Note: Descriptions are shown in the official language in which they were submitted.
' v' CA 02239791 1998-06-17 2.
Separation of middle boilers from a mixture of low,
middle and high boilers
The present invention relates to a process for separating
middle boilers from a mixture of low, middle and high
boilers which is separated into a fraction containing low
and middle boilers and a fraction containing low and high
boilers.
A problem frequently encountered in the chemical industry
is the need to separate the middle boilers in pure form
or with only traces of low boilers from a liquid multi-
substance mixture consisting of a low- (L), middle- (M)
and high-boiler (H) fraction.
To do this it is possible to employ the known distil-
lation methods, for example those described in Ullmann's
Encyclopedia of Industrial Chemistry Vol. B3, page 4-46
et seq. A common feature of the known' distillation
methods is that the high boilers are drawn off at the
bottom in pure form, or possibly with residual traces of
middle boilers, and that the middle-boiling component is
separated off at the top of the column at temperatures
determined largely by .the concentration of the high-
boiling component and its boiling temperature. Further-
more, with the known methods it is not possible to co-
separate a mixture of low and middle boilers while at the
same time separating out a mixture of low and high
boilers which is free from middle boilers. In many cases,
however, this would be desirable, especially if low and
high boilers are to be put to some further conjoint use
(sale, recovery, disposal).
Page 4-48 of the abovementioned publication describes the
use of side columns for separating middle boilers from
the mixture of low, middle and high boilers (L,M,H
mixture). In this case too, low and high boilers are
always separated. The same applies to the directly or
indirectly coupled columns describe on pages 4-62 and
CA 02239791 1998-06-17
- 2 -
4-63 in the abovementioned publication. In all of these
cases it is ultimately necessary to separate the middle-
boiling component from the high-boiling component by
means of distillation, which in every case requires
boiling temperatures which are at least equal to that of
the middle-boiling component and, in extreme cases, are
close to the boiling temperature of the high-boiling
component and are therefore very high. This is partic-
ularly the case a.f the middle-boiling component must be
separated completely from the high-boiling component.
Such high temperatures may arise that, even in the case
of relatively heat-stable substances, decomposition or
chemical conversion (polymerization etc.) of the sub-
stances involved may occur. For this reason, complex
distillation techniques, for example gentle distillations
carried out under reduced-pressure conditions (thin-film
_. evaporators, molecular jet distillation, etc.) are often
necessary far separation tasks of this kind. Such distil
lation techniques have the disadvantage that the through
puts are extremely low. This leads to high capital
investment and product costs, which may mean that a
distillative separation which per se is advantageous may
not be economic to carry out.
Also known are special techniques for separating
difficult-to-separate liquid mixtures. Special techniques
are only relevant if they are more cost-effective or when
other, common techniques have failed. They are frequently
employed with substances whose capacity to withstand
thermal stress is limited, ie. if the boiling point is
above or close to the decomposition temperature. A known
method of separating components of low volatility from
mixtures comprising immiscible components is that of
carrier-gas distillation. The basis for this method is
that, in a mixture of immiscible substances, each
substance behaves as if the other was not there; in other
words, at a given temperature, each substance possesses
a partial pressure which - independently of the
composition of the mixture -is equal to the vapor
CA 02239791 1998-06-17
- 3 -
pressure of the substance concerned. Consequently, the
pressure over such a mixture a.s equal to the sum of the
vapor pressures of the individual components. A known
example of this is the water/bromobenzene system. The
mixture boils at 95°C, whereas the pure substances boil
at 100°C (water) and 156°C (bromobenzene). Carrier-gas
distillation is suitable in particular for separating
miscible components of relatively high boiling point
(eg. glycerol), for separating substances which
polymerize or decompose even before reaching the boiling
point (fatty acids), and for separating substances which
are very difficult to handle and for which direct heating
to the boiling point may be hazardous (eg. turpentine).
The best-luiown example of carrier-gas distillation is
.15 steam distillation, ie. where steam is the carrier gas.
__ It is extensively employed, for example, in the pet-
roleum-processing industry. for removing light hydro-
carbons from absorber oils; in the coal industry, for the
steam distillation of hydrocarbon cuts from the coal
distillation operation; for separating off turpentine
from resins in the rubber industry; and a.n preparative
organic chemistry. Steam distillation is a special form
of azeotropic or extractive distillation, as described in
the abovementioned publication on pages 4-50 to 4-52. The
technical effect of this process is based on the finding
that, by adding a substitute substance (an entrainer),
the azeotropic point is surpassed and, consequently, the
desired concentration above the azeotropic point is
achieved.
All of these techniques have the disadvantage that an
additive (entrainer) is introduced into the system which
is to be distilled, and this entrainer then has to be
separated off from the system again by way of an add-
itional process step.
A further known method of removing relatively high-
boiling substances from a mixture of substances is
CA 02239791 1998-06-17
- 4 -
stripping. Stripping has the disadvantage that it always
produces only a highly dilute solution of the high-
boiling component or middle-boiling component a.n the
stripping medium, thereby necessitating a laborious and
costly separation. In general, the process is only
economical when the products can be separated by phase
separation, ie. when the mixture of substances exhibits
a miscibility gap.
It is an object of the present invention, therefore, to
provide a simple and gentle process for separating a
middle-boiling component or a fraction comprising low and
middle boilers from a mixture which includes low, middle
and high boilers.
We have found that this object can be achieved, sur
_. 15 prisingly, if the abovementioned mixture in a column is
treated in the bottom with low-boiler vapor.
The present invention consequently provides a process for
separating a fraction containing low and middle boilers
(L,M fraction) and a fraction containing low and high
boilers (L,H fraction) from a homogeneous mixture com-
prising low, middle and high boilers (L,M,H mixture),
which comprises treating the L,M,H mixture in a column
with low-boiler vapor and separating it into an L,M
fraction and an L,H fraction. The middle-boiling
component accumulates in the low-boiler vapor, so that
the L,M fraction can be recovered above the infeed site
of the mixture, and the L,H fraction is obtained in the
liquid phase.
The mixture to be separated is generally passed directly
to the top of the column. The treatment of the mixture
with the low-boiler vapor is preferably carried out in
countercurrent and, in particular, by passing low-boiler
vapor into the bottom of the column or by. supplying
liquid low-boiling component and boiling it up in the
bottom. The low-boiling component supplied to the column
' CA 02239791 1998-06-17
- 5 -
is usually the same as that present a.n the mixture.
It has been found particularly advantageous to carry out
the treatment with low-boiler vapor in a stripping
column. This can be a customary plate column, for example
a bubble-cap or sieve plate column, or can be provided
with a customary packing, for example Raschig rings, Pall
rings, saddles, etc., and preferably has a theoretical
plate number in the range from 5 to 100. Depending on the
separation task at hand, the plate number may even be
more than 100.
As a result of passing low-boiler vapor into the bottom
of the column, the middle-boiling component accumulates
in the low-boiler vapor. The L,M fxaction is
advantageously obtained at or above the vertical level of
-- 15 the infeed plate. Preferably, the L,M fraction is drawn
off from the top of the column.
The L,M fraction generally comprises the low-boiling
component in a large to very large excess. It is there-
fore particularly advantageous to concentrate the L,M
fraction in order to enrich it with middle-boiling
component. This can be done,' for example, by passing the
L,M fraction into a separate, multistage column, which
serves as a rectifying column, in which the low-boiling
component is separated off to give an L,M fraction richer
in middle-boiling component, or even a pure middle-
boiling component.
It is particularly preferable to provide the rectifying
column as a separate distillation column or to mount it
directly onto the column in which treatment with low-
boiler vapor takes place and to distill off the low-
boiling component from the top. The enriched L,M fraction
or the middle-boiling component can be removed via a
sidestream offtake of ,the column return flow. It is
particularly preferred in this context to employ an
essentially vertical dividing wall. In this case, the
' CA 02239791 1998-06-17
- 6 -
mixture which is to be separated is supplied approxim-
ately in the center of the stripping-rectifying column.
At the vertical level of this supply point, a dividing
wall is installed in the column over an extent of in
general from 1 to 10, from 1 to 5, theoretical plates in
such a way that the column is divided vertically into two
separate sections, infeed taking place approximately in
the center of the dividing wall. In this way, the frac-
tion enriched with medium-boiling component can be taken
off on the side opposite the infeed site, in the region
of the dividing wall. The dividing wall separates the
~offtake site from the infeed site. Equal concentrations
of medium-boiling component are present on either side of
the dividing wall, although high boilers are present in
the mixture only on the infeed-site side. The fraction
enriched with medium-boiling component is preferably
-.- taken off approximately at the vertical level of the
infeed or, if appropriate, somewhat below this point.
As an alternative to the embodiment including a dividing
wall, it is also possible to mount a side column up -
against the stripping-rectifying column, in such a way
that the side column communicates with the gaseous and
liquid phases of the stripping-rectifying column in each
case at one or more separating stages above and below the
infeed site, and the fraction richer in middle-boiling
component is taken off via the side column. The side
column is configured such that high-boiling component
cannot pass over to the offtake side of the side column.
Measures suitable for this purpose are familiar to the
skilled worker.
If desired, a droplet separator (demister or other
customary device) may be installed over the infeed plate
or in the vapor offtake in such a Way as to prevent the
high-boiling component being entrained by means of
droplets.
The L,M fraction enriched in middle-boiling component by
CA 02239791 1998-06-17
- 7 _
the abovementioned rectifying column'may, if desired, be a
separated or concentrated in a further column with a
rectifying section and a stripping section.
A further advantageous embodiment of the novel process
comprises passing the vapors from the stripping column or
stripping-distillation column, possibly after conven-
tional compression, as low-boiling components or low-
boiler vapor back into the bottom of the treatment
column. Since in the novel process direct heating takes
place with low-boiling component or low-boiler vapor, and
vapor compression need only overcome the differential
pressure over the column, it is possible to bring about
a drastic reduction in energy consumption and, at the
same time, in the input required for cooling.
_. 15 The treatment column and/or rectifier or distillation
column can be operated at atmospheric, subatmospheric or
superatmospheric pressure and continuously or batchwise.
Against this background, the conditions depend of course
on the mixtures to be separated, and can be determined by
the skilled worker i.n a conventional manner. A critical
factor is the temperature of the low-boiler vapor, which
must be high enough for the L,M fraction to be distilled
off and the L,H fraction to be obtained in the bottom of
the column.
The novel process has the advantage that it is easy to
carry out and that it manages without the addition of any
extraneous substance. The concentration of middle-boiling
component is low over the entire process range. The
residence time in the process, ie. in the columns, is
relatively short. Owing to the simplicity of the process,
the capital investment required is low. Moreover, the
process is almost infinite in its scale-up possibilities.
The novel process makes it possible to carry out ex-
tremely gentle separation of an L,M fraction or of the
middle-boiling component from a mixture comprising low,
CA 02239791 1998-06-17
- g -
middle and high boilers, at the temperature level of the
boiling point of the low-boiling component. The process
is therefore particularly advantageous if it is necessary
to separate as gently as possible a thermally sensitive
middle-boiling, component, which has a tendency, for
example, to undergo decomposition or polymerization, from
an L,M,Fi mixture. The process is, particularly
advantageous if the high-boiling component present in the
crude mixture is, in its pure or highly enriched form,
alternatively of high viscosity, precipitates as a solid,
or at a relatively high concentration has a tendency to
enter into a chemical reaction, for example a
polymerization. The novel process in fact ensures that
the high-boiling component dissolved in the low-boiling
component can be stripped off. As a result, it is
necessary only to handle solutions; in other words,
-- problems with viscosity, solids, etc., are avoided.
The novel process a.s particularly suitable for obtaining
thermally sensitive products. Examples of this appli
cation are:
- obtaining an aqueous hydroxylamine solution from an
aqueous solution of a hydroxylamine salt,
- obtaining polymerizable compounds, for example
recovering styrene from mixtures obtained in the
production of styrene,
- obtaining chlorinated hydrocarbons, for example
recovering dichloroethane from mixtures obtained in
the production of dichloroethane,
- recovering carboxylic acids and aldehydes from the
stripping acids of cyclohexane oxidation with air or
from the production of adipic acid,
- separating organic acids and aldehydes, such as
acetic acid, acrylic acid, methacrolein or
methacrylic acid, from production effluence which
may still contain high boilers, organic compounds,
salts (catalysts), etc., and
- separating amines from mixtures comprising ammonia
and high boilers.
CA 02239791 1998-06-17
- 9 -
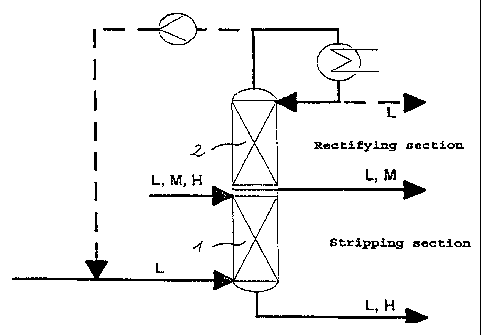
The novel process is illustrated in more detail with
reference to the layout shown in Figure 1:
Figure 1 shows a column for separating an L,M,H mixture,
comprising a stripping column 1 on which a rectifying
column. 2 is mounted. The mixture to be separated is
supplied directly to the top of the stripping column 1.
Low-boiler vapor L is passed into the bottom of the
stripping column 1 in countercurrent to this mixture. At
the bottom of the column, an L,H fraction is taken off,
while at the top of the column. an L,M fraction is
obtained which a.s essentially free from high-boiling
components. This fraction a.s concentrated, ie. enriched
with medium-boiling component, in the rectifying column.
The enriched L,M fraction is taken off somewhat above the
infeed site of the mixture to be separated. Low-boiling
. component is obtained at the top of the rectifying
column, and can if desired be condensed and passed on for
subsequent utilization. Alternatively, the low-boiling
component can be passed back, directly or after
compression, into the bottom of the stripping column 1.
The examples which follow illustrate the invention
without restricting it.
Example 1
Obtaining an aqueous hydroxylamine (HA) solution from a
hydroxylamine (HA)/ammonium sulfate (AS) solution using
a stripping column
An aqueous solution containing 218 g of HA/1 and 680 g of
AS/1 was supplied at a rate of 300 ml/h to the topmost
stage of a stripping column. The stripping column was
made of glass, had a height of 2 m and a diameter of
mm, and was packed over a vertical extent of 1.8 m
with 3 mm glass Raschig rings. 1000 ml/h of distilled
water were supplied to the bottom of the column. The
column was under a pressure of 40 kPa. The bottom
35 temperature was 84°C. 1000 ml/h of aqueous, salt-free HA
CA 02239791 1998-06-17
- 10 -
solution were distilled off at a rate of 39..0 g of HA/h
from the top of the column, corresponding to 59.6 of the
entire HA in the feed. 300 ml/h of ammonium sulfate
solution with an HA content of 86.0 g of HA/1 were taken
off from the bottom of the column. This corresponds to .
39.4 of the total HA in the feed.
The maximum concentration of FiA in the column was 100
g/1. The quantity of liquid in the column, depending on
the particular loading, was 20-225 ml. The residence time
of the liquid in the column was therefore only 1.5-10
minutes. The decomposition rate a.s low at this low
concentration and within the short time.
Further experiments are set out a.n the table below.
. - Table 1
Separation of an aqueous HA solution from an aqueous
HAAS solution
Fead HA coatnat8=O/- PressureColuma-headHA from HA at the
ml/h g/1 Steam kPa temper- the bottom
ature top
'C g/1 (k)
g/1 (~t)
318 Z22 11561 50.0 81.0 40.5 66.9 48.6 21.2
2 170 222 1060 70.0 90.5 22.8 65.6 45.2 17.2
0
370 219 1475A 100.4 100.9 32.4 62.2 75.6 47.8
179 105.5 153011100.8 100.6 9.0 70.5 29.0 27.6
245 220.0 1530A 100.8 100.6 28.0 73.3 54.0 42.2
150 4 990/1 100.8 100.0 0.4 68.1 0.8 15.7
2 150 5.6 990A 100.8 99.9 0.6 73.0 0.4 5.6
5
119 20.4 1063A 101.5 100.4 15.4 67.6 40.5 19.7
* The bottom of the column was heated by way of a
thermostat.
/1 For simultaneous bottom heating, the water was
30 supplied as superheated steam.
Example 2
CA 02239791 1998-06-17
- 11 -
Separation of an aqueous HA solution from an aqueous
HA/NazSO~ solution using a stripping column
The aqueous solution from Example 3, containing 11'~k by
weight of HA and 23.6 by weight of Na2S0~, was supplied
at a rate of 978 g/h to the topmost stage of a stripping
column. The stripping column was made of enamel, had a
height of 2 m and a diameter of 50 ~, and was packed
with 5 mm glass Raschig rings. The column Was at atmos-
pheric pressure. Steam at 2.5 bar absolute was passed
into the bottom of the column. The steam/feed ratio was
2.9:1. 985 g/h of sodium sulfate solution with an HA
content of 1.7 g of FiA/1 were taken off from the bottom
of the column. This corresponds to 1~ of the entire HA in
the feed. 3593 g/h of aqueous, salt-free HA solution
containing 36.8 g of HA/1 were distilled off from the top
-- of the column, corresponding to 99.2 of the entire HA in
the feed.
Further experiments are set out in the table below.
Table 2:
Separating an aqueous HA solution from an aqueous HA
sodium sulfate solution
CA 02239791 1998-06-17
- 12 -
Feed HA contentSteam/ PressureColumn-headHA from HA at the
thn
g/h g/1 Feed kPa temper- top bottom
kg/kg ature
'C g/1 ('t) g/1
945 135 2.6 200 125.4 34.0 84.07.8 17
970 136 2.7 101 106.3 35.5 96.23.3 2.5
980 80 2.8 101 107.0 2.1 95.5 0.45 5.7
Example 3
Obtaining an aqueous HA solution from an aqueous
HA/sodium sulfate solution using a stripping-distillation
column
An aqueous solution containing 221 g of HA/1 and 540 g of
AS/1 was supplied at a rate of 202 ml/h onto the 11th
_. tray of a glass bubble-cap column with a diameter of
35 mm, an overall height of 1.6 m and with 21 trays
(lowest tray - tray 1). 1300 ml/h of steam (at about
125°C) were supplied to the bottom of the column. The
pressure in the column was 99 kPa. At the top of the
column, 180 ml/h of substantially HA-free water (0.6 g
of HA/1) were taken off at a column-head temperature of
99.8°C and with a reflux ratio of 1:3 (return flow .
infeed). The aqueous HA solution (product solution) was
taken off at a rate of 1180 ml/h and with a concentration
of 44 g/1 via a sidestream, from tray 12. 400 ml/h of
salt solution were taken off at the bottom of the column.
Example 4
Obtaining an aqueous HA solution from an aqueous HA
sodium sulfate solution using a stripping-distillation
column, with concentration via a side offtake.
An aqueous HA solution as in Example 3, containing 11~ by
weight of HA and 23.6 by weight of Na2S04, was supplied
to the 11th theoretical plate of a glass bubble-cap
column with a diameter of 50 mm (tray number
corresponding to 30 theoretical plates). Steam at 2.5 bar
. ' CA 02239791 1998-06-17
- 13 -
absolute and at about 125°C was supplied to the bottom of
the column. The pressure in the column was 101 kPa.
Substantially HA-free water (0.05 g of HA/1) was taken
off at the top of the columa. The aqueous, salt-free HA
solution (product solution) was withdrawn in liquid form,
with a concentration of~8.3~ by weight, via a sidestream
from tray 12. The salt solution was taken off at the
bottom of the column with a residual HA content of 0.2~
by weight.
Example 5
Concentration of a salt-free aqueous hydroxylamine
solution by distillation
In a glass bubble-cap column with a diameter of 50 ~ and
30 bubble-cap trays, 1600 g/h of an 8.3~ strength by
.- 15 weight aqueous, salt-free stabilized hydroxylamine
solution were fed continuously onto the 8th tray. In
addition, a small quantity of stabilizer, dissolved a.n
hydroxylamine solution, was metered into the column at
the topmost tray, tray no. 30. The reflux ratio was set
at 0.5. Water was distilled off from the top of the
column. The distillate still contained a residual quan
tity of hydroxylamine of 0.07 by weight. About 240 ml/h
of a 50~ strength by weight hydroxylamine solution were
discharged by means of a pump from the bottom of the
column.