Note: Descriptions are shown in the official language in which they were submitted.
CA 02241093 1998-06-19
WO 97/23461 PCT/GB96/03196
TRI-SUBSTITUTED PHENYL DERIVATIVES USEFUL AS
PDE IV INHIBITORS
This invention relates to a novel series of triarylethanes, to processes for
their preparation, to pharmaceutical compositions containing them, and to
their use in medicine.
Many hormones and neurotransmitters modulate tissue function by
elevating intra-cellular levels of adenosine 3', 5'-cyclic monophosphate
(cAMP). The cellular levels of cAMP are regulated by mechanisms which
control synthesis and breakdown. The synthesis of CAMP is controlled by
adenyl cyciase which may be directly activated by agents such as forskolin
or indirectly activated by the binding of specific agonists to cell surface
receptors which are coupled to adenyl cyciase. The breakdown of cAMP
is controlled by a family of phosphodiesterase (PDE) isoenzymes, which
also control the breakdown of guanosine 3',5'-cyclic monophosphate
(cGMP). To date, seven members of the family have been described
(PDE I-VII) the distribution of which varies from tissue to tissue. This
suggests that specific inhibitors of PDE isoenzymes could achieve
differential elevation of cAMP in different tissues, [for reviews of PDE
distribution, structure, function and regulation, see Beavo & Reifsnyder
{1990) TIPS, 11: 150-155 and Nicholson et al (1991) TIPS, 12: 19-27].
There is clear evidence that elevation of cAMP in inflammatory leukocytes
leads to inhibition of their activation. Furthermore, elevation of cAMP in
airway smooth muscle has a spasmolytic effect. In these tissues, PDE IV
plays a major role in the hydrolysis of cAMP. It can be expected,
t rcfnraa~that gglg~tiyca iniiihit~r~ of PII~ I\/ wny;ld hays thrrratng~,~tin
he. ~..>
effects in inflammatory diseases such as asthma, by achieving both anti-
inflammatory and bronchodilator effects.
In our International Patent Specification No. W094/14742 we describe a
series of triarylethanes which are potent inhibitors of the PDE IV
isoenzyme at concentrations at which they have little or no inhibitory
action on other PDE isoenzymes. The compounds are of use in medicine,
CA 02241093 1998-06-19
WO 9/23461 PCT/GB96/03196
2
especially in the prophylaxis and treatment of asthma. An
enantioselective process for the preparation of these compounds is
described in our International Patent Specification No. W095/17386.
We have now found a particular series of triarylethanes which are potent
and selective PDE IV inhibitors and which also have other advantageous
pharmacological properties, including especially good oral availability and
improved metabolic stability.
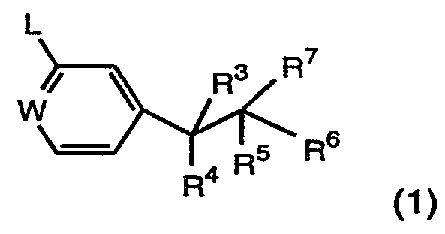
Thus according to one aspect of the invention, we provide a compound of
formula (1 )
L
/ ~ Rs R~
W
R5 R6
R4 (1)
wherein
=W- is (1 ) =C(Y)- where Y is a halogen atom, or an alkyl or -XRa group
where X is -O-, -S(O)p- [where p is zero or an integer of value 1 or 2], or
-N(Rb)- [ where Rb is a hydrogen atom or an optionally substituted alkyl
group] and Ra is a hydrogen atom or an optionally substituted alkyl group
or, (2) =N-;
L is a -XR, [where R is an optionally substituted alkyl, alkenyt, cycloalkyl
or
cyloalkenyl groupj, -C(Rjj)=C(R~)(R2) or [-CH(R1~)]~CH(Rj)(R2) group
where Ri ~ is a hydrogen or a fluorine atom or a methyl group, and R~ and
R2, which may be the same or different, is each a hydrogen or fluorine
atom or an optionally substituted alkyl, alkenyl, alkynyl, alkoxy, alkylthio,
-C02R8, [where R8 is a hydrogen atom or an optionally substituted alkyl,
aralkyl, or aryl group], -CONRsRio [where R9 and R'~o, which may be the
same or different is each as defined for R$], -CSNR9R'~~, -CN or -N02
group, or R1 and R2 together with the C atom to which they are attached
are linked to form an optionally substituted cycloalkyl or cycloalkenyl group
and n is zero or the integer 1;
R3 is a hydrogen or a fluorine atom, an optionally substituted straight or
branched alkyl group, or a hydroxyl group;
CA 02241093 1998-06-19
WO 97!23461 PCTJGB96/03196
3
R4 is a hydrogen atom or group -(CH2)tAr [where t is zero or an integer 1,
2 or 3 and Ar is a monocyclic or bicyclic aryl group, optionally containing
one or more heteroatoms selected from oxygen, sulphur or nitrogen
atoms] or a group -(CH2)t-Ar-(L1 )~-Ar' [where Lt is a divalent linking group
n is zero or an integer 1 and Ar' is -Ar, -CO(Alk)n,Ar, [where Alk is an
optionally substituted straight or branched C1 _s atkylene, C2_6 alkenylene
or C2_6 atkynylene chain optionally interrupted by one, two or three -O- or
-S- atoms or -S(O)q- (where q is an integer 1 or 2) or -N(Rb)- groups and
m is zero or an integer 1 ], -S02(Aik)~Ar, -S02NH(Alk)mAr,
-S02N(Alki){Alk)~Ar [where Alki is as defined for Alk] -S02N[(Alk)n.,Ar]2,
-CONH(Alk)mAr, -CON(Alki )(Alk),~,~,Ar,
-CON[(Alk)mAr]2, -N(Alk~ )SOZ(Alk)mAr, -NHS02(Alk)mAr,
-N[S02(Alk)mAr]2, -NHS02NH(Alk)mAr, -N(AIk1 )S02NH(Alk)r,.,Ar,
-NHS02N(AIk1)(Atk),nAr, -N(AIk1)S02N(Alki)(Alk)~,Ar,
-NHS02N[(Atk)mAr]2, -N(AIk1 )S02N[(Alk)mAr]2, -NHC(O)(Alk),nAr,
-N(Atk~)C(O)(Atk)mAr, -N[C(O)(Alk)mAr]2, - NHC(O)NH(Alk)~Ar,
-N(AIk1)C(O)NH(Alk),r,Ar, -NHC(O)N(Alky)(Alk)n,Ar, .
-N(Alk~)C(O)N(Alk~)(Alk)mAr, -NHC(O)O(A1k)mAr, -N(Alk'~)C(O)O(Alk),~.~Ar,
-C(S)NH(Alk)mAr, -C(S)N(Alk~)(Alk)mAr, -C(S)N(Alk~)(Alk),~,~,Ar,
-C(S)N[(Alk)r,.~Ar]2, -NHC(S)(Alk)~-,Ar, -N(Atk~ )C(S)(Alk)mAr,
-N[C(S)(Alk),~,~,Ar]2, -NHC(S)NH(Alk)mAr, -N(Alk~ )C(S)NH(Alk)mAr,
-NHC(S)N{AIk1)(Alk),~,~,Ar, -N(AIk1)C(S)N(Alki)(Alk)~,Ar, -S02(Alk)mNHet
[where -NHet is an optionally substituted C~_~ heterocyclic amino group
optionally containing one or more other -O- or -S- atoms or -N(Rb)-, -C{O)-
or -C(S)- groups], -CO(Atk}r,~,NHet, -CS(Alk)r,.,NHet, -NHS02(Alk)=,~,NHet,
-NHC(O)(Alk)r,.,NHet, -NHC(S)(Alk)mNHet, -S02NH((Alk)mHet') [where
Het' is an optionally substituted C~_~monocyclic carbocyclic group
optionally containing one or more -O- or -S- atoms or -N(R~)- groups],
-CC_~I~jHIIA~kI H~t'1 -~~,itl~llAlk) HAt') -NI-tSn~NN'(Atk)~,uot~)~
v iiil~ i~ w Wt r
-NHC{O)NH(Alk)~,.,(Het') or -NHC(S)NH(Alk),~,.,(Het') group] or R4 is a
-(CH2)tArN(R~)CX1 N{Rb)L2(Alk)mAr group where X is an oxygen or
sulphur atom and L2 is a divalent linking group;
R~ is a -(CH2)tAr, -(CH2)t-Ar-(L~ )n-Ar' o r
-(CH2)tArN(Rb)CX'~ N(Rb)L2(Alk),nAr group, provided that at least one of
R4 or R5 is a -(CH2)tArN(Rb)CX1 N(Rb)L2{Alk)mAr group;
CA 02241093 1998-06-19
WO 97/23461 1'CT/GB96/03196
4
Rs is a hydrogen or a fluorine atom, or an optionally substituted alkyl
group;
R~ is a hydrogen or a fluorine atom, an optionally substituted straight or
branched alley! group or an ORS group where R~ is a hydrogen atom or an
optionally substituted alkyl or alkeny! group, or an alkoxyalkyf, alkanoyl,
formyl, carboxamido or thiocarboxamido group; and the salts, solvates,
hydrates, prodrugs and N-oxides thereof.
it will be appreciated that certain compounds of formula (i ) may have one
or more chiral centres, depending on the nature of the groups L, R'~ , R2,
R3, R4, R5, R6 and R~. Where one or more chirat centres is present,
enantiomers or diastereomers may exist, and the invention is to be
understood to extend to al! such enantiomers, diastereomers and mixtures
thereof, including racemates.
Compounds of formula (1 ) wherein L is a -C(R1 i }=C(Ri){R2) group may
exist as geometric isomers depending on the nature of the groups R1, R2,
and R1 ~ and the invention is to be understood to extend to all such
isomers and mixtures thereof.
In the compounds of formula (1), when =W- is =C(Y}- and Y is a halogen
atom Y may be for example a fluorine, chlorine, bromine or iodine atom.
When W in the compounds of formula (1 ) is a group =C(Y)- and Y is -XRa,
Ra may be, for example, a hydrogen atom or an optionally substituted
straight or branched alkyl group, for example, an optionally substituted Ci _
salkyl group, such as a methyl, ethyl, n-propyl or i-propyl group. Optional
substituents which may be present on Ra groups include one or more
halogen atoms, e.g. fluorine, or chlorine atoms. Particular Ra groups
include for example -CH2F, -CH2CI, -CHF2, -CHCI2, -CF3 or -CCI3 groups.
When =W- in the compounds of formula (1) is a group =C(Y}- where -Y is
-N(Rb), =W- may be a =C(NH2)-, =C(NHCH3)- or =C(NHC2H5)- group.
CA 02241093 1998-06-19
WO 97/23461 PCT/GB96/03196
In compounds of formula (1 ), X may be an oxygen or a sulphur atom, or a
group -S(O)-, -S(O)2-, -NH- or C~_s alkylamino, for example a Cy_3
alkylamino, e.g. methylamino [-N(CH3)-] or ethylamino [-N(C2H5)-] group.
5 Alkyl groups represented by Y, R, R1, R2, or Rb in the compounds of
formula (1) include optionally substituted straight or branched Ci_s alkyl
groups optionally interrupted by one or more X atoms or groups.
Particular examples include C~_3 alkyl groups such as methyl or ethyl
groups. t~ptiona~ substituents on these groups include one, two or three
substituents selected from halogen atoms, e.g. fluorine, chlorine, bromine
or iodine atoms, or hydroxyl or C1_s alkoxy e.g. Ci_3 alkoxy such as
methoxy or ethoxy or -C02R8, -CONR9R», -CSNR9R~o or -CN groups.
Alkenyl groups represented by R, R1 or R2 in the compounds of formula
(1) include optionally substituted straight or branched C2_salkenyl groups
optionally interrupted by one or more X atoms or groups. Particular
examples include ethenyl, propen-1-yl and 2-methyipropen-1-yl groups.
Optional substituents include those described above in relation to alkyl
groups represented by the groups Rj or R2.
Alkynyl groups represented by Ry or R2 in compounds of formula (1 )
include optionally substituted straight or branched C2_salkynyl groups
optionally interrupted by one or more X atoms or groups. Particular
examples include ethynyl and propyn-1-yl groups. Optional substituents
include those described above in relation to alkyl groups represented by
the groups R1 or R2.
When R~ or R2 in compounds of formula (1 ) is an alkoxy or alkylthio group
it may be for example an optionally substituted straight or branched CI-s
alkoxy or Cy_salkylthio group optionally interrupted by one or more X
atoms or groups. Particular examples include C1 _3alkoxy, e.g. methoxy or
ethoxy, or C~ _3alkylthio e.g. methylthio or ethylthio groups. Optional
substituents include those described above i_n relation to alkyl groups
represented by the groups R1 or R2.
CA 02241093 1998-06-19
WO 97/23461 PCT/GB96/03196
6
When RI and R2 together with the carbon atom to which they are attached
in the compounds of formula (1 ) are linked to form a cycloalkyl or cyclo-
alkenyl group, the group may be for example a C3_8cycloalkyl group such
as a cyclobutyl, cyclopentyl or cyclohexyl group or a C3_$ cycloalkenyl
group containing for example one or two double bonds such as a 2-cyclo-
buten-1-yl, 2-cyclopenten-1-yl, 3-cyciopenten-1-yl, 2,4-cyclopentadien-1-
yl, 2-cyclohexen-1-yl, 3-cyclohexen-1-yl, 2,4-cyclohexadien-1-yl or 3,5-
cyclohexadien-1-yl group, each cycloalkyl or cycloalkenyl group being
optionally substituted by one, two or three substituents selected from
halogen atoms, e.g. fluorine, chlorine, bromine or iodine atoms,
straight or branched C~_salkyl e.g. C1-3atkyl such as methyl or ethyl,
hydroxyl or C1_6alkoxy e.g. C~_3alkoxy such as methoxy or ethoxy groups.
When R in the compounds of formula (1) is an optionally substituted
cyctoalkyl or cycloalkenyt group it may be for example a C3_$cycloalky!
group such as a cyclobutyl, cyclopentyl or cyclohexyl group or a C3-
8cyctoalkenyl group containing for example one or two double bonds such
as a 2-cyclobuten-1-yl, 2-cyclopenten-1-y1, 3-cyclopenten-1-yl, 2,4-
~yclopentadien-1-yl, 2-cyclohexen-1-yt, 3-cyclohexen-1-yl, 2,4-
cyclohexadien-i-yl or 3,5-cyclohexadien-1-yi group, each cycloalkyf or
cycloalkenyl group being optionally substituted by one, two or three
substituents selected from halogen atoms, e.g. fluorine, chlorine, bromine
or iodine atoms, straight or branched C~_safkyl e.g. C1_3alkyl such as
methyl or ethyl, hydroxyl or C1 _salkoxy e.g. C1 _3alkoxy such as methoxy or
ethoxy groups.
When the group R7 in compounds of formula (i) is an ORS group it may
be for example a hydroxyl group; or a group -ORS where R~ is an
optionally substituted straight or branched C~ _salkyl group, e.g. a C1
_3alkyl
group such as a methyl or ethyl group, a C2_satkenyl group such as an
ethenyl or 2-propen-1-yl group, a Cy _3alkoxyCy _3alkyl group such as a
methoxymethyl, ethoxymethyl or ethoxyethyl group, a C~_~alkanoyl, e.g.
C~ _3alkanoyl group such as an acetyl group, or a formyl [HC(O)-j,
carboxamido (COI~1R~2R'~2a) or thiocarboxamido (CSNR~2R12a) group,
where Ri2 and R'~2a in each instance may be the same or different and is
each a hydrogen atom or an optionally substituted straight or branched C1 _
CA 02241093 1998-06-19
WO 97/23461 PCT/GB96/03196
7
6alkyl, e.g. Cj_3aikyl group such as methyl or ethyl group. Optional
substituents which may be present on such R~, R12 or Rl2a groups
include those described below in relation to the alkyl groups R6 or R~.
w 5 Alkyl groups represented by R3, R6 or R7 in compounds of formula (1 )
include optionally substituted straight or branched C~_6 alkyl groups, e.g.
C1_3 alkyl groups such as methyl, ethyl, n-propyl or i-propyl groups.
Optional substituents which may be present on these groups include one
or more halogen atoms, e.g. fluorine, chlorine, bromine or iodine atoms, or
hydroxyl or C1 _salkoxy e.g. C j _3atkoxy such as methoxy or ethoxy groups.
When the group Rs in compounds of formula (1 ) is a halogen atom it may
be for example a fluorine, chlorine, bromine or iodine atom.
75 When R1 or R2 is a -C02R8, -CONR9R» or CSNR9R'~c group or these
groups appear as substituents, the groups may be for example a -C02H,
-CONH2 or -CSNH2 group or a group -C02R8, -CONR9R~o, -CSNR9R»,
-CONHR~a, or -CSNHR» where R8, R9 and R'~c where present is a C~_
3alkyl group such as methyl or ethyl group, a Cs_y2aryl group, for example
an optionally substituted phenyl, or a 1- or 2- naphthyt group, or a C6_
~2aryl C~-3alkyl group such as an optionally substituted benzyl or
phenethyl group. Optional substituents which may be present on these
aryl groups include R'~3 substituents discussed below in relation to the
group Ar.
In the compounds of formula (7 ), the groups -{CH2)tAr and
-(CH2)~Ar(L1 )~Ar' when present may be -Ar, -CH2Ar, -(CH2)2Ar, -{CH2)3Ar-,
-Ar-Ar', -Ar-L1-Ar', -CH2ArAr', -CH2ArL~Ar', -(CH2)2ArAr', -(CH2)2ArL~Ar',
-(CHz)3ArAr' or -(CH2)3ArL~ Ar' groups.
Monocyclic~or bicyclic aryl groups represented by the group Ar or Ar' in
compounds of formula (1) include for example C6_~2 optionally substituted
aryl groups, for example optionally substituted phenyl, 1-or 2-naphthyl,
indenyl or isoindenyl groups.
CA 02241093 1998-06-19
WO 97/23461 PCT/GB96/03196
8
When the monocyclic or bicyciic aryl group Ar or Ar' contains one or more
heteroatoms, Ar or Ar' may be for example a Cf_9 optionally substituted
heteroaryl group containing for example one, two, three or four
heteroatoms selected from oxygen, sulphur or nitrogen atoms. In general,
Ar or Ar' heteroaryl groups may be for example monocyclic or bicyclic
heteroaryl groups. Monocyctic heteroaryl groups include for example five-
or six-membered heteroaryl groups containing one, two, three or four
heteroatoms selected from oxygen, sulphur or nitrogen atoms. Bicyclic
heteroaryl groups include for example nine- or ten- membered heteroaryl
groups containing one, two or more heteroatoms selected from oxygen,
sulphur or nitrogen atoms.
Examples of heteroaryl groups represented by Ar or Ar' include pyrrolyl,
furyl, thienyl, imidazolyl, N-methylimidazolyl, N-ethylimidazolyl, oxazolyi,
isoxazolyl, thiazolyl, isothiazolyl, pyrazolyl, 1,2,3-triazolyl, 1,2,4-
triazolyl,
1,2,3-oxadiazolyl, 1,2,4-oxadiazolyl, 1,2,5-oxadiazolyt, 1,3,4-oxadiazolyl,
pyridyl, pyrimidinyl, pyridazinyl, pyrazinyl, 1,3,5-triazinyl, 1,2,4-
triazinyl,
1,2,3-triazinyl, benzofuryl, isobenzofuryl, benzothienyl, isobenzothienyl,
indolyl, isoindolyl, benzimidazolyl, benzothiazolyl, benzoxazolyl,
quinazolinyl, naphthyridinyl, pyrido[3,4-b]pyridyl, pyrido[3,2-b]pyridyl,
pyrido[4,3-b]pyridyt, quinolinyl, isoquinolinyl, tetrazolyl, 5,6,7,8-tetra-
hydroquinolinyl and 5,6,7,8-tetrahydroisoquinolinyl. Example of bicycfic
heteroaryl groups include quinolinyf or isoquinolinyl groups.
The heteroaryl group represented by Ar or Ar' may be attached to the
remainder of the molecule of formula (1 ) through any ring carbon or
heteroatom as appropriate. Thus, for example, when the group Ar or Ar' is
a pyridyl group it may be a 2-pyridyl, 3-pyridyl or 4-pyridyl group. When it
is a thienyl group it may be a 2-thienyl or 3-thienyl group, and, similarly,
when it is a furyl group it may be a 2-furyl or 3-furyl group. in another
example, when the group Ar or Ar' is a quinolinyl group it may be a 2-, 3-,
4-, 5-, 6-, 7- or 8- quinolinyi and when it is an isoquinolinyl, it may be a 1-
,
3-, 4-, 5-, 6-, 7- or 8- isoquinolinyl group.
When in compounds of formula (1 ) the Ar or Ar' group is a nitrogen-
containing heterocycle it may be possible to form quaternary salts, for
CA 02241093 1998-06-19
WO 97/23461 PCT/GB96/03196
9
example N-alkyl quaternary salts and the invention is to be understood to
extend to such salts. Thus for example when the group Ar or Ar' is a
pyridyl group, pyridinium salts may be formed, for example N-
alkyfpyridinium salts such as N-methylpyridinium.
The aryl or heteroaryl groups represented by Ar or Ar' in compounds of
formula (1 ) may each optionally be substituted by one, two, three or more
substituents [R13]. The substituent R~3 may be selected from an atom or
group R14 or -AIk2(R_ 14},r, wherein R~4 is a halogen atom, or an amino
(-NH2), substituted amino, vitro, cyano, hydroxyl (-OH), substituted
hydroxyl, cycloalkoxy, formyl [HC(O)-], carboxyl (-C02H), esterified
carboxyl, thiol {-SH), substituted thiol, -C(O)AIk2, -S03H, -S02AIk2,
-S02NH2, -S02NHAIk2, -S02N[AIk2]2, -CONH2, -CONHAIk2 , CON[AIk2]2,
-NHS02H, -NAIk2S02H, -NHS02AIk2, -NAIk2S02A1k2, -N[S02AIk2]2,
-NHS02NH2, -NAIk2S02NH2, -NHS02NHAIk2, -NAIk2S02NHAlk2,
-NHS02N[Alk2] 2 , -NAIk2S02N[AIk2]2, -NHC(O)H, -NHC(O)Aik2,
-NAIk2C(O)H, -NAIk2C(O)AIk2, -N[Cl0)Alk2]2, -NHC{O)OH,
-NHC(O)OAtk2, -NAIk2C{O)OH, -NAIk2C(O)OAIk2, -NHCONH2,
-NHCONHAlk2, -NHCON[AIk2]2, -NAIk2CON[AIk2]2, -NAIk~CONH[AIk2],
-NAIk2CONH2, - C(S)H, -C{S)AIk2, -CSNH2, -CSNHAIk2, -CSN[AIk2]2,
-NHC(S)H, -NHCSAlk2, -NAIk2C(S}H, -NAIk2C(S)AIk2, -N[C(S)AIk2]2,
-N[C(O}AIk2]S02H, -NHCSNH2, -NHCSNHAlk2, -NHCSN[Alk2]2r
-NAIk2CSN[Aik2]2, - N A I k2CSNHAlk2, - N A I k2CSNH2, o r
-N[C(O)AIk2]S02AIk2 group, AIk2 is a straight or branched Ci_6 alkylene,
C2_6alkenylene, or C2_salkynyfene chain optionally interrupted by one, two,
or three -O-, or -S- atoms or -S(O)p-, [where p is an integer 1 or 2] or
-N(R8)- groups; and m is zero or an integer 1, 2 or 3 .
When in the group -AIk2(R»),~ m is an integer 1, 2 or 3, it is to be
understood that the substituent or substituents R14 may be present on any
suitable carbon atom in -Alk2. Where more than one R~4 substituent is
present these may be the same or different and may be present on the
same or different carbon atom in AIk2. Clearly, when m is zero and no
substituent R14 is present or when AIk2 forms part of a group such as
-S02AIk2 the alkylene, alkenylene or alkynylene chain represented by AIk2
becomes an alkyl, alkenyl or alkynyl group.
CA 02241093 1998-06-19
WO 97/23461 PCT/GB96/03196
When R14 is a substituted amino group it may be a group -NH[AIk2(Rl4a}mj
[where Alk2 and m ace as defined above and Rla.a is as defined above for .
R14 but is not a substituted amino, a substituted hydroxyl or a substituted
5 thiol group] or a group -N[AIk2(Rl4a}mj2 wherein each -AIk2(Rl4a)m group ,
is the same or different.
When R14 is a halogen atom it may be for example a fluorine, chlorine,
bromine, or iodine atom.
When R14 is a cycloalkoxy group it may be for example a C5_~cycloalkoxy
group such as a cyclopentytoxy or cyclohexyloxy group.
When R14 is a substituted hydroxyl or substituted thiol group it may be a
i5 group -OAIk2(Rla.a}m or -SAIk2(Rl4a}m respectively, where AIk2, Rl4a and
m are as just defined.
Esterified carboxyl groups represented by the group R14 include groups of
formula -C02Aik3 wherein Alk3 is a straight or branched, optionally
substituted C1_$alkyl group such as a methyl, ethyl, n-propyl, i-propyl, n-
butyl, i-butyl, s-butyl or t-butyl group; a C6_l2aryIC1_8alkyl group such as
an
optionally substituted benzyl, phenylethyi, phenyipropyl, 1-naphthylmethyl
or 2-naphthylmethyl group; a C6_l2aryB group such as an optionally
substituted phenyl, 1-naphthyl or 2-naphthyl group; a C6_l2aryloxyCl_8alkyl
group such as an optionally substituted phenyloxymethyl, phenyloxyethyl,
1-naphthyloxymethyl, or 2-naphthyloxymethyl group; an optionally
substituted C1_$alkanoyloxyCl_$alkyl group, such as a pivaloyloxymethyl,
propionyioxyethyl or propionyloxypropyl group; or a Cs_l2aroytoxyCl _$alkyl
group such as an optionally substituted benzoyloxyethyl or benzoyloxy-
propyi group. Optional substituents present on the AIk3 group include R13
substituents described above.
It will be appreciated that the group Ar or Ar' may be attached to the
remainder of the molecule of formula (1) through either a ring carbon atom
or heteroatom.
CA 02241093 1998-06-19
WO 97/23461 PCT/GB96/03196
11
Particular examples of the chain AIk2 when present include methylene,
ethylene, n-propylene, i-propylene, n-butylene, i-butylene, s-butylene, t-
- butylene, ethenyiene, 2-propenylene, 2-butenytene, 3-butenylene,
ethynylene, 2-propynylene, 2-butynylene or 3-butynylene chain, optionally
interrupted by one, two, or three -O- or -S-, atoms or -S(O}-, -S(O)2- or
-N{Rb)- groups.
Particularly useful atoms or groups represented by R1
s include fluorine,
chlorine, bromine or iodine atoms, or C1_salkyl, e.g.
methyl or ethyl,
i0 C1_salkylamino, e.g. methylamino or ethylamino, C1_s
hydroxyalkyl,
e.g.
hydroxymethyt or hydroxyethyl, C1_6alkylthiol e.g. methylthiol
or ethylthiol,
C1 _salkoxy, e.g. methoxy or ethoxy, C5_7cycloalkoxy,
e.g. cyctopentyloxy,
haloCl _6alkyl, e.g. triffuoromethyl, C1 _salkylamino,
e.g. methytamino or
ethyiamino, amino (-NH2), aminoCl~alkyl, e.g. aminomethyl
or aminoethyl,
C1_sdialkylamino, e.g. dimethylamino or diethytamino,
nitro, cyano,
hydroxyl (-OH), formyl [HC(O)-], carboxyl (-C02H), -C02AIk3
[where Alk3 is
as defined above], C1_s alkanoyl e.g. acetyl, thiol (-SH),
thioCl_6alkyl, e.g.
thiomethyl or thioethyl, sulphonyl (-S03H), C1_salkylsulphonyi,
e.g:
methytsulphonyi, aminosuiphonyl (-S02NH2), C1_salkylaminosutphonyl,
e.g. methylaminosulphonyl or ethylaminosulphonyl, C1_sdialkylamino-
sulphonyl, e.g. dimethylaminosulphonyl or diethylaminosufphonyl,
carboxamido (-CONH2), C1_safkylaminocarbonyi, e.g. methylamino-
carbonyl or ethyfaminocarbonyl, C1_sdialkytaminocarbonyi,
e.g. dimethyl-
aminocarbonyl or diethylaminocarbonyl, sulphonyfamino
(-NHS02H),
G1_satkyisulphonylamino, e.g. methylsulphonylamino or
ethylsulphonyi-
amino, C1_sdialkylsulphonylamino, e.g. dimethytsulphonylamino
or diethyl-
sulphonylamino, aminosulphonylamino {-NHS02NH2), C1_salkyiamino-
sulphonylamino, e.g. methylaminosulphonylamino or ethylamino-
sulphonytamino, C1_sdialkylaminosulphonylamino, e.g. dimethylamino-
sulphonylamino or diethylaminosuiphonylamino, C1_~alkanoylamino,
e.g.
acetyfamino, C1 _6alkanoylaminoCl _6alkyl, e.g. acetylaminomethyl
or C1 _6
alkoxycarbonylamino, e.g. methoxycarbonylamino, ethoxycarbonylamino
or
t-butoxycarbonylamino thiocarboxamido (-CSNH2), C1_6 alkylamino-
thiocarbonyl, e.g. methylaminothiocarbonyl or ethylaminothiocarbonyl,
C1_sdialkylaminothiocarbonyl, e.g. dimethylaminothiocarbonyt
or diethyl-
aminothiocarbonyl, aminocarbonyiamino, C1_salkylaminocarbonylamino,
CA 02241093 1998-06-19
WO 97/23461 PCT/GB96/03196
'12
e.g. methylaminocarbonylamino or ethytaminocarbonylamino, C~-sdialkyla-
minocarbonylamino, e.g. dimethylaminocarbonylamino or diethylamino-
carbonylamino, aminothiocarbonylamino, C1_salkylaminothiocarbonyl-
amino, e.g. methylaminothiocarbonylamino or ethylaminothiocarbonyt-
amino, C~_s dtalkylaminothiocarbonylamino, e.g. dimethyfaminothio-
carbonylamino, or dtethylaminothiocarbonylamino, aminocarbonylCl _salkyl-
amino, e.g. aminocarbonylmethylamino or aminocarbonylethytamino,
amtnothiocarbonylCl _satkyiamino e.g. aminothiocarbonylmethylamino or
aminothiocarbonylethylamino, formylaminoCi_6 alkylsulphonylamino, e.g.
formylaminomethytsutphonylamino or formyl-aminoethylsulphonylamino,
thioformylaminoC~_6alkylsulphonylamino, e.g. thtoformytaminomethyl-
sulphonylamino or thioformylethylsulphonylamino, C1_gacylaminosulphonyt-
amino, e.g. acetylaminosulphonytamino, C1_6thio-acylaminosulphonyl-
amino, e.g. thioacetylaminosulphonylamino groups.
Where desired, two R'~ 3 substituents may be linked together to form a
cyclic group such as a cyclic ether, e.g. a C2_salkylenedioxy group such as
ethylenedioxy.
It wilt be appreciated that where two or more R~3 substituents are present,
these need not necessarily be the same atoms and/or groups. The R~ 3
substituents may be present at any ring carbon atom away from that
attached to the rest of the molecule of formula (1). Thus, for example, in
phenyl groups represented by Ar or Ar' any substituent may be present at
the 2-, 3-, 4-, 5- or 6- positions relative to the ring carbon atom attached
to
the remainder of the molecule.
In the compounds of formula (1 ), when the group -(CH2)tAr(L1 )nAr' is
present in R4 and/or R5, the linker group L1 may be any divalent linking
group. Particular examples of L~ groups which may be present in
compounds of the invention include groups of formula -(AIk4)r(Xa)S(Alk~)~-
where Alk4 and AIkS is each an optionally substituted straight or branched
C~_~alkylene, C2_salkenylene or C2_6aikynylene chain optionally interrupted
by one or more, e.g. one, two or three heteroatoms or carbocyclic or '
heteroatom-containing groups, Xa is an -O- or -S- atom or a -S(O)-, -S(O)2-
or -N(Rb)- group, r is zero or the integer 1, t is zero or the integer 1 and s
is
CA 02241093 1998-06-19
WO 97/23461 PCT/GB96/03196
13
zero or the integer 1, provided that when one of r, s, or t is zero at least
one
of the remainder is the integer 1.
The heteroatoms which may interrupt the AIk4 or AIkS chains include for
example -O- or -S- atoms. Carbocyclic groups include for example
cycloalkyl, e.g. cyclopentyl or cyclohexyl, or cycloalkenyl e.g. cyclopentenyl
or cyclohexenyl, groups. Particular heteroatom-containing groups which
may interrupt AIk4 or Alk5 include oxygen-, sulphur- or nitrogen-containing
groups such as -S(O)-, -S(O)2-, -N(Rb)-, -C(O)-, -C(S)-, -C(NRb)-,
-CON(Rb)-, -CSN(Rb)-, -N(R~)CO-, -N{Rb)CS-, -SON{R~)-, -S02N(Rb)-,
-N(Rb)SO-, -N(Rb)S02-, -N{Rb)S02N(Rb)-, -N{Rb)SON(R~)-, or
-N(Rb)CON(Rb)- groups. It will be appreciated that when the chains AEk4 or
AIkS are interrupted by two or more heteroatoms, carbocyclic or
heteroatom-containing groups, such atoms or groups may be adjacent to
one another, for example to form a group -N(Rb)-C{NRb)-N(R~)- or -O-
CONH-.
Optional substituents which may be present on AIk4 or AIk5 chains include
those described above in relation to the group R1 when it is an alkyl group.
The group -(L1 )~,Ar' may be attached to the group Ar through any available
carbon or heteroatoms present in the two groups. Thus, for example, when
Ar is a phenyl group, -(L1 )~Ar' may be attached through a carbon or
heteroatom in -(L1 )~Ar' to a carbon atom in Ar at the 2-, 3-, 4-, 5-, or 6-
position relative to the Ar carbon atom attached to the remainder of the
molecule.
In the group {L1 )~Ar' particular examples of Alk4 or AIkS include optionally
substituted methylene, ethylene, propylene, butylene, ethenylene, 2-
propenylene, 2-butenylene, 3-butenylene, ethynylene, 2-propynylene, 2-
butynylene or 3-butynylene chains, optionally interrupted by one, two or
three heteroatoms, carbocyclic or heteroatom-containing groups as
described above.
Particular examples of the group -(L1 )~Ar' include the groups -AIk4Ar',
-XAr', -AIk4XAr' and -XAlkSAr', especially for example -CH2Ar', -(CH2)2Ar',
CA 02241093 1998-06-19
WO 97/23461 PCT/GB96/03196
14
-(CH2)3Ar', -CH20CH~Ar', -CH2SCH2Ar', -CH2N(Rb)CH2Ar', -CH=CHAr',
-CH2CH=CHAr', -OAr', -SAr', -N(R~)Ar', -CH20Ar', -CH2SAr',
-CH2N(Rb)Ar', -CH20CH20Ar', -OCH2Ar', -O(CH2)2Ar', -SCH2Ar',
-S(CH2)2Ar', -N(Rb)CH2Ar' and -N(Rb)(CH2)2Ar'. In these particular
groups, Ar' may be as generally described herein and as particularly
described below.
In general, and in the particular groups just mentioned, Alk in A~' may be an
optionally substituted ~methylene, ethylene, n-propylene, i-propylene, n-
butylene, s-butytene, t-butylene, ethenylene, 2-propenylene, 2-butenylene,
3-butenylene, ethynylene, 2-propenylene, 2-butynylene, or 3-butynylene
chain optionally interrupted by one, two or three -O- or -S- atoms or -S(O)-,
-S(O)2- or -N(Rb)- groups. Optional substttuents which may be present
include one or more halogen atoms, e.g. #luortne, chlorine, bromine or
iodine atoms, or hydroxyl or Ci~alkoxy e.g. Cj_3alkOXy such as methoxy or
ethoxy groups. The group Alki when present in Ar' may also be as just
described #or Alk, but will clearly be an alkyl, alkenyl or alkynyl group,
rather than a corresponding alkylene, afkenytene or alkynylene chain.
Particular examples of the group Ar' include optionally substituted Cs_~2aryl
or C~_9heteroaryl groups, especiaEly optionally substituted phenyl or pyridyl
groups, or, in particular, -CO(Alk),~,~Ph ( where Ph is an optionally
substituted phenyl group), -SONH(Alk),~,~,Ph, -S02N(AIk1)(Alk)n,Ph,
-S02N[(Alk),~,~Ph]2, -CONH(Alk),~,~Ph, -CON(Alk~)(Atk),rPh,
-CON[(Alk}mPh]2, -NAIk1S02(Alk}mPh, -NHS02N(Alk~)(Alk),~,~Ph,
-NAIk~ SOzAIk~ (Alk),~,iPh, -NHS02Nj(Alk)~,Ph]2, - NAlki S02N[(Alk)~,Ph]2,
-NHC(O)(Alk)~,.~Ph, -NAIkrtCO{Alk)mPh, -NC(O)N[(Alk)mPh]2,
-NHC(O)NH(Alk}mPh, -NAIkyC(O}NH{Alk)mPh,
-NHC(O)N(Alk~)(Alk),~.~Ph, -NAIkIG(O)N(Alki)(Alk)~Ph,
-NHC(O)O(Alk)~.,Ph, -NAIkIC(O)O(Alk)r,~,Ph, -C{S)NH(Alk),~Ph,
-C(S)N(Alk~)(Alk)mPh, -N(S)N[(Alk),~,~,Ph]2, -NHC(S}(Alk)~,~,Ph,
-N(Atki}C(S)(Alk)mPh, -NjC(S)(Alk)mPh]2, -NHC(S)NH(Aik)~,Ph,
-NAIkIC(S)NH{Atk),r,Ph, -NHC(S)N{Alk'~){Alk)mPh, or
-N(Aik1)C(S)N(Alk~)(Alk)mPh groups. In these groups, the groups Atk and
Alk~ may in particular each be a methylene or ethylene, and a methyl or
ethyl group respectively and m may be zero or in particular 1.
CA 02241093 1998-06-19
WO 97/23461 PCT/GB96/03196
'15
When in R4 andlor R5 a -NHet group is present this may be for example a
pyrrolidinyl, pyrazolidinyl, piperidinyl, morpholinyl, piperazinyl or thiomor-
pholinyl group. Optional substituents that may be present in such groups
include R13 substituents described above in relation to Ar or Ar' groups.
When in R4 and/or R5 a Het' group is present this may be for example a
pyrrolidinyl, pyrazolidinyt, pipertdinyl, morpholinyl, piperazinyl, thio-
morpholinyl, cycfopentyl, or cyclohexyt group. Optional substituents that
may be present on such groups include R13 substituents described above.
In the compounds of formula (1 ), when an ester group is present, for
example a group C02R8 or -C02A1k3 this may advantageously be a
metabolically labile ester.
In the -(CH2)tArN(Rb)CXy N{R~)L2(Alk),~,~,Ar group present as R4 and/or R5
in compounds of formula (1) the divalent linking group represented by L?
may be for example a -S(O)-, -S(O}N{Rb)-, -S(O)2-, -S(O)2N(Rb)-, -C(O)-,
-C(O)N(Rb)-, -C(S)- or -C(S)N(R)- group. All the other groups represented
by -{CH2)t, Ar, Rb X1, and (Alk),~,~, may be as generally and particularly
discussed above.
Particular examples of R4 and/or R5 groups of these types include
-ArN{Rb}CONHS02(Alk}"~ Ar and -ArN(Rb)CONHS02N(R2)(Alk)r,-,Ar
groups, especially where Ar is an optionally substitued phenyl group.
The presence of certain substituents in the compounds of formula (1 ) may
enable salts of the compounds to be formed. Suitable salts include
pharmaceutically acceptable salts, for example acid addition salts derived
from inorganic or organic acids, and salts derived from inorganic and
organic bases.
Acid addition salts include hydrochlorides, hydrobromides, hydroiodides,
alkylsulphonates, e.g. methanesulphonates, ethanesulphonates, or
isethionates, arylsulphonates, e.g. p-toluenesulphonates, besylates or
napsylates, phosphates, sulphates, hydrogen sulphates, acetates,
CA 02241093 1998-06-19
WO 97/23461 PCT/GB96/03196
16
trif(uoroacetates, propionates, citrates, maleates, fumarates, malonates,
succinates, lactates, oxalates, tartrates and benzoates.
Salts derived from inorganic or organic bases include alkali metat salts
such as sodium or potassium salts, alkaline earth metal salts such as .
magnesium or calcium salts, and organic amine salts such as morpholine,
piperidine, dimethylamine or diethylamine salts.
Prodrugs of compounds of formula (1 ) include those compounds, for
example esters, alcohols or aminos, which are convertible in vivo by
metabolic means, e.g. by hydrolysis, reduction, oxidation or trans-
esterification, to compounds of formula (l ).
Particularly useful salts of compounds according to the invention include
pharmaceutically acceptable salts, especially acid addition
pharmaceutically acceptable salts.
in the compounds of formula (1 ) the group =W- is preferably a =C(Y)-
group. In compounds of this type Y is preferably a -XRa group where X ~is
-O- and Ra is an optionally substituted ethyl group or, especially, an
optionaEly substituted methyl group. Especially useful substituents which
may be present on Ra groups include one, two or three fluorine or chlorine
atoms.
One particularly useful group of compounds of the invention has the
formula (1 ) where L is a group -XR. In compounds of this type X is
preferably -O-. The group R in these compounds is preferably an
optionally substituted cycloalky! group, particularly an optionally
substituted cyclopentyl group, and is, especially a cyclopentyl group.
In another group of compounds of formula (1 ) L is preferably a
-CH=C(R1 )(R2) group. In compounds of this type R1 and R2 are
preferably linked together with the C atom to which they are attached to
form an optionally substituted cycloalkyl or cycloalkenyl group, especially a
substituted cyclopentyl or ' cyclohexyl or, especially, a cyclopentyl or
cyciohexyl group.
CA 02241093 1998-06-19
WO 97/23461 PCT/GB96/03196
'! 7
The groups R4 and R5 in compounds of formula (1 ) is each, independently,
preferably a CH2Ar, -CH2Ar(L~ )nAr' or -CH2ArN(Rb)CX1 N(Rb)L2(Alk),I,Ar
group or especially an -Ar, Ar-Ar', ArL1 Ar' or -ArN(Rb)CXi N(R5)L2(Atk),~.~Ar
group, with the proviso mentioned in connection with formula (1 ). In one
preference the group R4 is especially a -ArN(Rb)CX1 N(Rb)L2{Alk)mAr
group and R~ is an -Ar group. Particularly useful R4 or R5 groups of these
types include those groups in which Ar or Ar' is a monocyclic aryl group
optionally containing one or more heteroatoms selected from oxygen,
sulphur, or, in particular, nitrogen atoms, and optionally substituted by one,
two, three or more R~3 substituents. In these compounds, when the group
represented by Ar or Ar' is a heteroaryl group it is preferably a nitrogen-
containing monocyclic heteroaryl group, especially a six-membered
nitrogen-containing heteroaryl group. Thus, in one preferred example, the
groups R4 and R5 may each contain a six-membered nitrogen-containing
heteroaryl Ar or Ar' group. In another preferred example R4 may contain a
monocyclic aryl group or a monocyclic or bicyclic heteroaryl group Ar or
Ar' containing one or more oxygen, sulphur or nitrogen atoms and R5 rnay
contain a six-membered nitrogen-containing heteroaryt group Ar or Ar'. In
these examples, the six-membered nitrogen-containing heteroaryl group
may be an optionally substituted pyridyl, pyridazinyl, pyrimidinyl, pyrazinyl
or imidazolyl group. Particular examples include optionally substituted 2-
pyridyl, 3-pyridyl, 5-imidazolyt, or, especially, 4-pyridyl, 3-pyridazinyl, 4-
pyridazinyl, 5-pyridazinyl, 2-pyrimidinyl, 4-pyrimidinyl, 5-pyrimidinyl, 2-
pyrazinyt or 3-pyrazinyl. The monocyclic aryl group may be a phenyl
group or a substituted phenyl group, and the monocyctic or bicyclic
heteroaryl group containing one or more oxygen, sulphur or nitrogen atom
may be an optionally substituted 2-fury!, 3-fury!, 2-thienyl, 3-thienyl, 2-
thiazolyl, 2-benzo(b)thiophenyl, 2-benzo(b)furyl or 4-isoquinolinyl group.
In another preference relating to R4 groups of the just mentioned particular
types, Ar' is a -NHC(O}NH(Alk)mPh (where Ph is as just described,
-NHCH3C{O)NH(Alk},~,~Ph, -NHC(O)N(CH3)(Afk)~Ph,
-N{CH3)C{O)N(CHg)(Alk)mPh, -CO(Alk}mPh, -NHS02NH(Alk)mPh,
-N{CHg)S02NH{Alk},~,~,Ph, -N(CH3)S02N(CH3)(Alk)mPh, -NHCO(Atk),~,~,Ph,
-N(CH3)CO(Alk),~,~,Ph or -NHS02(Alk)mPh group.
CA 02241093 1998-06-19
WO 97/23461 PCT/GB96/03196
18
In general in compounds of formula (1 ) when R4 and/or R5 contains a
substituted phenyl group it may be for example a mono-, dl- or
trisubstituted phenyl group in which the substituent is an atom or group
R13 as defined above. When the R4 andlor R5 group contains a
monosubstituted phenyl group the substituent may be in the 2-, or
preferably 3-, or especially 4-position relative to the ring carbon atom
attached to the remainder of the molecule. When the R4 andlor R5 group
contains a disubstituted phenyl group, the substituents may be in the 2,6
position relative to the ring carbon atom attached to the remainder of the
molecule.
Particularly useful substituents R13 Which may be present on Ar groups in
R4 and R5, especially on phenyl groups, include halogen atoms or alkyl,
haloafkyl, amino, substituted amino, vitro, -NHS02NH2, -NHS02NHCH3,
-NHS02N(CHg)2, -NHCOCH3, -NHC(O)NH2, -NCH3C(O)NH2,
-NHC(O)NHCH3, -NHC(O)NHCH2CH3, or -NHC(O)N(CH3)2 groups, each
of said atoms or groups being optinally separated from the remainder of
the Ar group by a group AIk2 as defined above.
When in compounds of formula (1 ) R4 andlor R~ contains a substituted
pyridyl group it may be for example a mono-or disubstituted pyridyl group,
such as a mono- or disubstituted 2-pyridyi, 3-pyridyl or especially 4-pyridyl
group substituted by one or two atoms or groups R13 as defined above, in
particular one or two halogen atoms such as fluorine or chlorine atoms, or
methyl, methoxy, hydroxyl or vitro groups. Particularly useful pyridyl
groups of these types are 3-monosubstituted-4-pyridyl or 3,5-disubstituted-
4-pyridyl, or 2- or 4-monosubstituted-3-pyridyl or 2,4-disubstituted-3-
pyridyl groups.
A particularly useful group of compounds of formula (1 ) has the formula
(2):
CA 02241093 1998-06-19
WO 97/23461 PCTlGB96103196
19
L
Ra ~ ~ R3 R~
Rs Rs
Ra {2)
where -L is a OR, where R is an optionally substituted cycloalkyl group,
-CH=C{R1){R2) or -CH2CH(Ri){R2) group where R1 and R2 are linked
together with the carbon atom to which they are attached to form a
cycloalkyl group; Ra is an optionally substituted alkyl group and R3, R4,
R5, R6 and R~ are as defined for formula (1 ); and the salts, solvates,
hydrates, prodrugs and N-oxides thereof.
In the compounds of formutae {1 ) or (2) one preferred group of
compounds are those where the group R3 is a hydrogen atom; the group
R6 is a methyl group, or especially a hydrogen atom; the group R~ is a
methyl group, or especially a hydrogen atom; and R4 and R5 are as
defined for formula (1 ). In compounds of this type R6 and R7 is each
especially a hydrogen atom.
In general in compounds of formulae {1) or (2) R3, R6 and R~ is each
especially a hydrogen atom, R5 is in particular a -(CH2)tAr group,
especially an optionally substituted pyridyl group, especially a 4-pyridyl
group. The group R4 in compounds of these types is preferably an
-ArN(Rb)CX1 N(Rb)L2(Alk)~,Ar group, particularly where each Ar group is a
monocyclic aryl group optionally containing one or more heteroatoms
selected from oxygen, sulphur or, especially, nitrogen atoms. Particularly
useful R4 groups are those of formula -ArN(Rb)CXi N(Rb)S(O}2(Alk}r,-,Ar or
-ArN{Rb)CXi N{R~)S(O)2N{Rb)(Alk)r"Ar, especially where each Ar group is
an optionally substituted phenyl group. Particular examples of such
groups include -ArNHCONHS(O)2Ar, -ArNHCSNHS(O)2Ar,
-ArNHCONHS(O)2NHAr and -ArNHCSNHS(O)2NHAr, especially where in
each instance Ar is an optionally substituted phenyl group as defined
herein. in general in these compounds, when Ar is a phenyl group the
-N(R6)CXi N{Rb}L2{Alk)mAr group is preferably attached to this group at
CA 02241093 1998-06-19
WO 97/23461 1'CT/GB96/03196
the 3- or 4-position of the phenyl ring relative to the point of attachment of
the ring to the remainder of the molecule of formula (1 ).
In one particular group of compounds of formulae (1 ) or (2} R4 is
5 preferably a -ArNHCONHS(O)2Ar" group wherein Ar is a phenyl group and
Ar" is an optionally substituted phenyl group. In these compounds the
-NHCONHS(O)2Ar" group is preferably attached to the Ar group at the 3
or 4-position of the phenyl ring as explained above. In this particular
group of compounds the other groups W, L, R3, R5, R6 and R~ may be as
10 generally or particularly defined above.
Particularly useful compounds according to the invention are:
($)-,~I-[4-{i -(3-cyclopentyloxy-4-methoxyphenyl)-2-(4-pyridyl)ethyl}phenyl]-
,[y,'-(phenylsulphonyl) urea;
15 (~)-.~.-[4-{i-(3-cyclopentyloxy-4-methoxyphenyl)-2-(4-pyridyl)ethyl}phenyl]-
]~'-(methyiphenylsulphonyl) urea; and
($)-,C-[4-{'! -(3-cyclopentytoxy-4-methoxyphenyl}-2-{4-pyridyl}ethyl}phenyl]-
~'-{4-chlorophenyfsulphonyt) urea;
and the salts, solvates, hydrates, prodrugs and N-oxides thereof.
Compounds according to the invention are selective and potent inhibitors
of PDE IV and advantageously have improved metabolic stability. The
ability of the compounds to act in this way may be simply determined by
the tests described in the Examples hereinafter.
Particular uses to which the compounds of the invention may be put
include the prophylaxis and treatment of asthma, especially inflamed lung
associated with asthma, cystic fibrosis, or in the treatment of inflammatory
airway disease, chronic bronchitis, eosinophitic granuloma, psoriasis and
other benign and malignant proliferative skin diseases, endotoxic shock,
septic shock, ulcerative colitis, Crohn's disease, reperfusion injury of the
myocardium and brain, inflammatory arthritis, chronic glomerulonephritis,
atopic dermatitis, urticaria, adult respiratory distress syndrome, diabetes
insipidus, allergic rhinitis, allergic conjunctivitis, vernal conjunctivitis,
arterial restenosis and artherosclerosis.
CA 02241093 1998-06-19
WO 97/23461 PCT/GB96/03196
21
Compounds of the invention may also suppress neurogenic inflammation
through elevation of cAMP in sensory neurones. They are, therefore,
analgesic, anti-tussive and anti-hyperalgesic in inflammatory diseases
associated with irritation and pain.
- 5
Compounds according to the invention may also elevate cAMP in
lymphocytes and thereby suppress unwanted lymphocyte activation in
immune-based diseases such as rheumatoid arthritis, ankylosing
spondylitis, transplant rejection and graft versus host disease.
Compounds according to the invention may also reduce gastric acid
secretion and therefore can be used to treat conditions associated with
hypersecretion.
Compounds of the invention may suppress cytokine synthesis by
inflammatory cells in response to immune or infectious stimulation. They
are, therefore, useful in the treatment of bacterial, fungal or viral induced
sepsis and septic shock in which cytokines such as tumour necrosis factor
(TNF) are key mediators. Also compounds of the invention may suppress
inflammation and pyrexia due to cytokines and are, therefore, useful in the
treatment of inflammation and cytokine-mediated chronic tissue
degeneration which occurs in diseases such as rheumatoid or osteo-
arthritis.
Over-production of cytoktnes such as TNF in bacterial, fungal or viral
infections or in diseases such as cancer, leads to cachexia and muscle
wasting. Compounds of the invention may ameliorate these symptoms
with a consequent enhancement of quality of life.
Compounds of the invention may also elevate cAMP in certain areas of
the brain and thereby counteract depression and memory impairment.
Compounds of the invention may suppress cell proliferation in certain
tumour cells and can be used, therefore, to prevent tumour growth and
invasion of normal tissues.
CA 02241093 1998-06-19
WO 97/23461 PCT/GB96/03~96
22
For the prophylaxis or treatment of disease the compounds according to
the invention may be administered as pharmaceutical compositions, and
according to a further aspect of the invention we provide a pharmaceutical
composition which comprises a compound of formula (1 ) together with one
or more pharmaceutically acceptable carriers, excipients or dlluents.
Pharmaceutical compositions according to the invention may take a form
suitable for oral, buccal, parenteral, nasal, topical or rectal
administration,
or a form suitable for administration by inhalation or insufflation.
For oral administration, the pharmaceutical compositions may take the
form of, for example, tablets, lozenges or capsules prepared by
conventional means with pharmaceutically acceptable excipients such as
binding agents (e.g. pregelatinised maize starch, polyvinylpyrrolidone or
hydroxypropyl methylcellulose); fillers {e.g. lactose, microcrystaliine
cellulose or calcium hydrogen phosphate); lubricants (e.g. magnesium
stearate, talc or silica); disintegrants (e.g. potato starch or sodium
glycollate); or wetting agents {e.g. sodium lauryl sulphate). The tablets
may be coated by methods well known in the art. Liquid preparations for
oral administration may take the form of, for example, solutions, syrups or
suspensions, or they may be presented as a dry product for constitution
with water or other suitable vehicle before use. Such liquid preparations
may be prepared by conventional means with pharmaceutically acceptable
additives such as suspending agents, emulsifying agents, non-aqueous
vehicles and preservatives. The preparations may also contain buffer
salts, flavouring, colouring and sweetening agents as appropriate.
Preparations for oral administration may be suitably formulated to give
controlled release of the active compound.
For buccal administration_the compositions may take the form of tablets or
lozenges formulated in conventional manner.
The compounds of formulae (1 ) and (2) may be formulated for parentera!
administration by injection e.g. by bolus injection or infusion. Formulations
for injection may be presented in unit dosage form, e.g. in glass ampoule
CA 02241093 1998-06-19
WO 97/23461 PCT/GB96/03196
23
or multi dose containers, e.g. glass vials. The compositions for injection
may take such forms as suspensions, solutions or emulsions in oily or
aqueous vehicles, and may contain formulatory agents such as
suspending, stabilising, preserving and/or dispersing agents.
Alternatively, the active ingredient may be in powder form for constitution
with a suitable vehicle, e.g. sterile pyrogen-free water, before use.
In addition to the formulations described above, the compounds of
formulae (1 ) and (2) may also be formulated as a depot preparation. Such
long acting formulations may be administered by implantation or by
intramuscular injection.
For nasal administration or administration by inhalation, the compounds
for use according to the present invention are conveniently delivered in the
form of an aerosol spray presentation for pressurised packs or a nebuliser,
with the use of suitable propellant, e.g. dichlorodifluoromethane,
trichlorofluoromethane, dichlorotetrafluoroethane, carbon dioxide or other
suitable gas or mixture of gases.
The compositions may, if desired, be presented in a pack or dispenser
device which may contain one or more unit dosage forms containing the
active ingredient. .The pack or dispensing device may be accompanied by
instructions for administration.
The quantity of a compound of the invention required for the prophylaxis or
treatment of a particular inflammatory condition will vary depending on the
compound chosen, and the condition of the patient to be treated. In
general, however, daily dosages may range from around 100ng/kg to
100mglkg e.g. around 0.01 mg/kg to 40mg/kg body weight for oral or
buccal administration, from around l0ng/kg to 50mglkg body weight for
parenteral administration and around 0.05mg to around 1000mg e.g.
around 0.5mg to around 1 OOOmg for nasal administration or administratino
by inhalation or insufflation.
The compounds according to the invention may be prepared by the
following processes. In the reactions described below it may be
CA 02241093 2005-02-08
24
necessary to protect reactive functional groups, for example hydroxy,
amino, thin, or carboxy groups, where these are desired in the final
product, to avoid their unwanted participation in the reactions.
Conventional protecting groups may be used in accordance with standard
5 practice [see, for example, Green, T.W. in "Protective Groups in Organic
Synthesis" John Wiley and Sons, 1981 J.
Thus according to a further aspect of the invention a compound of formula
(1 ) may be prepared by reaction of a corresponding intermediate
10 compound of formula (1 ) wherein R4 and/or R~ is a -(CH2)tArNHRb group
with an isocyanate Ar(Alk)mL2N=C=O or isothiocyanate
Ar(Alk)mL2N=C=S. The reaction may be performed in a solvent, for
example an organic solvent such as a halogenated hydrocarbon, e.g.
dichloromethane, at around -O~C to around ambient temperature.
In a variation of this process the starting intermediate amine of formula (1)
may first be treated with phosgene in the presence of a base, e.g. an
organic amine such as triethylamine, and subsequently reacted with an
amine Ar(Alk)mL2NHRb to yield the desired compound of formula (1)
20 wherein R4 and/or R5 contains a urea group. The reaction may be carried
out in an organic solvent such as a halogenated hydrocarbon, e.g
dichloromethane, at from around O~C to ambient temperature. .
The intermediate amines of formula (1 ) for use as starting materials in
these reactions are either described in International Patent Spec'~'tcation
Nos. W094114742 and W095/17386 or may be
obtained using the processes described therein
from known starting materials. The intermediate isocyanates,
isothiocyanates and amines also for use in these processes are readily
30 available known compounds or may be prepared from known starting
materials using methods analogous to those used for the preparation of
the known compounds.
N-oxides of compounds of formula (1 ) may be prepared for example by
oxidation of the corresponding nitrogen base using an oxidising agent
such as hydrogen peroxide in the presence of an acid such as acetic acid,
CA 02241093 1998-06-19
WO 97/23461 PCTlGB96/03196
at an elevated temperature, for example around 70oC to 80oC, or
alternatively by reaction with a peracid such as peracetic acid in a solvent,
e.g. dichloromethane, at ambient temperature.
5 Salts of compounds of formula (1 ) may be prepared by reaction of a
compound of formuta (i ) with an appropriate acid or base in a suitable
solvent or mixture of solvents e.g. an organic solvent such as an ether e.g.
diethylether, or an alcohol, e.g. ethanol using conventional procedures.
10 Where it is desired to obtain a particular enantiomer of a compound of
formula (1 ) this may be produced from a corresponding mixture of
enantiomers using any suitable conventional procedure for resolving
enantiomers.
15 Thus for example diastereomeric derivatives, e.g. salts, may be produced
by reaction of a mixture of enantiomers of formula (1 ) e.g. a racemate, and
an appropriate chiral compound, e.g. a chiral acid or base. Suitable chiral
acids include, for example, tartaric acid and other tartrates such as
dibenzoyl tartrates and ditoluoyl tartrates, sulphonates such as camphor
20 sulphonates, mandelic acid and other mandelates and phosphates such
as 1,1'-binaphthalene-2,2'-diyl hydrogen phosphate. The diastereomers
may then be separated by any convenient means, for example by
crystallisation and the desired enantiomer recovered, e.g. by treatment
with an acid or base in the instance where the diastereomer is a salt.
In another resolution process a racemate of formula (1 ) may be separated
using chiral High Perfomlance Liquid Chromatography.
Alternatively, a particular enantiomer may be obtained by using an
appropriate chiral intermediate in one of the processes described above.
Chiral intermediates may be obtained in particular by use of the
enantioselective process described in International Patent Specification
No. W095/1738fi.
The following Examples illustrate the invention, and describe the
preparation of the following compounds:
CA 02241093 1998-06-19
WO 9'7123461 PCT/GB96/03196
26
OCp
CH30
R
NHCONHS(O)2Ar
where Cp is cyclopentyl, R5 is 4-pyridyl and Ar is:
5
Example 1:
hydrochloride salt
Example 2:
l ~ cH3 , hydrochloride salt
Example 3:
C~ , hydrochloride salt
i 5 EXAMPLE 1
~$)~ N f4 ~1 (3~'yrctoAentyrloxy-4 methoxxphen~L)~ 2 (4 ~,rridy~eth~CLt
hoe yrtl-N'-( hens Ii sutphonvt) urea, hyrdrochloride
Benzenesulphonyltsocyanate (205p,1) was added dropwise to a solution of
4-ji -(R)-(3-cyclopentyloxy-4-methoxyphenyl)-2-(4-pyridyl)ethyl]aniline
(500mg prepared as described in lntemational Patent specification No.
W095/17386) in dichloromethane (10m1) under nitrogen at room
temperature. The mixture was stirred or 30min at this temperature and the
solvent then removed in va~uo to yield a yellow gum. Trituration with
ether furnished a pate yellow solid (the free base of the title corn op sand
CA 02241093 1998-06-19
WO 97123461 PCT/GB96/03196
27
which was disolved in dichloromethane (5m1) and treated with ethereal
HCI to afford a yellow solid. Subsequent recrystallisation of the solid from
methanol yielded the title compound (450mg) as an off white solid 8H
{CD30D) 1.40-1.90 (8H, br m), 3.64 {2H, d, ,~ 9Hz), 3.72 {3H, s), 4.34
(1 H, t, ~ 9Hz), 4.72 (1 H, m}, 6.75 (3H, d+d+s), 7.20 {4H, d+d}, 7.50-7.75
(3H, m), 7.72 (2H, d, J_ SHz), 8.00 (2H, d, ~ 8Hz), and 8.55 (2H, d, J_ 8Hz).
The following compounds were prepared in a similar manner to the
compound of Example 1:
EXAMPLE 2
~Rl-N-[4-.(1-i(3- 'yctopentyrloxy-4-methoxyrphenyrl~-~4-plrridyl)~ethyrl~-
phen~ll-N'-~(methylahenyrtsulphonlrl) urea hyrdrochloride
From the starting aniline (1.0g) used in Example 1 and p-
toluenesulphonylisocyanate (507mg) in dichloromethane (20m#) at 0-5~C
to yield the free base of the title compound (1.29g) as a white solid
(CDCl3} i 49-1.95 (8H, m), 2.4 (3H, s), 3.28 (2H, d, ~ 7.9Hz), 3.6-3.9 (1 H,
br s), 3.78 (3H, s), 4.11 {1 H, t, ~ 6.OHz), 4.65 (1 H, m), 6.62-6.78 (3H, m),
6.95 (2H, d, ~ 6.06Hz), 7.1 {2H, d, J_ 8.57Hz), 7.26 (4H, m), 7.79 (2H, d,.~
8.37Hz}, and 8.40 (3H, m). r~/~ (ESI} (MH+586, 100%).
The solid on treatment with ethereal HCI gave the title compound as an off
white solid. (Found: C, 62.04; H, 5.90; N, 6.39. C33H35N3o5s~ HCI.
H20 requires C, 61.91; H, 5.98, N, 6.56%) 8H {CD30D) 1.45-1.85 (8H,
m), 2.38 (3H, s), 3.6 (2H, d, ~ 6.OHz}, 3.7 (3H, s), 4.3 (1 H, m), 4.68 (1 H,
br
s), 6.68-6.8 (3H, m), 7.1-7.45 {6H, m), 7.7-7.9 (4H, m) and 8.52 {2H, br s).
EXAMPLE 3
,(R)~-N-I[4-~'f -i(3-Cyctopentlrloxyr-4-methoxyrphenyl}~-2-i(4-
pyrridyi)iethvlJ~-
phenyl]-N'-(4-chlorophenyrlsulphonyrl} urea. hydrochloride
From the starting aniline (1.0g} used in Example 1 and 4-
chlorobenzenesulphonylisocyanate (0.384m1) in dichloromethane (20m1) at
0-5~C to yield the free base of the title cam ound (1.49g) as a white solid,
8H {CDco3) 1.43-1.9 (8H, m), 3.3 (2H, d, ~ 7.82Hz), 3.77 (3H, s), 4.09 (1 H,
t, ,~ 7.75Hz, ,~' 7.96Hz), 4.69 (1 H, m), 5.59 (1 H, br s), 6.62-6.8 {3H, m),
CA 02241093 1998-06-19
WO 97/23461 PCT/GB96103196
28
6.95-7.1 (4H, m), 7.15-7.4 (4H, m), 7.88 (2H, d, ,~ 7.1 Hz), 8.36 (2H, d, ~
6.OHz) and 8.50 (1 H, s); rr z_ (ESI) (MHO- 505 100%)
The solid on treatment with ethereal HCI gave the title compound as a light
yellow solid (Found: C, 58.33, H, 5.17, N, 6.12. C32H32CIN3O5S. HCt.
0.75H20 requires C, 58.58, H, 5.30, N, 6.41 %) SH (CD30D) 1.42-1.8
(8H, m), 3.i2 (2H. d, ~ 6.OHz), 3.68 (3H, s), 4.3 (1 H, m), 4.67 (1 H, m),
6.72 (3H, m), 7.1-7.25 (4H, m), 7.45-7.55 (2H, m), 7.77 (2H, d, ~j 3.OHz),
7.94 (2H, d, ~ 8.7Hz) and 8.55 (2H, d, ~ 3.OHz); ~n/~ (ESI) (MH+ 606
100%.
The advantageous pharmacological properties of the compounds
according to the invention may be demonstrated in the following inin tritro
and inin vivo tests:
1. isolated Recombinant ~luman PDE IVA Enzyrme
A gene encoding human PDE iV has been cloned from human monocytes
(Livi, et al., y990, Molecular and Cellular Biology, ~Q, 267. Using similar
procedures we have ctoned human PDE 1V genes from a number of
sources including eosinophils, neutrophils, lymphocytes, monocytes, brain
and neuronal tissues. These genes have been transfected into yeast
using an inducible vector and various recombinant proteins have been
expressed which have the biochemical characteristics of PDE IV (Beavo
and Reifsnyder, 1990, TIPS, ~, 150). These recombinant enzymes,
particularly the human eosinophil recombinant PDE IVA, have been used
as the basis of a screen for potent, selective PDE IV inhibitors.
The enzymes were purified to isoenzyme homogeneity using standard
chromatographic techniques.
Phosphodiesterase activity was assayed as follows. The reaction was
conducted in 150,1 of standard mixture containing (final concentrations):
50mM 2-[[tris(hydroxymethyl)methyl]amino]-1-ethanesulphonic acid (TES)
-NaOH buffer (pH 7.5), lOmM MgCl2, 0.1 p.M [3H]-cAMP and vehicle or
various concentrations of the test compounds. The reaction was initiated
by addition of enzyme and conducted at 30oC for between 5 to 30 min.
The reaction was terminated by addition of 50p,1 2% trifluoroacetic acid
CA 02241093 1998-06-19
WO 97/23461 PCT/GB96/03196
29
containing [14C]-5'AMP for determining recovery of the product. An aliquot
of the sample was then applied to a column of neutral alumina and the
(3H]-cAMP eluted with 10m1 0.1 TES-NaOH buffer (pH8). The [3H]-5'-AMP
product was eluted with 2m1 2M NaOH into a scintillation viat containing
10m1 of scintillation cocktail. Recovery of [3H]-5'AMP was determined
using the [14C]-5'AMP and all assays were conducted in the linear range of
the reaction. Results were expressed as ICSa values.
Using this procedure, the compounds according to the invention had ICSo
values of 4nM (compound of Example 1 ), 13.9nM (compound of Example
2) and l7.finM (compound of Example 3).
The compounds of the Examples had little or no activity against other
isolated PDE isoenzymes (specifically PDE l, 11, III or V - see WO
94/14742 for experimental details) at concentrations up to 100wM, thus
illustrating the selectivity of their action against PDE IV.
2. at Hepatocyrte Metabolism
The improved metabolic stability of the compounds according to the
invention was demonstrated in a conventional rat hepatocyte model in
which rat hepatocytes were cultured in the presence of test compound.
The quantity of compound remaining after a fixed period of time was then
detem~ined using mass spectroscopy.
Thus in one such test the compound of Example 1 was compared with a
reiated compound particularly described in International Patent
Specification No. W094/14742 in which the phenylsulphonyl group in the
compound of Example 1 is replaced by a hydrogen atom. After 3h the
percentage of each compound remaining was:
Compound of Example 1 - 56%
' WO 94/14732 comparison compound - 3%.
The W094/14742 compound had been extensively metabolised whereas
over 50% of the compound of the invention remained after 3h, illustrating
the advantageous in vitro metabolic stability of the compound.
CA 02241093 1998-06-19
WO 97/23461 PCT/GB96/03196
The same two compounds were also administered orally to squirrel
monkeys at l0mg/kg p.o,. and the plasma levels of each determined.
After 1 h the W094/14732 compound was present at 1 p.glml whereas after
5 30min the compound of Example 1 was present at 341ug/ml, thus ,
illustrating the good oral availability of the compound.
r