Note: Claims are shown in the official language in which they were submitted.
CLAIMS
1) process for crosslinking sulfonated polymers, characterized in that at
least some of the bonds linking
the chains bears au ionic charge and involve, partially or in their totality,
the sulfonyl groups through
interchain linkage of the following type:
P~SO2Y(M+)SO2~P'
P~SO2(M+)Y-SO2Y-(M+)SO2~P,
P~SO2(M+)Y-SO2QSO2Y-(M+)SO2-P'
where:
P and P' represents two different strands of the polymer backbone.
Y represents:
- N (Nitrogen)
- CH, CCN, CR where R represents an alkyl or alkylene with 1 to 20 carbons,
halogenated or
not, possibly bearing aza or oxa subtituents, or T or an alkyl- or an alkylene-
sulfonyl group,
with 1 to 20 carbons, halogenated or not, possibly bearing aza or oxa
subtituents, including
TSO2.
Q represents a divalent, alkyl, oxaalkyl, azaalkyl, aryl or arylalkyl or
alkylalyl radical containing 1
(inclusive) to 20 (inclusive) carbon atoms, possibly halogenated, is
particular perfluorinated and
-7-
optionally possibly including aza- or oxa- subtituents. When there is no
carbon atom, the
compound is a sulfamide or a sulfone
2) crosslinked sulfamide polymers, characterized in that at least some of the
bonds linking the chains
bears an ionic charge and involve, partially or in their totality, the
sulfonyl groups through interchain
linkage of the following type:
P~SO2Y-(M+)SO2~P
P~SO2(M+)Z-SO2-Z-(M+)SO2~P
P~SO2(M+)Z-SO2QSO2Z-(M+)SO2-P
3) process for crosslinking polymers according to claim 2 characterized in
that the sulfonated groups
are totally or partially under the form :
P~SO2L
where:
L = is a leaving group, like F, Cl, Br, au electrophilic heterocycle N-
imidazolyl, N-triazolyl,
R"SO3, R" being au organic radical, preferably halogenated, especially
fluorinated.
4) process for crosslinking polymers according to claim 2 characterized in
that the crosslinking agents
are of the general formula:
(M+)A2Z
(M+)AZSO2ZA(M+)
(M+)AZSO2QZA(M+)
5) process for crosslinking polymers according to claim 2 characterized in
that one of the following
reactions is used to form the crosslinks:
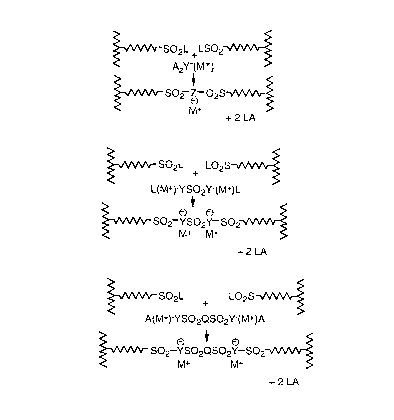
P~SO2L+(M+)A2Y + LO2S~P'~ P~SO2Z(M+)O2S~P'+2LA
P~SO2L+(M+)AYSO2ZA(M+) + LO2S~P' ~
P~SO2Z(M+)SO2Y(M+)SO2~P' + 2LA
P~SO2L + (NI+)AYSO2QZA(M+) + LO2S~P'~
P~SO2Y(M+)SO2QSO2Y(M+)SO2~P'+2LA
6) process for crosslinking polymers according to claim 3 characterized in
that the sulfonated groups
are totally or partially under the form:
P~SO2Y(M+)A
7) process for crosslinking polymers according to claim 4 characterized in
that one of the following
reactions is used to form the crosslinks:
-8-
P~SO2Y(M+)A + LSO2L + A(M+)Y-P' ~
P~SO2Z(M+)SO2Z(M+)SO2~P' + 2LA
P~SO2Y(M+)A + LSO2QSO2L + A(M+)Y~P' ~
P~SO2Z(M+)SO2QSO2Z(M+)SO2~P' + 2LA
8) process for crosslinking polymers according to claim 3 and 4 characterized
in that either M+ or A or
both is a proton and the reaction is conducted in presence of a tertiary or
hindered organic base, an
organometallic reagent, a metal amide.
9) process for crosslinking polymers according to claim 4 characterized in
that A is a trialkylsilyl
group, especially trimethylsilyl.
10) process according to claim 4 characterized in that A is a tertioalkyl
group and the condensation
reaction is conducted in the presence of a tertialy or hindered organic base.
11) process for crosslinking polymers according to claim 8 and 10
characterized in that the tertiary base
is triethylamine) di-isopropylamine, quinuclidine), 1,4-
diazobicyclo[2,2,2]octane (DABCO); a
pyridine (for example pyridine, alkylpryidines, dialkylaminopyridine); an
imidazole (for example
N-alkylimidazoles, imidazo[1,1-a]pyridine); as amidine (for example 1,5-
diazabicyclo[4,3,0]non-
5-ene (DBN), 1,8-diazabicyclo[5,4,0]undec-7-ene (DBU); a guanidine (for
example tetramethyl
guanidine) 1,3,4,7,8-hexahydro-1-methyl-2H-pyrimido[1,2-a]pyrimidine (HPP).
12) process according to claim 3 characterized in that enter A or M+ or both
are solvated by
dialkylethers or oligo-ethylene glycols or permethylaled oligo-ethylendiamines
(ex tetramethyl-
ethylene diamine TMEDA).
13) crosslinked polymers derived from at least one of the following monomers:
<IMG>
-9-
<IMG>
where:
- X represents F, Cl or CF3
- a being comprised between 0 (included) and 10
- E represents an ether -O-, sulfide -S-, sulfone -SO2- or nothing (direct
=C(Z)~aryl link).
- Z is either F or H.
14) crosslinked polymers according to claim 13 characterized is that L = F or
Cl.
15) crosslinked polymers according to claim 13 characterized in that n = 0
(included) or 1.
16) process according to claim 4 characterized in that the crosslinking agent
are chosen between:
Li3N C3AI4 [(CH3)3Si]2NLi,Na, K
NH3 + 3DABCO CF3SO2C[(CH3)3Si][Li(TMEDA)]2 (CH3)3CNH2 + 3TEA
NH2SO2NH2 + 4TEA {[(CH3)3Si](Li)N]2SO2 [(TMEDA)(Mg)N]2SO2
CH3Li (CH3)3Al NH2Li, Na, K
{[Si(CH3)3](Li)NSO2]2CF ((Li)[Si(CH3)3]NSO2CF2)2CF2 [(Li)Si(CH3)3NSO2CF2]2
2
{(Li)[Si(CH3)3]NSO2CF2CF2}2O
17) process according to claim 6 characterized in that the crosslinking agent
are chosen between:
SO2Cl2 + 3DABCO SO2(imidazole)2 [FSO2CF2]2 + 3TEA
(FSO2CF2CF2)2O + 3DABCO
-10-
18) sulfonated polymers according to claims 2 characterized in that the
uncrosslinked polymer
containing the P~SO2L is processed into its final shape and crosslinked in a
further step.
19) sulfonated polymers according to claims 2 characterized in that the
uncrosslinked polymer is
mechanically mixed with the cross-linking agent and pressed and heated,
preferably at temperatures
ranging from 0 to 200°C.
20) sulfonated polymers according to claims 2 characterized in that the
uncrosslinked polymer is
processed into its final shape and brought in contact with a solution of the
crosslinking reagent in
an inert solvent and reacted at temperatures ranging from -60 to 200°C.
21) sulfonate polymers according to claims 2 characterized in that the
crosslink density is controlled
by the immersion time, the temperature and the concentration of the reagent.
22) Method for preparing a membrane according to claim 20 characterized in
that the suitable solvent is
chosen among: lower aliphatic alcohols, polyhalocarbons, THF, the glymes,
tertiary alkylamides
including DMF, N-methyl-pyrrolidone, tetramethyl-urea and its cyclic analogs,
N-alkylimidazoles,
tetraalkyl sulfamides and mixtures thereof.
23) sulfonated polymers according to claims 2 characterized in that the
uncrosslinked polymer is is
processed into its final shape and brought in contact with the crosslinking
reagent and a non-crosslinking
ion-generating reagent to form ~SO3-(M+), or -[SO2YSO2R]-(M+) end groups, R"'
being an organic radical, preferably halogenated, especially perfluorinated.
24) sulfonated polymers according to claims 23 characterized in that the
uncrosslinked polymer is is
processed info its final shape and brought in contact sequentially wills the
crosslinking reagent and
the non-crosslinking ion-generating reagent.
25) sulfonated polymers according to claims 23 characterized is that the
uncrosslinked polymer is is
processed info its final shape and brought in contact simultaneously wish the
crosslinking reagent
and the non-crosslinking ion-generating reagent, the crosslink density being
controlled by the
immersion time, the temperature and the concentration of the reagents.
26) sulfonated polymers according to claims 23 characterized in that the non-
crosslinking
ion-generating reagent, is (CH3)3SiO-(M+) or [(CH3)3SiNSO2CF3]-(M+).
27) Method for preparing material according to claims 2 to 26 characterized in
that ion exchange to the
desired cation M+ is performed offer polymerization.
28) Material according to claim 2 to 26 characterized in that inorganic or
organic filler particles,
including fibers, filaments, woven or non woven cloth , are included in the
polymers while in the
processable form.
29) electrochemical cell characterized in that a membrane according to claims
1 to 28 is used as solid
electrolyte.
-11-
30) electrochemical cell according to claim 29 characterized in that it is a
fuel cell, an/or a water
electrolyser, a chlor-alkali cell, an electrochemical acid or salt recovery
cell, an ozone production
cell.
31) electrochemical cell according to claim 29 characterized in that at least
one electrode is in contact
with the membrane.
32) electrochemical cell according to claims 30 characterized in that at least
one electrode containing a
conductive additive, optionally a catalyst, optionally a pore forming agent
and the un-crosslinked
sulfonated polymer is coated on the pre-crosslinked electrolyte membrane, then
crosslinked.
33) electrochemical cell according to claim 23 characterized in that at least
one electrode containing a
conductive additive, optionally a catalyst, and optionally a pore forming
agent and the monomers of
claims 1 to 6, is coated on, or co-extruded with, the un-crosslinked
electrolyte membrane then the
assembly crosslinked.
34) electrochemical cell according to claim 30 characterized in that it forms
the element of a fuel cell
where M+ is an hydrated proton and the positive electrode contains au oxygen
reduction catalyst an
the negative electrode either an hydrogen, methanol, dimethoxymethane,
trimethoxymethane,
trioxane or ammonia oxidation catalyst.
35) fuel cell according to claim 34 characterized in that the electrodes are
applied onto the membrane
using the process or either claims 32 or 34
36) Material according to claims 1 to 11 characterized in that it is used for
chlor-alkali electrolysis.
37) Material according to claim 29 characterized in that it is used as a
separator in the electrochemical
preparation of organic or inorganic substances.
38) Material according to claims 29 characterized in that it is used a
separator between an an aqueous
phase and an organic phase.
39) Material according to claims 1 to 11 and 12 characterized in that the M+
ions associated with the
non-nucleophilic anionic centers of the backbone confer catalytic properties.
40) Material according to claims 1 to 11 and 20 characterized in that it is a
catalyst for Diels & Alder
additions, Friedel & Craft reactions, aldol condensations, cationic
polymerization, esterifications,
acetal formation.
-12-