Note: Descriptions are shown in the official language in which they were submitted.
CA 02239520 1998-06-03
f- WO 97/20560 - 1 - PCTIEP96/05132
Description
The use of 1-hydroxy-2-pyridones for the treatment of mucosal disorders
which are difficult to treat
The number of mucosal disorders which are difficult to treat has
considerably increased in recent times, and the increasing trend continues.
The mucosal disorders which are difficult to treat nowadays include
primarily candidoses of the oral and vaginal mucosa.
Candidoses are defined as infections usually caused by Candida albicans,
but also by a large number of other Candida species which grow
opportunistically (C. krusei, C. tropicalis, Candida glabrata and many
others). The yeast-like fungi which are often present anyway in the oral
cavity, the gastrointestinal tract and the vagina grow under particular
conditions and assume parasitic/pathogenic characteristics. Yeast-like
fungi are able in some circumstances to colonize the skin and its
appendages, all mucosae near the skin and several internal organs
(esophagus, lung etc.) and, in this event, induce a remarkably wide range
of disorders.
The occurrence of a candidosis may be favored in particular by pregnancy,
metabolic disorders, infectious diseases, tumors and immunodeficiencies.
Locally favoring factors are regarded as being mechanical irritation (for
example dentures), occlusion, moisture and moist heat.
The occurrence of extensive oral candidosis is nowadays regarded in most
countries as one of the principal clinical signs of impaired functioning of
the
immune system. Persistent oral candidoses indicate in many HIV patients
the transition to immunodecompensation. In advanced immunodeficiency
there are also erosive, and sometimes ulcerative, inflammations involving
the gingiva, and candidal balanitis, candidal vulvitis and Candida-related
CA 02239520 1998-06-03
-2-
anal eczema are not uncommon. Intestinal infections and candidal sepsis
are likewise observed.
In immunocompetent patients, nystatin is regarded as the agent of choice
for local treatment of Candida infections, but clinical experience shows that
nystatin therapy on its own is often insufficient in HIV-infected
immunodeficient patients. In these cases, systemic therapy with
antimycotics of the azole type is widely used. Candida strains resistant to
azoles were virtually unknown up to 1989. However, the treatment of
vaginal candidoses has often proven difficult due to the occurrence of
mixed infections with the protozoal strains Trichomonas vaginalis and
Entamoeba histolytica.
However, since the use of fluconazole for preventing recurrence of
oropharyngeal candidoses in HIV patients, the number of azole resistances
which have become known has increased dramatically. Up to and including
the 1 st half of 1995, 98 publications in the specialist literature reported
resistance of Candida strains to azoles.
The documents EP 0 241 918 or US 4 797 409 describe the preparation of
1-hydroxy-2-pyridones and their use for controlling mainly infections by
fungi and yeast, and pharmaceuticals containing these compounds.
It is therefore an object of the present invention to provide topical
pharmaceutical formulations which are suitable for breaking through
exsistent intrinsic and acquired resistances of Candida strains to azoles,
while simultaneously having activity on the problem organisms
Trichomonas vaginalis and Entamoeba histolytica.
It has now been found that compounds of the formula I are outstandingly
suitable for the treatment of candidoses caused by yeast strains with
intrinsic and acquired azole resistance. Said compounds are also
distinguished by their activity, which is sufficient for therapeutic purposes,
on the problem organisms Trichomonas vaginalis and Entamoeba
o- CA 02239520 1998-06-03
E_ -3-
histolytica which are often the cause of the development of mixed
infections in cases of vaginal candidosis.
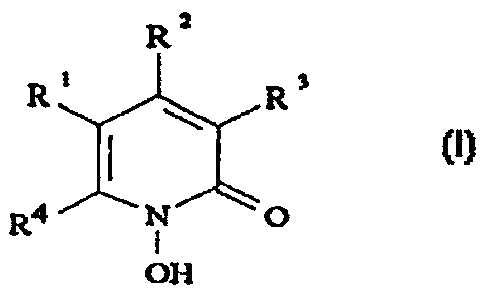
The invention relates to the use of the compound of the formula I
Ra
Ri R3
(n.
R4 N O
I
OH
in which R', R2 and R3, which are identical or different, are hydrogen atom
or alkyl with 1-4 carbon atoms, and R4 is a saturated hydrocarbon radical
with 6 to 9 carbon atoms or a radical of the formula II
i (~.
x-cHa
Y
where
X is S or O,
Y is a hydrogen atom or up to 2 halogen atoms such as chlorine
and/or bromine,
Z is a single bond or the divalent radicals O, S, -CR2- (R = H or C~-C4-
alkyl) or other divalent radicals with 2-10 carbon and, where
appropriate, oxygen and/or sulfur atoms linked to form a chain, and
- when the radicals contain 2 or more oxygen and/or sulfur atoms -
the latter must be separated from one another by at least 2 carbon
atoms, and where 2 adjacent carbon atoms can also be linked
together by a double bond, and the free valencies of the carbon
atoms are saturated by H and/or C~-C4 alkyl groups,
Ar is an aromatic ring system which has up to two rings and can be
substituted by up to three radicals from the group of fluorine,
chlorine, bromine, methoxy, C~-C4-alkyl, trifluoromethyl and
trifluoromethoxy,
for the preparation of a pharmaceutical for the treatment of fungal
r CA 02239520 1998-06-03
-4-
disorders caused by azole-resistant fungi.
The compounds according to the invention are furthermore suitable for the
treatment of trichomoniasis, one of the commonest causes, which is
spread around the world, of non-gonorrheal urethritis. The disorder is
caused by the pathogen Trichomonas vaginalis which is one of the
protozoa.
The carbon chain members in the "Z" radicals are preferably CH2 groups.
If the CH2 groups are substituted by C~-C4-alkyl groups, CH3 and C2H5 are
preferred substituents.
Examples of "Z" radicals are:
-O-, -S-, -CH2-, -(CH2)m (m=2-10), -C(CH3)2-, -CH20-, -OCH2-, -CH2S-,
-S CH2-, -S CH (CZHS)-, -CH=CH-CH20-, -O-CH2-CH=CH-CH20-, -O CH2-
CH20-, -O CHZ-CH2CH20-, -S CH2 CH2 CH2 S-, - S CH2 CH2 CH2 CH2
O-, -S CH2 CH2 O CHZ CH2 O-, -S CH2 CH2 O CH2 CH2 O-CH2 CHZ S-, or
-S-CH2-C (CH3)2-CH2-S-.
The radical "S" is a sulfur atom, and the radical "O" is an oxygen atom. The
term "Ar" means phenyl and condensed systems such as naphthyl,
tetrahydronaphthyl and indenyl, and isolated systems such as those
derived from biphenyl, diphenylalkanes, Biphenyl ethers and Biphenyl
thioethers.
Principal representatives of the class of compounds characterized by the
formula I are:
6-[4-(4-chlorophenoxy)phenoxymethyl]-1-hyd roxy-4.-methyl-2-pyridone;
6-[4-(2,4-dichlorophenoxy)phenoxymethyl]-1-hydroxy-4.-methyl-2-pyridone;
6-(4-biphenylyloxymethyl)-1-hydroxy-4.-methyl-2-pyridone;
6-(4-benzyl phenoxymethyl )-1-hydroxy-4.-methyl-2-pyridone;
6-[4-(2,4-dichlorobenzyloxy)phenoxymethyl]-1-hydroxy-4-methyl-2-
pyridone;
6-[4-(4-chlorophenoxy)phenoxymethyl]-1-hydroxy-3,4-dimethyl-2-pyridone;
r CA 02239520 1998-06-03
-5-
6-[4-(2,4-dichlorobenzyl)phenoxymethyl]-1-hydroxy-3,4-dimethyl-2-
pyridone;
6-[4-(cinnamyloxy)phenoxymethyl]-1-hydroxy-4-methyl-2-pyridone;
1-hyd roxy-4-methyl-6-[4-(4-trifluoromethylphenoxy)phenoxymethyl]-2-
pyridone;
1-hydroxy-4.-methyl-6-cyclohexyl-2-pyridone
or
1-hydroxy-4.-methyl-6-(2,4,4-trimethylpentyl)-2-pyridone.
The abovementioned compounds of the formula I can be employed both in
free form and in the form of salts, and use in free form is preferred.
The compounds of the formula I to be employed in the formulations can be
prepared, for example, by processes disclosed in US 2 540 218 or US
4 797 409.
The term "azole-resistant fungi" means all species of fungi or yeasts which
have become resistant to antimycotics, for example to antimycotics which
contain azole residues, such as fluconazole.
Immunosuppressed patients are preferably treated such as diabetics,
asthmatics, smokers, AIDS patients, patients before and after transplants,
cancer patients, patients chronically treated with antibiotics, cytostatics or
corticosteroids, patients with antimycotic-resistant fungi, in particular
patients with fluconazole-resistant fungi or elderly people.
Suitable for the use of said compounds according to the invention are
liquid, semisolid and solid pharmaceutical formulations, in particular
solutions, cream, ointment and gel formulations, and pastilles and
pessaries.
The active substance is incorporated into the formulations according to the
invention in amounts which are normally between about 0.05 and about
5%, preferably between 0.1 and 1 %.
CA 02239520 1998-06-03
-6-
Topical treatment of candidoses caused by yeast strains with intrinsic and
acquired azole resistance with the pharmaceuticals according to the
invention is able to achieve an effective cure.
The compositions according to the invention can also be employed
successfully for the treatment of vaginal candidoses with mixed infections
by the protozoal strains Trichomonas vaginalis and Entamoeba histolytica.
Example 1
A formulation according to the invention has the following composition:
6-[4-(4-Chlorophenoxy)phenoxymethyl]-1-hydroxy-4-methyl-0.50
2(1 H)-pyridone
Hydroxyethylcellulose 1.50
Polyethylene glycol 7 glyceryl cocoate 5.00
1,2-Propylene glycol 10.00
Isopropyl alcohol 20.00
Demineralized water 63.00
Example 2
A formulation according to the invention has the following composition:
6-[4-(4-Chlorophenoxy)phenoxymethyl]-1-hydroxy-4-methyl-1.00
2(1 H)-pyridone
2-Octyldodecanol 5.00
Liquid paraffin 5.00
Cetyl alcohol 5.00
Stearyl alcohol 5.00
Myristyl alcohol 5.00
Polyoxyethylene 20 sorbitan monostearate 3.00
Sorbitan monostearate 2.00
Demineralized water 69.00
CA 02239520 2004-07-13
-7-
Example 3
A formulation according to the invention has the following composition:
6-Cyclohexyl-1-hydroxyl-methyl-2(1 H)-pyridone 5 mg
Polyethylene glycol 1500 1500 mg
Polyethylene glycol 4000 1000 mg
Polyethylene glycol 6000 165 mg
Sodium bicarbonate 180 mg
Tartaric acid 150 mg
Example 4
A formulation according to the invention has the following composition:
6-[4-(4-Chlorophenoxy)phenoxymethyl]-1-hydroxy-4-methyl- 10 mg
2(1 H~pyridone
Tylose C 1000 P
30 mg
Polyethylene glycol 6000 500 mg
Mannitol 305 mg
Sodium stearyl fumarate 5 mg
Example 5
Test of efficacy
Determination of the efficacy of 6-[4-(4-chlorophenoxy)phenoxymethyl]-1-
hydroxy-4-methyl-2(1 H~pyridone (Compound 1 ) on fluconazole-resistant
strains of Candida aibicans
Fluconazole-resistant strains of Candida albicans are isolated from
patients who have been treated, for example, with fluconazole for more
than one year. For this purpose, samples are taken from the patients'
mouths and applied, undiluted or diluted 1:100, to an RPMI 1640 agar
(Gibco/BRL, Life Technologies GmbH, D-76339 Eggenstein) which
contains about 1.0 Ng/ml fluconazole. Resistant Candida albicans strains
are isolated, further purified on agar and stored isolated in
,.. CA 02239520 1998-06-03
_- _$_
peptone/dextrose slant agar tubes.
The activity of Compound 1 and fluconazole is determined by the microtiter
dilution technique in RPMI 1640 medium. The growth medium RPMI 1640
buffered with 0.165 M morpholinopropanesulfonic acid pH 7.0 is introduced
into 96-well microtiter plates. Serial dilutions by a factor of 2 are prepared
for Compound 1 and fluconazole to result in final concentrations of 256 to
0.002 Ng/ml of Compound 1 and fluconazole. The microtiter plates
prepared in this way are incubated with the Candida strains to be tested.
For comparison purposes, the finro Candida albicans strains ATCC 90028
and 90029 which are not resistant to fluconazole are included in the test.
The initial cell count is 1-5 x 103 colony-forming units per ml of growth
medium. The microtiter plates are incubated at 35°C for 48 hours. The
minimum inhibitory concentration is determined by photometry at 510 nm.
Table 1 shows the results:
CA 02239520 1998-06-03
r - _ 9
Table 1
Minimum inhibito
concentration MIC
ml
Strain Fluconazole Com ound 1
Candida albicans ATCC 900280.5 1
Candida aibicans ATCC 900291 0,5
Candida albicans 94/3 32 2
Candida albicans 94/14 32 2
Candida albicans 94/57 > 256 1
Candida albicans 94/62 > 256 1
Candida albicans 94/90 > 256 1
Candida albicans 94/118 > 256 2
Candida albicans 94/134 > 256 1
Candida albicans 94/138 > 256 1
Candida aibicans 94:/222 > 256 1
Candida albicans 94/231 > 256 1
Candida albicans B6 1 1
Candida albicans B70 > 256 1
Candida aibicans B75 2 1
Candida krusei B1 32 0.5
Candida krusei B4 16 1
Candida labrata B12 4 0.5
Candida labrata B14 2 0.5
Candida labrata B18 8 0.5
Candida labrata B21 8 1
Candida labrata B35 8 1
Candida labrata B37 16 1
Candida labrata B38 16 1
Candida labrata B39 8 0.5
Candida labrata B40 16 1
Candida labrata B50 16 0.5
Candida labrata B51 32 0.5
Candida uillermondii B47 4 1
CA 02239520 1998-06-03
-10-
Table 1 shows that Compound 1 impedes the growth of
Candida strains in a very narrow concentration range -
irrespective of existent fluconazole resistance.
Example 6
In vitro activity of the Compound 1 on protozoa
n = MIC /ml
Trichomonas vaginalis 5 31.2~3~ , 125~2~
Entamoeba histolytica 4 31.2 , 62.5~3~
n= number of strains investigated;
the number given in parentheses corresponds to the number
of tested strains with which the stated MIC was determined.