Note: Descriptions are shown in the official language in which they were submitted.
CA 02240196 1998-08-10
WO 97122592 PCTIDE96102161
PROCESS FOR THE PRODUCTION OF (METH)ACRYLIC ACID ESTERS
SPECIFICATION
Field of the Invention
The present invention relates to a new and improved process for the production
of
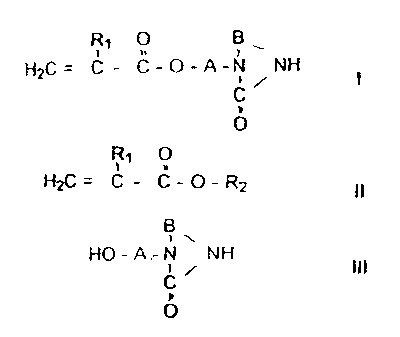
acrylic or methacrylic acid esters with the formula
R~ 0 B
.. , \
HZC= C - C-O-A-N NH
I
O
in which R, stands for hydrogen or a methyl group and A and B stand for
unbranched
or branched alkylene groups with 2 to 5 C atoms.
State of the Art
Compounds of Formula I can be obtained in accordance with the process
described in
the U.S. patent 2,871,223, by means of reaction of acrylic or methacrylic acid
chloride
with hydroxyalkyl imidazolidine-2-ones in the presence of tertiary nitrogen
bases, with
stoichiometric amounts of the hydrochlorides of the tertiary nitrogen bases
being
formed, along with other products.
CA 02240196 2005-06-10
WO S7I2239Z 2 PCT1DE961Q2161
tn the process known from EP 0 236 984 A1, for the production of acryl and
methacryt
esters of Formula t, acrylic or methacrytic acid esters are rearfed with 1-
(hydroxyaitcyl}
imidazotidine-2-ones in the presence of titanium atcohotates or chelafe
compounds of
the metals titanium, zirconium, iron, and zinc, with l,3-dicarbonyt compounds
as the re-
esterification catalysts.
In EP-A 0 433 'f 35 and EP-AO 453 638, diorgano tin oxide compounds are
claimed ~as
re-ester~cation catalysts for the re-esteTrfcation of acryl and methacryt
esters with
hydroxyattcyl imiciazotine-2-ones.
As a rule, the metal catalyst must be removed from batches after the reaction
is
complete. This is advantageously done by adding crater, fQr example when using
tetraalkyl titanates yr diatkyt tin oxides. tn this eonriection, the titanates
form metal
(hydr}oxides, such as Ti02; vsihich are removed by filtering or centrifuging
them off, for
example_ These hydwolyzed re-esfeirftcation Catalysts cannot be used again as
such
after being removed.
It is true that the dialkyt tin oxides can be removed as such by the addition
of
wafer, and can be used again as re-esterification catalysts. However, a
relatively
large amount of water has to be introduced, at first, and this has to be
removed
from the reaction product once again. According to the European patent No.
0 571 851, the reaction can also be carried out in the presence of mixtures of
alkaiilearth alkali metal compounds, which are essentially used as oxides,
hydroxides, carbonates, andlor as salts of carboxylic aEids. The ~afkati/earth
alkali
compounds present as catalysts can be removed without adding water. The
amount of catatyticalty active compound mixtures is 0.01 10 wt.-%, with
reference to the reaction mixture. In spite of the advantageously high
reaction
speed which is achieved with alkaGlearth alkali catalysts, these systems
stagnate
after approximately 80% hydroxyalkyl imidazotidine-2-one conversion, so that
the
residual alcohol content in the reaction mixture is relatively high.
CA 02240196 2005-06-10
VY0 9T12?592 g I'CTIDE951a2i6t
Also, the formation of N-(methacryloy) oxyefhyl)-N'-(rnetha~ryloyij ethylene
urea, a
bifunctiona! methacryt compound, which therefore has a cn~ss-linking effect
during
polymerization reactions, is very high, at approximately 't0°r'o' of
the re-esterifi~ation
compounds, aced must be irrzproved-to be at tower proportions.
_DE-t)S 3x13927 BASF) describes a polymer-anafog reaction with approximately
't00,000 ppm calcium hydroxide a5 the catalyst:
DE 2238208 describes the re-esterifcaf~on of bactericide quinoxaline
derivatives with
eaiciurr~ hydroxide or barium hydroxide catalysis.
SumrTaary of the Invention
the invention relates to a process for the production of (meth)acty! esters of
the
formula I
R~s O Bt.
G= C _ C_p_a_N NH
!.
C
b
in which R, stands for hydrogen or a methyl group and A and B stand fir
unbranched
or branched alkylene groups with 2 to ~ C atari~s, by reac~ian of an acr~Eic
acid ester or
methacrytic acid ester of Formula i1
p
H2C= C _ C_O_Rz
1t,
CA 02240196 2006-O1-04
in vrihich Rj is as defined above and R~ stands for an alkyl iradicai v~h'1 to
d'C, atoms,
with ~a heterocyciic compound of .the Formula Ill . '
B ~, .
a
. HO - A - H NH.
. . ~ ~ . . rig.
. .
0
wherein A and B are, as defined above, which is characterized by the fact that
the.
reaction of an ester in accordance.with Formuia~ it vfn'th a h~cyclic
ct~mpownd~~of
~Formute.lil is carfied out to pn~duce~.an acryl or xnethacrtrl est~t~of
Formula I in the
presence of a catalyst system comprising calcium hydroxide.
The invention was based on the task of finding a catalytic process for the
pr4duc~fon of
acrylic or methacryiic acid esters of i=armula ~I by alcoholysis of
(n9eth)adcylic:at~d~ alkyl
esters with'h~dr~xyalkyi irr~idaznlidine-~-pneg, which proceeds at a good,
reac~i~n speead
even in tfxe region of final re-esterificatiori, end in which the catalyst
used can ~b~
removed from. the reaction mixture vu4thout adding irvsiter and ~ used again -
as such, 'if
necessary. It was now found thvt the reaction can be carried out ~in
surprisingly
advantageous manner with~cait~um hydroxidie, in an amount of lees than .250
p~pm, with
referewce ~to the' tofal amount of the feactiort mixture:
yA particu[ar advantage of the new process is. thbt high rates of cx~eigiQn
are:gchieved,
ahd that tile catalyst.system which contains c~lciuii~.. which ie s~utrii~d'up
quanttiveiy:
~ ~ ~ Y
to a. grt~af ant, irr the reaction rni~cture.~~ui ~be rer#wved
witt~oWt.adding.~Wrtwe
added 7.~, for exafnpie by frltration. Tcl,.~ is used as an aid for removing.
diss~oived
catalyst (saaaptier: S~rdchemie MG).
Cornpvunds of~~omnula I are valuable corr~onome(s and are used, for exari~ple,
~in the
production of polymer dispersions ,from vinyl monomers, which are prrmartly
used as
binders. in paints, for example, or as~leathor processing aids:, Cor~ionomer~s
of
* trade~narJc
CA 02240196 2005-06-10
Wf3 97122582 5 ~F'GTtDE9gf021.69
Formula t impart a desired hydrophilic to copolymerizates, and can function as
formaldehyde scavengers in heat-curable resins with their irrride group.
The success of the process according to the invention is surprising, since NH
grouping
of a compound of Formula Itl,vsras to be expected in the presence of the
catalyst,
because of the bifunctionalities of t and l11, during their reaction In
further reactions,
such as addition reactions analogous to a Nfichae! addition to the double
bond, or in
amide formation by reaction of the acryl or methacryt ester of IFormuta t_
The. readtion
of acryl and methacryl esters of Formula ti with the alcohots cif Forri~uta
!1l, according
to the invention, proceeds very se3ectivety to produce compounds of Formula t:
According to the process according to the invention, process products of
Forrrlula E ate
obtained, vsrhich can be used without costly and quatitattvety burdensome
remova)
prQCesses, directly, for example as a solution in the acryt or methacryl ester
ft; for use
as comonorr~ers, particularly in the production of dispersion polyrnerizates.
Compounds
1 can also be produced as solids according to the present press; for example
by being
evaporated from solution.
~ettteti ties~ription t~f the Preferred Embodiments of the Invention
For production of the compounds 1 in accordance with the process acxording to
the
- inventipn, acrylic or methacrytic acid esters of Forrrt~la it ire used, iri
~rvhidh ~;2
particularly stands for an alkyl radical with 'I to 4 carbon a#oms. .As
exampi~s, propyl
acrytater, n-butyl acrytate, ethyl methacrytate, i-pwopyl methacrylate; i-
btjtyl methacrytate,
n-butyl methaoryiete, and particularly methyl methacrylate should be
mentioned.
As starting substarzees of Formula t!!, such compounds in which ~4 or B
represent a
branched or untrranched aikyiene group with 2 to b carbon atoms, e.g: -
(CHZ)2~, -(C~)3
-(CH~,~., -CHZCH(CH~)CW2-, -CHZC(Chi3)?CHZ-, are possibte_
... ._ _._ . .CA 02240196 2005-06-10
WO 97122592 g PCTIDI:96I0296'1
The number of ring elements of the heterocycle is preferably 5 and 6. It is
particularly
advantageous to use 1-(2-hydroxyethyl)-imidazolidine-2-vne, which can be
easily
produced on a technical scale, for example, in accordance with U.S: patent
3,254,075,
from aminoethyt ethanoiamine and urea, as compound iit_
As calcium compounds which are added to the reaction system as catalysts or
catalyst-
forming precursors, the bivalent calcium compounds, such as calcium hydroxide,
should
be mentioned. It is practical to ease the calcium edmpovnds which #orm the
catalyst, i.e.
the catalyst system, in catalytic amounts; in general not more than 250 ppm
with
referer?ce to the sum of the reaction partners i1 and 111. A high selectivity
of product l
with R, = CH3; A and 8 = -(Gl-i2}~= is achieved. for example, with 250 ppm
Ga(f3,H~ with
reference to the.total amount of reaction mixture, in the re-esterification of
methyl
rnethacrylate with the corresp4nding compound Ill.
It is advantageous if the catalysts are used in fine dispersion, for example
in powder or
mierocrystalline form.
The reaction of acryl esters and/or methacryl esters of Formula II with the
alcohols of
Formula )1l (alcoholysis) can be carried out at temperatures between 30 and
180 degrees C,
particularly between 50 and 130 degrees C, preferably in the presence of not
more than 250
ppm of the calcium compound, calculated on the basis of the weight of the
reaction mixture.
~trding t~ the equation, equimoiar amounts of the reaction partners ii and (1i
react
to fog the desired end products 1. In practice, however, it ha> proven to be
practical
to always keep the starting esters II in excess during the reaction. 'They are
preferably used
in amounts of 1 to 20, preferably 2 to 10, particularly 3 to 6 males per mole
Nl.
'lo avoid polymerization losses, it is preferable to carry out the reaction
and processing of the
reaction mixture in the presence of polymerization inhibitors such as
phenothiazine;
hydroquinone monomethyl ether, and particularly oxygen.
CA 02240196 1998-08-10
WO 97122592 7 PCTIDE96102161
The reaction can take place under standard pressure, greater pressure, or in a
partial
vacuum. It can take place discontinuously or continuously. The starting
substances II
and III, for example, are heated to boiling together, in the presence of
calcium
compounds, and in this connection, the alcohol R20H which is split off is
continuously
distilled off with the ester II, possibly in the form of its azeotrope.
Depending on the
reaction temperature, the catalyst, and the catalyst amount, the reaction
times range
from approximately 2 to 10J~ours. It is also possible to carry out the
reaction in the
presence of an inert solvent, for example toluene or cyclohexane, but this is
normally
not necessary.
After completion of the reaction, excess monomer ester II can be removed
completely
or partially, by distilling it off. The dispersed catalyst is usually removed
by filtration, and
it is advantageous to do so before distilling off the monomer ester II, which
is mostly
present in excess. However, it can also be removed only after partial or
complete
removal of excess monomer ester II. The catalyst, which is recovered in the
filtered
form, can then be used in other alcoholysis batches, if necessary after being
dried.
A preferred reaction product is one that is formed from methyl methacrylate
and 1-(2-
hydroxyethyl)-imidazolidine-2-one (hydroxyethyl ethylene urea) and therefore
corresponds to Formula I with R, = CH3, A = -(CH2)2- and B = -(CH2)2 .
EXAMPLES
Example 1
1100 g (11 mol) methyl methacrylate, 286 g (2.2 mol) hydroxyethyl ethylene
urea, and
0.35 g hydroquinone monomethyl ether as well as 0.09 g phenothiazine as
inhibitors
are placed in a 2 liter round flask with mechanical stirring, air
introduction, sump
temperature display, and a filling element column
CA 02240196 1998-08-10
WO 97122592 $ PCTIDE96102161
(diameter: 35 mm, height 55 cm, 8 x 8 mm - Raschig rings) set on it, as well
as an
automatic column head with reflux and distillate cooler.
The mixture is heated to boiling and first a methyl methacrylate water
azeotrope is
distilled off via the column, until the head temperature reaches 99 °C.
The batch is
cooled by about 10 °C, 0.35 g calcium hydroxide and the mass of methyl
methacrylate
which is equivalent to the azeotrope distillate are added.
Again, the mixture is heated fo boiling, and the resulting methyl methacrylate
methanol
azeotrope is distilled off at a reflux ratio of 2:1, up to a maximum head
temperature of
70 °C, later at a reflux ratio of 10:1, until a constant head
temperature (99 °C) is
reached. The reaction is terminated after 6 h. The batch is cooled to 80
°C and
adjusted to a 25% solution of the product in methyl methacrylate by adding
methyl
methacrylate up to a total mass of 1742 g. 3.5 g Tonsil L80FF (Sudchemie) are
added,
and the batch is clarified by pressure filtration (Seitz pressure filter,
diameter - 14 cm,
filter layer T 1000 (Seitz) p < 0.4 bar). The filtrate has the following
composition,
according to gas chromatography analysis:
methyl methacrylate: 72.5%
hydroxyethyl ethylene urea: 1.4%
methacryloyl oxyethyl ethylene urea: 23.7%
N-(methacryloyl oxyethyl)-N'-(methacryloyl) ethylene urea: 1.2%
Example 2
Carried out as Example 1, but leaving out the water removal step. Reaction
time: 5.3 h.
CA 02240196 1998-08-10
WO 97122592 g PCT/DE96102161
The product is composed as follows, according to gas chromatography analysis:
methyl methacrylate: 71.8%
hydroxyethyl ethylene urea: 1.7%
methacryloyl oxyethyl ethylene urea: 24.0%
N-(methacryloyl oxyethyl)-N'-(methacryloyl) ethylene1.2%
urea:
Platinum cobalt color number: 22
Acid number: _ 0.05
Example 3
Carried out as Example 2, but using 0.55 g calcium hydroxide. Reaction time:
5.5 h.
The product has the following composition, according to gas chromatography
analysis:
methyl methacrylate: 70.5%
hydroxyethyl ethylene urea: 1.0%
methacryloyl oxyethyl ethylene urea: 24.4%
N-(methacryloyl oxyethyl)-N'-(methacryloyl) ethylene urea: 2.0%
Example 4
Carried out as in Example 2, but using 0.28 g (200 ppm relative to the total
amount
weighed in) calcium hydroxide. Reaction time: 6.0 h.
The product has the following composition, according to gas chromatography
analysis:
methyl methacrylate: 71.3%
hydroxyethyl ethylene urea: 1.6%
methacryloyl oxyethyl ethylene urea: 25.1
N-(methacryloyl oxyethyl)-N'-(methacryloyl) ethylene urea: 0.7%