamp vacuum
applicator ("~VA") without loss of optical quality; adhere very strongly withoutany dPI~ ;Qn or "pop of~' failure; and be applicable to curved edge substrates
such as the .9.1~ . panels used for JspqnPce regulatory road signs.
Adhesives co---~ ;--g nitrile rubber and an acrylic polymer, ~1ierlosed in
JAr~ se p~lh!iched Patent Kokai No. 88056274-B, are heat-activatable adhesives
currently employed to adhere ~~ u~nective chPetingc to sll-mimlm sul.sl.~les in the
JsrsnPse traffic sign market. However, if this type of adhesive is applied to a cube
corner r~,l-ort;nective cheeting, the wluhuess of the .cheeting typically decleases
because the color of the adhesive is dark brown and the cheeting is trsnQI~lcPntFurther, when the heat-activatable adhesives of the prior art are Is-mins-ted to a cube
corner ~ ~,l- ol ~,nective ~I .F ~ WL.~-'e;ll the sealing film of the cheeting has a surface
l-- A~ e~-l such as corona or çhPm;~ ~l primer, the chPeting cannot hold on curved
edge sul,~ ales because of their low cohesiveness at elevated temperatures.
Because the heat activation tel--pe,~L-lre ofthe current heat-activatable adhesives is
CA 02234772 1998-04-15
W O97/17411 PCTAUS96/16399
~L
relatively high (about 82~ to 93~C), the bri~htnecs of the she~ x typically
de.,.~ scs due to thermal distortion of its r~lolcllective ~71C-"~ ~"Q Also, the nitrile
rubber-based heat-activatable adhesives have little or no tack at room tc.l,pe.~L~lre.
As a result, they are not appl.pliale for ~-lhPring cube corner lc~loreflective
S .~l,r~ y,~ to ~ mimlm be~,use the ~1~PeI;~ shifts before ~VA procçccing
A l)re~ lt; applicable cube-corner ~GI-vlt:nective cheeting is not easily
positic~ le be~lQe most pressurc-se.l~;liv-e adhesives have very high initial tack.
R~ ~~ the adhesive typically fails cohesively after applic~tiQn~ the chseting can
only be applied to curved S~S~ ,S that have a 127 mi11imPter ("mm") or greater
10 radius. Such adhesives cannot hold on the curved edges of regulatory ~ dPCi nc in
the JAI~A~ Se traffic sign market which typically have a 7 to 8 mm radius. Further,
~h~ applied using p,e,~,ul1-sens;~ive adhesives tend to trap air b~ een the
substrate and the cheeting during sign fabrication, so waste is typically high.
Similar IC~uilt;l~ S need to be met for the &ppli~l;on of optically clear
15 overlay filrns to traffic signs, Dinoc products and the l,,,,,;~AI;On of light
m~n~S~mPnt films to glass or clear plastic surfaces. Overlay films typically must be
d to their sub~ cs without ell~l~ll,ent of air bubbles and without
;..- e r~. ;i,g with the optics of the product. Such films typically provide such unique
pl.p~ ies as stain ~çc~ l~n~P, dew rçcict~n~e and the like. Light management
20 products, such as bl;g~ e5~enh~ncpmpnt films, light control fflms and privacy films
for col.lpu~ or ,llo~ or screens may be conQ;~red as special ~ ,les of overlay
fil-ns wl,~ the plill~hly fimctioll is control of the optical plupellies of the
product.
DccOlalive films, such as for tile, tables and countertops, also make use of
25 overlay films. In such decol~liv-e films, ease of application, in~ in~ positionability
and the ability to be bonded to the substrate without C.l~ lllC.ll of air bubbles are
of c~ nQ;d~rable illlpOl lance.
Pressurc-se.l~;~ive adhesives and tapes currently produced by ultraviolet
("W") polyl,l~,.i~lion do not meet all the above requilc.llelll~, especially with
30 respect to tack bccause the higher tack of known pressure-sensitive adhesivesmakes them very difficult to position. Known heat-activatable adhesives often lack
CA 02234772 1998-04-15
WO 97/17411 PCTrUS96/16399
the co~ ;ol- of optical clarity, high cohesive sl,~,ngll, and low te.,,~e,~lu,_ of
activation which is critical for l~ ;on of microstructured sul~ces such as thoseused in the l~,u,~ne~ e ~h~l;uB products ofthis invention.
Acrylic a~ ,s employing ivobo,,,yl acrylate are des~;,il,ed in JAP~nF,.,E.
5P~ .d Patent Kokai Nos. 5(1993)-310810 and 6(1994)-128544, but these
l~,f;~.ences do not teach or suggest their use in r~hF~ing r~,l,ùlt;nective .~l.e~ "C to
al~s.
Acrylic adhesives employing N,N-dialkyl substituted amides are described in
US 4,946,742, US 5,334,686 and EP 615 983 A2. While some of these rlicr,lose
10prevv~l,e-3e.lsili~e adhesives for PVC applir~tion~ none rlicr1ose heat-activatable and
optically clear adhesives used for the applic~tio~ of chPeting products.
A need exists for improved heat-activatable adhesives for adherence of the
.~I.ee~ to desired vul,vL,~les. A need also exists for heat-applicable .~ g
products that may be used, for; . '~, to e .~hAnce br ghtnF cs, control light, help
15",~ Ail. the privacy of a co"" ~ller screen, e~ Anr,c the al~pe~ce of s~sl,ales, or
create reflective areas on clothing or other articles to F-~h~nre the visibility of the
user.
The present invention provides a heat-activatable adhesive composition
co",~ i"g an acrylic copolymer, said copolymer co,~ i"g: (a) about 10 to 85
20 wt-% based on Illollolll~r weight of a ol-oe~ c~n~ g of an acrylate or
h~ ~ylate ester of a non-tertiary alkyl alcohol having a Tg of about 0~ C. or
lower; (b) about 10 to 70 wt-% based on monomer weight of a monomer consisting
of an acrylate or mrth~r.rylate ester of a non-tertiary alkyl alcohol having a Tg of at
least about 50~ C.; and (c) about 5 to 50 wt-% based on ",ono",~l weight of a
25filnr,ti~n~ nl~
The present invention also provides ,el,orenective articles having on the
rear, i.e., non-light-i"-pingil~g, surface thereof a low te",~ re heat-activatable
adhesive. The invention further provides light controlling and optically clear
overlay films having a low te.,.p.,.~ re heat-activatable adhesive on at least one
30 surface. The adhesive of the invention has high l-~-~a-t;"-,y both upon application
and a~er aging, rYcFI1~nt cohesive sl-t;ngLIl, high adhesion to polar sub~ tcs such
CA 02234772 1998-04-15
W O 97/17411 PCTAUS96/16399
as ~ , glass, PVC, PMMA and SIA;~ CS steel, and is obtained from a
solventless process
Retroreflective articles of the invention comrrice in order, a r~ ,.Gflective
shP~ 8 having a ,~ y flat surface and a structured surface, the structured
5 surface c0...~.;3cd of a plurality of plccisely shaped projections such as cube corner
~1~.". ~1~, a colored thermnFlsctic layer disposed on the structured surface andadhered thereto in a plurality of discrete lor~tionc, and a heat-activatable adhcs;~,
layer ~li~osed on the colored lLw~ûpl~slic layer. The heat activatable adhesive
layer may co---~.-;sc a c us~ -~ acrylic polymer having an elastic modulus
10 (...essul.,d by ~ly - ~..e~ 1 thermal analyzer, 6 28 rad/second, co---p-~,ss;on
mode) ranging from about S x 106 to about 1 x 10~' dyne/square CA ~; n~l~
(dyn/cm2) at 30~C, and preferably ranging from about S.0 x 10~ to about 1.0 x 1O7
dyn/cm2 at 70~C.
The invention further provides articles having decorative and optical
15 prop~,.lies cQ~ qh~ the heat-activatable adhesive ofthe invention.
The overlay film of the present invention is used to provide a barrier to
prevent foreign materials such as organic solvents, water, dirt, oil, dust, etc, from
rl~in~ the lel.u~efle~ e, e g, cube corner film The film can also be used to
protect various surfaces and sul.i,llal~,s from ~ ,.. such as graffiti. Thus, the
polymeric m~tPri~ic used in the overlay film should be generally I ~ to
degradation by weathering (e.g, heat, W light) and rhPm:c~l attack so that the
urt;nective chPetinE~ can be used for generally long-term outdoor applir~tir,nc
The polymeric materials should also have good adhesion to the cube-corner layer
and ink.
Adhesives of this invention meet the ~t~uir,.ll~nls of the JAI.A~eSe traffic
sign market, that is, they have high transpa cn.;y both initially and upon aging;
approj..idl~ initial room tt .Ipe ~l~re tack to position the shee~ P~ high adhesion to
minum, st~ steel and other cheeting substrates; low activation tc,~ re
(not more than about 70~C); they do not decrease the ~ lurt;nective bri~htnecc Of
30 the r~,l.olt;llective ,I.ç~,l;ny~, and they exhibit eYc~llent cohesive strength to hold the
ch~eting on curved substrates Col!~h.alions of all these plup~,llies are difficult but
CA 02234772 1998-04-15
W O 97/17411 PCTrUS96/16399
S
not i...po~;.ible to obtain from solvent-based heat-activatable adhesives. However,
sdhesives of the invention are advantageous from the standpoint of e-lVilV~
p-~l - because they can be made by a solventless process.
The heat applicable cube-corner ~GL-u-t:nective ~I.e.,l;.~p~, light control,
S overlay, and other filrns of the invention can be readily applied without pe- ...~ y
~ llapplllg air. Retroreflective articles provide ~Ycell~nt adhesion to round edge
~l,ales such as are used for JAp~ se re~ll~ted road signs, as well as to variousseverely curved s~ les useful in the construction work zone market.
The invention will be further ~Ypl~ined with ~_f~,.ences to the ~ w;--~s,
10 ~11~
Figure 1 is a cross-section~l view of a lel-o-t;nective chelo,ting article made in
acco..lallce with the present invention.
Figure 2 is a p~ ,e.;live view of a signage article made in accordance with
the present h~enliûll, also illu~ li--g s~ ;c~lly the round edge test.
Figure 3 is a cross-sectional view of a traffic control ~ -u-t;nective ~h~
with overlay film made accc,-dil-g to the invention.
Figures 4 and 5 are cross-section~l views of graphics and~or decol~Live
.e~ gc made accold;--g to the invention.
These figures, which are ide~li7e-l, are not to scale and are infP!nded to be
merely illusl.ali~e and non-lin iti-~g
DETAILED DEscRn~oN O~ PREFERRED ~NVE~ON E~DBOD~ENTS
An illu~ live lello~ ective article of the present invention is shown (in
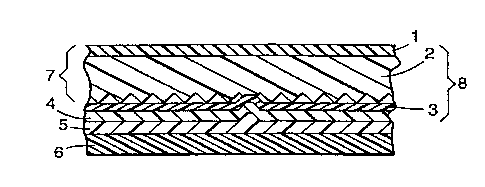
enlarged cross-section) in Figure 1. Overlay film 1 is plert.~bly disposed on a fiat,
smooth surface of layer 2, the co---l~ Lion of overlay 1 and layer 2 lerw.~d to as a
structured ~l.e~ g 7. Colored sealing film 3 is disposed on the rear or structured
surface of layer 2, and empty volumes 10 are defined between the recesses of layer
2 and colored sealing film 3 so as to impart ~t;l-orenectivity to the article. Sealing
film 3 is prt;re.~.bly sealed to layer 2 in a network of intercnnnection bonds such as
is tlicl~losed in U.S. Patent No. 4,025,159 (McGrath).
CA 02234772 1998-04-15
W O 97/17411 PCT~US96/16399
In Figure 1, .~re~ ce numeral 4 denotes an optional ~h~m:c~l primer layer
or a corona l,~J.~f-.l Iayer disposed on the surface of colored sealing film 3.
Ch~m~ and/or physical priming is pl~r~"~,d but not l~cces~y to the invention
The ~...bilu~lioll of layers co~ , of structured ;>~-f~ 7, colored sealing film
5 3, and primer and/or ~ 1ayer 4 is d~ign~ed as "~ .ort;flective ~l~e~ ~" 8.
Illu.A,a~ e; , '~ of primer layers include layers of materials that provide a
strong bond b~,L~ n sealing film 3 and adhesive layer 5. In another illus~ e
ernbor~ nf, sealing filrn 3 and/or adhesive layer 5 may be surface treated, for
~".~le by corona L~ r~1~ prior to being bonded tog~th~n
Layer 5 of a heat-activatable adhesive is disposed on the surface of the
primer layer or corona l~e~ .l Iayer 4 or directly on sealing film 3 if no primer
1 "~t ~ is used. Liner 6 is p~ tir~ bly disposed on the surface of heat-activatable
adhesive layer S so as to protect its surface. A sheet having .~-..h~,-s 1 to 6
des_.il,cd above is ref~,.ftd to herein as "heat-activatable le~,o~ene~;Li~ hl.eel;~
The inventive adhesive of the articles of the invention, and the articles
themselves are now desv,il,ed in more detail.
L Ac~ylic ~eat ~ ~ . ..table ~AI ~
Heat-activatable adhesives of the present invention exhibit ~ en.;y of
20 at least 85 percent in terms of the value measured by the method desc.ibed in the
F ,~~~ section inf~ra. If the adhesive ~.~n~ .~ is less than 85 percent, the
color of the adhesive is visible through the seal film and the structured surface
portions of the article, and the appe~ ce and visibility of the article is degraded.
A p-~r~ d range of 11~UIS~J~e.ICY is at least 88 percent and more preferably at least
25 90 percent, to ..,.~ , p.,.r~"",u~ce ofthe reflective sl~ee~
The glass transition te~ ,.al~lre (T8) of polymers is c~lc~ ted using the
glass transition t~"pe~ l t; of the homopolymers of each monomer and the weight
fraction of the monomers, as shown in the following equation of Fox, T. G., BullAm. Phys Soc (Ser 2) 1:123 (1956),
I/T8 = W./Tga + Wb/T8b + WJT8c
CA 02234772 1998-04-15
W O 97/17411 PCTAUS96/16399
v~,LGI~ T8, Tga, Tsb and Tsc deei~n~te the glass transition tel..pGI~lule (in ~K) of a
terpolymer of...ol-o,-.- D a, b, and c, a homopoly-mer of monnmer a, a homopolymer
of.~ol-o...~ b, and a homopolymer of ...l~no... l C, r~s~e-li~_ly. W., Wb, and Wc
S are the weight fractions ofmonomp~rs a, b, and c, l e~e~ cly~ where W, + Wb + Wc
~ --1. For the purposes of this ;Il~ ion~ the Tg of the heat r v ~. ble adhesive is
sul,~ ;slly equal to the T,~ ofthe wry-lic copolymer or tel~oly.llc..
To obtain the app-op,ide twk the glass l-~ ;on temperature of the heat-
~:: vall ble adhesive must be ilul_aDed to a value higher than that of normally used
10 prcODul~-sellDilive adhesives. This may be accomr!iehed by the use of monolllclD
which have higher homopolymer glass transition tC~IIpGIi ILUC;" or by r.hsnginf~ the
weight fractions of the cc,-npollelll mono--.e. D.
The glass tr-sneition temperature of adhesives useful in the present invention
is about 0~ to 40~C. When the glass trsneition telllpGIalulc is lower than about 0~C,
15 p.~ ~he~;on tack tends to becol..e excessively high, making poeitionin~ and
repositioning .liffiylh When it exceeds about 40~C, pre-s-~lh s;on tack tends tobecolllc e~.Ce;.D;~ IY IOW, making it difficult to keep the articles securely positioned
during heat-activation and bondillg. Further, the heat press tc.l.pc.àl~lre needed to
achieve a good bond tends to become high. The glass transition te..,~ re of the
adhesive is plGr~.~ly about 10 to 35~C and more p-GrG ~ly about- 15 to 30~C.
When the glass llallDilion l .--p_.alu.G is within such ranges, final bond.--g at a lower
heat press te...~ ule becomes easier and at the same time, tack within a suitable
range can be oblaillGd~
The tack value of the adhesive of the present invention is plGrGI~ly about
50 to about 1,000 grams-force/inch ("gf/inch") in terms of the value of the
"pre~Ah~~;on test" which is also de.,_-il,ed in the FYr-lF'es section, and more
p-.,f~ bly is about S00 to about 950 gp/inch.
The adhesives of the invention are comprised of three types of monomers: a
low Ts ac-~1~1e ...OI-O.~ , a filn~tion~l monomer, and a high Ts acrylate monomer.
30 The weight average Inole '- weight of the acrylic polymers is prGr~.~bly within
CA 02234772 1998-04-15
WO97/17411 PCT~US96/16399
the range of 10,000 to 5,000,000 and particularly p,~relably within the range of500,000 to 2,000,000.
Acrylic copolymers useful in the adhesive of the invention col"ll,ise from
about 10 to about 85 wt-% based on monorne~ weight, pl.,rel~bly about 20 to about
5 70 wt-% of at least one low T, acrylate or meth~crylate ".ono.,."l. Higher ~moof this monomer relative to the other comono-..c.~ will soften the heat-ailiv '-le
adhesive, while less than about 10 wt-% of this ...OIlO~..Pl will ~ignifir;~ntly reduce
or e~ le the tack. Useful low T~ no..._.~ include those selected from the
group col.~ ;ng of a ~OnOr~ ;ol~ c~ylale or mçth~rrylate ester of a non-
10 tertiary alkyl ~IriQhnl7 the alkyl group of which CO"~liS~S from 4 to about 12 carbonatoms, and ~ lules thereof. Such low Tg acrylate or m~th~rrylate esters generally
have, as homopolymers, glass l-~ ;l;o-- tC.~p~ ul~S below about 0~ C.
r~t;rt;"tid low T8 acrylate or m~ c~ylate ester nlol-o~ include ethyl
acrylate, n-butyl ac.ylàle (BA), isobutyl acrylate, 2-methyl butyl acrylate, 2-
15 ethylhexyl acrylate, 2-ethylhexyl meth~rrylate~ n-octyl acrylate, n-octyl
...c~l.zc.ylate, isooctyl acrylate (IOA), isooctyl m.eth~r,rylate, isononyl acrylate,
isodecyl acrylate, and mixtures thereo~
Particularly p~rt;ll~,d low T8 acrylate monomers include isooctyl acrylate, n-
butyl acrylate, 2-methyl butyl acrylate, 2-ethylhexyl acrylate, and mixtures thereof.
The copolymers of the invention also contain at least one filnrtion~l
lllono-~lel useful to r nh~nre specific ~lhrcion to certain surfaces and increase total
adhesion. For ;..~ nce, acid functional monomers such as acrylic acid will enh~nr,e
~tlh~;on to polar surfaces such as glass or metals, paint, and to basic surfaces.
Weakly basic ...ono...- .:i, Iike N,N-dill-~;lllyl acrylarnide and N-vinylpyrrolidone, will
25 ~-.h~ncc adhesion to surfaces such as pl~tir-i7ed and rigid PVC and to acidic
Sl Irf~ces.
Useful filnr,tion~l monoll,~.:, include those conli~;..;..P. polar functional
groups, like carboxylic, sulfonic and phosphoric acids; hydroxy groups, lactam and
lactone groups; N-sllbstit~lted amides, N-substituted amine, call.~ les and the like.
30 In general, the filnctio~l monomer rnay com~,lise about S to 50 urt-% based on
total mollompr weight of the copolyrner.
CA 02234772 1998-04-15
W O97117411 PCT~US96/16399
q
Moderately basic, fi~nCtion~ ono-,.~. ~ include N,N-dialkyl sub~Liluled
amides and ~--O1~O-.-- - ~ which behave as N,N-dialkyl s~bsti~l1ted amides. Fy~mples
include N,N-dimethyl ac,yl&--~de (NNDMA), N,N-di~lwLllyl mP~th~rrylamide~ N,N-
diethyl E~ , N,N-diethyl '- ylamide, N-vinyl pyrrolidone (NVP), N-
5 vinyl capro'-~t~m and the like.
Weakly basic copoly..~ hle monomers, such as N-octyl acrylamide can be
used in cQ...l~ n with a major amount of moderately basic nlonolller. Strongly
basic ...onol--e.~ (--.O1~O.~ ; having non-sterically hindered tertiary amine term~
groups) such as N,N-dhlleLllylàll~no~Lll~l meth~crylatê~ N,N-dimethyla...n~op,.,~yl
10 .... ~ ylate~ N~N-dimethyl~minoethyl acrylate, N,N-dimethylaminopropyl acrylate,
and the like, were found to be too basic when used as the sole basic ...onr,.... l,
dehydro-,l.lo,i"aling PVC upon aging and thereby possibly shortening the useful life
of PVC coated fabric and other PVC col,ll.o~ lls. If ~hongly basic ",onomc. ~ are
employed, it is pr~f.,.led that these monol-,e,~ be present in a minor amount and
15 that they are used in ~ u~j nn with a major amount of a moderately basic
""~1m~.f ~. If a :,Ll.,m2,1y basic mn~omer is used, it is present up to about 5 wt-%
based on total ...o1ln..~ I weight. More prere"ed are moderately basic polar
.... n~-.e~:~, alone or in cGIlll~ .liol- with other basic ...ono-..t;.~. About S to 45 wt-
% of moderately basic ll-onolll~l~ can be used, and about 15 to 30 wt-% of basic20 ...o,~rJ,..~ is especially plerclled~
Prere..~d acid functional monomers include acrylic acid, b-ca.l uAyeLl-yl
acrylate, meth~rirylic acid, itaconic acid, crotonic acid, fumaric acid and the like.
~ ,relled are moderately basic monomers such as N,N-dimethyl acrylamide, N,N-
dimethyl meth~ e, N,N-diethyl acrylamide, N,N-diethyl meth~crylamide~ N-
25 vinyl capro'- , N-vinyl pyrroMQrle, and the like. If an acidic functional
...O1-O-.-. . is used it plfrel bly coll.l..ises about 5 to 20 wt-% of the copolymer.
F-1m~,ti~n~l ...ono..-~-(s) are typically copolymerized with the rest of the
copolymer colllpol el-ls at levels from about 5 to 50 parts per hundred by weight of
the ...ono...~,. composition, more p-~rc.~bly from lO to 40 parts per hundred by30 weight of the m- nomP!r composition. These functional monomers can also be used
âS C,lu. " ~ sites for the polymer. For ~ .~...plç, acidic Illol Olllel~ can be reacted
CA 02234772 1998-04-15
W O 97/17411 PCTAJS96/16399
with c~u " ' ~ ~ agents that react with the acid group, for example m~ltifilnr,tiQnal
epoxies or iso~allales.
The acrylic copolymers useful in the heat-activatable adhesive of this
invention contain from about 10 to 70 parts per hundred by weight of ~o~
5 (wt-%), pl~ft. ~ly ~om about 20 to 60 wt-%, ~..~ r-d in the copolymer of at
least one l,lonol.l~,r which as a homopolymer has a high T,. As used herein, "high
T~" means the ~.I~ o~ ulg homopolymer has a Tg of at least 50~ C., pl~,felnbly of
at least 75~ C., and more preL ably at least 100~ C.
Typically, the higher the amount of the high Tg m~nomRr in the acrylate
10 copolymers ofthe heat-activatable adhesive ofthis invention, the lower the tack and
pre~lh~ n of the adhesive and the higher the heat-activation te~ alule. The
lower the amount of these high Tg ~--ono-n~ , the higher the pre~lhRciQn and thelower the activation t~llpel2~Lulê. The ~n-ollntc of this high Tg monomer and thê
low Tg monomRr are b~l~nced to provide the dêsired properties.
So long as the monomrr can be poly.. -t;-,Led with the rest of the Illollonlethat co...~ e the acrylic copolymer, any high Tg ..-- no...~l, inr~ linp styrene and
the like, can be used. However, the high Te ...ono...e~ is typically an acrylate or
1.9~.ylate ester. P'l~,fell~,d high Tg monomers are monofilnction~l acIylate or
mf~th~r.rylate esters of bridged cycloa1kyl ~lcoholc having at least 6 carbon atoms
and of a-u---~lic ~lroholc Both the cycloalkyl and aromatic groups may bê
subs~ituteA~ for ~ ?'-, by Cl~ alkyl, halogen, cyano, and the likê. Rcpeçi~lly
pl~L.Ièd high Tg monomers include 3,5-dimethyl~m~ntyl acrylate and
mPth~r~ylatê; isobornyl acrylatê and m~.th~rylate; 4-biphenylyl acrylatê and
meth~rrylate; phenyl acrylate and mPth~çrylate; and 2-naphthyl acrylate and
me~th~r.rylate.
Mixtures of high Tg monomers may also be used.
Pl~,f~,~ably, the acrylic polymers useful as the adhesives of the invention are
",oS~ A This i~ lu~,es cohesive strength of the adhesive, making it easier to
control the elastic rnodlll-lc, heat activation te"-pt,alu,~, and pre~lh~ciQn tack.
A cros~ agent may ~,~,fe.~ly be present in the heat-activatable
adhesive in an amount of about 0.05 to about 3 wt-%, more preferably about 0.1 to
CA 02234772 1998-04-15
WO 97/17411 PCT~US96/16399
JJ~
2 wt-%, based on the weight of the ",ono~,c.~ in the adhesive Depçn~1ing on the
mol~ r weight and the acrylate equivalent weight of the CC""pOl ents, as much asabout 20 wt-% of a cros~lin~in~ agent may be used
The c o~ B agent useful in the adhesives of the ;"~,e~,Lion is typically an
S organic co...~ A that reacts with the other ~.Ol-O . ~ by virtue of having a
y of ethylenically u"s~lu,~lcd groups, r~,rt;,.~,d to herein as mllltifi-nrtiQr~l
acrylates ~l~ ly, a croE~Iint~in~ agent is a co-..pou-~d which can directly
react with the polymeric backbone and result in cros~linl-in~ as, for; , '-, in a
pe, c Aide thermal cure or bP ~ophe ~one W cure
The adhesives of the present invention may be cros ' ' ~ before or after
bondi~, of the ~he~ to a substrate There are two major cl ~ t ing
,..~ç~ ..c for the acrylic polymer adhesives of the invention: free-radical
copoly. . ;~ -l;on of m~lhifimrtiQnal ethylenically ull~alu~led groups with the other
Illol o...e :i, and covalent or ionic cror-' I g through the filnction:~l Illonolll~
15 such as acrylic acid Another method is the use of W cros~lir'-çrs, such as
copoly...-, ;,- '-le bcnzoph~ ~on~s or post-added photocrosslinkers, such as
mllltifilncti~n~l be~vph~nollF,s and l,i ~i"es High energy irradiation, like electron-
beam or gamma is also useful With the exception of the use of mnltifilncti~nal vinyl
unsalulaled ~ , all the cros~ ;..g will be done after coating of the
20 polymers
Cr~ ,3 agents that are usable in the present invention may be sP1e~ed
from the group csn~ of triazine compounds; acrylated u~t;lhal~es such as the
diacrylated un,lh~es known under the trade de~ign~ti~n EBECRYL, especislly
EBECRYL 230 (a polyu-c;ll,&l e diacrylate available from Radcure Spe~i~lti~ Inc,25 Norfolk VA); hydrogen abstraction ~i~ u~ compounds inr~ n
copoly. .~ l le mono-ethylenically u~saLulaled aromatic ketones, particularly 4-acrylo,~il.e~ophenone (ABP), as described in U S Patent No 4,737,559 (Kellen et
al ), and post-added ~mlltifilnctional benzophenones as described in US Patent
No 5,407,971 (Everaerts et al ), both of which are inco"lo,ated by ~rerence; and30 ml l~;rl ~cl;on~l acrylates, such as 1,6-hexane diol diacrylate (HDDA)
CA 02234772 1998-04-15
WO 97/17411 PCTAUS96/16399
Crocclinling agents are s~e~,le~d acco,-ling to the poly...~ ;QIl method
employed. I'l~,f~ S'' 1' g agents for adhesives p~ ,d via
pholopoly.,~ ion on web are mllltifilnctionsl acrylates such as 1,6-k~ e~
~ ' ~I..Ie (EIDDA) 8S well as those tlic~ sed in U.S. Pat. No. 4,379,201 ~t~ilm9nn
S et al.), il,coll,o,~led by l.,rci,~,nce herein, such s L~ olpropane triacrylate,
pt~ntq ylh,ilol ~ c,y-late, 1,2-ethylene glycol diacrylate, and 1~l2-doclçç-s-ne ~ yl, le.
Also useful as crosslinkers are acrylate and ~~ ~e~ sc~ ylate filnctiQn~
nligomPrs, like EBECRYL 230 which, in view of their higher molc '- - weight,
10 have lower acrylate content than the lower n~nl~ s,r weight diacrylates, such as
1,6-k. -A~-etl;ol diacrylate and the like, mentioned above. To comr~nc-s-te for this
lower ac,ylale content~ higher weight pelCf~ 3f S of the oligomeric mllltifiln~ion
acrylates must be used in the adhesive composition.
~rltlitit n9l usefi~ ros~l;-.L;.~g agents include Lydlug~,n abstraction type
15 photocrocclin'-~rs such as those based on bel,~ophF-~oll~c, aceLophf-.ollf,c
anthr-s-tlllinonec, and the like. These crocclinlrin~ agents can be copolyl"~ ~le.or
non-copolyl~ .C F - .'es of non-copolymerizable hydrogen abstraction
c,v ' ' ~ agents include benzophenone; anthraquinones, and r~ tion-activatable
cros-~ ;n~ agents such as those described in U.S. Patent No. 5,407,971. Such
20 agents have the general rv",lula
lX--g~(w)2--(cH2)m--(Y) 21r~ Z
wl,~.~.n W ..,~,rese..Ls -O-, -N-, or -S-, X r~.ese.lls CH3- or phenyl; Y r~p.cscnLs a
ketone, ester, or amide filnctiorl~lity; Z .~ s_nls a polyfunctional organic s~~me-.l
25 that co..l~ c no hydrogen atoms more photo~bstractable than hydrogen atoms of a
polymer formed using the cros~ .g agent; m ~~.ese.lLs an integer from 0 to 6;
"a" r~res~ s 0 or 1; and n represe ,Ls an integer of 2 or greater. Fx~mples of
copolylll~ hydl ogell abstraction crocclinl~in~ compounds include mono-
ethylenically unsaturated aromatic ketones, particularly 4-acrylo~ybc~zophenolle
CA 02234772 1998-04-15
W O 97/17411 PCT~US96/16399
~3
(ABP), as described in U S Patent No. 4,737,559 (Kellen et al, inco~olaled herein
by r.,fer.,nce).
Copoly~ a-cleavage type photoh.;l;~r~ can also be employed, such
as LC y' -lo-filnctionsl fl:c ~b;,~ ted acetyl aryl ketQnPc~ such as those des.;lil ed
in a~:gl-2e's PCT Applirstion No. 94/10620, filed .Sept~mhP,r 16, 1994, which is~ i.,co.~ûlaled herein by ,.,f~,.ellce.
In n~l;tinn cC~ h~ ;nnc of mu1ti-fi~ l (meth)acrylates and the
l.~/.l~ogell al;~,t~ type Cl~ erg or copol~ h!e a-cleavage type photo
h lialu~:~ can be used Low intensity W light, such as "W black light", is
sllffici~nt to induce crosclinkin~ in most cases; however, when hydrogen abstraction
type cr~ Prs are used by II.~...selves, high h~Lel.siLy W CA~JO~Ult; iS neceC~ y to
achieve sllffiri~nt crosclin~ing at high line speeds Such exposure can be provided
by a ~-.~,..,u-y Iamp processor such as those available from PPG, Pill~ur~l~, PA,
Aetek and others.
Yet another method for crosclin'-ing that does not nece~c,.. ily require
n~l~iti~n of crosd~ agents is exposure to an elc~,l,oll-beam.
Other useful cr~ ' ' g agents include the substituted triazines, such as
those disclosed in U.S. Patent Nos. 4,329,384 and 4,330,590 (both to Vesley, both
illcG.~ led herein by rerelence), such as 2,4-bis(trichloromethyl)-6-p-
20 ~ l.uA~ .Iy~ne-s triazine and the cl~ ..opho.~ h~ mP,thyl_s-~ Pc
C-u ~- 1 - , agents useful in p-~,a i--g solution poly---c i~ed heat-
activatable adhesives of the invention are those which are free radically
copoly.. ;~hle and which effect crosclinkinE through exposure to r~ tion,
lllOi:.Lul~, or heat following polyl.w,i~lion Such crosslinkers include the above
25 mPntion~<l photoactive substituted triazines and hydrogen abstraction type
photocrosclinkPrs Hydrolyzable, free radically copolymerizable ~,.usslinkers such
as m~nop~thylenically unsaturated mono-, di-, and trialkoxy silane compounds
in~lu~in~, but not limited to, 3-meth~rryloA-ypropylLl;~ lo~ysilane (sold under the
trade name "Silane A-174" by Union Carbide Ch~omi~ls and Plastics Co ),
30 vinyl.l;.llcll-~/lethoxysilane, ~ yhu~,lllyldiethoAy~;lanc, vinyltriethoxysilane,
CA 02234772 1998-04-15
W O 97/17411PCT~US96/16399
~4
v.l,~e, vin~ .h&.loxysilane, and the like are also useful ,;,, ~ 1 ~ g
agents.
Heat activated copolyl,le.~al)le cros~linking agents, inçll~ing but not
limited to N-methylol ~. yl~unide and acrylamido glycolic acid, can also be used to
5 el-hA~ the shear ;,~ h of the pressure-sensitive adhesive composition of the
l;on.
;r~ ;O~O~ idines ~; .- ' 3 agents may also be employed.
nir~ c c~.,s~agents are more fully ~psc~ ed as compounds with the
general formula (I):
~ N--C--R2 C--N~
R R3
wl.~hl Rl and R3 are the same or di~elen~ and are independently selecte(l from the
group co~ g of H and C"H2~1, wl. .Ghl n is an integer ranging from 1 to about
5, and R2 is a divalent radical s~olected from the group con~ g of benzeno (-C6EI4-
15 ), s~bstit~te~ phenylene, and C~I2m~ where m is an integer ranging from 1 to about10. An ~ e of a useful ml~ltifilnctionol aziridine within general f~ la I is
N,N'-bis-1,2-propyl&.l_;sopl.ll.AI~mide, which has the following structure (general
r.,,.. l~ II):
CH ~ \~ CH3
aI)
Other .;los~l;..'-i..g agents can be used for the acid co..l~ g polyrners of
the invention. They include epoxies, isocyanates, and the like.
Adhesives of the invention can be poly,ll_.i~ed by convPnti~n~l free radical
pol~lll_.i~ion m.~?thnd~ whether thermally or radiation initi~ted, in~lutling solution
CA 02234772 1998-04-15
W O 97/17411 PCT~US96/16399
/~
and bulk poly".~ ,alion plocesses. P~,f~ d mPthor~c yield high mo1er,~1Or weightpolymer without the use of solvents, such as obtained from s~1~pFn~ion, emulsionand bulk poly...~ ;on Particularly p-t;rtlled is W curing on the web, which
yields the fini~hpd product in a single step.
S SUitp~b'~ thermal free radical inilialo,~ which may be utilized include but are
not limited to azo co---~ uu"ds such as 2,2'-azobis(isobuly,o. il,ile)~ Lyd~vpelo~ides
such as tert-butyl hydroperoxide, and peroxides such as benzoyl peroxide and
cynlok~ OI~F peroxide. pl.olo -. ~ js~o~ that are useful accolding to the invention
include but are not limited to those s~lp~led from the group con~ of benzoin
ethers such as benzoin methyl ether or benzoin isopropyl ether, sub~ ed benzoin
ethers such as anisole methy1 ether, sul,slilulcd acclophenû~e~ such as 2,2-
diethoxyacetophe~-ol-F and 2,2-~imethoxy-2-phenyl acetophF.~ol-~, s-1bstih-ted alpha
ketols such as 2-methyl-2-hydlu~-y propiophenol1e, aromatic ~ulrullyl chlorides such
as 2-1.~phll.o1F~nç sulfonyl chloride, and photoactive oximes.
For both thermal and M~ otion in~-1ced poly-~c ;~I;on~, an i- ilialvr is
present in an amount of about 0.01 to about 0.5 percent by weight based upon thetotal weight of the ~ no.. s of the instant heat-activatable adhesive compos~ ne
In one solution poly",e,i~lion method, the high Tg and low Tg mollo",c,~
and the functional .non(j-"er, along with a suitable inert organic solvent and free
20 radically copoly... ~i~nble cros~lin~Fr are charged into a four-neck reaction vessel
which is equipped with a stirrer, a thermometer, a condenser, addition funnel and a
thcll~ alch. APler this ...ono~ mixture is charged into the reaction vessel, a
conccllllnled thermal free radical initiator solution is added to the addition funnel.
The whole reaction vessel and od~lition funnel and their contents are then purged
25 with nitrogen to create an inert atmosphere. Once purged, the solution within the
vessel is heated to about 55~ C., the inilinlor is added, and the "~lu~c is stirred
during the course of the reaction. A 98 to 99 percent conversion should be
ob~;ned in about 20 hours.
Another polymc~i~lion method is a two step ultraviolet (UV) radiation
30 i.~ "d photopoly~c~i~lion of a 100% solids monomer mixture. In the first step,
the low viscos;ly ,nono---e.~ are mixed at the app,op,inle ratios and a photoinitiator
CA 02234772 1998-04-15
W O 97/17411 PCT~US96/16399
1~
is added to the ~ u~c. The mixture is purged with nitrogen to remove dissolved
oxygen. Short ~ -I'O:~"'G to W light results in a partially poly.-.~ ed syrup with
moderate v sc- ~ that can be coated easily. Further pholoi~ or and ~ s~ t~r
are added to the sy~up. The syrup is then coated while ~Y~ ns~ oxygen at a
desired l~. -L"- c~, usually about 0.5 to 10 mils (about 0.01 to 0.25 .. i~ tr-~)
During the coating process, the syrup is further ~Yposed to a bank of W lights to
,c - , 1~ e the poly...- ~ ;,Al;on and e~s .lil~ the adhesive.
An A~ e to the above two step method involves the use of an extruder.
In this m ~hnri, a plastic pouch is filled with nlono..._.~ and il ilialo.~, with the
10 ~ itiQn of chain llan:.rel agents to keep the molecular weight low enough after
pol~,.-.~.i~aLion so that the pol~vmer can be extruded. The filled pouch is exposed to
W, which produces the poly---~ ed composition inside the pouch. The pouch and
cont~nt~ are then fed to the extruder and the res ~lting molten composition hot melt
coated onto a liner, after which it is then exposed again to W or cle~,l.ull beam to
15 crosslink the adhesive, to yield a co~ ,o~ilion co~ hlg a high m~le ' weight
heat-acliv '~'~ adhesive having a small pelcenlage of such plastic polymer IllalGli
from the pouch therein, typically 3 weight percent or less.
Reactive extrusion, such as the contimlollc free radical poly..~ ;Ql
rngtho-l~ described in U.S. Pat. Nos. 4,619,979 and 4,843,134 (both Kotnour et al.,
20 both i--cc,-~o-~-led herein by .~rc.~.nce), may also be utilized to prepare the heat-
a.;Liv~lable adhesives of the invention. Reactive extrusion is a solventless
technology where the polylllGli~dlion is initiAted by thermal means as opposed to
W radiation. The monomers along with the i. iliator are fed to an extruder. The
h,.--i.c ~lule along the extruder is varied to control the poly---G-~lion. Chain25 lla--sr~,r agents are added to control the molec~ r weight and prevent gel
fo-l~alion The adhesive obL~....ed at the end of the extruder is hot melt coated and
cured either by W light or ele~,L.on beam in order to improve its cohesive strength.
The formulation of the heat-activatable adhesive of the invention is
S~ ed below:
CA 02234772 l998-04-l5
WO 97/17411 PCT~US96/16399
f~ ,d Preferred
Ingredient Useful (~cidic (basic
r A t i A _ ~ f ~
- m~ t )monomer)
Low T~ onnl~tl 10-85 10~0 10-40
FllnrtiQtt~ -~An.,.. -,r 5 50 5-20 15-30
High Ts .~-on- .~ 10-70 25-60 25-60
Crosslinker 0.05-0.5* 0.05-0.5* O.OS-0.5*
T.. :l;o~r 0.05-0.3 0.05-0.3 0.05-0.3
* Can be as high as 20 wt-%.
The elastic modlllllc of the acrylic heat-activatable adhesive (l.~easur~,d by
dynamic ...~-c1~A~ l thermal analyzer, 628 rad/sec, colu~ s;on mode) plertl~ly
ranges from about 5 x 106 to about 1 x 108 dyn/cm2 at 30~C. When the elastic
mn~ .lc is less than 5 x lo6 dyn/cm2 at 30~C the initial tack or "pre-~rlhec;ion",
which will be des~,lil)ed below, is very high, similar to that of a pLes~ule sensitive
10 adhesive, so that air is likely to be trapped between the adhesive and the substrate
during ;,~ g application. When the elastic modulus ~Ycee~ 1 x 108 dyn/cm2, it
beconles ~1iffir.lllt to keep the sheet positioned at the time of provisional bonding
even with the applirAtiQn of high pressure.
The.~,role, if the elastic mod~ ls is bt;~ween about 5 x 106 and about 1 x 108
15 dyn/cm2 at 30~C, optimal plopGllies for COIIvlF ~ ~ provisional bondil* are
provided. When the elastic mo~ has such a value, the heat-activatable adhesive
may be positionrd as desired upon a substrate without stirl~ing When the heat-
acLiv~lable adhesive is in the proper position, application of pressure results in a
weak provisional bond to ~ the position. If repositioning is desired, the
20 adhesive may be easily lifted from the substrate and repositioned. Application of
pl~S:,.llt; will again provide a tenll)ol~l~ bond, ,..~;..l~i,~i"g position until the
adhesive is heat-activated. A more pl-,f~ ,d range ofthe elastic modulus at 30~C is
CA 02234772 1998-04-15
WO 97/17411 PCT~US96/16399
from about 7 5 x 106 to about 6 0 x 10' dyn/cm2, particularly preferably from about
1 0 x 107 to about 3 6 x 107dyn~cm2.
In the present invention, the elastic mod~ c of the acrylic heat-a.;Lival~le
adhesive ~ ,r~.abl~r ranges from about 5 x 105 to about 1 x 107 dyn/cm2 at 70~C
When the elastic mn~ul~c is less than about 5 x 105 dy~lJcm2 at 70~C the cohesive
sL.~ tends to be low, the-erole, the adhesive tends to fail or stretch so severely
that the ~he~ g can not hold on severely curved substrates, i e pop-off is a
p.eb' On the other hand, when the elastic modulus ~.,eecls 1 x 10' dyn/cm2, it
is ~liffic~lt to carry out the final bon~ g at a heat press te...~ lu,c of less than
10 about 70~C If the t~ p~ re is higher than 70~C, the l~Llu-enecli~,ily of the
structured ~hf~ 3 tends to decrease because of thennal distortion of the
proje ~nc When the elastic nnot~ --c is within the range, the final bonding at the
low heat press tc.--~e.al~re beco~ easier and higher cohesive strength can be
obt~i.,ed A more p. ~,f~ e range of the elastic modulus at 70~C is from about 9 0
x 105 to about 8 0 x 106 dyn/crn2, particularly p~erc-~bly ranging from about 2 0 x
106 to about 6 0 x 106 dyn/cm2
The elastic mod~ c of the adhesives of the invention is a value measured at
30~C and 70~C by using dynamic ~e~ n;~~l thermal analyzer Model RSA II
available from ~h~o _I ics Co The conrlitinns for the measurement are as follows:
- sample shape: cylindrical (outer ~ mP,ter = 3 to 3 5 mm, ll.. cl çcc = 6 to 8
nun
- tc.llp~ ul~ range: -60 to 160~C
- frequency: 6 28 rad/sec
- mea;,u~ L mode: co-l.p-~s~;on mode
The elastic m-~d--l--c of the heat-activatable adhesives of the invention is
evaluated at 30~C ~o ~ the ability to heat press at 70~C and still also have
suitable pr~lh~ ;on tack at room temperature (i e, about 25~C) is .e4u..cd for
adhesives useful in the present invention The term "heat press tC.--~ re"
l~ples~ s a value of the surface te...p~ ure of the ~cl~u~cnective sheet measured
30 by using a therrno ~1~
CA 02234772 1998-04-15
W O 97/17411 PCT~US96/16399
~9
Other additives, such as an ultraviolet ("UV") absorber, an anti-oxi~snt a
visco~ y incleas;l.~ agent, trc~ifiers~ i--o,gaiuc particles, etc., can be added to the
heat-activatable adhesives of the invention to the extent they do not inlc r~rc with
poly"w,i~liol, s~ ly reduce the desired l~ ,~e.~;y, ;,ulJ,~ ;zlly adversely
S affect the glass l, _n ;t;~n t~ p_-~lu~c or the elastic mo~lulus of the adhesives.
The adh~ es of the invention are useful in the pro~luction of many dirrc~c!~l
c~ 2 or film-type products, particularly those wl,clc,;" optical clarity is desired.
One applice';oll ofthe adhesive is in a structured ~ as shown in Fig. 1.
In Figure 1, cube-corner chPeting 7 colul.~ises an overlay or cover film 1 and
10 structured layer 2 having cube corner PlPmPntc on the rear surface thereo~ The
overlay film is typically and pl ~,r~,. ~,ly poly.,,cll,y~ eth -,rylate ("PMMA")Cc~ g a W abso,l,el to prevent deg~dalion, while the ~ olcnective cube
corner el~ -,.F-~lc are p-crc-~bly made of polyc~lJonale resin. It is 32
understood that the present invention may be used with any cube corner optical
15 design approp,;ale for the desired spplirstiQn Illustrative; . Ics of some cube
corner P.l...,,...~l designs that can be used in the invention are disclosed in U.S.
Patent. Nos. 4,588,258 ~EIoopman); 4,775,219 (Appeldorn); and 5,138,488
(S7~',7P,..11). Structured !~ 7 may also co"""ise a S~ l;AllY totally i.,le".all~
rPflecting film cO-l" ~iSiilg a plurality of parallel prisms, such as described in U:S.
Patent Nos. 4,805,984 (Cobb); 4,906,070 (Cobb); 5,056,892 (Cobb); 5,175,030
(Lu); or 5,183,597 (Lu).
The plecisely shaped el-omPnte of structured layer 2 and sealing layer 3
define a plurality of concavities 10, filled with air or other fluid. ~cubst~-nt~ y
totally intPrn_lly rPflectin~" p~ ins to the optical quality of the film, and means that
the film has a T-Test Value of 5 percent or less, ~l.e.till the T-Test is as defined
below.
The optical quality of a lell ul~ nective film can be evaluated with app~lus
inclu~in~ a laser (such as a SPECTR~-PHYSICS Brand Model 117A) with a
spatial filter, a beam PYp_n~1~or, and a collim-tor. Two diaphragms or irises are
placed 18 and 38 cm from the laser, and an annular sample holder with an opening6.35 cm in ~ ,t~ ~ is placed 84 cm from the laser. Directly behind the sample
CA 02234772 1998-04-15
WO 97/17411 PCT~US96/16399
aO
holder is an i ~ g sphere (vvith a 3 cm ~iAmeter aperture) and a LABSPHERE
Brand ML 400 r~inm-otPr~
Using the diap~agms or irises, the laser is focused through the aperture to
obtain a clean circle of light of about 3 mm A~meter on a black surface mounte(l on
S the sample holder. A source ...lens;ly ~ .--CI~L of 100 percent is taken with no
sample in place. The TIRF (Totally Internally ~f~ne.,~ g Film) to be tested is then
..... u~.lec~ on the sample holder with its flat surface facing the laser and its grooves
..l....li.,,2 ~e.lic,~lly. Unless o~ ..~;se l~o.lcd, T-Test Values are l~e&S~1Cd at
' ~ t~.n~ u-e. RP9~;ng~ are then made at from 12 to 15 dirre~,.-l points on
10 the TIRF within a S cm di~metPr area while making sure that none of the lightstrikes the frame ofthe sample holder. The readings are ave.a~,ed and mllltirliP~d by
100 to give percent tr~nemie~;on which is the T-Test Value of the TD~F sample. T-
Test Value is a criterion of the fidelity of replication of the TlIRF. Smaller T-Test
Value pe-c~ agP~s i~ te better fidelity of replication than larger p~",~ ~Pe, and
15 a T-Test Value of S percent or less in-1ic~tes that the film is substAntiAlly totally
intP,rnAlly rPflPC,~ g
Overlay f~lm 1 ~ ,r~.bly conlp.ises an acrylic material having PYCPllpnt
durability, such as poly(methyl)met~-~rylate, a polyester such as, for ~ ~!e,
polyethylene t~ k~i~rl~t~ polyamide, polyc&ll,ol.d1e, poly(vinylchloride),
20 poly(vinyli~iinPrhloride), c~ ose acetate butyrate, cell~llose acetate propionz-tP,
poly(ethersulfone), polyul~Ll-alle, ionomer resins such as the metal ion croeelin'-Pd
polyethylene/acrylic acid iQrlom~ors known under the trade dçei~tion SURLYN,
and the like, and ~er~-~bly also co".p,ises a W absorber.
From the aspects of ...ccl~An:~Al strength and light reflectivity, layer 2
25 p~.,fe.~ly has a refractive index of about 1.6, which is possible if the layer is made
of a polyc~l,onale resin, an ionnmPr resin as described above, or an acrylic resin.
In the case of cube corner r~,L,olt:nective articles, the leng,th of the base of the
pyramidal cube corner ~ "~.,1 p~ert;,ably ranges from about 0.1 to about 3.0
millimetPr ("rnm"), and more plef~,.ably ranges from about 0.2 to about 1.0 mm, in
30 order to secure good ,~,ol~nectivity and wide angle property. For flexible articles
CA 02234772 1998-04-15
WO 97/17411 PCT~US96/16399
a~
of the invention such as those to be used on clothing, a length of up to 0.625 mm is
e
Structured sheeti~ 7 may be made as one integral material, e.g. by
l-..ho~ g a plero---.ed sheet with a d_s_lib_d array of cube-corner rl .,~ ; or
S casting a fluid mstPri~l into a mold; or they may be made as a layered product, e.g.
by casting the ~lr."...lc against a plefo-"-ed film as taught in U.S. Patent No.3,684,348, or by l~ a plercl~l.ed film over the front face of individual
molded plr~ ; Polyc~l,ol-~lPs and io--o-----~ are ~ ,f~,..td integral sheet
l c
The i' ~' ~ of structured ~l~ee~ 7 pler~,.ably ranges from about 50 to
about 500 miclom~lel~ in terms of the height from the apex of the cube corner
pl- " " or prism to the base of the base portion. If the 11~ ..PC~ is less than 50
ml( u.--~,lers, the ~ en~ of the ~l~e~ .P may not be s lffi~;ent and a
pre~ct~ -.Fd height is typically difflcult to obtain for the pyramids or prisms, such
15 that l~ r~nectivity decreases. If the th;~~nesc eYcee~lC 500 micl.,-l,~,le.~, on the
other hand, the total th;~~necs of the ~eL-u-t;llective sheet becQmes so thick that
h~nrlli-~g beco...Fs ~1iffic~llt and the amount of adhesive required increases.
As stated above, the overlay film should be light tran.emiscible and plérel~bly
is s~l,s~ ly l.a".,~ .,l. The polymer used in the overlay film plerèr~bly
20 co...~ e5 a moderate elastic modulus polymer for bending, curling, flexing,
co..r~....;.~, or stretching. The polymer used in the overlay film also plt;rel~bly has
ductility, which can be e,~.essed in terms of Youngs mod~ le The Youngs
modllllle can be about 0.7 x 105 to 5.7 x 105 psi, and is plerel~bly about 2.5 x 105 to
4.0 x 105 psi. The polymer should retain its physical inleg.ily at the te.npc,.~lults at
25 which it is applied to the cube-corner layer, and desirably has a Vicat sot~ning
e."~ re that is greater than about 50~C.
FY ~mples of poly ners that can be used in the overlay film in~ l~lde, but are
not limited to: fluc~lil,aled polymers such as poly(chlorotrifluoroethylene), which is
available, for PY~mrle, under the trade d~eif~n~tion KEL-F800 from 3M Co., St.
30 Paul, MN, poly(tetrafluoroethylene-co-h~Y~fllloropropylene), which is available, for
~ _ p!c, under the trade d~,;g,~l;on EXAC FEP from Norton Pelro~lllance,
CA 02234772 1998-04-15
WO 97/17411 PCTAUS96/16399
B. a...plOn, MA, poly(tetrafluoroell-ylene-co-perfluoro(alkyl)vinylether), which is
av '~'~e, for ~ , under the trade dPcignAti~n EXAC PEA from Norton
p~,.r,....A~.cç~ and poly(vll.~;de.le fluoride) or poly(vinylidene fluoride-co-
h~fl~lolopl~ ylelle)~ which are available under the trade ~ÇcignAtiQn KYNAR
S from Pennwalt Co",olalion, P' '~~lPlph;a, PA; ionn~ ic ethylene copolymers such
as poly(etktylene-co-methArrylic acid) with sodium or zinc ions, which are available
under the trade ~e~ ;Qne SURLYN-8920 and SURLYN-9910 from E I. duPont
de Nemours, Wi~ 5lo.-, DE; low density polyethylenes such as low density
pol~ .c, linear low density polyethylene, and very low density polyethylene,
10 plActiri7~d vinyl halide polymers such as pl~ctiri7ed poly(vinylchloride);
polyethylene copolymers inr1n~1ing acid functional polymers such as poly(ethylene-
co-acrylic acid) and poly(ethylene-co-m~oth~crylic acid), poly(ethylene-co-maleic
acid), and poly(ethylene-co-fumaric acid); acrylic fi-nr,tionAl polymers such aspol~,...cill.~l...~th~crylate, poly(ethylene-co-alkylacrylates) where the aLkyl group is
methyl, ethyl, propyl, butyl, etc, or CH3(CH2)n~ where n is 0-12, and poly(ethylene-
co-v;..y'-- te); and aliphatic and aromatic polyurethanes derived from
diisO~;y~al~,s such as dicyclohex-yl...~ e 1,4'-diisocyanate, isophorone
diisocyanate, 1,6-hPYAm~thylene diisocyanate, cyclohexyl diisocyanate,
d;~!,h~ d--sG.,yanale, and COI~ ;OnC of these diisocyanates, polydiols
20 such as polypenty" e- 'i, ~ glycol, polytetramethylene ether glycol, polyethylene
glycol, polycaprol~-,t~ne diol, poly-1,2-butylene oxide glycol, and colllbin&lions of
these polydiols, and chain ~yt~on~lers such as b~ltAnefliQI or ~. Anc.~iol
Coll..,l~;ally available urethane polymers include PN-03 or 3429 from Morton
InternAtionAl Inc, Seabrook, NH, or X-4 1 07 from B F Goodrich Colup~ly,
25 Cl~,~,.,l~d, OH Co...l~ ;on~ of the above polymers also may be used in the
overlay film.
~ .~f.,..~,d polymers for the overlay film include fluolillaled polymers such as
poly(vinylidene fluoride) (PVDF), acrylic fimctional polymers such as
polyl..~ l...ell.s-i.ylate (PMMA), and col,ll)h~alions thereo~ A particularly
30 pl~rtll_d group of polymers inchldes blends of PVDF and PMMA that contain
~out 60-95 weight percent PMMA and about 5-40 weight percent PVDF. In thése
CA 02234772 1998-04-1'
WO 97/17411 PCTnUS96/16399
blends, the PMMA contributes to the durability of the overlay film wheleaS the
PVDF co..~ fs to the ~hfm:~Al (e.g., organic solvent) stability and fl. ;l.~ y Of
the overlay film. P.~,rel~.bly, the PMMA is not impact modified. Such PMMA
-" are also r~f~ d to as "straight" PMMA. .S~itsJble sources of "straight"
PMMA include Rohm & Haas VOM, V045, V052, V081 and the like. PVDF is
available from Soltex Polymer Corp., TT~ lQl~, TX under the trade dçcigrAtion
SOLEF or SOLVEY, from Pennwalt Col~.c..~lion under the trade de ~Al;onc
KYNAR/l~ 1010 and 1008, and from FlrA-O.~ .,, North America Inc., PhilA 1flphia~PA under the trade ~e~ ;QIlS l~L)LAR 710 and 720. These polymers are
10 pl~f,l,ed or one or more of the following n,asons; suitable .~.CC1~ Al pl~._lLies;
good str~hPo;~n to the cube-corner film; clarity; f~ Al-.,ed solvent illt~ leS:j, and
e lVilt_t----~ Al stability.
The overlay film can be a single layer or a multi-layer film as desired. The
intf~ Cl adhesion between the overlay film and the cube-corner film can be
15 illlp~ovt;d by placing a thin tie-layer lhelel)elv~een. In addition, a surface ~ "~
metho-l such as an electrical discharge method (e.g., a corona or plasma ll~AI.. l)
can be used to further improve the ~slrlhP~;~n of tie-layer to the overlay film or the
tie-layer to the cube-corner layer. Typically, however, a tie layer or surface
l~ l - - -1 mPth~c are not required in the embo~irnpntc of the present invention.
The polymeric m~t~ c used in the cube-corner layer and overlay film can
include additives such as acid scavengers and W absorbers. These are particularly
useful to prevent degradation of the polymeric material during procçCcin~ and upon
exposure to en~ o.~ conditions (e.g., heat and W radiation). Examples of
W absolbel~ include derivatives of benzotliazole such as those available under the
trade df~:~.. Al;orc TrNUVlN 327, 328, 900, 1130, and TINUVlN-P from Ciba-
Geigy Col~ol~lion, Ardsley, NY; çh.orni~.Al derivatives of benzophenone such as
those available under the trade dçcignAtiQns UVINYL-M40, 408, and D-50 from
BASF Corporation, Clifton, NJ; and other related bel~ophenone derivatives such as
those available under the trade dÇcignAtiQnc SYNTASE 230, 800, 1200 from
30 Neville-Synthese 0-~, 'GS
CA 02234772 1998-04-15
WO 97/17411 PCTrUS96/16399
Colored sealing film 3 is l~...;..~le~l onto the structured surface of layer 2 and
is bonded thereto with heat and/or radiation at a plurality of loc~tion.e, thus forming
a plurality of sealed air pockets. When describing the pockets, "air" is used only as
an .~ . lc. Other fluids may be used, dep~n-ling on the atmosphere in which the
5 artides of the invention are produced, and provided that the fluid used is
cignifir~ tly dirr .~nl in refiractive index from layer 2, with a di~rèlGnce in refractive
indJces of about 0.5 or more pl~,f~ ,d. The procedures of U.S. Patent No
4,025,159 (McGrath) may be used to effect the bonding of colored sealing film 3 to
the structured second surface of layer 2.
Colored sealing film 3 is prertl~bly a plastic film Co~ ulg a plastic resin
such as polyester that cc,~ c a suitable amount of one or more pi~mRnte such as
-.. oxide, silica, red oxide, and the like, to impart desired color. Illusl.~livG
, 'e s of colors include white, gray, red, yellow, green, orange, blue, and brown.
Colorants such as dyes and p;g.~ ls may be used to impart desired color to sealing
15 layer 3 as is ap~.u~ le for the int~n~ed appli~tio~ Those skilled in the art will be
able to readily select suitable colorants and colorant lo~(ling.e for int~nrled
appli~ti~ne
White and gray are typically prGre..ed for the present invention bec~use
recop..;,~silily of the léLlulGnective articles of the invention is high when these
20 colors are used.
A particularly pr.,f~,.lGd resin for forming the colored sealing film layer is
often polyester resin because the p;g--~,.-l can be easily added to the resin.
However, bonding of polyester films to adhesive layers can be ~iffiC llt
In the present il.~e..l;on, an optional ~ 1 primer layer or a corona
25 l~ layer is pl~GrGI~bly disposed bG~ ,.,n colored sealing film 3 and heat-
activatable adhesive layer 5. When a ~h~mie~l primer layer and/or corona 11~.,l~.. ~.l
is employed, inter-layer adhesion between the colored sealing layer film 3 and heat-
activatable adhesive layer 5 can be improved, making possible high adhesion of
articles of the invention to a substrate.
Illu~ ive eY~mr~es of suitable ch~m;~l primer layer types include
ure~ ç~, silicones, epoxy resins, vinyl acetate resins, ethylçn~imines, and the like.
CA 02234772 1998-04-15
W O 97/17411 PCT~US96/16399
S~l~ction of a suitable primer layer or llc~ .l will be dependent in part upon the
dl~.c~ I;cs of sealing film 3, adhesive layer 5, and the co~ ;ol-c under which
the resl~lt~nt article will be used. Ur~ e and silicone types are particularly
,r~ ~~ 1 primers for polyester colored sealing films. One suitable silicone
S type of primer layer has a continllol~s gelled ~ olk structure of iLolgai-lc
particles, and is des~,.il,ed in Jopon~5e U--~ ed Patent Publication (Kokai) No.2-200476. This primer layer has a strong affinity for polyester resins and polyolefin
resins. Illu~ live; , '-~ of ~ 1 primers for vinyl and polyethylene
t~ kll.ol-ote films include the cloc~l;..~ed acrylic ester/acrylic acid copolymers
di~closed in U.S. Patent No. 3,578,622 (Brown).
The acrylic adhesives of the invention generally adhere well to many
slllfr-es However, in some cases it may be useful to ~nhon~.e the adhesion to a
;.~ ,I.ale by el~h~llrinB the ~Ç~ ir~~l interlocking of the adhesive with the
s~;,l,~le which can be done, for ~ ~'ç, by abrasion or etching of the substrate or
p.i.. ,g with a material which s:g,.;~ y In~ aSe,S the surface area for the
adhesive to adhere to, such as the Msol plilllh~g diecllcced below. The acrylic
adhesives used for this invention contain filn~tion~l mono..._l ~, such as acrylic acid
or N~N-dhl~elllyl&cryla--m-ide. These filnction~l monomers can strongly interact with
~~h~m;~~l primers by such l"e~ is~c as hydrogen bonding, acid-base interaction or
20 r~ P ction across the adhesive/primer interface.
The th~ n~CC of the ..h~nnic~l primer layer is suitably within the range of 10
to 3,000 nanometers ("nm"). If the thic~n~ss is less than 10 nm, the primer effect is
minim~l; if it ~yceeAs 3,000 nm, on the other hand, inter-layer peel is likely to occur
in the primer layer.
Corona l-~ .l is a pre~--cd physical priming that can be suitably applied
to the surface of the colored sealing film layer onto which is then coated the
adhesive of the present invention. Corona ~ "~,l not only improves the inter-
layer ~-lh~cion bc~ ,n the adhesive and the colored sealing film but provides anadvantage in the production process in that it can be separately applied after
30 structured ~hf~ g 7 and colored sealing film layer 3 are sealed.
CA 02234772 1998-04-15
WO 97/17411 PCT~US96/16399
:26
A surface ~ ............. ..................l 4 is plc~,._d to obtain strong adhesion b~ een the
sealing film and the heat-activatable adhesive layer S as illu~ Led in Figure 1. In
gPn~r~l surf~ce L.~ may be d_sc-il,ed as ~~hPmicAl ~ "l'; physical
and co-nl,;~ ;onc thereof, so that the following illustrative surface
L ~ may be app-up.iale:
1) AlirhAtiC polyu-_ll,~e primer coating (applied after corona l-_al,~,enl), an
e ~ A~..ple of which is as follows (~mollntS in parts by weight):
Table A
A moult CC ,~ ' t
58."~ .~EO~F7 R-960 aliphatic polyurethane (Zeneca Resins)
31. )9 ,~Pion 7ed water
1.,6 CX-100 mllltifim~.tional aziridine (Zeneca Resins)
0.0: CAAlco~lllor Dye (BASF) _ _
.~7 F.th lrt~l
.~7 FC q: fluorochesnical (Su",.lc,."o 3M Co.)
~.20 Bubr e Breaker 3056A (Witco Corp.)
2) Msol primer coating after corona ll~ The teçhnnlQgy of Msol
prirner is based on ~Acci~ee~s JAp~qn~se Patent J02200476-A, an example of
which is ples~, lled in Table B (Amollntc in parts by weight):
Table B
t ¦ CG , Q t
67.56 DPi~ni7ed water
31.-53 Nalco 2326, COllQi~5~l silica (Nalco ChPm:,~lCo.)
0.'1 A-l lO0 silane coupling agent (Nippon Yunika)
0.~0 Triton X-100 s~ ct~nt 10 weight percent ~ .eo-lc
solution (Rohrn & Haas)
. _ . .
3) Nitrogen corona 1,~ Al."~
Corona Ll- Al."e-~l of the surface in the present invention can be suitably
carried out in a nillu~ atmosphere becwse the duration of the improvement of
inter-layer ~lhPQ;on is high. The useful energy density of the nitrogen corono
CA 02234772 1998-04-15
W O 97/17411 PCTAJS96/16399
;~
1 ranges from about lS to 500 watts/meter2/min~tee, preferably about 80 to
250 watts/meter2/minute. The energy density can be cA~ Ated from the equation
Ehe~ y(wat~ eter2/mi~te)= NetP~wer(W)
Electrode ~dth(m)xL~ne Speed(cm/m~n)
wl.e.~ the electrode width is 0.035m and the net power and line speed can be
r ~Bed to obtain the desired energy density.
Corona l~ l of films is a well-known te~hn q~l-, and is des---;l-cd
generally in Cramm, R.H., and Bibee, D.V., The Theory and Practice of Corona
T~A~ .. I for Improving ~rlhP!eion, TAPPI, Vol. 65, NO. 8, pp 75-78 (August
1982).
The heat activatable adhesive of the invention may be used in the
mAn-lf~llre of a variety of di~elenl articles some of which are illustrated in Figs. 2
to 5. Fig. 2 depicts, in perspective view, a signage article made using the heat-
activatable adhesive of the invention. The article, having the desired thirl~nç~ss 11,
width 12 and flat length 13, also has a round edge 15. The culv~lu,t; ofthe round
edge is in~liCAt~d by the radius R. Retroreflective ~hçeting articles 14 are adhered to
the article by a layer of the heat-activatable adhesive of the invention and remain in
place will~oul "pop-of~' at the edge ofthe rel,o,t;nective ~hçeting articles 16.Fig. 3 shows a heat activatable traffic control ,el~orenective s~ , in
cross-section similar to the ,vl,o,~lective ehçetinge described earlier. In this article
overlay film 21 is bonded to p,;~",alic layer 22. The prism layer 22 has a sealing
film 23 bonded to its structured surface with a plurality of sealed air pockets formed
b~l~vv.l the two layers as desv,il,ed in detail for Fig. 1 above. The sealing film
layer is bonded to a primer layer 24 which may be, for eYAmrle, the Mso1 primer
layer des_,il,ed in Table B. A poly~ ll,ane type primer coating as described in
Table A, a corona l,e~ , and so on. The heat activatable adhesive layer 25 is
dis~.osed upon the primer layer 24 and a protective release liner 26 is disposed upon
the heat activatable adhesive layer to protect the adhesive and which is to be
removed prior to heat lAminAtion
CA 02234772 1998-04-15
WO 97/17411 PCTAUS96/16399
The ,~ .,nc;~ e ~heetingc ofthe invention may optionally also comprise
a further film layer 27 which has protective pl.,pc.lies. F -~.'es of suitable films
are those films having anti-graffiti, anti-dew, anti-moisture, ~hpm;c~l~ heat and/or
impact l-,..;s~ films. If such a film is used it is bonded to overlay film 21 with a
S layer of the heat-a~ ,..ldble adhesive of the ;"~,..Lion 25.
In r~ldition to the use in the pr~a.~lion of ~ J.Gne~ hc~ g~ the
heat a ilivatable adhesive of the invention may be used in the p, c~,&, alion of graphic
or decorative film as seen in Fig. 4. For ~ e, the heat activatable adhesive maybe used to adhere a stain or heat r-,;.;~l~ll overlay onto a deco~ali~re film product,
10 allowing it to be used in heavy use applic~tionc such as for table tops, bar cou,~h,.~,
kitchen cou"~ , and so on. An illustrative graphics film is d~ in cross-section
in Fig. 4.
The graphics film of Fig. 4 has a hard coat 31 that is a heat, stain and/or
r~h-~m:~~lly ~ alll film which prole~l~ the rpm~inrlpr of the ~hr..,~ g This hard
15 coat layer may be made up of any suitable polymeric m~t~ri~l but is pr~,rc,ably a
W curable acrylic resin. This hard coat 31 is followed by a lla,.~,~cl,L film layer
32, e.g., a polyester film, that is used to smooth the graphics film and obtain
~ -r~ haldness to~ fh~r with the hard coat. This ~ spal~,nl film is useful to
prevent pl~ctiri7pr migration so that ~xcellPnt stain reci~t~nce can be obtained. This
20 film is bonded by a layer of the heat-activatable adhesive 33 co~ a basicfiln~ion~l monomer such as NNDMA to a layer of clear PVC 36. A printed or
other deco.~ re layer 35 may be adhered to the clear PVC layer followed by a base
layer of colored PVC 36. A layer of pressure sensitive adhesive 37 is disposed on
the surface of the base colored PVC with p,.~le.;li~e release liner 38 on the surface
25 ofthe pressure sensitive adhesive.
In an nd~ition~ r~' application as shown in Fig. 5, a layer of clear
PVC 41, optionally with a pattern embossed into its surface, has a layer of
deco,aLi~re pli,l~ 42 att~ched to its back or smooth surface. A layer 43 of the
heat activatable adhesive of the invention is disposed on the surface of the p,i,~
30 layer. ~lthou~h optical clarity is not critical in this particular applic~tion, low heat
activation t~"pe.a~u~e is desirable in view of the heat sensitivity of PVC film. A
CA 02234772 1998-04-15
W O 97/17411 PCTAUS96/16399
2q
layer of ~ -..;..---.. or other metal foil 44 is disposed on the surface of the heat
acliv I b'~ adhesive. Upon this surface is a layer of pressure sens;live adhesive 45
to attach the article to the desired s~l-ale. On the surface of the pres~ulc
sensitive adhesive is a protcvlive release liner 46 to protect the surface of the
5 r"--
When ~ u~cplic~ed film with optical plOpv~ hes such as that desv.il,ed in
U.S. Patent No. 4,775,219 (Appledorn) must be ~ ed to typically glass
~ -~s, the adhesive used in the lqm;~l~tion must be optically clear so as to leave
the - "~e~ qtP,d films with the int~n-led optical quality and they must be heat
10 a~livalable at a moderate t~ pv.alulc to prevent distortion of the films and its
rçsulting optical cl~ gcs For example, the heat-activatable adhesive of the
invention may be used to l~min~te or adhere a film, such as a microreplic~te(l film,
directly to a glass surface such as a comp~t~r screen or other monitor. In otherapplil ~tion~ such as an automotive or other window type applic~tion~, the film will
15 typically be l .~ e;l in a "sandwich" configuration bvl~,en two glass panels.Clearly, the heat activatable adhesive of the invention is useful in a wide
range of ~l.P~ g or overlay products having a mllltitu~e of end uses ranging from
light or optics control to anti-graffiti film to rcl,u,cnective articles. The figures
des-.i1.ed above are not to scale and are int~nded to not limit but illustrate the
20 invention.
~LES
The invention will be further eYrlqinPd by the following illustrative
~; ~ 'es which are int~n~ecl to be non-limiti~ Unless otherwise inflic~teA, all
25 ~mollntc are c,~plcssvd in parts by weight.
In the c,.~ p'e~ which follow, the monomers of alkylacrylate and
filnction~l ",on~s",cr were mixed at l~,~ecli~re weight percentages in~lic~ted in each
~mp'ç, with 0.1 weight percent of the photoinitiator known under the trade
de ;~ n ESACURE KB-1, 2,2-~imetho,Y~y-2-phenylacetophenone available from
30 S&~lull~vr Co. The resulting sol~tion~ were deaerated for 10 min~ltes with nitrogen
CA 02234772 1998-04-15
W O 97/17411 PCTnUS96/16399
~ 0
gas, and then poly.l.~.~ed to 8-12 percent conversion by using low intensity W
lamps under nitrogen gas. Polymerization can be stopped by exposure to oxygen.
Various kinds of croselinL-~rs and ~d~iitiQn~l 0.2 percent of photo;..;~;~tor
were added into the sy-rup solution and then mixed co~ l~ely. The crosc1in'-~rs
S used in the ~ s were 1,6~ f~ diz._-ylaLe (HDDA), X~-353 triazine
&~ le from 3M, which is 2~4-bis(trichlorol~ yl)-6-(3~4 rlimetho~y-phenyl)-s-
tri~7..1~, 4-acryl~Ayb~n~oph~ )nlle (ABP), and u-ell~,e diacry-late (EBECRYL 230available from Radcure Spec~;slti~s)
The following two curing procedures were employed.
L OpeD Face Curi~g
The sy~rup sol~tiQn~ con~ g ~L-353 crosslirl~er were coated ollto
~,oni7ed paper liner at 4 mil (0.01 cm) bar setting and the web was irradiated with
a low illL~nsily W lamp (IJV B~ light from Sylvania, e ..;~ g b.;L~__n 300 and
400 nrn with a peak around 350nm and an intensity around 2 mW cm~2) under
nitrogen gas. The total dose of the W light was 420.7 millijoules/square
C~ ("mj/cm2").
II. Du~ll Liner Curing
The syrup s ~! ~tir~n~ c~ HDDA, ABP, and l,leLl,al e diacrylate were
coated onto ~ilicQni7ed paper liner at 4 mil (0.01 cm) bar setting and a siliconized
polyester film was 7sn~in~ted to the web. The sandwiched web ran through a low
si~y W lamp which total dose was 444.2 mj/cm2.
In the case of ABP cf~ syrup, the web was irradiated with a high
inL~.Isily W lamp after the low intensity W light irr~ tinn The total dose of
high il~Le.l:,;Ly W light was 429.0 mj/cm2.
The total dose was measured by WIMAP radiometer (Electronic
In;,Ll.. ~ .l;Qn and Technology, Inc.).
The heat-a~;Liv~L~ble adhesives can also be obtained from solvent
poly,.l_.i~Lion, h~ er, the adhesives may exhibit slightly di~relenL plopelLies as
those cured by W polylll~ ~lion. For ~ e, the holding power on highly
CA 02234772 1998-04-15
W O 97/17411 PCT~US96/16399
3 1
curved surfaces may be lower and the . '~- may be better suited for flatter
surface appli~ ti.~,ne
60 weight percent isooctyl acrylate, 10 weight percent acrylic acid, and 30
weight percent isobo,..rl acrylate were mixed with 01 weight percent of
pholo~ ;Alor (S~ol~c;r CO., ESCACURE KB-l). The le~ g so'-~tion was
dcze~aled for 10 ..; . ~les with nitrogen gas, and then was poly~eli~ed up to 8 7
percent COIlve~:~;Ol- by using a low i--len~ily W lamp under nitrogen gas The
10 poly..._,i~lion was sLopped by exposing the sollltiQn to air.
0.2 part by weight, based on total weight of monomers, of triazine (XL-
353) and 0.2 weight percent of ~d~lifion~l phot~ lor were added to 100 parts by
weight of the syrup solution and were mixed c~ ly~
The syrup solution was coated on the eiliconi7Pd paper liner at 4 mil (0.01
15 cm) bar setting and the web was irradiated with a low intensity W lamp under
il- ogen gas The total dose of the W light was 420.7 mj/cm2
The e1astic m.~,~l~llllc at 30~C and 70~C, h~l sp~ency, glass transition
le.ll~_.alul~, pre-adhesion, and post-adhesion are shown in Table 1 The test
methr~tle of those ...eas.~ nls were as follows:
- Elastic modlll~le: des~,~il,ed above
- T~ ,a.~ ;y
The cured heat activatable adhesive, which was coated at 4 mil bar setting,
is l~min~tP~d with 50 mm Toyobo polyester film A4100 on both sides ofthe adhesive
and the sample is .~._~u.ed by an integrating-sphere photometer accor-ling to
section 5 5 in JIS K7105
- Glass l-~n :~;on t~ re: des_-il,ed above
- Prc ~;~ih~~ ;on
A heat-activatable adhesive was coated on the siliconized paper liner at 4
mil (0 01 cm) bar setting and cured, and then 50 mm ~IIlminllm foil was l~min~ted
to the adhesive at 70~C by a heat l~min~tor The sample was cut to 1 inch (2 54
cm) width and the test piece permitted to equilibrate at 20 + 2~C and 65 + 5
W O 97/17411 PCT~US96/16399 3 ~
percent relative hurnidity (as per test standard JIS Z8703) for 24 hours. A 3 mmthick poly~,~l,ollale substrate was wiped with isop,opyl alcohol, and then the test
piece was l~ d to the s~L.aLe by means of an automatic l~min~tor with S
mrn/second l~ n speed as d~sc~ ed in JIS Z0237. Just after l~ ;on, the
S release force was ~~-easured by a tensile tester known under the trade deci~n~tiQn
INSTRON with 90~ peel and 300 rnmtminute peel rate. The pre-adhesion was
defined as the averaged release force of three mea~ul e.nc
- Post-~ rlh~ ;~-n
The same method as above was used to measure post-adhesion, except
10 with the following r~h~ngP~s use of 80 mm ~IIlmimlm foil and 1 mm of ~IIlmimlm
panel as the test substrate, and HLVA for final bonding before mcas~l1",.,.,l. The
heat applicable cube-corner ~ u~ellective ~heel;~ was made by the sarne method
des- ~;l,ed above except the use of Msol primed cube-corner rel,or~nective chee~ g
instead of the cilicQni7çd paper liner, that is direct coated onto the .chee~ The
15 prop~,.lies of these chpeting are shown in Table 2 below. The metho~lc of the mea~ul~,."~ s are des~;,;l,ed below:
The whi~Pnpcc was measured in terms of Cap Y(D65/2~) by use of Color
meter S 80 (Nlppill~, Dp-ncholnl Kogyoh). A higher Cap Y value means the
20 wl.;l- ...çcs is higher. The color of the white chPeting should be stay in the following
color box: l(x~.305, y=0.305), 2(x=0.355. y--0.355), 3(x=0.355, y=0.375),
4(x=0.285, y=0.325). See ASTM Standards on Color and Appearance
Mea~ule..,~.."s, Standard E308.
- Provisional bond",g pe~ru~ ce:
Ease of pocitioning of the cube corner .~,L~ur~nective !~h~el;~,3 in this
e~bodiment to a predele"",.led bonding site (an al--min--m substrate for a road
sign) was ev~hl~teA Where positioning was easily accomrlichçd and a provisional
or te",~o,~y bond was formed upon appli~ati~n of pressure, it was evaluated as
"Excer' for ~Yc~ nt, where the reflective cheeting did not adhere but could slide
along the ~IIlmimlm surface with low friction it was evaluated as "Slide" for
undes;. ~le sliding of the shçetinp~ and where the chçefin~ was aggressively tacky
CA 02234772 1998-04-15
W O 97/17411 PCT~US96/16399
33
so that it could not be peeled off easily by hand it WAS evaluated as "Tack" forpositioning of the ~heetinf~ was impossible due to excessively high tack.
- HLVA application te-llp~ re (heat press tell,l~e. ~L~Ire)
The le~ e of HLVA application when the cube corner
S ~ lùl'~lective .1.~ 3 was bonded to the ~ mimlm sllb~ le of the road sign was
,d by blill~-g a thermocoll!ple into contact with the surface of the ~1 .e~
- Retroreflectivity loss after ~VA app~ tiQn
The p~ age of the I~ll Ul~neCtiVity IOSS measured after the cube corner
g was bonded the ~IIlmin-lrn substrate for the road sign as described above
was evaluated using rt;llur.,llectivity before bonding as 100 percent. The
.,~lurenc~ ity was measured at ang1e co~ ;l;ons of 0.2~ observation angle and -4~
tlll- ~ce angle.
- Bonding Test:
90~ peel ~lhP~ion of the cube-corner lGllu-~nective ~h~e~ was carried
out after HLVA application based on JIS Z0237. The case where the peel force
was greater than 1.5 kilograms-force/inch ("kgf/in") or the ~heetin5~ could not be
peeled off without damage of chee~ was evaluated as "Excel" for ~Ycel1~ont, and
the case where peel occurred between the adhesive layer and the sheetin~ was
evaluated as "Delam" for dFl ~;s-l;on.
- Round Edge Test:
Test panels with radius ranging from 3 to 10 mm were made as shown in
Figure 2. The size ofthe panel was 1.5 x 70 x 110 mm. Two pieces of 1 inch (2.54cm) width sample of a heat applicable cube corner ~t;l-c,~eflective ~heetin~ were
applied by HLVA to the panels a~er wiping with an 2% aqueous solution of a non-
ionic ~ ;rler (all~ylphenylether of polyethylene glycol). The ~VA application
t~.llp~,.al~re was chosen as shown in Table 2 and 4. After each test piece (~ub~ e
having the salll~ s bonded thereto) was cooled, the edges ofthe test s&---ples which
protruded were l.i-..,..ed. In this way, the test samples were bonded to substrates
having the radii of curvature of 3 to 10 mm, and the environ...~ l aging test was
30 carried out in 14 cycles under the condition listed below so as to observe pop-off
failure of the ~~I-ul~nective sheet from the curved surface. As a result, the
CA 02234772 1998-04-15
W O97/17411 PCTAUS96/16399
3 5~!
min~ m value of the radius of curvature of each test piece substrate, in which pop-
off removal of the leL-or,nective sheet was not observed for two test s~ lPc, was
used as the test result. Radius = 3, 4, 5, 6, 7, 8, 9 and 10 mm.
S 1 cycle condition of ~I.v;rolu~ aging test*
1. -30~C, 0 percent RH (relative humidity) (2 hours) ~(1 hour)~
2. 23~C, 65 percent RH (0.5 hour) ~(0.5 hour)~
3. 40~C, 95 percent RX (2 hours) ~(0.5 hour)~
4. 23~C, 65 percent RX (0.5 hour) ~{0.5h)~
5. -30~C, 0 percent RH (1.5 hour) ~(lh)~
6. 23~C, 65 percent RH (0.5 hour) ~(lhour)~
7. 80~C, 50 percent RX (1 hour) ~(lh)~
8. 23~C, 65 percent RH (0.5 hour)
15 *The cycle contlitionc were originally used in the ~ltomotive industry to provide a
coll~lalioll to outdoor weath~,.al,ilily. The first time listed in each step is the length
of time the sample is permitted to stand at the inflir~ted con-lition~ The time
bc;l..~.ll two di~ con~itionc~ for .~ -i....ple ~(l hour)~, is an interval to change
to reach the next con-lition
The same procedure as FY~mrle 1 was pe-rc"ll-ed except 0.4 parts by
weight of triazine (XL-353) cro~cclin~er was added. The fin-lingc are shown in
Table 1 and 2.
F..~ 3
The same procedult; as FY~--Fle 1 was pe,r~""lcd except 0.6 parts by
weight of triazine (~-353) ~i~us~ t-Pr was added. The fin~lingc are shown in
Table 1 and 2.
CA 02234772 1998-04-15
WO 97/17411 PCT~US96/16399
E~mple 4
The same procedure as Example 1 was pe-ro--.~ed except 0.2 parts by
weight of ABP cros ' '-er was added and the dual liner process was used. This
time the ~ g wa~c primed with NEOREZ.
s
r ~;',~D A ~ 5
The same procedure as FY~nPIe 4 was pG-rullllcd except an additional 0.2
parts by weight of ABP c ..............................., ' ' was added instead. The fin-lingc are shown in
Table 1 and 2.
The same procedure as F.Y~mple 4 was pelrolllled except an additional 0.4
parts by weight of ABP crosslinker was added. The fin-lingc are shown in Table 1and 2.
The same procedure as FY~mp'c 4 was pelr~,lllled except n-butyl acrylate
was used instead of isooctyl acrylate and 0.1 parts by weight of HDDA crosclin~-~r
was added. The chee~ g was also primed with M-sol solution. The finr~ingc are
20 shown in Table 1 and 2.
Example 8
The same procedure as Example 7 was pe-rol---ed except 4.4 parts by
weight of urG~ e diacrylate (Ebecryl 230) crosclinker was added and the .$heeting
25 was primed using nitrogen corona. The fin-~ingc are shown in Table 1 and 2.
~.., . " 9
The same procedure as Example 8 was pe.ro....ed except 8 8 parts by
weight of u~e~ e diacrylate (EBECRYL 230) clos~ çr was added. The
30 r....~ gc are shown in Table 1 and 2.
CA 02234772 1998-04-15
W O 97/17411 PCTrUS96/16399
3~
, 'e 10
The same procedure as Example 8 was p~.r~,-,.led except 13.2 parts by
weight of ul- IL~e diz_lylale ~BECRYL 230) croselirl-er was added. The
l~...l;.~g~ are shown in Table 1 and 2.
E~mple 11
The same procedure as F-~ -ple 8 was p~lr..llllcd except 81 weight
percent isooctyl acrylate and 19 weight percent of acrylic acid were used and 0.2
parts by weight of HDDA CIO~ W8S added. The fin-ling~ are shown in Table
10 1 and 2.
~~ 'e 12
120 g of IllelLyl...eth~crylate, 40 g of N,N-dilllclllyl acrylamide and 40 g
isoo~ Iylac~ylate were charged to a reaction vessel co.~ P 300 g ethyl~cet~te and
0.6 g VAZO~' 64 ~DuPont Chemical). The vessel was purged with nitrogen,
sealed and ~t~ted for 24 hours in a water bath at 55 degrees Celsius. The
g polymer can be diluted with ethylacetate to 30% solids and coated to yield
an optically clear, non-tacky film.
This film adhesive is position~hle up to 70~C and can be heat-l~min~ted
around 110~C bel~en two pieces of p1~eti~i7~o~1 vinyl (PanaflexTM available from3M Colllp&lly) to yield a strong bond. After aging (9 days at 65~C), the two PVCpieces can no longer be sep&-~led without destruction of the vinyl.
F..~ '~ 13
The same charges and reaction con~1iti~n~ were used as in Ex~ le 12, but
we also ~I.~ged 0.2 g of carbon teL~ ~rc"nide to reduce the molecular weight of the
polymer. The polymer can be coated at 40% solids to yield a clear, non-tacky film
which le.l,&ins positionable to around 70~C, yet will heat-l~min~te to PVC around
110~C. Again, a very good bond was obtained.
CA 02234772 1998-04-15
WO 97/17411 PCTAUS96/16399
14
The same charges as in E~ ,le 12 were used, except the
mcll,yl~ hAcrylate was replaced with ~lhyl~ A~ rylate. Solution coating yields adear, non-tacky film which .~,.n&ins pocitionAble up to about 50~C, yet is heat-
5 ~ ble to pl~cl;~ d vinyl around 90~C and gives a strong bond.
E~mple 15
The same charges as in F ~~ , 'e 13 were used, but .lwlllyl~ h~-crylate
was r~ ed with cl~ s~ ' --rylate. Solution coating yields a clear film with very10 slight tack but pos;l;ol-~ble up to around 50~C.
Heat l~min~tion to pl~Cti~i7~d viny1 at 90~C yields a strong bond.
The ~_ "!e~ above dc,--o~ e that optically clear adhesives with good
heat-activatable prop~,. lies for PVC application can be obtained from solution.In order to d;.~ c~e solvents from the process, we can s~1cpçn~;on
15 poly...~ the n.onolllC.~ but we would need an additional step to convert polymer
beads into a thin co~Atin Bulk poly--.~ alion right on the web is highly p-crc--cd
because the free-st~n-ling adhesive film or adhesive coated article is obla---cd in one
step. Due to the high volatility and flAmm~bility of some monomers, the s~lection
of Illollolllc. :, for polS,---~, izalion on web is more limited. For example, mono...c~
20 like methylacrylate or ethylacrylate are too flAmmAble and odorous to be hAn~1ed
safely, and non-llA~.. Able monomers like isobornyl acrylate will have to be
I,sl;~ ed as a higher Tg yielding monomer. The eYAmr1e, below d~.--on~ e the
use of W initi~ted dual liner curing of adhesives useful for PVC application.
25 ~ 16
A mixture of 30 g isobo...yl acrylate, 30 g N,N-dimethyl acrylamide, 40 g
isooctyl acrylate and 0.3 g EsacureTM KB-l was purged with nitrogen and exposed
to low intensity UV light (IJV "b1~~l~1ight" from Sylvania) to make a coatable syrup.
Once coatable ViSCG~ity was oblahled, the reaction was stopped by turning off the
30 UV light and exposure of the syrup to oxygen. The syrup was then completely
polyll~1liGed as o~ ed under dual liner curing ~licc~ssed above.
CA 02234772 1998-04-15
W O 97/17411 PCTAJS96/16399
F~D .'r 17
This sample was made similar to c-~..rle 16 above, but 20 g isobornyl
aclyl~le, 30 g N,N-dinlell"~l n~ yl~...de and S0 g isooctyl acrylate was used
At room t~.. pw~u.e, both W cured ~ can easily be positioned on
a Panaflex~ le. Heat-~sminati~n st 80~C gives a good bond to the PVC
wilLvuL ~ .nl of gas l,.~'es The adhesive does not discolor and is optically
clear.
As ~ e~ileA, higher levels of isobomyl acrylate will increase the heat-
activation t-,.--~e ~ re but they will also lead to embrittlemf nt of the adhesive.
C'G p~rl~tive F-g ~
17.2 grams ("g") but~q~if ~f/a ~rlonitrile synthetic rubber (Nippon Zeon
Co., ~ipol N009) and 0.5 g zinc oxide (New Jersey Zinc Co. Inc., Protox 166)
were V~ i7f d by a rubber mill. 60.4 g of MEK (methylethyl ketone) and 10.4 g
phf nc!1ic resin ~f ;chhol~l Inc., Varcum 861) was added to the pPIlf,ti7f d synthetic
rubber and then the mixture was stirred completely.
The solution was coated on polyethylene l~min~ted release liner and was
dried in an oven at about 25~C for 5 min, 65~C for 5 min, and 93~C for 3 min to
yield a heat-activatable adhesive having a coating weight of 90.4 g/m2. The fin~1inge
are shown in Table 3.
The adhesive was l~min~ted to Msol primed p, i~.--aLic I ellur~;nective
~ ~e~ B at 75~C by using a heat 2~ l or The p. opc. Iies are shown in Table 4.
C'c r~r~ltive ~
0.23 g of 5 weight percent bisamide cro~eelin~-~r in toluene and a 15 g of
methyl ethyl ketone were added to 100 g of a 93:7 isooctylacrylate:acrylic acid
copolymer, and the sQ!~ltiQn was mixed c~m~letely.
The sQ' ~ti<~n was coated on a ~ coni7çd paper liner and was dried at room
te.llpt;~lul~e (about 75~C) for 5 min, 65~C for 4 min, and 95~C for 3 minl~tee- Ae a
CA 02234772 1998-04-15
W O 97/17411 PCT~US96/16399
result, a pressure sensitive adhesive (133.9 g/m2) was obtained and the fin~ing~ are
shown in Table 3.
The adhesive was lAminAted to a primed prismatic le~-olt;nective ~h~oeting
at room te.,.p~. al-lre by a lA~ or. The properties are shown in Table 4.
s
E~mple
The same procedure as F . 1~ 1 was done except the use of 90 weight
percent of isooctyl acrylate and 10 weight percent of acrylic acid and the addition of
0.18 parts by weight of triazine (~353) cro-~' ' . The finrling~ are shown in
10 Table 3 and 4.
Table 1
Heat-activatable Adhesives of ~.SAO rle 1-11
Elastic Elastic Trans- Glass Pre- Post-
Modulus atModulus atparencyTransitionr~lh~ n'- -
30~C 70~C (~/0)Temp (~C) ~in) (kg~in)
(dyn/cm2)(dyn/cm2)
r ~ 2.3xlO' 3.Sx10~ X9.5 23 140 2.2
F , '- 4 2.6x107 3.5x106 90.1 27 260 1.7
F , ' S 3.3x107 3.6x106 89.8 26 2S0 1.5
F , '- 6 3.6xlO' 4.0x106 89.9 26 230 l.S
3.0xlO' 4.2xlO~ 90.3 20 190 3.S
"~.lr~:~l.OxlO' 2.0x10~ 89.7 16 820 8.3
~ ~. l.lxlO' 3.6x10~ 89.4 lS 780 6.5
rF-,'-10 1.3xlO' 6.0xlOUl89.4 113 730 S.9
¦ F , '- 11 ¦¦ 1.4x1074.1x106 ¦ 90.4 ¦14 2S0 7.4
CA 02234772 1998-04-15
W O 97/17411 PCTrUS96/16399
Table 2
Heat A~ e ~ .' ~ ' ~ Relr~ ~n~ ~'~e ~ V,C li~,-u
Whiteness ~,v - ' E~VA Re~u~ll~livily Bonding Round
Cap Y (~/0)Bonding T~npLOSS afier TestEdge
Test (~C)*HLVA Test
~Iq~ (~/0) (mm)
¦ F ,' 2 ,. 47.2 I Exoel 1 70 S Excel S
~4*~ xoel /0 1 ~xoel S
~;~ 4S.9 Exoel 7() 3 Excel S
F ,'-S 46.3Exoel 70 3 Exoel S
F ,'-6 46.5Exoel 70 2 Exoel 5
F ~-7 47.2Exoel 70 2 Exoel 6
F ,'-8 46.1Exoel 70 1 Escel S
F ,'-9 4S,SExoel 70 4 Exoel 6
r ,'elO 46,7Exoel 70 2 Exoel 7
F , -11 46,3Exoel 70 2 Exoel 7
*Bake time was 1.~ min at 70~C
s
Table 3
C~ psrative F.-~ . 'er 1-3
ElasticElastic Trans- GlassTransi- Pre-adhe-Post-
ModulusatModulusae parency tionTemp sion (~in) adhesion
30~C 30~C(d~cm2) (~/0) ~C) (kg~
(d~cm2) in)
2.0xlO~ 2.0xlO' 40,5 42 0 1.8
' .. 2.SxlO 1.4xlO 92.1-13 2030 3.S
~ , 3.3x10~ 2,0x10~ 89.7 -7 17S0 4.9
Table 4
CC Pgrat;VeF~D pr~ ~ 1-3
Whiteness P uv ~ ' HLVARetro-reflec- BondingRound
Cap Y (~/0) Bonding Temp (~C)* tivity Loss after Test Edge
Test HLVA Test
" (~/~) (mm)
Comp 1 39.3 Slide 93 30 Delam >10
W liu, 46.1 Tack RT O Exoel>10
W~ 46,S Tack RT O Exoel>10
CA 02234772 1998-04-15
W O 97/17411 PCT~US96/16399
Various mo~ific~tion~ and alterations ofthis invention will become appd-~l.L
to those skilled in the art without departing from the scope and spirit of this
L~