Note: Descriptions are shown in the official language in which they were submitted.
CA 02235928 1998-06-18
- 1 -
PROCESS FOR OPERATING EQUILIBRIUM CONTROLLED REACTIONS
This application is a division of copending Canadian
Application Serial No. 2,173,809, filed April 10, 1996.
The subject matter presented in this patent application
was funded in part by the United States Department of Energy
(DOE) under Cooperative Agreement No. DE-FC36-956010059. The
DOE may possess certain rights under the claims appended
thereto.
The present invention is an adsorbent capable of
preferentially adsorbing carbon dioxide in a gaseous mixture
containing carbon dioxide and an excess of water at a
temperature ranging from 200°C to 500°C represented by the
formula:
~M9~-x) AIx (OH)z~~C03)~z ~ Y HzO ~ z M~2CO3
wherein 0.09 < x < 0.40;
0<y<3.5,
0<z<3.5;and
M' = Na or K.
CA 02235928 1998-06-18
_2_
BACKGROUND OF THE INVENTION
The chemical industry pertorms numerous equilibrium controlled reactions to
manufacture a wide range of chemical raw materials, intermediates and
products.
Product yield obtained in such equilibrium controlled reactions is typically
limited by the
thermodynamic equilibrium of the reaction. Therefore, such reactions are
typically
operated at an elevated temperature for endothermic reactions or at a reduced
temperature for exothermic reactions in order to shift equilibrium toward the
product
direction. Thus, the chemical industry has been searching for improved
processes for
operating equilibrium controlled reactions at reduced temperatures for
endothermic
reactions wherein product yield is not substantially diminished due to
unfavorable
thermodynamic equilibrium constants.
Representative equilibrium controlled reactions include methane and
hydrocarbon steam reforming reactions which are used to manufacture hydrogen
or
synthesis gas, the water gas shift reaction for converting CO to C02, as well
as the
reverse water gas shift reaction for converting COZ to CO. Some of these
reactions are
typically carried out at relatively high temperatures to shift the equilibrium
toward the
product direction as well as to obtain relatively faster reaction kinetics.
Signii~icant
efforts have been described in the literature to improve reaction kinetics by
identifying
new catalysts and by controlling process operating conditions. Additionally,
the concept
of removing a product from a reaction zone to increase product conversion is
well known.
CA 02235928 1998-06-18
-3-
Representative processes for operating equilibrium cohtrolled reactions
include
an article by Vaporciyan and Kadlec (AIChE Journal, Vol. 33, No. 8, August
1987)
which discloses a unit operation comprising a rapid pressure swing cycle in a
catalytic-
adsorbent bed to effect both continuous gas-phase reaction and separation. The
hybrid device combines features of a pressure swing adsorber with those of a
flow-
forced catalytic reactor.
Westerterp and coworkers (Hydrocarbon Processing) p. 69 (November 1988)
disclose two process schemes for improving conversion of hydrogen and carbon
monoxide to methanol. The first embodiment employs a Gas-Solid-Solid Trickle
Flow
Reactor (GSSTFR) wherein a solid adsorbent is trickled through a packed bed
reactor
to remove methanol from the reaction zone which results in increased
production of
methanol. The adsorbent saturated with methanol is collected on a continuous
basis
using multiple storage tanks wherein the methanol is desorbed by reducing the
75 pressure. The second embodiment employs a Reactor System with Interstage
Product
Removal (RSIPR) wherein methanol is synthesized in several stages and removed
utilizing a liquid solvent. High conversion of methanol per pass is achieved
in a series
of adiabatic or isothermal fixed bed reactors. Product is selectively removed
in
absorbers situated between the respective reactor stages.
Prior art processes for conducting simultaneous reaction and adsorption steps
have not achieved commercial success because product flow rates do not remain
sufficiently constant and the desired products are~present in unacceptably low
concentrations with respect to the undesired reaction products, unreacted
feedstock
and purge fluids. Industry is searching for a process for operating
equilibrium controlled
CA 02235928 1999-09-02
- 4 -
reactions which can be operated in continuous mode at reduced
reaction temperatures wherein a reaction product can be
produced in substantially pure form at high conversion, under
relatively constant flow rate and at feedstock pressure.
In accordance with an embodiment of the present invention
there is provided an adsorbent capable of preferentially
adsorbing carbon dioxide in a gaseous mixture containing carbon
dioxide and an excess of water at a temperature ranging from
200°C to 500°C represented by the formula:
[Mg~_,~ AIX (OH)zJ(C03],~,2 ~ y H20 ~ t M~sC03
wherein 0,09 < x _< 0.40;
0 _< y <_ 3.5,
0 < z < 3.5; and
Ml = Na or K.
Applicants have developed a cyclic process, operated under
isothermal conditions, which utilizes a plurality of reactors
operated in a predetermined timed sequence wherein the heating
and cooling requirements in a moving reaction mass transfer
zone within each reactor are provided by indirect heat exchange
with a fluid capable of phase change at temperatures maintained
in each reactor during sorpreaction, depressurization, purging
and pressurization steps. The following five steps of the
general embodiment are performed in each reactor during a
process cycle.
The first step of the process comprises reacting a
feedstock at a first pressure in a first reactor containing an
admixture of an adsorbent and a catalyst suitable for
conducting the equilibrium controlled reaction under reactions
conditions sufficient to
CA 02235928 1998-06-18
-5-
convert the feedstock into a more adsorbable product which is selectively
adsorbed by
the adsorbent and a less adsorbable product and withdrawing a stream which is
enriched in the less adsorbable product and depleted in the more adsorbable
product
as well as unreacted feedstock.
The second step comprises countercurrently depressurizing the first reactor to
a second pressure by withdrawing a mixture comprising unreacted feedstock, a
portion
of the less adsorbable product and a portion of the more adsorbable product.
The third step comprises countercurrently purging the first reactor at the
second pressure with a weakly adsorbing purge fluid with respect to the
adsorbent to
desorb the more adsorbable product from the adsorbent and withdrawing a
mixture
comprising unreacted feedstock, a portion of the more adsorbable product and a
portion of the less adsorbable. product.
The fourth step comprises countercurrently purging the first reactor at the
second pressure with the less adsorbable product to desorb the weakly
adsorbing
purge fluid and withdrawing a mixture comprising the weakly adsorbing, purge
fluid, a
portion of the more adsorbable product and a portion of the less adsorbable
product.
The fifth step comprises countercurrently pressurizing the first reactor from
the
second pressure to the first pressure with the less adsorbable product prior
to
commencing another process cycle within the first reactor.
CA 02235928 1998-06-18
-6-
The general embodiment can be readily adapted to utilize the following
additional step following the first step and prior to the second step wherein
the first
reactor is countercurrently purged at the first pressure with a weakly
adsorbing purge
fluid and a mixture comprising unreacted feedstock, a portion of the more
adsorbable
product and a portion of the less adsorbable product is withdrawn from the
first reactor
at the first pressure. Optionally, this mixture comprising the unreacted
feedstock, the
more adsorbable product and the less adsorbable product can be separated to
form a
stream comprising unreacted feedstock and the unreacted feedstock can be
recycled
for use as feedstock in the first step of the process.
Applicants' process can be readily adapted to perform a variety of additional
' steps in order to further separate the process streams by conventional
methods such as
distillation to yield higher purity products or a source process fluids which
may be
recycled. For example, the stream of the first step which is enriched in the
less
adsorbable product and depleted~in the more adsorbable product as well as
unreacted
feedstock can be separated to form a stream comprising the less adsorbable
product.
The stream of the third step comprising unreacted feedstock, a portion of the
more
adsorbable product and a portion of the less adsorbable product can also be
separated
to form a stream comprising the more adsorbable product. Finally, the mixture
of the
fourth step comprising the weakly adsorbable purge fluid, a portion of the
more
adsorbable product and a portion of the less adsorbable product can be
separated to
form a stream comprising the weakly adsorbable purge fluid and recycling'a
portion of
the stream for use as the weakly adsorbing purge fluid in the third step.
CA 02235928 1998-06-18
_ 7 _
The process can be utilized in any endothermic or
exothermic equilibrium controlled process including homogeneous
reactions involving solely gaseous reactants, and
heterogeneous, catalytic reactions involving gaseous reactants.
Moreover, the present process can be readily adapted for use
in equilibrium controlled reactions which are capable of
operation in the absence of a catalyst. The general embodiment
is followed with the exception that the reactors contain only
adsorbent for the more adsorbable product.
While Applicants' process is suitable for operating any
equilibrium controlled process, Applicants have identified
reactions which are particularly suited for operation using
their process. Preferred reactions include the reverse water
gas shift reaction for producing carbon monoxide, the steam-
methane reforming reaction for producing hydrogen, and methane
reforming with carbon dioxide to produce carbon monoxide and
hydrogen.
The general embodiment can be used to operate each of
these reactions by simply substituting the proper feedstock,
adsorbent, catalyst, the relative proportion of catalyst and
adsorbent residing in the reactor, the reaction conditions and
purge fluids to provide the desired products. For example, the
reverse water gas shift reaction for producing carbon monoxide
contemplates reacting a feedstock of carbon dioxide and
hydrogen to produce carbon monoxide and water. The more
adsorbable product with respect to the adsorbents is water such
that the less adsorbable product, carbon monoxide, can be
collected at feedstock pressure.
CA 02235928 1998-06-18
_8-
Suitable adsorbents for conducting the reverse water gas shift reaction to the
present invention include zeolites such as X, A, Y and the mordenites, silica
gel, and
aluminas such as Alcan AA300 and AA230 which are commercially available from
Alcan Corporation, Cleveland, Ohio. Suitable catalysts inGude conventional
water gas
shift catalysts such. as the iron-chromium high temperature shift catalyst
from ICI
Corporation, Oakbrook Terrace, Illinois, K&10 and K6-11 catalysts available
from BASF
Corporation, Geismer, Louisiana, and low and medium temperature shift
catalysts such
as R3-11 and K3-110 catalysts which are also commercially available from BASF
Corporation, Geismer, Louisiana. Reaction conditions include a temperature
ranging
from 200° to 600°C, the first pressure which ranges from 2 to 50
bar and the second
pressure which ranges from 0.05 bar to 2 bar. The weakly adsorbing purge fluid
is
selected from the group consisting of methane, hydrogen, nitrogen and
carbon dioxide.
The feedstock for the steam-methane reforming reaction comprises water and
methane with a ratio of water to methane ranging from 1.5 to 30 when the more
adsorbable product is primarily carbon dioxide and from 1 to 1.5 when the more
adsorbable product is primarily carbon monoxide. The less adsorbable product
is
hydrogen in both cases. Reaction conditions include a temperature ranging from
200°
to 600°C, the first pressure which ranges from 2 to 50 bar and the
second pressure
which ranges from 0.05 bar to 2 bar. The weakly adsorbing purge fluid is
selected from
the group consisting of methane, steam, hydrogen and nitrogen.
CA 02235928 1998-06-18
_g_
Suitable catalysts for conducting the steam-methane reforming reaction
include conventional steam-methane reforming and prereforming catalysts such
as
nickel-alumina, nickel-magnesium alumina and the noble metal catalysts. The
adsorbent can be selected to adsorb~carbon monoxide, carbon dioxide or a
mixture of
carbon monoxide and carbon dioxide. For example, adsorbents for carbon dioxide
include the metal oxides and mixed metal oxides of magnesium, manganese,
lanthanum and calcium and the clays minerals such as sepiolite and dolomite.
Adsorbents which are selective toward carbon monoxide inGude Cu" on silica-
alumina
and Ag''' on silica-alumina as described in U.S. Patents 4,019,879 and
4,019,880.
Applicants have discovered two new classes of materials described herein as
modified double layered hydroxides and non-modified and modified spinets which
are
capable of selectively adsorbing C02 from C02-containing streams containing
moisture
levels as high as 10 atmospheres of water vapor and which possess utility at
temperatures ranging from 200°C to 500°C, and possibly higher
temperatures. The
literature is not believed to teach adsorbents of the following types which
are capable of
selectively adsorbing C02 in the presence of large amounts of water under
operating
temperatures ranging from 200°C to 500°C and which are capable
of being regenerated
by purging with inert gas at such elevated temperatures.
The preferred modified double layered hydroxides which possess utility ~as C02
adsorbents under the above-referenced conditions are represented by the
formula:
CA 02235928 1998-06-18
-10-
[Mgp.~ Alx yl"1~2I[C03~x/2 ~ Y HzO ~ z M~2CO3
wherein 0.09 < x < 0.40;
0<_y<3.5,0<z<3.5;
M~ = Na or K.
The spinets and modified spinets which possess utility as COz adsorbents under
the above-referenced conditions are represented by the formula Mg [Alz]O,, ~ y
K2C03
wherein 0 < y < 3.5. The modified spinets are identified by the materials
wherein y is
greater than zero. Thus, the spinets which are not modified are represented by
the
formula wherein y equals zero.
Methane can be reformed with carbon dioxide to produce carbon monoxide
and hydrogen. Thus, the feedstock comprises carbon dioxide and methane, the
catalyst
comprises a methane reforming or prereforming catalyst such as nickel-alumina,
nickel-
magnesium alumina and the noble metal catalysts such as fiodium, ruthenium and
iridium. The more adsorbable product with respect to the adsorbent may be
hydrogen
and the less adsorbable product may be carbon monoxide. Alternatively, the
more
adsorbable product may be carbon monoxide and the less adsorbable product may
be
hydrogen. Suitable adsorbents for hydrogen include the hydrogen-metal alloys
such as
palladium, palladium-silver, magnesium-nickel, iron-titanium and lanthanum-
nickel, and
the like. Suitable adsorbents for carbon monoxide include Cu+ and Ag' salts.
Reaction
conditions comprise a temperature ranging from 200° to 600°C,
the first pressure which
ranges from 2 to 50 bar and the second pressure which ranges from 0.05 to 2
bar. The
weakly adsorbing purge fluid is selected from the group consisting of steam,
methane,
carbon dioxide, carbon monoxide, nitrogen, and hydrogen.
CA 02235928 1998-06-18
- 11 -
As shall become more apparent upon reading the Detailed
Description of the Invention, Applicants' process overcomes
problems associated with prior art processes by utilizing a
novel series of reaction, adsorption and desorption steps to
collect the less adsorbable product in substantially pure form
under a relatively constant flow rate at feedstock pressure.
BRTEF DESCRTpmrnN nF TgE DRAWIN~s
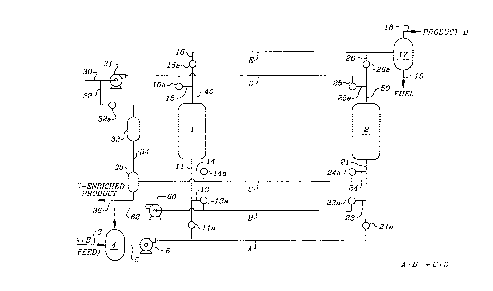
FIG. 1 is a process flow diagram of a process for
operating equilibrium controlled reactions which utilizes two
reactors containing an admixture of catalyst and adsorbent,
wherein the reactors are operated in a cycle of predetermined
sequence;
FIG. 2 illustrates the CO concentration profile in reactor
effluent versus time for the reverse water gas shift reaction
carried out according to the process at 57 psig and 275°C.
Carbon monoxide was used as the purge fluid and the
pressurizing fluid;
FIG. 3 illustrates the reactor effluent flow rate versus
time for the reverse water gas shift reaction carried out
according to the process at 57 psig and 275°C. Carbon monoxide
was used as the purge fluid and the pressurizing fluid;
FIG. 4 illustrates the CO concentration profile and the
flow rate of the reactor effluent for the reverse water gas
shift reaction carried out according to the process at 55 psig
and 250°C. Carbon monoxide was used as the weakly adsorbing
purge fluid and the pressurizing fluid;
CA 02235928 1998-06-18
- 12 -
FIG. 5 illustrates the CO concentration profile and the
flow rate of the reactor effluent for the reverse water gas
shift reaction carried out according to the process at 50 psig
and 250°C. Carbon dioxide was used as the purge fluid and the
pressurizing fluid:
FIG. 6 illustrates the CO concentration profile and the
flow rate of the reactor effluent for the reverse water gas
shift reaction carried out at 50 psig and 250°C. Hydrogen was
used as the purge fluid and the pressurizing fluid:
FIG. 7 illustrates one of a plurality of reactors suitable
for practicing the process for operating an equilibrium
controlled reaction which comprises a shell-and-tube
configuration wherein an admixture of an adsorbent for
preferentially adsorbing one reaction product over the other
reaction products and a catalyst for performing the desired
equilibrium controlled reaction are situated in the reactor
tubes. The heating and cooling requirements in the moving
reaction mass transfer zone of each reactor are provided by
indirect heat exchange with a fluid capable of phase change at
temperatures maintained in each reactor during sorpreaction,
depressurization, purging and pressurization steps during each
process cycle: and
FIG.8 presents a graph of COZ/HZO isotherms for potassium
carbonate modified double layered hydroxide, a preferred
adsorbent for use in the process for operating an equilibrium
controlled reaction wherein the feedstock comprises water and
methane, the more adsorbable product with respect to the
adsorbent is carbon dioxide and the less adsorbable product is
hydrogen.
CA 02235928 1998-06-18
- 13 -
Applicants will now discuss in greater detail their
process for operating equilibrium controlled reactions which
provides numerous benefits over prior art processes.
Specifically, greater conversion of feedstock to products per
unit volume of reactor is achieved: a more concentrated
reaction product is obtained than could be achieved using a
conventional process wherein the reaction stage is segregated
from the product adsorption stage: and the process can be
operated at less stringent conditions because very favorable
equilibrium may not be required.
The present process also provides another additional
benefit in that the less adsorbable product with respect to the
adsorbent residing in admixture with the catalyst within each
reactor may be collected at a relatively constant flow rate at
feedstock pressure. This result is accomplished in part by
countercurrently purging the reactor with the less adsorbable
product until breakthrough of the less adsorbable product
occurs at the feed end of each reactor. The extent to which
purge is required is dictated by the extent of purity required
in the less adsorbable product to be collected during the
reaction step. Moreover, the reactor is countercurrently
repressurized to the
CA 02235928 1998-06-18
-14-
initial process pressure with the less adsorbable product prior to commencing
the next
process cycle within each reactor.
While removing the more adsorbable product from the reactor immediately as it
is formed in the reaction zone does not change the equilibrium constant for
the
particular equilibrium controlled reaction, substantially increased conversion
of
feedstock to products is achieved by practicing the combined steps of the
process. In
order to effect this objective, two key requirements must be met: First, the
adsorbent
must be active at the reaction conditions meaning that the adsorbent must
retain its
capacity and selectivity for the more adsorbable product. Second, the
adsorbent must
be chemically neutral and must not act as a catalyst for undesirable side
reactions.
The general embodiment of Applicant's process for operating equilibrium
controlled reactions is described in FIG. 1 which utilizes two reactors each
containing an
admixture of catalyst and adsorbent chosen for the desired equilibrium
controlled
reaction. Such reactors are described in greater detail in the Specification
and FIG. 7.
The schematic according to FIG. 1 consists of reactors 1 and 2; numerous
control
valves; manifolds A through E; pumps 6, 31 and 60; separators 17 and 35; and
storage
tanks 4 and 33. Feedstock comprising the reactants to be subjected to the
desired
equilibrium controlled reaction is drawn from storage tank 4 having inlet line
3 and outlet
line 5 via pump 6 wherein the pressurized feedstock is introduced into
manifold A.
Manifold A is in flow communication with branch inlet lines 11 and 21 which
are
connected to the inlet ends of reactors 1 and 2. Lines 11 and 21 are equipped
with
valves 11 a and 21 a, respectively. Opening of the appropriate valve permits
the
CA 02235928 1998-06-18
-15-
pressurized feedstock to flow through manifold A into the selected reactor
being initially
placed on stream. Thus, by opening valve 11a, while valve 21a is closed,
feedstock
may be caused to flow from manifold A, through line 11 and into reactor 1.
Reactors 1 and 2 are fitted at their respective outlet ends with lines 40 and
50
respectively, each equipped with control valves 16a and 26a, respectively.
Lines 40
and 50 are operatively connected to manifold E.via lines 16 and 26 through
which a
stream containing a mixture of components withdrawn from reactors 1 and 2 can
be
collected in separator 17. The mixture can be separated such that a stream
containing
the less adsorbable product, referred to as Product D, can be collected via
line 18 and
residuals may be collected via line 19 for fuel value or recycle. Thus, by
opening the
appropriate valve 16a or 26a, a mixture containing the less adsorbable
product, product
D, is caused to flow from the corresponding reactor through lines 40 and 16 or
lines 50
and 26 into manifold E for passage into separator 17.
Reactors 1 and 2 are operatively connected to lines 11 and 21, each of which
is in flow communication with lines 13 and 23. Lines 13 and 23 are provided
with
control valves 13a and 23a, respectively, such lines being in flow
communication with
manifold B. Manifold B can be placed in flow communication with reactor 1 or 2
via
lines 13 and 23 upon opening valve 13a or 23a, respectively. Manifold B is
also in flow
communication with pump 60 which is connected to line 62 which can be used to
recycle feedstock to storage tank 4. -
Manifold C is in flow communication with reactors 1 and 2 via lines 14 and 24,
each line which is equipped with valves 14a and 24a, respectively. Reactor
effluent
CA 02235928 1998-06-18
-16-
from reactors 1 and 2 may be passed through lines 14 and 24 into manifold C
for
separation in separator 35 into a stream which is enriched in the more
adsorbable
product, referred to as Product C, and a stream comprising weakly adsorbing
purge
fluid which can be passed into storage tank 33 via line 34 for later use.
Manifold D is connected to pump 31 which receives various process fluids via
lines 30 and 32. Such process fluids pass through line 30 or line 32 and are
pressurized via pump 31. The pressurized fluids may be passed through manifold
D
which is in flow communication with reactors 1 and 2 via lines 15 and 25,
respectively.
Lines 15 and 25 are each fitted with valves 15a and 25a such that the flow of
streams
from Manifold D into reactors 1 and 2 can be controlled. Moreover, weakly
adsorbing
purge fluid from tank 33 can be transferred to pump 31 via lines 32 and 30 by
opening
valve 32a.
Operation of the embodiment represented in FIG. 1 will now be explained in
connection with an arbitrarily chosen cycle having eight timed periods of ten
minutes
per period as set forth in Table 1. Although not limited thereto, the process
as
illustrated in FIG. 1 utilizes two reactors which are operated in cycle
according to a
predetermined timed sequence. Other arrangements using fewer or a greater
number
of reactors and the associated gas manifolds and switch valves may be
employed,
optionally using interrupted or discontinuous operation (using idling) of
pumps. Other
an-angements using more than two reactors-may be employed by appropriate
sequencing of the individual steps or periods of the process cycle.
CA 02235928 1998-06-18
i I~. r
H
N N
O O C O O
UJ ~ N i'~ ~: ~ :~
'~ ~ ~ p '~ ~ v
O
~ b0 by N ~'' ~ u'~H
N N H ~"~ N t..
V~ A ~l1 ~ W V~ UJ C/~V~
~.,
cd
U U U U U U U U
N U U U U O O O O
U O O O U U U U
N
U O O U U U U U
cd
O U U U U U U U
U U U U O O O O
W
W
a
U U U U U U U U
M
cps
O O O O U U U U
U U U U U O O O
U U U U U O O U
U U U U O U U U
O O O O U U U U
N OM eh O~n Vr Ol~oo
.
O O O O O O O O
~ N ~M - N
-d
N
O O O O
N LL L~r H I~rH H N
V1 C~ C~ V7 A Q. Q1 ~
CA 02235928 1998-06-18
-18-
According to the general embodiment of FIG. 1, each of the reactors 1 and 2
undergo four periods of the reactioNadsorption step, referred to as the
sorpreaction
step, one period of the depressurization step, one period of the Purge I step,
one period
of the Purge II step, and one period of the pressurization step. As
illustrated in Table 1,
the steps undertaken at startup in each of reactors 1 and 2 are staggered to
enable at
least one of the two reactors to undergo the sorpreaction, step at all times
during the
process cycle. The operation of the invention described in FIG. 1 involves
principally
the following sequence of steps:
(a) SORPREACTION-feedstock at a first predetermined pressure is
passed through the reactor containing an admixture of catalyst and adsorbent
preferentially selective toward retention of the more adsorbable product,
referred to as
Product C, wherein an effluent stream enriched in the less adsorbable product,
referred
to as Product D, is withdrawn from the reactor. Product C is selectively
adsorbed by the
adsorbent and a reaction mass transfer zone (RMTZ) is formed inside the
reactor which
moves toward the outlet or discharge end of the reactor as more feedstock is
passed
through the reactor. The adsorbent at the leading edge of the RMTZ is
essentially free
of the more adsorbable product while the trailing edge of the RMTZ is
equilibrated with
the more adsorbable product at the local conditions. The sorpreaction step is
continued
until the adsorbent in the reactor is essentially saturated with Product C. In
other
words, the RMTZ has reached the effluent end of the reactor or somewhat short
of it.
The effluent gas, rich in Product D, is discharged from-the reactor.
CA 02235928 1998-06-18
-19-
(b) DEPRESSURIZATION-the reactor is countercurrently depressurized to
a second predetermined pressure by withdrawing a mixture comprising unreacted
feedstock, a portion of the more adsorbable product and a portion of the less
adsorbable product. The depressurization step is continued until the reactor
reaches
the second predetermined pressure.
(c) ~ PURGE I- the reactor is countercurrently purged at the second
pressure with a weakly adsorbing purge fluid with respect to the adsorbent to
desorb
Product C from the adsorbent and a mixture comprising unreacted feedstock, a
portion
of Product C and a portion of Product D is withdrawn from the reactor.
(d) PURGE II-the reactor is countercurrently purged at the second
pressure with Product D to desorb the weakly adsorbing purge fluid and a
mixture
comprising the weakly adsorbing purge fluid, a portion of Product C and a
portion of
Product D is withdrawn from the reactor.
(e) PRESSURIZATION--the reactor is countercurrently pressurized from
the second pressure to the first pressure with Product D prior to commencing
another
process cycle within the reactor.
The valve positions during the above-mentioned operating cycle are also set
forth in Table 1. The designation "O" indicates that a specified valve is open
while a "C"
represents that a specibed valve is closed. The operative sequence of steps
occurring
in reactor 1 during a complete process cycle will now be described in
exhaustive detail
CA 02235928 1998-06-18
-20-
so that operation of a continuous process will be fully understood. The
identical
sequence of steps according to Table 1 occurs in staggered sequence in reactor
2.
Again, referring to the embodiment disclosed in FIG. 1 and the sequence
periods and valve positions designated in Table 1, reactor 1 undergoes four
sequence
periods of the sorpreaction step. Feedstock comprising reactants A and B,
stored in
storage tank 4, is introduced into reactor 1 by opening valves 11 a and 16a
and closing
valves 13a, 14a and 15a thereby allowing feedstock to flow through manifold A,
line 11
and into reactor 1 which contains an admixture of a desired catalyst and an
adsorbent
preferentially selective toward the more adsorbable product, Product C.
The sorpreaction is continued until reactor 1 is essentially saturated with
adsorbed Product C. Product C is selectively adsorbed onto the adsorberit and
a
reaction mass transfer zone (RMTZ) is formed within reactor 1 which moves
toward the
discharge end of reactor 1 as more feedstock is passed. The sorpreaction is
completed
when the MTZ reaches the effluent end of the reactor or somewhat short of it
by a
predetermined set point.
A mixture which is enriched in the less adsorbable product and depleted in the
more adsorbable product as well as unreacted feedstock exits the discharge end
of
reactor 1 via lines 40 and 16 and flows into manifold E for collection in
separator 17.
Optionally, the mixture in separator 17 can be separated by c~ventional
techniques
such as pressure swing adsorption, thermal swing adsorption or distillation or
condensation to form a stream comprising the less adsorbable product, Product
D,
CA 02235928 1998-06-18
-21 -
which is discharged from separator 17 via line 18 and the remainder of the
components
of the mixture are discharged via line 19.
The process proceeds with one period of the depressurization step wherein
reactor 1 is countercurrently depressurized to a second predetermined pressure
by
withdrawing a mixture comprising unreacted feedstock; a portion of the more
adsorbable product and a portion of the less adsorbable product from the inlet
end of
reactor 1. Valve 13a is opened while valves 11a and 14a remain closed allowing
the
mixture to be passed through lines 11 and 13 into manifold B and in flow
communication with pump 60. The mixture exits the discharge end of pump 60
proceeding via line 62 for use as fuel (not shown) or recycle into storage
tank 4 for use
as feedstock in a subsequent process cycle. The depressurization step is
continued
until the reactor reaches the second predetermined pressure.
Reactor 1 is then subjected to one period of the purge I step. Reactor 1 is
countercurrently purged at the second pressure with a weakly adsorbing purge
fluid
with respect to the adsorbent. Upon opening valves 14a and 15a while valves
25a and
32a remain in the closed position, weakly absorbing purge fluid from an
external source
passes through pump 31 via line 30 and exits pump 31 at the second pressure to
proceed via manifold D, line 15 and line 40 into the exit end of reactor 1. A
mixture
comprising unreacted feedstock, weakly adsorbing purge fluid, a portion of
Product C
and a portion of Product D is withdrawn from reactor 1 via line 11, line 14
and manifold -
C and is collected in separator 35. This mixture may be used as fuel,
discharged for
use outside the process or separated in separator 35 to form a stream of
weakly
adsorbing purge gas. A portion of the weakly adsorbing purge fluid may be
transferred
CA 02235928 1998-06-18
_22_
through line 34 into storage tank 33 for future use. Upon demand via opening
valve
32a, weakly adsorbing purge fluid may be drawn to pump 31 via lines 32 and 30
for use
in subsequent process. cycles.
Reactor 1 is then subjected to one period of the purge II step wherein reactor
1
is countercurrently purged with the less adsorbable product, Product D. Upon
opening
valves 14a and 15a while valves 25a and 32a remain in the closed position, the
less
adsorbable product from an external source passes through pump 31 via line 30
and
exits pump 31 at the second pressure to proceed via manifold D, line 15 and
line 40
into the exit end of reactor 1. A mixture comprising unreacted feedstock, a
portion of
Product C and a portion of Product D is withdrawn from reactor 1 via line 11,
line 14
and manifold C and is collected in separator 35. This mixture may be used as
fuel,
discharged for use outside the process or separated in separator 35 to form a
stream of
the weakly adsorbing purge fluid which may be transferred through line 34 into
storage
tank 33 for future use. Upon demand via opening valve 32a, such weakly
adsorbing
purge fluid may be drawn to pump 31 via lines 32 and 30 for use in subsequent
process cycles.
The final step of the process cycle involves a single sequence of the
pressurization step wherein reactor 1 is countercurrently pressurized from the
second
pressure to the first pressure with Product D prior to commencing another
process cycle
within the reactor. Specifically, . upon opening valve 15a while valves-11 a,
13a, 14a,
25a and 32a remain in the closed position, the less adsorbable product passes
through
pump 31 via line 30 and exits pump 31 at the second pressure to proceed via
manifold
CA 02235928 1998-06-18
-23-
D, line 15 and line 40 into the exit end of reactor 1. This step is stopped
when reactor 1
reaches the first pressure.
The process proceeds through additional cycles according to the above-
mentioned steps enumerated in Table 1. While the sequence periods are depicted
as
being of equal length, this is neither required or necessary. The times will
be set by
allowable maximum gas flow rates, valve and line sizes and the properties of
the
adsorbent used. Alternate routines may be employed for establishing the
duration of
each of the cycle steps. For example, the end of a particular step may be
determined
by other techniques known in the art such as by analysis of the composition of
the
reactor effluent.
FIG. 7 presents a detailed description of reactors 1 and 2 as presented in
FIG. 1. The reactor design and operating scheme are particularly suited toward
use in
practicing the claimed invention. Reactor 201 depicts a conventional stainless
steel
shell-and-tube reactor. An admixture of the catalyst suitable for catalyzing
the desired
equilibrium controlled reaction and the adsorbent for preferentially adsorbing
the more
adsorbable reaction product is loaded into a plurality of tubes 202. A
plurality of shell
elements 204 formed between adjacent tubes 202 receives a heat transfer fluid
capable of phase change which is selected to undergo such phase change at the
desired operating temperature of the desired equilibrium controlled reaction
being
carried out in the tubes 202. _
The reactor 201 is preferentially designed to operate net endothermic
reactions
although the reactor is also well suited to operate net exothermic equilibrium
controlled
CA 02235928 1998-06-18
-24-
reactions. Net endothermic reactions are defined as those equilibrium
controlled
reactions in which heat must be added to the reactor to carry out the
sorpreaction step
because the endothermic heat of reaction of the equilibirum controlled
reaction is
greater than the energy liberated by the exothermic adsorption of the more
adsorbable product.
The feedstock for a particular equilibrium controlled reaction is fed into
port 111 of reactor 201 and into a gas distributor 206 at the desired feed
rate, pressure
and temperature before entering the tubes 202. The feed gas is contacted with
the
admixture of adsorbent for the more adsorbable product and desired catalyst
residing in
tubes 202 under reaction conditions sufficient to convert the feedstock to a
mixture of a
more adsorbable product, a less adsorbable product and unreacted feedstock.
The
feedstock reacts with the catalyst as the feedstock flows through tubes 202
and the
more adsorbable product is adsorbed onto the adsorbent.
The region of the tubes 202 in which simultaneous reaction and adsorption
occurs is referred to as the reaction mass transfer zone (RMTZ). The more
adsorbable
product is removed from the reaction zone by adsorption onto the adsorbent and
the
less adsorbable product exits tubes 202 into a gas collection zone 208. The
less
adsorbable product is collected until the RMTZ proceeds to a point wherein the
purity
of the less adsorbable product diminishes to a preset level at which time the
flow of
feedstock~into reactor 201 is stopped and diverted to an alternate reactor-~f
the plurality
of reactors being utilized in the process. The less adsorbable product is
withdrawn as
an essentially pure product from port 140.
CA 02235928 1998-06-18
-25-
When the overall sorpreaction step is endothermic (endothermic heat of
reaction is greater than the exothermic heat of adsorption), the RMTZ moving
through
the reactor requires heat input to maintain the reactor temperature. The heat
transfer
fluid (HTF) vapor which is capable of phase change under process operating
conditions
enters port 232 of reactor 201 and fills shells 204 which are isolated from
tubes 202.
The pressure within the shells is controlled to the saturation pressure of the
HTF at the
desired reaction temperature. When the reaction occurring within the tubes 202
is
endothermic, the temperature in the RMTZ within the tube begins to decrease as
the
reaction proceeds. Below the dew point of the HTF vapor, the vapor condenses
on the
outer wall of the tubes 202 into a liquid at the same temperature and the
vapor liberates
the heat of condensation. The heat released from condensation of the HTF vapor
w travels through the tube walls and heats the reactants, catalyst and
adsorbent within
the RMTZ contained in tubes 202.
As the RMTZ moves along the reaction tubes, HTF vapor continuously
condenses outside the tubes at the RMTZ thereby providing heat to maintain the
RMTZ
at an approximately constant temperature. The HTF vapor condenses only at the
location where heat is required (at the RMTZ). The condensed HTF is collected
at the
bottom of the shell side and is withdrawn periodically or continuously through
port 242
and line 244 by opening valve 246 into line 248. The liquid level in the
reactor shell
side is always maintained below the level of port 232.
A small amount of HTF vapor is continuously withdrawn from port 234 at the
top of the reactor and is transported to condenser 238 via line 236. The
condensed
HTF from the condenser 238 in :line 240 is combined with the condensed HTF
CA 02235928 1998-06-18
- 26 -
withdrawn from the reactor via line 248. The combined HTF liquid is pumped
through
a liquid pump 210 via line 212 into a HTF vaporizer drurt'~ 214 which is part
of the HTF
vaporizer system consisting of the vaporizer drum 214 and the heater or
furnace 218.
The HTF in drum 214 is continuously fed as liquid into the heater 218 via line
215.
Heater 218 is a conventional radiant heat exchange system in which heat is
provided by combusting natural gas via line 220 and air via line 222. The
combustion
of natural gas produces a flue gas which is discharged from vent 224. The
transfer
tubes or coils 215 and 216 are contained in the radiant section of the furnace
218. The
heat generated in the furnace is used to vaporize a portion of the HTF liquid
and the
vapor and liquid mixture is recirculated into the HTF vaporizer drum 214 via
line 216.
The HTF vapor in drum 214 is fed to the reactor shell side via lines 226 and
230 and
through port 232. A forward pressure regulator 228 maintains the HTF vapor on
the
reactor shell side at a fixed pressure. The vapor in the shells 204 is
maintained at 50-
500 psig and at a temperature typically in excess of 100°C. As the HTF
vapor
condenses due to heat flux requirements in the tubes 202, the HTF vapor
pressure on
the reactor shell side decreases,. and the pressure regulator 228 opens to
release more
HTF vapor via line 230 and port 232 into the shells. Thus, the reactor can be
maintained at nearly isothermal conditions by circulating the HTF liquid and
vapor as
previously described.
At the end of the reaction sequence, the feed to the reactor is discontinued
by
closing the inlet valve at port 111 and the outlet valve at port 140. The
valve
sequencing described in Table 1 is followed during regeneration of the
adsorbent in the
reactor. Regeneration of the adsorbent in reaction tubes 202 is done
countercun-ently
CA 02235928 1998-06-18
-27-
to the direction of the feedstock flow in the first step of the process. First
the reactor is
depressurized by opening port 111 so that the pressure in the reactor is
decreased from
the first pressure to the second pressure as described in the general
embodiment. The
HTF vapor provides the heat to compensate for the cooling associated with
conducting
the depressurization step of the process.
The purging step is conducted next wherein a weakly adsorbing purge fluid is
introduced through port 140 into the gas collection zone 208 and the tubes 202
to
desorb the more adsorbable product from the adsorbent. The step is endothermic
and
heat and mass transfer zones are formed which move through tubes 202 in a
direction
countercurrent to the feedstock flow in the first step of the process. The HTF
vapor
condenses on the tube surface at the location of the heat or mass transfer
zone and
provides heat to the reactor in order to maintain the tubes at essentially
constant
temperature during this desorption step. Once the mass transfer zone has
countercurrently exited tubes 202 , the flow of the weakly adsorbing fluid
from port 140
is stopped.
The product purge steps are then performed wherein the product gas is
introduced into the reactor tubes 202 from port 140. The product gas desorbs
the
weakly adsorbing gas from the adsorbent. As in all steps of the process, heat
and
mass transfer zones are formed in the reactor tubes 202 which move along the
length
of the reactor. Condensation of the HTF vapor in the shells 204 provides the
energy for
desorbing the weakly adsorbing fluid with the product gas. When the product
gas
mass transfer zone exits the reaction tubes, port 111 is closed and the
pressure in the
CA 02235928 1998-06-18
- 28 -
reactor tubes is allowed to increase to the reaction presssure with the
product gas. At
this point the regeneration cycle is complete and the reactor is ready for the
reaction cycle.
Several fluids capable of phase change under reaction conditions are
commericially available and can be selected by one of ordinary skill in the
art without
undue experimentation. Examples of such fluids include (i) * Sylthenn 800 from
Dow
Chemical Company (highly stable polydimethylsiloxane), (ii) *Dowtherm A from
Dow
Chemical Company (a eutectic mixture of biphenyl oxide and Biphenyl oxide) and
(iii) *Therminol VP-1 from Monsanto Company (eutectic mixture of 26.5%
biphenyl oxide'
and 73.5% Biphenyl oxide). These HTFs are capable of operating with film
temperatures in excess of 425°C. For lower temperature operation, steam
may be
used as the phase change fluid which is capable of phase change. The maximum
temperature for using steam in this application is about 300°C.
Applicants' cyclic process which draws its heating and cooling requirements
via
indirect heat exchange with a fluid capable of phase change under process
operating
conditions offers several advantages over prior art because (i) the condensing
HTF
vapor provides heat to the moving RMTZ allowing the SER reactor to operate
20 isothermally, (ii) the zones behind and ahead of the RMTZ do not overheat,
eliminating
problems associated with tube or catalyst overheating, (iii) the inventory and
circulation
of heat transfer fluid is significantly reduced compared with conventional
heat exchange
where no phase change occurs.
*Trade-mark
CA 02235928 1998-06-18
-29-
Applicants have identified reactions which are particularly suited for
operation
using their claimed process. Preferred reactions include the reverse water gas
shift
reaction for producing carbon monoxide, the steam-methane reforming reaction
for
producing hydrogen, and methane reforming with carbon dioxide to produce
carbon
monoxide and hydrogen. The general embodiment of this invention can be used to
operate each of these reactions by simply substituting the proper feedstock,
adsorbent,
catalyst, the relative proportion of catalyst and adsorbent residing in the
reactor, the
reaction conditions and purge fluids to provide the desired products. For
example, the
reverse water gas shift reaction for producing carbon monoxide contemplates
reacting a
feedstock of carbon dioxide and hydrogen to produce carbon monoxide and water.
The more adsorbable product with respect to the adsorbent is water such that
the less
adsorbable product, carbon monoxide, can be collected at feedstock pressure.
Suitable adsorbents for conducting the reverse water gas shift reaction to the
present invention include zeolites such as X, A, Y and the mordenites, silica
gel, and
aluminas such as Alcan AA300 and AA230 which are commercially available from
Alcan Corporation, Cleveland, Ohio. Suitable catalysts include conventional
water gas
shift catalysts such as the iron-chromium high temperature shift catalyst from
ICI
Corporation, Oakbrook Terrace, Illinois, K6-10 and K&11 catalysts available
from BASF
Corporation, Geismer, Louisiana, and low and medium temperature shift
catalysts such
as R3-11 and K3-110 catalysts which are also commercially available from BASF
Corporation, Geismer, Louisiana. Reaction conditions include a temperature
ranging
from 200° to 600°C, the first pressure which ranges from 2 to 50
bar and the second
pressure which ranges from 0.05 bar to 2 bar. The weakly adsorbing purge fluid
is
selected from the group.consisting of methane, hydrogen, nitrogen and carbon
dioxide.
CA 02235928 1998-06-18
-30-
The feedstock for the steam-methane reforming reaction comprises water and
methane in a stoichiometric ratio of water to methane ranging from 1.5 to 30
when the
more adsorbable product is primarily carbon dioxide and from 1 to 1.5 when the
more
adsorbable product is primarily carbon monoxide. The less adsorbable product
is
hydrogen in both cases. Reaction conditions include a temperature ranging from
200°
to 600°C, the first pressure which ranges from 2 to 50 bar and the
second pressure
which ranges from 0.05 bar to 2 bar. The weakly adsorbing purge fluid is
selected from
the group consisting of methane, steam, hydrogen and nitrogen.
Suitable catalysts for conducting the steam-methane reforming reaction
include conventional steam-methane reforming and prereforming catalysts such
as
nickel-alumina, nickel-magnesium alumina and the noble metal catalysts. The
adsorbent can be selected to adsorb carbon monoxide, carbon dioxide or a
mixture of
carbon monoxide and carbon dioxide. For example, preferential adsorbents for
carbon
dioxide include the metal oxides and mixed metal oxides of magnesium,
manganese,
lanthanum and calcium and the clays minerals such as sepiolite and dolomite.
Adsorbents which are selective toward carbon monoxide inGude Cui' on silica-
alumina
and Ag" on silica-alumina as described in U.S. Patents 4,019,879 and
4,019,880.
Applicants have discovered two new classes of materials described herein as
modified double layered hydroxides and non-modified and modified spinets which
are
capable of selectively adsorbing C02 from C02-containing streams containing
moisture
levels above stoichiometric with respect to C02 and as high as 10 atmospheres
of water
vapor and which possess .utility at temperatures ranging from 200°C to
500°C, and
CA 02235928 1998-06-18
-31-
possible higher temperatures. The literature is not believed to teach
adsorbents of the
following types which are capable of selectively adsorbing C02 in the presence
of large
amounts of water under operating temperatures ranging from 200°C to
500°C. and
which are capable of being regenerated by purging with inert gas at such
elevated temperatures.
The modified double layered hydroxides which possess utility as COZ
adsorbents under the above-referenced conditions are represented by the
general formula:
~M11~1_X'MxIII~OH~2~ Un~x/2 ~ Y H2~ ~ zM~2~~_r~MrnA, wherein
M' = Lid, Na+, K'', Cr' or Rb+, and mixtures thereof;
M" = Mg2', Ca2+, Mn2+, Fe2~, Co2', Ni2' or Zn2', and mixtures thereof;
M"~ = Mg2~, Ca2', Srz+, Ba2~, Zn2f
M"' = AI3~, Cr3+, Mn3', Co3+, Ni3' or La3', and mixtures thereof;
C"~ = NO3 , SO42 , CO32~, CH3CO2 , CI , Br , F' Or I and
A = OZ' or COa2-; wherein
n=1,2
x = 0.01 to 0.99;
y=Oto4;
z = 0.01 to 7; and -
r=Oto1.
CA 02235928 1998-06-18
-32-
Preferred modified double layered hydroxides are represented by the. formula:
IMg~-~ AlX UH~2~ICO3~x/Z ~ Y HzO ~ z M'2CO3
wherein 0.09 < x < 0.40
0<y<3.5,0<z<3.5and
M'=Naor K.
The modified and non-modified spinets which possess utility as C02 adsorbents
under the above-referenced conditions are represented by the generic formula:
Dra~~_~ ETdP Done E°n~_P~ ~4 ~ y M~Z~~.~ MxuA
in which "Td" indicates ration occupation of tetrahedral lattice site and "oh"
indicates ration occupation of octahedral lattice site,
DTd and ~D°" are metal rations or a combination of metal rations
chosen from
groups I-A, II-A, III-A, IV-A, V-A, I-B, II-B, IV-B, V-B, VI-B, VII-B and VIII
of the periodic
table of the elements;
ETd and E°" are individually selected metal rations selected from
groups I-A,
III-A, IV-A, V-A, I-B, II-B, IV-B, V-B, VI-B, VII-B, VIII of~the periodic
table of the elements;
such that the value of the octahedral to tetrahedral bond length ratio, R, of
the
metal rations DT°, ETd, D°" and E°" falls within the
range of 1.155 > R > 0.886, wherein
M' = Li', Na'', K+, Cs'', Rb
M° = Mg'2, Ca+2, Sr~2, Zn+2, Ba+z
X=OtoI;A=02-,C03'andY=Oto7.
CA 02235928 1998-06-18
-33-
Preferred spinets which are suitable for use as COZ adsorbent under high
temperatures in the presence of large amounts of water are represented by
the formula Mg [AIZ]O,, ~ y K2COa wherein 0 < y < 3.5.
Methane can be reformed with carbon dioxide to produce carbon monoxide
and hydrogen. Thus, the feedstock comprises carbon dioxide and methane, the
catalyst
comprises a methane reforming or prereforming catalyst such as nickel-alumina,
nickel-
magnesium alumina and the noble metal catalysts such as fiodium, ruthenium and
iridium. Suitable adsorbents for hydrogen include the hydrogen-metal alloys
such as
palladium, palladium-silver, magnesium-nickel, iron-titanium and lanthanum-
nickel, and
the like. Suitable adsorbents for carbon monoxide include Cu+ and Ag+ salts.
The more
adsorbable product with respect to the adsorbent may be hydrogen and the less
adsorbable product may be carbon monoxide. Alternatively, the more adsorbable
product may be carbon monoxide and the less adsorbable product may be
hydrogen.
Reaction conditions comprise a temperature ranging from 200° to
600°C, the first
pressure which ranges from 2 to 50 bar and the second pressure which ranges
from
0.05 to 2 bar. The weakly adsorbing purge fluid is selected from the group
consisting of
steam, methane, carbon dioxide, carbon monoxide, nitrogen, and hydrogen.
While removing the more adsorbable product from the reactor immediately as it
is formed does not change the equilibrium constant for the particular
equilibrium
controlled reaction, substantially increased reactor throughput is achieved by
practicing
the combined steps of the process. In order to effect this objective, two key
requirements must be met: First, the adsorbent must be active at the reaction
.
CA 02235928 1998-06-18
-34-
conditions meaning that such the adsorbent must retain its capacity and
selectivity for
the more adsorbable product. Second, the adsorbent must be chemically neutral
and
must not act as a catalyst for the desired equilibrium controlled reaction.
The ratio by weight of catalyst and adsorbent can be widely varied depending
upon the particular requirements of a particular catalyzed equilibrium
controlled reaction
to be operated under the present invention. As stated earlier in the
Specification, the
general and alternate embodiments of the present invention are suitable for
operating
equilibrium controlled reactions which are capable of being operated in the
absence of
a catalyst. No special techniques are required to prepare the admixture of
catalyst and
adsorbent to be placed in each reactor. The catalyst and adsorbent are simply
mixed
together by conventional means in order to disperse the catalyst and
adsorbent. The
catalyst and adsorbent to be mixed should desirably possess compatible average
particle sizes such that the catalyst and adsorbent do not segregate into
domains
during operation of the process.
. The term, weakly adsorbing fluid, refers to a fluid which is capable of
displacing the product.which is adsorbed by the adsorbent during operation of
the
process and which can then be desorbed by the less adsorbing product such that
subsequent process cycles can be conducted in each reactor. One of ordinary
skill in
the art can readily select one or a mixture of weakly adsorbing fluids
suitable for use in
the claimed invention. .
The general and alternate embodiments of the present invention can be
operated using conventional hardware. ~ For example, suitable reactors include
any
CA 02235928 1998-06-18
-35-
vessel which is capable of being subjected to the reaction conditions required
to
practice a particular equilibrium controlled process such as shell and tube
reactors.
Moreover, the separators enumerated in the process are readily selected by one
of
ordinary skill in the art based upon considerations such as the particular
mixtures to be
separated, the volume of fluids to be separated and the like.
The following examples are provided to further illustrate Applicants' process
for
operating equilibrium controlled reactions. The examples are illustrative and
are not
intended to limit the scope of the appended claims.
EXAMPLE 1
REVERSE WATER-GAS SHIFT REACTION
FOR PRODUCING CARBON MONOXIDE
The reverse water gas shift reaction for manufacturing carbon monoxide was
theoretically evaluated in order to test Applicants' process for controlling
equilibrium
controlled reactions. The process is represented by the reaction
COZ + HZ H CO + H20
and is especially of interest because the reaction is typically performed at a
high
temperature (>800°C) because it results in low equilibrium conversions
at lower
temperatures (especially at <500°C). The process is further complicated
by side
reactions at high temperatures which result in the .formation of carbon which
deactivates
the catalyst. Calculations performed using temperature versus thermodynamic
equilibrium constant data found in the literature indicate that if an
adsorbent such as
CA 02235928 1998-06-18
-36-
NaX zeolite could be used to remove 99.9% of the water formed in the reaction
zone,
conversions of greater than 80% would be possible at about 300°C.
Applicants experimentally tested the general embodiment of their invention
using the reverse water gas shift reaction as a representative equilibrium
controlled
reaction. The process was operated under the following conditions: Reaction
Temperature = 275°C; Reaction Pressure = 57 psig; H2 flow rate = 100
cc/min; COZ flow
rate = 100 ccJmin; 1:1 (by weight) physically admixed low temperature shift
catalyst and
NaX zeolite adsorbent pellets; Gas in reactor prior to admission of reactants
= CO at 57
psig and 275°C; Total moles CO in reactor system prior to introduction
of reactants =
0.17 gram mole.
In the first step of the process, the feedstock was introduced into the
reactor
which was pre-saturated with one of the product gases, carbon monoxide, at
275°C and
57 psig. The process was carried out under the above-mentioned conditions. For
the
first 70 minutes; the effluent stream consisted of CO only. FIG. 2 illustrates
the CO
concentration profile in reactor effluent versus time for the reverse water
gas shift
reaction. The hatched area of the diagram represents the carbon monoxide
effluent
from the reactor which is equivalent to the quantity of carbon monoxide
present in the
reactor at the start of the process. This amount of carbon monoxide left the
reactor in
the first 38 minutes of the operation. Furthermore, the diagram illustrates
that
essentially pure carbon monoxide was produced from the reactor during the time
span
between about 38 minutes and 78 minutes. This essentially pure carbon monoxide
effluent, which requires minimal cleanup to remove the small amount of
impurities in the
CA 02235928 1998-06-18
-37-
effluent, was the net essentially pure carbon monoxide produced by the concept
of the
present invention. This product CO is produced at the feedstock pressure of 57
psig.
FIG. 3 illustrates the reactor effluent flow rate versus time for the reverse
water
gas shift reaction of Example 1. The effluent flow rate was relatively
constant during
the period of 38 to 78 minutes when the carbon monoxide product is produced.
The
initial step may be carried on for varying amounts of time: the reaction step
is
preferably stopped after 71.7 minutes to provide a product stream containing
98%
carbon monoxide or 78.3 minutes to obtain a product stream containing 97%
carbon
monoxide. In the second step the reactor was countercurrently depressurized to
10 psig
to release a mixture of CO, COZ, HZ and H20. In the third step the bed was
countercurrently purged with a weakly adsorbing purge fluid, methane, at
275°C, at a
pressure of 10 psig and a flow rate of 1600 cGmin. in order to desorb the
remaining
water from the adsorbent-catalyst admixture. In the fourth step of the
process, the
reactor was countercurrently purged with carbon monoxide to desorb the weakly
adsorbing purge fluid and other fluids remaining in the reactor. Finally, in
the fifth step,
the reactor was countercurrently pressurized to 57 psig with carbon monoxide.
The usable conversion of carbon dioxide to carbon monoxide was 60.6% for
reactor effluent containing 97% carbon monoxide and 56.0% for reactor effluent
containing 98% purity.
CA 02235928 1998-06-18
-38-
EXAMPLE 2 (COMPARATIVE)
EQUILIBRIUM CONVERSION ESTIMATIONS FOR
CONVENTIONAL REVERSE WATER-GAS SHIFT REACTION
FOR PRODUCING CARBON MONOXIDE
Equilibrium conversion was estimated based on thermodynamic data for a
conventional reverse water gas shift reaction at different reaction
temperatures. The
calculations show that at the reaction temperature of 275°C according
to Example 1,
the equilibrium conversion in the absence of the adsorbent is only 11.0%. In
order to
achieve conversion of 60.6% according to Example 1, the reaction would need to
be
carried out at 1150°C. This points to the significant reduction in
reaction temperature
achieved in practicing the process of the present invention.
An additional major advantage of the present invention is that CO is obtained
as an essentially pure product as in Example 1. In conventional reverse water
gas shift
reactions, operated at a temperature of 275°C, the product composition
would be:
5.5% CO, 5.5% H20, 44.5% HZ and 44.5% C02. For reactions at 1000° and
1150°C,
the compositions are.(i)28% C0, 28% H20, 22% HZ and 22% C02 and (ii) 30.3% CO,
30.3% H20, 19.7% HZ and 19.7% CO2, respectively. Thus, the gas mixture would
need
to be separated by PSA/VSA technology, for example, to obtain a pure CO
product.
Yet another major advantage of the present invention is that CO is obtained at
feedstock pressure (eg. 57 psig in Example 1). With conventional PSA/VSA~
technology, the CO would be recovered at close to atmosphere pressure and
would
have to be recompressed for use.
CA 02235928 1998-06-18
-39-
EXAMPLE 3
EFFECT OF PRESSURIZATION GAS TYPE ON PROCESS PERFORMANCE
FOR REVERSE WATER-GAS SHIFT REACTION
FOR PRODUCING CARBON MONOXIDE
This example demonstrates the effect of varying the type of fluid used in the
fourth
and fifth steps of Applicants' process described in Example 1 on reactor
operation,
conversion of C02 to CO and product purity. The experiments were carried out
at the
following conditions: Reaction Temperature = 250°C; Reaction Pressure =
50 psig; H2
flow rate = 100 cdmin; COz flow rate = 100 cGmin; Catalyst and zeolite pellets
employed in Example 1 were physically admixed (1:1 ratio by weight); Gas in
reactor
prior to admission of reactants = CO or C02 or HZ at 50 psig. FIGS. 4, 5 and 6
illustrate
. the different results obtained when carbon monoxide, carbon dioxide, or
hydrogen were
used as the.purge and pressurization fluids, respectively.
FIG. 4 illustrates the CO concentration profile and the flow rate of the
reactor
effluent for the reverse water gas shift reaction when the process is carried
out using
carbon monoxide as the purge fluid and pressurizing fluid according to the
fourth and
fifth steps of the process according to Example 1. Again, the hatched area of
the
diagram represents the carbon monoxide effluent from the reactor which is
equivalent
to the quantity of carbon monoxide present in the reactor at the start of the
process.
This amount of carbon monoxide left the reactor.in just 38 minutes of the
operation.
FIG. 4 demonstrates that the claimed process provides a reactor effluent which
comprises essentially pure carbon monoxide and that the carbon monoxide can be
' conveniently collected at a constant flowrate.
CA 02235928 1998-06-18
-40-
FIG. 5 illustrates the CO concentration profile and the flow rate of the
reactor
effluent for the reverse water gas shift reaction when the process is carried
out using
carbon dioxide as the purge fluid and pressurizing fluid according to the
fourth and fifth
steps of the process according to Example 1. In contrast to the results
depicted in
FIG. 4 when carbon monoxide was used as the purge fluid, FIG. 5 shows that the
carbon monoxide concentration slowly rises, reaches a maximum and then
decreases
with practically no section of constant CO composition and flowrate when
carbon
dioxide was used as the purge fluid in the fourth and fifth steps of the
process
according to Example 1. Furthermore FIG. 5 shows that the CO composition of
reactor
effluent never exceeds 83% as opposed to essentially pure CO effluent obtained
in
FIG. 4 wherein carbon monoxide was used as the purge and pressurization fluid.
FIG. 6 illustrates the CO concentration profile and the flow rate of the
reactor
effluent for the reverse water gas shift reaction when the process is carried
out using
hydrogen as the purge fluid and pressurizing fluid according to the fourth and
fifth steps
of the process according to Example 1. In contrast to the results depicted in
FIG. 4
when carbon monoxide was used as the purge fluid, FIG. 6 shows that the carbon
monoxide concentration slowly rises, reaches a maximum and decreases with
practically no section of constant CO component and flowrate when hydrogen was
used
as the purge fluid in the fourth and fifth steps of the process according to
Example 1.
Further, FIG. 6 shows that the CO composition of the effluent gas never
exceeds 85%
as opposed to essentially pure CO effluent of FIG. 4.
CA 02235928 1998-06-18
-41 -
Several observations can be made upon reviewing the results depicted in
FIGS. 4, 5 and 6. Pure CO in the product stream is surprisingly observed only
in the
case where carbon monoxide is used as the purge and pressure fluid of steps 4
and 5
of the general embodiment. This result does not occur when carbon dioxide or
hydrogen is used as the purge and pressurization fluid. As evidenced in FIGS.
5 and 6,
a maximum carbon monoxide concentration of only about 80-85% is achieved when
H2
or COZ are employed as the purge fluid in the fourth and fifth steps of the
process
according to Example 1. Thus, if a high purity CO product is desired without
further
separation, the reactor must be pre-saturated with the less adsorbable
product,
carbon monoxide.
EXAMPLE 4
PREPARATION AND CHARACTERIZATION OF A
POTASSIUM CARBONATE MODIFIED DOUBLE LAYERED HYDROXIDE
Three samples of a potassium carbonate modified double layered hydroxide
were prepared respectively by impregnation of 0.5 M, 2.0 M and 5.0 M aqueous
solutions of K2C03 into individual 3g samples of HTC hydrotalcite powder (HTC)
supplied by La Roche Company; Baton Rouge, Louisiana. The HTC was activated at
400°C for 4 hours prior to impregnation. The carbonate solution was
added to test
tubes containing the hydrotalcite to the point of incipient wetness. After 1
hour, the
excess liquid was decanted and the resulting paste was placed into an oven and
dried
at 120°_C for 16 hours. Samples of the modified HTC were activated by
heating at 400-
500°C for 2 hours prior to measuring their C02 capacities. Samples
prepared from the
0.5 M, 2.0 M and 5.0 M solutions were characterized by elemental analysis to
contain
21.7, 57.3 and 77.0 weight percent K2C03, respectively with Mg to AI ratios of
CA 02235928 1998-06-18
-42-
approximately 2.1. These materials were analyzed by thermo gravimetric
analysis
(TGA) to possess capacities of 0.67, 0.35 and 0.15 mmol C02/g, respectively,
at 400°C
under dry C02/N2 cycling conditions (pCp2 = 500 Torr). At 500°C the
same materials
had capacities of 0.82 0.43 and 0.18 rnmol COZ/g, respectively, under similar
testing conditions.
EXAMPLE 5
EFFECT OF LOW HUMIDITY ON COa CAPACITY OF
POTASSIUM CARBONATE MODIFIED DOUBLE LAYERED HYDROXIDE
A sample was prepared by impregnating HTC powder with 0.5 M K2C03 as
described in Example 4. The effect of low moisture levels on the C02 capacity
of this
sample was determined by measuring its C02 capacity on the TGA under dry
C02/dry
N2 cycling conditions. These results were compared with the C02 capacity
measured
under humid C02/dry N2 cycling conditions. The humid C02 contained 20 Torr of
water vapor pressure and was generated by saturating the dry C02 stream with
room
temperature water vapor. In this manner, the sample was shown to have an
identical
capacity of 0.66 mmol C02/g whether tested under dry or low humidity
conditions at
400°C. This example demonstrates that low levels of humidity do not
adversely affect
the C02 capacity of the material at elevated temperatures.
EXAMPLE 6
PREPARATION AND CHARACTERIZATION OF A
POTASSIUM CARBONATE MODIFIED DOUBLE LAYERED HYDROXIDE
Three samples of potassium carbonate modified HTC were prepared by
treatment of Alcoa HTC (1/8" extruded pellets) with 0.5 M, 2.0 M and 5.0 M
aqueous
solutions of K2C03 as described in Example 4. Samples prepared from these
solutions
CA 02235928 1998-06-18
-43-
were characterized by elemental analysis to contain 3.66, 16.8 and 28.1 weight
percent
K2C03, respectively with Mg to AI ratios of approximately 3Ø These materials
were .
analyzed by TGA to have capacities of 0.28, 0.39 and 0.28 mmol C02/g,
respectively,
at 400°C under dry C02/N2 cycling conditions (pCpz = 500 Torr).
EXAMPLE 7
EFFECT OF STEAM ON CO~-CAPACITY OF
POTASSIUM CARBONATE MODIFIED DOUBLE LAYERED HYDROXIDE
Potassium carbonate modified HTC was prepared by impregnation of the
hydrotalcite with 2.0 M K2C03 as described in Example 4. This sample was
loaded into
a cell contained in a box furnace. The sample was activated at 400°C
for two hours
under a N2 purge, then saturated by purging with a binary gaseous mixture of
10
atmospheres of H20 and 0.3 atmospheres of C02 at 400°C for 1 hour. The
adsorbed
C02 and H20 were desorbed at 400°C and quantitated by an in-line
mass
spectrometer. From this experiment the potassium carbonate modified HTC was
determined to have a capacity of 0.69 mmol C02Ig of adsorbent in the presence
of 10
atmospheres of steam. In order to collect C02-H20 binary isotherm information
under
simulated process conditions, the C02 capacity was measured under C02 partial
pressures ranging from 0.1 to 1.5 atmospheres while holding the water vapor
pressure
constant at 10 atmospheres. Under these conditions the capacity ranged from
0.52 to
1.06 mmol C02/g despite the fact that adsorption occurred in a gaseous
environment
with a large molar excess of H20 over C02. The C02/H2 O binary isotherms are
, presented in FIG. 8.
CA 02235928 1998-06-18
-44-
Under 1 atmosphere of dry C02, the potassium carbonate modified HTC had a
capacity of 0.85 mmollg. The material had similar capacities for C02 whether
saturated
with 1 atm of dry C02 (0.85 mmol C02/g) or with a binary mixture consisting of
1 atm
C02 and 10 atm H20 (0.89 mmol C02/g). The presence of water had no significant
effect on the capacity of potassium carbonate modified HTC.
EXAMPLE 8
CO~-ADSORPTIVE PROPERTIES OF HYDROTALCITES AND KaC03
The C02 capacities of La Roche HTC, Alcoa HTC, and K2C03 were determined
by TGA under dry C02/dry N2 cycling conditions. The La Roche HTC and Alcoa HTC
had capacities of 0.31 and 0.21 mmol C02/g, respectively, at 400°C.
K2C03, as
described in Example 3, has a'capacity of <0.02 mmol C02/g under similar
conditions.
This demonstrates the synergistic effect that some of the modified
hydrotalcites have
with respect to C02 adsorption (Examples 4-6) relative to the adsorptive
properties of
the individual components.
EXAMPLE 9
PREPARATION. CHARACTERIZATION AND ADSORPTIVE
PROPERTIES OF MgAla,04-SPINEL
MgA1204 spinet was identified as one of the decomposition products formed
during the prolonged treatment of the potassium carbonate modified HTC with
high
temperature stem This spinet component is believed to be responsible for the
increased C02 capacity observed for samples which had undergone extensive
steam
stability testing. To confirm this hypothesis, pure MgA120,1 spinet was
prepared and
characterized. MgA1204 was made by the reaction of a ground solid mixture of
CA 02235928 1998-06-18
-45-
magnesium and aluminum acetate in the proper molar ratio at 500°C for 4
hours. X-ray
diffraction analysis confirmed the formation of a pure spinet product. A
portion of this
product was modified by treatment with a 2.0 M K2C03 solution and dried at
400°C.
The resulting MgA12O4 and K2C03 promoted MgA1204 had capacities of 0.32 and
0.62
mmoUg, respectively, at 400°C under dry C02 conditions (pC02 = 500
Torr), confirming
that the spinet component was responsible for the observed increased
capacities.
The Examples demonstrate that the present invention for operating equilibrium
controlled reactions overcomes problems associated with prior art processes
wherein
product flow rates do not remain constant and the desired products are present
in
unacceptably low concentrations in the reactor effluent while obtaining high
conversion
of the product at lower temperatures. Moreover, Applicants' unique series of
steps for
desorbing the more adsorbable product from the adsorbent residing in the
reactor and
for preparing the reactor for subsequent process cycles provides.outstanding
process
efficiency and control. Having thus described the present invention, what is
now
deemed appropriate for Letters Patent is set forth in the following Claims.