Note: Descriptions are shown in the official language in which they were submitted.
BASF' Aktiengesellschaft 950921 O.Z. 0050/46488
- a
Simultaneous preparation of caprolactam and hexamethylenediamine
The present invention relates to a process for the simultaneous
preparation of caprolactam and hexamethylenediamine starting from
adiponitrile.
The present invention furthermore relates to an improved process
for the simultaneous separation of 6-aminocapronitrile and
hexamethylenediamine from a mixture containing these substances.
The partial hydrogenation of adiponitrile to 6-aminocapronitrile
in the presence of ammonia and various catalysts has been
sufficiently described. For example, US 4 601 859 describes the
use of catalysts based on rhodium on magnesium oxide, US 2 762
835 describes the use of Raney nickel, US 2 208 598 describes the
use of nickel on alumina, DE-A 848 654 describes fixed-bed
catalysts based on copper/cobalt/zinc and iron/cobalt spinels,
DE-A 954 416 describes the use of cobalt on silica gel and DE-A 4
235 466 describes the use of iron sponges.
In the process described in WO 92/21650, aminocapronitrile yields
of 60~ at a conversion of 70~ and hexamethylenediamine yields of
9~ are achieved in the presence of Raney nickel. At a conversion
of 80$, the yield is 62~.
It is also known that 6-aminocapronitrile can be reacted with
water in the gas or liquid phase in the presence or absence of
catalysts with liberation of ammonia to give caprolactam. For
example, US 2 301 964 describes a process in which from 10 to 25$
strength solutions of 6-aminocapronitrile are converted in the
liquid phase at from 250 to 290°C into caprolactam a.n yields of up
to 76~.
Furthermore, the cyclization of from 25 to 35~ strength
6-aminocapronitrile solutions at 220°C in the liquid phase in
.tea-e... ..; i-i, +1,e nriri; t; r, T, ~,f ~,rnan; r~ cnl voni-c i n i-thP
nrPSAnCe Of
i'ncw.ci. Yr~.v.m a.aac aua.ai.v..iv.w.~. ....-7.......~.. ....~.....~.... .~--
..-- r__------ --
zinc compounds, copper compounds, lead compounds and mercury
compounds is described in FR-A 2,029,540. Caprolactam yields of
up to 83~ are achieved here.
The cyclization of 6-aminocapronitrile can also be carried out in
the gas phase (US 2 357 484): starting from 80$ strength aqueous
solutions, caprolactam yields of 92~ are achieved at 305°C using
alumina as a catalyst.
CA 02237727 1998-06-O1
BASF Aktieagesellschaft 950921 O.Z. 0050/46488
2
EP-A 150 295 describes the reaction of 6-aminocapronitrile in the
gas phase in the presence of copper/vanadium catalysts, hydrogen,
water and ammonia at 290°C with a 77~ yield of caprolactam.
Furthermore, DE-A 43 19 134 describes the conversion of
6-aminocapronitrile into caprolactam in water in the liquid phase
without the addition of a catalyst.
The abovementioned documents do not suggest a process by means of
which caprolactam is obtained from adiponitrile via
6-aminocapronitrile in an overall process comprising both process
steps.
It is an object of the present invention to provide a process for
the simultaneous preparation of caprolactam and
hexamethylenediamine starting from adiponitrile. Furthermore, it
was intended to provide a process which gives pure
6-aminocapronitrile and hexamethylenediamine in a continuous
process from the reaction mixture obtained in the partial
hydrogenation of adiponitrile, the 6-aminocapronitrile being
cyclized to caprolactam in a further process step. Moreover,
byproducts obtained in this process were as far as possible to be
reused, preferably recycled to an earlier process stage.
We have found that this object is achieved by a process for the
simultaneous preparation of caprolactam and hexamethylenediamine
starting from adiponitrile, wherein
(a) adiponitrile is partially hydrogenated to give a mixture
containing essentially 6-aminocapronitrile,
hexamethylenediamine, ammonia, adiponitrile and
hexamethyleneimine, and
(b) the mixture obtained in (a) is subjected to a distillation to
give ammonia as the top product and a bottom product I, the
distillation being carried out at a bottom temperature of
from 60 to 250°C and a pressure of from 5 to 30 bar in the
presence of a compound A which is inert under the
distillation conditions, or a mixture of inert compounds A,
which boils at from 60 to 250°C at 18 bar, and the ammonia
not being completely separated off, and
(c) the bottom product I, containing essentially
6-aminocapronitrile, hexamethylenediamine, adiponitrile,
hexamethyleneimine, inert compound or compounds A and
ammonia, the ammonia content being lower than that of the
mixture used in stage (b), is subjected to a second
CA 02237727 1998-06-O1
BASF Aktiengesellschaft 950921 O.Z. 0050/46488
3
distillation to dive a mixture of the inert compound A and
ammonia as the top product and a bottom product II, the
distillation being carried out at a bottom temperature of
from 100 to 250°C and a pressure of from 2 to 15 bar, with
the proviso that the pressures of the first and of the second
column are matched with one another so that, at a bottom
temperature of not more than 250°C in each case, a top
temperature of more than 20°C is obtained, or, in the
condenser thereof,
15
the condensation at the top of the second column is carried
out at lower temperatures, the top product, which consists of
pure or relatively highly concentrated ammonia, being
recycled to the first column, or
the top product of the second column is recycled to the first
column or to the condenser thereof in vapor form after the
pressure has been increased by means of a compressor,
(d) the bottom product II, containing essentially
6-aminocapronitrile, hexamethylenediamine, adiponitrile,
hexamethyleneimine and inert compound or compounds A, is
subjected to a distillation in a third column to give the
inert compound or compounds A as the top product and a bottom
product III, the distillation being carried out at a bottom
temperature of from 50 to 250°C and a pressure of from 0.05
to 2 bar, with the proviso that the inert compounds A
obtained as the top product are fed to the second column,
and, if desired, the distillation being carried out in the
presence of one or more compounds B which are inert under the
distillation conditions and boil at from 20 to 250°C at
0.3 bar,
(e) the bottom product III, containing essentially
6-aminocapronitrile, hexamethylenediamine, adiponitrile,
hexamethyleneimine and, if desired, inert compound or
compounds B, is subjected to distillation in a fourth column
to give a top product KP1, containing essentially
hexamethyleneimine, if desired inert compound or compounds B,
and a side stream SA1, containing essentially
hexamethylenediamine, the bottom temperature of the column
being from 50 to 250°C and the pressure from 0.05 to 1.5 bar,
and to give a bottom product IV, and, if desired, the column
is equipped with a dividing wall in the region between feed
and side take-off point (Petyuk [sic] column), so that the
hexamethylenediamine obtained is essentially free of
CA 02237727 1998-06-O1
~ '~ BASF Aktiengesellschaft 950921 O.Z. 0050/46488
- 4
hexamethyleneimine and inert compound or compounds B and of
other low boiler,
20
top product KP1 being fed to the third column or, if
5 required, only some of top product KP1 being fed to the third
column and the remainder being removed, and
(f) the bottom product Iv, containing essentially
6-aminocapronitrile and adiponitrile and possibly high
10 boilers, is subjected to a distillation in a fifth column to
give 6-aminocapronitrile having a purity of at least 95% as
the top product and a side stream consisting essentially of
adiponitrile and a bottom product V which consists of high
boilers and small amounts of adiponitrile, and,
if desired, the column is equipped with a dividing wall in
the region between feed and side take-off point, so that the
adiponitrile obtained contains relatively small amounts of
high boilers,
the distillation being carried out at a bottom temperature of
from 50 to 250°C and a pressure of from 10 to 300 mbar,
and the 6-aminocapronitrile thus obtained is then cyclized to
give caprolactam.
we have also found a process for the simultaneous separation of
6-aminocapronitrile and hexamethylenediamine from a mixture
containing these substances.
The partial hydrogenation of adiponitrile can be carried out by
one of the known processes, for example by one of the
abovementioned processes described in US 4 601 859, US 2 762 835,
US 2 208 598, DE-A 848 654, DE-A 954 416, DE-A 4 235 466 or
WO 92/21650, by effecting the hydrogenation in general in the
presence of nickel-, cobalt-, iron- or rhodium-containing
catalysts. The catalysts may be used in the form of supported
catalysts or unsupported catalysts. Examples of suitable catalyst
carriers are alumina, silica, titanium dioxide, magnesium oxide,
active carbons and spinels. Examples of suitable unsupported
catalysts are Raney nickel and Raney cobalt.
The catalyst space velocity is usually chosen in the range from
0.05 to 10, preferably from 0.1 to 5, kg of adiponitrile per 1 of
catalyst per hour.
CA 02237727 1998-06-O1
~ ~~ BASF Aktiengesellschaft 950921 O.Z. 0050/46488
- 5
Hydrogenation is carried out, as a rule, at from 20 to 200°C,
preferably from 50 to 150°C, and at hydrogen partial pressures of
from 0.1 to 20, preferably from 0.5 to 10, MPa.
The hydrogenation is preferably carried out in the presence of a
solvent, in particular ammonia. The amount of ammonia is chosen
in general in the range from 0.1 to 10, preferably from 0.5 to 3,
kg of ammonia per kg of adiponitrile.
The molar ratio of 6-aminocapronitrile to hexamethylenediamine
and hence the molar ratio of caprolactam to hexamethylenediamine
can be controlled by the adiponitrile conversion chosen in each
case. Adiponitrile conversions of from 10 to 90~, preferably from
30 to 80$, are preferably employed in order to obtain high
6-aminocapronitrile selectivities.
As a rule, the sum of 6-aminocapronitrile and
hexamethylenediamine is from 95 to 99~, depending on the catalyst
and reaction conditions, hexamethyleneimine being the most
important byproduct in terms of quantity.
In a preferred embodiment, the reaction is carried out in the
presence of ammonia and lithium hydroxide or a lithium compound
which forms lithium hydroxide under the reaction conditions, at
from 40 to 120°C, preferably from 50 to 100°C, particularly
preferably from 60 to 90°C; the pressure is chosen in general in
the range from 2 to 12, preferably from 3 to 10, particularly
preferably from 4 to 8, MPa. The residence times are essentially
dependent on the desired yield, the selectivity and the desired
conversion; usually, the residence time is chosen so that a
maximum yield is achieved, for example in the range from 50 to
275, preferably from 70 to 200, minutes.
The pressure and temperature ranges are preferably chosen so that
the reaction can be carried out in the liquid phase.
w...-...., ' ..7 ' ~~ in 4n ~mnt~nt ~ttr~_h_ t-h-at the weight
timuumtl3a is u$cu iia geiaerca.i
ratio of ammonia to dinitrile is from 9:1 to O.1:I, preferably
from 2.3:1 to 0.25:1, particularly preferably from 1.5:1 to
0.4:1.
The amount of lithium hydroxide is chosen as a rule in the range
from 0.1 to 20, preferably from 1 to 10, ~ by weight, based on
the amount of catalyst used.
CA 02237727 1998-06-O1
BASF Aktiengesellschaft 950921 O.Z. 0050/46488
6
Examples of lithium compounds which form lithium hydroxide under
the reaction conditions are lithium metal and alkyllithium and
aryllithium compounds such as n-butyllithium and phenyllithium.
The amount of these compounds is chosen in general so that the
abovementioned amount of lithium hydroxide is obtained.
Preferably used catalysts are nickel-, ruthenium-, rhodium-,
iron- and cobalt-containing compounds, preferably those of the
Raney type, in particular Raney nickel and Raney cobalt. The
catalysts may also be used in the form of supported catalysts,
carriers which may be used being, for example, alumina, silica,
zinc oxide, active carbon and titanium dioxide (cf. Appl. Het.
Cat. (1987), 106-122; Catalysis ,~. (1981), 1-30). Raney nickel
(for example from BASF AG, Degussa and Grace) is particularly
preferred.
30
The nickel-, ruthenium-, rhodium-, iron- and cobalt-catalysts may
be modified with metals of groups VIB (Cr, Mo, W) and VIII (Fe,
Ru, Os, Co (only in the case of nickel), Rh, Ir, Pd, Pt) of the
20 Periodic Table. Observations to date have shown that the use of,
in particular, modified Raney nickel catalysts, for example
modified with chromium and/or iron, leads to higher aminonitrile
selectivities (for preparation, cf. DE-A-2 260 978 and Bull. Soc.
Chem. ~ (1946), 208).
The amount of catalyst is chosen in general so that the amount of
cobalt, ruthenium, rhodium, iron or nickel is from 1 to 50,
preferably from 5 to 20, $ by weight, based on the amount of
dinitrile used.
The catalysts may be used as fixed-bed catalysts by the liquid
phase or trickle-bed procedure or as suspended catalysts.
In a further preferred embodiment, adiponitrile is partially
hydrogenated to 6-aminocapronitrile at elevated temperatures and
high pressure in the presence of a solvent and of a catalyst by
using a catalyst which
(a) contains a compound based on a metal selected from the group
consisting of nickel, cobalt, iron, ruthenium and rhodium and
(b) from 0.01 to 25, preferably from 0.1 to 5, $ by weight, based
on (a), of a promoter based on a metal selected from the
group consisting of palladium, platinum, iridium, osmium,
copper, silver, gold, chromium, molybdenum, tungsten,
manganese, rhenium, zinc, cadmium, lead, aluminum, tin,
CA 02237727 1998-06-O1
~~ BASF Aktiengesellschaft 950921 O.Z. 0050/46488
y" __.._ ._.___.
phosphorus, arsenic, antimony, bismuth and rare earth metals,
and
(c) from 0 to 5, preferably from 0.1 to 3, % by weight, based on
(a), of a compound based on an alkali metal or an alkaline
earth metal,
with the proviso that, if a compound based on only ruthenium or
rhodium or ruthenium and rhodium or nickel and rhodium is chosen
as component (a), the promoter (b) can, if desired, be dispensed
with.
Preferred catalysts are those in which the component (a) contains
at least one compound based on a metal selected from the group
consisting of nickel, cobalt and iron, in an amount of from 10 to
95% by weight and ruthenium and/or rhodium in an amount of from
0.1 to 5% by weight, based in each case on the sum of components
(a) to (c),
component (b) contains at least one promoter based on a metal
selected from the group consisting of silver, copper, manganese,
rhenium, lead and phosphorus, in an amount of from 0.1 to 5% by
weight, based on (a), and
component (c) contains at least one compound based on the alkali
metals and alkaline earth metals, selected from the group
consisting of lithium, sodium, potassium, cesium, magnesium and
calcium, in an amount of from 0.1 to 5% by weight.
Particularly preferred catalysts are:
catalyst A, containing 90% by weight of cobalt oxide (Co0), 5% by
weight of manganese oxide (Mn203), 3% by weight of phosphorus
pentoxide and 2% by weight of sodium oxide (NayO),
catalyst B, containing 20% by weight of cobalt oxide (Co0), 5% by
weight of manganese oxide (Mn203), 0.3% by weight of silver oxide
(Ag20), 70% by weight of silica (Si02), 3.5% by weight of alumina
(A1203), 0.4% by weight of iron oxide (Fe203), 0.4% by weight of
magnesium oxide (Mg0) and 0.4% by weight of calcium oxide (Ca0),
and
catalyst C, containing 20% by weight of nickel oxide (Ni0),
67.42% by weight of silica (Si02), 3.7% by weight of alumina
(A1a03), 0.8% by weight of iron oxide (Fe2o3), 0.76% by weight of
magnesium oxide (Mg0), 1.92% by weight of calcium oxide (Ca0),
CA 02237727 1998-06-O1
'~ BASF Aktiengesellschaft 950921 O.Z. 0050/46488
,_
8
3.4% by~weight of sodium oxide (Na20) and 2.0% by weight of
potassium oxide (Ra0).
The catalysts which may be preferably used may be unsupported
Catalysts or supported catalysts. Examples of suitable carrier
materials are porous oxides, such as alumina, silica,
aluminosilicates, lanthanum oxide, titanium dioxide, zirconium
dioxide, magnesium oxide, zinc oxide and zeolites, and active
carbon or mixtures thereof.
The preparation is carried out as a rule by a procedure in which
precursors of the components (a) are precipitated together with
precursors of the promoters (components (b) and, if desired, with
precursors of the trace components (c) in the presence or absence
of carrier materials (depending on the catalyst type desired), if
desired the resulting catalyst precursor is processed to give
extrudates or pellets and is dried and then calcined. Supported
catalysts are generally also obtainable by impregnating the
carrier with a solution of the components (a), (b) and, if
desired, (c), it being possible to add the individual components
simultaneously or in succession, or by spraying the components
(a), (b) and, if desired, (c) onto the carrier by a method known
per se.
Suitable precursors of the components (a) are as a rule readily
water-soluble salts of the abovementioned metals, such as
nitrates, chlorides, acetates, formates and sulfates, preferably
nitrates.
Suitable precursors of the components (b) are as a rule readily
water-soluble salts or complex salts of the abovementioned
metals, such as nitrates, chlorides, acetates, formates and
sulfates and in particular hexachloroplatinate, preferably
nitrates and hexachloroplatinate.
Suitable precursors of the components (c) are as a rule readily
water-soluble salts of the abovementioned alkali metals and
alkaline earth metals, such as hydroxides, carbonates, nitrates,
chlorides, acetates, formates and sulfates, preferably hydroxides
and carbonates.
The precipitation is generally effected from aqueous solutions,
alternatively by adding precipitating reagents, by changing the
pH or by changing the temperature.
CA 02237727 1998-06-O1
~ '~ BASF Aktiengesellschaft 950921 O.Z. 0050/46488
9
The catalyst precursor thus obtained is usually dried, generally
at from 80 to 150°C, preferably from 80 to 120°C.
The calcination is usually carried out at from 150 to 500°C,
preferably from 200 to 450°C, in a gas stream comprising air or
nitrogen.
After calcination, the catalyst material obtained is generally
exposed to a reducing atmosphere (activation), for example by
exposing it for from 2 to 24 hours to a hydrogen atmosphere or a
gas mixture containing hydrogen and an inert gas, such as
nitrogen, at from 80 to 250°C, preferably from 80 to 180°C, in
the
case of catalysts based on ruthenium or rhodium as component (a)
or at from 200 to 500°C, preferably from 250 to 400°C, in the
case
of catalysts based on one of the metals selected from the group
consisting of nickel, cobalt and iron as component (a). The
catalyst loading here is preferably 200 1 per 1 of catalyst.
Advantageously, the activation of the catalyst is carried out
directly in the synthesis reactor, since this usually dispenses
with an otherwise necessary intermediate step, ie. the
passivation of the surface, usually at from 20 to 80°C, preferably
~~ +.~, Z~°r. l~,v mcaanc of oxvaen/nitrocren mixtures, such as
a_r.vau ~..r w .r.n ..., ...1 ~..r--~._. _- -__f ~-__. _.~
air. The activation of passivated catalysts is then preferably
carried out in the synthesis reactor at from 180 to 500°C,
preferably from 200 to 350°C, in a hydrogen-containing atmosphere.
The catalysts may be used as fixed-bed catalysts by the liquid
phase or trickle-bed procedure or as suspended catalysts.
If the reaction is carried out in a suspension, temperatures of
from 40 to 150°C, preferably from 50 to 100°C, particularly
preferably from 60 to 90°C, are usually chosen; the pressure is
chosen in general in the range from 2 to 20, preferably from 3 to
10, particularly preferably from 4 to 9, MPa. The residence times
are essentially dependent on the desired yield, the selectivity
and the desired conversion; usually, the residence time is chosen
so that a maximum yield is achieved, for example in the range
from 50 to 275, preferably from 70 to 200, minutes.
In the suspension procedure, preferably used solvents are
ammonia, amines, diamines and triamines of 1 to 6 carbon atoms,
such as trimethylamine, triethylamine, tripropylamine and
tributylamine, or alcohols, in particular methanol and ethanol,
particularly preferably ammonia. A dinitrile concentration of
from 10 to 90, preferably from 30 to 80, particularly preferably
CA 02237727 1998-06-O1
hASF Alstiengesellschaft 950921 O.Z. 0050/46488
from 40 to 70, % by weight, based on the sum of dinitrile and
solvent, is advantageously chosen.
The amount of catalyst is chosen in general in the range from 1
5 to 50, preferably from 5 to 20, % by weight, based on the amount
of dinitrile used.
The suspension hydrogenation may be carried out batchwise or,
preferably, continuously, as a rule in the liquid phase.
The partial hydrogenation can also be carried out batchwise or
continuously in a fixed-bed reactor by the trickle-bed or liquid
phase procedure, a temperature of from 20 to 150°C, preferably
from 30 to 90°C, and a pressure of, as a rule, from 2 to 30,
preferably from 3 to 20, MPa usually being chosen. The partial
hydrogenation is preferably carried out in the presence of a
solvent, preferably ammonia, an amine, a diamine or a triamine of
1 to 6 carbon atoms, such as trimethylamine, triethylamine,
tripropylamine or tributylamine, or an alcohol, preferably
methanol or ethanol, particularly preferably ammonia. In a
preferred embodiment, an ammonia content of from 1 to 10,
preferably from 2 to 6, g per g of adiponitrile is chosen. A
catalyst space velocity of from 0.1 to 2.0, preferably from 0.3
to 1.0, kg of adiponitrile per 1 per h is preferably chosen. Here
too, the conversion and hence the selectivity can be controlled
by changing the residence time.
The partial hydrogenation can be carried out in a conventional
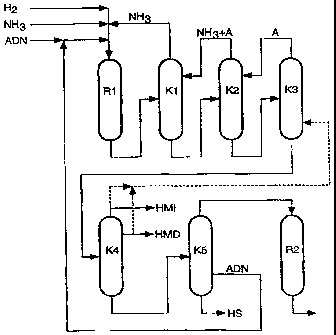
reactor suitable for this purpose (R1 in the drawing).
The distillation in the first column (stage (b); R1 in the
drawing) is carried out according to the invention by a method in
which the mixture from stage (a), containing essentially
6-aminocapronitrile, hexamethylenediamine, ammonia, adiponitrile
and hexamethyleneimine, preferably a mixture containing
essentially from 1 to 70, preferably from 5 to 40, % by weight of
6-aminocapronitrile,
from 1 to 70, preferably from 5 to 40, % by weight of
adiponitrile,
from 0.1 to 70, preferably from 1 to 40, % by weight of
hexamethylenediamine,
from 0.01 to 10, preferably from 0.05 to 5, % by weight of
hexamethyleneimine and
from 5 to 95, preferably from 20 to 85, % by weight of ammonia,
CA 02237727 1998-06-O1
BASF Aktiengesellschaft 950921 O.Z. 0050/46488
11
is carried out as a rule in a conventional distillation
column at a bottom temperature of from 60 to 250°C,
preferably from 100 to 200°C, and a pressure of from 5 to 30,
preferably from 12 to 25, bar in the presence of one or more
compounds A.which are inert under the distillation conditions
and boil at from 60 to 220°C at 18 bar, to give ammonia as
the top product and a bottom product I, the ammonia not being
completely separated off.
According to the invention, suitable compounds A are substances
which are inert under the distillation conditions and have a
boiling point of from 60 to 250°C, preferably from 60 to 150°C,
at
18 bar. Examples are alkanes, cycloalkanes, aromatics,
naphthenes, alcohols, ethers, nitriles and amines having the
abovementioned properties, in particular CS-C8-alkanes and
C2-C4-alkanols, particularly preferably n-pentane, cyclohexane,
triethylamine, ethanol, acetonitrile, n-hexane, di-n-propyl
ether, isopropanol, n-butylamine and benzene, very particularly
preferably ethanol.
Compound A is usually added in an amount of from 0.1 to 50,
preferably from 1 to 10, ~ by weight, based on the bottom product
I.
In stage (c), the bottom product I, containing essentially
6-aminocapronitrile, hexamethylenediamine, adiponitrile,
hexamethyleneimine, inert compound or compounds A and ammonia,
the ammonia content being lower than that of the mixture obtained
from stage (a) and used in stage (b), is subjected to a second
distillation to give a mixture of the inert compound or compounds
A and ammonia as the top product and a bottom product II, the
distillation being carried out at a bottom temperature of from
100 to 250°C, preferably from 140 to 200°C, and at from 2 to 15,
preferably from 4 to 12, bar, with the proviso that the pressures
of the first and of the second column (K2 in the drawing) are
matched with one another so that a top temperature of more than
20°C is obtained at a respective bottom temperature of not more
than 250°C. It may also be advantageous to carry out the
condensation at the top of the second column at lower
temperatures, the top product, which consists of pure or
relatively highly concentrated ammonia, being recycled to the
first column, or
to recycle the top product of the second column in vapor form,
after increasing the pressure by means of a compressor, to the
first column or to its condenser.
CA 02237727 1998-06-O1
BASF Aktiengesellschaft 950921 O.Z. 0050/46488
12
In stage (d), the bottom product II, containing essentially
6-aminocapronitrile, hexamethylenediamine, adiponitrile,
hexamethyleneimine and inert compound or compounds A, is
subjected to a distillation in a third column (R3 in the drawing)
to give the inert compound or compounds A as the top product and
a bottom product III, the distillation being carried out at a
bottom temperature of from 50 to 250°C, preferably from 140 to
200°C, and at from 0.05 to 2, preferably from 0.2 to 1, bar, with
the proviso that the inert compound or compounds A obtained as
the top product is or are fed to the second column, and, if
desired, the distillation is carried out in the presence of one
or more compounds B which are inert under the distillation
conditions and boil at from 20 to 250°C, preferably from 60 to
170°C, at a given pressure of 0.3 bar.
Examples of compounds B are alkanes, cycloalkanes, aromatics,
naphthenes, alcohols, ethers, nitriles and amines having the
abovementioned properties, in particular di-n-butyl ether,
valeronitrile, n-octane, cyclooctane, n-hexylamine,
hexamethyleneimine and hexamethylenediamine, preferably
hexamethyleneimine and/or hexamethylenediamine, particularly
preferably hexamethyleneimine.
In a preferred embodiment, hexamethyleneimine and/or
hexamethylenediamine are chosen as compound B or, particularly
preferably, no further compound B is added.
Compound B is preferably added to the column K3 in an amount of
from 0.01 to 50, preferably from 0.5 to 10, ~ by weight, based on
the bottom product II.
In stage (e), the bottom product III, containing essentially
6-aminocapronitrile, hexamethylenediamine, adiponitrile,
hexamethyleneimine and, if desired, inert compound or compounds
B, is subjected to a distillation in a fourth column (K4 in the
drawing) to give a top product KP1, containing essentially
hexamethyleneimine, if desired inert compound or compounds B and
a side stream SA1, containing essentially hexamethylenediamine,
the bottom temperature of the column being from 50 to 250°C and
the pressure from 0.05 to 1.5 bar, and to give a bottom product
IV.
If desired, the column is equipped with a dividing wall in the
region between feed and side take-off point (Petlyuk column) so
that the hexamethylenediamine obtained is essentially free of
CA 02237727 1998-06-O1
'' BASF Aktiengesellschaft 950921 O.Z. 0050/46488
13
hexamethyleneimine and inert compound or compounds B and of other
low boilers,
top product KP1 and/or HMD from the side stream SA1 being fed, if
required, to the third column or, if required, only a part
thereof being fed to the third column and the remainder being
removed.
In stage (f), the bottom product IV, containing essentially
6-aminocapronitrile and adiponitrile and possibly high boilers,
is subjected to a distillation in a fifth column (K5 in the
drawing) to give 6-aminocapronitrile having a purity of at least
95%, preferably from 99 to 99.9%, as the top product and a side
stream consisting essentially of adiponitrile and a bottom
product V which consists of high boilers and small amounts of
adiponitrile.
If desired, the column is equipped with a dividing wall in the
region between feed and side take-off point, so that the
adiponitrile obtained contains relatively small amounts of high
boilers, the distillation being carried out at a bottom
temperature of from 50 to 250°C and at from 10 to 300 mbar.
Adiponitrile (ADN) from side stream 2 (SA2) is recycled to the
reactor R1.
According to the invention, the 6-aminocapronitrile obtained is
converted into caprolactam. This cyclization can be carried out
by known processes in the liquid or gas phase, for example by a
process disclosed in US 2 301 964, US 2 357 484, EP-A I50 295 or
DE-A 43 19 134, usually by reacting the 6-aminocapronitrile with
water in the liquid phase to give caprolactam and ammonia.
In the reaction without a catalyst, a temperature of from 200 to
375°C and reaction times of from 10 to 90, preferably from 10 to
30, minutes are chosen. As a rule, water is used as the solvent,
the 6-aminocapronitrile content generally being chosen in the
range of less than 30, preferably from 10 to 25, % by weight,
based on the water.
In the reaction in the liquid phase in the presence of a
catalyst, a temperature of from 50 to 330°C, an amount of water of
from 1.3 to 50, preferably from 1.3 to 30, mol per mol of
6-aminocapronitrile and a reaction time of from 10 minutes to
several hours are usually chosen. When an organic solvent is
CA 02237727 1998-06-O1
BASF Aktiengesellschaft 950921 O.Z. 0050/46488
14
used, in particular an alcohol, the amount of water generally
chosen is from 1.3 to 5 mol per mol of 6-aminocapronitrile.
The reacted mixture obtained in the cyclization is usually first
forked up by distillation, ammonia, water and any organic solvent
being separated off. If a catalyst is used, the catalyst present
in the bottom product is generally separated from the caprolactam
by one of the conventional methods and recycled to the
cyclization reactor (R2 in the drawing). The crude caprolactam is
generally converted into pure lactam by purification operations
known per se, such as distillation, and is then available for
polymerization to polycaprolactam.
In a preferred embodiment, 6-aminocapronitrile is reacted with
water in the liquid phase with the use of heterogeneous
catalysts.
The reaction is carried out in the liquid phase at in general
from 140 to 320°C, preferably from 160 to 280°C; the pressure is
in general from 1 to 250, preferably from 5 to 150, bar, and it
should be ensured that the reaction mixture is predominantly
liquid under the conditions used. The residence times are in
general from 1 to 120, preferably from 1 to 90, in particular
from 1 to 60, minutes. In some cases, residence times of from 1
to 10 minutes have proven completely sufficient.
In general, at least 0.01, preferably from 0.1 to 20, in
particular from 1 to 5, mol of water are used per mol of
6-aminocapronitrile.
Advantageously, the 6-aminocapronitrile is used in the form of a
1-50, in particular 5-50, particularly preferably 5-30, %
strength by weight solution in water (in which case the solvent
is simultaneously a reactant) or in water/solvent mixtures.
Examples of solvents are alkanols, such as methanol, ethanol,
n-propanol, isopropanol, n-butanol, isobutanol and tert-butanol,
and polyols such as diethylene glycol and tetraethylene glycol,
hydrocarbons such as petroleum ether, benzene, toluene and
xylene, lactams, such as pyrrolidone and caprolactam, and
alkyl-substituted lactams, such as N-methylpyrrolidone,
N-methylcaprolactam and N-ethylcaprolactam, and carboxylates,
preferably of carboxylic acids of 1 to 8 carbon atoms. Ammonia
may also be present in the reaction. Mixtures of organic solvents
may of course also be used. Mixtures of water and alkanols in a
water/alkanol weight ratio of 1-75/25-99, preferably 1-50/50-99,
have proven particularly advantageous in some cases.
CA 02237727 1998-06-O1
- ~' BASF Aktiengesellschaft 950921 O.Z. 0050/46488
It is in principle just as possible to use the
6-aminocapronitrile as a reactant and simultaneously as a
solvent.
5 Examples of heterogeneous catalysts which may be used are:
acidic, basic or amphoteric oxides of the elements of the second,
third or fourth main group of the Periodic Table, such as calcium
oxide, magnesium oxide, boron oxide, alumina, tin oxide or silica
in the form of pyrogenic silica, silica gel, kieselguhr, quartz
10 or mixtures thereof, and oxides of metals of the second to sixth
subgroups of the Periodic Table, such as titanium oxide, in
amorphous form or as anatase or rutile, zirconium oxide, zinc
oxide, manganese oxide or mixtures thereof. Oxides of the
lanthanides and actinides, such as cerium oxide, thorium oxide,
15 praseodymium oxide and samarium oxide, rare earth mixed oxide and
mixtures thereof with the abovementioned oxides may also be used.
Examples of further catalysts are:
vanadium oxide, niobium oxide, iron oxide, chromium oxide,
molybdenum oxide, tungsten oxide and mixtures thereof. Mixtures
of the stated oxides with one another are also possible. Some
sulfides, selenides and tellurides, such as zinc telluride, tin
selenide, molybdenum sulfide, tungsten sulfide and sulfides of
nickel, of zinc and of chromium, may also be used.
The abovementioned compounds may be doped with compounds of main
groups 1 and 7 of the Periodic Table or may contain these
compounds.
Furthermore, zeolites, phosphates and heteropolyacids and acidic
and alkaline ion exchangers, for example Naphion~, are examples
of suitable catalysts.
If required, these catalysts may contain up to 50~ by weight in
each case of copper, tin, zinc, manganese, iron, cobalt, nickel,
ruthenium, palladium, platinum, silver or rhodium.
Depending on the composition of the catalyst, the catalysts may
be used as unsupported catalysts or supported catalysts. For
example, titanium dioxide may be used as titanium dioxide
extrudates or as titanium dioxide applied in a thin layer on a
carrier. All methods described in the literature may be used for
applying titanium dioxide to a carrier such as silica, alumina or
zirconium dioxide. Thus, a thin titanium dioxide layer can be
applied by hydrolysis of titanium organyls, such as titanium
isopropylate or titanium butylate, or by hydrolysis of TiCl4 or
CA 02237727 1998-06-O1
BASF Aktiengesellschaft 950921 O.Z. 0050/46488
16
other inorganic titanium-containing compounds. Sols containing
titanium dioxide may also be used.
Further suitable compounds are zirconyl chloride, aluminum
nitrate and cerium nitrate.
Suitable carriers are powders, extrudates or pellets of the
stated oxides themselves or of other stable oxides, such as
silica. The carriers used may be rendered macroporous to improve
the mass transfer.
In a further preferred embodiment, 6-aminocapronitrile is
cyclized in the liquid phase with water at elevated temperatures
without a catalyst by heating an aqueous solution of
6-aminocapronitrile in the liquid phase without the addition of a
catalyst in a reactor to give a mixture I consisting essentially
of water, caprolactam and a high-boiling fraction (high boiler).
In this preferred embodiment, an excess of water is preferably
used, particularly preferably from 10 to 150, in particular from
20 to 100, mol of water per mol of 6-aminocapronitrile, to give
an aqueous solution of 6-aminocapronitrile. In a further
preferred embodiment, from 5 to 25 mol of water are usually used
per mol of 6-aminocapronitrile, and the solution can generally be
further diluted to 5-25~ by weight of 6-aminocapronitrile by
adding an organic solvent.
Examples of suitable solvents are:
C1-C4-alkanols, such as methanol, ethanol, n-propanol, isopropanol
and butanols, glycols, such as ethylene glycol, diethylene
glycol, triethylene glycol, tetraethylene glycol, ethers, such as
methyl tert-butyl ether and diethylene glycol diethyl ether,
C6-Clo-alkanes, such as n-hexane, n-heptane, n-octane, n-nonane
and n-decane, and cyclohexane, benzene, toluene, xylene, lactams,
such as pyrrolidone and caprolactam, and N-C1-C4-alkyllactams,
such as N-methylpyrrolidone, N-methylcaprolactam and
N-ethylcaprolactam.
In a further embodiment, from 0 to 5, preferably from 0.1 to 2,
by weight of ammonia, hydrogen or nitrogen may be added to the
reaction mixture.
The reaction is preferably carried out at from 200 to 370°C,
preferably from 220 to 350°C, particularly preferably from 240 to
320°C.
CA 02237727 1998-06-O1
BASF Aktiengesellschaft 950921 O.Z. 0050/46488
17
The reaction is usually carried out under superatmospheric
pressure, the pressure chosen being as a rule from 0.1 to 50,
preferably from 5 to 25, MPa, so that the reaction mixture is
preferably in the liquid phase.
The duration of the reaction depends essentially on the chosen
process parameters and, in the continuous process, is generally
from 20 to 180, preferably from 20 to 90, minutes. As a rule,
lower conversion is obtained in a shorter reaction time, and
observations to date have shown that troublesome oligomers form
during longer reaction times.
The cyclization is preferably carried out continuously,
preferably in a tube reactor, in stirred kettles or in
combinations thereof.
The cyclization may also be carried out batchwise. The reaction
time is then usually from 30 to 180 minutes.
The discharge is, as a rule, a mixture consisting essentially of
from 50 to 98, preferably from 80 to 95, ~ by weight of water and
from 2 to 50, preferably from 5 to 20, ~ by weight of a mixture
consisting essentially of from 50 to 90, preferably from 65 to
85, $ by weight of caprolactam and from 10 to 50, preferably from
15 to 35, ~ by weight of a high-boiling fraction (high boilers).
In a further embodiment, after the partial hydrogenation and
after removal of ammonia.and inert compound or compounds A
(bottom product of column 3), any abraded catalyst material
present and high boilers are removed by evaporation or
distillation, by obtaining the undesired substances as bottom
product and feeding the top product to the column R4.
In a further embodiment, adiponitrile and high boilers are taken
off via the bottom of column R5 and fed to stage (a). In this
case, it is also possible to remove a stream from the bottom of
column K5.
It is also possible to purify ADN from the side stream of column
K5 in a further column.
The hexamethylenediamine obtained according to the invention can
be further purified by conventional methods and used for the
preparation of polymers and copolymers, such as polyamide 66.
CA 02237727 1998-06-O1
BASF Aktieagesellschaft 950921 O.Z. 0050/46488
18
According to the invention, a part of the process for the
preparation of caprolactam from adiponitrile may also be used for
the simultaneous separation of 6-aminocapronitrile and
hexamethylenediamine by distillation of a mixture essentially
containing these compounds, wherein
(a) a mixture containing essentially 6-aminocapronitrile,
hexamethylenediamine, ammonia, adiponitrile and
hexamethyleneimine is subjected to a distillation to give
ammonia as the top product and a bottom product I, the
distillation being carried out at a bottom temperature of
from 60 to 250°C and at from 5 to 30 bar in the presence of a
compound which is inert under the distillation conditions, or
a mixture of inert compounds A, which boils at from 60 to
250°C at 18 bar, and the ammonia not being completely
separated off, and
(b) the bottom product I, containing essentially
6-aminocapronitrile, hexamethylenediamine, adiponitrile,
hexamethyleneimine, inert compound or compounds A and
ammonia, the ammonia content being lower than that of the
mixture used in stage (a), is subjected to a second
distillation to give a mixture of the inert compound A and
ammonia as the top product and a bottom product II, the
distillation being carried out at a bottom temperature of
from 100 to 250°C and a pressure of from 2 to 15 bar, with
the proviso that the pressures of the first and of the second
column are matched with one another so that, at a bottom
temperature of not more than 220°C in each case, a top
temperature of more than 20°C is obtained, or,
the condensation at the top of the second column is carried
out at lower temperatures, the top product, which consists of
pure or relatively highly concentrated ammonia, being
recycled to the first column, or
the top product of the second column is recycled to the first
column or to the condenser thereof in vapor form after the
pressure has been increased by means of a compressor,
(c) the bottom product II, containing essentially
6-aminocapronitrile, hexamethylenediamine, adiponitrile,
hexamethyleneimine and inert compound or compounds A, is
subjected to a distillation in a third column to give the
inert compound or compounds A as the top product and a bottom
product III, the distillation being carried out at a bottom
temperature of from 50 to 250°C and a pressure of from 0.05
CA 02237727 1998-06-O1
BASF Aktiengesellschaft 950921 O.Z. 0050/46488
____ _ _
19
to 2 bar, with the proviso that the inert compound or
compounds A obtained as the top product are fed to the second
column, and, if desired, the distillation being carried out
in the presence of one or more compounds B which are inert
under the distillation conditions and boil at from 20 to
250°C at 0.3 bar,
(d) the bottom product III, containing essentially
6-aminocapronitrile, hexamethylenediamine, adiponitrile,
hexamethyleneimine and, if desired, inert compound or
compounds B, is subjected to distillation in a fourth column
to give a top product KP1, containing essentially
hexamethyleneimine, if desired inert compound or compounds B,
and a side stream SA1, containing essentially
hexamethylenediamine, the bottom temperature of the column
being from 50 to 250°C and the pressure from 0.05 to 1.5 bar,
and to give a bottom product IV, and, if desired, the column
is equipped with a dividing wall in the region between feed
and side take-off point (Petlyuk column), so that the
hexamethylenediamine obtained is essentially free of
hexamethyleneimine and inert compound or compounds B and of
other low boilers,
top product KP1 being fed to the third column or, if
required, only some of top product KP1 being fed to the third
column and the remainder being removed, and
(e) the bottom product IV, containing essentially
6-aminocapronitrile and adiponitrile and possibly high
boilers, is subjected to a distillation in a fifth column to
give 6-aminocapronitrile having a purity of at least 95$ as
the top product and a side stream consisting essentially of
adiponitrile and a bottom product V which consists of high
boilers and small amounts of adiponitrile, and,
if desired, the column is equipped with a dividing wall in
the region between feed and side take-off point, so that the
adiponitrile obtained contains relatively small amounts of
high boilers,
the distillation being carried out at a bottom temperature of
from 50 to 250°C and at from 10 to 300 mbar.
The novel process has the advantage that it provides a
continuous process for obtaining caprolactam starting from
adiponitrile, hexamethylenediamine simultaneously being
obtained.
CA 02237727 1998-06-O1
'' BASF.Aktiengesellschaft 950921 O.Z. 0050/46488
Examples
(a) Hydrogenation of adiponitrile to 6-aminocapronitrile
5 ' A tube reactor having a length of 2 m and an internal
diameter of 2.5 cm was filled with 750 ml of (1534 g) of
catalyst consisting of 90~ by weight of CoO, 5~ by weight of
Mn2o3, 3% by weight of Pa05 and 2~ by weight of NazO, and the
catalyst was then activated in the course of 48 hours in a
10 stream of hydrogen (500 1/h) by increasing the temperature
from 30°C to 280°C under atmospheric pressure. After the
temperature had been reduced to 45°C (inlet) or 85°C
(outlet), a mixture of 380 g/h of adiponitrile, 380 g/h of
ammonia and 500 1/h of hydrogen was fed to the reactor at
15 200 bar. In addition, four times the feed quantity (about
3 kg/h) was circulated for removal of heat. 70~ of
adiponitrile were converted under these conditions. The
reaction mixture consisted of 50~ by weight of ammonia, 15$
by weight of ADN, 17.5$ by weight of aminocapronitrile (ACN),
20 17.4 by weight of HMD and 0.1$ by weight of other substances
(ACN selectivity: 50$, ACN+HMD selectivity: > 99$).
(b) Working up the discharged hydrogenation mixture by
distillation
The discharged hydrogenation mixture from (a) was freed from
catalyst and fed to the top of a first column having two
theoretical plates. 5.0 kg of ammonia were separated off with
200 ppm of ethanol via the top at 47°C and 19 bar and used
for the hydrogenation (stage (a)).
The bottom product of the first column, a reaction mixture
containing ethanol and small amounts of ammonia, was fed at a
bottom temperature of 180°C to a second column having 10
theoretical plates.
1.22 kg of a mixture of 30~ by weight of ammonia and 70$ by
weight of ethanol and traces of hexamethyleneimine (HMI),
hexamethylenediamine (HMD), ACN and ADN were recycled via the
top of this column, at 50°C and 10 bar, to the first column.
The bottom product of the second column, which contained 39~
of ethanol and 100 ppm of ammonia and was at 177°C, was fed
to a third column having 10 theoretical plates. 3.2 kg of
ethanol were taken off via the top of this column at
47°C/300 mbar and recycled to the second column.
CA 02237727 1998-06-O1
BASF Aktiengesellschaft 950921 O.Z. 0050/46488
.. _..____ _ __. __ _ __
21
5.0 kg of product were fed from the bottom of the third
column, which was at 166°C, to a fourth column having 35
theoretical plates. 5 g of HMI containing 19$ of HMD were
taken off via the top of this column at 67°C/90 mbar. 1.74 kg
of HMD containing 155 ppm of HMI and 100 ppm of ACN were
taken off as a liquid side stream from the 30th theoretical
plate of this column and removed from the process.
3.25 kg of product, consisting essentially of ACN and ADN,
were removed from the bottom of the fourth column, which was
at 181°C, and fed to a fifth column having 20 theoretical
plates. 1.75 kg of ACN containing 185 ppm of HMD and 100 ppm
of ADN were obtained via the top of this column at 20 mbar
and 120°C.
20
1.39 kg of ADN containing 800 ppm of ACN were taken off as a
vapor side stream in the lower part of the fifth column.
0.11 kg of ADN containing about 10$ by weight of high boilers
was taken off at 190°C via the bottom.
(c) Cyclization of 6-aminocapronitrile to caprolactam
A solution of 2 kg of ACN (from (b)), 0.64 kg of water and
17.4 kg of ethanol was passed, at 220°C and 70 bar and with a
residence time of 15 minutes, through an oil-heated tube
reactor having a length/diameter ratio of I00 and containing
4 mm titanium dioxide extrudates. The discharged reaction
mixture contained 1.7 kg of caprolactam, 0.06 kg of ethyl
6-aminocaproate, 0.1 kg of 6-aminocapronitrile (determined by
gas chromatography) and 0.1 kg of 6-aminocaproic acid and
oligomers and polymers of caprolactam (determined by means of
HPLC). 1.6 kg of caprolactam were obtained therefrom by
fractional distillation.
40
CA 02237727 1998-06-O1