Note: Descriptions are shown in the official language in which they were submitted.
CA 02236925 1998-OS-06
WD 97/17063 PCT/GB96/02770
-1_
MICROENCAPSULATED DNA FOR VACCINATION AND GENE THERAP'~f
The present invention relates to microencapsulated DNA, to vaccines comprising
microencapsulated DNA, to methods of vaccination and to methods of gene
therapy
comprising administration of DNA in microparticles, to methods of preparing
microparticles containing DNA and to dried compositions comprising DNA-
containing
particles.
The bio-degradable polymer poly (DL-lactide-co-glycolide) (PLG) has been used
for
many years by the pharmaceutical industry to deliver drugs and biologicals in
microparticulate form in viva. The United States FDA has recently approved a
PLG
microsphere 30-day delivery system for leuprolide acetate (Lupran Depot
(registered
trade mark)) to be used in the treatment of prostate cancer. A useful review
of the
potential of polymer microencapsulation technology for vaccine use is found in
Vaccine, 1994, volume 12, number 1, pages 5-11, by William Morris et al.
As an alternative to encapsulation, it is also known to deliver antigens in
phospholipid
vesicles called liposomes, as described for example by Eppstein, D.A et al in
Crit.
Rev. Ther. Drug Carrier Syst. 1988, 5(2), pages 99-139. It is reported that a
number
of antigens have been delivered intraperitoneally using liposomes, including
cholera
toxin, malaria sporozoite protein and tetanus toxoid, and that influenza
antigen has
been delivered intra-nasally.
It is also known that, in certain circumstances, injection of naked DNA into
tissue can
lead to expression of a gene product coded by that DNA. For example, in 1984,
work
at the United States NIH reported that intrahepatic injection of naked, cloned
plasmid
DNA for squirrel hepatitis produced both viral infection and the formation of
anti-viral
antibodies in the squirrels.
. WO-A-95/05853 describes methods, compositions and devices for administration
of
naked polynucleotides which encode biologically active peptides. This
published
application describes, inter alia, the injection of naked DNA coding for an
immunogenic
SUBSTITUTE SHEET (RULE 26)
CA 02236925 1998-OS-06
WO 97/17063 PCT/GB96/02770
-2-
antigen with the aim of raising antibodies in the recipient of the naked DNA.
Liposomal delivery of DNA is also known, and is described, for example, in EP-
A-
0475178.
An alternative method for obtaining expression of a desired gene product is
described
in EP-A-0161640, in which mouse cells expressing bovine growth hormone are
encapsulated and implanted into a cow to increase milk production therein.
EP-A-0248531 describes encapsulating linear poly (I:C) in microcapsules and
using
these to induce production of interferon.
WO-A-94/23738 purports to describe a microparticle containing DNA in
combination
with a conjugate that facilitates and targets cellular uptake of the DNA. In
working
examples, bombardment of cells by microparticles containing Tungsten is
described.
These examples appear little different to conventional bombardment of cells
with
DNA-coated metal particles. Furthermore, sonication is proposed in
microparticle
manufacture, a step that is known to risk DNA damage but the presented data is
inadequate and inappropriate to determine the integrity of the encapsulated
DNA.
In the present invention, it is desired to deliver, in vivo, DNA encoding
proteins with
immunogenic, enrymatic or other useful biological activity, usually under the
control
of an active eukaryotic promoter. Objects of the invention include improvement
on
vaccination therapies known in the art and improvement upon prior art gene
therapy
methods.
Improvement of or alternatives to existing compositions and methods are
desirable as
these existing methods are known to contain a number of drawbacks.
WO-A-95/05853 describes administration of naked polynucleotides which code for
desired gene products. However, the compositions and methods in this
publication
are suitable only for injection, requiring sterile procedures, being in itself
an unpleasant
SUBSTITUTE SHEET (RULE 26)
CA 02236925 1998-OS-06
WO 97/17063 PC'd'/GB96/02770
-3-
and awkward route of administration.
WO-A-94/23738 purports to provide a process in which encapsulated DNA is
released from the particles in the body of the recipient and then taken up by
cells,
' although no accomplished in vivo examples are presented.
The invention seeks to provide novel compositions and methods of
administration
thereof that improve upon existing vaccination and gene therapy techniques and
are
effective in vivo, or at least overcome some of the problems or disadvantages
identified in known compositions and methods.
It is Known that DNA is readily damaged so that it is no longer capable of
inducing
expression of a gene product. Surprisingly, the inventors have succeeded in
devising
a technique for encapsulation of DNA within polymer particles, such that the
DNA
retains sufficient integrity to induce expression of a gene product coded
thereby. The
inventors have also succeeded in devising a DNA-containing microparticle
suitable for
mammalian vaccination or for gene therapy.
Accordingly, a first aspect of the invention provides a pharmaceutical
composition
comprising DNA encapsulated in a polymer, said DNA comprising a sequence
coding
for a polypeptide and wherein the composition is adapted to induce expression
in a
recipient of the coding sequence. Preferably, the coding sequence is
accompanied
by a promoter promoting expression of the sequence. Where the pharmaceutical
composition is for use on mammals, it is convenient to use a eukaryotic
promoter and
especially a promoter that operates in a wide variety of tissue types. In
particular
embodiments of the invention, a tissue - or cell type - specific promoter is
used.
In use, the pharmaceutical composition is orally administered, and the coding
sequence is expressed leading to desired therapeutic effects.
A composition of the invention suitable for vaccination contains a sequence
coding for
an immunogen. Following administration of the composition, expressed immunogen
SUE3STITUTE SHEET (RULE 26)
CA 02236925 1998-OS-06
WO 97/17063 PCT/GB96/02770
-4-
elicits production of antibodies within the recipient, thereby contributing to
vaccination
of the recipient.
A specific embodiment of the invention, described in an example below,
contains a
DNA sequence coding for a protein. It has been administered in vivo and has
been '
found to induce expression in a mammal of that protein. The expression was
detected
by measurement of production of antibodies specific for that protein. The
composition
of the specific embodiment comprises microparticles in the size range up to
about 10
~cm.
C,enerally, the microparticles of the invention are intended to enter cells of
the recipient
by phagocytosis, for example phagocytosis by macrophages or other antigen
presenting cells. Subsequently, the body of the microparticle breaks down in
the
intracellular space and the DNA is released. It is preferred that the
microparticles of
the invention are in the size range 0.01 arm to 30 ~Cm, with 1 ~Cm to 10 ~Cm
being a
more preferred range. These sizes have been found to be suitable for reliably
achieving in vivo expression of the DNA. It is also to be noted that agents
promoting
uptake of the DNA are not needed in microparticles of the invention - as the
microparticie size determines its uptake.
An alternative composition, according to the invention, contains a sequence
coding for
a non-immunogenic product. Such a composition is particularly useful for gene
therapy, wherein it is desired to express in a recipient a gene product that
is non-
expressed, or expressed at a level that it is desired to increase. In this
case, a
composition of the invention comprises a DNA sequence coding far a desired
product,
and wherein following administration of the composition expression of the
coding
sequence results in an increased level of the desired product, giving the gene
therapy
effect.
It is again a feature of the invention that microparticles for gene therapy
applications
have sizes in the range 0.01 ~Cm to 30 ~m , preferably 1 ~tm to 10 ~Cm, to
target their
uptake by phagocytosis.
SUBSTITUTE SHEET (RULE 26)
CA 02236925 1998-OS-06
WU 97/17063 PCT/GB96/02770
-5-
Specific embodiments of the invention, for use in vaccination, comprise a DNA
sequence coding for a protein or an immunogenic component thereof, or an
immunogenic fragment or variant thereof, of a virus, bacterium or other
pathogenic
microorganism. The protein is, for example, an HIV protein, an influenza virus
protein,
. a measles virus protein, a hepatitis virus protein (such as hepatitis
surface antigen)
or a pertussis protein. Further, where the composition is for oral use, it can
conveniently also contain a taste - enhancing agent. The term "taste -
enhancing
agent" is intended to encompass sweeteners, flavourings and agents that mask
any
unpleasant taste from other components of the composition. It can conveniently
be
enterically coated or co-administered with an appropriate antacid formulation.
In a specific embodiment described below, a preparation of microparticles
contains a
DNA sequence coding for a measles protein. Oral administration of the
microparticles
elicited an increase in antibodies specific for that protein. Likewise,
another
microparticle preparation contains a DNA sequence coding for a rotavirus
protein.
Oral administration of these microparticles preparation elicited anti-
rotavirus protein
antibodies and a protective effect against challenge by the virus.
Specific embodiments of the invention, suitable for gene therapy, comprise a
DNA
sequence coding for an enzyme or another protein needed for the treatment of
genetic
disease. For example, a DNA sequence coding for glucocerebrosidase is suitable
for
the treatment of Gaucher's disease.
The inventors have thus provided DNA encapsulated within a polymer such that
the
ability of DNA to code for a desired gene product is substantially not
affected by the
encapsulation process. It is known that DNA can readily be damaged by
emulsifying
and other steps necessary for production of polymer particles. The inventors
have
provided for encapsulation of DNA such that sufficient operative DNA is
encapsulated
for a biological effect to be obtainable upon administration of the
encapsulated DNA.
The invention offers advantages, in that encapsulated DNA is suitable for oral
administration, avoiding the unpleasant and awkward aspects associated with
having
SUE~STITUTE SHEET (RULE 26)
CA 02236925 2004-07-15
WO 97/17063 PGT/GB96/02770
-6-
to inject DNA preparations described in the prior art. Specfic embodiments in
examples described below have been successful in inducing immunogen-specific
antibodies in response to oral administration of a composition of the
invention. In .
addition, the encapsulated DNA formulation is suitable for drying, e.g. freeze
drying,
into a form that is stable over long periods and is suitable for storage.
Further, for .'
many vaccine applications it would be advantageous if, as well as a systemic
humoral
and cell - mediated immune response, immunity at mucosal surfaces could also
be
evoked. Specific embodiments of the invention, described below, have been
demonstrated to elicit significant increases in specific IgA antibodies,
following oral
administration. The invention thus provides a pharmaceutical composition
comprising
DNA within a polymer particle, the DNA encoding a polypeptide, and the
composition
being adapted to induce mucosal polypeptide specific IgA antibodies in a
recipient.
The polymer of the microparticle of the invention preferably is both
biodegradable and
non-toxic. Suitable polymers include lacctide containing polymers and
glycolide
containing polymers and copolymers of 0-100:100-0 lacctide:glycolide. In a
specific
embodiment of the invention, the polymer comprises poly (DL-lactide-co-
glycolide),
otherwise referred to as PLG, chosen as it has been approved for human and
veterinary use.
The products of the invention are typically for in vivo use on animals, in
particular
animals. The polymer of the microparticle should therefore be non toxic in
vivo and
suitable for pharmaceutical use. The polymer should further be biodegradable -
either
by consisting of or comprising biodegradable polymer - so that It releases its
DNA in
the recipient. There exists in the art an extensive literature on polymers
suitable for
human and animal oral use and a person of skill in the art will be able to
adapt
polymers of the art into the microparticles of the present invention without
difficulty.
The DNA contained within the particle will typically comprise double stranded
DNA.
The construction of a suitable DNA sequence for use in the invention will be
SUBSTITUTE SHEET (RULE 26) _
CA 02236925 1998-OS-06
WO 97/17063 PCT/GB96/OZ770
- 7 -
appreciated by persons of skill in the art. It is preferred that the sequence
comprises
both a transcriptional promoter and a gene coding sequence. It is further
preferred
that the DNA sequence provides for a transcription termination and
polyadenylation
downstream of the coding sequence.
It is particularly preferred that the DNA be double stranded, circular and
super coiled.
It has been observed that during manufacture of particles the DNA is subjected
to
severe shear forces. Using particular mild particle manufacturing conditions,
the
inventors have managed to retain functional DNA, though have observed that
previously supercoiled DNA may become partly converted to the open circular
form
in the process.
Plasmid DNA is particularly suitable and is used in the specific embodiments
of the
invention described below. As there is extensive literature relating to
plasmid
manufiacture a person of skill in the art will readily be able to prepared a
pfasmid
suitable for the microparticle of the invention. In general, plasmids
incorporating any
eukaryotic promoter sequence are suitable.
A further optional feature of the invention is that DNA - containing polymer
particles
can be manufactured so as to have different half-lives in vivo. When
administering
an antigen during vaccination, it may be advantageous for the antigen to be
delivered
over as long a time frame as possible. A particular embodiment of the
invention
provides a vaccine comprising first and second vaccine components, the first
vaccine
component comprising polymer - encapsulated DNA wherein the DNA includes a
sequence coding for an immunogen and wherein the polymer has a first half life
in
vivo, and a second vaccine component comprising polymer - encapsulated DNA,
wherein the DNA contains a sequence coding for an immunogen and wherein the
polymer has a second half - life in vivo. The respective half-lives could be
up to 5
days and more than 5 days. On one example, the immunogen of the first and
second
vaccine components are the same. Alternatively, the respective vaccine
components
can contain DNA sequences coding for different immunogens.
SUE3STITUTE SHEET (RULE 26)
CA 02236925 1998-OS-06
WO 97/17063 PCT/GB9C/02770
- g -
In an embodiment of the invention, the half-lives of the respective first and
second
vaccine components are up to two days, and more than two weeks. In a further
embodiment, the first and second half-lives differ by at least an order of
magnitude.
In use of a specific embodiment of the invention, described in an example
below, a
plasmid encoding luciferase is under control of the human cytomegalovirus
immediate
early promoter and is encapsulated within PLG in particles around two ~Cm in
size.
This encapsulated DNA was administered to mice and elicited anti-luciferase
antibodies that were detected over a period of several weeks. The production
of
antibodies in response to encapsulated DNA according to the invention was
compared
with antibody production in response to administration of naked DNA
intraperitoneally
and orally. In both cases, encapsulated DNA elicited equivalent or
significantly higher
amounts of IgG and IgM, and also evoked a significant IgA response.
A second aspect of the invention provides a method of encapsulating DNA in a
polymer such that biological activity of the DNA is retained to a significant
extent. In
an embodiment of the second aspect, a method for encapsulating DNA within a
polymer particle, said DNA being capable of inducing expression of a coding
sequence
within said DNA, comprises preparing a (water-in-oil)-in-water emulsion to
form
microparticles and separating subsequently produced DNA-containing
microparticles
by centrifugation. Resultant microparticles preferably have sizes in the range
0.01 ~Cm
to 30 Vim, more preferably 1 ~m to 10 Vim.
The method of the invention is carried out under conditions that ensure at
least a
portion of the DNA is not damaged during manufacture of the particles and
thereby
retains its ability to induce expression of its gene coding sequence.
It is essential that DNA is incorporated into the microparticles, and DNA
incorporation
is increased by preparing a solution of DNA plus an alcohol, adding
microparticle
polymer and forming microparticles therefrom. The alcohol content of the
solution
suitably varies between 1 % and 60% and preferably between 5% and 40%. In
specific
embodiments of the invention the alcohol content is around 15-35%, more
particularly
SUBSTITUTE SHEET (RULE 26)
CA 02236925 1998-OS-06
WO 97/17063 PCT/GB96/OZ770
-9-
20-30% for microparticles made from PLG, producing DNA incorporation of 25%
and
above, up to 50-60%. Ethanol is particularly suitable; methanol and propanol
and
other alcohols that do not denature DNA are also suitable, and the alcohol is
preferably a straight chain or branched C2-C10 alcohol.
It is also preferred that the emulsification step or steps of the method be
carried out
under conditions of reduced shear stress, and this is optionally achieved by
use of an
emulsifying speed that is suf6icient to obtain an emulsion and to form
microparticles
in the desired size range but not so high that all DNA is damaged by excessive
shear.
In an embodiment of the invention described below the emulsifying mixer speed
is
modified so that at least 25% DNA activity (assayed by transformation of
competent
bacteria or transfection of cultured cells) is retained in the resultant
microparticles that
contain DNA. Suitable speeds are below 8000 rpm, preferably below 6000 rpm,
and
in a specific embodiment described below the speed is about 3000 rpm.
The method may be performed at ambient temperature, which is convenient for
laboratory and industrial purposes, and may also be performed at below ambient
temperature improves the stability of the plasmid DNA during the encapsulation
procedures. The temperature of the method may be reduced to below 20°C,
below
10°C or even below 5°C. In an embodiment of the invention, the
method is carried
out at below ambient temperature using a reduced amount of microparticle
precursor
compared to the amount used at ambient temperature.
The parameters of the method are thus chosen to promote formation of
microparticles
of 1 O,um diameter or less and to promote incorporation of DNA into
microparticles, and
to avoid damage to the DNA such that the DNA can not be expressed in the
recipient.
- For any particular choice of polymer and DNA variations in the method may be
necessary to obtain best results. The efficiency of a method can be assessed
by
' transformation or transfection assays. In the transformation assay used by
the
inventors, DNA is recovered 'from microparticles by dissolution with organic
solvent,
quantitated and used to transform bacteria - ampicillin selection determines
successful
SUBSTITUTE SHEET (RULE 26)
CA 02236925 1998-OS-06
WO 97/17063 PCT/G1396/02770
- 10 -
transformants. In the transfection assay, recovered DNA is used to transfect
eukaryotic cells in culture, which culture is then assayed for presence of the
antigen
or gene therapy product. These assays have demonstrated that DNA recovered
from
microparticles produced by the method of the invention can retain 50-60% and
up to
80% of the activity of the original DNA, indicating high efficiency of
incorporation of
functional DNA into microparticles.
In a further embodiment of the invention there is provided a method of making
a
pharmaceutical composition comprising preparing a DNA construct for expression
of
a coding sequence within the construct, and forming around the construct a
polymer
particle of size between 0.01,um and 30~m, wherein the construct remains
capable of
inducing expression ofi the coding sequence. In use, when the construct is
separated
from the particle, it induces expression of the coding sequence. The particle
is
preferably formed by emulsifying a solution of a polymer plus DNA plus
alcohol.
The method of the invention is adapted to produce pharmaceutical compositions
of the
first aspect of the invention. The steps of the method are adapted so that, in
a
resultant composition which contains many DNA containing polymer particles, a
useful
proportion of particles contain active DNA, i.e. DNA that has not been damaged
by the
method such that its ability to induce expression of its coding sequence is
lost. DNA
activity is measured as a percentage of activity prior to the particle forming
step.
An acceptable level of DNA biological activity is at least 10% and preferably
at least
25%, though for particularly fragile DNA a lower percentage may be acceptable
so
long as, in use, a therapeutic effect is demonstrated by the composition.
In a specific embodiment of the invention, a composition is made by preparing
a
solution of a plasmid of double stranded, supercoiled DNA comprising a coding
sequence and a eukaryotic promoter. Separately, a polymer solution is
prepared. The
two solutions are mixed together and emulsified at a speed between 1000 and
4000
rpm. A solution of a stabilizing agent is then added and the new mixture
emulsified
at a speed between 1000 and 4000 rpm. After centrifugation and resuspension of
SUBSTITUTE SHEET (RULE 26) ,_
CA 02236925 1998-OS-06
WO 97/17063 PC'T/GB96/02770
- 11 -
particles the DNA within retains 25% of its activity.
A third aspect of the invention provides a pharmaceutical composition
comprising
polymer - encapsulated DNA and having a reduced water content, such as less
than
~ 5% by weight. This composition is suitable for long term storage while
retaining the
ability of the DNA, upon administration to a recipient, to induce expression
of a coding
sequence within said DNA.
A method of preparing a pharmaceutical composition for storage, is to dry,
such as by
freeze drying, a pharmaceutical composition according to the first aspect of
the
invention. It is preferred that the dried composition has a water content of
less than
5%, though the precise water content will be determined by the period of
drying used.
A fourth aspect of the invention provides a method of vaccination comprising
administering a vaccine according to the first aspect of the invention.
Vaccination can
thus be obtained by eliciting .antibodies to the immunogen expressed from the
gene
coding sequence. As will be appreciated, the immunogen can be a component of a
virus or bacterium or other pathogenic microorganism, or can be an analogue of
said
immunogen such that antibodies against the analogue are efFective against the
pathogen itself.
In a fifth aspect of the invention there is provided a method of gene therapy,
comprising identifying a gene product that is to be expressed in an
individual,
preparing a composition according to the first aspect of the invention for
expression
of the desired gene product and administering said composition. The gene
product
desired to be expressed is typically a gene product that is previously not
fully or not
adequately expressed, or not expressed at all, in the patient receiving the
gene
therapy.
The gene therapy can conveniently be achieved by administration orally or
intranasally, or by injection.
SUBSTITUTE SHEET (RULE 26)
CA 02236925 1998-OS-06
WO 97/17063 PCT/GB96/02770
-12-
According to a sixth aspect of the invention there is provided use of a
microparticle
according to the first aspect of the invention in manufacture of a medicament
for
inducing production of IgA antibodies.
According to a seventh aspect of the invention there is provided use of a
microparticle
according to the first aspect of the invention in manufacture of a medicament
for gene
therapy.
In a specific embodiment of the invention described in an example below, the
particle
material is PLG. The size of particles produced by the method of the invention
are
generally in the range of 0.01-30 arm, preferably 1-10 Vim. Other suitable
polymer
formulations for DNA - containing particles according to the present invention
include
poly-lactic acid, poly - hydroxybutyrate, poly hydroxyvalerate, poly
(hydroxybutrate/valerate), ethyl cellulose, dextran, polysaccharides,
polyalkylcyanoacrylate, poly-methyl-methacrylate, poly(e- caprolactone) and
mixtures
of all of these components.
In use of a specific embodiment of the invention, described in an example
below, a
preparation of microparticles according to the invention comprises DNA coding
for the
protein luciferase. As will be appreciated by a person of skill in the art, a
wide range
of DNA sequences and constructs are suitable for use in this invention. In
particular.
the invention can be practised incorporating a wide range of plasmid vectors
already
well known and characterised in the art. Typically, a plasmid vector used in
this
invention will include a cDNA that codes for the desired gene product. The
selection
of additional components for the DNA sequence, such as promoters, reporter
genes
and transcription termination sequences can be made by a person of skill in
the art
according to common general knowledge concerning construction of known plasmid
vectors.
The preferred administration route for compositions of the invention is the
oral route, '
meaning that compositions of the invention should preferably be designed to
avoid
significant degradation while passing through the stomach with its high acid
levels.
SUBSTITUTE SHEET (RULE 26)
CA 02236925 1998-OS-06
WO 97/17063 PCT/GB96/OZ770
-13-
It is known that uptake of microparticles of less than 10 ~tm in size occurs,
inter alia,
in the M cells of the intestine, and thus inclusion of DNA containing
particles in this
size range can be advantageous in promoting uptake at this intestinal
location. Other
modifications to the nature and character and components of the polymer can be
made within the concept of the invention.
There now follows description of specific embodiments of the invention,
accompanied
by figures in which:-
Fig.1 is a schematic diagram of the components of a protein - expressing
plasmid suitable for incorporation into a DNA-containing particle according to
the invention;
Fig. 2 illustrates the results of example 3, namely induction of luciferase -
specific serum antibodies by PLG - encapsulated plasmid DNA, and in which
the left column indicates antibody titre after 3 weeks and the right column
indicates antibody titre after 6 weeks (i.m. represents intra muscular, i.p.
represents intra peritoneal, the bottom portion of each column represents IgG
levels, the top portion represents IgM levels);
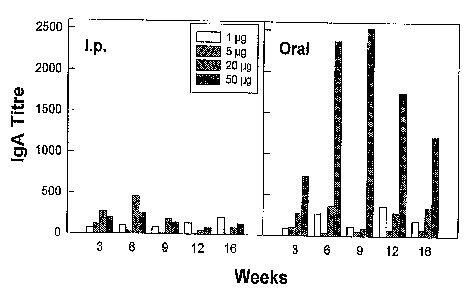
Fig. 3 shows dose-response data for injected and oral doses of encapsulated
DNA, with the titre of IgG, IgM and IgA in each case.
Fig. 4 shows: A - agarose gel electrophoresis of plasmid DNA following
homogenisation in a Silverson mixer at 2000 and 8000 rpm for 0 - 300
seconds; and B - agarose gel electrophoresis of DNA before and after
encapsulation;
Fig. 5 shows stool anti-luciferase IgA response to DNA within PLG
microparticles.
Fig. 6 shows the results of oral administration of PLG - encapsulated DNA
SUI3STITUTE SHEET (RULE 26) _
CA 02236925 1998-OS-06
WO 97/17063 PCT/GB96/02770
-14-
expressing measles virus N protein; and
Fig. 7 shows the results of oral administration of PLG - encapsulated DNA ..
expressing rotavirus VP6 gene: A - faecal rotavirus - specific IgA response,
B - rotavirus shedding after challenge of orally immunized mice.
Example 1
Method for Encapsulation of Plasmid DNA in PLG microparticles
Equipment:
1 ) Silverson Laboratory mixer with 3/4" probe fitted with emulsor screen.
2) High speed centrifuge.
3) Normal laboratory glassware, beakers, measuring cylinders, stirrers etc.
Reagents:
1 ) Poly(lactide-co-glycolide) (PLG) solution - 500 mgs in 3 ml
dichloromethane.
2) Plasmid DNA (12 mg/ml in water).
3) Polyvinyl alcohol (PVA) solution (8% w/v in water).
4) Absolute ethanol.
5) TEN buffer (10 mM tris pH 8.0 + 1 mM EDTA + 50 mM NaCI).
Method:
1 ) Mix 200 SCI plasmid DNA solution with 250 SCI of TEN and add 150 SCI
ethanol with
stirring. Mix well.
2) Add this mixture to 3 ml PLG solution and emulsify in the Silverson mixer
at 3000
rpm for 2 min.
3) Add this emulsion to 100 ml PVA and emulsify at 3000 rpm for 2 min.
4) Add the double emulsion to '1 litre of water and stir vigorously for 1 min.
5) Distribute the suspension of microparticles in centrifuge containers and
centrifuge
at 10,000 x gay for 30 mins.
6) Resuspend the microparticle pellet in 25m1 of water and homogenise with a
hand
homogeniser with large clearance (0.5mm) to make a homogeneous suspension.
SUBSTITUTE SHEET (RULE 26)
CA 02236925 1998-OS-06
WO 97/17063 PCT/GB96/02770
-15-
Dilute with 200 ml of water and recentrifuge as above.
7) Repeat step 6 three times.
8) Resuspend the microparticle pellet in 25 ml of water as above, transfer to
a vessel
suitable for freeze drying, shell freeze in an isopropanol/dry ice mixture and
lyophilise
far 48 h.
In this method , steps 1-3 are carried out at ambient temperature. DNA was
incorporated into the microparticles with an efficiency of about 25%.
Example 2
Plasmids for the Expression of Proteins after In Vivo Delivery
Suitable plasmids for use in microparticles according to the invention consist
of the
following components (see fig. 1):-
1. Plasmid Backbone The pfasmid backbone has an origin of replication and an
antibiotic resistance gene or other selectable marker to allow maintenance of
the
plasmid in its bacterial host. Backbones providing a high copy number will
facilitate
production of plasmid DNA. An example would be from the pUC piasmid vectors.
2. Transcriptional Promoter Sequence Expression of the desired protein will be
driven by a, typically eukaryotic, transcriptional promoter initiating the
synthesis of
mRNA. Generally, strong promoters functioning in a wide variety of tissue
types and
animal species are to be used, e.g. the human cytomegalovirus immediate early
(hCMV IE) promoter. However, particularly for gene therapy applications, a
tissue- or
cell type-specific promoter may be more appropriate.
3. Coding Sequence The coding sequence contains the DNA sequence encoding the
protein of interest. It contains the translational start codon ATG in sequence
context
favourable for initiation of protein synthesis. The coding sequence ends with
a
translational termination codon. Proteins to be expressed include a) reporter
enzymes
(e.g. luciferase, (3-gafactosidase); b) components of pathogenic
microorganisms
SUBSTITUTE SHEET (RULE 26) _
CA 02236925 1998-OS-06
WO 97/17063 PCT/GB96/02770
-16-
capable of inducing protective immune responses (e.g. the NS1 protein of tick-
borne
encephalitis virus, the N, H or F proteins of measles virus, the gp120 protein
of human
immunodeficiency virus 1 ); c) enzymes or other proteins intended for the
treatment of .
genetic disease (e.g. glucocerebrosidase for the treatment of Gaucher's
disease).
4. Transcription Termination Sequence Improved expression levels are obtained
under some circumstances when sequences causing termination of mRNA
transcription are incorporated downstream of the coding sequence. These
sequences
frequently also contain signals causing the addition of a poly A tail to the
mRNA
transcript. Sequences which can be used in this role can be derived from the
hCMV
major immediate early protein gene or from SV40 viral DNA or elsewhere.
Example 3
We have constructed a plasmid encoding the insect protein luciferase, under
the
transcriptional control of the human cytomegalovirus immediate early (hCMV IE)
promoter, and demonstrated luciferase activity in cells transfected in vitro.
We encapsulated purified piasmid DNA in PLG microparticles around 2 ~Cm in
size with
moderate (about 25%) efficiency using the protocol of Example 1. Agarose gel
electrophoresis indicates that a proportion of the initially closed circular
supercoiled
DNA undergoes conversion to a more slowly migrating form, probably relaxed
circles,
as a result ofi shear stresses in the encapsulation process. The encapsulated
DNA
was released from the particles and shown to retain a significant fraction of
its in vivo
biological activity in assays of bacterial transformation by electroporation,
and
luciferase expression after transfection into cultured cells.
Microencapsulated DNA (50 erg) was administered to mice by intraperitoneal
(i.p.)
injection and orally. Control animals received unencapsulated DNA by the same
routes, and as a positive control by standard intramuscular (i.m.) injection.
Luciferase-specific serum antibodies were analyzed by ELISA three and six
weeks
after DNA administration. Results are presented in fig. 2.
SUBSTITUTE SHEET (RULE 26)
CA 02236925 1998-OS-06
W~ 97/17063 PCT/GB96/02770
-17-
As shown in fig. 2, modest specific IgG and IgM responses were seen after i.m.
injection, as might be expected. Encapsulated DNA evoked strong IgG and IgM
responses after i.p. injection, while unencapsulated DNA pave much weaker
responses. Similarly, orally administered encapsulated DNA evoked a good IgG
response which was not matched by unencapsulated DNA. The IgG and IgM antibody
responses indicate that luciferase expression and presentation to the immune
system
occur after administration of plasmid DNA encapsulated in PLG microparticles
with
efficiencies exceeding that seen with the standard i.m. route, and exceeding
that seen
in comparative administration of unencapsulated DNA.
Example 4
In further experiments, microencapsulated DNA, made by the method of Example
1,
in a range of doses (1-50 erg DNA) was administered to groups of outbred mice
by
intra-peritoneal (i.p.) injection or orally. Luciferase-specific serum
antibodies of IgG,
IgM and IgA classes were analysed by ELISA at 3,6 and 9 weeks after DNA
administration.
In Fig. 3, it can be seen that i.p. injection of PLG-encapsulated DNA evoked
good IgG
and- IgM responses, and a modest IgA response. Orally administered
encapsulated
DNA evoked good responses in all three antibody classes. There is a trend far
the
antibody titres to increase with time after DNA administration, and the
responses are
also dose-related to a greater or lesser extent. It is apparent that
quantities of DNA
as low as 1 E.cg are able to evoke significant responses, especially at longer
times after
administration. These antibody responses again confirm that luciferase
expression
occurs after administration of plasmid DNA encapsulated in PLG microparticles,
either
by i.p. injection or orally. They also demonstrate that antigen is presented
to the
immune system by these means in such a fashion as to evoke IgG, IgM and IgA
classes of antibody.
SUl3STITUTE SHEET (RULE 26)
CA 02236925 1998-OS-06
WO 97/17063 PCT/GB96/02770
-18-
Example 5
We examined the effect of high-speed homogenisation steps, used to generate
the -
required water-oil-water emulsions which are intermediates in the
encapsulation
process, on the physical integrity and biological function of plasmid DNA. '
In initial experiments, supercoiled plasmid DNA was adjusted to concentrations
and
volumes similar to those to be used in microencapsulation experiments, and
homogenised with a Silverson laboratory homogeniser. Samples were removed at
intervals from 0 to 300 sec for analysis by agarose gel electrophoresis (Fig.
4A). Such
an analytical procedure is capable of distinguishing between supercoiled (sc)
DNA,
open circular (oc) DNA, where a single strand has been nicked, and linear (I)
DNA,
where both strands have been cut at adjacent points (see, for example, Fig. 2C
in
Garner and Chrambach 1992. Resolution of circular, nicked and linear DNA, 4.4
kb
in length, by electrophoresis in polyacrylamide solutions. Electrophoresis 13,
176-
178). It is clear that exposure to such conditions for periods as short as 10
sec results
In conversion from sc to oc form. At 8000 rpm, further conversion to the
linear form
and eventually more extensive degradation occur. However, at the reduced speed
of
2000 rpm the oc form of DNA is relatively stable over the time period
typically required
for formation of the emulsion intermediates involved in PLG encapsulation.
These
studies thus show that plasmid DNA is vulnerable to shear-induced damage, and
careful attention is required to the precise conditions to obtain
encapsulation of
minimally altered DNA.
From this basis, we have developed conditions for the encapsulation of
purified
plasmid DNA in PLC, microparticles around 2 ~Cm in size with moderate (about
25%)
efficiency. Agarose gel electrophoresis (Fig. 4B) indicates that the initially
closed
circular supercoiled DNA undergoes conversion to the oc form, as a result of
shear
stresses in the encapsulation process. Biological activity of DNA released
from
microparticles has been assessed in assays of bacterial transformation by
electroporation, and luciferase expression after transfection into cultured
cells. DNA
released from the particles retains a significant fraction (about 25%) of its
in vitro
SUBSTITUTE SHEET (RULE 26) _.
CA 02236925 1998-OS-06
WO 97/17063 PCT/GB96/02770
-19-
activity in both these assays.
Example 6
" PLG-encapsulated DNA coding for luciferase, made by the method of Example 3,
is
also able to evoke a mucosal immune response to the expressed protein. Levels
of
IgG, IgM and IgA antibodies specific for luciferase were assessed by ELISA in
stool
samples from mice which received i.p. or oral doses of 1, 5, 20 or 50 ug of
PLG-
encapsulated DNA. No significant levels of IgG or IgM antibodies were found in
stool
samples from any group of mice. Rather limited IgA responses were seen in the
i.p.-
injected mice; however, oral administration resulted in significant levels of
luciferase-
specific igA antibodies in the stool samples (Fig. 5). These reached
extraordinarily
high levels in those mice which received 50 ~g PLG-encapsulated DNA. These
results
indicate that oral administration of a single dose of PLG-encapsulated plasmid
DNA
is capable of evoking a mucosal, as well as a systemic antibody response. This
may
be a useful attribute of a PLG-encapsulated DNA vaccine in applications where
protection against infection at mucosal surfaces is desirable, as for measles
or AIDS.
Example 7
We have exploited a plasmid expressing the measles virus (MV) nucleocapsid
protein
(N) to extend our observations that the oral administration of encapsulated
plasmid
DNA expressing luciferase is capable of eliciting a systemic antibody
response. The
N-expressing construct is identical to that expressing luciferase (described
in example
3), except for the replacement of the coding sequence with the Edmonston
strain MV
N coding sequence. The purified plasmid DNA was PLG-encapsulated (using the
method as described in example 1).
Inbred C3H mice were immunized with two doses suspended in 0.1 M Sodium
bicarbonate administered by oral gavage, 13 days apart; each dose contained 50
ug
DNA. Control groups of mice received PBS alone or PLG particles containing
plasmid
vector DNA containing no coding sequence. Mice were bled at intervals and
serum
SUIBSTITUTE SHEET (RULE 26) _
CA 02236925 1998-OS-06
WO' 97/17063 PCT/GB96/02770
-20-
levels of IgG specific for MV N determined by ELISA, using recombinant MV N
expressed in insect cells as antigen. As shown in Fig. 6A, immunization with
PLG-
encapsulated DNA expressing MV N resulted in significant levels of N-specific
antibody; results shown are mean absorbances in 1/100 diluted sera taken 53
days
after the second DNA administration. There seems to be a considerable degree
of
variability in the response of individual mice to DNA immunization in these
experiments
(see Fig. 6B), but very high levels of antibody (reciprocal titres exceeding
104,
determined in follow-up experiments) are present in some animals. These
results
demonstrate that oral delivery of PLG-encapsulated DNA is an effective method
for
inducing an immune response against an important pathogen.
Example 8
A Further Method for Encapsulation of Plasmid DNA in PLG microparticles
Equipment:
1 ) Silverson Laboratory mixer with 3/4" probe fitted with emulsor screen.
2) High speed centrifuge.
3) Normal laboratory glassware, beakers, measuring cylinders, stirrers etc.
Reagents:
1 ) Poly(lactide-co-glycolide) (PLG) solution - 500 mgs in 3 ml
dichloromethane.
2) Plasmid DNA (>10 mg/ml in TE buffer).
3) Polyvinyl alcohol (PVA) solution (8% w/v in water).
4) Absolute ethanol.
5) TE buffer (10 mM tris pH 8.0 + 1 mM EDTA + 50 mM NaCI).
Method:
1 ) Mix 450 SCI plasmid DNA solution with 150 ~I ethanol with stirring. Mix
well.
2) Add this mixture to 3 ml PLG solution and emulsify in the Silverson mixer
at 2000
rpm for 2'/z min.
3) Add this emulsion to 100 ml PVA and emulsify at 2000 rpm for 2~/2 min.
4) Add the double emulsion to 1 litre of water and stir vigorously for 1 min.
SUBSTITUTE SHEET (RULE 26) "
CA 02236925 1998-OS-06
WO 97/I7063 PCT/GB96/02770
- 21 -
5) Distribute the suspension of microparticles in centrifuge containers and
centrifuge
at 10,000 x gay for 30 mins.
6) Resuspend the microparticle pellet in 25m8 of water and homogenise with a
hand
homogeniser with large clearance (0.5mm) to make a homogeneous suspension.
Dilute with 200 ml of water and recentrifuge as above.
7) Repeat steps 5 and 6 four times.
8) Resuspend the microparticle pellet in 25 ml of water as above, transfer to
a vessel
suitable for freeze drying, shell freeze in an isopropanol/dry ice mixture and
lyophilise
for 48 h.
In this method, steps 1-3 are carried out at ambient temperature. The
efficiency was
improved compared to example 1, up to 30-4~0% efficiency.
Example 9
A Further Method for Encapsulation of Plasmid DNA in PLG microparticles
Equipment:
1 ) Silverson Laboratory mixer with 3/4" probe fitted with emulsor screen.
2) High speed centrifuge.
3) Normal laboratory glassware, beakers, measuring cylinders, stirrers etc.
Reagents:
1 ) Poly(lactide-co-glycolide) (PLG) solution - 400 mgs in 3 ml
dichloromethane.
2) Plasmid DNA (>10 mg/ml in TE buffer).
3) Polyvinyl alcohol (PVA) solution (8% w/v in water).
4) Absolute ethanol.
5) TE buffer (10 mM tris pH 8.0 + 1 mM EDTA.
Method:
1) Mix 450 ul plasmid DNA solution with 150 SCI ethanol with stirring. Mix
well.
2) Add this mixture to 3 ml PLG solution and emulsify in the Silverson mixer
at 2000
rpm for 2~/2 min.
SUBSTITUTE SHEET (RULE 26)
CA 02236925 1998-OS-06
WO 97/17063 PC'T/GB96/02770
3) Add this emulsion to 100 ml PVA and emulsify at 2000 rpm for 2'/i min.
4) Add the double emulsion to 1 litre of water and stir vigorously for 1 min.
5) Distribute the suspension of microparticles in centrifuge containers and
centrifuge .
at 10,000 x gay for 30 mins.
6) Resuspend the microparticle pellet in 25m1 of water and homogenise with a
hand '
homogeniser with large clearance (0.5mm) to make a homogeneous suspension.
Dilute with 200 ml of water and recentrifuge as above.
7) Repeat steps 5 and 6 four times.
8) Resuspend the microparticle pellet in 25 ml of water as above, transfer to
a vessel
suitable for freeze drying, shell freeze and lyophilise for 48 h.
In this method, steps 1-3 are carried out at 4°C. The efficiency of
incorporation of
DNA into microparticles was 50-60%.
Example 10
Plasmid DNA (pCMVIA/VP6) expressing the VP6 gene of murine rotavirus
(epizootic
diarrhoea of infant mice (EDIM) virus) was constructed at the University of
Massachusetts Medical Center. The gene encoding VP6 was inserted into a vector
(pJW4303) downstream of sequences of the immediate early transcriptional
promoter
and intron A of human cytomegalovirus and a tPA-derived secretory signal
sequence.
The gene was followed by transcriptional termination and polyadenylation
sequences
derived from the bovine growth hormone gene. The purified plasmid DNA was PLG-
encapsulated using the method described in example 1.
Inbred Balb/c mice were immunized by oral administration with a dose
containing 50
micrograms VP6-expressing DNA encapsulated in PLG microparticles. A control
group of mice received a similar dose of encapsulated vector DNA. Mice were
examined for intestinal rotavirus-specific IgA at fortnightly intervals by
ELISA, using
EDIM virus as antigen. Nine weeks after immunisation, the animals were
challenged
with EDIM virus, and monitored for excretion of virus in stool using a
monoclonal
antibody-based ELISA.
SUBSTITUTE SHEET (RULE 26)
CA 02236925 1998-OS-06
WO 97/17063 PCT/GB96/02770
- 23 -
As shown in Fig. 7A, immunisation with PLG-encapsulated DNA expressing VP6
resulted in signifiicant levels of rotavirus-specific intestinal IgA antibody
when
_ compared with the control animals. This is particularly noteworthy, since
other routes
of administration of VP6-expressing plasmid DNA do not elicit detectable
levels of
intestinal virus-specific IgA before virus challenge. After challenge with
EDIM virus,
there was a reduction in rotavirus shedding in mice which had received PLG -
encapsulated VP6-expressing plasmid DNA, compared with the control group (Fig.
7B).
These results demonstrate that orally administered PLG-encapsulated plasmid
DNA
is capable of inducing a) a specific intestinal IgA response, and b)
protection against
virus challenge, as manifested in a reduction in virus shedding.
The compositions and methods of the invention have application in slow release
systems for delivery of DNA vaccines; prolonged expression of immunogen
potentially
results in efficient single dose priming and boosting, with consequent
efficient induction
of long term memory responses. Another application is in a vehicle for the
oral
delivery of vaccines; simple and acceptable means for vaccine administration
are likely
to improve vaccine uptake rates; in addition, freeze-dried encapsulated
plasmid DNA
is likely to be very stable and insensitive to environmental conditions. A
further
application is in a slow release system for gene therapy; prolonged release of
DNA
and subsequent expression potentially reduces the need for repeated treatment.
SUBSTITUTE SHEET (RULE 26)