Note: Descriptions are shown in the official language in which they were submitted.
CA 022378~6 1998-0~-13
WO 97/18285 PCT/US96/l~iS88
IMP~OVED COMPOSITIONS CONTAINING ORGANIC COMPOUNDS
The present invention relates to improvements in cleaning compositions. More particularly,
the present invention is directed to improved pine oil type cleaning compositions and concentrates
S thereof, which find particular use in hard surface cleaning applications.
One particular category of cleaning compositions are those which are classed as pine oil type
cleaning compositions, such typically contain one or more resins or oils derived from coniferous
species oftrees, and typically generate a milky or cloudy appea~ance when diluted with water in
dilutions useful for cleaning applications. Such pine oil type cleaning compositions are generally
provided in a concentrated composition which is subsequently diluted with water by an end
user/consumer to forrn a cleaning composition therefrom. However, pine oils also are known to leave
undesirable surface residues, particularly on hard surfaces and are further known irritants particularly
to the eyes, skin and mucocus tissues. Also, pine oil, while known to have cleaning efficacy is not
generally considered useful as a broad spectrum antib~ct~rial or c~nti7ing agent.
It is among the objects of the present invention to provide improved pine oil type cleaning
compositions in a "concentrate" form which exhibit one or more of the identifying chal a~ " ;stics
outlined above, especially a pronounced blooming effect, and an effective sanitizing effect and a long
lasting scent while having reduced amounts of pine oiJ in such compositions. According to a further
aspect of the invention there are provided "cleaning compositions", i.e., aqueous dilutions of these
improved pine oil type cleaning compositions which exhibit good blooming upon being produced,
and which provide an effective cleaning and/or s~niti7ing benefit to hard surfaces.
These and other objects of the invention will become appale~l from the following detailed
description of the invention.
The compositions according to the invention comprise the following constituents:A) a pine oil ~ clJ~lalion cont~ining at least about 60% alpha-terpineol;
B3 a co-solvent;
C) a non-ionic surfactant system which desirably includes two or more non-ionic
~ul r~ wherein at least one of which exhibits a cloud point of 20~~ or less in water;
D) at least one cationic quaternary ammonium surfactant which exhibits germicidal
activity;
E) fragrance/fragrance enh~ncer; and,
F) water.
~n addition to the above described conctituentc, the compositions according to the invention
may optionally further include known art additives in conventional amounts.
CA 022378~6 1998-0~-13
WO 97/18285 PCT/US96/15588
- 2 --
The inventors have found that it is now possible to produce certain concentrate compositions
utilizing these selected constituents in particular formulations which provide pine oil type cleaning
compositions in a concentrated liquid form which unlike many known prior art composition further
include a germicidal effect, good blooming and a high concentration of fragrance and/or fragrance
solubilizer constituents. Surprisingly however, these inventive compositions still exhibit many of the
desirable characteristics of pine oil type cleaning compositions described above, especially
"blooming", notwithstanding the relatively high levels of fragrance and/or fragrance solubilizer
constituents which they comprise. This is an important and surprising feature of the invention as the
use of relatively higher amounts of fragrance and/or fragrance solubilizer cnnc1i~nts, which are
known to be organic constituents, would be expected to significantly diminish or deactivate the
desirable "blooming" effect when such concentrates are further diluted with water. The ~'blooming"
observed may be described as the change of the water's appearance from essentially colorless and
~,allsl,a,e"L to that of a milky white or milky yellowish white, cloudy apl.e~d"ce. That such
behaviour is achieved in the compositions according to the present invention, which contain
relatively high amounts of fragrance and/or fragrance solubilizer constituents, is surprising to say the
least.
Constituent A) Compositions according to the invention comprise a pine oil conctihltent
Pine oil is an organic solvent, and is a complex blend of oils, alcohols, acids, esters, aldehydes and
other organic compounds. These include terpenes which include a large number of related alcohols
or ketones. Some important constituents include terpineol, which is one of three isomeric alcohols
having the basic molecular formula C ~ oH 1 70H. Useful pine oils include synthetic pine oil, and also
include steam distilled and sulfate pine oils, and will generally contain a higher content of turpentine
alcohols. Other important compounds include alpha- and beta-pinene (turpentine~, abietic acid
(rosin), and other isoprene derivatives.
Particularly effective pine oils which are presently co~ ;ially available include Glidco
Pine OilTM 60 (believed to contain approximately 60% terpene alcohols), Glidco(~ Pine Oil 80
(believed to contain approximately 80% terpene alcohols) Glidco(~) Pine Oil 150 (believed to contain
lo~i,..ately 85% terpene alcohols); Glidco(~) Terpene SW (believed to contain approximately 75%
terpene alcohols); as well as Glidcog~ Terpineol 350 (believed to contain ap~ xi-,-ately 100%
terpene alcohols). Each of these may be obtained from available from Glidco Organics Corp.,
Jacksonville, FL (USA). Other products which can contain up to J 00% pure alpha-terpineoll may
also be used in the present invention.
The pine oil constituent may be present in the concentrate compostions in amounts of from
about 0.00~ % by weight to up to about 15% by weight, preferably about 4 - 12 % by ~eight, most
,
CA 022378~6 1998-0~-13
WO 97tl828S PCT/US96/15588
preferably in amount of between 6 - 10 % pine oil by weight. Preferred of these are pine oil
pa~lions which comprise at least about 60% terpene alcohols, and more preferably those which
comprise at least about 80% terpene alcohols. As with all of the weight percentages of the
constituents described, the weight percentages are indicative of tlle weight percentages of the actives
in a constituent containing p.c?ala~ion.
Constituent B) A further constituent according to the invention is a co-solvent which is
present in addition to the pine oil which is itself known to be an organic solvent and assists in
improves the dispersability and/or miscibility of the water insoluble pine oil in water. The co-solvent
may also improve the miscibility of further constituents according to the present invention, including
any water insoluble or poorly soluble constituents. Many useful co-solvents which are known to be
useful in dispersing pine oil in water may be used as Constituent B, especially those based on are
based on organic solvents; virutally any may be used as long as it does not undesirably disrupt the
favorable characteristics of the invention, especially the blooming characteristic. Mixtures of two or
more co-solvents may also be used as Constituent B.
Exemplary co-solvents useful as Constituent B include certain glycols and glycol ethers
which exhibit the above described properties. Examples of such glycol ethers include those having
the general structure Rg-O-RIo-OH, wherein Rg is an alkoxy of I to 20 carbon atoms, or aryloxy of
at least 6 carbon atoms, and Rl o is an ether condensate of propylene glycol and/or ethylene glycol
having from one to ten glycol monomer units. Examples of such useful glycol ethers include
propylene glycol methyl ether, dipropylene glycol methyl ether, tripropylene glycol methyl ether,
propylene glycol isobutyl ether, ethylene glycol methyl ether, ethylene glycol ethyl ether, ethylene
glycol butyl ether, diethylene glycol phenyl ether, propylene glycol phenol ether, and mixtures
thereof. Preferred are ethylene glycol n-butyl ether, diethylene glycol n-butyl ether, and mixtures
thereof . Such glycol ethers are presently commercially available from a number of sources
including in the DOWANOLTM glycol ether from The Dow Chemical Company, Midland MI (USA).
~urther exemplary co-solvents useful as Constituent B include Cl-Cg alcohols, especially
Cl-C3 alcohols, of which isop.~panol is preferred.
It has generally been found the addition of only a minimum effective amount which is found
to be effective in dispersing or solubilizing the pine oil constituent and any other aqueous insoluble
or poorly soluble constituents in the concentrate compostions is desirably usedl. Such is due to desire
to reduce the amount of volatile organic constituents in the concentrate compositions of the
invention, which volatile organic constituents are desirably minimi7Pd from an environmental
standpoint. The present inventors have found that inclusion of the solubilizing agent according to
Constituent B in amounts of about 0.001% by weight to about 15 % by weight have been found to be
CA 022378~6 1998-05-13
W O 97/18285 PCTAUS96/15588
effective to solubili~e the pine oil, as well as in solubilizing other less water soluble constituents
present in the concentrate compositions of the invention. Preferably, the solubilizing agent of
Constituent B is present in amounts 4 - 12% by weight, and most preferably 8 - 10% by weight .
Constituent C) The concentrate compositions according to the invention include a nonionic
surfactant system which comprises a mixture of two or more nonionic surfactants which includes a
first nonionic ~u- ra~ -l constituent which is a single or is a mixture of noninonic surfactants which
exhibit a cloud point of 20~C or less in water, and a second nonionic surfactant c~-nctit7~ent which
includes a single nonionic surfactant or mixture of surfactants which are useful in solubilizing the
first nonionic surfactant constituent in water. The first said nonionic surfactant constituent is
generally selected to be one or more aqueous insoluble or poorly soluble nonionic, which optionally,
but further very desirably exhibit a cloud point of 20~C or less in water. The second nonionic to
solubilize the first nonionic surfactant. Such a solubilizing effect aids in the long term shelf stability
of prepared concentrated compositions, and in ensuring the optical clarity of concentrated
compositions especially during the shelf life of prepared concentrated compositions.
Generally, suitable nonionic surface active agents which may be used in the nonionic
surfactant system according to Constituent C includes condensation products of one or more alkylene
oxide groups with an organic hydrophobic compound, such as an aliphatic or alkyl aromatic
compound. Exemplary suitable nonionic surface active agents include surfactant compositions
based upon polyethoxylated, polypropxylated, or polyglycerolated alcohols, alkylphenols or fatty
acids.
One exemplary class of nonionic surfactants useful in Constituent C according to the instant
invention include certain alkoxylated linear aliphatic alcohol surfactants which are believed to be the
condP~c~tion products of a C8-CIo hydrophilic moiety with polyethylene oxide/polypropylene oxide
moieties. Such alkoxylated linear alcohol surfactants are presently commercially available under the
trsdename Poly-Tergent~) (Olin Chemical Co., Stamford CT) and of these particularly useful are
those which are marketed as Poly-Tergent~) SL-22, Poly-Tergent~ S~42, Poly-Tergentg) SL-62 and
Poly-Tergent/g) SL-29, of which Poly-Tergent~ SL-62 is particularly advantageous. Poly-Te.~en
S~42 is described as being a moderately foaming, biodegradable alkoxylated linear alcohol
surfactant having on average 5 moles of oxyethylene groups per molecule. Poly-Tergent &) SL-62 is
described as being a moderately foaming, biodegradable alkoxylated linear alcohol surfactant having
on average 8 moles of oxyethylene groups per molecule. These alkoxylated linear alcohol surfactsnts
provide good detersive action in the removal of many types of fats and greases such as are frequently
found in soils on hard surfaces, as well as providing a further solubilizing effects
CA 022378~6 1998-0~-13
WO 97/I 8285 PCT/~596~15~i88
A further exemplary class of nonionic surfactants which finds use are alkoxylated alcohols
especially alkoxylated fatty alcohols. These include ethoxylated and propoxylated fatty alcohols, as
well as ethoxylated and propoxylated alkyl phenols, having both with alkyl chains of about 7-16,
more preferably about 8-13 carbon chains in length. Exemplary alkoxylated alcohols include certain
ethoxylated alcohol compositions presently commercially available from the Shell Chemical
Company, lHouston, TX) under the general trade name Neodol~, which are described to be linear
alcohol ethoxylates. Of these, those exhibiting a cloud point of 20~C or less may be used. Specific
compositions include: Neodol~) 91-2.5 which is described as an ethoxylated alcohol having an
average molar ratio of 2.7:1 ethoxy groups/alcohol groups per molecule; a molecular weight of 281,
and a cloud point in water of 20~C and less; Neodol(~ 23-3 which is described as an ethoxylated
alcohol having an average molar ratio of 2.9:1 1 ethoxy groups/alcohol groups per molecule; a
molecular weight of 322, and a cloud point in water of 20~C and less.
Exemplary alkoxylated alcohols further include compositions commercially available from
the Union Carbide Co., (Danbury, CT) under the general trade name Tergitol~), which are described
to be secondary alcohol ethoxylates. Again, those exhibiting a cloud point of 20~C and less may be
used. Specific compositions include: Tergitol~ 1 5-S-3 described as an ethoxylated secondary
alcohol having an average molar ratio of 3.2:1 ethoxy groups/alcohol groups per molecule, and a
cloud point in water of less than 20~C; Tergitol~ I S-S-S described as an ethoxylated secondary
alcohol having an average molar ratio of 5:1 ethoxy groups/alcohol groups per molecule, and a cloud
point in water of less than 20~C.
Further exemplary nonionic surfactants which may be used in Constituent C include certain
alkanolamides including monoethanolamides and diethanolamides, particularly fatty
monoalkanolamides and fatty dialkanolamides. Commercially available monoethanol amides and
diethanol amides include those marketed under the trade names Alakamide~ and Cyclomide(~ by
2~ Rhône-Poulenc Co., (Cranbury, NJ). These include surfactants based on coconut diethanolamides;
coconut monoethanol~mides, 2:1 modified coconut monoethanolamide; fatty acid diethanolamides;
lauric/linoleic diethanol~mides; linoleic diethanol~mid~s, lauric monoethanolamides; lauric
diethanolamides; lauric/myristic diethanolamides; oleic diethanolamide; stearic diethanol~mi~es;
coconut diethanolamides; lauric monoisopropanolamides; stearic monoethanolamides;
diethanolamides of ulls~ulated fatty acids; alkanolamides which may be used singly, or in mixtures.
Particularly useful are lineolic diethanolamides and lauric diethanolamides.
l~xemplary alkoxylated alkyl phenols useful in Constituent C include certain compositions
presently commercially available from the Rhône-Poulenc Co., ~Cranbury, NJ) under the general
trade name Igepal~, which are described to be octyl and nonyl phenols. Again, those exhibiting a
CA 022378~6 1998-0~-13
WO 97118285 PCT/US96/15588
eloud point of 20~C or less may be used. These include Igepal(g) CA-2 10 an ethoxylated octyl phenol
having an average of 1.5 ethoxy groups groups per molecule and a cloud point in water of less than
20~C and, Igepal(~) CA-420 an ethoxylated octyl phenol having an average of 3 ethoxy groups groups
per molecule and a eloud point in water of less than 20~C.
Especially preferred for use as the first nonionic surfactant constituent whieh Cu~ ises
Constituent C aeeording to the instant invention is Neodol~) 91-2.5 whieh is described as an
ethoxylated alcohol having an average molar ratio of 2.7:1 ethoxy groups/alcohol groups per
molecule, a molecular weight of 281, and a cloud point in water of 20~C and less.
Of course, a mixture of two or more nonionic surfactants having a cloud point of 20~C or less
may be ineorporated into the inventive compositions. Other known nonionic surface active agents
not particularly enumerated here may also be used.
The cloud point of the first nonionic surfacant constituent aeeording to Constituent C of the
present invention may be determined by known methods, such as by ASTM D2024 (reapproved
1986) for "Standard Test method for Cloud Point of Nonionic Surfaetants". An even simpler test
1~ method for effectively determining which nonionic surfactants may be used as the first nonionic
surfactant conctituent in the eompositions of the invention is as follows: to a elean beaker or other
glass vessel is added 99 parts by weight of deionized water at 20~C +0.5~C, and I part by weight (by
weight of the aetives) of a surfaetant eomposition to be tested. This test sample is stirred and the
temperature permitted to drop to 20~C; if this test sample is observed to be murky or eloudy in
appea~ ce as the test sample's temperature achieves 20~C and drops below 20~C, it is considered to
have a suitable cloud point of 20~C and less and may be used as Constituent B in the concentrate
compositions aceording to the invention.
Constituent C, may be present in any effective amount, but desirably is present in the
eoneentrate eompositions from about 0.001 % by weight to about 25% by weight, preferably 0.1 -
20% by weight, and most preferably from 8% and 15% by weight. Desirably, partieularly where
Constituent C ineludes an alkanolamide, dialkanolamide or trialkanolamide, C~ nct;hlent C) is present
in a weight pe~ ge about equal to, or greater than the amount of Conctihlent E) present in the
eoneentrate eomposition.
Conctitnent D) The eoncentrate eompositions aeeording to the invention include as a
necesc~ry eonstituent at least one eationic 4u~L~IIlal y ammonium surfaetant which is found to
provide a broad antibacterial or s~niti7ing function. Such materials are per se, known to the art.
Exemplary useful and preferred eompounds are quarternary ammonium compounds and salts
thereof, which may be eharaeterized by the general struetural formula:
-
CA 022378~6 1998-0~-13
WO 97/18285 PCTJUS96J}5588
R2--N--R3 X~
R4
where at least one or Rl, R2, R3 and R4 is a hydrophobic, aliphatic, aryl aliphatic or aliphatic aryl
}adical of from 6 to 26 carbon atoms, and the entire cation portion of the molecule has a molecular
weight of at least 165. The hydrophobic radicals may be long-chain alkyl, long-chain alkoxy aryl,
long-chain alkyl aryl, halogen-substituted long-chain alkyl aryl, long-chain alkyl phenoxy alkyl, aryl
alkyl, etc. The rçm~inin~ radicals on the nitrogen atoms other than the hydrophobic radicals are
substituents of a hydrocarbon structure usually containing a total of no more than 12 carbon atoms.
The radicals Rl, R2, R3 and R4 may be straight chained or may be branched, but are preferably
straight chained, and may include one or more amide or ether linkages. The radical X may be any
salt-forming anionic radical.
Exemplary qua.l~;...a~y ammonium salts within the above description include the alkyl
ammonium halides such as cetyl trimethyl ammonium bromide, alkyl aryl ammonium halides such as
octadecy I dimethyl benzyl ammonium bromide, N-alkyl pyridinium halides such as N-cetyl
pyridinium bromide, and the like. Other suitable types of qua. 1~1 lld~ y ammonium salts include those
in which the molecule contains either amide or ether linkages such as octyl phenoxy ethoxy ethyl
dimethyl benzyl ammonium chloride, N-(laurylcocoaminoformylmethyl)-pyridinium chloride, and
the like. Other very effective types of quarternary ammonium compounds which are useful as
germicides include those in which the hydrophobic radical is characterized by a substituted aromatic
nucleus as in the case of lauryloxyphenyltrimethyl ammonium chloride, cetylaminophenyltrimethyl
ammonium methosulfate, dodecylphenyltrimethyl ammonium methosulfate, dodecylbenzyltrimethyl
ammonium chloride, chlorinated dodecylbenzyltrimethyl ammonium chloride, and the like.
Especially Preferred 4U~ laly ammonium cornpounds which act as germicides and which
are be found useful in the practice of the present invention include those which have the structural
formula:
CH3
R2--1~1--R3 X-
CH3
' 25
wherein R2 and R3 are the same or different Cg-C12alkyl, or R2 is C12 16alkyl, Cg Igalkylethoxy,
C8 1 galkylphenolethoxy and R3 is benzyl, and X is a halide, for example chloride, bromide or
CA 022378~6 1998-0~-13
WO 97/18285 PCT/US96/15588
iodide, or methosulfate. The alkyl groups recited in R2 and R3 may be straight chained or branched,
but are preferably subst~nti~lly linear.
Such quartenary germicides are commercially available either as single quaternary
ammonium compounds or as mixtures of two or more different quartenaries. Suitable materials
include germicidal quaternary ammonium compounds include those sold under the tradenames
BARDAC, BARQUAT and HYAMINE (Lonza Inc., Fairlawn NJ (USA)), as well as those
designated "BTC" (Stepan Co. Northfield IL (USA)).
The quaternary ammonium compound of Conctituent C) is desirably present in a minimum
amount to provide the desired germicidal and sanitizing effects, as the blooming effect of the
concentrate coll~posilions when added to a larger volume of water have been found to be hindered by
the inclusion of excessive amounts of the such quaternary ammonium compounds in the concentrate
compositions. Generally, Constituent C is present in the concentrate compostions in amounts of up
to 5 % by weight and less, preferably from 0.5 - 2 % by weight, and most preferably from 0.8 - 1.2 %
by weight. It has also been found by the inventors that the preferred amounts are in part dictated by
toxicological considerations as an excess of the cationic component may pose an increasing risk of
irritation to the eyes, skin and mucocous tissues of a consumer.
Constituent E) A further constituent of the concentrate compositions according to the
invention are fragrances and/or fragrance enhancers which provide a characteristic pine oil scent and
and scent longevity, particularly wherein the concentrate compositions are diluted to forrn cleaning
compositions therefrom. As is described in the specification under claims, the term "fragrance" is
used to refer to and to include any non-water soluble fragrance substance or mixture of such
substances including those which are naturally derived (i.e., obtained by extraction of flower, herb,
blossom or plant), those which are artificially derived or produced (i.e., mixture of natural oils and/or
oil constituents), and those which are synthetically produced substances (odiferous substances).
Generally fragrances are complex mixtures or blends various organic compounds inclu~inp, but not
limited to, certain alcohols, aldehydes, ethers, aromatic compounds and varying amounts of essenti~l
oils such as from about 0 to about 85% by weight, usually from about 10 to about 70% by weight, the
eccenti~l oils themselves being volatile odiferous compounds and also functioning to aid in the
dissolution of the other components of the perfume. In the present invention, the precise composition
31) of the perfu~ne is of no particular consequence to cleaning performance so long as it may be
effectively included as a constituent of the compositions. Generally however, one or more fragrances
characteristic of pine oil type compositions, such as natural or synthetically produced fragrance
compositions, especially those which are intended to mimic the scent of one or more resins or oils
derived from coniferous species of trees, viz., a scent characteristic of pine oil type cleaning
CA 022378~6 1998-0~-13
WO 97/18285 PCTJlJS9tif255~
concentrates are used. Fragrance effects atypical of pine oil type cleaning concentrates may be used
as well. Fragrance adjuvants for enhancing the scent effect of a fragrance, and/or for improving the
miscibility of such fragrance compositions include known art fragrance adjuvants, for example,
fenchol.
S In any type of composition, the pine oil scent is the cha~ dCL~,. islic scent which is emitted by
pine oil, and the longevity of such a pine oil scent is understood to be closely re~ated to the pine oil
content of a concentrate composition or a cleaning composition as described in this specification.
Thus, it is normally expected that an increase in the pine oil provides an increase in the characteristic
pine oil scent and in the scent longevity, however, for reasons noted earlier in this specification, the
inclusion of increased amounts of pine oil is not always desirable from other standpoints. To provide
a desired characteristic pine oil scent and pine oil scent longevity without re~uiring an increased
amount of pine oil, the present inventors have found that by careful selection of fragrances and/or
fragrance enhancers a reduction in the amount of pine oil may be achieved while m~int~ining a
characteristic pine oil scent and a scent longevity. At the same time, this has been achieved without
undesirably effect the blooming behavior of pine oil cleaning compositions taught herein. Also, a
further undesired characteristic is an expectation of increased irritancy especially to the eyes, skin
and mucocous tissues which an increase in an organic solvent such as a fragrance/fragrance
solubilizer which is known to emit a volatile fraction has been avoided.
Constituent Fl Water is added in order to provide 100% by weight of the ConcG~ a~
composition. The water may be tap water, but is preferably distilled and/or deionized water. Water
is added in amounts which are sufficient to form the concentrated compositions which amount is
sufficient to ensure the retention of a substantially clear characteristic when produced as a
concentrate, but at the same time ensuring good blooming upon the addition of the concentrated
composition to a further amount of water, or upon the addition of further water to the concentrate.
Generally, water is present in the concentrate compositions in amounts in excess of about 80% by
weight, preferably in amounts of in excess of 75% by weight, but most preferably in amount of
between 60 -70 % by weight based on the total weight of Constituents A - F in the concentrate
compositions taught herein.
Optional constituents:
Further optional, but advantageously included constituents are one or more coloring agents
which find use in modifying the appea- dnce of the concentrate co---po~ilions especially to impart to
concentrate compositions an appearance characteristic of a pine oil type concentrate composition.
However, other colors atypical of pine oil type cleaning concel~l~ ales may be used as well. Known
art light stabilizer constituents which act to retain the appea~ ance characteristics of the concentrate
CA 022378~6 1998-0~-13
WO 97/18285 PCT/US96/15588
- 10-
compositions over longer intervals of time may also be used. Further optional constitutents may also
be used which, by way of non-limiting example include p~ adjusters, pH buffering agents, foaming
agents, further surfactants including anionic, cationic, non-ionic, amphoteric and zwitterionic
surfactants, especially those useful in providing further detersive effects, and water softening agents.
These optional, i.e., non-ecsen~i~l constituents should be selected so to have little or no detrimental
effect upon the desirable characteristics of the present invention, namely the blooming behaviour,
cleaning efficacy, disinfectant activity, and low toxicity as provided by the inventive compositions,
an in most cases anionic surfactants which may deactivate the quaternary cationic surfactant are to be
avoided. Generally the total weight of such further conventional additives may comprise up to 10%
by weight ~ f a concentrated composition formulation.
The term "concentrate" as used in this specification is the pre-consumer dilution and the
composition of the cleaning composition which is the typically the form of the product prepared for
sale to the consumer or other end user. The terrn "cleaning cornpositions" are the water diluted
compositions which are expected to be prepared by the consumer or other end user by mixing a
measured amount of the "concentrate" with water in order to form an ap~ U~l iately diluted cleaning
composition which is suitable for use in cleaning applications, especially in the cleaning of hard
surfaces. Such may be easily prepared by diluting measured amounts of the concentrate compositions
in water by the consumer or other end user in certain weight ratios of concentrate:water, and
optionally, agitating the same to ensure even distribution of the concentrate in the water. As noted,
the concentrate may be used without dilution, i.e., in concentrate:water concentrations of 1:0, to
extremely dilute dilutions such as 1:10,000. Desirably, the concentrate is diluted in the range of 1:0.1
- 1:1000, preferably in the range of 1:1 - 1:500 but most preferably in the range of 1:10 - 1:100. It is
to be understood however that the "concentrate" composition without any further dilution and it may
be used "as is."
It is also to be understood, that the pc:l~;e~L~ges ofthe constituents have been generally
referred to in this specification as percent by weight or as parts by weight based on a measure of 100
% by weight, unless other~vise indicated.
Exam~le Formulations:
Preparation of Example Formulations:
Exemplary formulations as illustrated on Table I following according to the instant invention
were plepaled in accordance with the following general procedure.
Into a suitably sized vessel, the following constituents were added in the following sequence:
pine oil, co-solvent, nonionic surfactant system, fragrance/fragrance enhancer, cationic surfactants,
water and lastly, optional constituents. The order of mixing is not critical in order to achieve
CA 022378~6 1998-0~-13
WO 97/1828~; PC~J~tS96J~558Y
concentrate compositions exhibiting the desired results. All of the constituents were supplied at as
weight percentages, as room temperature, and mixing of the constituents was achieved by the use of a
magnetic stirrer. Mixing, which generally lasted from l minute to 15 minutes, was m~int~ined until
the particular exemplary formulation attained uniform color and uniform clarity. Each of the
S formulations exhibited the following physical characteristics: lla~ alclll appearance, light orange to
medium orange-brown color, and a noticeable pine oil odor. The exemplary formulations were
readily pourable, and retained well mixed characteristics (i.e., stable mixtures) upon st~n-1ing at room
temperature (about 68~F) for periods in excess of several weeks. The stability of the concentrate
formulations was also evaluated at by heating to 120~F determine if clouding or phase separation
occurred; none was observed.
Table 1: Fonnulations ,'
Constituent: E1 E~ E3 E4 E5 E6 E7 E8
PineOil 1 8 -- -- -- -- 8 8 8
Pine Oil 2 -- 8 5 5 6
Isopropanol 9.25 9 5 5 4 9.25 9.25 9.25
diethyiene gîycol n-butyl ~ -- 6 6 5 -- -- --
ether
Poly-Tergent~ SL-62 8 8 8 8 8.75 8 8 8
Neodol~ 91-2.5 5 5 4 4 1.75 5 5 5
alkyldiethanolamide 1.4 1.2 1 1 1.18 1.5 1.8 D
BTC-8358 0.9 0.9 1 1 1 0.9 0.9 0.9 ~
BTC-818 05 05 05 05 05 05 05 05 ~,
Fragrance 1 1.2 -- -- -- -- -- 1.2 1.2
Fragrance ll -- 0.1 0.1 0.1 0.1 -- --
F,~y,~llce lll -- -- - -- -- 0.6 -- --
fenchol 0.1 0.1 0.1 0.1 0.1 0.1 0.1 0.1
coloring agent 0.0010.0008 0.0008 0.0008 0.0008 0.0008 0.0008 0.0008
Deionized water 65.65 67.2 69.29 70.29 71.8 66.47 65.55 65.25
Pine Oil 1 is a pine oil preparation c~"laining at least about 60% terpene alcohols
Pine Oil 2 is a pine oil p.~pa,dlioll col~ .g at least about 80% terpene alcohols
Poly-Tergent~ SL-62 is a nonionic alkoxylated linear alcohol su,fh~ "l
Neodol~) 91-25 is is a nonionic ~u,r~la"l cu,l,posilion based on linear alcohol ethoxylates featuring a cloud point < 20~C
BTC-8358 is an alkyl benyl dimethyl ammonium chloride (80% active) available from Stepan Chemical Co.
BTC-818 is a dialkyl dimethyl ammonium chloride (50% active) available from Stepan Chemical Co.
ul
CA 022378~6 1998-0~-13
WO 97/18~85 PCT/US96/1 s588
- 13 -
The deterrnination of the amount of a solubilizing agent, here isopropyl alcohol, required in
order to clarify the formulations of Table I provides a useful indication of the amount of required co-
solvents which are required in typical concentrate formulations. The weight ]percent of isopropyl
alcohol (100%) which was added to each of the formulations is also indicated on Table 1. It is to be
S noted that the values indicated on Table 1 are on a 100% total weight basis of the actual weight
p~,. cen~ges of the constituents added.
Preparation of Cleanin~ Compositions:
Cleaning testing was performed utilizing on~ or more of the exemplary compositions within
the scope of the invention as illustrated on Table I, and cleaning compositions prepared from Icnown
I0 commercially avai]able cleaning products, which are indicated as comparative examples.
ComParative Example "Cl":
A cleaning composition was formed by forming an aqueous dilution of one part by weight of
Lysol~ Pine-Action Cleaner, a commercially available cleaning concentrate with 64 parts by weight
of water at approximately 20~C and subsequently manually stirring the same to form a uniform
mixture.
ComParative Example "C2":
A cleaning composition was formed by forming an aqueous dilution of one part by weight of
Spic and Span~ Ultra Pine Deodorizing Cleaner, a commercially available cleaning concentrate with
128 parts by weight of water at room temperature (approx. 20~C) and subsequently manually stirring
the same to form a uniform mixture.
Com~arative Example "C3":
A cleaning composition was formed by mixing one part of a commercially available cleaning
formulation, PineSol(g) Cleaner, a pine oil type cleaning concentrate, with 64 ~parts of water at room
temperature, approximately 2~~C, and manually stirring the same to form a cleaning composition
thel ~rl u,.. .
Cleanin~ Evaluations:
Cleaning evaluations were also performed in accordance with the testing protocol outlined
accc,lding to ASTM D4488 A2 Test Method, which evaluated the efficacy of the cleaning
compositions on masonite wallboard samples painted with wall paint. The soil applied was a greasy
soil sample cont~ining vegetable oil, food shortening and animal fat. The sponge (water dampened)
of a Gardner Abrasion Tester alJpLal dlUS was squirted with a 15 gram sample of a tested cleaning
composition, and the ap~al~lus was cycled 10 times. The evaluation of cleaning compositions was
"paired" with one side of each of the test samples treated with a composition according to the
invention, and the other side of the same sample treated with a comparative e~ample's composition,
CA 022378~6 1998-0~-13
WO 97/18285 PCT/US96/15588
thus allowing a "side-by-side" comparison to be made. Each of these tests were duplicated on 20
wallboard tiles and the results statistically analyzed and the averaged results reported on Table 2,
below. The cleaning efficacy of the tested compositions was evaluated utili7ing a Minolta Chroma
Meter CF- I 10, with Data Processor DP- 100, which evaluated spectrophotomic characteristics of the
sample. The results are reported on Table 2 following.
Table 2
composition I comparative E1 I C1 E1 I C2 E1 i C3 E2 I C3
composition
reflectance reading: 69.04 1 63.68 1 70.931 65.63
composition I comparative 67.1g 62.22 69.82 65.14
composition
With respect to the results reported on Table 2 a value of "100" is indicative of a white
(unsoiled3 background, and a "0" value is indicative of a black background. A soil laden (uncleaned)
surface generally provided a result of about 20-30.
As can be seen from the results of l able 2, the cleaning efficacy of the composition
according to the invention provided favorable results with those of known art cleaning products.
I . Evaluation of Li~ht Transmittance ("Bloomin~"~ of Forrnulations:
Each of the formulations described on Table I was evaluated to determine the degree of light
transmittance, a measure of the opacity of each of these concentrated formulations. Also evaluated
~5 were comparative formulations designated as " Cl" and "C2" as described above.
These aqueous dilutions were prepared to evaluate the degree of light transmittance, a
measure of the opacity as well as of the blooming of each of the a~ueous dilutions. Certain of these
aqueous dilutions were also evaluated to determine the antimicrobal efficacy of the a~ueous dilution.
The results of the light transmittance evaluation was determined as a percentage of light transmitted
through a sample of a particular aqueous dilution wherein the tr~n~miccon of a like sample of water is
assigned a percentage of 100%. Testing was performed by mixing a 3 g aliquot of a particular
example formulation with 192 g of tap water (with approx. 100 ppm hardness) which formed a 1:64
dilution of the example formulation:water, after which the sample was mixed for 60 second and a
tranc~mitt~nce reading was taken using a Brinkman model PC801 dipping probe colorimeter, which
was set at 620 nm to determine the light transmission of each of the samples. Samples of each
formulation at 20~C and at 40~C were evaluated, as well as the reference (pure tap water) sample
used to calibrate the colorimeter to the reference 100% light transmission sample outlined above.
The resulting determined values, reported in Table 3 below provide an empirical evaluation, reported
in percent transmittance ("%T") of the degree of transparency of a diluted example formulation
wherein 0% indicates complete opacity and 100% the transparency of a water sample as noted above.
CA 02237856 1998-05-13
WO 97/18285 PCT/US96/155fl8
Accordingly, a lower %T of a particular aqueous dilution provided a more desirable indication of the
blooming characteristic of the particular aqueous dilution.
Table 3 - Percentage Light Transmittance (%T)
Light E1 F3 E4 F5 E7 E8 C2 C3
~ransmittance:
koT at 20~C 30-40 30-40 >80 >80 20-30 10-20 ~B0~80
/oT at 40~C 10-20 3040 -- -- 10-20 ~10 ~80>80
As may be seen from the results desc}ibed on Table 3, the formulations according to the invention
were comparable to, but in most cases better than the known art formulations le~ sellted by the
Comparative examples, C1 and C2.
2. Evaluation of Ei~ht Transmittance ("Bloomin~") of Formulations:
Further formulations according to the invention as well as further "c~lllpa~d~ e"
formulations were produced; their constituents are noted on Table 4 below. The individual
constituents were the same as used for the formulations of Table 1, but for the use of a different
coloring agent (1% ;ndicates weight actives) and a different fragrance.
Table 4
C4 C5 E9 E 10
Constituents
Pine Oil 80 .'~0 .00 .00 .0Q
Isopropanol ~.~5 .25 ~.25 ~.. 5
Poiytergen~ SL-62 .CI0 .00 .00 .~0
Neodol~ 91-2.5 .00 .00 .r 0 .00
~Ikyldiethanoiamide 0.00 0. 0 ._0 .. 00
enchol 0. 0 0. 0 0. 0 0. 0
ragr; ~:e 1 .r 0 1.' 0 1.' 0 1
BTC- ., 8 0. 0 0.~'0 0. 0 0."~
BTC- ~ ~ ~ ~ ~ ~ ~ ~
coloring agent (1 %) 0. 1 0.~ 1 0.~ 1 0.~ 1
Deionized Water 66. 34 66.44 65.44 64. 34
1~ In a similar manner to that described above, the blooming characteristics of the further
compositions according to the invention (designated as "E9" and "E10") as well as further
comparative examples (designated as "C4", "C5") were evaluated as to their blooming characteristics
by forming formed a 1:64 dilution of a respective example or compartive formulation in water, after
which the sample was mixed for 60 seconds and a transmittance reading was taken using a Brinkman
model PC801 dipping probe colorimeter set at 620 nm to determine the light trancmicc;on of each of
CA 022378~6 1998-0~-13
WO 97/18285 PCT/US96/15588
- 16 -
the samples. Samples of each formulation at 20~C and at 40~C were evaluated and the percentage
light transmittance is reporLed on Table 5 belo~s
Table 5 - Percentage Li~3ht Transmittance (%T)
C4 C~ E~ E 10
Bloom at 20~C 97 97 2 13
Bloom at40~C 86 92 1- 2
As may be seen from Table 5, the formulations according to C4 and C5 having low amounts
of diethanolamide and high levels of fragrance (1 .2%wt.) exhibited high percentages of light
transmitted, indicating very poor blooming charateristics. In sharp contrast, the formulations
according to E9 and E10 having the same level of fragrance, but including at least about an
equivalent amount of (in %wt.) of the diethanolamide as the fragrance featured surprisingly and
subst~nti~lly improved blooming characteristics as demonstrated by low high pc:lce~ ges of light
1 0 transmitted.
Evaluation of Scent Lon~evitY:
An evaluation of the scent longevity of formulations of Example formulation "~1" described
on Table I above, compared to a comparative formulation "C l " was performed.
Individual cleaning composition based on each of these formulations was produced by
1!5 mixing 4 grams of a respective formulation with 256 grams of water, which formed a lc~ sentative
1:64 dilution, typical of the dilutions used for such pine oil type products in domestic environments
Next, each of these cleaning compositions was poured onto a floor surface in separate rooms and
mopped with a sponge mop to cover approximately 40 square feet of floor surface with a cleaning
composition. Circulation fans were turned on for 5 minutes and then turned off. Within 1/2 hour of
mopping, two groups each consisting of 20 test panelists were asked to enter one of the rooms, stay
in the room for 45 seconds, and then exit the room. Subsequently, each panelist was asked to
evaluate the scent em~n~ted by the floor, and remain outside of both test rooms in order to allow for
their nasal passages to clear and become accustomed to the ambient. Arl~;l w~,ds, the members of the
group were asked to enter the other of the two rooms, again stay in the room for 45 seconds, exit the
2~ room, and evaluate the scent ern~n~ed by the floor in the room just visited.
After a time interval of 7 hours after mopping, and again after a 24 hour interval after
mopping, each of the members of the groups repeated the above protocol, and again evaluated the
scent em~n~ted by the two treated flooring surfaces at these two later time periods. In such a manner,
a me~nin~ful evaluation of the relative pine scent, especially after the longer time periods of 7 and 24
hours after application could be obtained, and compared to a presently commerically available pine
oil type cleaning composition.
CA 022378~6 1998-0~-13
WO 97/18285 PCT/US96~5~;88
Table 6 - Scent Duration Cha~a~ ristics
Cleaning Strength o~ pine Strength ~f pine Strength of pine
Composition: scent: scent: scent:
1 part 1/2 hour after 7 hours after 24 hours aFter
formulation: application application application
64 parts water
C1 44% 16% 33%
E1 56% 84% 67%
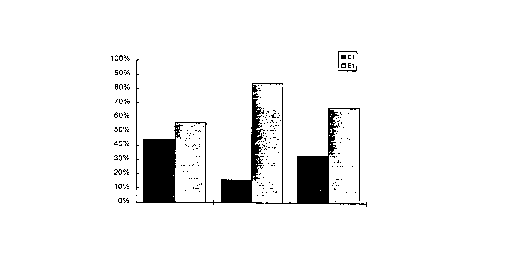
The numbers indicated on Table 6, as well as graphically illustrated on Figure 1, the total
percentages of the group who indicated preferance for one formulation's scent over that of the other.
As can be seen, while the results following the first one-half hour following application are not
dissimilar, the strength of the pine oil scent left by the formulations according to the invention as
exemplified by E1 are significantly stronger, and exhibit a greater duration than that of the prior art
formulation, Cl.
Evaluation of Ocular Irritation:
The occular irritation characteristics of formulations according to the invention were
evaluated using the known Draize ~ye test protocol. Evaluation was performed on a formulation
according to Example E7 of Table 1 above in an "as is" composition, namely without any further
dilution. As known, the Draize Eye Test measures eye irritation for the grading of severity of ocular
lesions, with separate scores obtained for the cornea, iris and conjunctiva. For the cornea, after
exposure to the composition, A the cornea opacity is grated on a scale from 1-4; B the area of cornea
involved is graded on a scale from 1-4 ~where the score = A x B x S may be a total maximum of 80).
For evaluation of the iris, after exposure the composition, A the involvement of the iris is graded on a
scale of 1-2 ~where the score = A x 5 may be a total maximum of 10). For a evaluation of the
conjunctive, A Redness is graded on a scale of 1-3; B Chemosis is graded on a scale of 1-4; and C
Discharge is measured on a scale of 1-3 [where the score = (A + B + C) x 2 may be a maximum of
20]. The maximum total score is the sum of all scores obtained for the cornea, iris and conjunctive ~a
maximum of 110~.
The Draize test score on day I of the test was 19.33, and it was further observed that all signs
of opacity cleared in all of the 6 subjects by day 7 of the test, although conjunctival irritation was
observed in 1 of the 6 test subjects by day 7. The Draize score on day 7 of the test was 0.33. By day
14, all signs of any irritation, opacity or conjunctival irritation was observed to have cleared. The
results ofthe Draize test indicated than an EPA Tox Category "0" was ap~"u~.;ate. That these results
were achieved with a product Icnown to have a significant content of constituents which individually
considered are known irritants was particularly surprising.