Note: Descriptions are shown in the official language in which they were submitted.
CA 02237912 1998-05-14
W O 97/19667 PCT~US96/18777
~ s~q~T ~ TUBE PI~(~
R~CK(~l~OUND A~D ~U~MA~Y OF T~I13 INV~TION
In many patients, gastrointestinal feeding is the preferred route
of nutrient delivery with either the stQm~h or the small intestine being
the areas of major importance. Proper positioning of the feeding end of
an enteral feeding tube in the desired area of the gastrointestinal tract
has always been a problem. Even after proper posit.ioning of the feeding
end of a feeding tube in either the stomach or the small intestine, it is
possible that the feeding end of the tube may unknowingly migrate from
the selected area, whereupon the patient may be subjected to a risky
feeding sitll~tiorl
A common method of initially positioning and then monitoring
the position of the feeding end of such a gastrointestinal feeding tube has
been to use an x-ray. To repeatedly verify proper pl~cement in this
mAnner is not only cumbersome, time consuming, and expensive, but it
also subjects the patient to llnnecessary x-ray exposure.
Post-pyloric feeding is often desirable in critically ill patients.
Some studies have shown that only about 5 to 15% of feeding tubes pass
spontaneously into the small bowel in critically ill p~ti~nt~. Post-pyloric
feeding tube placement is difficult, frequently requiring time consuming
ao blind attempts, transport to radiology for fluoroscopic guidance or a
bedside endoscopic procedure. Proper placement and verification of a
feeding tube can take an hour or more depending on all of the
;umstances involved.
Erythromycin is a motilin analog which promotes gastric motility
by stimulating the gastric migrating motor comple~. Use of
erythromycin has been demonstrated to facilitate spontaneous
post-pyloric passage in patients.
Recording of an electromyogram (EMG) from the wall of the
gastrointestinal tract allows differentiation between gastric and small
CA 02237912 1998-05-14
W O 97/19667 PCT~US96/18777
bowel location. The results of an EMG recorded from the stomach
compared to the results of an EMG recorded from the duodenum will
show a sharp contrast. For e~mrle, signals originating from the
stom~rh will have a flomin~nt. frequency of a~o~ tely 3 cpm (cycles
per minute) whereas si~n~l~ ori~in~ting from the duo~çnnm will have
a rln~ninsmt frequency of about 11 or 12 cpm.
The present il~v~ tion takes advantage of the contrast between the
electrical ~ign~l~ that can be detected from the stomach and the sign~l~
that can be detected from the small bowel. The present invention
Co",~l ises a feeding tube having at least one electrode secured on an
end of the feeding tube. By detecting signals received from the
electrode(s) on the end of the feeding tube, a physician can know the
location of the feeding tube without resorting to x-rays or other
cumbersome procedures.
Erythromycin may be ~mini~tered to enhance gastric motor
activity during insertion of the feeding tube. With the electrode~s)
placed at the distal tip of the feeding tube, the feeding tube is first guided
into the stomach. The si~n~l.q obtained from the stom~h are generally
about 3 cpm in frequency and have relatively large amplitude. As the
ao distal tip of the feeding tube passes into the small bowel, the resident
Rign~ls increase generally to about 10 to 13 cpm in frequency at a much
lower amplitude.
Frequency and amplitude of the si~n~l~ can be monitored in
sllhst~ntiP.lly real time at the bedside of the patient using, ~or ~ mrle~ a
2~i computer monitor or a printer to show the gr~phic~l representation of
the frequency and/or amplitude of the ~ign~
The present invention offers several advantages. First, the
present invention allows for substantially real time feedback of the
location of the feeding tube tip as it is being guided into the patient's
body. Second, the present invention minimi7.es radiation exposure,
since x-rays are no longer needed or can be minimi7:ed by use of the
present invention. A third advantage of the present invention is that the
elapsed time from the be~nning of the insertion of the feeding tube to the
CA 02237912 1998-05-14
W O 97/19667 PCTrUS96/18777
time in which feeding can begin may be subst~nti~lly less than when
using prior methods, especially if the present invention is used along
with a prokinetic agent. It should also not be overlooked that the present
invention is bPT-efici~l in that it may cause less discomfort to the p~tient
since the feeding tube may be placed much more quickly. A final
consideration is cost, which may be si~nificantly lower using the
method of the present invention.
The ~si~nee of the present invention is also the owner of U.S.
Patent No. 4,921,481 which issued on May 1, 1990, and is entitled Enteral
0 E'eeding System Ut.ili7in~ Gastrointestinal Myoelectrography. U.S.
Patent No. 4,921,481 is hereby incorporated by reference into the present
application.
Other objects and advantages of the present invention will become
more apparent when considered in view of the following detailed
description and drawings.
RRT~,F DESCRIPTION OF THE DRAWIN(~S
Figure 1 is a plan view of the feeding tube of a preferred
ao embodiment of the present invention;
Figure 2 is an end view taken in the direction of lines 2-2 of Figure
l;
Figure 3 i8 a perspective view of one embodiment of a data
acquisition system of the present invention;
Figure 4A is a view of an electromyogram signal from the
stomach of a patient, showing, generally, a 3 cpm frequency;
Figure 4B is a view of an ele.;l~om~ogram signal from the small
bowel of a patient, which generally reveals a 10 cpm frequency;
Figure 4C is a view of an electromyogram signal in transition,
reflecting the real time contrast between Figures 4A and 4B;
Figure 6 shows a schematic representation of a portion of a
patient's digestive tract;
Figure 6 is a schematic representation of the feeding tube of the
present invention with its distal end located within a patient's stomach;
CA 02237912 1998-05-14
WO 97/19667 PCT/US96/18777
Figure 7 is a diagrAmm~ticAl represent~tion of a preferred signal
acquisition system of the present invention; and
Figure 8 is a diagrAmmAtical representation of another
embodiment of the present invention in which a large physiological
signal acquisition monitor system incorporates the signal acqu~sition of
the present invention.
:D~,T~TT.~.T) nF.~CRIPTION OF P~P',FF~l~RED EMBOI~IMENT(~)
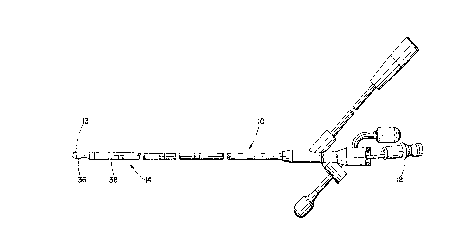
Referring now to the drawings, in Figure 1 a feeding tube 10 is
shown that may be used in the present invention. A Flexiflo 10F feeding
tube with internal stylet 12 such as made by Ross Products Division of
Abbott Laboratories in Columbus, Ohio, is shown modified by the
plAcement of three silver wire electrodes 14, preferably arranged at four,
six and eight c~ntimeterS from the tip 13 ~shown in Figure 2) to record
1~ EMG ~;~nA1S by contact with the mucosa. As shown in Figure 7, the
signal transmitted from the electrode(s) is filtered and Amplified by an
Amplifier, such as a R1000 research amplifier 16 made by Ross Products
Division of Abbott Laboratories. The signal may be conditioned by a
bandpass filter that may operate from 0.Q3 to 15 Hz with a 40 dB per
~o decade roll-off. Signal gain may be controlled by an internal switch. A 2
pole high pass filter may be incorporated with a cut off frequency of 0.03
Hz and a 6 pole low pass filter may be set with a cut off frequency of 1 Hz.
A variety of electrode configurations may be used cont~inin~,
preferably two or more electrodes 36, 38 to obtain a signal. One of the
2Ei electrodes would be used to provide a reference. Three or more
electrodes may be used at the distal end of the tube to offset naturally
occurring noise levels in the gut.
The myoelectrical gastrointestinal signal may be digitized,
preferably, by a 12 bit A/D board on a personal con~uler 20 and can be
30 stored on disc or printed as a real time amplitude-time plot. Gastric
~;~nA1~ 40 in the stomA~h are generally of relatively high amplitude with
a frequency of 3 cycles per minute as shown in Figure 4A, while the
duodenal ~i~nAl.~ 42 are generally low amplitude with frequencies of 10
CA 02237912 1998-0~-14
W O 97/19667 PCTAJS96/18777
to 13 cycles per minllte as shown in Figure 4B.
Erythfo~ycin lactobionate may be infused at initiation of the
procedure of inserting the iEeeding tube 10 into a p~tiçnt 11, at a preferred
dose of 3 mg/Kg given over ten minutes. The erytl.lo~y~in enh~nceR the
5 gastric migratory motor complex activity and accelerate gastric
emptying, which may result in a more rapid duodenal pl~cemçnt of the
feeding tube tip.
The feeding tube 10 may be of a nasoenteric type to be ul*ms.tely
located in the str m~h 30 or small bowel 32, and its position confirmed
0 by auscultation and EMG real time printout. The tube is then slowly
advanced into the patient until the duodenal EMG is detected on a
continuous record. If the small bowel 32 signal is not detected, the
feeAing tube 10 is withdrawn and advanced again until it is successfully
located in a postpyloric position. Figures 5 and 6 show the plAcPment of
1~ a feeding tube 10 within a patient 11.
A medical care provider, such as a physician, may carefully
monitor the progression of the feeding tube into the patient, by viewing a
display monitor 26 or a continuous printout 26 from a chart printer 24
for P~rAmple~ as shown in Figure 3. The monitor 26 or printer 24 may be
ao placed on a mobile cart 27 and moved to a patient's bedside prior to
introducing the feeding tube. The feeding tube is electrically connected
to the monitor or printer so that Ri~ detected by the electrodes on the
feeding tube are received by the display device. The medical care
provider would be trained to look for the characteristic Rign~lR on the
display monitor or printout which reveal the location of the fee~ing tube
during the insertion procedure. As the feeding tube enters the patient's
stom~h, the medical care provider will be able, simultaneously, to see
the frequency and ~mplitude characteristics of stomach ~ign~lR 40, on
the monitor or real-time printout.
Figure 4A is a representation of what the medical care provider
would see on a monitor or printout as the feeding tube enters the
patient's stom~rh
As the feeding tube continues to be inserted, it will arrive in the
CA 02237912 1998-05-14
W O 97/19667 PCTAUS96/18777
duodenD. The medical care provider will be able, simultaneously, to
see the frequency and amplitude characteristics of duodenum sign~
42, on the monitor or real-time printout.
Figure 4B i9 a representation of what the medical care provider
would see on a Inonitor or printout as the feeding tube enters the
patient's duodenum.
Figure 4C shows the transition over time (i.e. - over several
seconds or a few minutes, depen-ling on the rate at which the medical
care provider is inserting the feeding tube) as the feeding tube moves
from the patient's stom~ch to the patient's duo-lçnllm The notice~hle
change in the frequency and amplitude of the signal shown in Figure 4~
is an indication that the feeding tube has moved from the stom~-~h to the
duodenum. With this live source of up-to-the-minute, accurate, bedside
information, medical care providers can quickly and properly place a
feeding tube within a p~t.içnt,
The feeding tubes may be initially inserted into a patient 11
through the nose, but may also be inserted through the mouth or even
through the skin in the abdomin~l region of the patient. An enteral tube
can be used for feeding the patient, for checking food absorption levels,
a~ as a means for inputting drugs, and as a means for deg~s~in~ the
stom~h, among other uses known to those of skill in the art. In a
preferred embodiment of the present invention erythromycin is used as
a motility agent to assist in the adv~ncement of the tube into the small
bowel of the patient; however, other prokinetic agents may be used
2~ which would also stimulate the gut.
The tube may be physically advanced by a medical care provider
carefully guiding the tube into the patient until the distal end 13 of the
tube 10 arrives at its intended location. The tube may also be inserted
into the patient and then allowed to naturally migrate into the region
where it is inteIl~ed to supply its function. t
Figure 8 shows the present invention as a part of a physiological
patient monitor system in which multiple ~ are obtained from a
plurality of different data monitors. A total patient condition record may
CA 02237912 1998-05-14
W O 97/19667 PCTAJS96/18777
be stored at a rh~nnel bank 50 which forms a part of the system.
While there has been shown and described several possible
embo~iment~ of the inven~ion~ it will be obvious to those of skill in the art
that changes and modifications may be made without departing from
the inv~.n~ion, and it is inten~ell by the appended claims to cover all such
changes and modifications as fall within the true spirit and scope of the
invention.