Note: Descriptions are shown in the official language in which they were submitted.
CA 02238530 1998-05-25
WO 98/1411r, PCTIUS96/15643
ABNORMAL DYSPNEA PE~CEPTION DETECTION SYSTEM
E3ACICGROUND OF THE~ INVENTION
This invention relates to improved methods and apparatus to detect
patients with an abnormally altered perception of dyspnea. Of particular concern5 are asthma patients at risk for fatal asthmatic attaclcs. An unexpected risingincidence of fatal asthmatic attaclcs in recent years has been of concern to themedical profession.
The sensitivity of the testing procedure is enhanced by the test being
performed under controlled conditions by having the patient breathe in a
0 precisely defined manner by visual biofeedback means, with the subject
following a preclet.o~nined breathing pattern on a computer CRT or similar
means. Hillsman incorporates by reference his U.S. Patent No. 3,991,304 which
describes a sophisticated method to prompt patients to desired breathing
patterns by visual biofeedback means.
Prior art has indicated patients who have survived a near fatal asthma
attack have decreased dyspnea awareness to increased inspiratory resistance (See:
LOWERED CHEMOSENSITIVITY AND PERCEPTION OF DYSPNEA IN
PATIENTS WIT~I NEAR-F~TALASTHMA - Mlcuchi, ~. et all, Respiratory and
Critical Care Medicine, Supplement, Volume l 49, Number 4, April 1994). The
20 cited investigators demonstrated decreased dyspnea awareness in near fatal
asthma patients by imposing graded inspiratory respiratory resistance from zero
to minus 30 cm. water/liter/second gauge pressure. But no attempt was made b~
these investigators to further control the experimental conditions by defining the
testing tidal volume to the patient's available lung volume as reflected in the
CA 02238530 1998-05-25
WO 98/1411~; PCT/US96/15643
patient's vital capacity, or the testing inspiratory resistance load to the patient's
available maximum inspiratory pressure capability. Further, no attempt was made
to otherwise precisely control the patient's breathing pattern or the precise timing
of the breathing stages under the testing conditions, or to otherwise detect
5 whether or not the subjects were performing as required under the testing
conditions. The~crol~, absent col~ chensive controlled breathing conditions the
testing achieved was relatively crude and therefore less sensitive to defining and
detecting dyspnea awareness as measured by the commonly used Borg scale of
dyspnea, and likewise there was no assurance as to patient performance and
0 therefore data reliability.
~ n addition, there are many patients vvith Hyperventilation Syndrome,
who perceive they ha~e dyspnea when in fact their respiratory function is normal,
and definition and quantifying this abnormality and normalization with
treatment is of value in the patient therapeutic program.
Further, many patients with dyspnea related to Chronic Obstructive
Pulmonary Disease (Emphysema and Chronic Bronchitis) and other respiratory
conditions undergo comprehensive Pulmonary Rehabilitation, including various
measures to improve dyspnea distress. These measures include various
medications, breathing exercises and breathing retraining in proper breathing
2 o patterns, respiratory muscle reconditioning and strengthening by various means,
and general body reconditioning and strengthening. Present methodology to
quantify dyspnea and measure improvement with the various treatment
modalities has generally been controversial and unsa~isfactory. Therefore, thereis a need to properly define and quantify the dyspnea abnormality and an~
CA 02238530 1998-05-25
WO 98/1411~ PCTIUS96115643
normalization with the various treatments in the patient therapeutic program7
both to guide therapy and to document improvement for administrative needs.
The instant invention to comprehensively define the testing conditions
relative to the patient's vital capacity and~or maximum inspiratory pressure
5 capability, and to further define the testing conditions by having the patientbreathe in a precisely controlled manner using predefined breathing patterns by
visual biofee~1h~flc means, and to precisely control the sequence and timing of the
testing events. Therefore, by establishing the breathing testing conditions the
sensitivity, accuracy and reproducibility of the diagnostic methodology will be
0 enhanced. In addition, by placing definable plus and minus error limits abo~reand below the desired breathing analogs, with suitable audio and~or visual alarms
to indicate if the patient breathing performance is outside acceptable limits, the
diagnostician may determine whether or not the subject is performing in an
acceptable manner to the testing methodology as defined by the operator Çor the
5 particular subject and thereby generating reliable testing data.
CA 02238~30 1998-0~-2~
WO 98/14115 PCT/US9611~i643
SUMMAR~ OF TH}~ INVENTION
It is therefore one object of the present invention to enhance the
sensitivity of testing for dyspnea awareness by the testing procedures being
precisely controlled as to breathing patterns with defined elements of respiratory
5 rate, inspiration to expiration time ratio, and inspiration and expiration
breathing waveforrn analogs by visual biofeedback prompting means.
It is another object of the invention to enhance testing sensitivity in a first
mode of operation by relating the testing tidal volume breath to a defined
percentage of the patient's vital capacity capability, and to observe the patients
0 dyspnea awareness under controlled respiratory stress conditions to detect
abnormal response.
It is yet another object of the invention to enhance testing sensitivity in
a first mode of operation by relating the testing inspiratory restive load to a
defined percentage of the patient's maximum inspiratory pressure capability. A
15 variable resistive load can be imposed by either a so-called non-linear adjustable
"pinhole" orifice restrictive device or a so-called inspiratory "threshold" loading
device, the non-linear resistive device being ~rcrcllcd in the present embodiment.
It is still another object of the invention to insure data integrity in all
testing procedures by having the patient's breathing performance during testing
2 o tal~e place between definable percentage plus and minus tidal volume error limits,
and to indicate on the patient's breathing signal what zone of reliabilit~ they are
operating within, and to indicate with alarm means when breathing performance
is unsatisfactory.
CA 02238530 l998-05-25
WO 98/14115 PCT/US96/Lr,643
It is a further object of the invention to have the patient indicate freely on
a sliding electro-mecnanical device their Borg Unit level of dyspnea awareness,
on a scale of zero to ten (0 to l0), with automatic input of same to computer
J display and storage means.
It is an additional objective of the invention to perrnit testing other
perceptual abnormality of patients for excessive dyspnea awareness, such as may
occur in the Hyperventilation Syndrome.
It is a further additional objective of the invention to permit testing other
perceptual abnormality of patients for excessive dyspnea awareness, such as may
occur in Chronic Obstructive Pulmonary Disease7 such as Emphysema and/or
Chronic Bronchitis, and/or other respiratory conditions.
It is a final object of the invention to enhance testing sensitivity in a
second mode of operation b~ controlling the testing breathing pattern by visual
biofrr-lh~rlc means while introducing progressive inspiratory loads precisely and
automatically at prt .~et~rrnined time intervals.
These objectives are achieved by a computer based controlling system that
displays the desired patient breathing patterns and real time patient pclr~lmance
for patient biofeedback breathing control, and the patient indication of Borg
defined units of dyspnea level. Inspiratory pressure is sensed7 input to the
computer and automatically adjusted to prr-let-ormined levels. Data integrity isassured by automated detection of the patient's ~reathing pattern exceeding plusor minus tidal volume percentage error limits, with a~ riate indicating alarms.
CA 02238530 l998-05-25
WO 98/14115 PCT/US96/15643
In the first mode of operation the patient follows the prescribed breathing
pattern and tidal volume based on a percentage of the patient's vital capacity at
a constant inspirato~r pressure predetermined as a percentage of the patient's
n~xim~l inspiratory pressure. The resultant data is plotted on a graph with the
5 ~3org IJnit dyspnea level plotted on the vertical "y" ordinate axis verslls Tirne on
the horizontal "x" abscissa axis. Also numerically indicated at one minute
intervals are the number of times the patient's breathing performance failed to
remain within acceptable plus and minus defined parameter error limits.
In the second mode of operation the patient follows the prescribed
10 breathing pattern and tidal volume based on a percentage of the patient's vital
capacity at progressively increasing inspiratol~ resistive loads, starting a zero load
and then automatically increasing by suitable increments, e.g. minus 5 cm. waterpressure at suitable time intervals, e.g. every two mimltes. The resultant data is
plotted on a graph with the Borg Unit dyspnea level on the vertical "y" ordinate5 axis versus Time on the Horizontal "~" abscissa axis, and in addition the
inspiratory pressures are plotted on the vertical "y" axis. Also numerically
indicated at one minute inter~rals are the number of times the patient's breathing
performance failed to remain within acceptable plus and minus defined parameter
error limits.
These and other objects of the invention will be seen in the following
description and in the drawing.
CA 02238530 1998-05-25
WO 98/14115 PCT/US96/1~;643
T~IE D~WING
Fig. l is a simple schematic diagram of the overall system;
Fig. 2 is a schematic diagram of the system and patient interactive devices;
Fig. 3 is a schematic diagram of the Inspiratory Resistive Device, where
5 Fig. 3a is a side view of the Airway Resistor/Stepping Motor Assembly, and
Fig. 3b is a top view of the ~irway Resistive Device, and
Fig. 3c is a side view of the Airway Resistor/Stepping Motor Assembly;
Fig. 4 is a schematic diagram of the breathing Visual Biofeedbaclc Display, where
Fig. 4a is a display of the patient ~ Ling program breathing analogs, and
10 Fig. 4b is a display of proper patient breathing performance matching the
prompting analog display, and
Fig. 4c is a display of inadeqllate patient breathing performance, not achievingthe cursor prompted analog display~ and
Fig. 4d is a display of plus and minus Phantom Line error limits, and the
15 detection of inadequate patient performance, and
Fig. 4e is a display of the prompting breathing analog and the patient real timebreathing performance, with changing symbols and!or color depending on which
error limit the patient's breathing is operative, and
Fig. 4f is display of an exhausted patient ~mable to maintain performance
2 o requirements and therefore termination of the test;
Fig. 5 is a display of the patient data, where
Fig. 5a is a display of Constant Inspiratory Resistance plotting the Borg Dyspnea
Scale and Inspiratory Pressure against time~ and
Fig. 5b is a display of Incremental Inspiratory ~esistance plotting the Borg
~ 2~ Dyspnea Scale and Inspiratory Pressure against time.
CA 02238530 l998-05-25
WO 98/1411~; PCTIUS96/15643
DESC~IPTION OF P~EF~ED EMBODIMENTS
In the following description, metric units and standard respiratory
terminology as defined by the American College of Chest Physicians are
employed unless othervvise stated. Particular attention is directed toward the
5 testing of human subjects for susceptibility to fatal asthmatic attaclcs by detecting
a decreased awareness of dyspnea distress during the imposition of an inspiratory
resistance load. This has been found to be a valid method to test asthmatic
patients in this regard, but the lcnown ~nethods have employed relatively simplemethodology that fails to standardize the testing conditions adequately. The
10 method and apparatus may alternatively be used to test subiects for e~cessive dyspnea awareness as may be present in the abnormal perception-related
condition of Hyperventilation Syndrome, or to define and quantify the dyspnea
of patients with Chronic Obstructive Pulmonary Disease and!or other respiratory
conditions.
The underlying ob~ect of this invention is to define testing conditions for
all a~ iate pulmonaIy function testing procedures in a more precise manner
by visual biofeedbadc means, where the subject is encouraged to follow preciselydefined inspiration and expiration visual analogs and thereby malce the
sensitivity, accuracy and reproducibility of the tested parameter optimally
2 o standardized. This is based on the general observation that the sophistication of
modern pulmonary function testing equipment is usually more accurate than the
methodology and the physiologic parameter being tested, due to the natural
variability of native patient breathing pattems, and the alteration of these
breathing patterns under testing conditions. Therefore, to improve the accuracy
2 5 of the relevant pulmonary function test, the variable patient breathing patterns
must be standardized and quality controlled in order the measuring equipment
CA 02238=730 lsss-o=7-2=7
WO 98/14115 PCT/US96115643
and the measuring methodology produce more valid data on the tested patient
functional parameter.
The underlying concept of the instant invention relates to precise
breathing control using defined visual inspiration and expiration analogs for the
5 subject to follow7 with the subject's respiratory Tidal Volume defined as a
pref~et~orrnined ~-elltage of their Vital Capacity, and the Inspiratory Resistance
load a pr~let.orn~ined percentage of the subject's Maximum Inspiratory Pressure;or alternatively the Inspiratory Resistance being predetermined time incrementalsteps of predetermined resistance loads7 and to automate the procedure.
0 In the ~lere~led embodiment the patient sees a visual analog of inspiration
and expiration on a computer CRT or TV display7 and with a simultaneous
display also visualizes their real time breathing performance analog7 with the
Tidal Volume breath indicated on the vertical ~lly~l ordinate axis plotted against
Time on the horizontal ~Ixll abscissa axis. The patient is instructed to match their
real time breathing performance to the desired performance analog as indicated
by flashing cursor means in the a~ropfiate time domain using so-called visual
biofeedback means; thereby conforming the patientls breathing to a defined
standard breathing pattern. The Tidal Volume breath is determined as a defined
standard percentage7 generally between 25% and 50% of an independently
2 o measured Vital Capacity breath. The Inspiratory Resistive negative pressure load
is determined as a defined standard percentage7 generally between 25% and 75%
of an independently measured Maximum Inspiratory Pressure. I'he ~espiratory
Rate is defined generally between 5 and l 5 breaths per minute. The Inspiration
to Expiration Time Ratio is defined generally between 1: l and l :3. The
Inspiratory Pause Time is defined as a percentage of the Inspiratory Time
CA 02238530 1998-05-25
WO 9811411S PCT/US96tl5643
generally between zero and l 0%, and the Expiratory Pause Time is defined as a
percentage of the Expiratory Time generally between zero and 25%. The
Inspiration and ~ild~ion waveforms are defined as linear or various curvilinear
forms. Thus all the components of the breathing cycle may be defined precisel~
5 and displayed as a visual analog of breath volume plotted against time, and with
the patient following the breathing analog at the flashing cursor the method
therefore becomes an instantaneous breath flow controller as dictated by the
fundamental ec~uation Volume = Flow X Time. In another mode of operation
multiple plus and minus analog error limits as a percentage of the Tidal Volume
10 may be defined and optionally displayed, to detect patient performance falling
outside defined limits, with suitable auditory and/or visual alarms to indicate
deficient performance. Optionall~ the error limit analogs ma~ be hidden from
display, with the displayed patient breathing signal changing shape andlor colordepending on wnich error limit the patient's breathing performance is operative.
In the ~refc~l~cd embodiment the Inspiratory ~esistive Load remains
constant, with the patient attempting to maintain the desired breathing pattern
until unable to maintain said standardized breathing pattern due to fatigue or
excessive r~spir~tory distress. At one minute intervals the patient is prompted to
indicate their perceived dyspnea level in standard Borg numeric units on a scale2 o of zero to ten (0 to 10), zero indicative of no perceived dyspnea and ten being
indicative of maximal perceived dyspnea, by sliding a pointer along a linear
potentiometer or similar device for data input. In an alternate mode of operation
the Inspiratory Resistive Load is progressively incremented in predetermined
negative pressure loads ~ressed as cm. water, for example zero, -2, -S, -10, -15,
-20, -25, -30, etc. cm. water at time intervals between l and 3 minutes.
Inspiratory pressures, the integrated respiratory flow~ridal ~olume, and Borg
CA 02238~30 1998-0~-2~
WO 98/14115 PCT/[rS96/15643
Dyspnea Units are stored in Cc)lllpuL~ memory, and are reported in graphic form7the Borg Dyspnea Units and Inspiratory Pressure plotted on the vertical "y" axiscoordinate against Time on the horizontal "x" axis coordinate. Optionally the
rldal Volumes may be .simil~rly displayed on the vertical "~' axis coordinate. In
5 this manner the patient's level of dyspnea may be plotted against a standardized
breathing pattern and inspiratory load stress, the normal subject indicating
progressive dyspnea, and those subjects susceptible to asthmatic hazard and
potential fatality indicating a minimal dyspnea response to progressive
inspiratory muscle resistive stress, and with Hype~ventilation Syndrome patients0 and those subjects with Chronic Obstructive Pulmonary Diseases and/or other
pulmonary pathological conditions indicating excessive dyspnea at
inappropriately low inspiratory work loads.
In the ~lcrt:llcd embodiment the testing process is automated in the
a~lu~liate time domain, by feedback computer control of inspiratory pressure
15 adjusting a variable inspiratory resistance device by means of a computer
feedback controlled stepping motor. Inspiration Pressures, Tidal Volume, Borg
Unit data and the minute by minute frequency of patient failure to achieve
acceptable breathing performance is stored in computer memory for display and
analysis. In an alternate mode of operation the operator may manually adjust the20 inspiratory pressure with reference to a separate mechanical pressure gauge.
This invention is general as to means and method to control breathing
during breathing testing, and specific as to means and method to control
breathing in a standardized manner while test;ng for dyspnea awareness with
- increasing inspiratory resistance loading and stress of inspiratory musdes, to
25 thereby re~real patients with ina~n~liate and reduced breathing awareness that
11
CA 02238530 1998-05-25
WO 98/14115 PCT/US96115643
might subject them to asthma hazard and potential fatality, though the inventiveconcept would not be limited to specific testing for d~spnea awareness with
inspiratory loading. This invention could also be used specifically to test patients
for excessive dyspnea awareness as may be present in the condition of
5 Hyperventilation S~,rndrome and a variety of pulmonary pathologic conditions,
including Chronic Obstructive Pulmonary Diseases and/or pulmonary Restrictive
Diseases.
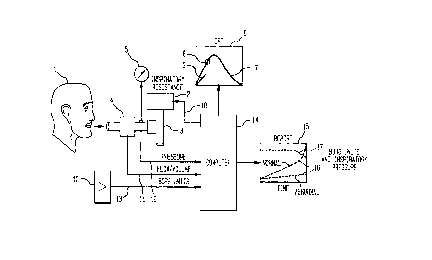
Referring to the simplified schematic diagram in Fig. l the Patient (l)
inspires air through Inspiratory Resistance Device (2) via Directional Respiratory
0 Valve (3). Inspiratory and expiratory air is sensed by Plowrneter (4) and
Mechanical Pressure Meter (5). The Patient (l) observes CRT (6) to visualize
Prescribed Breathing Pattem (7) and by visual biofeedback means following
Prompting Cursor (8) attempts to place their real time Breathing Signal (9) on
the Prescribed Breathing Patten (7). At one minute intervals the patient is
15 prompted to indicate their perceived level of dyspnea on an electro-mechan;cal
Linear Potentiometer ( l 0) calibrated in zero to ten Borg Dyspnea Units. Suitable
differential pressure transducer and integrator means in conjunction with
Flowmeter (4) senses respiratory flow which is integrated into Tidal Volume
Signal (1l), and suitable pressure transducer means provides Respiratory
20 Pressure Signal (12) and Linear Potentiometer (l0) provides Borg Units Signal(13), all of which are input to Computer ~14) data storage and control means,
to be output to Graphic Report (15) of Borg Dyspnea Display (16) and
Inspiratory Pressure Display ( l 7) plotted against Time. Computer ( l 4) provides
Inspiratory Resistance Feedbaclc Control Signal (l 8) to Inspiratory Resistive
25 Device (2).
CA 02238~30 1998-0~-2~
WO 98/14115 PCT/US96115643
The schematic diagram in Fig. 2 is a more detailed overall description of
the system design and the patient interactive devices. Patient ( 1 ) breaths through
Flowmeter ~4) which provides a differential pressure due to Flowmeter
Restrictions ( l 9). The flow generated differential pressure is sensed on each side
5 of Flowmeter Restrictions ( 19) and fletecte~l by Differential Pressure Transducer
(20) with subsequent signal conditioning and analog to digital conversion by
suitable hardware and/or software means for input of Tidal Volume Signal ( 1 1 )to Computer ( 14) . Optionally the differential pressure detection, signal
conditioning and analog to digital conversion may be within Computer (14) or
10 by external devices. Respiratory pressure is sensed by Mechanical Pressure h~eter
(S) and Pressure Transducer (21 )with subsequent signal conditioning and analog
to digital conversion by suitable hardware andlor software means for input of
Respiratory Pressure Signal (12) to Computer (14). Optionally the pressure
detection, signal conditioning and analog to digital conversion may be within
5 Computer ( 14) or by external devices. Within Computer ( 14) all inspiratory and
expiratory pressures at suitable sampling rates, for example 100 Hz., are storedbreath by breath in suitable computer memory array means, and pattern
recognition algorithms detect and similarly store Peak Inspiratory Pressure and
~verage Inspiratory Pressure. Patient ( 1 ) in response to perceived dyspnea level
20 manipulates sliding scale Pointer (22) on Linear Potentiometer (10) to provide
Borg Units Signal (13) to Computer (14). l~espiratory flow is directed by
Directional Respiratory Valve (3) by Inspiration Valve (23) and ~xpiration Valve(24) which vents the patient's unobstructed exhaled breath to roorn air. Patient(1) inspires through Directional Respiratory Valve (3) which is attached to
~ Inspiratory Resistive Device (2). Variable inspiratory respiratory resistance is
- provided by ~espiratory Resistance Plate (25) which exposes a variable sized
orifice to Inspiration Chamber (27) b~r the operator rotating Respiratory
13
CA 02238530 1998-05-25
WO 98/14115 PCT/US96115643
Resistance Plate (25) manually by Handle (26) while observing Mechanical
Pressure Meter (5). In the preferred embodiment Respiratory Pressure Signal
(12) is compared to a pr~letf rrnined desired inspiratory pressure and Computer
(14) generates an appropriate ~nspiratory E~esistance Feedbadc Control Signal
(18) to Stepping Motor (28) and Reduction Gear (29) to turn Respiratory
Resistance Plate (25) to achieve the desired inspiratory pressure. Patient (1)
observes CRT (6) to visualize Prescribed 33reathing Pattern (7) and by visual
biofeedback means following Prompting Cursor (8) attempts to place their real
time Breathing Signal (9) on the Prescribed Breathing Patten (7). ~t one minute
intervals the patient is prompted to indicate their perceived level of dyspnea by
Pointer (22) on electro-mechanical Linear Potentiometer ( 10) calibrated in zeroto ten l~org Dyspnea Units. ~t the conclusion of the test, or optionally
dynamically on a second CRT, data display and Graphics Report (15) are
generated for direct ~riewing or hard copy report. The Borg Dyspnea Display ( 16)
units and Pealc Inspiratory Pressure Display ( 17) units in cm. water, or optionally
the A~verage In~ toly Pressure, is plotted on the vertical '~' axis ordinate versus
Time on the horizontal "x" axis abscissa.
The schematic diagram in Fig. 3 is a ~ore detailed overall description of
the Inspiratory Resistive Device (2). Fig. 3a.) is a side view of Inspiratory
2 o Resistive De~rice (2) and Stepping Motor (28) with Reduction Gear (29) meshing
wit~l Respiratory Resistance Plate (25). ~espiratory Resistance Plate (25) rotates
about a central mount on Respiratory Resistance Device (2) and has a ~andle
(26) to assist manual rotation to permit a variable sized orifice to be exposed to
Inspiration Chamber (27). Fig 3~.) is a top view of Inspiratory l~esistive Device
2 5 (2) and Inspiration Chamber (27~ with centrally mounted Respiratory Resistance
Plate (25) containing Variable Orifice (30). As Respiratory ~esistance Plate (25)
14
CA 02238.730 1998 - 0.7 - 2.7
WO 98114115 PCT/US96/15643
is rotated the opening of Inspiration Chamber (27) will be constricted to a
greater or lesser degree, thereby producing a greater or lesser degree of inspiratory
resistance. Fig 3c.) is a top view of Inspiratory ~esistive Device (2) and Stepping
Motor (28) with Reduction Gear (29) meshing with ~espiratory ~esistance Plate
5 (25), thereby perrnitting motor adjustment of Variable Orifice (30) to
automatically adjust airway resistance by computer controlled feedback means.
The schematic diagrams in Fig. 4 describes various visual biofeedback
images seen on CRT (6). Fig. 4a.) shows Prescribed Breathing Pattern (7)
displayed where Tidal Volume is depicted on the vertical "~' ordinate axis plotted
0 against Time on the horizontal "x" abscissa axis7 indicating Inspiration in anupward direction and Expiration in a downward direction. Fig. 4b.) indicates
proper patient biofeedbaclc breathing performance with the patient Breathing
Signal (9) superimposed on Prescribed Breathing Pattern (7) at Prompting
Cursor (8). Fig. 4c.) indicates inadequate patient biofeedback breathing
15 performance with the patient Breathing Signal (9) falling below Prescribed
Breathing Pattern (7) and Plu~ Ling Cursor (8). Fig. 4d.~ is identical to Fig. 4c.)
and in addition shows plus and minus Phantom Line Error Lirnits (3 l ) above andbelow Prescribed Breathing Pattern (7) with a Negative Error Limit Detection
(32) to trigger a~ iate audio and!or visual alarms. Not shovvn are multiple
20 Phantom Iine error detection limits, for example error limits of plus and minus
10% of Tidal Volume, plus and minus 20%, plus and minus 30%, etc. Fig. 4e.)
is a display of patient pclro~lllance error detection without the display of thePhantom ~ines, wherein only Prescribed Breathing Pattern (7) and patient
Breathing Signal (9) appear. In this option the patient Breathing Signal (9)
25 changes to different graphic characters andlor colors, depending on which zone
of error detection the patient performance is operative. For example, perfect
CA 02238530 1998-05-25
WO 98114115 PCT/US96/15643
matching o~ the patient's breathing pclro~mance with Prescribed Breathing
Program (7) might be indicated by a Small Closed Circle (3dr), acceptable
breathing performance within plus and minus 10% indicated by a Small Open
Cirde(35), and unacceptable patient performance in excess of plus and minus
5 25% indicated by Small Closed Squares (36). Fig.4 f.) is a display of patient
exhaustion wherein the Patient Breathing Signal (9) is unable to follow
Prescribed Breathing Program (7) and is unable to achieve a minimal Tidal
Volume as depicted by Negative Error Limit (33) and thus indicating the need
to terminate the testing procedure.
The schernatic diagrams in Fig. 5 describes various CRT graphic displays
and/or hard copy printed reports of the deri~red data. Fig. 5a.) is the ~tiefe~ d
embodiment wherein the testing procedure has been with constant prescribed
inspiratory resistance load, as determined by a predetermined percentage of the
Maximum Inspiratory Capacity. Graphics ~port (15) plots Borg Dyspnea ~Jnits
and Pealc Inspiratory Pressure on the vertical r ordinate axis, against Time on
the horizontal "x" abscissa axis. Optionally Average Inspiratory Pressure may besubstituted for Peak Inspiratory Pressure. Inspiratory Pressure Display ( I 7) in
this mode of operation is a generally a straight line throughout most of the
testing procedure, reflecting the ability of the patient to inspire the prescribed
20 Tidal Volume breath within the defined parameters of Prescribed Breathing
Pattern (7). Near the end of the testing procedure the inspiratory pressure tends
to diminish, reflecting patient exhaustion and the inability thelcfule to inspire
the full prescribed Tidal Volume breath, though in some cases the patient may
maintain their ability to breathe as prescribed despite fatigue and severe dyspnea.
25 Numeric Parameter Limit Failure (40) is accumulated and indicated at one
minute intervals, and as indicated with increased failure of breathing control as
16
CA 02238530 1998-05-25
WO 98/14115 PCT/US96/lS643
the patient becomes exhausted. ~ Normal Borg ~esponse (37) to progressive
fatigue is indicated. Also shown is abnormal Diminished Borg Response (38)
thereby indicating the patient to be susceptible of developing severe or
potentiaUy fatal asthmatic exacerbations as such patients are relatively unaware5 of the severity of their condition and therefore may not p~omptly seek
a~p~ ,iate urgent medical attention. ~lso shown is Excessive Borg Response
(39~ as may be seen in subjects susceptible to the condition of HyperventilationSyndrome, or subjects with pulmoriary pathologic conditions such as Chronic
Obstructive Pulmonary Disease. Fig. 5b.) is an alternate testing method wherein
10 the inspirato~y resistive load is applied in incremental steps at prescribed times,
e.g. two minute intervals, and with prescribed resistive loads at each incremental
step, e.g. zero, -2, -5, -10, -15, -20, -25, -30, etc. cm. of water pressure, with the
patient breathing in a prescribed manner according to Prescribed Breathing
Pattern (7). Displayed are examples of Normal Borg Response (37), Diminished
5 Borg Response (38) and Excessive Borg Response (39) and numeric Parameter
Limit Failure (40) events.