Note: Descriptions are shown in the official language in which they were submitted.
CA 02239907 1998-06-08
W O 96/39942 PCTrUS96/07970
LESS INVASIVE DEVICES AND M~ DS FOR
TREATMENT OF CARDIAC VA~VES
Field of the Invention
The present invention relates generally to devices
and methods ~or performing surgery on the heart. More
specifically, the invention relates to less-invasive devices
and methods for the surgical treatment of diseased heart
valves.
R~R~OUND OF THE lNvh:NllON
Heart valve disease is a widespread condition in
which one or more of the valves of the heart fails to ~unction
properly. Diseased heart valves may be categorized as either
stenotic, wherein the valve does not open sufficiently to
allow adequate forward flow of blood through the valve, or
incompetent, wherein the valve does not close completely,
causing excessive backward flow o~ blood through the valve
when the valve is closed. A heart valve may also be both
stenotic and incompetent. Valve disease can be severely
debilitating and even fatal if left untreated, particularly if
the diseased valve is the mitral valve (between the le~t
atrium and left ventricle) or the aortic valve (between the
left ventricle and the aorta). According to recent estimates,
more than 80,000 patients are diagnosed with aortic or mitral
valve disease in U.S. hospitals each year.
Various surgical techniques may be used to repair a
diseased or damaged valve. One repair technique which has
been shown to be ef~ective in treating incompetence,
particularly of the mitral and tricuspid valves, is
annuloplasty, in which the ef~ective size of the valve annulus
- is contracted by attaching a prosthetic annuloplasty ring to
an interior wall of the heart around the valve annulus. The
annuloplasty ring comprises an inner substrate of a metal such
as stainless or titanium, or a flexible material such as
CA 02239907 1998-06-08
W O 96~9942 PCTrUS96/07970
slllcone rubber or Dacron cordage, covered with a
biocompatible fabric or cloth to allow the ring to be sutured
to the heart tissue. The annuloplasty ring may be stiff or
flexible, may be split or continuous, and may have a variety
of shapes, including circular, D-shaped, C-shaped, or
kidney-shaped. Examples are seen in U.S. Patent Nos.4,917,698,
5,061,277, 5,290,300, 5,350,420, 5,104,407, 5,064,431,
5,201,880, and 5,041,130, which are incorporated herein by
reference.
Annuloplasty rings may also be utilized in
combination with other repair techniques such as quadrangular
resection, in which a portion of a valve leaflet is excised,
the remaining portions of the leaflet are sewn back together,
and a prosthetic annuloplasty ring is then attached to the
valve annulus to maintain the contracted size of the valve.
Other valve repair techniques in current use include
commissurotomy (cutting the valve commissures to separate the
valve leaflets), shortening mitral or tricuspid valve chordae
tendonae, reattachment of severed mitral or tricuspid valve
chordae ten~o~e or papillary muscle tissue, and
decalcification of the valve leaflets or annulus.
Annuloplasty rings may be used in conjunction with any repair
procedures where contracting or stabilizing the valve annulus
might be desirable.
In cases where a cardiac valve is not suited to
repair, the valve may be replaced, by excising the valve
leaflets of the natural valve, and securing a replacement
valve in the valve position, usually by suturing the
replacement valve to the natural valve annulus. Various types
of replacement valves are in current use, including mechanical
and biological prostheses, homografts, and allografts, as
described in Bodnar and Frater, Replacement Cardiac Valves
1-357 (1991). A comprehensive discussion o~ heart valve
diseases and the surgical treatment thereof is found in
Kirklin and Barratt-Boyes, Cardiac Surgery 323-459 (1986).
Using current techniques, most valve repair and
replacement procedures require a gross thoracotomy, usually in
the form of a median sternotomy, to gain access into the
CA 02239907 1998-06-08
W O 96~9942 . PCTrUS9G/~7970
patlent~s thoracic cavity. A saw or other cutting 1nstrument
is used to cut the sternum longitudinally, allowing two
opposing halves of the anterior or ventral portion of the rib
cage to be spread apart. A large opening into the thoracic
cavity is thus created, through which the surgical team may
directly visualize and operate upon the heart and other
thoracic contents. Alternatively, a thoracotomy may be
performed on a lateral side of the chest, wherein a large
incision is made generally parallel to the ribs, and the ribs
are spread apart and/or removed in the region of the incision
to create a large enough opening to facilitate the surgery.
Surgical intervention within the heart generally
requires isolation of the heart and coronary blood vessels
from the r~m~;n~r of the arterial system, and arrest of
cardiac function. Usually, the heart is isolated from the
arterial system by introducing an external aortic cross-clamp
through a sternotomy and applying it to the aorta to occlude
the aortic lumen between the brachiocephalic artery and the
coronary ostia. Cardioplegic fluid is then injected into the
coronary arteries, either directly into the coronary ostia or
through a puncture in the ascending aorta, so as to arrest
cardiac function. In some cases, cardioplegic fluid is
injected into the coronary sinus for retrograde perfusion of
the myocardium. The patient is placed on extracorporeal
cardiopulmonary bypass to maintain peripheral circulation of
oxygenated blood.
Of particular interest in the present application
are techniques for the repair and replacement of the mitral
valve. The mitral valve, located between the left atrium and
left ventricle of the heart, is most easily reached through
the wall of the left atrium, which normally resides on the
posterior side of the heart, opposite the side of the heart
that is exposed by a median sternotomy. Therefore, to access
the mitral valve via a sternotomy, the heart is rotated to
bring the left atrium into an anterior position accessible
through the sternotomy. An opening, or atriotomy, is then
made in the right side of the left atrium, anterior to the
right pulmonary veins. The atriotomy is retracted by means of
CA 02239907 1998-06-08
WO 96~9942 PCTAUS96/07970
sutures or a retraction device, exposing the mitral valve
directly posterior to the atriotomy. One of the forementioned
techniques may then be used to repair or replace the valve.
An alternative technique for mitral valve access may
be used when a median sternotomy and/or rotational
manipulation of the heart are inappropriate. In this
technique, a thoracotomy is made in the right lateral side of
the chest, usually in the region of the fourth or fifth
intercostal space. One or more ribs may be removed from the
patient, and other ribs near the incision are retracted
outward to create a large opening into the thoracic cavity.
The left atrium is then exposed on the posterior side of the
heart, and an atriotomy is formed in the wall of the left
atrium, through which the mitral valve may be accessed for
repair or replacement.
Using such open-chest techniques, the large opening
provided by a median sternotomy or right thoracotomy enables
the surgeon to see the mitral valve directly through the left
atriotomy, and to position his or her hands within the
thoracic cavity in close proximity to the exterior of the
heart for cannulation of the aorta and/or coronary arteries to
induce cardioplegia, manipulation of surgical instruments,
removal of excised tissue, and introduction of an annuloplasty
ring or a replacement valve through the atriotomy for
attachment within the heart. However, these invasive,
open-chest procedures produce a high degree of trauma, a
significant risk of complications, an extended hospital stay,
and a painful recovery period for the patient. Moreover,
while heart valve surgery produces beneficial results for many
patients, numerous others who might benefit from such surgery
are unable or unwilling to undergo the trauma and risks of
current techniques.
What is needed, therefore, are devices and methods
for carrying out heart valve repair and replacement as well as
other procedures within the heart and great vessels that
reduce the trauma, risks, recovery time and pain that
accompany current techniques. The devices and methods should
facilitate surgical intervention within the heart or great
CA 02239907 1998-06-08
WO 96~9942 PCTrUS96/07970
vessels without the need for a gross thoracotomy, preferably
through small incisions within intercostal spaces of the rib
cage, without cutting, removing, or significantly deflecting
the patient's ribs or sternum. In particular, the devices and
methods should allow for removal of tissue from the thoracic
cavity, as well as for introduction of surgical instruments,
visualization devices, annuloplasty rings, replacement valves,
and the like into the thoracic cavity, to facilitate heart
valve repair and replacement. The devices and methods should
enable the implantation of annuloplasty rings of various
shape, size, and stiffness. In addition, the devices and
methods should facilitate replacement of a heart valve with
various types of prostheses, including mechanical and
biological prostheses, homografts, and allografts.
SU~IARY OF THE lNvt~:N-LloN
The invention provides devices and methods for
performing less-invasive surgical procedures within an organ
or vessel, and particularly, within the heart and great
vessels of the thoracic cavity. The devices and methods of
the invention facilitate intervention within the heart and
great vessels without the need for a median sternotomy or
other form of gross thoracotomy, substantially reducing
trauma, risk of complication, recovery time, and pain for the
patient. Using the devices and methods of the invention,
surgical procedures may be performed through percutaneous
penetrations within intercostal spaces of the patient~s rib
cage, without cutting, removing, or significantly displacing
any of the patient's ribs or sternum. The devices and methods
are particularly well-adapted for heart valve repair and
replacement, facilitating visualization within the patient~s
thoracic cavity, repair or removal of the patient's natural
valve, and, if necessary, attachment of an annuloplasty ring
or a replacement valve in the natural valve position. The
invention facilitates valve repair with a variety of different
annuloplasty rings, as well as valve replacement with any of a
variety of replacement valves, including mechanical
prostheses, bioprostheses, homografts, and allografts.
CA 02239907 1998-06-08
WO 96/39942 PCTAUS96/07970
According to the invention, access into the chest
cavity and into the heart is obtained by means of small
incisions, punctures, cannulae, trocars, or other percutaneous
penetrations of minimal size positioned in the intercostal
spaces between adjacent ribs of the rib cage. In this
application, these percutaneous penetrations within
intercostal spaces will be referred to as "intercostal ports."
The intercostal ports utilized in the present invention will
not require removal, cutting, or other modification of the
ribs or sternum, and will generally avoid any significant
retraction of the ribs, other than the incidental deflection
of the ribs which may occur when a cannula, trocar, or other
means of tissue retraction is placed in an intercostal space.
Such retraction of ribs will generally be avoided entirely,
and if occurring at all, will be limited to deflection of less
than about one centimeter. Preferably, all such intercostal
ports will have a width (or diameter, if round) of less than
30 mm in order to fit within an intercostal space without
significant rib retraction, and in many cases will have a
width of less than 12 mm so as to m;n;m; ze trauma.
In a first aspect, the invention provides a method
of closed-chest repair of a heart valve. Utilizing the method
of the invention, the patient's heart is arrested and
cardiopulmonary bypass is established. The interior of the
patient's chest cavity is viewed by means of a thoracoscope or
by directly looking through a c~nnl]la or other retracting
means positioned in an intercostal space. A knife or scissors
is introduced through an intercostal port into the patient~s
chest, and the cutting means is used to first form an opening
in the pericardium, then to form a cardiac penetration in a
wall of the heart. One or more percutaneous c~nnl~lae,
trocars, or other means of retracting tissue may be positioned
in an incision or puncture within an intercostal space through
which various instruments may be introduced into the chest
cavity. These instruments may be positioned through the
cardiac penetration to perform, for example, annuloplasty,
quadrangular resection of valve leaflets, commissurotomy,
reattachment of chordae tendonae or papillary muscle tissue,
CA 02239907 1998-06-08
W O 96/39942 PCTAJS96/07970
shortening of chordae tendonae, decalcification, and the like.
Advantageously, all of these steps may be performed without
cutting, removing, or substantially retracting the ribs or
sternum, eliminating the pain, trauma, long recovery time, and
complications associated with gross thoracotomy.
The patient's heart is preferably arrested by
occluding the patient's aorta between the patient's coronary
ostia and the patient's brachiocephalic artery with an
expandable member on a distal end of an endovascular aortic
catheter introduced through a peripheral artery such as a
femoral artery. Cardioplegic fluid is then delivered through
a lumen in the catheter into the patient's aorta upstream of
the expandable member to arrest cardiac function.
Alternatively, or in addition to such antegrade cardioplegic
fluid delivery, cardioplegic fluid may be delivered in a
retrograde manner by means of a catheter positioned in the
coronary sinus of the patient's heart. In an alternative
approach, an external cross-clamp may be placed
thoracoscopically on the aorta through a small incision or
cannula in the patient's chest. Cardioplegic fluid may be
delivered either through a c~nnllla introduced
thoracoscopically and inserted through the aortic wall, or
through an endovascular aortic catheter extending from a
peripheral artery into the ascending aorta upstream of the
cross-clamp.
In a preferred embodiment of the method, a
prosthetic annuloplasty ring is introduced through an
intercostal port and into an internal chamber of the heart,
and the ring is attached to the heart wall around the annulus
of the valve within the internal chamber. Usually, the valve
will first be sized by introducing a sizing device through the
intercostal port and into the heart through the cardiac
penetration, and positioning the sizing device adjacent to the
valve to measure its size. A valve sizing disk attached to an
elongated shaft or handle may be used for this purpose. Once
the valve size has been determined, sutures are inserted in
the native valve annulus and an annuloplasty ring of
appropriate size is selected. The ring is attached to an
CA 02239907 1998-06-08
W O 96~9942 PCT~US9G/~7Y70
elongated handle and the ring is introduced through an
intercostal port and through the cardiac penetration into the
heart. The annuloplasty ring is then secured to the annulus
of the heart valve, by tying knots in the sutures
extracorporeally and pushing the knots into the heart with an
elongated knot-pusher. The sutures are preferably applied to
the annuloplasty ring outside of the chest cavity, and the
ring is slid along the sutures through the intercostal port
and cardiac penetration up to the valve annulus. The sutures
are then tied and trimmed using thoracoscopic instruments.
One advantage of the method of the invention is that
it allows the surgeon to obtain access to the valve through an
intercostal port and a cardiac penetration, assess the nature
and extent of valve disease, and then decide whether to repair
or replace the valve. If the disease is such that repair is
inappropriate, the surgeon may elect to replace the valve with
any of a variety of replacement valves. A valve replacement
method according to the invention may include the step of
removing all or part of the patient's natural heart valve by
means of a cutting tool introduced through an intercostal port
and through the cardiac penetration into the heart. The
method ~urther comprises the step of introducing a replacement
valve through an intercostal port and through the cardiac
penetration into the internal chamber of the heart. The
replacement valve is then fastened within the heart, usually
by means of a suturing instrument introduced through an
intercostal port and through the cardiac penetration. As with
the annuloplasty method described above, sutures are usually
applied to the valve outside of the chest, and the valve is
slid along the sutures into the heart. The sutures are then
tied and trimmed. The method may further include the step o~
sizing the patient's heart valve before the replacement valve
is introduced. In an exemplary embodiment, a sizing
instrument is introduced through an intercostal port and
through the cardiac penetration to measure the size of the
valve annulus and to determine the size of the replacement
valve.
CA 02239907 1998-06-08
W O 96/39942 PCT~US96/07970
- 9
In order to suture the annuloplasty ring or
replacement valve to the interior of the heart, the sutures
are preferably applied to the heart tissue, drawn out of the
patient's body through an intercostal port and then applied to
the annuloplasty ring or replacement valve. The sutures may
be radially arranged in spaced-apart locations about an
organizer ring disposed outside of the patient's body. The
sutures are then held in tension as the annuloplasty ring or
replacement valve is introduced into the interior of the heart
and positioned in the natural valve position. The
annuloplasty ring or replacement valve may be introduced by
means of a specialized holder attached to an elongated handle,
or simply pushed along the sutures into the chest cavity by
means of the surgeon's hands, then into the native valve
position using conventional thoracoscopic instruments such as
forceps or needle drivers.
In a particularly preferred embodiment, the heart
valve comprises a mitral valve which is disposed between the
left atrium and left ventricle of the patient's heart. An
intercostal port is created within an intercostal space in a
right lateral portion of the patient's chest, usually within
the third, fourth, or fifth intercostal space. From this
intercostal port, a cardiac penetration may be formed in the
wall of the left atrium at a location which is generally
aligned with the intercostal port. In this way, surgical
instruments may be introduced from the intercostal port in the
right chest to form the cardiac penetration, repair or excise
the patient's natural valve, and/or introduce and attach an
annuloplasty ring or replacement valve.
In a ~urther aspect of the invention, a system is
provided for repairing a heart valve. The system includes an
annuloplasty device and a device holder for releasably holding
the annuloplasty device to facilitate introducing it through
an intercostal port and into the heart. The device holder
includes connection means ~or connecting the holder to an
elongated handle. The connection means is configured to
connect to the handle such that the handle, holder, and
annuloplasty device together have a profile with a profile
CA 02239907 1998-06-08
W O 96/39942 PCTAJS9G~'~7970
height smaller than the width of the intercostal space,
usually less than about 30 mm and preferably less than about
25 mm. The annuloplasty device has a bottom side which i8
positioned in contact with the wall of the heart around the
heart valve when the device is implanted. The bottom side
defines a first plane which is generally perpendicular to a
longitudinal (axial) axis of the annuloplasty device. In an
exemplary configuration, the connection means connects to the
handle such that the longitll~inAl axis of the handle forms an
angle with the first plane selected so that handle may be used
to introduce the annuloplasty ring through the intercostal
port without contacting or retracting the ribs adjacent the
intercostal port. The angle will usually be about 0~ +/- 45~,
and preferably 0~ +/- 20~, but could also be outside of this
range if the annuloplasty device is small relative to the size
of the intercostal space.
The holder also includes a means for retaining the
annuloplasty device on the holder, such as retention sutures,
a retaining clip, or a pivoting leaf on the holder. The
system may further include means for releasing the
annuloplasty device from the holder, such as a cutting device
for cutting the retention sutures which hold the annuloplasty
device on the holder, or other device for releasing the
mechanism which secures the annuloplasty device to the holder.
The annuloplasty device may be any of the
commercially-available annuloplasty rings, may be either stiff
or flexible, split or continuous, and may have any of a
variety of shapes, including C-shaped, D-shaped,
kidney-shaped, saddle-shaped racetrack-shaped, oval,
semi-circular, and circular. The annuloplasty device may also
be malleable or shapable into a desired shape, or may be
flexible and resilient and secured in the heart in a shape
which differs from its natural, unstressed shape.
The valve repair system may further include an
elongated handle having a distal end mounted to the device
holder and a proximal end opposite the distal end. The handle
is configured to introduce the annuloplasty device into the
CA 02239907 1998-06-08
W O 96/39942 PCTrUS96/07970
11
patient' 8 heart through an intercostal port. Preferably, the
handle is at least about 20 cm in length to allow positioning
the annuloplasty device in the left atrium of the heart from a
right lateral portion of the patient's chest.
The handle may also include means for pivoting the
annuloplasty device from a first orientation ~or introduction
through the intercostal space to a second orientation for
attachment in the patient's heart. The pivoting means is
configured for actuation from a proximal end of the handle.
In this way, the annuloplasty device may be introduced
edge-first through the intercostal space, then pivoted about
an axis generally perpendicular to the handle into an
orientation suitable for attachment within the patient~s
heart, pre~erably wherein the first plane is perpendicular to
the longitudinal axis of the handle.
While a variety of mechanisms may be utilized for
connecting the holder to the handle, in an exemplary
embodiment, the handle has a tongue pivotably coupled to its
distal end, a movable actuator coupled to its proximal end,
and a rod or cable extending through a lumen in the handle
connecting the actuator to the tongue. The tongue is received
in an aperture in the device holder, and includes a spring
catch or other means ~or retaining the tongue in the aperture.
The aperture has an open prox;m~l end, a distal end opposite
the proximal end, and an axis therebetween defining the
direction in which the tongue is received in the aperture.
The aperture is preferably oriented so that the axis forms an
angle of 0~ +/- 45~ relative to the first plane of the
annuloplasty device, facilitating introduction through an
intercostal port. In this way, the tongue may be aligned with
the longitll~; n~l axis o~ the handle for edge-first
introduction of the annuloplasty device through the
intercostal port, then pivoted to an appropriate angle,
usually about 90~, relative to the handle so that the first
plane o~ the annuloplasty device is generally parallel to the
interior wall of the heart to which it is to be attached.
As an alternative to pivoting the annuloplasty
device, the annuloplasty device and device holder may be
CA 02239907 l998-06-08
W O 96/39942 PCTAUS96/07970
12
flexible, collapsible, or compressible so that it may be
deformed or constrained into a shape which allows the device
and holder to be introduced through an intercostal space into
the thoracic cavity.
The system of the invention may also include a
retraction means for retracting the chest wall tissue in a
percutaneous penetration within an intercostal space, to
facilitate introduction of instruments, visualization devices,
valve sizers, annuloplasty devices, and replacement valves
through the penetration without interference and without
damaging tissue. The retraction means displaces the tissue
around the percutaneous penetration to create a small opening,
but does not significantly retract or deflect the ribs. The
retraction means may comprise any of various types of tissue
or wound retractors, but in a preferred embodiment comprises a
cannula having a distal end positionable through the
intercostal space and an inner lumen of sufficient size and
shape to allow a replacement valve or annuloplasty device to
be positioned through the cannula into the chest cavity
Preferably, the inner lumen has a width of between about 12 mm
and about 30 mm, in order to allow the cannula to be
positioned within the intercostal space with the ribs
unretracted, while allowing the annuloplasty device or
replacement valve to pass through the lumen with sufficient
clearance. The inner lumen has a height of at least 25 mm,
and usually at least 35 mm, to permit introduction of the
annuloplasty device or replacement valve. Usually, the height
is larger than the width, in a preferred embodiment, at least
about 1. 5 times the width. In this way, the annuloplasty
device or replacement valve may be introduced in an edge-first
manner through the lumen of the cannula, then pivoted 90~ into
a face-first orientation for attachment within the heart.
Because the annuloplasty device or replacement valve
may be attached within the heart with a plurality of
individual sutures, the system may further include means for
organizing sutures outside of the chest cavity. The suture
organizing means preferably is attached to the proximal end o~
the access cannula described above, and comprises a plurality
CA 02239907 l998-06-08
WO 96/39942 PCTrUS96/07970
- 13
of slots arranged radially about the inner lumen of the
cannula. In this way, as each suture is placed in the heart
tissue, the free ends of the suture may be withdrawn through
the lumen of the access cannula and placed in one of the
slots. The free ends may then be placed through the sewing
ring of the annuloplasty device or replacement valve, and the
device or valve advanced through the inner lumen of the
cannula and into the heart by sliding along the suture
threads.
Preferably, the annuloplasty device is premounted to
the device holder and the two are sterilized and packaged
together in a sterile pack. In this way, the pack may be
opened in the sterile operating room environment with the
annuloplasty device and holder ready for immediate use. In
some embodiments, the elongated delivery handle, sizing disks,
access cannula or other retraction means, suture organizer,
and/or other system components may be included in the sterile
pack with the annuloplasty device and holder. Alternatively,
the annuloplasty device could be packaged separately from the
device holder and the device mounted to the holder in the
operating room at the time of the valve repair procedure.
The delivery handle of the invention is configured
not only for introducing the annuloplasty device through an
intercostal port into the heart, but for introducing valve
sizing devices and/or a replacement valve as well. In this
way, the same handle may be used to first size the native
valve, then to introduce an annuloplasty device to repair the
mitral valve, or to introduce a replacement valve to replace
the native valve.
Accordingly, the invention also provides a device
for sizing a valve which includes both an elongated handle and
a sizing disk attached to the distal end of the handle. The
sizing disk is configured to connect to the handle in an
orientation in which the handle and the sizing disk together
have a profile with a profile height smaller than the width of
the intercostal space through which the sizing disk is
introduced, usually less than about 30 mm and preferably less
than about 25 mm. In a preferred embodiment, the sizing disk
CA 02239907 l998-06-08
W 096/39942 PCTrUS9G/~7970
14
is pivotably attached to the handle so that it may be
introduced through the intercostal space in an edge-first
orientation, and then pivoted into a face-first orientation
for sizing the valve. The handle may have, as described above,
a tongue pivotably mounted to its- distal end which is received
in an aperture on the sizing disk, allowing the sizing disk to
be oriented with its face generally parallel to the
longitll~; n~l axis of the handle for introduction, then
perpendicular to the longitudinal axis for sizing the valve.
For sizing a valve for an annuloplasty repair, the sizing disk
usually has a shape corresponding generally to the natural
shape of the native valve annulus, which is roughly oval,
kidney-shaped or D-shaped. The sizing disk also includes
notches or markings to measure the spacing between the
trigones or commisures of the valve. For valve replacement
procedures, the sizing disk is preferably round, corresponding
to the shape of the replacement valve sewing ring.
The invention further provides a holder for a
prosthesis for repairing or replacing a heart valve. The
holder may be adapted for holding either an annuloplasty ring
or a prosthetic heart valve. The holder includes a holder
body having a top, a bottom, and a holder axis. A holding
means is included on the holder body for releasably holding a
prosthesis such that the central axis of the attachment ring
of the prosthesis is approximately parallel to the holder
axis. The holder further includes a connection means for
connecting to an elongated handle for introducing the holder
and prosthesis through an intercostal space. The connection
means has a proximal end, a distal end, and a connection axis
therebetween. The connection means is positioned on the
holder body such that the connection axis is oriented at an
angle relative to the holder axis selected so that, when the
prosthesis is held by the holding means, the profile of the
prosthesis and holder perpendicular to the connection axis has
a height less than the width of the intercostal space, usually
less than about 30 mm and preferably less than about 25 mm.
In a preferred embodiment, as described above, the
handle has a pivotable tongue on its distal end, and the
CA 02239907 l998-06-08
W O 96/39942 PCTrUS96/07970
connection means comprises an aperture for receiving the
tongue. The aperture has an open proximal end through which
the tongue is received in a direction parallel to the
connection axis. Alternatively, the connection means may
comprise a threaded hole, snap fitting, luer fitting, threaded
shaft, or tongue configured to connect to a complementary
connector on the handle. Preferably, the connection means is
removable from the handle to allow valve sizers, annuloplasty
rings, and replacement valves to be interchanged on the same
handle. However, the handle and holder may alternatively be
permanently inseparably interconnected for dedicated use with
a single annuloplasty device or replacement valve.
A further understanding of the nature and advantages
of the invention may be realized by reference to the r~;n;ng
portions of the specification and the drawings.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 is a perspective view of a delivery handle
for a cardiac valve sizer, annuloplasty ring, or valve
prosthesis constructed in accordance with the principles of
the present invention.
Figure 2 is a perspective view of a distal portion
of the delivery handle of Figure 1.
Figure 3 is a side cross-sectional view of the
delivery handle of Figure 1 with a holder coupling at the
distal end of the delivery handle in a longitll~;n~1ly-aligned
orientation.
Figure 4 is a side cross-sectional view of the
delivery handle of Figure 1 with a holder coupling at the
distal end of the delivery handle pivoted into a transverse
orientation.
Figures 5A-5C are perspective, front and side views
respectively of an annuloplasty ring holder constructed in
accordance with the principles of the invention.
Figures 6A, 7A, and 8A are top elevational views of
various embodiments of an annuloplasty device constructed in
accordance with the principles of the present invention.
CA 02239907 1998-06-08
W O 96/39942 PCTAJS96/07970
- 16
Figures 6B, 7B, and 8B are perspective views of
various holders for the annuloplasty devices of Figures 6A,
7A, and 8A, respectively.
Figures 6C, 7C and 8C are perspective views of the
holders of Figures 6B, 7B, and 8B holding the annuloplasty
devices of Figures 6A, 7A, and 8A, and attached to a distal
portion of the delivery handle of Figure 1.
Figures 9A, 9B and 9C are side cross-sectional views
of various alternative embodiments of annuloplasty device
holders constructed according to the principles of the
invention, schematically illustrating the introduction of the
holder through an intercostal space between two ribs.
Figures lOA-lOD are bottom, side transverse
cross-sectional, front transverse cross-sectional, and top
views, respectively, of an annuloplasty ring and holder
according to the invention in an alternative embodiment
thereof, a pair of ring retention leafs of the holder being in
a closed position.
Figures lOE-lOF are bottom and front transverse
cross-sectional views, respectively, of the annuloplasty ring
and holder of Figures lOA-lOD with the ring retention leafs in
an open position.
Figure lOG is a perspective view of a leaf actuation
instrument for moving the ring retention leafs of the holder
of Figures lOA-lOF.
Figure llA is a perspective view of an annuloplasty
ring and holder according to the invention in a further
alternative embodiment thereof, with a ring retaining leaf of
the holder in an open position.
Figure llB is a perspective view of the annuloplasty
ring and holder of Figure llA with the ring retention leaf in
a closed position.
Figure llC is a front view of the holder of Figures
llA-llB with the ring retention lea~ in a closed position.
Figures 12A-12B are perspective views of two
embodiments of an annuloplasty ring holder assembly according
to the invention.
CA 02239907 l998-06-08
W096/39942 PCTrUS96/07970
- 17
Figures 13A-13C are front, top and side views,
respectively, of an adaptor for an annuloplasty ring holder in
accordance with the invention.
Figures 14A-14C are front, top and side views,
respectively, of an additional embodiment of an adaptor for an
annuloplasty ring holder in accordance with the invention.
Figures 15-18, l9A and 20A are side views of an
annuloplasty ring on a holder and a distal portion of a
delivery handle in various alternative embodiments thereof.
Figures l9B and 20B are top views of the distal
portion of the delivery handle of Figures l9A and 2OA,
respectively.
Figures 21 and 22 are perspective views of an
annuloplasty ring sizing disk and sizing disk assembly,
respectively, constructed in accordance with the principles of
the invention.
Figure 23 is a perspective view of a replacement
valve sizing disk constructed according to the invention.
Figures 24A-24B are perspective views of the sizing
disk of Figure 23 attached to the delivery handle of Figure 1,
in two alternative orientations.
Figure 25 is a perspective view of a sizing disk
aRsembly constructed according to the invention.
Figure 26A-26C are perspective, top and front views,
respectively, of a holder for a prosthetic valve constructed
in accordance with the principles of the invention.
Figure 27 is a front view of the prosthetic valve of
Figures 26A-26C illustrating the pivoting of distal and
proximal pieces thereof.
Figure 28 is a perspective view of a prosthetic
valve which may be held by the holder of Figures 26A-26C.
Figure 29 is a perspective view of the prosthetic
valve of Figure 28 held on the holder of Figure 27.
Figures 30A-30B are front views of the holder and
prosthetic valve of Figure 29 attached to the delivery handle
of Figure 1, in two alternative orientations.
CA 02239907 1998-06-08
W096/3994Z PCT~US96/07970
18
Figures 31-34 are side views of a prosthetic valve
holder and a distal portion of a delivery handle in various
alternative embodiments thereof.
Figure 35 is an anterior partially cut-away view of
a patient's chest illus~rating forming an opening in the
pericardium according to a cardiac valve treatment method o~
the invention.
Figures 36A-36D are perspective, side, front and top
views, respectively, of an oval port constructed in accordance
lo with the principles of the invention.
Figure 37A is a side view of an obturator for the
oval port of Figures 36A-36D.
Figure 37B iS a side view of the obturator of Figure
37A positioned in an inner lumen of the oval port of Figures
36A-36D.
Figure 38 is an elevational view through the oval
port of Figures 36A-36D positioned in a right lateral location
of a patient~s chest showing the formation of an atriotomy
according to the method of the invention.
Figure 39 iS a perspective view of a patient
illustrating the refraction of the atriotomy and positioning
of a valve sizing according to the method of the invention.
Figure 40 is an elevational view through the oval
port of Figures 36A-36D positioned in a right lateral location
oi~ a patient~s chest showing the placement of sutures near the
mitral valve according to the method of the invention.
Figure 41 is a perspective view of a patient
illustrating the placement of sutures through the annuloplasty
ring according to the method of the invention.
Figure 42 is a transverse cross-section of a
patient~s thorax showing the introduction of the annuloplasty
ring through an intercostal port according to the method of
the invention.
Figure 43 is a transverse cross-section of a
patient's thorax showing the pushing of suture knots through
an intercostal port for securing the annuloplasty ring within
the heart according to the method of the invention.
CA 02239907 1998-06-08
WO 96/39942 PCTAUS96/07970
19
Figure 44 is an elevational view through the oval
port of Figures 36A-36D positioned in a right lateral location
of a patient's chest showing the pushing of suture knots
against the annuloplasty ring and trimming the ends of the
sutures according to the method of the invention.
Figures 45A-45B are side elevational views of a
valve seating device and a leaflet testing device,
respectively, constructed in accordance with the principles of
the invention.
Figure 46 is an elevational view through the oval
port of Figures 36A-36D positioned in a right lateral location
of a patient's chest showing the closure of the atriotomy
according to the method of the invention.
DETATT.T.'n DESCRIPTION OF THE ~ :r~ KKED P'MRODIMENT
The present invention provides various devices and
systems for less-invasive surgical treatment of cardiac
valves, and methods of using the devices and systems. The
systems may be adapted either for cardiac valve repair,
wherein a prosthetic annuloplasty ring is attached to an
internal wall of the heart around the native valve annulus, or
for cardiac valve replacement, wherein the native valve is
replaced with a replacement valve, usually a prosthetic valve.
The system includes, as described in detail below, a delivery
handle for positioning the repair or replacement prosthesis
through an intercostal space and into the interior of the
heart, and a prosthesis holder, preferably attached to the end
of the delivery handle, for releasably holding the repair or
replacement prosthesis. In various embodiments, the system
may include the repair or replacement prosthesis itself,
devices for sizing the native valve, devices for retracting
tissue within an intercostal space to facilitate introduction
of the prosthesis, devices for organizing the sutures used to
attach the prosthesis within the heart, and other components.
Each of these components will now be described, followed by a
description of a preferred method of using the system in a
patient.
CA 02239907 1998-06-08
WO 96~9942 PCTAJS96/07970
. 20
The system and method of the invention facilitate
repairing or replacing a cardiac valve without requiring a
median sternotomy or other gross thoracotomy, and without the
substantial retraction of the ribs common in conventional
open-chest valve treatment procedures. To accomplish this,
the system is configured to operate through "intercostal
ports," which, as discussed above, is used herein to include
small incisions, punctures, or other types of percutaneous
penetrations positioned in the intercostal spaces of the rib
cage with the ribs in their natural, substantially unretracted
positions. C~nnlllae, trocars, or other types of tissue
retraction devices may be positioned in the percutaneous
penetrations to facilitate introduction of instruments,
visualization devices, prostheses, and the like, but these
will generally be limited in size to the width of the
intercostal space (e.g. less than about 30 mm), and will not
re~uire retraction of the ribs. In some cases, a slightly
oversized cannula or retractor may be used, but even in these
cases retraction of the ribs will be limited to less than
about one centimeter. In this way, the pain, trauma, and
complications associated with rib ~e.l.o~dl and/or gross rib
retraction may be eliminated.
Figs. 1-4 illustrate a delivery handle for
delivering a prosthesis mounted on a holder through an
intercostal space and into the interior of the heart. As
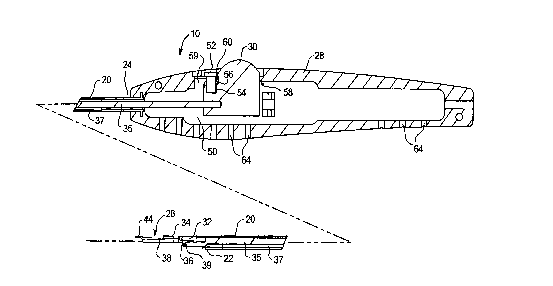
shown in Fig. 1, delivery handle 10 comprises a shaft 20
having a distal end 22 and a proximal end 24. A holder
coupling 26 is mounted to distal end 22, and a handle 28 is
mounted to proximal end 24. A slidable actuation button 30 is
mounted to handle 28 and is linked to holder coupling 26 as
described below, so that moving actuation button 30 pivots
holder coupling 26.
As shown in Figure 2, in a preferred embodiment,
holder coupling 26 comprises a base 32 mounted to distal end
22 of shaft 20. A bifurcated tongue 34 is pivotably mounted
to base 32 by a transverse pin 36. A rod 35 extends through a
lumen 37 in shaft 20 and is pinned to tongue 34 by a second
transverse pin 39. A leaf spring 38 has a proximal crosspiece
CA 02239907 l998-06-08
W 096~9942 PCTrUS96/07970
21
40 attached to tongue 34, and a free distal end 42 to which i8
attached a catch 44 stepped outwardly and upwardly from leaf
spring 38 to define a proximally-facing surface 46. Catch 44
serves to retain a prosthesis holder on holder coupling 26, as
described below.
Shaft 20 and holder coupling 26 are configured for
positioning through an intercostal port into the chest cavity
(without retracting the ribs), and preferably have a
cross-sectional of width less than about 30 mm. In an
exemplary embodiment, shaft 20 iS about 4-8 mm in diameter,
and tongue 34 has a transverse width of about 4-6 mm and a
transverse height of about 0. 5-2.0 mm. Shaft 20 has a length
selected so that holder coupling 26 may be positioned within
the heart near the native valve to be repaired or replaced,
with shaft 22 extending through the desired intercostal port
and handle 28 disposed outside of the patient's chest. In a
preferred embodiment, shaft 20 iS configured to reach the
mitral valve, disposed between the left atrium and left
ventricle of the heart, from an intercostal port in the right
lateral side of the patient's chest between the second and
sixth intercostal spaces. For most cases, shaft 20 has a
length of at least about 20 cm, and preferably at least about
30 cm, but may vary according to patient size and according to
the valve to be repaired and the approach taken to access the
valve. Shaft 20, handle 28, and holder coupling 26 are
preferably made of stainless steel, titanium, aluminum, or a
stiff biocompatible polymer.
Referring now to Figs. 3-4, rod 35 extends
proximally from holder coupling 26 through lumen 37 of shaft
20 into the interior 50 of handle 28, where it is attached to
a lower portion of actuator button 30. Rod 35 iS thus axially
movable in tandem with actuator button 30. A lock button 52
is slidably mounted within a bore 54 in actuator button 30 and
is biased upward by a coil spring (not illustrated). Lock
button 52 includes an annular flange 56 having a tapered upper
edge which engages an inner surface 58 of handle 28, holding
lock button 52 in a downward position when actuator button 30
is in the prox~ m~l position of Fig. 3. When actuator button
CA 02239907 l998-06-08
W 096~9942 PCT~US96/07970
22
30 i5 slid distally to the position of Fig. 4, flange 56 iS
aligned with an aperture 59, allowing lock button 52 to be
urged upward. In this position, actuator button 30 iS
prevented from moving proximally due to the engagement of
flange 56 with a proximal sur~ace 60 of aperture 59 (best seen
in Fig. 3). When it is desired to return actuator button 30
to the proximal position, lock button 52 iS pushed downward
until flange 56 clears pro~;m~l surface 60, allowing actuator
button 30 to slide proximally. It should be noted that
various types of actuators may be used for translating rod 35,
such as levers, rotatable knobs, and push buttons. Moreover,
the provision of lock button 52 iS optional, and in some cases
it may be more desirable to eliminate lock button 52 so that
actuator button 30 iS ~ree to move distally and proximally
without locking. Lock button 52 could also be configured to
lock actuator button 30 in both the proximal and distal
positions, or in various intermediate positions.
When actuator button 30 iS in the pro~c;m~l position
of Fig. 3, holder coupling 26 iS preferably longitudinally
aligned with the longitll~; n~l axis of shaft 20. In this
position, holder coupling 26 and shaft 20 have a transverse
profile small enough that a prosthetic annuloplasty ring or
valve on a holder, when mounted to holder coupling 26 as
described below, may be positioned through an intercostal port
into the chest cavity. Depending upon patient anatomy,
patient size, prosthesis holder configuration, and prosthesis
size, holder coupling 26 could be oriented at a range of
angles between about 0~ and 45~ relative to the longitudinal
axis of shaft 20 and still allow the prosthesis and holder to
introduced through an intercostal port without significant
retraction of the ribs.
When rod 35 iS translated distally by moving
actuator button 30, holder coupling 26 pivots about pin 36
through an angle ~ relative to the longitudinal axis of shaft
20, as shown in Fig. 4. In order to orient a prosthetic
annuloplasty ring or valve optimally for attachment within the
heart, angle ~ is preferably about 90~, however, angle ~ may
be any angle between about 45~ and 135~, depending upon the
CA 02239907 1998-06-08
W O 96/39942 PCTAJS96/07970
23
particular valve being repaired or replaced, the location and
size of the intercostal port through which delivery handle 10
i8 introduced, and the anatomy of the patient.
A cleaning port 62 is disposed in shaft 20 in
communication with inner lumen 37 to facilitate delivery of a
cleaning fluid into the interior 50 of shaft 20 and handle 28.
A plurality of drain holes 64 are provided in the lower side
of handle 28 to allow cleaning fluid to drain from the handle.
Holder coupling 26 is adapted for attachment to a
valve sizing device or to a holder for a prosthetic
annuloplasty ring or replacement valve. An exemplary
embodiment of a holder for a prosthetic annuloplasty ring
according to the invention is illustrated in Figs. 5A-5C.
Holder 70 includes a holder body 72 having an outer edge 74
with a shape selected to match that which the annuloplasty
ring is to assume when secured within the heart, such as
D-shaped, C-shaped, kidney-shaped, semicircular, oval, or
circular. A groove or channel 76 having an upper flange 73
extends around outer edge 74 on the lateral side of holder
body 72 and has a size and shape selected to receive the
annuloplasty ring in order to hold the annuloplasty ring on
holder body 72. One or more suture holes 77 extend through
holder body 72 through which a suture may be threaded and tied
around the annuloplasty ring to secure it to the holder. A
groove or ridge 75 extends across top surface 78 transverse to
the direction in which the retention sutures would be tied to
holder 70. In this way, a knife may be guided by groove or
ridge 75 to cut the sutures to release the ring from holder
70. Holder body 72 has a top surface 78 on which is disposed
a handle coupling 80. In an exemplary embodiment, handle
coupling 80 comprises a slot 82 configured to receive holder
coupling 26 on delivery handle 10. Slot 82 has an open
proximal end 84 and an open distal end 86. Holder coupling 26
is received into slot 82 through proximal end 84 and slides
into slot 82 until catch 44 extends outside of slot 82 through
distal end 86, as described more fully below.
Various exemplary annuloplasty rings which may be
utilized in conjunction with holder 70 are illustrated in
CA 02239907 1998-06-08
W O 96~9942 PCT~US96/07970
~ 24
Figures 6A, 7A, and 8A. The annuloplasty rings preferably
comprise a flexible, stiff, or deformable support ring covered
by a fabric or mesh suitable for suturing the annuloplasty
ring to heart tissue. The support ring may be a biocompatible
metal such as stainless steel or titanium or a flexible
material such as silicone rubber or Dacron cordage, depending
upon the structural and performance characteristics desired in
the ring. The overlying fabric or mesh may be a polyester
knit fabric, polyester velour cloth, expanded
polytetrafluoroethylene, or other biocompatible porous
material with sufficient structural integrity to resist
tearing when a suture is passed through it and secured to the
heart. Holder 70 may be adapted for use with any of the
various commercially available annuloplasty rings, including
the Carpentier-Edwards~ Mitral Ring, the Carpentier Physio~
Ring, or Cosgrove~ Ring available from Baxter Healthcare
Corp., Edwards CVS Div., Irvine, California, the Sculptor~ or
Duran~ Ring available from Medtronic, Inc. of Minneapolis,
Minnesota, the Puig Massana~ Ring available from Sorin
Biomedica of Salaggia, Italy, or the Biflex ~ Ring available
from St. Jude Medical, Inc. of St. Paul, Minnesota. Holder 70
is configured to hold annuloplasty rings of various shape and
size. Figure 6B illustrates a holder 70' adapted for holding
the D-shaped split annuloplasty ring 90' shown in Figure 6A,
such as the Baxter, Inc. Carpentier-Edwards Mitral Ring. In
Figure 6C, annuloplasty ring 90' is mounted to holder 70',
which is attached to holder coupling 26 of delivery handle 10.
Figure 7B illustrates a holder 70'' adapted for holding the
D-shaped continuous annuloplasty ring of Figure 7A, such as
the Baxter Carpentier Physio~ Ring, or the Medtronic Sculptor~
Ring. Figure 7C shows annuloplasty ring 90''' mounted to
holder 70~, which is attached to holder coupling 26 of
delivery handle 10. Figure 8B illustrates a holder 70'''
adapted for holding the C-shaped split or open annuloplasty
ring 90''' of Figure 8A, which may be the Baxter Cosgrove~
Ring. Figure 8C illustrates holder 70''' holding annuloplasty
ring 90''' and mounted to holder coupling 26 of delivery
handle 10. Annuloplasty rings of various other shapes may
CA 02239907 1998-06-08
WO 96/39942 PCTAUS96/07970
also be used with the holder of the invention, including
kidney-shaped, saddle-shaped racetrack-shaped, semicircular,
circular, and others. In some cases, the annuloplasty ring 90
may be flexible and may have a shape in a natural, unstressed
condition which is different than the shape of holder 70. For
example, a circular ring could be held by a D-shaped holder.
In this way, the ring conforms to the shape of holder 70 and
is held in the shape it will be in when secured within the
heart. Ring 90 may also be shapable or malleable so that it
may shaped into the shape of holder 70 and/or reshaped when
secured within the heart.
As shown in Figure 6A, annuloplasty ring 90' has a
transverse height RH and a transverse width RW. In many
cases, both transverse height RH and transverse width RW will
be larger than the width of the intercostal space through
which they are to be introduced. When mounted to holder 70,
handle coupling 80 is adapted to receive holder coupling 26
such that an annuloplasty ring 90 can be attached to delivery
handle 10 and introduced through an intercostal port without
retraction of the adjacent ribs. In a preferred embodiment,
slot 82 is parallel to a bottom side 88 of holder body 72,
which i8 generally parallel to the plane contacting the bottom
side of the annuloplasty ring when attached to holder 70.
Such a configuration is illustrated in Figure 9A, which
schematically illustrates shaft 20 of delivery handle 10
positioning holder 70, to which is mounted an annuloplasty
ring 90, within an intercostal space I between two ribs R
(chest wall tissue is not shown for simplification). It may
be seen that, when holder coupling 26 is longitll~;n~lly
aligned with shaft 20, the bottom side 88 of holder 70, along
with the plane containing the bottom side of annuloplasty ring
90, are parallel to the longitudinal axis of shaft 20
Alternatively stated, the longitll~; n~l (or axial) axis of
annuloplasty ring 90 is perpendicular to the longitudinal axis~ 35 of shaft 20. In this configuration, holder 70, annuloplasty
ring 90 and delivery handle 10 have a transverse profile of
minimum size to facilitate introduction through intercostal
space I. In most adult patients, intercostal space I will
CA 02239907 1998-06-08
W O 96/399~2 PCT~US9G1~7970
26
have a width W between about 20 mm and 30 mm in the right
lateral chest at the locations suitable for approaching the
mitral or tricuspid valve. Thus, with annuloplasty ring 90
mated to holder 70, the transverse height H between the bottom
and top sides of holder 70, including the diameter shaft 20,
will be less than about 30 mm, and preferably less than about
20 mm.
In addition to the configuration shown in Figure 9A,
handle coupling 80 of holder 70 may have various alternative
configurations, two of which are shown in Figures 9B and 9C.
In the embodiments of Figures 9B-9C, slot 82 is oriented at an
angle ~ relative to bottom side 88 of holder body 72 (and the
plane containing the bottom of annuloplasty ring 90 and
perpendicular to the longitudinal axis of annuloplasty ring
90. Angle ~ is selected so that holder 70, with annuloplasty
ring 90 mounted to it, may be attached to holder coupling 26
on shaft 20 and introduced through intercostal space I without
retracting ribs R. Angle a may be either positive or negative
relative to slot 82 (and the longitudinal axis of shaft 20),
and is usually within a range of -45~ to +45~, and preferably
-20~ to + 20~. The height H of holder 70 perpendicular to
bottom surface 88 will be substantially less than intercostal
width W, usually less than about 25 mm, and preferably less
than about 20 mm, so that some clearance is provided between
holder 70 and the ribs R defining the intercostal space I.
Once the holder and annuloplasty ring are through the
intercostal space, delivery handle 10 may be manipulated and
holder coupling 26 pivoted so that annuloplasty ring 90 is in
an orientation suitable for advancement into and attachment
within the heart, as described more fully below.
It should be noted that in some cases intercostal
width W may be sufficiently large and the annuloplasty ring
diameter (or width across the ring) sufficiently small that
angle ~ could be as great as 90~--that is, bottom surface 88
(or the plane of ring 90) could form a right angle relative
to the longitudinal axis of shaft 20--and ring 90 could still
be positioned through the intercostal space without retracting
the ribs significantly. However, in most cases it will be
-
CA 02239907 l998-06-08
W 096~9942 PCTAJS96/07970
27
advantageous to orient the ring at an angle somewhat less than
90~ relative to the longitudinal axis of shaft 20 to provide
maximum clearance relative to the adjacent ribs and to allow
the ring to be introduced through an intercostal port of
minimum size.
Figures lOA-lOG illustrate a further embodiment of
an annuloplasty ring holder according to the invention. In
this embodiment, holder 420 includes a holder body 422 having
a top surface 424 and a bottom surface 426. A handle coupling
428 is mounted to top surface 424, and includes an axial slot
430 for receiving holder coupling 26 on delivery handle 10. A
flange 432 extends around the top lateral edge of holder body
422. An annuloplasty ring 434, which may have various shapes,
stiffnesses, and materials, is positionable around the lateral
edge of holder body 422 abutting flange 432. A pair of ring
retention leafs 436 are rotatably coupled to holder body 422
by a bearing 438 SO as to be rotatable about an axis parallel
to the longitudinal axis of annuloplasty ring 434. Each ring
retention leaf 436 has a pair of apertures 440 in a top
surface thereof for engagement by a leaf actuation instrument
442, shown in Figure lOG. Leaf actuation instrument 442 has
an elongated shaft 444 long enough to reach the native valve
position in the heart from outside of the chest cavity (e.g.
about 30 cm), and a pair of prongs 446 at its distal end for
insertion into apertures 440 in ring retention leafs 436. A
stop 448 extends downwardly from bottom surface 426 of holder
body 422 to limit the rotation of ring retention leafs 436
beyond the open and closed positions. A lip 449 extends from
bottom surface 426 to help retain ring 434 against flange 432.
In this way, ring retention leafs 436 may be placed in the
open position of Figures lOE-lOF for placement of annuloplasty
ring 434 on holder 420, and, using leaf actuation instrument
442, leafs 436 may be rotated into the closed position of
Figures lOA-lOC to trap ring 434 between leafs 436 and flange
432. After annuloplasty ring 434 has been secured around the
native valve within the heart, leaf actuation instrument 442
may be introduced through an intercostal port and inserted
CA 02239907 l998-06-08
W 096~9942 PCT~US~G~79'/0
28
into apertures 440 to rotate ring retention leafs 436 into the
open position, releasing ring 434 from holder 420.
An additional embodiment of an annuloplasty ring
holder according to the invention is shown in Figures llA-llC.
Holder 450 comprises a holder body 452 having a top surface
454 and a bottom surface 456. A handle coupling 458 is
mounted to top surface 454 and includes an axial slot 460 for
receiving holder coupling 26 on delivery handle 10. A flange
462 extends around the top lateral edge of holder body 452,
and a groove or channel 464 extends around the lateral side of
holder body 452 on a rearward portion thereof. An
annuloplasty device 466, which again could be of various
shapes, stiffnesses, and materials, may be positioned around
holder body 452 in channel 464, so as to abut flange 462. A
ring retention leaf 468 is hingedly mounted to bottom surface
456 by a suitable coupling means, such as a living hinge 470
or by a pinned hinge joint, whereby ring retention leaf 468
may be pivoted between the open position of Figure llA to the
closed position of Figures llB-llC. A pair of tabs 472 extend
from an outer edge of ring retention leaf 468 such that, when
leaf 468 is in the closed position of Figure llB, ring 466 iS
trapped between tabs 472 and flange 462. Ring retention leaf
468 iS retained in the closed position by a pair of flexible
catches 474 which may be deflected toward each other by
applying a laterally-directed force. Each catch 474 has a
tapered distal end 476 leading proximally to a step 478. Ring
retention leaf 468 has a central opening 480 through which
catches 474 may extend when leaf 468 is in the closed
position, and a shelf 482 for engaging steps 478 to retain
lea~ 468 in the closed position. Thus, when leaf 468 is
pivoted from the open position to the closed position, the
tapered distal ends of catches 474 engage leaf 468 at the edge
of opening 480 and are urged inwardly as the leaf is closed.
When leaf 468 is completely closed, steps 478 clear shelf 482
and catches 474 spring outwardly so that steps 478 engage
shelf 478, maintaining leaf 468 in the closed position. After
annuloplasty ring 466 has been secured around the native valve
in the heart, thoracoscopic forceps or other elongated
CA 02239907 1998-06-08
W O 96/39942 PCTrUS96/07970
29
grasping device may be introduced through an intercostal port
and used to squeeze catches 474 together, allowing leaf 468 to
open and releasing annuloplasty ring 466 from holder 450.
Two additional embodiments of an annuloplasty ring
holder assembly according to the invention are illustrated in
Figures 12A-12B. In these embodiments, holder assemblies 100,
101 comprise a holder 102, 103 similar to the holders used
with commercially-available annuloplasty rings, and an adaptor
104, 105 for attaching holder 102 to delivery handle 10.
Holder 102,103 is in some respects similar to holder 70 of
Figures 5A-C, with the exception that, in place of handle
coupling 80 of holder 70, holder 102,103 has a hole 106, as in
Figure 12A, or a post 108, as in Figure 12B, adapted for
attachment to a conventional handle for use in open heart
surgery. Hole 106 and post 108 are designed to attach to such
a conventional handle in an orientation in which at least a
distal portion of the handle is perpendicular to the top
surface 110, 111 and bottom ~urface 112, 113 of holder 102,
103 (and the plane of the annuloplasty ring held by the
holder). The longitudinal axes of hole 106 and post 108 are
thus perpendicular to surfaces 110, 111, 112, 113. A groove
or channel 114, 115 extends around the lateral edge of each of
holders 102, 103 and is configured to receive an annuloplasty
ring .
Adaptors 104, 105, illustrated more clearly in
Figures 13A-C and 14A-C, facilitate the attachment of a
conventional angioplasty ring holder to delivery handle 10 of
the invention. Adaptor 104 includes a downward-extending
distal fitting 116 configured for insertion into a handle
attachment hole in a holder like holder 102 of Figure 12A.
Adaptor 104 further includes a pro~;mAlly-extending proximal
fitting 118 for attachment to holder coupling 26 of delivery
handle 10. Distal fitting 116 comprises a cylindrical member
120 with an annular groove 122 in which an O-ring 124 is
disposed. Cylindrical member 120 may be inserted into hole 106
in holder 102 and is retained therein by O-ring 124.
Alternatively, for annuloplasty ring holders having a threaded
handle attachment hole, cylindrical member 120 may have
CA 02239907 1998-06-08
W096/39942 PCTAJS96/07970
external threads to couple to the threaded hole. In a
preferred embodiment, pro~;m~l fitting 118 comprises a slot
126 having an open proximal end 12 8 through which holder
coupling 26 iS received and an open distal end 130 through
which catch 44 may extend. As shown in Figure 13C, the
longit-l~;n~l axis of slot 126 i5 preferably perpendicular to
the longitudinal axis of cylindrical member 120. However, as
with holder 70 described above in connection with Figures 10
and 11, slot 126 may be at a variety of angles relative to
cylindrical member 120 so long as the annuloplasty ring held
on holder 102 may be positioned through an intercostal space
without significant retraction of the adjacent ribs. Usually,
slot 126 is between about -45~ and +45~, and preferably
-20- to +20 , relative to the longitudinal axis of cylindrical
member 120.
Referring now to Figs. 14A-14C, adaptor 105
comprises a distal fitting 132 and a proximal fitting 134. In
this embodiment, distal fitting 132 is adapted to attach to
post 108 on holder 103, and comprises a cylindrical aperture
136 for receiving post 108. Cylindrical aperture 136 may
include an internal O-ring (not shown) or may be tapered so as
to frictionally engage post 108. Alternatively, if post 108
is externally threaded, aperture 136 may include internal
threads to retain post 108 therein. Prox;m~l fitting 134
preferably comprises a slot 138 having an open proximal end
140 and an open distal end 142 so as to receive holder
coupling 26 of delivery handle 10 as described above in
connection with adaptor 104. Again, slot 138 is preferably
perpendicular to the longitudinal axis of aperture 136, but it
may be at various other angles depending upon the size and
shape of holder 103 and the annuloplasty ring it is designed
to carry.
While only two configurations of the adaptor and
holder assembly of the invention are shown in Figures 12-14,
it will be understood by those of ordinary skill in the art
that various other configurations are possible to adapt
virtually any of the annuloplasty ring holders currently
available for attachment to delivery handle 10 of the
-
CA 02239907 l998-06-08
W O 96/39942 PCT~US96/07970
31
invention. In most cases, this will simply require adapting
the distal fitting of the adaptor for the particular handle
attachment means utilized on the ring holder.
Although the foregoing embodiments of holder 70 and
adaptors 104, 105 have been shown as having a handle coupling
in the form of a slot for receiving tongue 34 of delivery
handle 10, various other types of handle/holder couplings may
also be utilized. Various exemplary embodiments are
illustrated in Figs. 15-20. In the embodiments of Figs. 15
and 16, holder coupling 26 on delivery handle 10 comprises a
threaded aperture 150, and handle coupling 80 on holder 70
comprises a threaded shank 152 which may be threaded into
aperture 150. In Figure 15, the longitudinal axis of threaded
shank 152 is parallel to holder body 72, while in Figure 16,
the longitudinal axis of threaded shank 152 is disposed at an
angle relative to holder body 72, preferably between -45~ and
+45~. In Figures 17 and 18, holder coupling 26 comprises a
threaded shank 154, and handle coupling 80 comprises a
threaded aperture 156. The longitll~; n~l axis of threaded
aperture 156 may be parallel to holder body 72 as in Figure
17, or at an angle similar to threaded shank 152 of Figure 16.
Alternatively, the longitudinal axis of threaded aperture 156
may be perpendicular to holder body 72 as in Figure 18, and
threaded shank 154 may be mounted to shaft 20 so that the
longitudinal axis of threaded shank 154 is perpendicular to
shaft 20.
In the embodiment of Figures l9A-B, holder coupling
26 comprises a jaw 158 having a pair of resilient, arcuate jaw
members 160 forming a C-shape. Handle coupling 80 comprises a
cylindrical post 162 with an annular channel 164 formed
therein which is configured to receive jaw 158. In this way,
jaw 158 is attached to post 162 by sliding jaw members 160
around post 162 within annular channel 164. Jaw 158 is
slightly undersized relative to post 162, such that jaw
members 160 flex outwardly as they are inserted into annular
channel 164 and exert an inward force on post 162 to maintain
a tight grip thereon.
CA 02239907 l998-06-08
W O 96/39942 PCT~US96/07970
32
In still another embodiment, holder coupling 26
comprises a bayonet fitting 166 having a cylindrical body 168
and a pair of radially extending tabs 170. Handle coupling 80
comprises a cylindrical receptacle 172 having a pair of
helical slots 174 in its sidewall for receiving tabs 170. In
this way, bayonet fitting 166 may be inserted into receptacle
172 and twisted to form a tight attachment.
It should be understood that the above are only some
of the possible configurations for holder coupling 26 and
handle coupling 80, and should not be taken to limit the range
of possible interconnections between holder 70 and delivery
handle 10. In addition to those described above, other
possible connection means include luer fittings, snap
fittings, spring-loaded catches, magnetic attachments, movable
jaws on delivery handle 10 for grasping holder 70, and various
others. In addition, holder 70 may be permanently and
non-removably attached to delivery handle 10.
In order to select an annuloplasty ring of the
correct size for the valve being repaired, the native valve
must be sized. Sizing disks may be used for this purpose. As
will be described more fully below, sizing disks of various
sizes are positioned adjacent the native valve to be repaired
until a disk of the proper size is identified. An
annuloplasty ring of a corresponding size is then selected for
attachment around the native valve. A similar technique is
used for sizing a native valve for replacement with a
prosthetic valve. Advantageously, the present invention
provides devices and methods for sizing a native valve which
may be utilized through an intercostal port, without
retraction or removal of ribs. Figure 21 illustrates a
preferred embodiment of a sizing disk according to the
invention. Sizing disk 180 comprises a disk body 182 shaped
similarly to the native valve annulus and having and having an
upper face 183 and a lower face 185. Disk body 182 is
preferably a transparent material such as polysulfone or
polycarbonate such that the native valve is visible when the
sizing disk is positioned in front of it. Two or more notches
184 or other markings may be disposed along a side of sizing
CA 02239907 1998-06-08
W O 96/39942 PCT~US96/07970
33
disk 180 to facilitate measuring the spacing of the native
valve trigones or commissures. Sizing disk 180 also includes
a handle coupling 186 for attaching the sizing disk to
delivery handle 10. Handle coupling 186 comprises, in a
preferred embodiment, a slot 188-having an open proximal end
190 and an open distal end 192. Holder coupling 26 of
delivery handle 10 is received into slot 188 through proximal
end 190 and catch 44 extends out of slot 188 through distal
end 192. Slot 188 is disposed at an angle relative to disk
body 182 selected to allow sizing disk 180 to be introduced
through an intercostal space using delivery handle 10 without
retraction or removal of ribs. Usually the longitn~; n~l axis
of slot 188 is between -45~ and +45~, and is preferably
parallel to, upper and lower faces 183, 185 of disk body 182.
With this configuration, sizing disk 180 may be attached to
holder coupling 26 of delivery handle 10 and introduced
through an intercostal port with disk body 182 generally
parallel to the longitll~;n~l axis of shaft 20. Once within
the chest cavity, sizing disk 180 may be pivoted relative to
shaft 20 into a perpendicular orientation such that the disk
face is parallel to the native valve for the measurement
thereof. Of course, like holder 70, handle coupling 186 may
have a variety of other configurations, such as those of
handle coupling 80 described above in connection with Figures
15-20.
A second embodiment of a native valve sizer
according to the invention is illustrated in Figure 22. In
this embodiment, sizing disk 194 may be any of a variety of
commercially-available sizing disks for use with the
annuloplasty rings currently used in open heart surgery.
Sizing disk 194 has a disk body 196 with an upper face 198 and
a lower face 200. Two or more notches 202 or other markings
may be provided on a side of disk body 196 for measurement of
a native valve leaflet. A hole 204 is disposed in a central
region of disk body 196 for attachment of the sizing disk to a
conventional handle for use in open heart surgery. An adaptor
206 is further provided for attaching sizing disk 194 to
delivery handle 10. In a preferred embodiment, adaptor 206
CA 02239907 1998-06-08
W O 96~9942 PCTAUS96/07970
34
has a configuration like adaptor 104 described above in
connection with Figures 12-14. Adaptor 206 has a cylindrical
member 208 extending downwardly and configured for insertion
into hole 204. Cylindrical member 208 may include an O-ring
210 for securing the cylindrical member within hole 204.
Alternatively, if hole 204 has internal threads, cylindrical
member 208 may have external threads. Other types of
interconnections may also be used, such as a cylindrical
aperture on adaptor 206 designed to receive a post or shank on
sizing disk 194, like that described above in connection with
holder 103 of Figure 12B. Adaptor 206 also has a handle
coupling 212, which preferably comprises a slot 214 for
receiving holder coupling 26 of delivery handle 10. Slot 214
is preferably perpendicular to the longitudinal axis of
cylindrical member 208, so as to be parallel to the upper and
lower faces 198, 200 of sizing disk 194 when connected to it.
Slot 214 may be disposed at other angles as well, so long as
sizing disk 194, when connected to adaptor 206 and delivery
handle 10, may be introduced through an intercostal port
without removing or significantly retracting the ribs. As
with sizing disk 180 of Figure 21, various other handle
coupling configurations may also be utilized on adaptor 206.
For cases in which valve repair is inappropriate,
the invention also provides devices and methods for sizing a
native valve which is to be replaced with a prosthetic valve.
A replacement valve sizing disk according to the invention is
illustrated in Figure 23. Valve sizing disk 220 includes a
disk body 222 having an upper face 224 and a lower face 226.
Disk body 222 has a shape corresponding to that of the
prosthetic valve to be used for replacing the native valve,
and is usually circular. A handle coupling 228 is mounted to
upper face 224. Handle coupling 228 preferably comprises a
slot 230 configured to receive holder coupling 26 of delivery
handle 10. Slot 230 has an open proximal end 232 through
which coupling member 26 is received, and an open distal end
234 through which catch 44 may extend. The longitudinal axis
of slot 230 is preferably parallel to upper and lower faces
224, 226 of disk body 222, but may be at other angles, usually
CA 02239907 1998-06-08
W O 96t39942 PCTtUS9GtO7~70
between about -45~ and +45~, so long as sizing disk 220, when
attached to coupling means 26 of delivery handle 10, may be
introduced through an intercostal space without retraction or
removal of ribs. A multitude of alternative configurations
for handle coupling 228 are also possible, including those
described above in connection with Figures 15-20.
When sizing disk 220 is attached to delivery handle
10 and positioned in the orientation of Figure 24A, upper and
lower faces 224, 226 are parallel to the longitudinal axis of
shaft 20, and the combined profile of sizing disk 220 and
shaft 20 is minimized to facilitate introduction through an
intercostal port. Once positioned within the chest cavity,
sizing disk 220 may be pivoted into the orientation of Figure
24B, wherein faces 224, 226 are generally perpendicular to the
longitudinal axis of shaft 20. In this orientation, sizing
disk 220 may be positioned so that lower face 226 is facing
the native valve to allow sizing disk 220 to be pushed in and
out of and/or positioned within the native valve annulus to
compare the native annulus size to the sizing disk size. This
process is repeated using sizing disks of various diameters
until the proper size is determined.
Figure 25 illustrates an alternative embodiment of a
sizing disk assembly according to the invention, wherein an
adaptor 236 is utilized for attaching a conventional sizing
disk 238 to delivery handle 10. Sizing disk 238 may be any of
a variety of sizing disks currently in use in open heart valve
replacement surgeries, and has a shape corresponding to the
shape of the prosthetic valve to be used for the replacement.
Sizing disk 238 has a hole 240 of rectangular cross-section
suitable for attachment to a conventional handle utilized in
open heart surgery. Adaptor 236 includes a rectangular tongue
242 configured for insertion into hole 240. A leaf spring
catch 244 similar to catch 44 on delivery handle 10 is
provided on tongue 242 to retain it within hole 240.
Alternatively, sizing disk 238 may have a post or shank
extending upwardly from it, in which case cylindrical member
242 may have an internal aperture for receiving the post or
shank. Adaptor 236 further includes a handle coupling 246,
CA 02239907 1998-06-08
WO 96/39942 PCTAJS96/07970
36
which preferably comprises a slot 248 having an open proximal
end 250 for receiving holder coupling 26, and an open distal
end 252 through which catch 44 may extend. The longitudinal
axis of slot 248 is preferably perpendicular to the
longitudinal axis of tongue 242 so that, when adaptor 236 is
connected to sizing disk 238, the slot is generally parallel
to the face of the sizing disk. In this way, coupling member
26 of delivery handle 10 may be inserted through slot 248 and
sizing disk may be oriented so that it is parallel to the
longitudinal axis of shaft 20, thereby having a m; n; mllm
profile for introduction through an intercostal port.
Once the native valve has been sized for
replacement, delivery handle 10 of the invention may also be
used to deliver the replacement valve into the heart for
attachment at the native valve position, using techniques
described in detail below. In order to facilitate introducing
the replacement valve through an intercostal space without
retracting or removing ribs, the invention provides a
replacement valve holder having an extremely small profile and
adapted for attachment to delivery handle 10. A preferred
embodiment of a valve holder according to the invention is
shown in Figs. 26A-26C. Valve holder 260 is adapted for
holding a mechanical bileaflet valve prosthesis as shown in
Figure 28, which may be, for example, a bileaflet mitral or
aortic valve prosthesis available from St. Jude Medical, Inc.
of St. Paul, Minnesota, CarboMedics, Inc. of Austin, Texas, or
Sorin Biomedica of Saluggia, Italy. In the example of Figure
28, valve prosthesis 262 has an annular frame 264 and a sewing
ring 266 attached to frame 264 for attachment to an interior
wall of the heart at the native valve position. Sewing ring
266 is covered by a fabric or mesh of e.g. Dacron to allow the
prosthesis to be sutured to the heart tissue. A pair of
parallel uprights 268 extend axially upward from frame 264. A
pair of leaflets 270 having curved outer edges 272 and
straight inner edges 274 are pivotably mounted to uprights
268. Leaflets 270 are movable between a closed position, in
which straight inner edges 274 are contacting each other and
curved outer edges are contacting the inner wall of annular
CA 02239907 1998-06-08
WO 96/39942 PCTrUS96/07970
37
~rame 264, and an open position in which leaflets are spaced
apart from each other and ~rom annular frame 264. Frame 264,
uprights 268 and leaflets 270 are made of a rigid
biocompatible polymer, metal or graphite coated with a
thrombolytic material such as pyrolytic carbon.
Referring again to Figures 26A-26C, valve holder 260
comprises a distal piece 276 and a proximal piece 278
pivotably coupled together by two transverse pins 280,
allowing distal piece 276 to pivot about a transverse axis
relative to pro~;m~l piece 278, as shown in Figure 27. Distal
piece 276 has a top portion 282 which is bifurcated into two
parallel side sections 282A, 282B. Proximal piece 278 has a
single top portion 284 disposed between side sections 282A,
282B. Pins 280 extend through side sections 282A, 282B and
into top portion 284. An axial slot 286 extends through top
portion 284 in an axial direction, and is configured to
receive holder coupling 26 on delivery handle 10. Axial slot
286 has an open proximal end 288 through which tongue 34 is
inserted and an open distal end 290 through which catch 44 may
extend. A pair of transverse suture holes 287, 289 extend
through sides sections 282A, 282B and through top portion 284
for tying a retention suture, as described below. Distal
piece 276 has a distal leg 292 extending downwardly from top
portion 282 and having a distally-facing annular channel 294
for receiving a portion of annular frame 264 of valve
prosthesis 262. Proximal piece 278 has a proximal leg 296
extending downwardly from top portion 284 and having a
prox~m~lly-facing annular channel 298 for receiving a portion
of annular frame 264. When legs 292, 296 are pivoted into the
position of Figure 27, valve prosthesis 262 may be positioned
over the legs, which are disposed between each valve leaflet
270 and annular frame 264. Legs 292, 296 may then be pivoted
outwardly to seat annular frame 264 in channels 294, 298, as
shown in Figure 29. A suture 300 may then be tied through
suture holes 287, 289 to maintain distal piece 276 and
proximal piece 278 in an outward position to retain valve
prosthesis 262 thereon.
CA 02239907 1998-06-08
W O 96/39942 PCTrUS96~i9/0
38
Figures 30A-30B illustrate valve prosthesis 262
mounted to holder 260, which is attached to holder coupling 26
of delivery handle 10. In the introduction position of Figure
30A, holder coupling 26 is aligned with the longitudinal axis
of shaft 20, such that the plane containing the lower surface
of sewing ring 266 is generally parallel to the longitll~; n~l
axis of shaft 20. Alternatively stated, the central
longitll~n~l axis of valve prosthesis 262 is generally
perpendicular to the longitll~i n~l axis of shaft 20. In this
position, the overall height H of holder 260, valve prosthesis
262, and shaft 20 is m;n;m;zed to facilitate introduction
through an intercostal port, with height H usually being less
than about 30 mm and preferably less than about 25 mm. Of
course, some deviation from this orientation may be possible
without hindering introduction through the intercostal port.
For example, depending upon the size of the valve prosthesis
relative to the width of the intercostal space, valve
prosthesis 286 may be positioned as much as about +/-45~ from
the position of Figure 3OA during introduction. Once valve
prosthesis 262 has been introduced through the intercostal
port, it may be pivoted into an orientation suitable for
attachment at the native valve position within the heart. In
the attachment orientation, the central longitudinal axis of
valve prosthesis 262 will preferably be parallel to the
longitudinal axis of shaft 20, such that the lower surface of
sewing ring 266 is facing and parallel to the interior wall of
the heart to which the valve will be attached. A variety of
other angular orientations may also be used where a
non-perpendicular approach to the valve has been taken, or in
other appropriate circumstances. Advantageously, delivery
handle 10 allows the valve prosthesis to be pivoted into a
wide range of angular orientations according to the needs of
each particular case.
Figure 3OA also illustrates an important advantage
of holder 260 of the invention. It may be seen that distal
leg 292 and proximal leg 296 extend below the lower ends of
valve leaflets 270. In this way, when valve prosthesis 262 is
CA 02239907 1998-06-08
W O 96/39942 PCTrUS96/07970
~ 39
mounted to holder 260, leaflets 270 are protected from damage
during introduction and placement.
In the embodiment of Figures 26-30, valve holder 260
is attached to delivery handle 10 by means of axial slot 286
which receives holder coupling 26 on the handle. However, it
will be understood to those of ordinary skill in the art that
a variety of other handle attachment means may be used on the
valve holder of the invention. Four exemplary alternative
handle attachment means are shown in Figures 31-34. In Figure
31, holder coupling 26 on handle 10 comprises an
internally-threaded aperture 302, and holder 260 is attached
to handle 10 by a threaded shank 304 extending proximally from
proximal piece 278 which may be threaded into aperture 302.
Shank 304 may be at a variety of angles relative to holder
260, but is preferably perpendicular to the longitudinal axis
of the holder. In Figure 32, a threaded shank 306 on holder
260 on the top of proximal piece 278 is generally parallel to
the longitn~inAl axis of holder 260 for connection to a
laterally-oriented internally-threaded aperture 308 on holder
coupling 26. In Figure 33, a threaded shank 310 on holder
coupling 26 couples to a threaded hole 312 in holder 260 which
is perpendicular to the longitll~; nA 1 axis of the holder. In
Figure 34, a threaded hole 314 is parallel to the longitll~;n
axis of the holder and connects to a laterally oriented
threaded shank 316 on holder coupling 26. Various other
handle connection mechanisms are also possible, including
bayonet fittings, luer locks, spring-loaded catches,
holder-gripping jaws on handle 10, and permanent,
non-detachable linkages. The particular type of attachment
means is not critical, so long as valve holder 260 may be
connected to delivery handle 10 in an orientation which allows
valve prosthesis 262 to be held on holder 260 and introduced
~ through an intercostal port without removing or retracting the
ribs.
In addition to the bileaflet valve prosthesis
illustrated in Figure 28, the prosthesis holder and delivery
system may also be adapted for use with a variety of other
types o~ prosthetic valves, both mechanical and bioprosthetic.
CA 02239907 1998-06-08
W O 96/39942 PCT~US96/07970
Various types of prosthetic valves useful with the invention
are described in Jamieson, "Modern Cardiac Valve
Devices--Bioprostheses and Mechanical Prostheses: State of the
Art," ~. Card. Surg. 8:89-98 (1993). Mechanical valves which
may be used include the caged-ball type such as the
Starr-Edwards~ valve (Baxter Healthcare Corp., Edwards CVS
Division, Irvine, CA), the tilting disk type such as the
Medtronic-Hall~ valve (Medtronic, Inc., Minneapolis, MN), the
Bjork-Shiley Monostrut~ valve (Shiley, Inc., Irvine, CA), the
Omniscience~ valve (Omniscience Medical, Inc., Grove Heights,
MN), as well as the bileaflet type such as the Baxter
Duromedics~ Valve (Baxter Healthcare Corp., Edwards CVS
Division, Irvine, CA), St. Jude valve (St. Jude Medical Inc.,
St. Paul, MN), Carbomedics valve (CarboMedics, Inc., Austin,
TX), or Sorin valve (Sorin Biomedica, Saluggia, Italy).
Bioprosthetic valves which may be placed using the devices and
techniques of the invention include porcine aortic valves such
as the Hancock II~ bioprosthesis (Medtronic, Inc., Minneapolis
MN) ! the CarDentier-Bdwardc~ supr~n~lllar biop~osthe~i~
(Baxter Healthcare Corp., Edwards CVS Division, Irvine, CA),
the Carpentier-Edwards~ stentless bioprosthesis (Baxter
Healthcare Corp., Edwards CVS Division, Irvine, CA), the St.
Jude Bioimplant~ bioprosthesis (St. Jude Medical Inc., St.
Paul, MN), or the Medtronic Intact~ bioprosthesis (Medtronic,
Inc., Minneapolis, MN). Other valves which may be used
include the Mitroflow~ bioprosthesis (Mitroflow International,
Inc., Richmond, British Columbia, ~n~ ), and the
Carpentier-Edwards~ pericardial bioprosthesis (Baxter
Healthcare Corp., Edwards CVS Division, Irvine, CA). The
invention also facilitates valve replacement with homografts
and allografts, polymeric valves, and a variety of mechanical
and bioprosthetic valves not specifically listed here.
The methods of repairing and replacing a diseased
heart valve according to the invention will now be described
with reference to Figures 35-46. The patient must first be
prepared for surgery by inducing general anesthesia,
establishing cardiopulmonary bypass, and inducing cardioplegic
arrest. Devices and techniques for inducing cardioplegic and
CA 02239907 l998-06-08
W O 96/39942 PCTrUS96/07970
41
establishing cardiopulmonary bypass which may be used in
conjunction with the method of the present invention are
described in co-pending application Serial Nos. 08/282,192,
filed July 28, 1994, 08/159,815, filed November 30, 1993, and
08/173,899, filed December 27, 1993, which are incorporated
herein by reference. As described in those applications,
after general anesthesia has been induced, cardiopulmonary
bypass is initiated by placing a venous cannula in a major
peripheral vein such as a femoral vein, and placing an
arterial cannula in a major peripheral artery such a femoral
artery. The venous and arterial cannulae are connected to a
cardiopulmonary bypass system, which includes an oxygenator
for oxygenating blood withdrawn from the patient through the
venous cannula, a filter for removing emboli from the blood,
and a pump for returning the blood to the patient's arterial
system through the arterial cannula.
With cardiopulmonary bypass established,
cardioplegic arrest may be induced. Although conventional,
open-chest external aortic cross clamping and aortic
cannulation through the aortic wall may be utilized,
closed-chest cardioplegia techniques are preferred. As
described in the forementioned copending applications,
cardioplegia may be induced on a closed-chest patient by
introducing an aortic catheter into a femoral artery or other
major peripheral artery, translllm;n~lly positioning the distal
end of the aortic catheter in the ascending aorta, and
expanding an expandable member such as a balloon on the distal
end of the aortic catheter to occlude the ascending aortic
lumen between the coronary ostia and the brachiocephalic
artery. A cardioplegic agent, preferably comprising a
potassium chloride solution mixed with blood, is then
delivered through a lumen of the aortic catheter into the
ascending aorta, where the cardioplegic fluid flows into the
coronary arteries, perfusing the myocardium and arresting
cardiac function. A venting catheter may also introduced into
the right side of the heart or into the pulmonary artery from
a peripheral vein, as described in copending application
Serial No. 08/415,238, filed March 30, 1995, which is
CA 02239907 1998-06-08
WO 96~9942 PCTrUS96/07970
42
incorporated herein by reference. In addition, a retrograde
cardioplegia catheter may be introduced from another
peripheral vein into the coronary sinus for delivering
cardioplegic fluid into the coronary sinus under sufficient
pressure to flow in a retrograde manner through the coronary
veins to perfuse the myocardium, as described in copending
application Serial No. 08/372,741, filed January 12, 1995,
which is incorporated herein by reference.
As an alternative to these endovascular techniques,
cardioplegic arrest may be induced by occluding the ascending
aorta with a thoracoscopic cross-clamp positioned externally
on the aorta through an intercostal port in the anterior
chest. Cardioplegic fluid may then be delivered upstream of
the clamp with a cannula intrall1mlnAlly positioned in the
aorta from a peripheral artery, or by penetrating the aortic
wall with a cannula introduced thoracoscopically. Such
techniques are described in copending application Serial No.
08/173,899, filed December 27, 1993, which has been
incorporated herein by reference.
In order to obtain access to the heart from the
right lateral side of the chest, the right lung must be
collapsed. This may be accomplished by inserting an
endotracheal tube into the right main stem bronchus and
applying a vacuum so as to deflate the lung.
With cardiac function arrested and the patient's
circulation supported by extracorporeal cardiopulmonary
bypass, the patient is ready for the valve repair or
replacement procedure. Referring to Figure 35, a number of
percutaneous cannulae, hereinafter referred to as "ports, n are
positioned in the anterior chest and right lateral chest to
provide access into the chest cavity. In most cases, 3 to 5
ports are required, including a retraction port 332 located in
the anterior chest over the right lateral wall of the right
atrium, an oval port 334 located in the right lateral chest in
the second, third, fourth, fifth or sixth intercostal space,
and at least one instrument port 336 in the right lateral
chest or anterior chest for introduction of instruments or
visualization devices. Retraction port 332 and instrument
CA 02239907 l998-06-08
W 096~9942 PCT/U~C/~/~70
- 43
ports 336 are configured for placement within an intercostal
space without requiring retraction of the ribs, and are
usually 5-12 mm in diameter. To introduce the ports, a small
puncture or incision is made in the intercostal space at the
desired location, and, with an obturator positioned in the
lumen of the ports, they are advanced through the puncture or
nclsion.
Oval port 334, illustrated in Figure 36A-36D, is
also configured for placement within an intercostal space
without retraction of ribs, and has a width of less than about
30 mm, and preferably less than about 25 mm. Oval port 334 has
a percutaneous tube 340 having a flange 342 at its proximal
end to engage the outside of the chest when the oval port is
introduced. A plurality of tie-down holes 343 are provided in
flange 342 to facilitate securing oval port 334 to the patient
by means of sutures or other tie-down means passed through
holes 343. Percutaneous tube 340 has a length sufficient to
extend from outside of the chest, through the intercostal
space, and into the chest cavity ~ust beyond the interior of
the chest wall, the length typically being in the range of
30-50 mm. Percutaneous tube 340 has an inner lumen 338 with
shape and ~;m~n~ions selected to allow an annuloplasty ring or
replacement valve on a holder to be introduced through it
using delivery handle 10. Inner lumen 338 usually has a width
of about 10-30 mm, and preferably 15-25 mm, and a height of
about 25-75 mm, preferably 30-50 mm. The exact width and
height will be determined by the width (or diameter) and
height of the particular annuloplasty ring or replacement
valve and holder being used in the procedure. It is usually
desirable to begin the procedure with an oval port 334 of the
m;n;ml~m size necessary to assess the condition of the native
valve and to allow introduction of valve sizing disks. For
example, an oval port 334 having a width of about 15-20 mm may
be used initially. When the size of the annuloplasty ring or
~ 35 prosthetic valve to be used has been selected, the smaller
oval port may be replaced, if necessary, with a larger oval
port to accommodate the prosthesis.
. ..
CA 02239907 1998-06-08
W O 96/39942 PCT~US~G/0/~/0
- 44
Oval port 334 may also include a suture organizing
ring 344 attached to flange 342 so as to surround inner lumen
338. Organizing ring 344 has a plurality of
circumferentially-spaced radial slots 346 in which a suture
thread may be received and retained by friction. Slots 346
have tapered upper ends 348 to allow a suture thread to be
easily guided into the slot. Suture organizing ring 344
allows sutures placed in the heart for attachment of a
prosthesis to be drawn through inner lumen 338 and temporarily
placed in slots 346 so as to keep the sutures individually
separated and untangled, as described more fully below.
In order to facilitate introducing oval port 334
through a puncture or small incision between the ribs, an
obturator 350 may be slidably inserted into inner lumen 338,
as illustrated in Figures 37A-B. Obturator 350 includes an
oval shaft 352 positionable within inner lumen 338 and a
tapered or pointed distal end 354 which extends distally of
the distal end of percutaneous tube 340. A handle 356 is
attached to the prox~m~l end of oval shaft 352 and has a
distal face 358 for engaging flange 342 on oval port 334.
Handle 356 has shape and dimensions suitable for grasping in
the hand of the user and applying a distally-directed force
for percutaneous introduction. Once oval port 334 has been
introduced through an intercostal space, obturator 350 is
removed from inner lumen 338.
In addition to the oval configuration shown, oval
port 334 may have an inner lumen of various other shapes,
including race-track, rectangular, trapezoidal, elliptical or
circular. Alternatively, oval port 334 may be made of a
flexible or deformable material to allow it to be shaped by
the user or to conform to the shape of the intercostal space.
In addition, other means of tissue retraction may be used in
place of oval port 334, such as a 3-sided channel-shaped
member, or a wound retractor having a pair of adjustable
parallel blades which can be placed in an intercostal incision
and used to create a space by widening the distance between
the blades. All of these may fall within the scope of the
invention to the extent they facilitate introduction of a
CA 02239907 1998-06-08
W O 96/39942 PCT~US9G~'~7~7u
~ 45
prosthetic annuloplasty ring or valve through an intercostal
space without significant retraction or removal of the ribs or
sternum.
Referring again to Fig. 35, with ports 332, 334, 336
in position, surgery within the chest cavity may begin. Much,
if not all of the procedure may be carried out under direct
vision by illuminating the chest cavity with a light source or
light guide positioned in an instrument port or in the oval
port and looking through the inner lumen of oval port 334 or
through one of the instrument ports. Head-mounted surgical
loupes specially designed for looking through a small incision
or cannula may be utilized to facilitate direct vision through
a port, such as the devices described in U.S. Patent Nos.
4,836,188, 4,196,966, and 4,807,987, which are incorporated
herein by reference. A fiberoptic bundle may also be attached
to or embedded in the wall of one of instrument ports 336 or
in percutaneous tube 340 of oval port 334 to transmit light
into the chest from a light source outside the chest, in the
manner disclosed in copending application Serial No.
08/227,366, filed April 13, 1994, which is incorporated herein
by reference. In most cases, however, it will be desirable to
introduce a thoracoscope 360 through an instrument port 336 to
provide additional illumination and visualization of the chest
cavity, preferably by means of a video camera mounted to
thoracoscope 360 which transmits a video image to a monitor
(not shown in Fig. 35). ThoracoRcope 360 may comprise a rigid
thoracoscope with a straight end or an angled end such as
those available from, for example, Olympus Corp., Medical
Instruments Division, Lake Success, NY. Alternatively, a
thoracoscope with an articulated end steerable by means of an
actuator at the proximal end of the device may be used, such
as the Welch Allyn DistalVu~ (formerly Baxter DistalCam~ 360),
available from Welch Allyn, Inc., of Skaneateles Falls, NY.
Thoracoscopic surgical instruments are then
introduced in order to form an opening in the pericardium,
which surrounds the heart. If the right lung is not
Rufficiently collapsed, atraumatic retraction instruments may
be introduced through one of the ports to push the lung
CA 02239907 l998-06-08
W 096/39942 PCTrUS9G/~/Y70
~ 46
posteriorly such that the pericardium is visible by looking
through oval port 334 or through one of instrument ports 336.
Thoracoscopic scissors 362 and graspers 364 are then
introduced through oval port 334 or instrument port 336 and
used to cut an opening in the pericardium P. Suitable
thoracoscopic instruments for use in the method of the
invention are described in copending application Serial Nos.
08/281,962, filed July 28, 1994, and Serial No. 08/194, 946,
filed February 11, 1994, which are incorporated herein by
reference.
With an opening formed in the pericardium, the right
lateral wall of the left atrium is in a direct line of sight
from the right lateral chest looking through inner lumen 338
of oval port 334. An atriotomy incision AI is then made in
the left atrial wall W by means of thoracoscopic scissors 362
and graspers 364 introduced through instrument ports 336
and/or through oval port 334, as illustrated in Figure 38.
Atriotomy AI is located between and just anterior to the
pulmonary veins PV.
Before making atriotomy incision AI, it may be
advantageous to flood the thorax with cool carbon dioxide
(C02) and to maintain this C02 blanket around the heart
throughout the procedure in order to help exclude air from the
chest cavity and heart, thereby reducing the risk of trapped
air embolism in the heart. In such cases, retraction port
332, oval port 334 and instrument ports 336 may include
gaseous seals like those used in laparoscopic trocar sleeves
to prevent 1088 of C02 from the chest cavity and/or
introduction of air into the chest cavity. C02 may be
introduced through an insufflation tube introduced through one
of these ports, or through an insufflation lumen extending
through one of the ports.
Referring to Figures 39-40, an endoscopic atrial
retractor 366 is then inserted through retraction port 332,
positioned in atriotomy AI, and pulled anteriorly so as to
retract atriotomy AI open. A rake-type retractor with several
collapsible blades 368 (best seen in Figure 40), coupled to
the end of an elongated handle 370 may be used for this
CA 02239907 1998-06-08
W O 96~9942 PCTrUS96/07970
- 47
purpose, as described in copending application Serial No.
08/281,962, filed July 28, 1994. Alternatively, a retractor
having a single larger transverse blade which is attachable
and removable from an elongated handle may be used, as
described in copending application Serial No. 08/294,454,
filed August 23, 1994, which is incorporated herein by
reference. The single blade may be introduced through inner
lumen 338 of oval port 334 while the handle is introduced
through retraction port 332, the blade then being attached to
the handle within the chest cavity, and positioned within
atriotomy AI to facilitate retraction. With atriotomy AI
retracted, direct visualization of mitral valve MV is possible
through inner lumen 338 of oval port 334, as shown in Figure
40.
Under either direct visualization through a port or
video-based viewing using thoracoscope 360, the condition of
mitral valve MV is then assessed to determine whether the
valve may be repaired, or whether replacement of the valve is
necessary. If the surgeon determines that repair is the more
suitable option, a number of repair procedures may be
performed, including annuloplasty, wherein an annuloplasty
ring is attached around the native valve to contract the
annulus, quadrangular resection, in which a portion of a valve
leaflet is excised and the r~m~;n;ng portions of the leaflet
are sewn back together, commissurotomy, wherein the valve
commissures are incised to separate the valve leaflets,
shortening of the chordae tendonae, reattachment of severed
chordae ten~on~e or papillary muscle tissue, and
decalcification of the valve leaflets or annulus. Several of
these procedures may also be performed on the same valve. In
particular, annuloplasty rings may be used in conjunction with
any repair procedures where contracting or stabilizing the
valve annulus might be desirable.
In a preferred method of annuloplasty according to
the invention, a prosthetic annuloplasty ring is introduced
through oval port 334 and attached to an interior wall of the
heart around the native valve annulus VA of mitral valve MV.
In order to select an annuloplasty ring of the proper size,
CA 02239907 1998-06-08
WO 96~9942 PCTrUS9G/~7Y70
48
the native valve must be measured using a sizing device such
as sizing disk 180 or 194 described above in connection with
Figures 21-22. As illustrated in Figure 39, sizing disk 180
is attached to coupling member 26 on the distal end of
delivery handle 10, and pivoted relative to shaft 20 into an
orientation appropriate for introduction through inner lumen
338 of oval port 334. Preferably, in this orientation, the
lower face 185 of sizing disk 180 will be generally parallel
to the longitudinal axis of shaft 20. Sizing disk 180 is then
introduced through oval port 334 and through atriotomy AI
using delivery handle 10, until the sizing disk is within the
left atrium LA. Sizing disk 180 is then pivoted using
actuator button 30 on handle 28 such that lower face 185 is
facing mitral valve MV, approximately perpendicular to the
longitll~;nAl axis of shaft 20. Under visualization with
thoracoscope 360 and/or direct vision through a port, sizing
disk 180 is positioned adjacent or against mitral valve MV and
the size of the native valve is measured, usually by measuring
the width of the anterior leaflet AL (Figure 40) by comparing
the width of sizing di~k 180, and by measuring the spacing
between the native valve commissures or trigones using notches
184 or other markings on sizing disk 180. Sizing disks of
various sizes may be interchanged on delivery handle 10 and
positioned adjacent mitral valve MV until the proper size has
been determined.
With an annuloplasty ring of the appropriate size
identified, sutures are placed in or just outside of the
native valve annulus VA, as illustrated in Figure 40.
Double-armed sutures 372 of braided polyester or Nylon and
having a length of about 30-36 cm are preferred.
Thoracoscopic needle drivers 374 may be used to grasp a curved
suture needle 376 on one end of a suture 372, position the
suture in left atrium LA through oval port 334 or an
instrument port 336, and drive needle 374 through valve
annulus VA in the manner shown in Fig. 40. Appropriate
thoracoscopic needle drivers are described in copending
application Serial Nos. 08/281,962, filed July 28, 1994, and
Serial No. 08/194, 946, filed February 11, 1994, which have
CA 02239907 1998-06-08
W O 96~9942 PCTrUS96/07970
~ 49
been incorporated herein by reference. Suture placement is
visualized either by direct vision through oval port 334 or by
using thoracoscope 360. Usually between 8 and 20 double-armed
sutures 372 are placed in valve annulus VA. After being
placed, suture needles 376 are drawn out of the chest cavity
through inner lumen 338 of oval port 334, and sutures 372 are
inserted into slots 346 in organizing ring 344.
Sutures 372 are next placed through annuloplasty
ring 90 on holder 70, as illustrated in Figure 41. Holder 70
i8 attached to holder coupling 26 on delivery handle 10, which
optionally may be held in a clamping fixture 380 attached to
the operating table 382. Each suture needle 376 is grasped in
a needle driver 374 and passed through annuloplasty ring 90.
Sutures 372 may then be placed in a suture organizer, or a
pair of hemostats (not shown) may be clamped onto each needle
376 and suspended from annuloplasty ring 90 to maintain
tension on the sutures and prevent tangling.
Annuloplasty ring 90 is then introduced through
inner lumen 338 of oval port 334, as illustrated in Figure 42.
Holder 70 is pivoted relative to shaft 20 so that annuloplasty
ring 90 will pass through inner lumen 338 without
interference, preferably in an orientation in which a plane
containing the lower surface of annuloplasty ring 90 is
parallel to the longitudinal axis of shaft 20. As
annuloplasty ring 90 is advanced into the left atrium LA,
tension is maintained on sutures 372 by organizer ring 344 or
by individual hemostats (not pictured) clamped onto each pair
of needles 376 on sutures 372 so that the annuloplasty ring
slides along the sutures up to the mitral valve MV. Once
through oval port 334 and into the chest cavity, actuator
button 30 on delivery handle 10 may be actuated so that
annuloplasty ring 90 pivots into an orientation suitable for
attachment within the left atrium LA, preferably in an
orientation in which the lower surface of the annuloplasty
ring is parallel to the mitral valve and perpendicular to the
longit~ n~l axis of shaft 20. Annuloplasty ring 90 is
positioned in contact with the interior wall of the left
CA 02239907 l998-06-08
W 096~9942 PCT~US96/07970
~ 50
atrium in which sutures 372 have been placed so as to surround
the native valve annulus VA.
Holder 70 may then be removed from annuloplasty ring
90 by cutting any sutures used to retain ring 90 on the
holder, and urging ring 90 out of ch~nnel 76 on the side of
holder 70. Holder 70 and delivery handle 10 may then be
removed from the chest cavity.
Needles 376 are then trimmed from each suture 372,
and, as illustrated in Figures 43-44, knots 384 are tied in
each suture 372 and pushed into left atrium LA and against
annuloplasty ring 90 by an endoscopic knot pusher 386. Knot
pusher 386 preferably comprises a knot pusher with a rounded
distal end 388 and a single lateral eyelet 390, as disclosed
in copending application Serial No. 08/288,674, filed August
10, 1994, which is incorporated herein by reference. This
knot pusher is particularly well-adapted for the method of the
invention due its long length and low profile, and due to the
quickness with which knots can be tied and the ease with which
they can be slid into the left atrium from. outside of the
chest cavity. After each suture 372 is tied securely against
annuloplasty ring 90, the suture ends are trimmed off using
thoracoscopic scissors 362.
If neither annuloplasty nor any other repair
procedure will adequately treat the diseased valve, the
2~i surgeon may elect to replace the native valve with a
replacement valve. The techniques for introducing and
securing a replacement valve within the heart will be
analogous to those described above for annuloplasty ring 90,
and are further described in copending application Serial No.
08/281,962, filed July 28, 1994, which has been incorporated
herein by reference. The native valve may be sized for
replacement using valve sizing disks 220 or 238, shown in
Figures 23-25, which are introduced into left atrium LA using
delivery handle 10 by techniques similar to those described
above for sizing mitral valve MV for an annuloplasty ring.
Once a prosthetic valve 262 of the appropriate size is
identified, thoracoscopic needle drivers 274 may be used to
place sutures around the native valve annulus, in much the
CA 02239907 l998-06-08
W 096/39942 PCT~US96/07970
51
same way as described above for annuloplasty ring 90, using a
mattress stitch or everted mattress stitch. As in the case of
annuloplasty, the sutures are withdrawn from the body cavity
through inner lumen 338 of oval port 334 and placed in slots
346 of organizing ring 344. Each suture is then placed
through sewing ring 266 of prosthetic valve 262 outside of the
chest cavity. Optionally, prior to suture placement, the
valve leaflets of the native valve may be removed using
thoracoscopic scissors 362.
Prosthetic valve 262, held on valve holder 260
(described above in connection with Figures 26-34), is then
attached to delivery handle 10 to facilitate delivery of the
replacement valve through oval port 334 into left atrium LA.
During introduction, prosthetic valve 262 is pivoted into an
orientation in which it will pass through inner lumen 338 of
oval port 334 without interference, preferably with the
longitudinal axis of sewing ring 266 approximately
perpendicular to the longitudinal axis of shaft 20 a8 shown in
Fig. 30A. Once within the chest cavity, prosthetic valve 262
is pivoted into an orientation suitable for attachment at the
native valve position in the heart, preferably with the
longitudinal axis of sewing ring 266 perpendicular to the
interior wall of the heart to which the valve will be
attached, and parallel to the longitudinal axis of shaft 20 as
shown in Fig. 30B. Prosthetic valve 262 may then be removed
from holder 260 by cutting retaining suture 300, and holder
260 and delivery handle 10 are removed from the chest cavity.
It may be necessary to seat the prosthetic valve
firmly against the native valve annulus after holder 260 and
delivery handle 10 have been removed. A valve seater 400,
illustrated in Fig. 45A, may be utilized for this purpose.
Valve seater 400 comprises an elongated rigid shaft 402, and a
valve engaging tip 404 at its distal end. Valve engaging tip
404 has a concave end 406 radiused so as to match sewing ring
266, and is made of a soft polymer such as silicone rubber or
thermoplastic elastomer (TPE) with a durometer in a range of
20 to 70 Shore A so that it can contact the prosthetic valve
without damaging it. Valve seater 400 has a length of at
. .
CA 02239907 l998-06-08
W096/39942 PCTAJS96/07970
- 52
least about 20 cm and usually at least 30 cm so as to reach
the mitral position from outside the chest cavity via an
intercostal port, and a diameter of less than about 25 mm,
usually less than about 8 mm, so as to be positionable through
an intercostal port. In this way, valve seater 400 may be
positioned through an intercostal port (e.g. oval port 334)
and tip 404 can be used to push against sewing ring 266 to
seat the prosthetic valve against the native valve annulus.
Knots are then formed in the sutures and pushed into
the left atrium using thoracoscopic knot pu~her 386. The
sutures are then trimmed off above the knot using
thoracoscopic scissors 362, as described above.
Be~ore or after the prosthetic valve has been
secured in the heart, it may be necessary to test its leaflets
to ensure they are functioning properly. A leaflet testing
device as illustrated in Fig. 45B may be used for this
purpose. Leaflet testing device 408 comprises a rigid sha~t
410 and a leaflet-engaging tip 412 attached to the distal end
of shaft 410. Shaft 410 has a length of at least about 20 cm
usually at least 30 cm, and a diameter of less than about 25
mm, usually less than about 8 mm, to reach the mitral position
from outside the chest via a right lateral intercostal port.
Tip 412 has a tapered distal end 414 configured to push
lightly on each valve leaflet 270 (Fig. 28) to fully open the
leaflet without inter~erence with annular frame 264. Because
valve leaflets 270 may be fragile and susceptible to damage,
tip 412 is made of a soft polymer such as silicone rubber or
thermoplastic elastomer (TPE) with a durometer in a range of
20 to 70 Shore A. In this way, leaflet testing device 408 may
be introduced through oval port 334 and each leaflet of the
valve prosthesis pushed gently distally to ensure the leaflets
are opening properly.
For certain types of heart valves prostheses, it may
also be desirable to rotate the annular frame and valve
leaflets relative to the sewing ring after the prosthesis has
been secured in the heart. For this purpose, a specially
designed valve rotator may be used. The valve rotator has an
atraumatic rotator head for engaging the valve frame and/or
CA 02239907 1998-06-08
WO 96/39942 PCTrUS96/07970
53
leaflets similar to that disclosed in U.S. Patent No.
5,403,305, which is incorporated herein by reference.
However, rather than a socket which connects the head to a
handle such that the face of the head is perpendicular to the
handle, the rotator head used in the method of the present
invention has a handle coupling like handle coupling 80 of
Figures 5A-5C. In this way the rotator head may be connected
to holder coupling 26 of delivery handle 10 so that it may be
positioned in an edge-first orientation for introduction
through an intercostal port, then pivoted into a face-first
orientation for rotating the valve prosthesis.
When annuloplasty ring 90 or replacement valve 262
has been secured within the heart, atriotomy AI may be closed,
as illustrated in Figure 46. Thoracoscopic needle drivers 374
may be used to grasp a curved needle 392 on a suture 394,
introduce suture 394 into the chest cavity through oval port
334 or an instrument port 336, and drive needle 392 through
the left atrial wall to create a series of stitches across
atriotomy AI. Alternatively, an endoscopic stapling device
such as an AutoSuture~ Powered Multifire Endo TA60, available
from United States Surgical Corp. of Norwalk, CT, or an
endoscopic fascia stapler, may be inserted through an anterior
instrument port 336 and positioned around atriotomy AI to
drive a series of staples into the atrial wall to close the
atriotomy.
The opening formed in the pericardium may be closed
with sutures or staples in a manner similar to that used for
closing atriotomy AI. However, in most cases, closure of the
pericardium is not necessary, and the opening may be left in
it without adverse effect.
To complete the operation, cardiac function is
restored by discontinuing delivery of cardioplegic fluid,
terminating occlusion of the ascending aortic lumen, and
perfusing the myocardium with warm blood. Preferably, where
an aortic catheter has been used for aortic occlusion and
cardioplegic fluid delivery, the expandable member on the
distal end of the aortic catheter is deflated and warm blood
is allowed to flow into the coronary arteries. If sinus
CA 02239907 1998-06-08
WO 96~9942 PCTAJS96/07970
~ 54
rhythm is does not return immediately, electrical
defibrillation may be used to stimulate the heart and/or
pacing leads may be placed through a port into the heart
muscle to pace the heart for a period of time >
post-operatively. Once the heart is beating normally, the
aortic catheter may be removed from the patient, along with
any venting catheter or retrograde cardioplegia delivery
catheter which may have been used. Chest tubes may be
inserted into the chest to provide drainage. The patient is
then weaned from cardiopulmonary bypass, and the arterial and
venous cannulae are removed from the patient. All venous and
arterial punctures or cut-downs are closed. Any endotracheal
tubes used for ventilation are removed. Retraction port 332,
oval port 334, and instrument ports 336 are removed from the
chest, and all intercostal incisions and punctures are closed.
The patient is then recovered from anesthesia.
It will be understood to those of ordinary skill in
the art that, while the invention has been described
specifically in the context of mitral valve repair and
replacement, the devices and methods disclosed herein will
have equal application to a number of other cardiac valves,
including the aortic valve, the tricuspid valve, and the
pulmonary valve. While the specific locations of these valves
and the surgical approaches utilized to access these valves
2S may differ from those described in detail above, the devices
and methods described herein are easily adapted for use on
valves other than the mitral without departing from the scope
of the invention. For example, the tricuspid or pulmonary
valves may be accessed similarly to the mitral valve through
an intercostal port in the right lateral chest to access the
right atrium (for the tricuspid valve) or the right ventricle
(for the pulmonary valve), on the other hand, the aortic valve
can be accessed from an intercostal port in the upper anterior
chest via an incision in the ascending aorta. Moreover,
although the devices and methods of the invention have been
described in connection with specific types of prosthetic
annuloplasty rings and replacement valves, these are given by
way of example only. It should be understood that a wide
CA 02239907 1998-06-08
W O 96/39942 PCT~US96/07970
variety of prostheses may be implanted using the devices and
methods of the invention with little if any modification to
the specific embodiments described above.
Using the devices and methods of the invention, a
cardiac valve may be repaired or replaced using
minimally-invasive techni~ues which eliminate the need for a
median sternotomy or other gross thoracotomy involving
cutting, removal, or substantial retraction of the ribs or
sternum. As a result, patient recovery is accelerated, pain
and trauma are greatly reduced, and the morbidity and
mortality of valve repair and replacement procedures may be
decreased. Not only may this result in better outcomes and
reduced costs for the thousands of patients who undergo
cardiac valve surgery each year, but may allow thousands more
suffering from valve disease to receive surgical treatment who
would otherwise be unable or unwilling to tolerate the pain
and trauma of open-heart valve surgery.
While the above is a complete description of the
preferred embodiments of the invention, various alternatives,
modifications, additions, and substitutions are possible
without departing from the scope thereof. Therefore, the
above should not be taken as limiting the scope of the
invention, which is defined by the following claims.