Note: Descriptions are shown in the official language in which they were submitted.
CA 02241034 1998-08-12
TITLE
MULTICAPSULE INTRALUMINAL GRAFTING SYSTEM AND METHOD
BACKGROUND OF THE INVENTION
This is a divisional application to Canadian Patent application
2,125,258 filed June 6, 1994 and allowed February 18, 1998.
This invention relates to a system for emplacing a prosthesis and,
more particularly, to a catheter system for placement of a graft having
attachment
means within a corporeal lumen.
It is well established that various fluid conducting body or corporeal
lumens, such as veins and arteries, may deteriorate or suffer trauma so that
repair
is necessary. For example, various types of aneurysms or other deteriorative
diseases may affect the ability of the lumen to conduct fluids and in turn may
be
life threatening. In some cases, the damaged lumen is repairable only with the
use of prosthesis such as an artificial vessel or graft.
For repair of vital vessels such as the aorta, surgical repair is
significantly life threatening. Surgical techniques
-1-
62948-191 D
CA 02241034 1998-08-12
Docket No. ENDOV-34033
-2-
known in the art -involve major surgery in which a graft
resembling the natural vessel is spliced into the diseased or
obstructed section of the natural vessel. Known procedures
include surgically removing or bypassing the damaged or
diseased portion of the vessel and inserting an artificial or
donor graft inserted and stitched to the ends of the vessel
which were created by the removal of the diseased portion.
It is known within the art to provide a prothesis for
intraluminal repair of a vessel, such as an abdominal aorta
to having an aneurysm. The art has taught to provide a prothesis
positioned in a vessel then securing the prothesis within the
vessel with hooks or staples that are mechanically extended
by the user. The early prior art devices were large in
diameter, mechanically complex and in turn were susceptible
to mechanical failure. Prior intraluminal grafting systems
have embodied capsule catheters or balloon catheters, but were
relatively stiff and of a relatively high profile. Similarly,
the prior art systems were configured in such a way that the
graft was relatively difficult to deploy the prothesis. In
addition, prior systems having capsule catheter means were
usually configured such that the prothesis was disposed within
a unitary capsule.
In recent years, several devices have been developed
to attempt to treat an aortic aneurysm through intraluminal
repair. For example, U. S. Pat. No. 4, 140, 126 (Feb. 20, 1979) ,
Choudhury, discloses a method and article for performing an
aneurysm repair, wherein a prosthetic graft is utilized to
replace the damaged segment of the blood vessel. A plurality
CA 02241034 1998-08-12
Docket No. ENDOV-34033
-3-
of radially spaced anchoring pins are located adjacent each
end of the graft and provide means for securing the graft to
the wall of the vessel. Means is provided for moving the graft
within the vessel and permanently anchoring the graft to the
wall of the vessel.
U.S. Pat. No. 4,562,596 (Jan. 7, 1986), Kornberg,
discloses a bifurcated aortic graft constructed for
intraluminal insertion having a plurality of struts having
angled hooks with barbs at,their superior ends. Means for
inserting the graft and implanting the hooks into the vessel
lumen is also disclosed.
U.S. Pat. No. 4,787,899 (Nov. 29, 1988), Lazarus,
discloses an intraluminal grafting system including a hollow
graft having an attachment means located at one end of the
graft. The system includes positioning means for moving the
graft within the vessel, the positioning means having a capsule
positioned at one end for covering the graft attachment means.
The disclosed positioning means further includes an inflatable
member for securing the attachment means within the lumen.
EPO Pub. No. 0 461 791 A1 (Dec. 18, 1991), Barone et
al. discloses an aortic graft and apparatus for repairing an
aneurism. The disclosed system includes a tube graft secured
within the aorta and an attachment means at each-'end of the
graft. Intraluminal delivery is accomplished using a catheter
having a balloon for expanding and securing the attachment
means. The graft and attachment means are preferably enclosed
by a sheath which covers the entire graft and attachment means.
CA 02241034 1998-08-12
i
-4-
EPO Pub. No. 0 466 518 R3 (Jan. 15, 1992), Lazarus
et al., discloses an int raluminal grafting system including a
catheter having a capsule formed of a helical wrap of metal
ribbon. A tubular graft having attachment means at both ends
is removably disposed within the capsule. Means is provided
for moving the graft from the capsule, and an inflatable
member is provided for securing the attachment means within a
vessel lumen.
U.S. Patent No. 5.,104,399 (Apr. 14, 1992), Lazarus,
discloses an int raluminal grafting system including a tubular
graft having attachment means positioned at both ends. The
system includes a positioning means for transporting the graft
through a vessel lumen and for deploying the graft within the
lumen. The positioning means includes an inflatable member, a
capsule and means for removing the graft from the capsule.
The capsule is disclosed as a rigid cylindrical member
covering the entire graft.
EPO Pub. No. 0 508 473 A2 (Oct. 14, 1992), Piplani
et al., discloses an int raluminal grafting system including a
catheter having a capsule formed of a helical wrap of metal
ribbon. A bifurcated graft having attachment means is
removably disposed within the capsule. Means is provided for
moving the graft from the capsule, and an inflatable member is
provided for securing the attachment means within a vessel
lumen.
62948-191
CA 02241034 2000-02-07
62848-191D
-5-
To provide consistency with the common usage of terms
used in the medical surgical arts in the United States, the
terms "proximal, distal, inferior and superior" are used with a
certain regularity within the present specification. Proximal
refers to parts of the system, such as catheters, capsules and
wires, which are closest to the user and closest to the portion
of the system outside or exterior of the patient. Distal
refers to the point farthest from the user and typically most
interior to the corporeal lumen. The term superior refers to a
location situated above and is used herein in description of
the graft and attachment means. Inferior refers to the point
situated below and again is used herein with the graft and
attachment means. Thus, for applications in the abdominal
aorta which use a femoral approach, the superior end of the
graft resides within the most distal portion of the balloon
catheter. Likewise, the inferior end of the graft resides
within the proximal capsule which is on the most distal portion
of the capsule catheter.
SUMMARY OF THE INVENTION
In general, it is an object of the present invention
to provide an intraluminal grafting system and method which
overcome the disadvantages of the prior art systems.
In brief, this invention seeks to provide a balloon
catheter comprising: an elongate tubular member having a
proximal end and a distal end; a first lumen extending from the
proximal end to the distal end of said elongate tubular member,
said first lumen configured to receive a guidewire; a second
lumen extending from the proximal end to proximate the distal
end of said elongate tubular member; an inflatable member in
fluid communication with said second lumen and positioned
proximate the distal end of said elongate tubular member; and a
capsule assembly for removably retaining a prosthesis, said
CA 02241034 2000-02-07
62848-191D
-5a-
capsule assembly positioned proximate the distal end of said
elongate tubular member.
The present system has several advantages over prior
art systems. For example, the over the wire configuration of
the balloon catheter enables traversing the aneurysm with a
guidewire. Using a guidewire in this manner minimizes the risk
of dislodging thrombus in the aneurysm, since the placement
CA 02241034 1998-08-12 .--.
1
- 6 -
means follows the guidewire thereby preventing the distal tip
from contacting the vessel wall. In addition, using a
guidewire allows for traversing more difficult anatomy. Also,
the guide wire lumen may function as a through lumen for real
time angiograms during the emplacement procedure or to insert
intravascular probes such as intravascular ultrasound systems.
As another advantage, the smaller diameter and lower
profile of the capsule assemblies of the present invention
permit use of the invention in a larger patient population
because the variances in iliac vessel diameter. Slmilarly,
the smaller device diameter relative to the iliac diameter may
allow for easier navigation inside the corporeal lumen
especially with more difficult anatomy. Likewise, the two
capsule segments of the present invention permit a wider range
of graft lengths than available with a single capsule design.
The single capsule systems also require capsules slightly
longer than the graft, which imposes certain manufacturing and
deployment problems. Moreover, the shorter capsule segments
provide a more flexible device, thereby allowing traversing
more difficult anatomy.
The present invention comprises an intraluminal
placement means for securing a prosthesis within or between
vessels or corporeal lumens of an animal, such as a human.
The preferred embodiment of the placement means is configured
for introducing a graft into a corporeal lumen and positioning
62948-191
CA 02241034 1998-08-12
-. .
- 6a -
the graft in the area of the aort is bifurcat ion. The
placement means includes a balloon catheter and a capsule
catheter. In the preferred embodiment, the balloon catheter
and
62948-191
CA 02241034 1998-08-12
Docket No. ENDOV-34033
capsule catheter include capsule means for retaining the graft,
including a proximal capsule means and a distal capsule means.
The capsule means are movable relative to each other to allow
the graft to be emplaced at the desired location in the
corporeal lumen.
Preferably, the placement means includes a balloon
catheter having a multilumen hollow tube or shaft having a
proximal end provided with means for accepting a guide wire
and with means for inflating a balloon or similar inflatable
member. The balloon catheter shaft is of sufficient length
that the proximal end remains exterior the corporeal lumen
while the distal end of the balloon catheter shaft may be
positioned proximate the portion of the corporeal lumen to be
repaired. The balloon catheter further has means for inflating
and deflating the balloon. In addition, the balloon catheter
is coupled to control means and a distal capsule for retaining
and releasing the superior end of the graft. In the preferred
embodiment, the control means includes a control wire and
handle mechanism which provides movement of the distal capsule
relative to the balloon catheter shaft.
The placement means also includes a capsule catheter
shaped and sized for positioning within the corporeal lumen.
The capsule catheter comprises a hollow tube or shaft slidably
mounted on the balloon catheter shaft, having a proximal end
exterior the corporeal lumen for manipulation by the user.
The capsule catheter includes a proximal capsule secured to
the distal end of the capsule catheter shaft for retaining the
inferior end of the graft. The placement means is configured
CA 02241034 1998-08-12
f.
Docket No. ENDOV-34033
_g_
to provide relative movement between the proximal capsule of
the capsule catheter and the distal capsule of the balloon
catheter for removing the graft from the capsule means and for
subsequently urging the attachment means into engagement with
the wall of the corporeal lumen.
The placement means further includes a capsule jacket
for providing a smooth transition between the parts of the
balloon catheter and capsule catheter. The capsule jacket
comprises a double walled jacket or sheath configured coaxially
with the balloon catheter and capsule catheter, having a
proximal end exterior the corporeal lumen for manipulation by
the user. The distal end of the capsule jacket flares
outwardly to a size which is slidably retained over the distal
capsule when the placement means in deployed into the corporeal
lumen.
The present invention includes a prosthesis or graft
for intraluminal placement in a fluid conducting corporeal
lumen. For most applications the prothesis is a hollow graft
of preselected cross-section and length. The. graft is
deformable to conform substantially to the interior surface
of the corporeal lumen or other body part to be repaired.
Preferably, the graft is made of a material suitable for
permanent placement in the body such as polytetrafluroethylene
or a polyester. During emplacement, the superior and inferior
ends of the graft are positioned within the corporeal lumen
and the graft is configured such that the graft traverses the
diseased or damaged portion of the vessel. To anchor the graft
_ CA 02241034 1998-08-12
Docket No. ENDOV-34033
_g_
to the wall of the corporeal lumen, attachment means are
secured to the superior and inferior ends of the graft.
The preferred attachment means.has wall engaging members.
The wall engaging members of the superior attachment means are
angled toward the inferior end of the graft. Similarly, the
wall engaging members of the inferior attachment means are
angled slightly toward the superior end of the graft. The wall
engaging members of both attachment means have sharp tips for
engaging the corporeal lumen wall. The preferred attachment
means are formed into a V-shaped lattice or framework. The
frame of the attachment means allows for elastic radial
deformation resulting in a spring-like effect when a compressed
attachment means is allowed to expand as the graft is released
from the capsule means. In addition, radiopaque markers are
secured to the longitudinal axis of the graft to facilitate
orientation of the graft using f luoroscopy or x-ray techniques .
Deployment of the graft comprises a series of steps which
begins with introducing the placement means into the corporeal
lumen using well known surgical techniques. As a single
system, the balloon catheter, capsule catheter and capsule
jacket are manipulated to position the capsule means containing
the graft and attachment means to a desired location within
the corporeal lumen. Once the graft is in the desired
location, the capsule jacket is withdrawn to expose the distal
capsule and a portion of the graft. The distal capsule is then
moved relative to the balloon catheter shaft and capsule
catheter to expose the superior attachment means.
CA 02241034 1998-08-12
Docket No. ENDOV-34033
-10-
After the superior portion of the graft is removed from
the capsule means, the inflatable member is moved to within
the circumference of the superior attachment means and inflated
to urge wall engaging members into the wall of the corporeal
lumen. The capsule jacket and capsule catheter are then moved
relative to the graft and balloon catheter shaft to expose the
inferior attachment means. The balloon catheter shaft is then
moved to position the inflatable member proximate the inferior
attachment means. The inflatable member is then expanded to
seat the wall engaging members of the inferior attachment
means. The placement means is then removed from the corporeal
lumen.
Other features and advantages of the present invention
will become apparent from the following detailed description,
taken in conjunction with the accompanying drawings, which
illustrate, by way of example, the principles of the invention.
_.
CA 02241034 1998-08-12 ,
Docket No. ENDOV-34033
-11-
BRIEF DESCRIPTION OF THE DRAWINGS
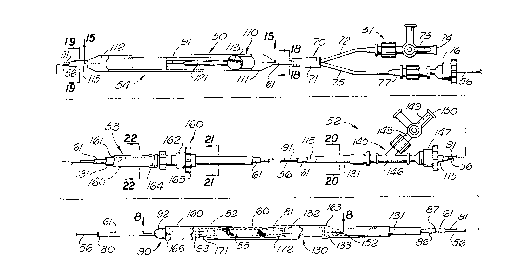
FIGURE 1 is a top plan view of an intraluminal grafting
apparatus and system incorporating the present invention.
FIG. 2 is a top plan view of a guidewire to be used with
the endovascular grafting system of the present invention.
FIG. 3 is a top plan view of the balloon catheter of
the present invention.
FIG. 4 is a top plan view of the distal cap, control
wire, guiding tube and control wire handle assembly of the
l0 present invention.
FIG. 5 is a top plan view of the proximal capsule and
capsule catheter assembly of the present invention.
FIG. 6 is a top plan view of the capsule jacket assembly
of the present invention.
FIG. 7 is a top plan view of a graft for use with the
system incorporating the present invention.
FIG. 8 is a partial cross-sectional view of the distal
end of the balloon catheter, capsule catheter and capsule
jacket assemblies taken along the line 8-8 of FIG.1.
FIG. 9 is an enlarged perspective view showing an
embodiment of the distal capsule, distal end of the control
wire and distal cap insert.
FIG. 10 is an enlarged cross-sectional view of one
embodiment of the distal capsule means of the balloon catheter.
FIG. 11 is a cross-sectional view taken along the line
11-11 of FIG. 10.
CA 02241034 1998-08-12
Docket No. ENDOV-34033
-12-
FIG. 12 is an enlarged perspective view showing an
embodiment of distal capsule, distal end of the control
the
wire and dista l
cap
insert.
FIG. 13 is an enlarged cross-sectional view of an
embodiment of he
t distal
capsule
means
of
the
balloon
catheter.
FIG. 14 is
a
cross-sectional
view
taken
along
the
line
14-14 of FIG. 13.
FIG. 15 is a partial cross-sectional view of the control
wire and control
handle mechanism
taken along
the line 15-15
of FIG. 1.
FIG. 16 is a cross-sectional view taken along the line
16-16 of FIG. 15.
FIG. 17 is a cross-sectional view taken along the line
17-17 of FIG. 15.
FIG. 18 is a cross-sectional view taken along the line
18-18 of FIG. 1.
FIG. 19 is a cross-sectional view taken along the line
19-19 of FIG. 1.
FIG. 20 is a cross-sectional view taken along the line
20-20 of FIG. 1.
FIG. 21 1s a cross-sectional view taken along the line
21-21 of FIG. 1.
FIG. 22 is a cross-sectional view taken along the line
22-22 of FIG. 1.
FIG. 23 is a partial cross-sectional view of the graft
and attachment
means of the
present invention.
FIG. 24 is a cross-sectional view taken along the line
24-24 of FIG. 23.
CA 02241034 1998-08-12
Docket No. ENDOV-34033
-13-
FIG. 25 is an enlarged perspective view showing a
superior attachment means.
FIG. 26 is an enlarged perspective view showing an
inferior attachment means.
FIG. 27 is a partial cross-sectional view of the intra-
luminal grafting system shown positioned within the corporeal
lumen.
FIG. 28 is a partial cross-sectional view of the
intraluminal grafting system, wherein the distal capsule has
been removed from the superior end of the graft.
FIG. 29 is a partial cross-section view of the
intraluminal grafting system, wherein the inflatable member
has been expanded to seat the attachment means of the graft.
FIG. 30 is a partial cross-sectional view of the
intraluminal grafting system, wherein the capsule jacket and
capsule catheter have been withdrawn from the inferior end of
the graft.
FIG. 31 is a partial cross-sectional view of the
intraluminal .grafting system, wherein the inflatable member
has been positioned and inflated to seat the inferior
attachment means.
FIG. 32 is a partial cross-sectional view of the
intraluminal grafting system, wherein the inflatable member
has been moved proximate the proximal capsule.
FIG. 33 is a partial cross-sectional view of the
intraluminal grafting system, wherein the balloon catheter,
capsule catheter and capsule jacket have been placed in a
position for withdrawal from the corporeal lumen.
CA 02241034 1998-08-12
Docket No. ENDOV-34033
-14
DESCRIPTION OF THE PREFERRED EMBODIMENTS
As shown in the drawings and for purposes of
illustration, the invention is embodied in an intraluminal
grafting system of the type having a balloon catheter, a
capsule catheter, and a protective sleeve or capsule jacket.
One of the novel features of the present system is the use
of a proximal capsule and a distal capsule to cover the
inferior and superior ends of a graft to be implanted in the
vessel. This feature provides.the capability of deploying the
interior end of the graft before the superior end or visa
versa. Another novel feature of the present invention is the
use of a sleeve or capsule jacket to create a smooth transition
between the proximal capsule and the distal capsule. The
uniqueness of the system is accentuated by the control wire
and associated handle which provide relative movement between
the distal capsule and the balloon catheter.
In the present system, the graft is comprised of a
monoluminal tubular member having superior and inferior
extremities. Attachment means are secured to the superior and
inferior ends of the tubular member. The attachment means are
provided with lumen piercing means which are covered by the
proximal and distal capsule assemblies. The balloon catheter,
capsule catheter and capsule jacket are configured coaxially
so that relative movement between them provides for deployment
of the graft. The inflatable member of the balloon catheter
is used to firmly implant the graft in the lumen.
In more detail, the intraluminal grafting system 50 is
shown in FIGS. 1-7. As shown in FIG. 1, the system includes
CA 02241034 1998-08-12
Docket No. ENDOV-34033
-15-
a balloon catheter assembly 51, which is coaxially disposed
within capsule catheter assembly 52, which is coaxially
disposed within capsule jacket assembly 53. The capsule
catheter and a distal capsule catheter assembly 90 are used
to contain the graft 55. A control wire assembly 54 is
coaxially disposed within a lumen of the balloon catheter
assembly and configured to move the distal capsule assembly
in relation to the other system components. In the preferred
embodiment, the system is used as an over-the-wire device, such
that the balloon catheter is further configured with a lumen
for a guide wire 56. It is contemplated, however, that the
system can also be used with well known fixed wire delivery
system configurations.
As shown in FIGS. 1 and 3, the intraluminal grafting
system 50 also includes a balloon catheter assembly 51 which
consists of an inflatable member or balloon 60 secured to a
flexible elongate element or balloon catheter shaft 61. As
shown in FIG. 18, the balloon catheter shaft is preferably
configured with three lumens; however, the balloon catheter
may be configured with a single, dual or similar multilumen
shaft. A guide wire lumen 63 extends the length of the balloon
catheter shaft. Similarly, a balloon inflation lumen 64
extends from the proximal end 70 of the balloon~catheter to
the inflatable member 60, wherein an inflation port 83, FIG.
8, is provided to allow inflation fluid to enter and exit the
inflatable member. The third lumen 65 is provided for the
control wire assembly 54.
CA 02241034 1998-08-12
Docket No. ENDOV-34033
-16-
The flexible elongate element or balloon catheter shaft
61 is preferably formed of a material suitable for intraluminal
use, such as irradiated polyethylene tubing. The three lumen
balloon catheter shaft is preferably extruded to an outside
diameter of 0.08 inches (2.03 mm). The guide wire lumen 63
has an inner diameter of 0 . 042 inches ( 1. 07 mm) . The inflation
lumen 64 and the control wire lumen 65 have identical inner
diameters of 0.022 inches (0.56 mm) . However, the lumen inside
diameter may range from 0.015 to 0.06 inches (0.381-1.52 mm)
and the outside diameter may range from 0.035 to 0.1 inches
(0.889-2.54 mm) for a multilumen balloon catheter shaft. The
balloon catheter may vary in length to suit the application,
for example, from fifty to one hundred-fifty centimeters.
Referring to FIG. 1, the proximal extremity 70 of the
balloon catheter shaft 61 is secured to a splitting adapter
71 which splits the guide wire lumen 63 from inflation lumen
64. The side arm 72 of the adapter 71 has a stop cock 73
mounted at its proximal end which is movable between open and
closed positions. The stop cock is provided with a Luer
fitting 74 which is adapted to be secured to a syringe for
injecting inflation fluid. The side arm 75 of the adapter 71
is connected to female Luer fitting 77 for distal tip injection
and to a Touhy Borst adapter 76 which is configured to
removably and slidably receive the guide wire 56.
The inflatable member or balloon 60 is preferably secured
twelve centimeters from the distal extremity 80 of the balloon
catheter shaft 61. The balloon is positioned proximal of the
distal capsule assembly 90 and the superior end of the graft
- CA 02241034 1998-08-12
Docket No. ENDOV-34033
_17_
55. For shorter grafts of four to seven centimeters in length,
the inflatable member may be positioned distal of the distal
capsule assembly. The balloon is formed of suitable material
such as polyethylene. The balloon can vary in diameter from
twelve to forty-five millimeters in diameter and can have a
wall thickness ranging from 0.001 to 0.005 inches (0.0254-0. 127
mm). The polyethylene utilized for the balloon is irradiated
to achieve an appropriate balloon size. The preferred balloon
made in accordance with the present invention has an outside
diameter of twenty-four millimeters, a diameter equal to the
inner diameter of the graft, and had a wall thickness of
approximately 0.003 inches (0.076 mm). In addition, the_
balloon is pleated along its axis for a low profile which
facilitates its introduction. into a corporeal lumen of a
patient as hereinafter described. Further, the deflated
balloon is heated to provide it with a memory of its low
profile configuration.
Balloon catheter shaft 61 is provided with an inflation
lumen 64 which is in fluid communication with the inflation
port 74. The inflation lumen is used to inflate and deflate
the balloon 60 by introducing and withdrawing a gas or liquid
through the inflation port. The balloon is secured
approximately twelve centimeters from the distal extremity 80
of the balloon catheter shaft. The balloon proximal stem 81
and balloon distal stem 82 are heat sealed to the balloon
catheter shaft to form a fluid tight seal. The length of the
proximal stem may vary from 0.5 to 5.0 centimeters so that the
CA 02241034 1998-08-12
Docket No. ENDOV-34033
-18-
balloon will span the distance between the inferior and
superior attachment means of the graft 55.
The balloon catheter shaft 61 has an inflation port 83
located approximately ten millimeters distal the balloon
proximal stem 81. In addition, a radiopaque marker 84 is
embedded in the balloon catheter shaft approximately two
millimeters distal the balloon inflation port. Preferably,
the radiopaque marker is a platinut~ or tungsten coil one
centimeter long with an outer. diameter of 0.02 inches (0.508
mm) and is located proximate the center of the balloon 60.
It should be appreciated that although a separate inflatable
member has been described in the drawing, an integral coaxial
inflatable member may be provided which is formed of the same
tubing from which the balloon catheter shaft is made. This
can be readily accomplished, as is well known to those skilled
in the art, by using an additional radiation dose for the
balloon region of the shaft.
As shown in FIGS . 2 and 8 , a pusher button 8 5 is s 1 idably
mounted on the balloon catheter shaft 61 and ,is located
proximal the balloon proximal stem 81, for example, a distance
of five to eight centimeters. The pusher button is retained
from moving proximal on the shaft by an annular bulb 86 which
is formed by localized heating of the shaft. A radiological
marker band 87 is placed on the shaft just proximal the annular
bulb. The pusher button is preferably cylindrical having a
preferred outside diameter of 0.22 inches (5.588 mm) , ranging
from 0.15 to 0.25 inches (3.81-6.35 mm). The pusher button
CA 02241034 1998-08-12
Docket No. ENDOV-34033
-19-
is formed of a suitable material such as 300 series stainless
steel to achieve radiopacity.
The balloon itself can also be observed under x-rays
because the blood in the patient's vessel is more opaque than
the gas used for inflating the balloon. In addition, increased
visibility of the balloon can be obtained by inflating the
balloon with a diluted radiopaque contrast solution. In
addition, radiopaque bands of a suitable material such as
platinum or a platinum-tungsten alloy can be placed on the
proximal and distal balloon stems 81 and 82 to aid in
ascertaining the position of the .balloon. Similarly,
radiopaque rods may be inserted in the distal balloon shaft
tubing, in the control wire or balloon inflation lumens.
The intraluminal grafting apparatus also includes a
control wire assembly 54, which is shown in FIGS. 1 and 4.
The distal end of the control wire assembly consists of a
distal capsule assembly 90. As shown in more detail in FIGS.
9-14, the distal capsule assembly comprises a control wire 91
secured within a distal cap 92. A hollow distal capsule 93
is secured to the distal cap and coaxially surrounds the
control wire.
The control wire 91 is threaded through aperture 94 in
the balloon catheter shaft 61 and is slidably disposed in the
balloon catheter shaft lumen 65. Distal end 95 of the control
wire is embedded in distal cap insert 96 which is secured to
the distal cap 92 by means of an adhesive, solvent bonding,
ultrasonic welding or by heat shrinking. As shown in FIG. 9,
the distal cap insert is formed with radiopaque markers 97 and
CA 02241034 1998-08-12
Docket No. ENDOV-39033
-20-
98 . Preferably, a 0 . 04 inch ( 1. O1 mm) wide, 0 . 005 inch ( 0 . 127
mm) thick and 2 millimeters long ribbon is disposed in slots
formed in the circumference of the insert at a 90 degree angle
from the control wire.
As shown in FIG. 10, balloon catheter proximal cap 100
is secured to the balloon catheter shaft 61 at a position
distal the balloon distal stem 82 and proximal the aperture
95. The proximal cap is secured to the balloon catheter shaft
by adhesive and by means of a- retaining bump 101. Retaining
sleeve 102 slidably secures the control wire 91 against the
balloon catheter shaft. Thus, as the control wire is moved
in a longitudinal manner, the distal end of the control wire
95, distal cap insert 96, the distal cap 92 and distal capsule
93 each move as a single assembly. The proximal edges 103 of
the distal capsule are rolled, curved or crimped inward so that
the proximal cap will lock into the distal capsule when the
distal capsule is advanced.
Referring to FIG. 11, the distal cap 92 may be formed
from polycarbonate or other suitable material for insertion
through the body lumen. The distal cap is formed with a bore
104 of approximately the same diameter as the outer diameter
of balloon catheter shaft 61. Similarly, the distal cap insert
96 may be formed of the same material as the distal cap,
wherein the distal cap insert is provided with a bore 105 for
receiving the balloon catheter shaft. The distal cap is
further provided with a recess 106 or other means for receiving
the distal end of the distal capsule 93. The distal capsule
is preferably formed of stainless steel, but may be formed of
CA 02241034 1998-08-12
Docket No. ENDOV-34033
-21-
other suitable biocompatable material, such as a nickel
titanium. The distal cap recess is angled to allow crimping
of the distal capsule to the distal cap.
The outside diameter of the distal cap 92 and capsule
93 may range from 4 to 9 millimeters and is preferably 0.282
inches (7.16 mm) in outer diameter and 0.276 inches (7.01 mm)
inner diameter. Similarly, the balloon catheter proximal cap
100 outer diameter is comprised of stainless steel and has an
outside diameter slightly less that of the distal capsule so
as to provide a smooth transition. The proximal end of the
proximal cap is preferably rounded to minimize trauma in the
vessel and to facilitate balloon retraction into the graft 55
during the emplacement process. The distal capsule may range
in length from one to five centimeters, and preferably is three
centimeters long so as to adequately retain the distal end of
the graft 55.
FIGS. 12-14 show an alternative and embodiment of the
distal cap assembly 90. In this embodiment, the core wire 91
is threaded through an opening 107 in the distal cap insert
108. A longitudinal slot 109 is cut out in the balloon
catheter shaft 61 to expose the core wire lumen 65. The
control wire is formed in a U-shaped bend over the opening in
the distal cap insert and is configured to slide=within the
slot and the core wire lumen of the balloon catheter shaft.
The distal end of the control wire resides in the portion of
the core wire lumen beyond the end of the slot. This
configuration allows the distal cap assembly to move axially
along the balloon catheter shaft. The U-shaped bend of the
CA 02241034 1998-08-12
Docket No. ENDOV-34033
-22-
control wire through the distal cap insert, however, prevents
the distal cap assembly from rotating in relation to the
balloon catheter shaft. As described above, the balloon
catheter insert is firmly secured within the distal cap. To
prevent rotation of the distal cap, a three centimeter length
of the control wire extends distal of the distal cap and is
slidably disposed in the balloon catheter shaft lumen 65.
As shown in FIGS. 15-17, a handle assembly 110 is secured
to the proximal end of the control wire 91. The handle
assembly comprises a proximal body 111, a distal body 112, a
control knob 113 with threaded shaft 114 and a guiding member
115. The two handle body parts have a central bore for
receiving the balloon catheter shaft 61. The guiding member
is coaxially disposed over the balloon catheter shaft and
extends distally from the central bore in the distal handle
body. The proximal end of the guiding member is secured to
the balloon catheter shaft approximately one centimeter
proximal from the distal end of the distal handle body by means
of a polyethylene sealing tube 116 which is heat shrunk over
the proximal end of the guiding member. An adhesive may be
used to fix the distal handle body to the guiding member.
Guiding member 115 consists of a rigid thin wall tube
formed of a suitable material such as stainless steel, e.a.,
a catheter hypotube. The guiding member has a length of about
45 centimeters and has an outside diameter of 0.095 inches
(2.41 mm) and an inside diameter of 0.087 inches (2.21 mm).
The control wire 91 preferably consists of an elongate solid
flexible stainless steel wire having a FEP or lubricating
CA 02241034 1998-08-12
Docket No. ENDOV-34033
-23-
coating. The coated control wire is about 0.02 inches (0.508
mm) in diameter so as to provide sufficient strength to move
the distal. capsule assembly 90 without breaking or kinking.
Referring to FIG. 15, the control wire resides in the balloon
catheter lumen 65 and extends from the distal capsule assembly
to an aperture 117 located in the lumen just proximal of the
proximal end of the guiding member.
The proximal end of the control wire 91 is disposed
within a longitudinal central bore in a retaining tube 120
l0 approximately six centimeters long. The retaining tube's
proximal end is slidably disposed within a longitudinal guiding
slot 121 in proximal handle body 111. Similarly, the retaining
tube's distal end is slidably disposed within an longitudinal
upper slot 122 in the distal handle body 112. A transverse
threaded bore 123 is located approximately 0.25 inches (6.35
mm~ from the proximal end of the retaining tube for receiving
the threaded shaft 114. The threaded shaft is turned down in
the threaded bore to engage the control wire to secure the
control wire firmly in the retaining tube.
The threaded shaft 114 extends upwardly from the
retaining tube 120 through the proximal handle body 111 and
is movable within the guiding slot 121. The control knob 113
is threaded onto the threaded shaft, wherein the control knob
can be turned down and tightened onto the proximal handle body,
thereby locking the retaining tube and control wire 91 in a
fixed position relative to the balloon catheter. When the
control knob is loosened, any longitudinal movement of the
' CA 02241034 1998-08-12
_.
i .
Docket No. ENDOV-34033
-24-
control knob causes corresponding movement of the distal
capsule assembly 90.
As shown in FIGS . 1 and 5 , the capsule catheter assembly
52 consists of a proximal capsule catheter assembly 130 secured
to the distal end of a flexible elongate tubular member 131
formed of a suitable plastic material such as polyether block
amide available under the trademark "PEBAX", available from
Atochem Polymers, Glen Rock, N.J. The capsule catheter
elongate tubular member is,of a suitable length as, for
l0 example, 40 to 100 centimeters and preferably approximately
60 centimeters for the abdominal. aortic artery and
approximately 80 centimeters for the thoracic aortic artery.
The elongate tubular member has a preferred outside diameter
of 0.187 inches (4.75.mm) and an inside diameter of 0.125
inches (3.175 mm) . The elongate tubular member can be produced
in a certain color such as blue. To render the elongate
tubular member radiopaque under x-rays, its material of
construction may contain a radiopaque material, such as twenty
percent by weight of bismuth subcarbonate or barium sulfate.
The elongate tubular member may have markings or bands distal
of the wye adapter 145 at predetermined positions to indicate
capsule jacket retraction and locking points.
The proximal catheter assembly 130 has a proximal capsule
132 mounted on the distal extremity of the capsule catheter
elongate tubular member 131. The proximal capsule has a
preferred diameter ranging from four to nine millimeters, which
may be configured to accommodate different size grafts. The
proximal capsule is configured to match the size of the distal
CA 02241034 1998-08-12
Docket No. ENDOV-34033
-25-
capsule assembly 90. Thus, the proximal capsule is preferably
made of stainless steel or similar impermeable and rigid, or
semi-flexible material. The elongate tubular member also
serves as a shaft for advancing the proximal capsule, as
hereinafter described. Thus, the elongate tubular member
should have a diameter which is less than that of the proximal
capsule, preferably having an outside diameter ranging from
three to seven millimeters.
Referring to FIG. 8, the proximal capsule 132 is secured
to the distal extremity of the elongate tubular member 131 by
means of a capsule adapter assembly 133. The capsule adapter
assembly comprises a housing 134 and an inner sleeve 135, which
may be constructed from polycarbonate. The capsule adapter
housing distal extremity 136 is secured in the proximal
extremity of the capsule, for example, by crimping, by using
a press fit swaging or an adhesive such as a cyanoacrylate
ester. The capsule adapter housing distal extremity may be
angled to facilitate securing the housing to the proximal
capsule.
The proximal extremity of the capsule adapter housing
134 is secured to the distal extremity of the elongate tubular
member 131 by means of an cyanoacrylate ester adhesive, or
other suitable means. To facilitate a mechanical lock, the
elongate tubular member distal extremity is molded to form a
flange 137, wherein the capsule adapter housing is configured
so as to close around the flange. The capsule adapter housing
is further provided with a recess for receiving the capsule
adapter inner sleeve 135. The inner sleeve is provided with
CA 02241034 1998-08-12
Docket No. ENDOV-34033
-26-
r
a bore of a suitable diameter so as to allow the balloon
catheter shaft 61 to reside therein. The inner sleeve may
further be provided with radiopaque marker rods or f lat ribbon
138 for detection of the capsule adapter assembly 130 during
fluoroscopy.
A wye adapter 145 is secured to the proximal extremity
of the flexible tubular member 131 of the capsule catheter.
The central arm 146 of the wye adapter is connected to a Touhy
Borst adapter 147 which tightens around the guiding member 115
disposed in the central arm of the wye adapter. The side arm
148 of the wye adapter has a stop cock 149 mounted therein
which is movable between open and closed positions. The stop
cock is provided with a Luer fitting 150 which is configured
to accept a syringe for injecting a dye. Air may be purged
from the capsule jacket assembly 53 by injecting fluid through
the Luer fitting. The injection fluid will exit purge ports
151 and 152, thereby filling the capsule jacket assembly with
injection fluid. The Luer fitting may be attached to a saline
drip line during the operative procedure.
Referring to FIGS. 1, 6 and 8, the capsule jacket
assembly 53 is slidably disposed coaxially over the capsule
catheter assembly 52 and the balloon catheter assembly 51.
The capsule jacket assembly is comprised of a main=sheath 160,
a capsule jacket support sheath 161 and a locking connector
162. The main and support sheaths are coaxial from their
proximal end, to a point approximately 15 centimeters from the
distal end 163 of the main sheath, depending on the length of
the graft. At the point where the support sheath terminates,
CA 02241034 1998-08-12
Docket No. ENDOV-34033
-27-
the main sheath flares to an expanded diameter to cover the
proximal capsule 132 and the distal capsule 93. The diameter
of the main sheath is about 0.263 inches (6.68 mm) at its
proximal end and about 0.3 inches (7.62 mm) at the distal end
163. The proximal ends of the sheaths may be secured to the
sheath adapter 164 of the locking connector by mechanical means
and by adhesive. The distal end of the main sheath of the
capsule jacket is provided with radiopaque marker 166.
When the capsule jacket assembly 53 is in its most distal
position, the distal end 163 of the capsule jacket main sheath
extends to cover at least a portion of the distal capsule
assembly 90. Similarly, the capsule jacket locking connector
162 is thereby positioned just proximal the proximal capsule
catheter purge port 151. Prior to insetion into the lumen,
locking ring 165 is turned down to hold the capsule jacket
assembly firmly in place, thereby maintaining a smooth
transition surface along the length of the intraluminal
grafting system 50 which resides in the body vessels. When
the locking ring is released, the capsule jacket assembly may
be moved to a furthermost proximal position, wherein at least
a portion of the proximal capsule catheter assembly is exposed.
Thus, the locking connector is positioned just distal the
capsule catheter wye adapter 145. The locking ring may be
tightened at any intermediate position to firmly secure the
capsule jacket assembly at the desired location. In addition,
a radiopaque marker 166 is provided at the distal end of the
main sheath to facilitate proper linear positioning of the main
sheath.
CA 02241034 1998-08-12
Docket No. ENDOV-34033
-28-
As shown in FIGS. 1 and 7, the intraluminal grafting
apparatus 50 also includes an expandable intraluminal vascular
graft 55 for implanting in a body vessel. Referring to FIG.
23, the graft consists of a deformable tubular member 170 which
is provided with superior end 171, inferior end 172 and a
cylindrical or continuous wall 173 extending between the
' superior and inferior ends of the graft. The tubular member
may have a length in the range of 8 to 20 centimeters, where
l0 centimeters is suitable for most patients. The tubular
member may have a maximum expandable diameter ranging from 14
to 40 millimeters and a minimum diameter in a collapsed
condition of 0.175 to 0.3 inches (4.44-7.62 mm). The
continuous wall can be woven of any surgical implantable
material such as polytetrafluroethylene or a polyester fiber
made from polyethylene terephthalate (PET), such as "DACRON"
type 56. One material found to be satisfactory is "DEBAKEY"
soft woven "DACRON" vascular prosthesis (uncrimped) sold by
C.R. Bard of Billerica, Mass. In order to prevent unraveling
of the woven material at the ends, the ends can be melted with
heat to provide a small melted bead of material on each end.
Referring to FIG. 23, expandable attachment means 175
is secured adjacent the superior end 171 of the tubular member
170. Similarly, expandable attachment system 176 is secured
adjacent the tubular member's inferior end 172. Each
attachment system serves to yieldably urge the tubular member
from a first compressed or collapsed position to a second
expanded position. Each attachment system is formed of a
plurality of vees 177 with the outer apices 178 and inner
CA 02241034 1998-08-12
_-.
Docket No. ENDOV-34033
-29-
apices 179 of the vees being formed with helical coil springs
180 to yieldably urge the long legs 181 and short legs 182 of
each of the vees outwardly at a direction approximately at
right angles to the plane in which each of the vees lie.
As shown in more detail in FIG. 25, the superior
attachment system 175 is comprised of a single piece of wire
which is formed to provide the vees 177 and also to define the
helical coil springs 180 between the legs 181 and 182. The
two ends of the single piece of wire can be welded together
in one of the legs to provide a continuous spring-like
attachment means. In the construction shown in FIG. 23, it
can be seen that the attachment means have apices lying in four
longitudinally spaced-apart parallel planes which are spaced
with respect to the longitudinal axis of the tubular member
170.
The superior and inferior attachment means 175 and 176
are secured to the superior and inferior ends 171 and 172,
respectively, of the tubular member 170 by suitable means such
as a polyester suture material 190. As shown in FIG. 23, the
suture material is used for sewing the attachment means onto
the wall 173 of the tubular member. Knots 191 are preferably
formed on each of the legs or struts 181 and 182 to firmly
secure each leg to the graft. The legs may be secured so that
the apices lying in each plane are staggered to provide for
the minimum profile when the attachment means is placed in its
collapsed condition. The inferior attachment means 176 may
be attached to the inferior end of the graft 172 in a similar
manner.
CA 02241034 1998-08-12
Docket No. ENDOV-34033
-30-
As shown in FIGS. 25 and 26, wall engaging members 195
and 196 are preferably secured to the legs 181 and 182 in the
vicinity of the outer apices 178 by suitable means such as
welding. The wall engaging members have a diameter ranging
from 0.01 to 0.018 inches (0.254-0.457 mm) and a length from
0.5 to 5.0 millimeters. The wall engaging members are
preferably sharpened to provide conical tips, and should have
a length which is sufficient for the tip to penetrate into and
perhaps through the corporeal lumen wall.
The superior attachment means 175 and the wall engaging
members 195 secured thereto are formed of a corrosion resistant
material which has good spring and fatigue characteristics.
One such material found to be particularly satisfactory is
"ELGILOY" which is a cobalt-chromium-nickel alloy manufactured
and sold by Elgiloy of Elgin, Illinois. The wire can have a
diameter ranging from 0.008 to 0.016 inches (0.203-0.406 mm) ,
with a smaller diameter wire being utilized for the smaller
diameter grafts. For example, 0.012 to 0.016 inch (0.305-0.406
mm) diameter wire for the frame and wall engaging members may
be used in the larger grafts of eighteen to twenty-eight
millimeters diameter, and 0.008 to 0.012 inch (0.203-0.305 mm)
diameter wire may be used in the smaller grafts being eight
to sixteen millimeters in diameter. -
It has been found that the spring force created by the
helical coils 180 at the apices 178 and 179 is largely
determined by the diameter of the wire. The greater the
diameter of the wire, the greater the spring force applied to
the legs 181 and 182 of the vees. Also, the longer the
CA 02241034 1998-08-12
. . ~.. ,,
a
Docket No. ENDOV-34033
-31-
distances are between the apices, the smaller the spring force
that is applied to the legs. It therefore has been desirable
to provide a spacing between the outer extremities of the legs
of approximately one centimeter, although smaller or larger
distances may be utilized.
To facilitate securing the graft 55 in the corporeal
lumen, the wall engaging members 195 and 196 of the superior
attachment means 175 and inferior attachment means 176 may be
angled with respect to longitudinal axis of the tubular member
170. The wall engaging members face outwardly from the tubular
member to facilitate holding the graft in place. Preferably,
the wall engaging members on the superior attachment means are
inclined from the longitudinal axis and toward the inferior
end of the graft 172 by 55° to 85° and preferably about
75°.
Likewise, the wall engaging members of the inferior attachment
means may be inclined towards the superior end of the graft
175 by 30° to 90° and preferably 85°. By angling the wall
engaging members so that they resist the force of the blood
f low, the implanted wall engaging members oppose migration of
the graft.
The helical coil springs 180 placed at the apices 178
and 179 serve to facilitate compression of the graft 55 to
place the superior and inferior attachment means'175 and 176
within the capsule assemblies 90 and 130, as hereinafter
described. The compression of the graft is accomplished by
deformation of the coil springs within their elastic limits.
Placing the apices in different planes and staggering or
offsetting the wall engaging members 195 and 196 significantly
CA 02241034 1998-08-12
,,:
Docket No. ENDOV-34033
-32-
reduces the minimum compressed size of the graft. Having the
apices in different planes also helps to prevent the wall
engaging members from becoming entangled with each other. The
natural spring forces of the helical coil springs serves to
expand the graft to its expanded position as soon as the
attachment means is free of the capsule means.
The graft 55 preferably contains radiopaque marker means
for locating the graft and for detecting any twisting of the
graft during deployment. The radiopaque marker means takes
the form of two sets of radiopaque markers 197 and 198. The
radiopaque markers are made of a suitable material such as a
platinum tungsten alloy wire of a suitable diameter such as
0.004 inches (0.102 mmj which is wound into a spring coil
having a diameter of 0.04 inches (0.102 mmj. The radiopaque
markers are secured to the tubular- member 170 by sutures 199,
using the same material sued to secure the attachment means
to the graft.
As shown in FIG. 23, the radiopaque markers 197 and 198
are located on the tubular member 170 of the graft 55 in a line
parallel to the longitudinal axis of the tubular member. Each
tubular member preferably has at least two sets of markers such
that the first marker is positioned 0.5 centimeters from the
superior attachment system 175. Additional markers are
positioned every one centimeter thereafter for the length of
the tubular member. The last marker in each set is 0.5
centimeters away from the inferior attachment system 176.
Thus, the total number of markers in each set depends upon the
length of the graft.
CA 02241034 1998-08-12
Docket No. ENDOV-34033
-33-
Referring also to FIG. 24, each of the first set of
radiopaque markers 197 has a preferred length of 3 millimeters.
Each of the second set of markers 198 preferably has a smaller
length, for example 2 millimeters, and are positioned along
the longitudinal axis of the tubular member 170 at a position
180° from the first set of markers. By placing markers of
different lengths along the axis of the tubular member, it is
possible to ascertain the position of the graft 55 and to
determine whether the graft has twisted between its superior
and inferior ends 171, 172. Under fluoroscopy, the two sets
markers will be exhibited as two relatively straight lines for
an untwisted graft, wherein a twisted graft will be revealed
by a non-linear pattern of markers. By placing the markers
at equal increments apart, it is possible to use fluoroscopy
to ascertain longitudinal compression or tension on the graft.
The sizing of the graft 55 may be performed on a patient
by-patient basis, or a series of sizes may be manufactured to
adapt to most patient needs. For the repair of an aortic
aneurism, the length of the graft is selected so to span
approximately one centimeter superior and one centimeter
inferior of the aneurysm, wherein the wall engaging members
195 and 196 of the graft can seat within normal tissue of the
vessel on both sides of the aneurysm. Thus, the g-raft should
be about two centimeters longer than the aneurysm being
repaired. During the preimplant fluoroscopy procedure, a
conventional pigtail catheter is used to determine the
locations of the renal arteries to ensure the renals will not
be covered by the implanted graft. Similarly, the diameter
CA 02241034 1998-08-12
Docket No. ENDOV-34033
-34-
t'~
of the tubular member 170 is selected by measuring the
corporeal lumen which will receive the graft by conventional
radiographic techniques and then selecting a tubular member
having a diameter one millimeter larger than that measured.
FIG. 8 depicts the distal end of the intraluminal
grafting system 50 assembled for deployment. The graft 55 is
disposed within the proximal capsule assembly 130, the distal
capsule assembly 90 and the capsule jacket assembly 53. The
superior end of the graft 171 and superior attachment means
175 are removably retained within the distal capsule 93.
Likewise, the inferior end of the graft 172 and inferior
attachment means 176 are removably retained within the proximal
capsule 132. The distal cap 92 is in its retracted or proximal
position adjacent to proximal cap 100. Similarly, core wire
91 is locked via control knob 113 in its retracted or proximal
position. During initial deployment, capsule catheter tubular
member 131 is in its most distal position in relation to
balloon catheter assembly 51 and is locked in place by the
locking ring on the Touhy Borst adapter 147.
During initial deployment, the distal end of the balloon
catheter 80 is positioned such that the balloon 60 resides
within the tubular member 170 of the graft 55, as shown in FIG.
8. The proximal cap 100 is positioned just proxima-1 the distal
cap 92 and is disposed within the distal capsule 93. Likewise,
pusher button 85 is disposed just proximal the inferior
attachment means 176 of the graft. The capsule jacket assembly
53 is positioned such that the distal end of the capsule jacket
main sheath 160 overlaps at least a portion of the distal
CA 02241034 1998-08-12
Docket No. ENDOV-34033
-35-
capsule 93. During deployment, capsule jacket locking
connector 162 secures the main sheath in place. Thus, when
any movement or force is applied to the handle assembly 110,
the entire apparatus 50 moves as a single unit.
By way of example, the following describes a method of
repair of an aortic aneurism using the method comprising the
present invention for intraluminal placement of a graft in an
aorta. First, a patient is prepared in a conventional manner
by use of a guide wire 56, a dilator and sheath (not shown)
l0 to open the femoral artery or vessel of the patient. The
distal end of the intraluminal grafting apparatus 50 is then
inserted into the sheath, which has previously been placed in
the femoral artery. In the preferred embodiment of the present
invention, balloon catheter lumen 63 is provided for receiving
the guide wire 56. However, the following procedure may also
be used when the guiding member is constructed as part of the
balloon catheter.
As shown in FIG. 27, the guide wire 56 is introduced
by the physician into the femoral artery and advanced to the
desired location in the aorta 200 and adjacent to the diseased
or damaged portion of the vessel 201. The balloon catheter
assembly 51, the capsule catheter assembly 52, the capsule
jacket assembly 53 and the control wire assembly- 54 are all
configured for deployment as shown in FIGS. 1 and 8. Thus,
the assemblies are advanced by the physician as a single unit
over the guide wire. The physician uses the handle assembly
110 and the proximal end of the balloon catheter 70 to guide
the distal end of the assemblies over the guide wire.
CA 02241034 1998-08-12
Docket No. ENDOV-34033
-36-
Typically, the desired position for implanting the graft 55
will be within the abdominal aorta with the superior extremity
171 of the graft at least one centimeter inferior to the lower
renal artery.
When the distal capsule assembly 90 and the superior
attachment means 175 are in the desired position, the superior
end of the graft 55 is implanted. First, the locking ring 165
of the capsule jacket assembly 53 is loosened to allow movement
of the capsule jacket main sheath 160. While using one hand
to firmly grasp the capsule catheter assembly 52 and hold it
stationary, the physician grasps the sheath adapter 164 with
the other hand and gently pulls the sheath adapter proximally
towards the capsule catheter wye adapter 145, as shown in FIG.
28. The capsule jacket assembly is moved about 6 centimeters
to fully expose the distal capsule assembly, and partially
expose the graft. The locking ring is then tightened to hold
the capsule jacket assembly in place.
At this point in the procedure, the superior end of the
graft 171 is disposed in the distal capsule 93, the inferior
end of the graft 172 is securely retained with in the proximal
capsule 132 and the distal end of the capsule jacket main
sheath 160 is located about midway between the ends of the
graft. The control knob 113 is then loosened to permit
relative movement between the distal capsule assembly 90 and
the balloon catheter assembly 51 to expose superior end 171
of the graft 55. Again using one hand to firmly grasp the
handle assembly 110, the physician slides the control knob
distally toward the wye adapter 145. Since the distal cap 92
CA 02241034 1998-08-12
Docket No. ENDOV-34033
-37-
and distal capsule 93 are secured to the control wire 91, they
move in a about a 1:1 ratio with the movement of the control
knob. The control knob is moved distally approximately 3
centimeters, which moves the distal capsule from engagement
with the superior attachment means 175 and exposes the balloon
catheter proximal cap 100. The control knob is then locked
in place. As soon as the distal capsule has cleared the
superior attachment means 175, the superior extremity of graft
expands outwardly under the force of the self-expanding
attachment means which springs into engagement with the vessel
wall 202.
Once the superior attachment means 175 is exposed, steps
are taken to firmly seat or urge the wall engaging members 195
an 196 in the vessel lumen. First, locking ring 165 is
loosened, and the capsule jacket assembly 53 is moved
proximally to a point where the distal end of the main sheath
160 is positioned just proximal the distal end of the proximal
capsule 132 (FIG. 29). The capsule jacket assembly is then
locked in place. Then, the locking ring on the capsule
catheter Touhy Borst adapter 147 is loosened to permit relative
movement between the capsule catheter assembly 52 and the
balloon catheter 51. While the physician uses one hand to hold
the capsule catheter assembly stationary, the hand-le assembly
110 is grasped by the other hand and pushed distally to
position the center of the balloon 60 into the. superior
extremity of the graft 171. The radiopaque marker 84 is used
to align the balloon and attachment means.
CA 02241034 1998-08-12
Docket No. ENDOV-34033
-38-
Thereafter, a conventional hand operated syringe or
inflation assembly (not shown) is attached to the balloon
catheter inflation port 74. The balloon is then expanded by
introducing a suitable gas such as carbon dioxide or a dilute
radiopaque liquid from the syringe to urge the wall engaging
members 195 and 196 outwardly to firmly emplace the attachment
system within the vessel wall 202. The balloon may be deflated
and inflated repeatedly to ensure the superior attachment means
is firmly implanted in the vessel. The balloon may be then
deflated or may remain in an inflated position during the next
steps of the procedure.
As shown in FIG. 30, the next step is to implant or
anchor the inferior attachment system 176. With the handle
assembly 110 held firmly in place, the capsule catheter
assembly 52 is moved proximally until the inferior attachment
system and inferior end of the graft 172 are completely clear
of the proximal capsule 132. Once the inferior extremity of
the graft is free of the proximal capsule, the inferior
attachment system will spring open and the wall engaging
members 195 and 196 will engage the vessel wall 202 . Leaving .
the balloon 60 inflated while the capsule catheter assembly
is moved ensures that the superior attachment system 175 will
remain firmly secured in place. Thereafter, the-balloon may
be deflated and the capsule catheter locking ring 147 secured
to the guiding member 115, thereby securing the capsule
catheter assembly 52 to the balloon catheter assembly 51.
In certain circumstances, an alternate method of
deploying the inferior attachment system 176 may be employed.
- CA 02241034 1998-08-12
Docket No. ENDOV-34033
-39-
Such an alternate method may be advantageous when a graft has
been chosen that is of a length which exceeds the length of
the aorta, thereby jeopardizing a secure emplacement of the
inferior extremity of the graft. In such cases, the balloon
60 is deflated so that the balloon catheter assembly 51 can
be freely moved within the corporeal lumen. Next, the capsule
catheter assembly 52 is advanced in a distal direction so that
the inferior attachment system is positioned in the desired
location of the aorta 200.
With the inferior attachment system 176 and the inferior
extremity of the graft 172 in the portion of the corporeal
lumen where the wall engaging members 196 are to engage the
vessel wall 202, the balloon catheter shaft 61 is advanced so
that the pusher button 85 and retaining bump 86 engage the
inferior attachment system. Then the user holds the handle
assembly 54 and balloon catheter assembly 51 fixed while moving
the capsule catheter assembly 52 in a proximal direction. When
the proximal capsule assembly 130 moves sufficiently distally,
the inferior attachment system will be released from the
proximal capsule 132. The inferior attachment system and
inferior wall engaging members may then be secured with the
balloon 60.
As shown in FIG. 31, the handle assembly 110 is moved
proximally so that the balloon 60 is then retracted further
into the graft 55 and placed adjacent the inferior attachment
system 176. Again, the balloon radiopaque marker 84 is used
to align the center of the balloon with the attachment means .
The balloon is then inflated, and perhaps deflated and
-- CA 02241034 1998-08-12
Docket No. ENDOV-34033
-40-
inflated, to expand the attachment system and ensure that the
wall engaging members 195 and 196 are firmly urged and
implanted in the vessel wall 202. Thereafter, the balloon is
finally deflated.
As shown in FIG. 32, the proximal capsule assembly 130
and balloon 60 are moved proximal the graft 55. First the
locking ring 147 is loosened. Then, while holding the capsule
catheter assembly 52 in place by grasping the wye adapter 145
with one hand, the balloon catheter assembly 51 is moved
proximally by gently pulling the handle assembly 110 with the
other hand. Thus, the capsule catheter assembly and balloon
catheter are in the same relative position as they were just
prior to deployment (FIG. 8). Also, the proximal end 103 of
the distal capsule 93 has been mated with the proximal cap 100
for smooth transition.
Finally, as shown in FIG. 33, the capsule jacket locking
ring 165 is loosened. While holding the capsule jacket sheath
adapter 164 in place, the balloon catheter and capsule catheter
assemblies 51 and 52 are moved proximally and in unison by
gently pulling the wye 145 of the capsule catheter assembly.
The catheter assemblies are moved until the distal end of the
main sheath 163 covers the distal capsule 93 or until the
proximal capsule adapter housing 134 mates with=the flared
transition of the capsule jacket, thereby creating a smooth
transition along the entire length of the intraluminal grafting
apparatus 50. Thereafter, the balloon catheter assembly,
capsule catheter assembly, capsule jacket assembly 53 and
control wire assembly 54 are removed from the aorta through
' CA 02241034 1998-08-12
Docket No. ENDOV-34033
-41-
the femoral artery. The graft 50 and attachment means 175 and
176 remain secured to the vessel wall 202, thereby sealing the
aneurism 201 from blood flow.
The entire procedure described herein can be observed
under fluoroscopy. The relative positioning of the graft 55
and the balloon 60 can be readily ascertained by the radiopaque
attachment systems 175 and 176, radiopaque markers 197 and 198
provided on the graft, and the radiopaque marker 84 on the
balloon shaft 61. If any twisting of the graft has occurred
between placement of the superior attachment system and the
inferior attachment system, then the twisting can be readily
ascertained by observing the series of markers 197 and 198.
Adjustments to eliminate any twisting which may have occurred
can be made before exposing the graft's inferior extremity 172
by rotation of the capsule catheter assembly 52. Any excessive
graft compression can be ascertained by observing the
radiopaque markers 197 and 198 under fluoroscopy. Adjustments
to eliminate graft compression can be made before exposing the
inferior extremity of the graft by applying tension on the
capsule catheter assembly 52.
Post implant fluoroscopy procedures can be utilized to
confirm the proper implantation of the device by the use of
a conventional pigtail catheter or by injecting dye into the
guide wire lumen of the balloon catheter shaft. Thereafter
the sheath can be removed from the femoral artery and the
femoral artery closed with conventional suturing techniques.
Tissues should begin to grow into the graft within two to four
weeks with tissue completely covering the interior side of the
,. CA 02241034 1998-08-12
Docket No. ENDOV-34033
-42-
graft within six months so that no portion of the graft
thereafter would be in communication with the blood circulating.
in the vessel. This establishes a complete repair of the
aneurysm which had occurred.
While several particular forms of the invention have
been illustrated and described, it will be apparent that
various modifications can be made without departing from the
spirit and scope of the invention. For example, references
to materials of construction and specific dimensions are also
not intended to be limiting in any manner and other materials
and dimensions could be substituted and remain within the
spirit and scope of the invention. Accordingly, it is not
intended that the invention be limited, except as by the
appended claims.