Note: Descriptions are shown in the official language in which they were submitted.
CA 02237956 1998-05-15
WO 97/19947 PCT/US96118550
2'~ 4'-HALO~ '-BIPHENYLl-4-YLlMETHY1 1-5'-METHYL-
SPIROlCYCLOPENTANE-1 ,7'(8'H)-l3HllMlD~70~2~1-blPURlNl-
4'(5'H)-ON ES
P~CKGF~oUNn
The present invention relates to 2'-[[4'-halo-[1,1'-biphenyl]-4-
yl]methyl]-5'-methyl-spiro[cyciopentane-1,7'(8'H)-[3H]imidazo[2,1-b~purin]-
4'(5'H)-ones, their use in treating cardiovascular and pulmonary
disorders, and pharmaceutical compositions comprising them.
Phosphodiesterase inhibitory compounds of this invention were
generically but not specifically disclosed in PCT publication WO91/19717,
published December 26, 1991, and related compounds were generically
and specifically disclosed in WO94/19351, published September~, ~994.
We have found that the compounds of the present invention show
unexpectedly superior plasma levels compared to the compounds of the
prior publications when administered intravenously, subcutaneously or
2~ orally.
~5UMMARY OF THF INVFNTION
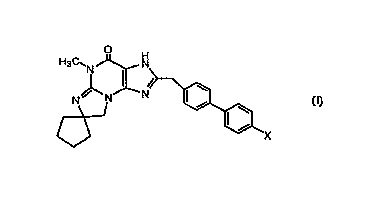
The present invention is directed to 2'-[[4'-halo-[1,1'-
biphenyl3-4-yl]methyl]-5'-methyl-spiro[cyclopentane-1 ,7'(8'H)-[3H]imidazo-
[2,1-b~purin]-4'(5'H)-ones of the formula ~:
H3C'N ~--~
<~ X
or a pharmaceutically acceptable salt thereof, wherein:
X is fluoro, chloro or bromo.
CA 022379~6 1998-0~
WO 97/19947 PCT/US96/18550
- 2 -
The compounds of formula I are useful as antihypertensive,
bronchodilating and blood platelet inhibiting agents. Compounds of the
invention are useful in inhibiting phosphodiesterase enzymes; the
inhibition of vascular phosphodiesterase is associated with vasodilation r
and vasorelaxation, and therefore is expected to induce antihypertensive
and antianginal activity. Compounds of formula I can also serve as
smooth muscle relaxants and are therefore useful in the treatment of
bronchoconstriction. Such compounds also can inhibit smooth muscle
proliferation, vascul~r growth and platelet function and are useful in
treating conditions such as restenosis post angioplasty, atherosclerosis
and conditions which benefit from inhibiting platelet function. Through
one or more of the above physiological mechanisms, compounds of
formula I are also useful in treating ischemia and peripheral vascular
diseases.
1~ Compared to previously known phosphodiesterase inhibitors
of similar structure, the compounds of the present invantion are not as
readily metabolked. They demonstrate good selectivity of inhibition of
Type I and Type V phosphodiest~rase isozymes while maintalning high
blood levels and demonstrating antiplatelet and vasodilator activity.
The present invention is also directed toward a
pharmaceutical composition containing a compound of formula I in an
amount effective to inhibit phosphodiesterase enzymes, smooth muscle
proliferation, vascular growth or platelet function, or to relax smooth
muscle. The present invention is also directed toward a pharmaceutical
composition containing an anti-hypertensive, an anti-anginal, a
bronchodilating or a platelet inhibiting effective amount of a compound of
formula I.
The present invention is also directed toward a method for
treating hypertension, angina, bronchoconstriction, restenosis post
angioplasty, atherosclerosis, ischemia, peripheral vascular diseases, or
diseases benefitting from platelet inhibition in a mammal comprising
administering to a mammal in need of such treatment an amount of a
compound of formùla I effective to treat any of the above diseases. The
present invention is also directed toward a method for maintaining
guanosine 3',5'-cyclic monophosphate (cGMP) levels in a mammal by
administering an amount of a compound of formula I effective to maintain
or increase cGMP levels.
CA 022379~6 1998-0~
WO 97/19947 PCT/US96/18550
- 3 -
DETAILED DESCRIPTION OF THF INVENTION
Compounds of the invention have a basic nitrogen
containing moiety, and can form pharmaceutically acceptable salts with
organic and inorganic acids. Examples of suitable acids for such salt
5 formation are hydrochloric, sulfuric, phosphoric, acetic, citric, oxalic,
- malonic, salicylic, malic, fumaric, succinic, ascorbic, maleic,
methanesulfonic and other mineral and carboxylic acids well known to
those ski~led in the art~ The salts are prepared by contacting the free base
form with a sufficient amount of the desired acid to produce a salt in the
10 conventional manner.
The compounds of the present invention can be prepared by
several routes, such as those described in WO91/19717 and
WO94/19351~ The following examples show typical procedures, but those
skilled in the art will recognize that the preparation of these compounds is
15 not limited to these procedures~ In the examples below, Me refers to
methyl, Bn refers to benzyl, Ph refers to phenyl and SEM refers to
trimethylsilylethoxymethyl.
EXAMPLE 1
MC ~ N ~ ~
,~
20 Step 1:
1~ ~ICI3 ~
a 2. CH3COCI H3C~
To a stirred ice-cold solution of 4-chlorobiphenyl (115 g, 0.61
mol) in CH2CI2 (1200 ml) add AICI3 (97~5 g, 0.73 mol) in portions over 0.5
h. Add acetyl chloride (48 ml, 0.68 mol) dropwise over 0.75 h and stir the
resultant red reaction mixture at 0-5 ~C. After 3.5 h, allow the reaction
mixture to warm to 10 ~C, then pour onto a slurry of ice/1 M HCI (1000 ml)
with vigorous stirring. Allow the mixture to stand overnight, then wash the
~ organic layer with sat'd NaCI, dry (MgSO4), filter and evaporate to give a
pale yellow solid. Triturate the solid with ether and collect to give the
ketone 1 (122 g, 87%)~ 1H NMR CDCb ~ 8.01 m, 2H; 7.62 m, 2H; 7.53 m,
2H; 7.41 m, 2H; 2.62 s, 3H.
CA 02237956 1998-05-15
WO 97/19947 PCT/US96/18550
- 4 -
Step 2:
O 1. mt~ hr~ P, S
H3C~ 2. aq. NaOH reflux HO2C~
Heat a mixture of the ketone 1 (60 9, 0.26 mol), sulfur (12.5
g, 0.39 mol) and morpholine (38 ml, 0.43 mol) at r~flux. After 28 h, add the
resultant brown solid to a solution of NaOH (240 g) in water (1200 ml).
Reflux the mixture for 24 h, cool, collect the precipitate, wash with water
and air-dry. Suspend the solid in hot water (2000 ml) and acidify to pH 2
with approximately 200 ml 1 M HCI. Allow the mixture to cool, collect the
solid, wash with water and air-dry. Take up the solid into EtOAc (1000 ml),
wash with sat'd NaCI, dry (MgSO4), filter and evaporate to dryness to give
the acid 2 (54.9 g, 86%) as a brown solid. 1H NMR CDCI3 ~ 7.52 m, 4H;
7.40 m, 4H; 3.72 s, 2H.
Step 3:
lJ~ DEC, DMAP, DMF ~ H
O N NH2 HO2C~ ~ H NH2 ~
To a stirred solution of the diaminouracil 3 (33.3 g, 0.21 mol),
acid 2 (58.0 g, 0.24 mol) and 4-dimethylaminopyridine ~2.6 g, 0.021 mol)
in DMF (1000 ml), add 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide
methiodide (76 g, 0.26 mol). Stir overnight at room temperature, then pour
the reaction mixture onto an ice water slurry (3000 ml). Collect the solid,
20 wash with water and ethanol, and dry in vacuo to give the amide 4 (69.9 g,
87%) which is used without further purification. lH NMR DMSO-d6 ~
10.51 s, 1H; 8.64 s, lH; 7.70-7.40 m, 8H; 6.01 s, 2H; 3.65 s, 2H; 3.02 s, 3H.
~tep 4:
Me~ POC13 M 'N~N~
4 c~ 5
Heat a stirred suspension of the amide 4 (45.0 g, 0.117
mole) in POCI3 (1500 ml) at 90-95 ~C for 43 h, then at 100 ~C for 16 h,
then at 110 ~C for 8 h. Remove the POC13 by distillation at reduced
CA 02237956 1998-05-15
WO 97/19947 PCT/US96/18550
- 5 -
pressure and cool the resultant oil in an ice bath. Neutralize residual
POCI3 by careful addition of saturated NaHCO3 and collect the resultant
solid by filtration, wash several times with water, air-d~, then dry in vacuo
at 50 ~C. Triturate the solid (53 g~ with 9:1 CH2CI2/CH3OH (1000 ml~,
5 collect and then triturate further with 1:1 CH3OH/CH2CI2 with gentle
heating. Concentrate the filtrate in vacuo to obtain the chloropurine 5
(25.6 g) as a brown solid. Concentrate the filtrate from the 9:1
CH2CI2/CH3OH trituration in vacuo and take up in the minimum volume of
approximately 4:1 CH2C12/CH3OH. Add hexanes to the resultant solution
10 until solids begin to separate. Collect the brown solid and wash with
CH2CI2 to obtain additional chloropurine 5 (6.0 g). Use the chloropurine
(combined yield after drying in vacuo 3t .0 g, 69%) in the next step without
further purification. 1H NMR CDCI3 ~ 7.53-7.46 m, 4H; 7.41-7.37 m, 4H;
4.31 s, 2H; 3.75 s, 3H.
15 Step5:
N~CN~_~ H~OH 1~
~ N,N~ ,u~,y~ yl~.i.. c r\ ~
5 ~ N~, 120 ~C ~> OH 6 ~_2
a Cl
Heat a suspension of the chloropurine 5 (11.7 g, 0.030 mole), 1-
amino-1-cyclopentanemethanol (7.1 g, 0.06 mole) and N,N-diisopropylethyl-
amine (13.2 ml, 0.076 mole) in NMP (25 ml) at 120 ~C under argon for 30 h.
20 Allow the reaction mixture to cool, then pour onto a stirred slurry of ice/water
(1000 ml). Collect the precipitate, wash with water, air-dry and dry in vacuo at50 ~C. Triturate the solid (13.3 g) with warm 1:1 CH2CI2/CH3OH, then filter.
Adsorb the filtrate onto silica gel (75 g) and flash chromatograph (24:1 CH2CI2/CH30H, then 19:1 CH2CI2/CH3OH when the product begins to elute) to obtain
25 the alcohol 6 (7.5 g, 53%) as an off-white solid. Calcd for C2sH26NsO2CI-0.75H2O: C, 62.11; H, 5.73; N, 14.49. Found: C, 62.38; H, 5.59; N, 14.30%. MS
(FAB) m/~ 466.1, 464.1 (M~H)+.
Step 6:
Me~NJ ~N SOCIZ, MeOH, 1J~t
HN 1N N~ ~ N N~
~~H 6 ~ 7
a Cl
CA 02237956 1998-05-15
WO 97/19947 PC~T/US96/18550
~ 6 ~
To a stirred suspension of the alcohol 6 (12.1 g, 0.026 mol~
in CH2CI2 (750 ml), add SOCI2 (5.7 ml, 0.078 mol) rapidly dropwise. Stir
the reaction mixture vigorously for 3 h, then evaporate in vacuo. Dissolve
the rcsidue in CH30H (100 ml), filter and pour the filtrate into a rapidly
5 stirred solution of 10% NaHCO3. Collect the pre~ipit~te, wash with water
(1000 ml) and CH30H ~150 ml), then dry. Recrystallize from CH30H/
CH2CI2 to obtain the product 7 as an off-white powder (10.30 g, 88%).
Calcd for C2sH2~NsOCI: C, 67.33; H, 5.42; N,15.70; Cl, 7.95 Found: C,
67.41; H, 5.66; N,15.41; Cl, 8.24%. MS (FAB) m/z 448.3, 446.3 (M+H)+.
10 Step 7:
o o
~ICINl~Cl
To a suspension of the tetracyclic guanine 7 (10.30 g, 0.023
mol) in EtOH (125 ml) warmed on a steam bath, add conc. HCI (1.9 ml,
0.023 mol). Filter the resultant cloudy mixture ~nd wash the filter pad with
15 warm EtOH (75 ml). To the combined filtrate and washings, add hot water
(100 ml) and allow the solution to stand. Collect the crystaliine mass,
wash with 1 :1 EtOH/H2O, air-d~y and dry in vacuo at 50 ~C to obtain the
hydrochloride 8 (7.0 g, 63%). A second crop of product 8 (3.0 g, 27%) is
obtained from the mother liquor. Combine the two crops and dry in vacuo
20 at 50 ~C over P2Os. Calcd for C2sH2sNsOCI2.1.75H2O: C, 58.43; H, 5.59;
N,13.63; Cl,13.80. Found: C, 58.45; H, 5.24; N,13.45; Cl,13.70%.
1H NMR CD30D ~ 7.58-7.56 m, 4H; 7.43-7.37 m, 4H; 4.38 s, 2H; 4.19 s,
2H; 3.46 s, 3H; 2.10-1.79 m, 8H.
EXAMPLE 2
N Jl~[ N
NlN N~
I \ I ~
~ a
Br
~te~ t:
Br 2 CH3COCI H3CJ~? Br
CA 02237956 1998-05-15
WO 97/19947 PCT/US96/18550
- 7 -
Uso the procedure o~ Example 1, Step 1, with bromobiphenyl
(5t.36 g, 0.22 rnol) to obtain ketone 9 ~50.94g, 84%) as a light yellow
solid. 1H NMR CDCI3 ~ 8.03 dd (J = 6.6 and 2.2 Hz), 2H; 7.65 dd (J - 6.6
r and 2.2 Hz), 2H; 7.55 dd (J = 6.6 and 2.2 Hz), 2H; 7.49 dd (J = 6.6 and 2.2
5 Hz), 2H; 2.64 s, 3H.
Step 2:
O ~ IolillC, S
~13CJ~ 2. aq. NaOH reflux HO2C~
Br Br
Use the procedure of Example 1, Step 2, hea~ing with sulfur
and morpholine for 4 h, to convert ketone 9 (50.93 ~, 0.19 mol) to acid 10
(51.3 g, 9~%), a white solid. 1 H NMR DMSO-d6 ~ 7.6~-7.59, m, 6H; 7.35 d
(J - 8.2 Hz), 2H; 3.60 s, 2H.
~tep 3:
N Jl~DEC, DMAP, DMF ~ E~
H HO2C ~ ~ H NH~ Br
Br
Treat acid 10 (51.3 g, 0.18 mol) using the procedure of
1~ Example 1, Step 3, to obtain amide 12 (61.2 g, 82%) as a yeliow solid.
1H NMR DMSO-d6 ~ 10.~2 s, 1 H; 8.66 s, 1 H; 7.66-7.59 m, 6H; 7.43 d (J =
8.2 Hz), 2H; 6.01 s, 2H; 3.61 s, 2H; 3.04 s, 3H.
Step 4:
~Br
POC13
Me'NJ~N Me~N ~N
CIlN N)~ + OlN N~
13 ~ 14 ~
Br Br
CA 02237956 1998-05-15
WO 97/19947 PCT/US96/18550
-8-
Heat a stirred suspension of the amide 12 ~40.0 9, 0.093
mole) in POCI3 (2100 ml) at 90-100 ~C for 6 days. Remove the POCI3 by
rotovaporation at reduced pressure (c45 ~C) and dissoive the resultant oil
in a minimal amount of CH2CI2 (~400 ml). Neutralize the residual POCI3
5 by carefully pouring the CH2CI2 solution into cold saturated NaHC,O3
(2200 ml) over a period of 1 h. Stir the mixture overnight at room
temperature, collect the resultant solid by filtration, wash several times with
water, air-dry and dry in vacuo at 60 ~C. The solid (28.47 g, 71%) is a 26:1
mixture of chloropurine 13 and xanthine 14, respectively (1H NMR
analysis). 1H NMR CDCI3 ~ 7.64-7.58 m, 6H; 7.38 d (J = 8.2 Hz), 2H; 4.13
s,2H;3.61 s,3H.
Ste~ 5:
J~tH H~ OH Me'NJ~
Cli N N>~ Br ' ~Br
Heat a suspension of the chloropurine 13 (28.57 g, 0.066
15 mole), 1-amino-1-cyclopentanemethanol (19.77 g, 0.17 mole) and N,N-
diisopropylethylamine (30 ml, 0.17 mole) in NMP (96 ml) at 120 ~C under
argon for 18 h. Cool the reaction mixture, then pour onto a stirred slurry of
ice/brine (1000 ml). Collect the precipitate, wash with water, air-dry and
dry in vacuo at 50 ~C. Triturate the solid (42.36 g) with CH2CI2-CH3OH
20 (4:1), and then filter to give 17.48 g of a solid consisting of alcohol 15 and
xanthine 14. Adsorb the filtrate onto silica gel and subject to flash
chromatography [gradient CH2CI2/CH3OH = 32:1 to 19:1 (product begins
eluting) to 11.5:1] to obtain alcohol 15 (7.4 g, 22%) as an off-white solid
and impure 15 (5.79 g~ containing xanthine 14. 1H NMR CDCI3 ~ 7.64-
25 7.58 m, 6H; 7.37 m,2H; 5.76-5.63 m,1 H; 5.09, 4.89 t,t (J = 5.8 Hz),1 H;
4.02,3.97s,s,2H;3.64d(J=5.8Hz),2H;3.36s,3H;2.05m,2H; 1.72m,
4H; 1.53 m, 2H.
Step 6:
o o
Me'NJ~N SOCI2, MeOH ,~
N N~ N N N~
OH 15 U ~> 16 ~
Br Br
CA 02237956 1998-05-15
WO 97119947 PCT/US96/18550
_ 9 _
Treat alcohol 1~ (10.1 g, 0.020 mol) according to the
procedure of Example 1, Step 6, to obtain tetracyclic guanine 16 (9.6 g,
95%) as a white solid. 1H NMR DMSO-d6 ~ 7.65-7.58, m, 6H; 7.37, d (J =
8.2Hz),2H;4.02s,2H;4.80s,2H;3.18s,3H; 1.75-1.62m,8H.
Step 7:
o H o
HCI
N N N~Z ~1 17 3
3~r Br
Using a procedure similar to Example 1, Step 7, except
recrystallizing twice from CH30H and then twice from ethanol-water, treat
the amine 16 (11.7 g, 0.024 mol) to obtain the hydrochloride 17 (10.22 g,
1081 %) as a white solid. Calcd for C2sH2sNsOBrCI 3H2O: C, 51.69; H, 5.39;
N,12.06; Br,13.75; ~::I, 6.10. Found: C, 51.58; H, 5.63; N,11.84; Br,13.55;
Cl, 5.77%. MS (FAB) m/z 490.1, 491.1 ~M+H). 1H NMR DMSO-d6 + D20
7.63-7.56, m,6H;7.37,d(J=8.2Hz),2H;4.28s,2H;4.12s,2H;3.31 s,
3H; 1.98-1.87 m, 4H; 1.79-1.61 m, 4H.
15EXAMPLE 3
N~D[ N
N N
Step 1:
J~ Pd(OH)2/C ~J~ ~>
N NH4HCO2 N~ I
~5J CH30H O
18
To a suspension of Pd(oH~2 (~g) in CH30H, add HNH4CO2
(14 g, 223 mmol) followed by a solution of 5'-methyl-3'-(phenylmethyl)-
spiro~cyclopentane-1,7'(8'H)-[3'H]-imidazo[2,1-b]purin]-4'~5'H)-one (15 g,
44.7 mmol) in CH30H (total volume 1000 ml) at room temperature. Heat
the mixture at reflux for 6h, filter and concentrate the filtrate to obtain a
white solid. Stir the catalyst in hot 10% CH3OH/CH2CI2, filter and
concentrate. Combine the concentrated filtrates, dissolve in 10%
CA 02237956 1998-05-15
WO 97/19947 PCT/US96/18550
~ 10 ~
CH3OH/CH2CI2 (400 ml) and wash with aqueous NaHC03. Extract the
aqueous solution with 10% CH30H/ CH2CI2 (200 ml x 2), dry the
combined extracts over MgSO4, filter and concentrate to obtain compound
18 as a white solid (9.7g, 88.5%). 1H NMR CDCI3 ~ 7.62, s, 1H; 3.95, s,
2H; 3.40, s, 3H; 1.8-2.0, m, br, 4H; 1.6-1.8, m, br, 4H.
Step ?:
1 NaHtrHF ~ N~
18 19 20
Add NaH (3.16 g, 60%, 79 mmol) to a mixture of 18 (9.7 g,
39.5 mmol) in THF (400 ml) at room temperature. Stir the mixture at room
10 temperature for 1.5h, add trimethylsilylethoxymethyi chloride (9.88g, 59.25
mmol) and stir at room temperature for 1.5h. Quench the resulting yellow
mixture with ice, extract with EtOAc (200 ml x 2), combine the EtOAc
extracts and wash with brine, dry over MgSO4, filter and concentrate.
Purify the crude product by flash chromatography on sio2 (1% to 10%
15 CH3OH/CH2CI2) to obtain compounds 19 (11.4 g), 2Q (2.37 g) and a
mixture of 19 and 20 (1.1 g) (98% ). 1 H NMR of 19 CDCI3 ~ 7.61, s, 1 H;
.65, s, 2H; 3.91, s, 2H; 3,62, t, 2tl; 3.38, s, 3H; 1.8-2.0, m, 4H; 1.6-1.8, m,
4H; 0.95, t, 3H; 0.00, s, 9H. 1 H NMR of 20 CDCI3 d 7.26, s, 1 H; 5.32, s,
2H; 4.00, s, 2H; 3.53, t. 2H; 3.40, s, 3H; 1.8-2.0, m, 4H; 1.6-1.8, br, 4H;
20 0.92, t, 2H; 0.00, s, 9H.
~Step 3:
Br F
Pd(PPh3)4 F~ CHO
CHO B(OB)2 Toluerle 21
To a mixture of Pd(PPh3)4 (1.5 g, 1.3 mmol) in toluene (52
ml), add p-bromobenzaldehyde (4.8 g, 25.98 mmol), aqueous Na2CO3
(26 ml, 2.0M, 52 mmol), and a solution of p-fluorophenylboronic acid (4g,
28.58 mmol) in EtOH (13 ml) and CH30H (2 ml). Heat the mixture at reflux
for 6h, cool to room temperature, pour into a~ueous NaHCO3, extract with
EtOAc (200 ml x 3), dry over MgSO4, filter and concentrate to obtain a
dark, thick oil. Purify the crude product by flash column chromatography
on siO2 (1:9, 1:7 EtOAc/hexane~ to obtain compound 21 (4.88 g, 93.8%).
CA 02237956 l998-05-l5
WO 97/19947 PCT/US96/18550
- 1 t -
1H NMR CDCI3 ~ 10.06, s,1H; 7.96, d,2H; 7.72, d,2H,7.62, dd,2H;
7.18, t, 2H.
~tep 4:
~ SEM O SEM
NJIXN~ 1. LDA/rHF N~N~OH
~N N 2- 21~ ~ d~N N
19 22
Add n-BuLi (6.65 ml,2.5M,16.62 mmol) to a solution of
diisopropylamine (1.68 g,16.62 mmot) in THF (30 ml) at 0 ~C. Stir at 0 ~C
for 1h, cool to -70 ~C and add a solution of compound 19 (4.8 g, 12.78
mmol) in THF (60ml) dropwise. After the addition is complete, stir the
mixture at -70 ~C for 1 h, then add a pre-cooled solution of 21 (3.869,
~ 10 19.17 mmol) in THF (1 ~ ml). Stir the mixture at -70 ~C for 3h, quench with
AcOH(4 ml), warm to 0 ~C and treat with aqueous NH4CI (30 ml). Extract
the mixture with EtOAc (200 ml x 3), wash with aqueous NaHCO3 and
brine, dry (MgSO4) and concentrate. Purify the crude product by flash
column chromatography on SiO2 ~0% to 2% CH30H/~tOAc) to obtain
compound 22 ~5.34 g, 72.6%). 1 H NMR CDCI3 ~ 7.55, m, 4H; 7.54, 2H;
7.1~,t,2H;6.05,d,br, 1H;5.56,ABq,2H;3.97,s,2H;3.60,t,2H;3.38,s,
3H; 1.8-2.0, m, 4H; 1.6-1.8, m, 4H; 0.90, m, 2H; 0.00, s, 9H.
~tep 5:
~ SEM o
22~ HSiEb ~ ~F
Add EtSiH (5.34 g, 45.4 mmol) to a solution of 22 (5.23 g,
9.08 mmol) in TFA (24 ml) at room temperature. Stir the reaction mixture
at room temperature over night, carefully neutralize with aqueous
NaHCO3, extract with 10% CH3OHICH2CI2, dry over MgSO4, filter and
concentrate. Purify the crude product by flash column chromatography on
SiO2 (3% to 10% CH3OH/CH2CI2) to obtain compound 24 (3.35 g).
1 H NMR CDCI3 ~ 7.51, m, 4H; 7.49, d, 2H; 7.1 1, t, 2H; 4.18, s, 2H; 3.92, s,
2H; 3.39, s, 3H; 1.8-2.0, m, 4H; 1.6-1.8, m, 4H.
CA 02237956 1998-05-15
WO 97/19947 . PCT/US96/18S50
- 12 -
~:;tep 6:
o o
N~[N~ 1. LDAITHF lJ~[ ~<
N 2. 2}/l~:JF > ~N N ~ F
Using compound 20 (8 g, 21.7 mmol) in the procedure of
Example 3, Step 4, prepare compound 23 (4.6 g, 37%). lH NMR CDCI3
~7.50,m,6H;7.10,t,2H;6.18,s,1H;5.21,s,2H;3.99,ABq,2H;3.39,s,
3H; 3.10, m, 2H; 1.8-2.0, m,4H; 1.6-1.8, m, 4H; 0.65, t, 2H; -0.10, s, 9H.
~Step 7:
N~[ ~ , NJ'~CN~F
Treat compound 23 according to the procedure of Example
3, Step 5, to obtain compound 24. 1 H NMR CDCb ~ 7.51, m, 4H; 7.49, d,
2H; 7.11, t,2H; 4.18, s,2H; 3.92, s, 2H; 3.39, s, 3H; 1.8-2.0, m, 4H; 1.6-1.8,
m,4H.
Ste~ 8:
o o
N~iCN HCI N~[N
~/ 24~ EtOH H~N N~ F
To a hot suspension of 24 ~17.5 g, 40.74 mmol) in EtOH, add
conc. HCI (5.1 ml, 61.2 mmol~ dropwise to give a clear solution. Stir the
solution at room temperature for 3Q min, evaporate the EtOH to give a
white solid and dry the solid in vacuo at 50~C over P2Os. Calcd for
C2sH24NsOF.1.05HCIØ75H2O: C, 62.39; H, 5.56; N,14.55; Cl, 7.73, F,
3.95. Found: C, 62.44; H, 5.71; N,14.38; Cl, 7.80; F, 4.00%. lH NMR
DMSO-d6 ~ 10.65, d, br,1H; 7.7, m,2H; 7.65, d, 2H, 7.40, d, 2H; 7.26, t,
2H;4.32s,2H;4.19s,2H;3.39s,3H;1.6-2.1 m,8H.
CA 022379~6 1998-0~
WO 97/19947 PCT/US96/18550
- 13-
Pharm~utical Preparations
The compounds of formuia I can be combined with a suitable
pharmaceutical carrier to prepare a pharmaceutical composition suitable for
parenteral or oral administration. Such pharmaceutical compositions are
5 useful in the treatment of cardiovascular and puimonary disorders such as
~ mammalian hypertension and bronchoconstriction
The effec~ive daily antihypertensive dose (EDso) of the present
compounds will typically be in the range of about 1 to about 1û0 mg/kg of
mammalian body weight, administered in single or divided doses. The exact
10 dosage to be administered can be determined by the attending clinician and
is dependent upon where the particular compound lies within the above cited
range, as well as upon the age, weight and condition of the individual.
Generally, in treating humans in need of treatment for
hypertension or bronchoconstriction, the present compounds can be
15 acl",inislered in a dosage range of about 1û to about 500 mg per patient
generally given a number of times per day, providing a total daily dosage of
from about 10 to about 2000 mg per day.
The compositions of the present invention can be administered
orally or parenterally. Typical inJectable formulations include solutions and
20 suspensions. Typical oral formulations include tablets, capsules, syrups,
suspensions and elixirs. Also contemplated are mechanical delivery
systems, e.g. transdermal dosage forms.
The typical acceptable pharmaceutical carriers for use in the
formulations described above are exemplified ~y sugars such as lactose,
25 sucrose, mannitol and sorbitol; starches such as cornstarch, tapioca starch
and potato starch; cellulose and derivatives such as sodium carboxymethyl
cellulose, ethyl cellulose and methyl cellulose; calcium phosphates such as
dicalcium phosphate and tricatcium phosphate; sodium suifate; calcium
sulfate; polyvinylpyrrolidone, polyvinyl atcohol; stearic acid; alkaline earth
30 metal stearates such as magnesium stearate and calcium stearate, stearic
acid, vegetable oils such as peanut oil, cottonseed oil, sesame oil, olive oil
and corn oil; non-ionic, cationic and anionic surfactants; ethylene glycol
polymers; beta-cyclodextrin; fatty alcohols and hydrolyzed cereal solids; as
well as other non-toxic compatible fillers, binders, disintegrants, buffers,
35 preservatives, antioxidants, lubricants, flavoring agents, and the like
commonly used in pharmaceutical formulations.
CA 022379~6 1998-0~
WO 97/19947 PCT/US96/18S50
- 14 -
Following are typical examples of oral and parenteral
formulations, wherein the term "Active Ingredient" refers to a compound
of formula I.
C~sule Amount (m~)
Active Ingredient250.0 125.0
Lactose 173.0 86.5
Corn Starch 75.0 37.
Magnesium Stearate.2.Q 1.0
TOTAL 500.0 250.0
Blend the active ingredient, lactose and corn starch until
uniform; then blend the magnesium stearate into the resulting powder.
Encapsulate the mixture into suitably sized two-piece hard gelatin
capsules.
Tablet Amount (mg)
Active Ingredient 250.0 12~.0
Lactose 1 6i .0 80.5
Corn Starch 12.0 6.0
Water (per thousand tablets) 120 ml 60 ml
(evaporates) (evaporates)
Corn Starch 75.0 37.5
~/l~nesium Stearate ~Q 1.0
TOTAL 500.0 250.0
Blend the active ingredient with the lactose until uniform.
25 Blend the smaller 4uantity of corn starch with the water and add the
resulting corn starch paste then mix until a uniform wet mass is formed.
Add the remaining corn starch to the remaining wet mass and mix until
uniform granules are obtained. Screen the granules through a suitable
milling machine, using a 3/4 inch stainless steel screen. Dry the milled
3~ granules in a suitable drying oven until the desired moisture content is
obtained. Mill the dried granules through a suitable milling machine
using a 16 mesh stainless steel screen. Blend in the magnesium
stearate and compress the resulting mixture into tablets of desired shape,
thickness, hardness and disintegration.
CA 022379F76 1998 - OF7 - lF7
WO 97/19947 PCT/US96/18550
- 15 -
Injectable Solutlon mg/ml
Active Ingredient 5.00
Methyl p-hydroxybenzoate 0.80
Propyl p-hydroxybenzoate û.10
Disodium Edetate 0.10
Citric Acid Monohydrate 0.08
Dextrose 40.0
Water for injection qs. ad. 1.0 ml
Dissoive the p-hydroxybenzoates in a portion of water for
10 injection at a temperature of between 60~C - 70~C and cool the solution
to 20~C - 30~C, Charge and dissolve all other excipients and the active
ingredient. Bring the solution to final volume, filter it through a sterilizing
membrane and fill into sterile containers.
Biological Activity of 2'-[r4'-halo-[1.1'-biphenyl]-4-yl~methyl~-S'-methyl-
spiro[cyclopentane-1 .7'(8'H)-~3H]imidazo-[2.1 -b]purin-4'(5'H)-ones
The present compounds are useful in inhibiting
phosphodiesterase enzymes, in particular phosphodiesterase isozymes
Types I and V. These phosphodiesterase enzymes are known to
20 hydrolyze cGMP in smooth muscle. High levels of cGMP are associated
with the relaxation of vascular smooth muscle, with a consequent
subsequent reduction in blood pressure. Thus, it is believed that by
inhibiting these phosphodiesterase enzymes, cG \/IP levels in muscle will
be either maintained or increased, with a subsequent reduction in blood
25 pressure. In vivo antihypertensive activity is determined orally in
spontaneously hypertensive rats (SHR).
Phosphodiesterase inhibition in vitro:
Compounds are evaluated for inhibition of two
30 phosphodiesterase enzymes which hydrolyze cyclic guanosine
monophosphate (cGMP). The first enzyme, calcium-calmodulin
dependent phosphodiesterase (CaM-PDE), is a partially pure enzyme
obtained from bovine aorta homogenates and purified by DEAE-cellulose
and calmodulin-affinity chromatography. The enzyme is activated several
35 fold by Ca-calmodulin and is selective for cGMP, although it will also
hydrolyze cAMP. The second enzyme, cGMP phosphodiesterase (cGMP-
PDE), is a homogeneous enzyme obtained from bovine lung and purified
by ion-exchange chromatography, gel filtration, and sucrose gradient
CA 022379~6 1998-0~
WO 97/19947 PCT/US96/18550
- 16 -
centrifugation. cGMP-PDE is highly selective for cGMP. Bovine aorta
homogenates and primary cultures of bovine aortic endothelial and
v~sc~ r smooth muscle cells contain an enzyme with properties very
similar to the lung isozyme.
The enzyme assay is performed using a Biomek Automated
Pipetting Station. Compounds are dissolved in distilled water or DMSO
and diluted with 10% DMSO. Compounds are tested at several
concentrations at log intervals, typically 0.1, 1.0, 10, and 100 ,llM final
concentration.
Assays contain the following components:
1 ,uM substrate 3H-cGMP
50 mM Tris-HCI, pH 7.5, 5 mM MgCI2
0.5 mg/ml snake venom alkaline phosphatase
0.1 ~lM Calmodulin and 1 mM CaCI2 (for CaM-PDE only)
Assays are initiated by addition of enzyme and stopped by addition
of 10 mM isobutylmethylxanthine, a ~eneral phosphodiesterase inhibitor.
Assays are performed for 25 minutes at room temperature to achieve 5-
10% hydrolysis of substrate. The negatively charged substrates are then
separated from guanosine by binding to an anion-exchange resin (AG1-
20 X8) and centrifugation or filtration, and the product is quantitated by
scintillation counting in counts per minute (cpm) of the remaining soluble
material. Percent inhibition is calculated as follows:
% inhibition= 1 00-~(cpm compound-blank)/(cpm control-blank)X100]
Activity is expresssed as the lcso value, ie. the concentration
25 required to inhibit activity of enzyme by 50 per cent.
Antihypertensive activity in rats
The ability of the compounds of the present invention to
lower blood pressure can be assessed in vivo in conscious spontaneously
30 hypertensive rats ~SHR). SHR males are purchased from Taconic Farms,
Germantown New York and are approximately 16-18 weeks old when
anesthetized with ether. The caudal (ventral tail) artery is cannulated with
polyethylene tubing (PE50) and blood pressure and heart rate are
recorded as described by Baum, T. et. al, J. Cardiovasc. Pharmacol. Vol 5,
35 pp. 655-667, (1983). Rats are placed into plastic cylindrical cages where
they rapidly recover consciousness. Blood pressure and heart rate are
allowed to stabilize for approximately 90 minutes prior to compound
administration. Compounds are administered orally as solutions or
CA 02237956 1998-05-15
WO 97/19947 PCT/US96/18550
- 17-
suspensions in 0.4% aqueous methylcellulose vehicle via a feeding
needle. The compound or 0.4% aqueous methylcellulose vehicle are
given in a volume of 4 ml/kg to SHRs that had been ~asted overnight.
f Activity is expressed as the fall in mean blood pressure (MBP) in
millimeters of mercury (mm Hg~. Compound-induced changes are
compared with the changes in an appropriate placebo group.
Plasma levels:
SHRs dosed orally with test compound at 10 mpk and
10 plasma levels were subsequently measured at intervals of 0.5, 1, 2, 3 and
4 hours. Plasma levels (~lg/ml) were plotted versus hours post dosing and
the arsa under the curve (AUC"ughr/ml) was calculated.
The following test results are compared to a previously-
known compound, 2'-~[(4'-methoxy-1,1'-biphenyl)-4-yl]methyl]-5'-methyl-
15 spiro~cyclopentane-1.7'(8'H)-[3H]imidazo-[2,1-blpurin]-4'(5'H)-one (Ref.
Cpd.).
ACTIVITY
PDE C~o SHR Antih~/pertensive 'lasma Level
Example Type I Type V Dose (po) Fall in MBP AUC
Number (nM) (nM) (mp~) (mmHg) (llghr/ml)
1 1 170 10 -24 4.80
2 12 210 10 -11 5.89
3 9 520 10 -35 7.73
Ref. Cpd. 5 500 10 -35 3.34