Note: Descriptions are shown in the official language in which they were submitted.
CA 02239296 1998-06-02
Glycol Coniu~ates as Viral Cell Adhesion Inhibitors
Description
This invention concerns new multivalent derivatives of Neu5Aca2~Gal~1 4GlcNAc
and the production of these compounds. It also concerns the use of these compounds as
viral cell adhesion inhibitors of unadapted human influenza viruses.
The first essential step in a viral infection consists of the adhesion of a virus to the
surface of the host cell. In the case of type A and B influenza viruses, this occurs through
the bonding of viral hemagglutinins (HA) to terminal glycoprotein and glycolipidSialyloligosaccharides on the surface of the host cell. One possible strategy for
preventing influenza infections would be to use cell adhesion inhibitors which would bond
with viral hemagglutinins and limit their reactions with host cells.
In the literature, there is already a range of known influenza virus adhesion
inhibitors. Compounds could be found, especially through the development of multivalent
compounds, that can successfully prevent the adhesion of a particular strain of influenza
in in vitro tests. (See Table 2).
One disadvantage of these known inhibitors is their restricted effective range, the
compounds currently being highly effective only against a single strain and thus not well
suited for practical prophylactic or therapeutic use.
A further disadvantage is that these known inhibitors are currently only being
shown to be effective against adapted influenza strains that are cultivated from the
embryos of hens' eggs. It is known that the cultivation of human influenza viruses from
hens' eggs leads to amino acid mutations in the region of the receptor bond area of
hemagglutinins (Robertson, 1993). It is true that the inhibitors found in this procedure
have a high affinity to the altered hemagglutinins of these mutated viruses but, in many
cases, their effectiveness against wild influenza strains is less (see sialoside BGN-PAA
polymers in Table 1).
The purpose of this invention was to find new inhibitors to viral cell adhesion of
human influenza viruses. These inhibitors were to have a wide effective range against
wild virus strains and thus be as effective as possible.
CA 02239296 1998-06-02
It has been found that multivalent conjugates of the sialosidligands Neu5Aca2-
6Gal~1 4GlcNAc (6'-sialyl-N-acetyllactosamine, 6SLN) are new, highly active viral cell
adhesion inhibitors.
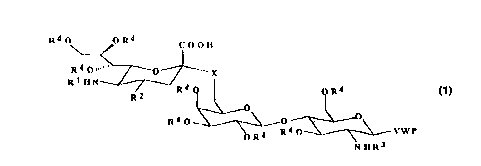
The object of the invention are Formula I compounds
R4 OyOR~ COOEI
R4 Oll~' / ~~J~ X
/2 R4 (~ . . o R4
R4 o~R4~ YW
N ~3
where R' means acyl or thioacyl groups, primarily acetyl, thioacetyl, propionyl or
thiopropionyl group; R2H means hydroxyl, Z-alkyl, substituted Z-alkyl, Z-aryl or substituted
Z-aryl, and Z O co~ I esponds to S or NH; R3 means an acyl or thioacyl group; R4represents H or acyl; X O means S or a linear C,-C4 alkyl; Y NH is O, S, CH2 or a sugar;
W is a bifunctional spacer;
P a multivalent carrier consisting of one of the following substances: polyacrylate,
polyacrylamide, N-substituted polyacrylamide, Metacrylamide, N-substituted
metacrylamide, polyacrylic acid, polycarbonate, polyester, polyamide, polyanhydride,
polyaminocarbonate, polyorthoester, polydioxanon, polyphosphazene, polyhydroxycarbic
acid, polyamino acid, polysaccharide, protein, dextran, chitosan, glucan, liposome,
microparticles.
P can also be a submolecular multivalent carrier such as C(CH2NH-An-)4, where A
is a neutral or negatively charged amino acid.
2s Surprisingly, it also happens that Formula I compounds very efficiently inhibit cell
adhesion in all known type A and B human influenza strains. These inhibitors areparticularly effective against authentic influenza viruses in which the structure of the
receptor bond area of the hemagglutins of influenza viruses is unaltered. In order to
ensure an unaltered hemagglutinin receptor bond area structure, influenza viruses from
clinical isolates were used, cultivated solely from MDCK (Madin-Derby canine kidney)
CA 02239296 1998-06-02
cells. As a PCR comparison of viral RNA sequences from MDCK cultivated cells with
RNA sequences from viruses from clinical isolates has indicated, the MDCK cultivated
viruses show no mutation in the region of hemagglutinin receptor bonds (Robertson,
1 993).
Table 1 shows a comparison of the inhibition of viral cell adhesion through the new
6SLN-PM compound and through the glycol conjugate BGN-PM, which were developed
earlier against H3N2 A/Sichuan/1/87 type hen egg cultivated strains. While this 6SLN-
PM compound is an outstanding inhibitor of all authentic influenza strains, BGN-PM has
a narrower effective range and has very little eMect on many strains.
The advantages of the invention-related Formula I compounds lie particularly in their
effective range as inhibitors of viral cell adhesion in all known Type A and B human
influenza viruses and in the possibilities these compounds open up for prophylactic and
therapeutic purposes for counteracting influenza infections.
A further advantage lies in the easy ~ccess of Formula I compound derivatives, which
carry an additional marker, examples of suitable markers being biotin, dyes, fluorescent
dyes or radioactive markers. These derivatives can be used as components of new,hitherto inaccessible screening systems for the discovery of new viral cell adhesion
inhibitors.
Table 1
Inhibition of influenza viral cell adhesion through the polymers sialoside BGN-PM
and 6SLN-PM, measured through the inhibition of viral bonding to fetuin, as in Example
8 as described by Gambaryan and Matrosovich in 1992.
BGN is Neu5Aca 2-OCH2C5H4NHCOCH2NH-,
6SLN is Neu5Aca2-6Gal,B1 4GlcNAc,B1 -NHCOCH2NH-
CA 02239296 1998-06-02
1. Clinical Human Isolates, Reproduced in MDCK Cells
Virus Affinity of the bonding on Virus Kaff ~M Neu5Ac
BGN-PM 6SLN-PAA
H3N2
AINIBI47/89M 0.03 0.01
A/NIB/3/90 M 0.01 0.01
A/NIB/44/9OM 0.01 0.01
H1 N1
AlEngland/157/83 M >100 0.03
A/NIB/12/89 M >6 0.1
AINIB/23/89 M >6 0.02
A/N IB/50189 M >5 0.02
B
B/England/222/82 M 0.05 0.04
B/NIB/48/90 M 0.05 0.04
B/NIBI15189 M 0.03 0.02
Il. By Cultivation in Hen Egg Adapted Strains
H3N2
X31 (AlAichil2168 100 3
A/Englandl321/77 0.03 0.01
AIUSSRJ3185 0.06 0.1
AlSichuan/1/87 0.02 0.1
H2N2
A/England/12/64 10 0.1
H1 N1
AIFW/1/50 5 3
AIUSSR/90177 6 0.3
A/Chile/1/83 30 6
B
B/USSR/100/83 5 2
B/Ann Arbor/1/86 >100 2
BlYamagata/11/88 >100 100
CA 02239296 1998-06-02
The following is a more detailed explanation of the invention using examples.
Example 1
Production of Neu5Aca2-6Gal,~1-4GlcNAc~1-NHCOCH2NH2 (modified method based on
Likhosherstov et al.,1986; and Manger et al., 1992)
At room temperature, 13.8 mg of 6-Sialyllactosamine NH4-Selz (20 ,uM, isolated
from human urine) was dissolved in a 1 ml saturated ammonium bicarbonate solution and
stirred for 170 hours during the incubation, solid ammonium bicarbonate was added
10 several times to the solution until it was saturated. The reaction solution was diluted with
2 ml water, frozen and Iyophilized. 13.7 mg of residue was obtained (the weight of the
obtained product was greater than the saccharide used, so the residue was again
absorbed in 2 ml of water and Iyophilized), this was absorbed in 0.6 ml 1 M NaHCO3. After
stirring at 0~C, 34 mg of chloracetic acid anhydride (200 I~M) in 0.34 ml ethylacetate were
15 added. After 1 hour, the solution was neutralized with acetic acid and concentrated in a
vacuum. The residue thus obtained was dissolved in a small quantity of water andchromotographed using a Sephadex G-25 column (1x50 cm). The sugarbearing part was
concentrated and the residue was absorbed in 1 ml of saturated ammonium carbonate.
After 48 hours, the solution was diluted with 2 ml of water, frozen and Iyophilized. The
20 residue was dissolved in 2 ml 1 % acetic acid and, after 15 hours, processed using a
column with 3 ml Dowex AG 50W-X4 (H~ form). The column was washed with 30 ml water
and then eluted with a 1 M ammonium hydroxide solution. After concentration of the
ammonium hydroxide eluate, 9.8 mg (67%) of the 6-Sialyllactose N-glycyl derivate was
obtained.
'H-NMR-spectrum (D2O, o, ppm): 5.16 (d, 1H, J2 9 HZ, H-1 Gal), 4.47 (d, 1H, J2 8 HZ, H-1
GlcNAc), 4.00-3.55 (Gal, GlcNAc, Neu5Ac), 3.46 (s, 2H, COCH2NH2), 2 69 (dd, 1 H, J3ax1
12 HZ, J4 4.5 HZ, H-3eq Neu5Ac), 2.04 (s, 6H, 2CH3CO), 1.72 (dd, 1 H, J4 12.5 Hz, H-3ax
Neu5Ac).
CA 02239296 1998-06-02
The reaction process was monitored by thin-layer chromotography (silica gel 60,
Merck):
Eluant 1: 2-Propanol/AcetoneNVater 4:3:2:
6-SLN R1, 0.61
6-SLN-NH2 0.49 ninhydrin positive
6-SLN-NHCOCH2CI 0.66
6-SLN-NHCOCH2NH2 0.09 ninhydrin positive
Eluant 2: Methanol/AcetoneNVater 1:1:1;
6-SLN-NHCOCH2NH2 0.73 ninhydrin positive
Example 2
Production of Neu5Aca2~Gal~14GlcNAc,~1-NHCOCH2NHCO(CH2~4COO-p-C6H,,NO2
At room temperature, 39 mg Bis(4-nitrophenyl)-adipat (100 ~M) in 0.6 ml DMF was
added to a 7.3 mg solution of Neu5Aca2~Gal~14GlcNAc~1-NHCOCH2NH2 (10 ,uM) from
Example 1 in 0.2 ml DMSO. After 3 hours, the reaction mixture was chromatographed
using a Sephadex LH-20 column (1.8x30 cm, eluant - acetonitrile/water 1:1). The sugar
bearing part was concentrated, absorbed in water, frozen and Iyophilized. 7.4 mg of the
glycoside (78%) was obtained.
Example 3
Production of Tetrakis (N-tert-butyloxycarbonyl-~lycyl-amidomethyl) methane
A mixture of 1 g tetrakis (aminomethyl) methane (Fleischer et al., 1971 )
tetrahydrochloride (3.6 mmol), 8.529 BocGlyONph (28.8 mmol) and NEt3 (2.5 ml,
18'mmol) in 2 ml DMF was stirred for 120 hours at room temperature. The reactionmixture was concentrated, suspended in 100 ml ethylacetate, and washed in a 2% H2SO4,
water, saturated NaHCO3 solution and water and dried using Na2SO4. The solution was
concentrated, the residue was dissolved in trifluorethanol, a mixture of
CHCI3/EtOAc/MeOH (9:3:1 ) and Et2O was added. 2.1 g of a crystalline product was
CA 02239296 1998-06-02
obtained (78%).
DC: R,=0.5 in CHCI3/EtOAc/MeOH/AcOH 9:3:3:0.2 (ninhydrin positive, after 5 minutes
processing of the plate with HCI-Gas).
'H-NMR-spectrum in D8-DMSO (o, ppm): 7.97 (br t, 1 H, CCH2NH), 7.37 (t, 1 H, NHGIY), 3.49
(d, 2H, JNH 6 Hz, CH2G'7, 2.76 (br d, 2H, CCH2),1.37 (s, 9H, OCMe3). Mass spectrum: 783
(M+Na).
Example 4
Production of Tetrakis (N-tert-butyloxycarbonyl-tri~lycl-amidomethyl) methane
A solution of 0.76 9 tetrakis (N-tert-butyloxycarbonyl-glycyl-amidomethyl)-methane
(1 mmol) from Example 3 was stirred into 8 ml of CF3COOH for 2 hours at room
temperature. The reaction mixture was mixed with 16 ml toluol and concentrated. The
residue was absorbed in 3 ml water, 0.35 ml of concentrated HCI (12M) was added, the
solution was concentrated and the residue was vacuum dried. The tetraamine thus
formed was suspended in 18 ml DMF and 1.45 9 BocGlyGlyONsu (4.4 mmol) and 0.6 El3N
was added. The reaction solution was stirred for 24 hours at room temperature,
concentrated in a vacuum (0.5-1 Torr) and the residue thus obtained was
chromatographed using a silica gel column (silica gel 60, Merck). Elution with
Me2CO/MeOH/H2O between 30:1:1 and 15:1:1 gave 0.94 9 (77%) of the [illegible] product.
DC: R,=0.79 in Me2CO/MeOH/H2O 15: 1 :1 (ninhydrin positive, after 5 minutes treatment of
the plate with HCI gas).
'H NMR spectrum in D5-DMSO (o, ppm): 8.53 (t, 1 H, NHGIY3), 7.98 (t, 1 H, CCH2NH), 7.81
(t,1 H, NHG'Y2), 6.98 (t,1 H, NHG'Y'), 3.85 (d, 2H, JNH 5.5 HZ, CH2GIY2), 3.73 (d, 2H, JNH 5 5
Hz, CH2GIY3), 3.59 (d, 2H, JNH 6 Hz, CH2GIY'), 2.69 (br. d, 2H, JNH 6.5 Hz, CCH2), 1.38 (s, 9H,
OCMe3). Mass spectrum: 1239 (M + Na).
Example 5
Poly-N-(2-Hydroxethyl) - acrlyamide with 20% mol, 6SLN (6SLN-PM)
CA 02239296 1998-06-02
1.32 mg of poly(4-nitrophenylacrylate) (6.84 I~M) and 7 ml Et3N in 0.132 ml DMF
were added to a 1 mg solution of 6SLN-Gly (1.37 ~M) from Example 1 in 0.1 ml DMSO).
The reaction mixture was incubated at 40~C (The conjugation process was monitered by
DC: the disappearance of sugar spots of the educt), after 24 hours 23 ~l of ethanolamine
5 were added. After a further 15 hours at room temperature, the reaction solution was
chromatographed using a Sephadex LH-20 column (1.5x25, eluent + acetonitril/water 1: 1).
The sugar bearing portion was concentrated, absorbed in water, frozen and Iyophilized.
1.6 mg of conjugate were obtained (90%).
10 Example 6
Polyacryl acid (Ne+ - salt) With 20 % mol 6SLN
1.32 mg of poly(4-nitrophenylacrylate) (6.84 ,uN) in 0.132 ml DMF and Et3N
(7 ,ul) were added to a solution of 1 mg 6SLN-Gly (1.37 ,uM) in 0.1 ml of DMSO. The
15 reaction mixture was incubated at 40~C (The conjugation process was monitored by DC:
the disappearance of sugar spots of the educt), after 24 hours, 230 ,ul of 0.1 M NaOH
were added. After 15 hours at room temperature, 15 ~l of 1 M HCI were added and the
reaction solution was chromatographed using a Sephadex LH-20 column (1.5x25 cm,
eluent - acetonitril/water 1: 1). The sugar bearing portion was concentrated, absorbed in
20 water, frozen and Iyophilized. 1.5 mg of conjugate were obtained (93%).
Example 7
(Neu5Aca2~Gal~1 4GlcNAc~1 -NHCOCH2NHCO(CH2)~CO(NHCH2CO)3NHCH
A 1.2 mg solution of tetrakis (N-tert-butyloxycarbonyl-triglycyl-amidomethyl)
methane (1 IJM) from Example 4 in 0.1 ml CF3COOH was stirred at room temperature.
After 15 hours, 1 ml of toluol was added and the solution was concentrated. The residue
was dissolved in 0.5 ml of water, 5 ,ul of concentrated HCI was added, the solution was
concentrated and the residue was vacuum dried. The tetraamine thus obtained was
suspended in 0.2 ml DMF and a 4.9 mg solution of Neu5Aca2~Gal,B1 4GlcNAc,~1 -
CA 02239296 1998-06-02
NHCOCH2NHCO(CH2)4COO-p-C6H4NO2(5 ~M) in 0.3 ml DMF and 5 ,ul of NEt3 was added.
The reaction mixture was stirred for 15 hours at 20~C. 10 ,ul of concentrated ammoniac
solution was added and after 1 hour the reaction solution was processed using a
Sephadex G-50 (1.2x50 cm) (eluant 0.05 M ammoniac solution).
The sugar bearing portion was concentrated, absorbed in water, frozen and Iyophilized.
2.9 mg of conjugate were obtained (68%).
Example 8
Cultivation of influenza viruses and determination of the inhibition of viral bondin~ to fetuin
The unadapted human influenza viruses AlEngland/157/83 M, AINIB/12189 M,
AINIB/23/89 M, AINIB/50/89 M (H1N1), AJNIB/47/89 M, AINIB/3/90/M, A/NIB/44/90 M
(H3N2), B/England/222/82 M, B/NIB/48/90 M and B/NIB/15/89 M were obtained from the
National Institute for Biological Standardisation and Control (NIBSC, Potters Bar/UK).
They were isolated from clinical samples and cultivated exclusively in MDCK cells.
The influenza viruses that were cultivated in hen eggs were obtained from the virus
collection of the D.l. Ivanovsky Institute for Virology in Moscow. These viruses were
incubated in 9-10 day old embrionic hens' eggs.
For the study of viral bonding, the virus bearing culture stocks and the allentoissh
fluids were separated from any cell fragments by centrifugation. These solutions were,
without further cleaning, either immediately used or stored for up to 4 weeks at 4~C.
The coating of the microlilralion plates with cow fetuin (Fluka, CH) was carried out
as follows: the cavities of a 95er EIA polystyrol micro titration plates (Flow, USA) were
incubated for 2 hours at 37~C with 0.1 ml of a fetuin solution in PBS (10 mg/ml). The plate
was washed with a 0.01 % Tween 20 (Serva, D) solution in PBS and then with distilled
water, and either used directly or air-dried and stored at -20~C until used. In order for the
virus to be adsorbed specifically to the feutin coated plates, the viral cultures were diluted
with PBS to a hemagglutinin titration of 1 :50-1 :200. The plates were then incubated with
0.1 ml of this solution per cavity for 2 hours at 4~C.
The inhibition of the bonding of the horseradish-peroxidase-fetuin conjugate
CA 02239296 1998-06-02
(produced according to the standard Perjodat activation method) to the adsorbed viruses
was determined as follows:
The plates were washed with a 0.01% Tween 20 solution in PBS, then 0.1 ml of a
0.02 ~M solution of the peroxidase-fetuin conjugate along with various concentrations of
the inhibitors to be tested were added. After a 1-hour incubation period at 4~C, the plates
were washed with a cold PBStTween solution.
The peroxidase activity was determined by the addition of 0.1 ml of a substrate
solution (0.4 mg/ml o-phenylenediamine plus 0.02 % H2O2 in a 50 mM Natriumacetalbuffer, pH 5.5). After the addition of the substrate solution, the plates were incubated for
30 minutes in the dark at room temperature, then 0.05 ml of 5% H2SO4 was added and the
absorption was determined at 492 nm with a Titertek Multiscan Reader (Flow, Finland).
For the determination of unspecified bonding, several cavities without the addition
of viruses were incubated on each plate. Only very low levels of unspecified bonding
were found (A492 values of 0.05-0.2).
For the determination of the maximum bonding Ama,~, the bonding of the peroxidase-
fetuin conjugate without inhibitor was measured for each plate. As a further control, each
separate virus strain was replaced with a peroxidase-fetuin conjugate that had previously
been replaced with neurominidase from vibrio choleras: in all cases the conjugate lost its
affinity to viruses, thus proving that the conjugate-virus interaction is determined by
sialyloligosaccharide.
This bonding affinity was calculated according to the following formula:
Cl x Al (Ama~(-Ao)
~ff
Ama,~ (A1-Ao)
where KaffiS the dissociation constant of the virus-inhibitor complexes; AmaX is the
meaured absorption in the absence of inhibitors; C, and A1 are the inhibitor
concentrations used and the corresponding measured absorption; Ao is the absorption
30 measured at inhibitor saturation.
CA 02239296 1998-06-02
The results of the inhibition of the cell adhesion of the new conjugate 6SLN-PM
from Example 5 are summarized in Tables 1 and 2.
Table 2:
Inhibition of viral cell adhesion in influenza viruses using sialoside inhibitors. The
literature data has been su"lnlari~ed and the highest inhibiting activity described above
has been explained. The activity is expressed in ,uM concentrations on Neu5Ac inhibitor
groups that cause 50% inhibition.
The test system used: Hemagglutination inhibition (HAI), inhibition of virus
adsorption to erythrocyte (Al), decrease in viral reproduction in cell cultures (Ml),
decrease in viral bonding to fetuin (FBI).
For comparison purposes, the 6SLN-PM bonding data from Example 5 are
presented at the bottom of the table.
Bonding type Test ~uM Neu5Ac groups Reference
at 50% inhibition
BivalentSialoside HAI30(X31) Glick& Knowles, 1991
HAI180(X31) Sabesanetal., 1992
Cluster Sialoside HAI20 (X31 ) Roy et al., 1993
Liposome sialoside HAI 0.02 (X31 ) Kingery-Wood et al., 1992
HAI 0.33(unknown) Spevaketal., 1993
~ 11
CA 02239296 1998-06-02
Sialylpolymer FBI 0.3 (A/TX177, AIBK/79) Matrosovich etal., 1990
HAI 0.2 (X31 ) Spaltstein & Whitesides,
1991
HAI 0.1 (X31 ) Sparks et al., 1993
HAI 0.2 (X31) Lees et al., 1994
Ml 0.2(X31, H3N2) Itoh etal., 1995
5 (WSN/33, H1 N1 ) Itoh et al., 1995
~400 (SG/57, H2N2) Itoh et al., 1995
FBI >50 (X31, H3N2) Mochalova et al., 1994
0.03(USSR185, H3N2) Mochalovaetal., 1994
10 (Chile/83, H1 N1 ) Mochalova et al. , 1994
10 (B/USSR~83) Mochalova et al., 1994
Ml ~8 (X31, H3N2) Mochalovaetal., 1994
0.2 (USSR/85, H3N2) Mochalova et al., 1994
4 (Chile/83, H1N1) Mochalova et al., 1994
HAI 0.001 (X31, H3N2) Maman etal., 1995
6SLN-PM as FBI 0.02 (A/NIBI23189M, H1 N1 )
described in Example 0.01 (A/NIBI44/9OM, H3N2)
5 of this invention 0.02 (BINIBI15/89M)
CA 02239296 1998-06-02
.
- L~-terature
- Bovln, N.V., Byramova, N.E., ~uzikov, A.B., Mstrosovich, M.N., Mioch~lova, i .V.,
and Gambaryan, A.S. (1992) Viral attachrnent inhlbi~ors~ Patent PCT No.
5 PCT/US92109746.
Byramova, N.E., Mochalovsl L.Y., B~lyanchikov, I.M., ~atrosovich~ t~i.N., and
~ovln, N.V. ~1991 ) Synthecls of slalic acid pseudopolysaccharides by coupling of
cpacer-connected NsuSAc with actlvat~d polymer. J.Carbohydr.C~em. 10, 691-700.
- Fl~ischer, E.i3., Gebala, A.E.~ LOYOY~ A., Tasker, P.~. (1971). Conversion of
alipi~atic and ~licyclic polyalcohols to ths corr~sponding primary polyamlnes. J.Organic
Chem., Vol. 36, No. 20, 3042-3044.
Gambaryan~ A.S. and Matrosovlch~ M.N. (1992) A solid-phase enzyrne-llnked
assay for influenza vlrus receptor-bindlng actlvily. J.Virol,Methods, 39, 111-123,
Gllck, G.D., 2nd Knowlss, J.R. (199~). Molecular recognition of blval~nt
sialosides by infiu~nza ~Irus. J.Amer.Chem.Soc. 113, 47014103.
Gottsche!k, A., Belyavln, G., i3iddl~, F. (1972). Glycoprot~ins as influenz~ virus
haemagglutlnln inhibitors and as cellular virus recep~ors. In "Glycoproteins. Thelr
composition, structure and function", (A.Gottschalk, Ed.), 2-d edition, part B, pp.1082-
1096. ~1tevier Publishing Company, Amsterdam-~ondon-Ne~4-York.
Itoh, M., i~etterlchj P., Isecke, R., Btossmer, R., and Kl~nk, ti~-D. (1995).
- Suprcssion of influen~a virus infection by an N-thioacetylneuraminic aoid acrylamlde
copolymer~reslstant ~o neurzminidase. ~./lrology 212, 340-347 (1995).
Kln~ery-Wood, J.E., \Nilllams, K.W" Sl~al~ G.B., and White~ldes, G.M~, ~1992).
The sgglytlnatlon of erythrocytes ~y influ~nza virus is strongly inhibited by llposomcs
- incorpora~in~ an analog of siàlyl ~arigliosides. J.Amer.Chem.Soc. 114, 7303-7305.
Krizanova, O., and RatnoYa, V. t1969). Serum inhibitors of myxoviruses.
Curr.~op.Microblol.lmmunot. 47,'125-151.
Lees, W.J., Spaltenstein, A., Kln~ery-Wood, J.E., and \~Yhi~eslde9, G.M. (1994).Polyacrylamldes b~arin3 pendant a-si~loside gro~lps strongly Inhlbl~ agglu~ination of
erythrocytes by Influenza A virus: m~ ltlvalency ~nd steric st~bili a~lon of partlculate
blolo~ical syst~ms. J.Med.Chem. 3~, 3419~3433.
13
. CA 02239296 1998-06-02
.
Likhoshorsto~, L.M., ~Jovikova. O.S., I:)ereYitskaJa, V.A, l~ochetkov, N K (1986).
A n~w si~pl~ synthesis of amino s-l~ar b-D-~lycosylarnines. Carbohydralo ~search,
146, C1 -C5.
Mammen, M., Dahmann, G.~ Whltesldes, G.M. (1995) Effective inhlbltors of
5 hemagglutln~tlon by tnfluenza virus syn~hesized from polymers havin~ active ester
~ groups; Insl~hl Into mechanism of Inhibitlon. J.Mod.Chem, 38, 4179~190.
Manger, I.~)., Rademacher, T.W., Dwek, R.A. (1992). 1-N-Glycyl b-
~- oligosaecharide deriYa~lves as stabl~ intermediates for the formatlon of glycoconjugate
probes Eiochemistry, 31, 10724-10732. .
Matrosovlch, M.N., l~iochalova, L.V., Marinina, V.P., Byramo~a~ N.E., and Bovin,N.~/. (1993). Syn~hetlc polymerlc sialosid~ inhlbitors of the Influenza Yirus recepior-
bindin~ activity FEBS Letters 272, 209-212.
Matrosovlch M.N., Gambaryan A S, Tuzikov A.B., Byramova N E, Mochalova
L.V., Golbralkh A.A., Shenderovlch l\l.D., Finne J., and BoYin, N.V. (1993) Probing of
~ 15 ~hc reccpIor-binding si~es of the 1 11 end H3 influenza A ~nd.influcnza B virus
hemag~lutinin~ by synthetlc and.naturei sialosldes. Virolo~y 196, 111~121.
Mochalo~a, L.V., TuzikoY, A.B., Marinina, V.P., Gambaryen, A.S., Byramo~.~a,
N E., Bo-~ln, ~I V., 2nd Matroso~.~lc~l, M.N, (1994) Synthctic potymeric Inhibi'~ors of
influenza vlrus receptor-binding activity suppress virus replication. Antiviral Research
20 23, 1 79-1 90
Robertson, J.S. ~9g3). Clinlcal Influenza virus and the embryonated hen's eg~
~ ReY~Med;Virol. 3, 97-106.- .
Roy, R.! and Lafcrrlere, C.A. (1988). Synlh~sis of antl~enic copolymers of t~i-
acetyl~n~uremlnic acid and their bindlng to wheat çerm a~lutinin and antlbodies.25 Carbohydr.Res. 177, c1 -c4.
Roy, R., 2~nlnl, D., M~unier, S.J., and Romanowska, A. (1993). Solld-phase
synthesis of dendrl~lc slaloside tnhibi~ors of Influ~nza A virut~ herna~glutinin.
J.Chem.Soc., Ch~m. Comrnun. 1993,1869-1872.
Sabesan, S., DUUS, J.O., ~elra, S., Domaille, P., Kelm, S., Palllson, J.C, and
30 ~ock, K. (1992). Clu3ter aialoside inhibitors for Influenza virus: synth~sls, NMR, and
blolo~ical studles. J.Am~r.Chem Soc 114, 8363-8375.
14
~A 02239296 1998-06-02
'
,
Sauter, N.K., Hanson, J.E., Gilck, G.D., Brown, J.H., Crowther, R.L., Par~ S.-J.,
. Skehel, J.J., and Wiley~ ~.C. (1992) Bindin~ of influenza vlrus h~mag~lutlnln to
- analogs of Its cell-surfece recoptor, slalic 2cid; analysis by proton nuclear ma~netlc
resonancs spectloscopy and X-ray crystallo~r2phy. Biochemistry 31, 9609-962l.
Spaltenstein, A r ~nd Whltesides, G ~l. (1931). Polyecryamides b~aring pendanl
a-sialosld~ ~1fOUpS stron~ly inhii~lt a~giutination of erythrocytes by Influenza vlrus.
J.Amer.Ch~m.Soc; 113, 686~87.
Spsvak, W., Na~y, J.O., Charychl D.i~., Schaefer, ~.E ~ Gilbort, J.~., end
8ednarskl, M D. (~993). Polymeri2ed llposome containin~ C-~lycosides of si~lic acld:
potent inhlbltors of influen~a YlrUS in vitro infectlvl~y. .I.Am~r.Chem.Soc. 115, 1146-
1147.
Too~ood, P.L, Gallikerl P.K., Glick, G.D. and Knowles, J.R. (19g1) Monovalen~
slalosides ~hat bind tightly to influen~a A vlrus. J.Med.Chem. 34, 3138-3140.
Watowloh, S.J., Skehel, J.J., and Wiiey, D.C. (1994) Crystal s~ruclures of
influenza Yirus hemagslLJtinln In complex wlth hl~h-affinlty receptor analo~s. Structure
2, 719-~31.
W~lnhold, E.G. and Knowl2s, J.R. (1992) Design and evzluation of a tlgh~ly
bindin~ fluorescent li~and for In~luenza A h~ma~glutlnin. J.Am.Chern Soc. 114, 9270-
~27S.
Wlley, D.C. and Skehel, J.J. (1987) The structure and functlon of the
hemagglu~lnin ~ ,niirar,e Qlycopro~sin of influsnza virus. Ann.Rev.eiochem. 56, 365-
394.