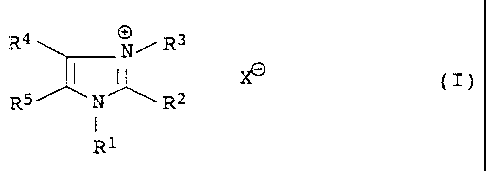Note: Descriptions are shown in the official language in which they were submitted.
0050/46588 CA 02234844 1998-OS-OS
Use of quaternized imidazoles as corrosion inhibitors for non-
ferrous metals, and coolant compositions and antifreeze concen-
trates comprising them
The present invention relates to the use of specific quaternized
imidazoles as corrosion inhibitors for preventing the corrosion
of nonferrous metals. The invention also relates to antifreeze
concentrates and to ready-to-use aqueous coolant compositions
comprising these quaternized imidazoles, and to a method of
treating aqueous liquids with these quaternized imidazoles in
order to reduce the corrosion of nonferrous metals.
Antifreeze for the cooling circuits of internal-combustion
engines, as in automobiles, generally contains an alkylene gly-
col, especially ethylene glycol or propylene glycol, as the
principal component. For use in the cooling system these are
diluted with water and are intended to provide not only for
protection from frost but also for good heat dissipation. Alkyl-
ene glycol/water mixtures, however, are highly corrosive at the
operating temperatures of internal-combustion engines, and there-
fore the various metals and their alloys present in the cooling
system must be provided with sufficient protection against a wide
variety of types of corrosion, such as pitting, crevice corro-
sion, erosion or cavitation. For use as corrosion inhibitors in
such cooling systems the prior art already includes a multi-
plicity of individual chemicals.
As far as temperature stress on the heat transfer faces, pres-
sure, flow rate and the selection of appropriate materials are
concerned, the operating conditions in modern internal-combustion
engines nowadays place very much greater demands on the anticor-
rosion capacity of the coolant than was previously the case. In
addition to the known materials, such as copper, brass, soft sol-
der, steel and gray cast iron, aluminum alloys are being used to
an increasing extent. Consequently, more of the recent patent
literature describes specific combinations of long-known active
substances, each claimed to have its own specific spectrum of ac-
tion.
For instance, EP-B 229 440 (1) describes the combination of the
salt of an aliphatic monobasic C3-Cls acid, the salt of a dibasic
Cs-his-hydrocarbon acid and a hydrocarbon-triazole as an effective
inhibitor formulation in a liquid-alcoholic freezing-point
reducer for protecting aluminum alloys against pitting. It is
additionally possible to employ other customary inhibitors, such
0050/46588 CA 02234844 1998-OS-OS
_ 2
as alkali metal borate, silicate, benzoate, nitrate, nitrite or
molybdate, and/or a hydrocarbazole.
EP A 564 721 (2) discloses antifreeze compositions, in particular
for car radiator protection, which comprise a combination of the
salt of an aliphatic monobasic C5-C1s acid, a hydrocarbon-triazole
and imidazole as effective inhibitor formulation in a liquid-
alcoholic freezing-point reducer. The imidazole concerned
includes unsubstituted imidazole and also alkyl- and aryl-
substituted imidazole.
A deficiency of the known inhibitor combinations is that, as far
as nonferrous metal protection is concerned, they lose marks at
elevated temperatures. For example, the hydrocarbon-triazoles
which are employed predominantly in this context, for example
benzotriazole and toluotriazole, lose their effectiveness above
about 130°C.
It is an object of the present invention, therefore, to find sub-
stances which raise the high-temperature protection afforded to
nonferrous metals by customary inhibitor systems without a drop
in protection for the other metals, especially aluminum alloys.
we have found that this object is achieved by the extensive
replacement, in customary inhibitor systems, of the hydrocarbon-
triazoles, such as benzotriazole or toluotriazole, by quaternized
imidazoles, resulting in a marked improvement in the protection
afforded to nonferrous metals, especially copper, brass and
bronze, under high temperature stress.
It has been found that quaternized imidazoles of the general
formula I
R4 N/ R3
X~ (I)
RS N R2
I
R1
where
R1 and R3 independently of one another are saturated or unsatu-
rated hydrocarbon radicals of 1-12 carbon atoms which
can be substituted by oxygen-functional groups or
interrupted by nonadjacent oxygens,
0050/46588 CA 02234844 1998-OS-OS
3
R2, R4 and R5independently of one another are hydrogen or satura-
ted or unsaturated hydrocarbon radicals of 1-12
carbon atoms which can be substituted by oxygen-
functional groups or interrupted by nonadjacent
oxygens, it being possible for R4 and R5, together
with the corresponding imidazole carbons, to form a
five- or six~nembered ring, and
Xe is an anion,
20
are generally suitable as corrosion inhibitors in connection with
the corrosion protection of nonferrous metals, especially copper,
brass and bronze.
15 Particularly suitable oxygen-functional groups for R1 to RS are
hydroxyl, carbonyl, carboxyl and C1-C4-alkoxycarbonyl. Depending
on the size of R1 to R5 it is possible for each to carry 1-6, in
particular 1-3, of such groups. Similarly, 1-6, especially 1-3,
ether oxygens can be present per radical.
Preference is given in this context to quaternized imidazoles I
where
R1 is C1-C4-alkyl, Ca-C4-a.lkenyl, C2-C4-hydroxyalkyl,
25 phenyl-C1-C4-alkyl, phenyl or tolyl,
RZ is hydrogen or C1-C4-alkyl,
R3 is C1-C4-alkyl or benzyl,
R4 and RS are hydrogen, C1-C4-alkyl or together are a
benzo-fused ring, and
Xe is a mono-C1-C4-alkyl sulfate anion, half the
stoichiometric amount of sulfate or of hydrogen
phosphate (HP04ae), dihydrogen phosphate (H2P04e), a
third of the stoichiometric amount of phosphate
.. zR. ...~., ,. ; .a.. ~., i.,-r"n; ao
( PU4''" ) , o= nitr a to , c.:li.ivr i.uc yr ui v.a.~...... .
Particularly preferred quaternized imidazoles I are 1-methyl-
imidazole, 1-ethylimidazole, 1-(~-hydroxyethyl)imidazole, 1,2-di-
methylimidazole, 1-phenylimidazole, benzimidazole and, in par-
ticular, N vinylimidazole, which are quaternized with benzyl
chloride, benzyl bromide, methyl chloride, methyl bromide, ethyl
chloride, ethyl bromide, diethyl sulfate or, in particular,
dimethyl sulfate.
0050/46588 CA 02234844 1998-OS-OS
4
The anions mentioned, where not obtained via the quaternizing
agent, can also be obtained by customary anion exchange methods
on the quaternized system. In the case of quaternization with
dialkyl sulfates it is possible for one or two alkyl groups to be
transferred, resulting in the anions R30S03e or S04ze (in half the
stoichiometric amount).
For the present invention, very special interest attaches to
N vinylimidazole quaternized with dimethyl sulfate.
The present invention additionally relates to antifreeze con-
centrates based on alkylene glycols or derivatives thereof and
comprising 0.0005-2$ by weight, preferably 0.01-1~ by weight,
especially 0.05-0.5$ by weight, based on the overall amount of
concentrate, of one or more of the abovementioned quaternized
imidazoles I.
The hydrocarbon-triazoles of unfavorable temperature stability,
such as benzotriazole or toluotriazole, which are commonly
present in such antifreeze compositions, can~be largely or com-
pletely replaced by the quaternized imidazoles I described. Since
under certain circumstances, however, advantages are possible in
respect of the protective action for nonferrous metals, owing to
synergistic effects between small amounts of hydrocarbon-
triazoles and quaternized imidazoles I, a preferred embodiment of
the present invention relates to novel antifreeze concentrates
which additionally comprise up to 0.3$ by weight, in particular
0.001-0.1~ by weight, especially 0.005-0.05 by weight, based on
the total amount of concentrate, of at least one hydrocarbon-
triazole and/or hydrocarbon-thiazole.
Since in some cases it is also possible to find synergistic
effects between the quaternized imidazoles I and nonquaternized
imidazoles as described in (2), a further preferred embodiment of
the present invention relates to novel antifreeze concentrates
which additionally comprise 0.001-5~ by weight, in particular
0.01-2~ by weight, especially 0.05-1~ by weight, based on the
total amount of concentrate, of nonquaternized imidazole and/or
at least one nonquaternized alkyl- or aryl-substituted imidazole,
for example a 1-(C1-C4-alkyl)imidazole or 1-phenylimidazole.
In addition, the novel antifreeze concentrates may also comprise
all other customary inhibitor components for antifreeze formula-
tions, including in particular:
0050/46588 CA 02234844 1998-OS-OS
- up to 5~ by weight, especially 0.05-3$ by weight, based on
the total amount of concentrate, of alkali metal borates, eg.
sodium tetraborate (borax), and/or alkali metal phosphates,
eg. disodium hydrogen phosphate or trisodium phosphate;
5
- up to 5~ by weight, especially 0.05-4$ by weight, based on
the total amount of concentrate, of at least one aliphatic or
aromatic dicarboxylic acid of 4-16 carbon atoms, in par-
ticular 8-12 carbon atoms, in the form of its alkali metal
salts, ammonium salts or amine salts, eg. the disodium salts
or dipotassium salts of suberic, azelaic, sebacic, undecane-
dioic, dodecanedioic, dicyclopentadienedicarboxylic, phthalic
or terephthalic acid;
- up to 5$ by weight, especially 0.05-4~ by weight, based on
the total amount of concentrate, of at least one aliphatic or
aromatic monocarboxylic acid of 3-16 carbon atoms, in par-
ticular 5-12 carbon atoms, 7 carbons being the minimum for
aromatic monocarboxylic acids, in the form of its alkali
metal salts, ammonium salts or amine salts, eg. the sodium
salts or potassium salts of pentanoic, hexanoic, octanoic,
2-ethylhexanoic, nonanoic, decanoic, undecanoic, dodecanoic,
benzoic or methylbenzoic acid;
- one or more corrosion inhibitors from the group consisting of
alkali metal silicates, molybdates, nitrites and nitrates,
and magnesium nitrate, each in amounts of up to 1~ by weight,
especially 0.05-0.8~ by weight, based on the total amount of
concentrate; examples are sodium metasilicate, sodium
nitrite, sodium nitrate and sodium molybdate.
When alkali metal silicates are also used, they are advantageous-
ly stabilized using customary organosilicophosphonates or organo-
silicosulfonates in customary amounts.
In addition to the abovementioned inhibitor components it a.s also
possible, for example, to employ hydrocarbazoles in customary
amounts.
The overall corrosion inhibitor component can be up to 15~ by
weight, in particular up to 10~ by weight, of the total amount of
concentrate, where the concentration of individual constituents
can be up to 5~ by weight.
The novel antifreeze concentrates can additionally comprise up to
1~ by weight, in particular 0.01-0.5~ by weight, based on the
total amount of concentrate, of hard-water stabilizers based on
0050/46588 CA 02234844 1998-OS-OS
6
polyacrylic acid, polymaleic acid, acrylic acid-malefic acid
copolymers, polyvinylpyrrolidone, polyvinylimidazole, vinyl-
pyrrolidone-vinylimidazole copolymers and/or copolymers of
unsaturated carboxylic acids and olefins.
The pH of the novel antifreeze concentrates is commonly 6-11,
preferably 7-9, in particular 7.1-7.3, and is generally estab-
lished at the desired level by adding alkali metal hydroxide,
ammonia or amines to the formulation; solid sodium hydroxide and
potassium hydroxide, and aqueous solutions thereof, are particu-
larly suitable for this purpose. Any aliphatic or aromatic mono-
and/or dicarboxylic acids used are advantageously added directly
as the corresponding alkali metal salts so as to establish the
desired pH range automatically, but these carboxylic acids can
also be added as the free acids, after which the formulation is
neutralized with alkali metal hydroxide, ammonia or amines and
the desired pH range is established.
Suitable liquid-alcoholic freezing-point reducers, which normally
make up the major constituent (generally at least 80$ by weight,
in particular at least 90~ by weight) of the novel antifreeze
concentrates, are alkylene glycols or derivatives thereof, espe-
cially propylene glycol and, in particular, ethylene glycol. How-
ever, higher glycols and glycol ethers are also suitable, exam-
ples being diethylene glycol, dipropylene glycol and monoethers
of glycols, such as the methyl, ethyl, propyl and butyl ethers of
ethylene, propylene, diethylene and dipropylene glycols. It is
also possible to use mixtures of said glycols and glycol ethers.
The present invention also relates to ready-to-use aqueous
coolant compositions of reduced freezing point, especially for
car radiator protection, which comprise water and 10-90~ by
weight, preferably 20-60~ by weight, of the novel antifreeze
concentrates.
The present invention relates, moreover, to a method of treating
aqueous liquids comprising a water-soluble freezing-point reducer
based on alkylene glycols or derivatives thereof in order to
reduce the corrosion of nonferrous metals in contact with the
aqueous liquids, which comprises admixing to the aqueous liquids
an effective amount of one or more quaternized imidazoles I. For
the corrosion-inhibiting imidazoles I, this amount is normally
0.0005-2~ by weight, preferably 0.01-1$ by weight, especially
0.05-0.5~ by weight, based on the total amount of freezing-point
reducer and all associated corrosion inhibitors and other
additives.
0050/46588 CA 02234844 1998-OS-OS
7
Examples
The present invention is illustrated using Examples A, B, D and G
of the invention and Comparison Examples C, E, F and X. Table 1
shows the composition of the inhibitor mixtures, in ethylene
glycol, which are used to illustrate the invention. These anti-
freeze concentrates were diluted with water and subjected to
standard corrosion tests.
The results obtained in the ASTM D 1384 corrosion test (Table 2)
first of all show clearly that the quaternary structure of the
imidazoles constitutes the principle of action of the novel non-
ferrous metal protector. Thus Examples C and D illustrate, in
direct comparison between l~nethylimidazole and the same compound
quaternized with dimethyl sulfate (DMS), the marked difference in
the amount of material lost, for copper and brass.
Examples B and G also show clearly the good protective action of
the quaternary structure, which is at least at the level of that
afforded by, for example, benzotriazole (the hydrocarbon-triazole
commonly used), as shown by Comparison Example X. Furthermore,
the quaternized imidazoles also exhibit an at least equal protec-
tive action for other metals (in this case soft solder, steel,
gray cast iron and cast aluminum) to that of benzotriazole.
The enhanced protective effect for nonferrous metals of the
quaternized imidazoles I employed in accordance with the inven-
tion, in comparison with the hydrocarbon-triazoles which are com-
monly employed, becomes particularly evident when a test under
elevated temperature stress is considered (Table 3). This is the
MTU (German Engine and Turbine Union) hot chamber corrosion test,
which is normally carried out using specimens of aluminum alloy.
Examples A and B demonstrate the enhanced protective effect rela-
tive to Comparison Example X; also evident is the synergy between
the quaternized imidazole I and small amounts of hydrocarbon-
triazole, which is not so marked in the ASTM D 1384 corrosion
test. Once again, the effect of quaternization is revealed when
Examples A and B are compared with C and E.
The variables and conditions specified in the examples serve to
illustrate the invention and do not constitute any restrictions.
0050/46588
CA 02234844 1998-OS-OS
8
o ~n o 0 0 0
V, O N ~ O N ~O1 1 1 1 1 1
*
r-~1O N .-iO O ~-1
O tf1 O O O O
1
O N ~ O N ~O1 1 1 i 1 ' 1
r-iO N ~-1O O 3-I
e n O
W O N * O N ~O1 1 1 1 ~ 1 1
r-1O N r-1O O 1-i
O tf1 O O O O
A O N ~ O N ~O1 1 1 ~ 1 1 1
*
r-1O N r'1O O S-1
O tn O O O O
'-1
V O N ~ O N ~D1 1 ' I 1 1 1
*
O N e-1O O S-i
O In O O O O
~
~ O N * O N ~D1 ' 1 1 1 1 1
~-iO N .-IO O 1-i
N
~f O tf7 O O O M O
.Ll ~ O N ~ O N ~DO '"'~1 1 1 1 1 ~ U
dP ~-1O N ~-1O O O S-1
U
td
O
o ~n o o o o
-1
x o N ~ o N ~o 1 I 1 1 1 1
r-iO N ~ O O ~1
O
N
-,..i
-r-i Cl~
W
O
x
~ Q'
U ~ - .i~
-I
-~ 3
O 3 3
,~
N -,-1
r1 --r-I N
ri
-r-1 -ri t~ -,-.I~ .U
U ~ ~ N
y. ~ +~ o
,~ U~
O O U1 +~ cri .N,.i O
U
~ O
N CT O'~
N U
N ~ ~ .p
N N
U U 0 0 ~
S-1 'LfU I r O O r r r-I y-,1
-~- -I -I-I
4-t O O .,~-,~ ~ O N N O O O
>~ -ri
'-1 .~+~ >CO -riN N f6 c~ N N U
-r-I
+~ cac~3O C 'Otne-Ictj'O 'O ~ tCS>,
~
i-aS-IS.-I~d -r-1ctSO 'O-.~i-r-I 'L3'O
+.~+~ 'ax U +~N -'-IE ~ -~-.~as
o~
O 'r-1'J~N c0O ACS~..-rl-r-IN .lr.E 1..1
1 +~'t~'.C.~" .~.,-r'I-rlr-1r-.Ir-I-r-I-r-1O
r-IU S-t~ ~r ~rO ri'-IS~ O
II
D, -r.i~ +.>>t~ .>~N ~,~ N
~ ~ ~ ,~ U ~ O C .i~+.~ctS~ .Crl p
O r-I-r-I-rl.1-1tLS-r-IN -r-Ip N 'O .1-~+~
'-I 'Lf'O 'L7W .~'bC ~ '~ ~ -r-IW W .C
N
O O O I N O O I i I E 1 1 +~
~-1
CI~U1 U1N U1U1!xt.-I'-iriH N r-fW
*
H
0050/46588
CA 02234844 1998-OS-OS
9
O O ~-t.-1N N
O O O O O O
~ ~ o o
+~~ + + + y n
rl W
x
U I
.,1
L~
ri M .-iN O
~-1O 01O O rii..1
W O
~-iO O O O O ~..7
I I 1 + + + 4-I
cV M
N
0 0 0 .-1~ N ~ U
00o m o 0 0 ~, I
W
o O o o +
~ f +
y r[
is
N
O r-1.-1O N 01
O O O O O O
O
p U
o o o + o o M
I ~
0
i
U .ri
N
+~ N M r N ~ ~r3
o ~ 0 0 0
C~ U
.-i O O O O o O
I I I I + +
"'I N
va
'
'a
. 0
Lf 3
rlO <-1O M N O ~ O
O O O O O O ~ ~ I
o o o o
+ t~ + +~ + +
O ~ .-I
.,..I
O +~ -
~
O ~-ItnO M O O O
V r-1O O O O O
~ 0 0 o
0 0 o o ~
l ~ x
p
t
u~ o
rl r-IN r-1O O v-i47 dP O
b1 O O O O O O
N rl Lf~
O O O O O O
OO + + I -h~+~I
M U
Ca U
~ N
O
H ~
..
~
~ N O ~ U
S-~ .r~ -~-
-i i
O +~ S-1
N ~ ~ M ~ 0
O ~
S-~ U ~ . 1
rl N T3 -ii
N N r-I +~
'" -"1 ~ W +~ i
W n CLW ~ U ~riu W t U
n
H U U ~ ~ ~ ~ O ~ N U
t c C.U E.W
H n f~ 7 H v1 v1 E-~ .,