Note: Descriptions are shown in the official language in which they were submitted.
CA 0223~X41 1998-04-23
W O 97/16067 PCT/G B96/02622
COMPO~NDS FQR CONTRQT, OF V~ LY
This invention relates to the control of whitefly and is particularly directed to the
control of resistant strains of whitefly.
Within the last decade whitefly, in particular tobacco whiLeny(Bemi~i~ tabaci), has
become a major pest of many crops in many countries. As a pest it causes direct feeding
damage, exudes copious honeydew (which is a ~ub~LldLe for fungi), creates harvesting
difficulties (especially in cotton) and Lld~llliL'i a large number of plant viruses. It has
become established in glasshouse horticulture in co.,~ ."~l Europe and poses a threat to
agriculture in the UK despite efforts at ~lu~ulLille. The problem is exacerbated by the
10 dissemination of resistant strains which are highly fecund, have a wider host range and
which are resistant to the major groups of insecticides, i.e. organochlorine,
organophosphorus, carbamate and pyrethroid insecticides.
There is thus an urgent need for the provision of new methods of whitefly control
directed in particular against the resistant wllileny strains.
Accordingly the present invention provides a method of cnmb~ting a resistant strain
of whitefly at a locus infested by the .esi~L~lL strain of whik;ny compri~ing applying to the
locus an amount effective to combat the whiLeny of at least one compound of formula I.
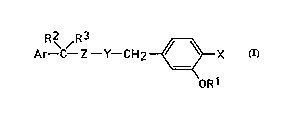
R 2~ / R 3 _~X (I)
OR1
in which forrnula
X is hydrogen or fluorine;
Y is CH2, CHF or O and Z is CH2, or Y is CH or CF and Z is CH, Y and Z forming
a double bond, or Y is CH2 and Z is CO;
R1 is optionally substituted phenyl;
R2 is hydrogen and R3 is CF3, isopropyl or cyclopropyl, or R2 and R3 are methyl,25 or R2 and R3 together form a cyclopropyl ring; and
SUBSTlTUrE SHEEr (RULE ~6
CA 02235841 1998-04-23
W O 97/16067 PCT/GB96/02622
Ar is a phenyl or naphthyl group optionally substituted by one or more halogen,
alkoxy, haloalkoxy, methylenedioxy, Cl -C6 alkyl or haloalkyl groups.
Preferably Ar in forrnula I ,e~.~s~llL~ a phenyl group, preferably sllbstit~lte~l at the
para position. Preferred substituents are halogen, particularly chlorine and fluorine and
S aL~coxy, especially ethoxy.
R1 is preferably an ull,ub,liluLed phenyl group.
Those compounds where X is fluorine are especially preferred.
It will be appreciated tnat, dependent on the sll~stit l.ont~, optically active carbon
atoms may be present. It is int~n~efl to include optically active as well as racemic forms
10 of such compounds. When Y and Z form a double bond, the compounds preferably have
the two carbon-co. .I ;~i . . i . .g substituents in a ~ans configuration.
A particularly ~ler~ ,d group of compounds according to the invention are those
of formula II
W ~f
Ar~ ~ORI (II)
15 in which Ar, X and R1 are as defined above and W is hydrogen or fluorine. Compounds
of forrnula II where W is hydrogen are described and claimed in UK Patent No. 2167749.
Compounds of formula II where W is fluorine are the subject of co-pending
UK Application No. 9219612Ø
A further ~lG~ll~,d group of colll~oullds are those of formula III
R2~R3 ~OR1 (III)
wherein Ar, X and R1 are as defined above, W is hydrogen or fluorine and either R2 is
hydrogen and R3 l~pleselll~, a cyclopropyl group or R1 and R2 each l~ sent a methyl
group. Compounds of formula III where W is hydrogen are disclosed in US Patent
--2--
CA 02235841 1998-04-23
WO 97/16067 PCT/GB96/026Z2
No. 4975451 and J~p~nl~se Patent Publications Nos. 60115545, 60193902 and 60193940.
Compounds of formula III where W is fluorine are the subject of co-pending
UK Application No. 9308626.2.
Preferably X is fluorine.
An additional group of preferred compounds are those of formula IV
R2 R3 I~X
Ar>~O ~OR1 (IV)
wherein Ar, X and Rl are as defined above, R2 is hydrogen and R3 is CF3 or R2 and R3
are methyl. Such compounds and their ~lc~aldLion are disclosed in Baydar et al., Pestic. Sci. 1988, ~, 247-257, or UK Patent No. 2118167.
Also included within the present invention are compounds of forrnula V
A ~ ~ OR1
where Ar, X and Rl are dS defined above and W is hydrogen or, more preferably, fluorine.
Compounds of formula V where W is fluorine are novel and may be p~c~u~ed by the
alaLion route generally described in co-pending UK Application No. 9308626.2 and15 exemplified herein.
Also included within the present invention is the use of compounds of formula VI
R2 R3 W I~X
A~--~ORl (VI)
where Ar, X and Rl are as defined above. R2 and R3 are methyl or R2 is hydrogen and R3
is cyclopropyl or R2 and R3 together forrn a cyclopropyl ring, and W is hydrogen
CA 0223~841 1998-04-23
W O 97/16067 PCT/GB96/02622
or fluorine. Certain of such compounds and their pl~pdldlion are described in
UK Patent No. 2120664.
Although the compounds of form~ ? I to VI as defined above have been generally
proposed for use as insecticidal agents and, for example, UK Patent No. 2167749 m~ntion~
5 activity against whitefly in a list of a very large number of insect orders and individual
species, no biological data showing effectiveness against whitefly has been given in the
above reference docllm~nt~ It was therefore ~ulyfl~ g to find that compounds of formula
I had generally good activity against whiL~ny and even more surprising to find that that
good activity was largely m~ ;..r~l against rc, ,i~L~IL whitefly strains. This m~ le~ e
10 of activity is in clear contrast to the majority of the comm~rcially available pyrethroid ester
insecticides, and an organochlorine insecticide such as DDT, which. while showing
reasonable levels of activity against non-resistant strains, show very much reduced activity
against resistant strains. This difference is illustrated in the following examples.
The compounds of formula I to VI as described above can be form~ tP~i in many
15 ways for use in comhating resistant whitefly. They can therefore be employed in a
p~ 1 composition comrri~in~ a compound of formulae I to VI as an active ingredient
together with an inert carrier or diluent.
Suitable diluents include both solid and liquid fliln--nt~ so as to provide
compositions which can be form~ te~l for example as granules, dusts or em~ ifi~hle
concentrates. Exarnples of diluents suitable for the ~ d~ion of granular compositions
are porous materials such as pumice, gypsum or corn cob grits. Suitable diluents for the
p.cp~hdLion of dusts include kaolin, bentonite, kieselguhr or talc. For the ~ ~d~ion of
em~ ifi~ble concc--LIdles, various solvents, such as ketones and aromatic solvents, may be
employed together with one or more known wetting agents, dispersing agents or
emulsifying agents.
Solid compositions especially granules, preferably contain from 0.5 to 15% by
weight of active ingredient, while liquid compositions, as applied to the crop, may contain
as little as from 0.0001 to 1% by weight of active ingredient. A composition such as a
wettable powder however may contain as much as 75% by weight of active ingredient.
CA 0223~841 1998-04-23
W O 97/16067 PCT/GB96/02622
Dependent on the mode of use, the compositions may conveniently be applied to
the locus of whitefly il~LdLion at an application rate of from 1 to 500 g of active
ingredient per hectare.
It will be appreciated that the compositions may include a mixture of compounds
5 of formula I and/or other ingredients, including another pesticidal material, eg. an
insecticide, acaricide or fungicide, or a synergist.
It is int~n~led that the co,.lposilions may be applicable to foliage soil and/or seeds
during cultivation of a wide variety of foliage, horticultural and agricultural crops such as
maize, sugar beet, potatoes, tobacco and cotton.
The compositions are particularly useful in comhating resistant strains of
Bemisia ~i, but are also contemplated for use to combat other resistant whitefly strains
such as Trialeurodes vaporariorum and abutilonea.
The following examples illustrate the invention.
A) Pl~dLion of compounds of Formula I.
Compounds of Formula I were ple~ ;d by the methods referenced in Table 1
below or in accordance with ~l~dLi~e Examples 1 to 5 below in which Examples 1 to 3
relate to the p.~,~dLion of intermediates and Examples 4 and 5 to compounds in
accordance with the invention.
CA 0223~841 1998-04-23
W O 97/16067 PCT/GB96/02622
Table1
R2 R3 ~ X
Z~ ~ OPh
R4
CompoundNo X Z-Y R2 R3 R4 Pl~d~ion
1 H CH=CH -CH2-CH2- Cl A
2 F CH=CH -CH2-CH2- Cl A
5 3 H CH=CF -CH2-CH2- Cl B
4 F CH=CF -CH2-CH2- Cl B
S H CH=CH -CH2-CH2- EtO A
6 F CH=CH -CH2-CH2- EtO A
7 H CH=CF -CH2-CH2- EtO B
10 8 F CH=CF -CH2-CH2- EtO B
9 H CH=CF -CH2-CH2- F B
F CH=CF -CH2-CH2- F B
11 H CH=CF CH3 CH3 Cl E
12 F CH=CF CH3 CH3 Cl E
15 13 H CH=CF CH3 CH3 EtO E
14 F CH=CF CH3 CH3 EtO E
H CH=CF H iPr Cl G
16 F CH=CF H iPr Cl G
17 H CH=CH H -~ Cl F
20 18 H CH=CF H -~ Cl E
19 F CH=CF H -~ Cl E
H CH=CF H -~ EtO E
CA 02235841 1998-04-23
WO 97/16067 PCT/GB96/026Z2
Table 1 (continued)
Compound No X Z-Y R2 R3 R4 Pl~a dlion
21 F CH=CF H - ~ EtO E
22 H CH2-O CH3 CH3 EtO H
23 F CH2-O CH3 CH3 EtO C
24 F CH2-O -CH2-CH2- Cl C
F CH2-~ H CF3 EtO D
26 H CH2-O H CF3 EtO D
27 F CH2-cH2 CH3 CH3 EtO
P~ lions were carried out in accordance with
A UK Patent No. 2167749
B UK Application No. 9219612.0
C Baydar et al. Pestic. Sci. 1988, ~, 247-257
D UK Patent No. 2178739
E UKApplicationNo. 9308626.2
F US Patent No. 4975451
G As shown below
H UK Patent No. 2118167
UK Patent No. 2120664
CA 02235841 1998-04-23
W O 97/16067 PCT/GB96/02622
In the following Exarnplest 1 3C NMR peaks are listed as ~ igne-l peaks in the
order indicated by the following diagram:
~I~r ; ~ !o~21
cl
Equivocal ::15~ are indicated by ~ 5. - ;~ , a, b. Peaks not ~letected above the noise
level are indicated by N. Coupling cor~lL, to fluorine are given in brackets, and are
S in Hz.
Fx~rnrle 1
Methyl 4-(4-chloropherlyl!-2-fluoro-5-methylhex-2-enoate
To a stirred mixture of acid-washed zinc powder (2.33 g), copper (I)
chloride (0.38 g) and molecular sieve 4A (2.6 g) in dry tetrahydrofuran (36 ml) under
10 nitrogen, 2-(4-chlorophenyl)-3-methylbutanal (2.26 g) was added slowly, followed by
acetic anhydride (1 ml). After the mixture had been warmed to 50~, methyl
dichlorofluoro~cet~te (2.3 g) was added dropwise, and stirring continued for 4 h at 50 .
After cooling, the mixture was diluted with diethyl ether (150 ml), filtered through a bed
of celite, and the filtrate was concentrated under reduced ~Jlc, 7ulc. The residual oil was
15 chromatographed on silica gel using diethyl ether/hexane ( 1:9) to yield methyl
4-(4-chlol~he.lyl)-2-fluoro-5-methylhex-2-enoate 1.08g 34%.
Fx~mple 2
4-(4-Chloropher~l)- 1 -t2-fluoro-5-methylthex-2-enol
Methyl 4-(4-chlorophenyl)-2-fluoro-5-methylhex-2-enoate prepared as described
20 in Example 1 (1.08 g) in dry diethyl ether (20 ml) was added dropwise to a stirred
suspension of lithium aluminium hydride (0.3 g) in dry diethyl ether at 0 C. Stirring was
c- ntinn~cl during 40 min, while the mixture warmed to room telll~c~dl lre. - Water (20 ml)
was added, and the mixture was extracted with diethyl ether (3 x 20 ml). The combined
organic layers were washed with water (3 x 10 ml), dried and evaporated under reduced
--8 -
CA 0223~84l l998-04-23
W O 97/16067 PCT/~5G/'~2622
pressure. The residue was chromatographed on silica using diethyl ether/hexane ( 1:2) to
yield 4-(4-chlorophenyl)-2-fluoro-5-methylhex-2-enol 0.78g 81%.
Fxarr~ple 3
4-(4-chlorophenvl!-2-fluoro-5-methvlhex-2-enyl acetate
Acetyl chloride (0.84 ml) was slowly added to a stirred solution of
4-(4-chlorophenyl)-2-fluoro-5-methylhex-2-enol (Example 2) (0.39 g) in benzene (20 ml)
and pyridine (0.17 ml) at 0 C, and stirrmg was continued for 24 h while the mixture
warmed to room tt~ eldLIlre. After addition of water ( 10 ml), the llli~lwe was ~tr~c t~-l
with diethyl ether (3 x 20 ml) and the combined organic layers were washed with water
10 (3 x 10 ml) and evaporated under reduced ~lcs~ulc. The residue was chromatographed on
silica using diethyl ether/hexane (1:9) to yield 4-(4-chlorophenyl)-2-fluoro-5-methylhex-
2-enylacetate (0.4 g, 87%).
Fx~mr~le 4
4-(4-Chlorophenyl)-2-fluoro-5-methvl- 1 -(3-~henoxyphenyl)hex-2-ene
A Grignard reagent, ~,c~cd from 3-phenoxyphenyl bromide (0.47 g) in dry
tetrahydrofuran (3 ml) and m~ (34 mg) under nitrogen using iodine as an initiator
at ca 40 C for 50 min, was cooled to room tcl~ dLulc then treated with cuprous bromide
(ca 20 mg) for 10 min. After cooling to -78 C, a solution of 4-(4-chlorophenyl)-2-fluoro-5-methylhex-2-enyl acetate (Example 3) (0.14 g) in tetrahydrofuran was added
slowly with stirring, then the mixture was allowed to warm to room LclllpcldLLu., overnight.
The ~ Lu~c was treated with water (4 ml), then extracted with diethyl ether (3 x 20 ml).
The combined organic extracts were washed with water (2 x 10 ml), dried, and evaporated
under reduced plC~ulc. The residue was purified by ~.cp~Live thin layer cl.. ~ Lography
(solvent: diethyl ether/hexane; 1:9) and then ~lc~a,dlive high performance liquid
25 chromatography (column: Cl 8; solvent: methanol; flow rate: 3 ml/min) to afford
4-(4-chlorophenyl)-2-fluoro-5-methyl-1-(3-phenoxyphenyl)hex-2-ene (36 mg, 18%).
3C NMR spectrum:
142.7, 128.5a, 129.1a, 131.6, 47.3(3), 33.5, 20.9,20.2. 109.5(15), 158.0(256), 38.5(29),
138.4,117.3, 157.5b, 119.1, 129.8,123.6, 157.0b, 118.9, 129.8,123.3
g
CA 0223~841 1998-04-23
W O 97/16067 . PCT/GB96/02622
Fxam~le S
4-(4-Chlorophenyl)-2-fluoro- 1 -(4-fluoro-3-phenoxvphenyl)5-methvlhex-2-ene
The method of Example 4 was repeated using a Grignard reagent, prepared from
4-fluoro-3-phenoxyphenyl bromide (0.3 g), tetrahydrofuran (2 ml) and m~gnpcium (28 mg)
5 and 4-(4-chlorophenyl)-2-fluoro-5-methylhex-2-enol (Example 3) (0.96 g). The residue
after evaporation was purified by p~ Jaldli~re thin layer cllloll,dl{~graphy
(solvent: diethyl ether/hexane; 1:9) to afford 4-(4-chlorophenyl)-2-fluoro-1-(4-fluoro-
3-phenoxyphenyl)-5-methylhex-2-ene (27mg; 19.4%)
13C NMR spectrurn:
10 142.7, 128.5a, 129.0a, 131.6, 47.3(3), 33.4, 20.9, 20.2, 109.6(15), N, 38.9(29), 136.7(3),
121.8,N,N, 117.0(18), 124.8(7), 157.1, 117.3, 129.9, 123.3
B) Biological Data
The compounds 1 to 26 idPntifi~cl in Table 1 were tested against susceptible andull strains of whitefly (Bemici~ tabaci~. The susceptible strain "SUD-S" was collected
15 from the Sudan in 1978 by Ciba-Geigy and subsequently labold~ / cultured to provide the
standard laboratory ~llsc~lible strain. The resistant strain "BELZ" was collected from
broccoli in Belize in November 1991. It is an example of this "poinsettia" strain of
E~mi~i~ tabaci which is the biotype causing control difficulties in American field crops and
glasshouses and in European glasshouses at the time of m~king the present patent20 application.
The tests were carried out as follows:
Acetone solutions (100 rnl) ofthe test compounds were placed in glass vials and
evaporated with rotation to deposit a film of the compound. I~irty adult whiteflies were
placed inside the vial, then after 60 mim-tçs, the treated insects were transferred onto
25 untreated cotton leaf discs which were kept moist on a bed of agar gel. The temperature
was m~int~inlod at 25~C and mortalitv ~c~e~ed after 48 hours. Three replicates were used
at each of S to 7 dose levels per compound. LCso values were calculated by using a
computer software package ("Polo-PC" available from LeOra Software, Berkeley,
California).
-10-
CA 0223~841 1998-04-23
W O 97/16067 PCT/~5G/02622
The LCso values are given in ppm (i.e. concentration of the acetone solution used) in Table
2 below. Several commercially available ~ylGLhluid esters and one organochlorineinsecticide (DDT) are included in the Table for reference.
- Table 2
Compound SUD-S LC50 BELZLC50
1 .1
2 0.8 6.7
3 2.2 7.7
4 0.67 30
S 0.94
6 0.42 1.1
7 0.35 4.2
8 1.1 2.6
9 1.3 17
lS 10 0.64 3.6
11 80 670
12 0.65 0.95
13 2.6 44
14 0.7 10
16 23 61
17 1.6 14
18 0.78 90
19 1.1 5.7
8.3
21 39.4
22 0.86 3.5
23 0.28 0.3
24 1.6
0.24 0.49
SUBSmUTE SHEEr (RULE 26~
CA 02235841 1998-04-23
O 97/16067 PCT/GB96/02622
Table 2 (ct ntinllPcl)
Co.l.~oulld SW-S LC50 BELZ LCsO
26 0.82 1.05
27 0.67 l.S
Cyp~rmPthrin 9.1 170
Bir~.. Ll.. il~ 0.66 1.2
Fe.l~ dL~in 4.3 48
Tenul~lli-l 1.9 40
Fel~lullllill 2.0 22
Fenvalerate 3.9 83
Flucythrinate 5.9 480
Delt~mPthrin o 4 > 1000
PermPthrin 9.S 600
DDT 5.9 11 0
SUBSTITUTE SHEET (RULE 2~