Note: Descriptions are shown in the official language in which they were submitted.
CA 02236959 1998-OS-06
WO 97/17039 PCT/US96/17721
1
SELF-EXPANDING ENDOLUMINAL STENT-GRAFT
~ BACKGROUND OF THE INVENTION
1. Field of the Invention
The invention relates to an implantable prosthesis. In
particular, the invention relates to a self-expanding endoluminal
graft material for use with a stmt as a stmt-graft. The
invention is particularly suited for repairing the aortic artery
and daughter arteries, although it is not limited thereto.
2. State of the Art
Two types of implantable prostheses utilize tubular graft
materials. These prostheses are known as vascular grafts and
endoluminal stmt-grafts. The endoluminal stmt-graft typically
includes tubulargraft material which is affixed (usually with
sutures) to the inside or outside of a woven metallic stmt and
is delivered to the damaged site via a catheter, whereas the
vascular graft does not utilize a stent and is sutured in place
using traditional open surgical techniques.
Vascular grafts are most often used to treat aneurysms and
are typically made of tightly woven polyester (polyethylene
terephthalate - "PET") fibers. PET fibers are chosen because
they have a history of satisfactory long term use in the human
body and because they can withstand relatively high hoop stress
which is imparted by blood pressure in large diameter vessels.
The fibers are tightly woven to limit the porosity of the graft
in order to prevent blood loss during the initial stages of
implantation and to facilitate preclotting with blood. As a
result, these grafts are relatively non-compliant tubes which
exhibit very little change in dimension when stressed in either
the axial or radial directions. To further appreciate the lack
of distensibility of these grafts, when using these types of
grafts in a joint area, such as across a knee joint, these grafts
must be mechanically and thermally crimped or corrugated in order
that it be able to flex and change length when the recipient
bends the knee joint. Such corrugations or crimps are also
CA 02236959 2004-03-16
70238-18
2
helpful during implantation of the graft so that the graft, if
inadvertently cut too short, may be axially elongated during
implantation. Although well-suited for open surgical procedures
these bulky vascular graft constructions are difficult to use in
an endoluminal application where the graft must be folded down
within a deployment catheter.
An elastomeric vascular graft is disclosed in U.S. Patent
Number 4,475,972 to Wong.. As disclosed in Wong,
polyurethane fibers are drawn from a viscous solution and
extruded from a transversing spinnerette onto a rotating mandrel.
As the filaments or fibers are wet as they are wound, they bond
to each as they dry. The resulting graft is a porous tube having
elastomeric properties. While the graft disclosed by Wong would
appear to have many advantages, polyurethane grafts are not noted
for their strength and it is believed that polyurethane may
degrade over time. Therefore, it is believed that the prosthesis
disclosed by Wong may be unsuitable for long term use in the
human body and is not well suited for treating aneurysms. The
graft taught by Wong may have greater elastomeric compliance than
the vessel to which the graft is attached. This can result in an
aneurism if a portion of the graft balloons after implantation.
Thus, when treating aneurysms, the relatively non-compliant PET
grafts are generally preferred.
My prior U.S. Patent Number 5,163,951 discloses an
improvement to the Wong graft wherein a PET mesh is adhered to
the outside of the elastomeric Wong graft. The PET mesh is woven
in a loose knit pattern (e. g. tricot or double tricot warp knit,
atlas or modified atlas warp knit, jersey or double jersey
patterns, etc.) to provide it with compliance. The PET mesh is
adhered to the Wong graft material using an intermediate material
(e. g. an aliphatic polycarbonate urethane) which has a melting
point substantially lower than both the PET mesh and the Wong
graft material. The intermediate material is placed between the
PET mesh and the Wong graft material and the three component
tubular structure is heated so that the intermediate material
CA 02236959 2004-03-16
70238-18
3
melts and mechanically bonds the PET mesh and Wong graft material
during cooling. The compliance and porosity of the three
component graft may be adjusted by adjusting the knitting
parameters, the size and number of strands, and the angle at
which the strands are drawn. The PET mesh gives the graft
greater load bearing ability and also facilitates retention of
sutures within the graft while maintaining some of the compliance
of the Wong graft material.
Endoluminal stents are most often used to repair blood
vessels affected by a variety of lesions which can compromise
circulation of blood through the vessel, i.e. stenoses. A
typical prior art stent, shown in Figures 1 and 2, is a metallic
structure 10 made of braided wire 12 such as stainless steel,
cobalt-chromium-nickel super alloys and combinations, co-
extrusions or braised combinations of the above with tantalum,
gold, platinum and the like. Stents are also made from memory
alloys such as nitinol and the like. Typical stents are
disclosed in U.S. Patents Numbers 9,655,771 and 4,954,126 to
Wallsten and in U.K. Patent Number 1,205,743 to Didcott.
Generally, the wires 12 are braided
with a large pick size, i.e. with relatively large interstices
14 between the wires, so that axial expansion of the stent
causes a diametrical compression of the stent as shown in prior
art Figure 2. Most often the braiding and/or the metal chosen
for the wires yields a resilient stent which is self-expanding.
The ends of the stent are axially displaced during delivery so
that the stent has a reduced diameter and can be easily located
in the vasculature via a relatively small catheter. This is
important since the location of the stent is typically severely
narrowed by the stenosis. Upon locating the stent in the
vessel, the stent is released so that the stent self-expands, as
shown in prior art Figure 1, and fixes itself to the interior of
the vasculature thereby opening a passageway for blood
circulation. While endoluminal stents have been used without
any graft material, it is now preferred to use a graft material
CA 02236959 1998-OS-06
WO 97/17039 PCT/US96/I7721
4
with the stmt in order to prevent the growth of lesions through
the picks (voids) in the stent and thus re-stenosis of the
vessel.
The graft material most often used in endoluminal grafts is
a PET or polytetrafluroethylene (PTFE) material which is folded
to reduce its size and which is attached to one or both ends of
a radially expandable stmt by means of sutures. When the stmt
self-expands or is balloon expanded, the graft unfolds around
the stmt. A disadvantage of the non-elastic folded graft
material is that it cannot be delivered through a small
catheter. It is known to provide a porous endoluminal graft
which is made of a spun matrix of polyurethane combined with a
self-expanding stent. (See, Kato and Dereume et al.,
Transactions of the 21st Meeting of the Society of Eioma rig
March 18-22, 1995,, San Francisco CA, page 81.) The
elastomeric polyurethane fibers allow the graft to compress with
the stmt and thereby permit delivery of the stent-graft through
a relatively small catheter. However, as mentioned above
polyurethane fibers may degrade over long term use in the human
body.
While the primary use of endoluminal stem s is to treat
stenoses, stems are also sometimes used in conjunction with
graft material to bridge aneurysms. The advantage of using a
stmt in bridging aneurysms is that the expanded stmt helps to
fix the graft in place, can eliminate the need for sutures, and
may provide some additional resistance to hoop stress. As
mentioned above, however, the preferred graft material for the
treatment of aneurysms is relatively non-compliant tightly woven
PET. In order to use this graft material, it must be folded,
attached to the stmt with sutures, and delivered through a
relatively large catheter.
CA 02236959 2004-03-16
70238-18
SUN~1ARY OF THE INVENTION
It is therefore an object of the invention to provide a
graft material for use with an endoluminal self-expanding stent.
It is also an object of the invention to provide a self-
expanding graft material for use with an endoluminal stem .
It is another object of the invention to provide a self-
expanding graft material for use with an endoluminal stent where
the graft material includes PET fibers.
It is a further object of the invention to provide a self-
expanding PET graft material for use with an endoluminal stent
wherein the graft material and the stent are attached to each
other without sutures.
Another object of the invention is to provide a stent-graft
where the graft and stent are intimately adhered to each other
and where the graft is self-expanding in tandem with the stent.
It is yet another object of the invention to provide a
stent-graft which has enhanced biocompatability.
A further object of the invention is to provide a stent-
graft which has improved potency.
It is still another object of the invention to provide a
stent-graft which has improved hoop stress resistance.
Yet a further object of the invention is to provide a
stent-graft which facilitates the grafting of a major vessel and
two branches of the major vessel.
CA 02236959 2004-03-16
70238-18
5a
In accord with these objects which will be
discussed in detail below, there is provided a
self-expanding endoluminal stmt-graft, comprising: a) a
self-expanding wire stmt having a geometry such that when
ends of said self-expanding wire stmt are pulled apart, the
diameter of said self-expanding wire stmt decreases to
between approximately one half to approximately one tenth of
the original diameter of said wire stmt; and b) a tubular
deformable textile material constructed of filaments and
having a density and geometry such that when ends of said
tubular deformable textile material are pulled apart, the
diameter of said tubular deformable material decreases to
between approximately one half to approximately one tenth of
the original diameter of said tubular deformable material,
wherein said tubular deformable material is arranged
substantially coaxially with said self-expanding wire stmt
and is affixed to said self-expanding wire stmt.
CA 02236959 2004-03-16
70238-18
6
According to one preferred aspect of the
invention, the tubular deformable material is affixed to the
interior of the stent with an elastomeric adhesive. According
to another preferred aspect of the invention, a biocompatible
porous elastomeric liner is affixed to the interior of the
deformable member with an elastomeric adhesive. The tubular
deformable member according to the invention is preferably a
warp-knit comprised of PET fibers. A presently preferred
embodiment of the deformable member for a ten millimeter
diameter vessel is a PET mesh using seventy denier, thirty-four
filament, false twist fiber which is knit in a double tricot
pattern with twenty-seven courses per inch and six needles per
side. The presently preferred elastomeric adhesive is a melt
adhesive used in conjunction with a fibrous or porous layer
which is applied to the exterior of the stent and applied with a
padding applicator to push the adhesive through the interstices
of the stent onto the graft material. Additional aspects of the
invention include providing a second biocompatible porous
elastomeric layer on the exterior of the stent, and providing a
second stent interior of the graft material.
A second embodiment of the invention provides a stent with
a partially bifurcated or Y-shaped graft material. The
bifurcated embodiment of the invention is particularly useful in
repairing a major vessel and two branch vessels. The
bifurcation of the graft material may be achieved using zig-zag
sutures preferably using radiopaque suture material.
Additional objects and advantages of the invention will
become apparent to these skilled in the art upon reference to
the detailed description taken in conjunction with the provided
figures.
CA 02236959 1998-OS-06
WO 97/17039 PCT/US96/17721
7
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 is a side elevation view of a prior art stent in a
~ radially expanded state;
Figure 2 is a side elevation view of the prior art stem of
Figure 1 in an elongated radially compressed state;
Figure 3 is a side elevation view of a first embodiment of
a self-expanding endoluminal graft according to the invention in
a radially expanded state;
Figure 4 is a side elevation view of the graft of Figure 3
in an axially elongated and radially compressed state;
Figure 5 is a side elevation view of the graft of Figure 3
affixed to the stent of Figure 1 in a radially expanded state;
Figure 6 is a side elevation view of the stent graft of
Figure 5 in an axially elongated and radially compressed state;
Figure 7 is an enlarged cross sectional view of stmt graft
of Figures 5 and 6;
Figure 8 is an enlarged cross sectional view of a first
alternate embodiment of a stent graft according to the
invention;
Figure 9 is an enlarged cross sectional view of a second
alternate embodiment of a stmt graft according to the
- invention;
Figure 10 is an enlarged cross sectional view of a third
alternate embodiment of a stmt graft according to, the
invention;
CA 02236959 1998-OS-06
WO 97/17039 PCT/US96/17721
8
Figure 11 is an enlarged cross sectional view of a fourth
alternate embodiment of a stmt graft according to the
invention;
Figure 12 is an enlarged cross sectional view of a fifth
alternate embodiment of a stent graft according to the
invention;
Figure 13 is a view similar to Figure 3 of a bifurcated
graft according to the invention;
Figure 14 is'a view similar to Figure 4 of the bifurcated
graft of Figure 13;
Figure 15 is a view similar to Figure 5 of the bifurcated
graft of Figures 13 and 14 in conjunction with a stent;
Figure 16 is a view similar to Figure 15 wherein the
bifurcated portion of the graft extends beyond the stent;
Figure 17 a view similar to Figure 16 wherein the
bifurcated portion of the graft comprises two independent legs;
and
Figure 18 is a schematic view of a bifurcated stent graft
according to the invention bypassing the aortic artery and the
iliac arteries.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
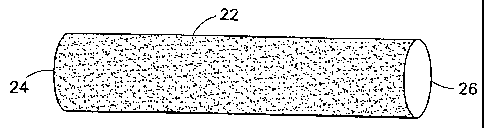
Referring now to Figures 3 to 5, a self-expanding stent-
graft 20, according to the invention includes a textile tube 22
which is axially and radially deformable. That is, when the
ends 24, 26 are pulled apart, as shown in Figure 4, the graft
will compress radially as it is expanded axially. Preferably,
when the ends 24, 26 are moved together, the graft will expand
radially to the configuration shown in Figure 3.
CA 02236959 1998-OS-06
WO 97/17039 PCT/US96/17721
9
The textile tube 22 is preferably a warp-knit or atlas-knit
of PET fibers. According to a presently preferred embodiment,
the fibers are knitted using a double bar rochel knitting
machine or a tubular warp-knit knitting machine. Alternatively,
the tube 22 may be knit using a weft-knit where a single spool
of fiber is knitted in a jersey stitch circular pattern. As a
further alternative, the tube 22 may be braided in a manner
similar to the stems disclosed by Didcott and Wallsten, i.e. as
a one-over-one-under or two-over-two-under using PET fibers or
the like. The braids can be made with single end filaments or
with multiple end filaments and the cross-over points can then
be sintered or glued such that the filaments do not unwind when
the tube is cut or removed from the mandrel. As still a further
alternative, the tube 22 may be constructed as a wound structure
where filaments are wound back and forth along a rotating
mandril and then sintered or glued at each cross-over point such
that the filaments do not unwind when the tube is removed from
the mandril.
According to the invention, the density of the textile
material used to form the tube 22 is significant. Textile tubes
which are knitted, woven, or braided with a high density will
not have the expansion and compression deformability required by
the invention. That is, if the pick size is too small, or there
are too many filaments or ends present, the fibers will jam
against each other when the ends of the tube are pulled apart
and prevent radial compression of the tube. In a knitted
textile tube, the picks are defined by stitches in the axial
direction called courses and stitches along the circumference of
the tube called wales. If the number of courses per inch is too
high, the tube will not deform as described above. On the other
hand, if the number of courses per inch is too low, the tube
will be an open macroporous structure which will be ineffective
- as a graft since the structure will not prevent blood from
flowing through the picks. Similarly, if the number of wales
per inch is too low, the graft will not seal blood flow. If the
number of wales per inch is too high, the graft will dilate with
time. The number of picks per square inch is approximately
CA 02236959 2004-03-16
70238-18
equal to the product of the number of courses per inch and the
number of wales per inch. While the number of courses per inch
chosen according to the invention is largely independent of the
dimensions of the graft, the number of wales per inch is
directly related to the diameter of the graft.
It has been discovered that a textile tube 22 which is
knitted from seventy denier filament PET should have between ten
and forty courses per inch and between ten and forty wales per
inch in order to be radially compressible to between one half
and one tenth of its original diameter. The number of picks per
square inch should therefore be between one hundred and one
thousand six hundreds preferably between one hundred and four
hundred.
Referring now to Figures 5-7, the stent-graft 20 according
to the invention includes a conventional self-expanding stent 10
which i.s affixed to the textile tube 22 with sutures or
adhesive. According to a preferred embodiment of the invention,
the textile tube 22 is attached to the stent 10 using an
elastomeric adhesive 28. Suitable adhesives include
polycarbonate urethanes such as described in U.S. Patent Number
5,229,431, Silicone rubber adhesives may also be used.
Silane priming agents may be used to enhance the bond of the
adhesive. The adhesive 28 may be applied in several ways. The
adhesive 28 may be applied to the stent 10 by dipping or
spraying, after which the stent 10 is placed over the textile
tube 22 and the adhesive is cured. Alternatively, the adhesive
28 may be applied to the textile tube 22 prior to placing it
inside the stent 10. As another alternative, the stent 10 can
be placed over the textile tube 22, after which the adhesive 28
is applied as a fibrous, or porous, layer over the stent 10 with
the aid of a padding applicator whereby the adhesive 28 is
pushed through the picks in the stent 10 and bonded to the
textile tube 22. As still another alternative, either the stent
10 or the tube 22, or both, may be coated with a melt adhesive
prior to placing the tube inside the stent. After assembling
CA 02236959 1998-OS-06
WO 97/17039 PCT/US96/17721
11
the stent and the textile tube, the stent-graft 20 is heated to
melt the adhesive. The presently preferred embodiment of the
- invention utilizes a melt adhesive in combination with the
padding technique described above. It is noted with respect to
Figure 7 (as well as Figures 8-12) that the adhesive 28 is shown
as separate layers. However, those skilled in the art will
appreciate that in many cases, the adhesive component of the
stmt-graft flows through the porous tube and macro-porous
stmt, especially after heating.
The invention will be better understood in conjunction with
the following examples.
The self-expanding endoluminal stent-graft 20 shown in
Figure 7 includes a textile tube 22 which is knitted using
seventy denier, thirty-four filament, false twist PET fibers
having a melting point of approximately 240°C. The knit
construction is a double tricot design having twenty-seven
courses per inch, six needles per side. The tube 22 is expanded
over a twelve millimeter mandril from a resting diameter of
approximately four millimeters and coated with a thin fibrous
(porous) layer of polycarbonate urethane 28 having a melting
point of approximately 160°C. A Didcott-type stmt 10 is
provided. The stmt 10 has twenty-four wire filaments, each
being approximately 0.006 inches in diameter, which are braided
one-over-one at an approximately 45° angle relative to the axis
of the stem . The wires of stent 10 are spray coated with a
thin layer of polycarbonate urethane 28, having a melting point
of approximately 160°C, dissolved in dimethylacetamide solvent
so that the polycarbonate urethane is approximately three and
ten percent by weight. Both the stmt 10 and the tube 22 are
. dried. The stmt 10 is placed over the tube 22 and both are
heated to approximately 160°C suchthat the polycarbonate
urethane 28 melts and bonds the stmt 10 to the tube 22. The
demolded stent-graft 20 can be pulled down from twelve
CA 02236959 1998-OS-06
WO 97/17039 PCTlUS96/17721
12
millimeters in diameter to four millimeters in diameter without
delamination of the tube 22 from the stmt 10.
Seventy denier PET fiber is pulled through a bath of 10~
polycarbonate urethane with a melting point of approximately
160°C dissolved in dimethyl acetamide and then through an oven
where the polyurethane is dried. The coated fiber is then re-
spooled onto forty-eight carrier spools and braided on a
braiding machine with a 90° pick angle on a twelve millimeter
mandril. The stent 10 coated with polycarbonate urethane 28 as
described in Example 1 is placed over the PET braid and both are
heated to approximately 160°C at which point the polyurethane
melts and bonds the stmt and the PET braid to each other at
each crossover point in the braid. The demolded stent-graft can
be pulled down from twelve millimeters in diameter to three
millimeters in diameter without delamination of the PET braid
from the stmt 10.
The coated PET fiber described in Example 2 is placed on a
transversing shuttle which reciprocates back and forth along the
longitudinal axis of a twelve millimeter diameter rotating
mandril. The speed of the shuttle and rotation of the mandril
are controlled such that the angle at which the fiber crosses
underlying fibers on the mandril is 45° as measured in respect
to the longitudinal axis of the mandril. The twelve millimeter
polycarbonate urethane coated stmt described in Example 1 is
placed on top of the wound structure and both are heated to
160°C at which point the polyurethane component melts and bonds
the stmt to the wound structure and the PET fibers to each
other. The assembly is then demolded and stretched
longitudinally to demonstrate a reduction in diameter from
twelve millimeters to three millimeters without delamination.
CA 02236959 1998-OS-06
WO 97/17039 PCT/US96/17721
13
The warp-knit PET mesh 22 described in Example 1 is placed
on a mandrel. A stent 10 is dip coated with a room-temperature
- vulcanizing silicone rubber adhesive. The wet stmt 10 is
placed over the mesh 22 on the mandril and the adhesive is cured
at 100°C in the presence of moisture for twenty minutes. The
assembly is then demolded and stretched longitudinally to
demonstrate a reduction in diameter from ten millimeters to
three millimeters without delamination.
Each of the above Examples 1-4 has a cross-sectional
structure substantially as shown in Figure 7. However, those
skilled in the art will appreciate that the relative locations
of the stmt 10 and the tube 22 may be reversed to form a stent-
graft 220 as shown in Figure 8 with the tube 22 located outside
the stmt 10. The stmt-graft 220 has similar characteristics
as described above. Further modifications of the basic
structure of the invention are described in the Examples which
follow.
Referring now to Figure 9, a stmt-graft 320 according to
the invention has an inner layer 30 of polycarbonate urethane
having a melting point of approximately 240°C. The inner layer
30 is spun on a mandril into a non-woven tube or vascular graft
in the manner described by U.S. Patent Number 4,475,972 to Wong.
An additional ten layers of fiber 32 are spun over the non-woven
mesh 30 with a polycarbonate urethane having a melting point of
approximately 160°C. The warp knit PET mesh 22 described in
~ Example 1 is placed over the polycarbonate urethane 32 and an
additional ten passes of polycarbonate urethane 32, having a
melting point of approximately 160°C, are applied over the PET
mesh 22. The stent 10 which is coated with polycarbonate
urethane 28 as described in Example 1 is placed over the
polycarbonate urethane 32 and the entire assembly is heated to
approximately 160°C at which point the layers 28 and 32 melt and
CA 02236959 1998-OS-06
WO 97/17039 PCT/US96/17721
14
bond the stmt 10 to the mesh 22 and the mesh 22 to the
polycarbonate tube 30. The assembly is demolded and stretched
longitudinally to demonstrate a reduction in diameter from
twelve millimeters to 4.5 millimeters without delamination.
Turning now to Figure 10, a stent-graft 420 according to
the invention is manufactured according to Example 1 and further
bonded by placing the stmt-graft 20 (Figure 7) on a mandril and
coating the outside of the stmt-graft with twenty passes of
polycarbonate urethane fiber 30 using the Wong method. As the
fibers are laid down, the fibers are padded through the picks in
the stmt 10 forming a bond between the polycarbonate urethane
36 and the stent 10 thereby increasing the bond strength of the
stent 10 to the PET mesh 22. It will be appreciated that the
additional outer polycarbonate urethane fiber layer 36 may be
used in conjunction with Example 5 or in any embodiment wherein
the stent 10 is the outermost layer.
Still another stmt-graft 520 according to the invention is
shown in Figure 11 where the stmt-graft 520 includes the stent
which is coated with low melting point polycarbonate urethane
28 as described in Example 1. The coated stmt is placed on a
TEFLON-coated mandrel and twenty passes of polyurethane 32,
having a melting point of approximately 160°C is spun over the
stent 10. Alternatively, a high melting point (e.g., 240°C)
polycarbonate urethane tube 30 such as taught by Wong may be
spun over the stmt 10. A PET sheath 22 is then placed over the
covered stmt or stmt-graft, and twenty layers of 160°C melting
urethane 32, or alternatively, another non-woven tube such as
taught by Wong is spun over the PET sheath 22. Another stmt -
10' coated with polycarbonate urethane 28 as per Example 1 is
then placed over the coated sheath and the entire assembly
heated in an oven at 160°C for 15 minutes, then cooled and
demolded. The resultant endoluminal stmt-graft consists of,
CA 02236959 1998-OS-06
WO 97/17039 PCT/US96/17721
from the lumen outward, a stmt 10, a non-woven polyurethane
tube layer 30 (if utilized in lieu of a low temperature
polyurethane 32), a PET tube 22, a second polyurethane layer 30
(again, if utilized in lieu of a low temperature polyurethane
- 32) and a second stent 10', with the layers bound together by
polycarbonate urethane 28 and 32. The advantage of this
embodiment is that if the polyurethane component were to
degrade, the graft material remains essentially trapped between
the wire stems .
Turning now to Figure 12, another stmt-graft 620 according
to the invention includes a polycarbonate urethane having a
melting point of 240°C, which is spun on a mandril into a non-
woven tube or vascular graft 30 in the manner described by Wong.
An additional ten layers of fiber 32 are spun over the non-woven
mesh 30 with a polycarbonate urethane having a melting point of
160°C. The stmt-graft 520 (Figure 11) described in Example 7
(with the low or high melting point polyurethane 30, 32) is then
placed over the fiber layer 32 and the entire assembly heated to
160°C where the layers are bonded to one another. The resultant
endoluminal stent-graft 620 includes, from the lumen outward, a
porous polyurethane graft 30, a stent 10, another porous
polyurethane graft 30 (when utilized in lieu of the low melting
point polyurethane 32), a PET tube 22, and a second stent 10'
with the layers bound together by low melting point
polycarbonate urethane 28 and 32. The advantages of this
embodiment are the same as Example 7. However, the polyurethane
graft 30 on the inside of the device in this example, provides a
good scaffold for tissue ingrowth and therefore a better flow
surface than the PET or wire surfaces.
It should be noted that the inner stents 10 in Examples 7
and 8 need not be entirely coated with polyurethane melt
adhesive 28, and that only the center or one end of the stmt
need be coated. Excessive adhesion between the inner stent and
the outer layers may restrict the ability for the device to pull
CA 02236959 1998-OS-06
WO 97/17039 PCT/US96/I7721
16
down in the event that there is a slight mismatch in geometry of
the stems 10 and 10'. Partial bonding of the system allows for
some mismatch in constructions by enabling slippage between the
layers. It should be noted that the inner stent 10 in Example 7
and the inner stmt 10 in Example 8 with the polyurethane graft -
adhered to it may be inserted into the stent grafts to form
composite stent graft structures 520 and 620 after stent grafts
are deployed into the body cavity. This permits deploying the
composite endoluminal graft into the body cavity using smaller
diameter introducers.
The self-expanding endoluminal stmt-grafts described above
are utilized by pulling the ends apart from each other with the
aid of an "introducer" to radially compress the graft, by
fitting the graft into a catheter, and by maneuvering the graft-
containing catheter through a vessel to the site of
implantation. Once the graft is located at the implantation
site, it is deployed at this site by pushing it out through the
end of the catheter and releasing the ends of the graft from the
introducer so that the graft expands radially and bridges the
underlying defects in the vessel.
As mentioned above, a second embodiment of the invention
provides a bifurcated stmt-graft which is particularly useful
in repairing a major vessel and two branches of the major
vessel, such as the aorta and the iliac arteries. A bifurcated
graft according to the invention is shown in Figures 13 and 14,
with bifurcated stmt-grafts shown in Figures 15-18.
Turning now to Figures 13 and 14, a first bifurcated graft
720 according to the invention is constructed in a manner
described by any one of Examples 1 through 8 and includes a
warp-knit PET textile tube 722 as described above, which is
radially compressible when its ends 724, 726 are pulled apart as
shown in Figure 14. According to the invention, one end 726 of
the tube 722 is bifurcated with sutures 727 to create two lumens
726a, 726b. The sutures 727 are preferably sewn in a zig zag
manner so as to allow axial elongation as shown in Figure 14.
CA 02236959 1998-OS-06
WO 97/17039 PCT/US96/17721
17
Alternatively, the sutures 727 may be made from an elastomeric
material such as a polycarbonate urethane. Still alternatively,
individual sutures can be used instead of a continuous zig-zag
suture to form the bifurcation and still allow axial elongation.
- As another alternative, an elastomeric adhesive such as a low
melting point polycarbonate urethane or silicone rubber adhesive
can be used to form the bifurcation; or the bifurcation can be
formed by heat fusing the bifurcated area. It is desirous that
the suture 727 forming the bifurcation be preferably made from a
radiopaque material, such as barium or bismuth-filled PET, gold,
tantalum, platinum or other radiopaque wire. Alternatively, the
suture line can be painted with a radiopaque paint such as a
tungsten, tantalum, or bismuth-filled silicone rubber to enable
visualization of the bifurcation under fluoroscopy. The
bifurcated tube 722 is affixed to one or more stems 10 as
described in the Examples given above.
As shown in Figure 15, in the first version of this stent-
graft embodiment of the invention, the entire tube 722 including
the bifurcation is contained within the stent 10.
Alternatively, and as shown in Figure 16, a bifurcated graft 820
is provided wherein the bifurcated portion of the tube 722
defined by the sutures 727 is left uncovered by the stmt 10.
According to a second alternative, as shown in Figure 17, a
stent-graft 920 is provided wherein bifurcated tube 922 is
formed with independent legs 926a, 926b at one end 926 of the
tube. The legs are formed by providing parallel sutures and
cutting between them. The tube 926 is affixed to one or more
stents 10 as described above in the Examples. The bifurcated
graft 920 may be formed with the legs 926a, 926b extending
- beyond the stent 10 as shown, or the entire tube 922 may be
covered by one or more stem s in the manner shown in Figure 15,
for example.
Figure 18 shows how a bifurcated stmt-graft 720 according
to the invention is useful in repairing an abdominal aortic
aneurysm and iliac aneurysm. As shown in Figure 18, the
CA 02236959 1998-OS-06
WO 97/17039 PCT/US96/17721
18
bifurcated graft 720 is located in the abdominal aortic artery
50 just above the iliac arteries 52, 54 with its bifurcated end
726 closest to the arteries 52, 54. The graft 720 effectively
bypasses an aneurysm 50a in the aortic artery 50 and, as
mentioned above, provides a radiopaque bifurcated guide to the -
iliac arteries 50, 52. Once the bifurcated graft 720 is
deployed, two additional grafts 20 according to the invention,
may be deployed in each of the iliac arteries 52, 54 to bypass
aneurysms 52a, 54a. The additional grafts 20 are preferably
deployed with the aid of guide wires (not shown) which are
maneuvered into legs 726a, 726b of aortic trunk graft 720. The
guide wires direct two introducers (not shown) into each iliac
artery 52, 54 wherein the additional grafts 20 are deployed.
The bifurcated legs 726a, 726b of the graft 720 provide separate
fluid couplings for the two additional grafts 20 so that blood
can flow from the aortic artery to both iliac arteries.
There have been described and illustrated herein several
embodiments of a self-expanding endoluminal graft. While
particular embodiments of the invention have been described, it
is not intended that the invention be limited thereto, as it is
intended that the invention be as broad in scope as the art will
allow and that the specification be read likewise. Thus, while
the endoluminal stmt-grafts have been discussed in connection
with vascular applications, the stmt-grafts according to the
invention can be used to graft other vessels such as biliary
ducts, the esophagus, ureters, urethra, the trachea, the
intestines, other visceral cavities and the like. In addition,
the stmt-grafts according to the invention can be constructed
with larger or smaller diameters depending upon the intended
application and size vessel to be grafted. It will therefore be
appreciated by those skilled in the art that yet other
modifications could be made to the provided invention without
deviating from its spirit and scope as so claimed.