Note: Descriptions are shown in the official language in which they were submitted.
CA 02241320 1998-06-24
P~T/~ U ;i 2 3
IPE~ Q 6 AUG 1997
A METHOD FOR REDUCTION OF SE~ ECTED ION INTENSITIES IN
SCONFINED ION BEAMS
FIELD OF THE INVENTION
The present invention relates generally to a method for producing an ion
10 beam having an increased plupollion of analyte ions con~pa,~,d to carrier gas ions.
More specifically, the method has steps resulting in selectively neutralizing carrier
gas ions. Yet more s~cirlcally, the method has the step of ~dAitic!n of a charge~la~l gas to the carrier analyte combination that accepts charge from the carrier
gas ions yet minimqlly accepts charge from the analyte thereby sel~li~ly
15 neutralizing the carrier gas ions.
BACKGROUND OF THE INVENTION
Many analytical or i~hl~hi~l processes require the generation of beams of
20 ions of particular ~b~t~nces or analytes. For e~ le, ion beams are used in ion
guns, ion ill pl~t~ ,~, ion thrusters for attitude control of satellites, laser ablation
plumes, and various mass ~holl-et~l~ (MS), inrlu~Aing linear quadmpole MS,
ion trap quadrupole MS, ion cyclotron I~SOn~'~r~ MS, time of flight MS, and
electric and/or magnPtil~ sector MS. Scveral s k~ are known in the art for
25 genelaling such ion beams in~ nAing el~holl impact, laser irradiation, io Lst,lay,
ele~;hv~ldy, th~mospray, ~ ,ly coupled plasma sources, glow dischalges
and hollow cathode discha,ges. Typical arlA~ colllbi~ the analyte with a
carrier or support gas w~ elJy the carrier gas is utilized to aid in h~olling,
ionizing, or both hA~lh~g and ioni7ing, the analyte.
For example, in a typical al,A~&.-.. ~ an analyte is coll~biLx:l with the
carrier gas in an elc~hical field, ~I~.~ poll the analyte and the carrier gas are
ionized in a strong electric or magn~tic field and later used in an analytical or
industrial process. In another typical allA~e~ the carrier gas is first ionized
~IE~ S~
CA 02241320 1998-06-24
PCT/US 9 7 / O ~ 3 2 3
IPE~ O6 AU~i1997
in a strong electric or magnetic field whereupon the analyte is then introduced into
the ionized carrier gas. Electric fields are ge~ ted by a variety of m~thodc well
known in the art including, but not limited to, capacilive and inductive coupling.
In an inductive coupling arrangement, a radio frequency (RF) voltage is
5 applied to a coil of a con~lucting material, typically brass. In the interior of the
coil, one or more tubes supply a carrier gas, such as argon, and an analyte, which
may be any substance. The analyte may be supplied in a variety of forms
inrlulling but not limited to a gaseous form, as a liquid, as a droplet form as in an
aerosol, or as a laser ablated aerosol. A large el~;~.ical field is ge~.at~d within
10 the coil. Within this field, any free el~llons will initiate a chain reaction in the
analyte and the carrier gas causing a loss of el~llons and thus ioni7~tion of the
carrier gas and the analyte. Several ...- Ih~c well known in the art, inrhl~ing but
not limited to the introduction of a Tesla coil, the introduction of a g~ rod,
or thermal emission of ele~lons, will provide free el~l,ons causing initiation of a
15 chain ~ io" The result is a weakly ioni_cd gas or plasrna con~; ~;ng of both
free cle~LIons and charged and uncha~g~ species of the carrier gas and the
analyte. The species of both the carrier gas and the analyte in the plasrna may be
- in the form of particles, atoms or molx~ s, or a ll~ e of particles, atorns and
molecules, :le~ ~ing on the particular species s~l~t~l for use as the carrier gas
20 and analyte.
The carrier gas and the analyte may be combined by a wide variety of
methods well known in the art. For example, as described above, the analyte and
the carrier gas in an aerosol form are coll,~ii~d and are then dh~x~l to the
interior of a coil in an inductively coupled plasma. Another typical a~,~nge~elll
25 employs a needle which l~Ci~,~,S a liquid sample of analyte from a source such as
a liquid chrollla~ograph. Su~ u~iing thc needle is a tube which supplies a carrier
gas such as argon as a high velocity atomi7.ing carrier gas. Both the needle andthe tube empty into a chall,bcr. Upon disch~ge from the needle, the analyte
liquid is evaporated and atomi_ed in the argon carrier gas. Ions of both the
30 evaporated liquid analyte and the argon carrier gas are produced by cleaLing an
CA 02241320 1998-06-24
~CT/US ~ - f ~ V ~ 2 3
IPE~J O 6 hl~G 1997
- 3 -
electric field within the chamber. The electric field may be produced by creating
a voltage dirr.,le.lce between the needle and the chamber. A voltage dirr~lel~cemay be created by applying a voltage to the needle and grounding the chamber.
The resultant plasma genel~ted by any of the foregoing methods is typically
5 directed towards either an analytical appal~lus or towards a reaction zone wheleill
the carrier gas and analyte ions are analyzed or otherwise reacted or utilized in
some fashion. The resultant plasma is typically directed by means of an electricor m~gn~tir field, or by means of a plcs~ule dirr~elllial~ or both. As the plasma
is directed, the plasma is converted from a plasma to an ion beam. As used
10 herein, the term "ion beam" re&rs to a stream co~i~li..g primarily of positively
charged and neutral species. The bulk of the ~eg~ ly charged species in the
plasma are typically electrons, which are rapidly di~ ed as the plasma is
directed by either electric or magnPtic fields or by a pleS~ dirr~lenlial.
However, even after sigl-iri~ di,~ dl of the ion beam, the ion beam may not
15 be co-l,lctcly void of negatively charged species. As the plasma progresses
folw~d, the free clecllulls, due to their low mass relative to the po~ ely charged
ions, tend to disperse from the plasma, thus CG~ g the plasma to an ion beam.
Also, the ion beam itself will tend to dis~.~ due to several effects. Most
plUIIIi~l~lll among these effects is the repulsive forces of ch~,~ species within the
ion beam. The beam is also dis~l~ through free jet eAl~n~ion. The effect of
dispersion of the con~tituent species in the ion beam is charge separation amongthose species and is well known in the art. The reSlllt~nt ion beam is thus typically
chal~t~ ed by high net positive charge density which is primarily alllibulable to
the relatively high abu~ re of posilivcly charged carrier gas ions.
In many applications, the ab~ln~l~nre of positively charged carrier gas ions
and/or the resultant high charge density may be ullde6irdble. For e~ le, it is
often desildble that the ion beam be focused through a small apellure, for
example, if the analyte ions were to be analyzed in a mass ~ ter. In such
an arrang~ll.ent, where the ion beam is dh~ted through an apcllul~, the high
charge density will p,esclibe a space charge limit to the amount of the ion beam
CA 02241320 1998-06-24
7~6 hU~(i319~97
that may be passed through a given apcllulc. When the space charge lirnit is
reached, the rernqin~r of the beam is unable to pass through the a~l~w~ and is
thus lost. In many applications, the portion of the beam which is lost includes
analyte ions. Indeed, a loss of a portion of the beam may result in a
S disproportionate loss of some or all of the analyte ions because the analyte ions
may not be evenly disllilwLed throughout the ion beam or may not respond to the
various dis~rsil g forces in the same manner as the carrier gas ions.
Another example where the ~r~scnce of carrier gas ions is unde.,ildblc is in
an ion trap mass s~ ,Llul.l~,t~,. where the ion trap has a limited ion storage
10 capacily. In an ion beam dil~t~d at an ion trap, the carrier gas ions cull.~L~. with
analyte ions for the limited storage capacity of the ion trap. Thus, to the extent
that carrier gas ions can be sele~Li~ eliminated from the ion beam, the storage
capacity for analyte ions~ in the ion trap is thereby IllCl~dSOd.
A third example where the pl~ ce of carrier gas ions is undesirable is any
15 application where the analyte ions are to be used in a process or l.,a_Lion where
the carrier gas ions might interfere with such process. By way of further example,
in many integratcd circuit and chip "~ -r;~ g plocesses, ion bcams may be
direct~d tow~ds a targeted material such as a silicon wafer to impart elecl,ical or
physical p.~pe.lies to the material. The desired prope.lics are typically highly20 ~ fk..L on thc specific ions di.~xt~ at such materials. Thus, carrier gas ions
may cause ul~desilablc effects in the ~2ct~ materials.
Thus, in an ion beam having a carrier gas and an analyte, there exists a need
for a method of selectively el;...;n ~ing carrier gas ions without eli...;n~;ng or
neutralizing the analyte ions.
SUMMARY OF THE INVENTION
Accordingly, it is an object of the invention in one of its aspects to provide amethod for producing an ion beam with i~ eased propollion of analyte ions and a
30 co~res~nding dc~ scd number of carrier gas ions by neutralizing carrier gas
CA 02241320 1998-06-24
~CT/US 9 ' / o o o 2 3
UEAI~ O 6 AUG 1997
- 5 -
ions while minim~lly removing or neutralizing the analyte ions. This is
accomplished by providing the ion beam at a desired kinetic energy and dil~ling
the ion beam through a volume of a reagent gas thereby allowing the carrier gas to
selectively ~ rer charge to the reagent gas rendering the reagent gas a charged
5 species and the carrier gas a neutral species. As used herein, "selectively" means
that the transfer of charge from the carrier gas ions to the reagent gas proceeds at
a rate at least ten times, and preferably over one thousand times, the rate of the
transfer of charge from the analyte gas ions to the reagent gas. After this charge
lr~f~,l, the charged reagent gas is then sclecli~ly di~l~d, leaving an ion beam
10 having a greater fraction of analyte ions to total ions. As used hcrein, charge
l-~L,r~,. refers to any pa~ a~ wh~.~m thc net effect is that charge is exc~
bet..xn a charged species and a neutral species. The pathway may involve step
which are not charge L,ar~r~,l reactions. Steps within the pàlll~.a~ may include but
are not limited to rh~olnir~l reaction(s), alone or in series, such as ,~so~t charge
15 ~.~r~,l(s), cl~n llal~Ç~l, proton ll~f,l, and Auger neutralization. As used
herein, analyte ions refers to any ions gen~ldt~d by any means in-~lu-lir~ but not
limited to thermal ionization, ion beams, el~l-~n impact ionization, laser
~- irradiation, ions~ y, cle.,lr~ o~l~, inductively coupled pl~"~c,
microwave plas~ds, glow discharges, arc/spark dischalE,~s and hollow cathode
20 discharges. As used herein, reagent gas refers to any gas suitable for ar~t;.~g
charge ll~Ç,l provided by any means i~ li~ but not limited to coll.l...,lcially
available ~vb~ s provided in gaseous form and ~lules thereof and gases
generated by evaporation of con~ nced ~ es or laser ablation of con~
substances. Further, reagent gas as used herein may include neutral species of
25 analyte ions gel~l~t_d by any of the folegohlg ~ sdc. Also, as will be appa,~to those skilled in the art, the method of the present invention is not limited to
,llls co.~ g a carrier gas per se. Typically, the two gas species are an
analyte and a carrier gas. However, the method of the present invention will work
equally well in any system having two or more ion speciPs, even if none of the
30 species were provided as a carrier gas. For example, in applications where
~E~ ~
CA 02241320 1998-06-24
~US ~ ~ 0 3 ~ 2 3
IPE~ 06 AU~1997
- 6 -
ghter ions gen~ tcd by tble dissociation of any charged species are undesirable,suitable reagents may be selected to remove or neutralize those ~ gb-- ions by
charge ~ fer. Similarly, a particular analyte may contain a substance of interest
in mixture with a separate inte.r~.ing subst~n~e. Suitable leage.l~ may be se!ected
S to remove or neutralize the s~l~araLc hltc.~ g ~lb~l~,~e by charge ~ r~,r.
In a plcr~ d embodiment of the invention, the carrier gas selected is argon
and the reagent gas selected is hydrogen. Accoldi~gly, it is an object of the
invention in one of its aspects to provide a method for selectively ~educillg the
charge density of an ion beam by neutralizing the ions of an argon carrier gas,
10 without çl;...in~;n~ or neutralizing the analyte ions. This is accolllJlished by
dhe~,~ing the ion beam Lllro~gh a volume of hydrogen at kinetic ene.gies ..L.,.cm
the argon ions selectively ll~r~r charge to the L~dro6en. In this manner, it is
theorized that the bulk of the ion beam is sel~li~ely shifted from a mass to charge
ratio (m/z) of 40 (Ar+) to m/z 3 (H3+) and m/z 2 (H2+). It is Il~ .,fol., a
15 further object of the invention in one of its aspects to provide a method allowing
the sele~ c transfer of charge from Ar+ to H2. Due to l~dlo~n's lower
molecular weight, in many applications it is possible to rapidly and selectively- eject H3+ and H2+ from an ion beam without eje.,~ing analyte ions where it
would have been ~liffi~l-lt or hJI~ssible to sele~ ,ly eject Ar+ ions from the ion
20 beam without also eje~ g or removing analyte ions. Thus, it is ll-cl~r~, a
further object of the invention in one of its aspects to provide a method for rapidly
eje~ling H3+ and H2+ from an ion beam, yet minim~lly re(hlcing or ejcc~ g
analyte ions.
Thus, it is a further object of the invention in one of its aspects to provide a25 method for providing a beam selectively d~pleted in Ar+, and thel~rol~ having a
much lower total ion density, yet minim~lly reduced ion density of analyte.
The subject matter of the present il.~e.-tion is particularly pointed out and
distinctly cl~im~d in the conrluding portion of this specffication. However, both
the orga~ ion and method of operation, together with further advantages and
30 objects thereof, may best be u~del~lood by ~fc.e~e to the following de~li~)tion
~r ~
CA 02241320 1998-06-24
PN/tJS ~ / 0 ~ 0 2 3
!P~ G 1997
taken in connection with accompanying drawings wherein like reference charactersrefer to like elemPntc.
BRIEF DESCRIPIION OF THE DRAWINGS
FIG. 1 is a sch~nnArir drawing of the apparatus used in the first plcfcl~d
embodiment of the present invention.
FIG. 2 contains two mass spectra from e~ elll~ p~ .rollllcd in the ap~ us
used in the first p,~ f .,ed embodiment of the present i.l~enl,on.
10 FIG. 3 is a s~ ic drawing of the app~atlls used in the second pl.f~ d
e.llbo~l;...- -n of the present invention.
FIG. 4 is a scl-f-..At;~ drawing of the _ppaldlus used in the third p-~fe.,~
embodiment of the present invention.
FIG. 5 contains two mass spectra from e~ ~ellts pe.rollllcd in the dppallllUS
15 used in the third 1"~ f~ .nho~ of the present invendon.
FIG. 6 co,l~ins two mass spectra from e~.~nls ~lro""ed in the ap~,~dlus
used in the third pl. f~ .lcd e-nho~ n of the present invention.
~- FIG. 7 is a schematic dlawil)g of the apparatus used in the fourth p~fe"~d
embodiment of the present invention.
DESCRIPTION OF THE PREFERRED EMBODIMENT(S)
The method of lr~r. .ling charge from s~ec~xl ions in an ion beam having
more than one species of ions to a reagent gas and thereafter pl._fe.e.lLially
25 di~ g the charged reagent gas was demol~tlat~d in inductively coupled plasma
mass s~~ te.~ ..art~. called ICP/MS). An ICP/MS is a device wh~ a
plasma co~ g of a carrier gas (typically argon) and an analyte is generated in
an inductively coupled plasma (ICP) and a mass s~ u,,,~t, . is employed to
Sepa~alt~ and di~ ;uish con~ atoms and isotopes. For both co~ ience of
30 operation and to m~int~in a desirable temperature in the plasma, the ICP is
CA 02241320 1998-06-24
~CT/IIS ~ ~ / O ~ ~ 2 3
IP~ 6 Al~G 1997
typically operated at atmospheric p~s~ur~. In order to transfer ions from the
plasma to a mass s~llo~lRter, the plasma is directed through two a~.lul~,s and
then through a lens stack. The plasma is thereby converted into an ion beam
cont~ining analyte ions and carrier gas ions. A lens stack typically consists of a
5 series of metal pieces, typically plates and/or cylindrical tubes which have
potentials applied to them and which have apclluies through which the ion beam is
directed. The ion beam is dil~.,t~ through these charged plates which focus the
ion beam into a narrow stream which is directed to a ion discrimin~ing unit,
typically a linear quadrupole. As used herein, ion discrimin~ti~ unit refers to
10 any ~)pala~us which se~,a,dt~,s charged species accol~g to their m/z and/or
kinetic energy. Ion discl.ll~ating units include but are not limited to a linear4ua~ole, an ion trap, a time-of-flight tube, a maenPti~ sector, an electric sector,
a combination of a ma~tir sector and an electric sector, a lens stack, a DC
voltage plate, an rf/dc multipole ion guide, and an rf multipole ion guide.
15 Modified ICP/MS systems have been built which use a three ~ cionql RF
quadrupole ion trap, either alone or in combi~tion with a linear RF ~lua~pole asan ion dis~ ating unit. Upon exiting the lens stack, the ion beam is dil~;t~
~- into the ion di~fl-.~ e unit. Ions are scl~li~ly emitted from the ion
discrimin~i~ unit according to their mass to charge ratio (m/z) and/or kinetic
20 energy. These selectively emitted ions are then dil~ct~d to a ch~g~i particle~etec~or. In this manner, the ICP/MS is able to ~le~ the pl~se~e of select~
ions in an analyte according to their (m/z) and/or kinetic energy. It is critical to
mq-intqin the ion discli...i.~lin~ unit in a V~;uulll because collisions or reactions
between the ions and any gases present in the ion discl;.~ g unit will tend to
25 deflect ions away from the charged particle detector or neutralize the ions of
analyte. It is critical to mqintqin the chal~ particle d&t~lor in a ~CUU1l~
because the high potential across the detector will cause an el~l,icdl di~ch&~ in
any gas present in sufficient pressure, typically above 104 Torr. One or more
pumps are thus typically utilized to evacuate a series of ch~mbers in ~t~.~n the30 ICP and the charged particle d-,t~lor. The chambers are scp~dt~d by one or
CA 02241320 1998-06-24
~C~US ~ ~ ~ r,~ 3
lPE~ O 6 AllG 1997
more a~.lules to achieve the transition from atmospheric pr~s~ure at the ICP to
high vacuum at the charged particle detector (typically between about 10-7 and 10-
4 torr). To effect the large dirf~ lial in plCS~ul~" ICPIMS systems typically
employ apc~ s between approximately 0.5 mm to approximately 2 mm.
In operation, the reagent gas is introduced within an ion beam having a
carrier gas and an analyte to allow the charge of the carrier gas ions to be
transferred to the reagent gas, wl~..,u~ll the now charged reagent gas may be
-- selectively di~l~d from the ion beam. The extent of reaction or compl~t~--fc.c
of this charge Ll~rcl will be driven by at least four factors. First, any two
10 species s~lecte ;1 will have an i~lel rate of reaction which will affect the
compll t~ ~ss of charge ll~f~r over a ghen period of time, all other things heldconstant. Second, lower velocities of the carrier gas ions will provide a longerresidence time for carrier gas ions in the reaction zone and thereby provide a
greater extent of reaction. Third, there is a velocity depen~len~ for the reaction
cross section which is in general dirr.,.c;~ for any given l~eacling species so tha
for any given reaction the OptilllU L~ veloc;l~l~ may be low or high. Thus, the
compl~ t~"nfSS of charge l~f~-~ in a given time period is incl~d as the
probability of a collision ~h.~n carrier gas ions and reagent gas species is
,ased. Thc~fole, the co~ ,lPt~ s of charge ~r~. is dcpf.~..~1 upon the
20 prcs;.~u~, of the reagent gas and the time that the two gases are in contact. If the
reagent gas species is present at low concc.~ ,lion or pll,S;~ " the carrier gas ions
must have s ~rl;~i- .1 oppollul~i~s to come into contact with the reagcnt gas, i.e., a
long l~ r~ time must be employed.
As will be ap~ to those skilled in the art, although the present ill~ lion
25 has been desclil,cd as employed in an ICP/MS, the method of the present
invention may be adv~nt~ol~cly applied in any system having a carrier gas and ananalyte gas where it is desired to remove or neutralize the carrier gas ions. The
ICP/MS system, as well as the ~hulllents de~libcd in the p~f~ ,d embo~l;...P-
~
which follow, both practice and are demo~llali~ of the present invention bccause
~AE~ S~
CA 02241320 1998-06-24
PCT/IIS ~ ' ~ O ~ 0 2 3
IPEA/~ O 6 AUG 1997
- 10 -
they contain detection methods to verify the selective neutralization or removal of
carrier gas ions.
THE FIRST PREFERRED EMBODIMENT
In a first p~cfell~d embodiment shown in ~G. 1, a conventional ICP/MS
mqn-lfa~hlred by VG Flem~nt-q-l, now Fisons (Winsford, Cheshire, F.nglqn l; model
PQ-I) was modified by l~lac~g the linear quadrupole and its associated
eleckonics (not shown) with n RF 4ua.11u~Jole ion trap 10 and its qes~iqt~d
10 electronics (not shown). The ion trap 10 was inet~qllPd with the ion input and
output ends reversed to maximize the ion L~f~.r ~rr~-~iC n~ from the lens stack 60
into the ion trap 10. The ion trap 10 used was removed from an ion trap mass
S~;IIUI11C~I mqmlf~ctllred by Finnigan MAT (San Jo~, California). The CI~IIOn
gun (not shown) and injection gate electrode as~mbly (not shown) were removed
15 to allow lla~Çe~ of ions from the lens stack 60 into the ion trap 10. The ~aCUUlll
system was m~ifi~ from a standard Fisons vacuum system and consisted of three
vacuum regions separated by two d~.lules. The~ vacuum regions are evacuated
- by ~ti~ vacuum pumps (not shown). The first va UUIll region 15 is co.. ~i.~l
in between a first a~ ule 20 and ~cond a~.lul~ 30 and is typically opc._~ed at
0.1 to 10 Torr. The second vacuum region 25 is cc~ en the second
a~x.lule 30 and a third ape.l~ 40 and is typically opelat~ at 10-5 to 10-3 Torr.The third apellule 40 is located within the lens stack 60 at ~llb~ lly the same
position as employed in the ~da.d Fisons ICP/MS. The third vacuum region 35
is ~p&l~t~ from the second vacuum region 25 by the third ape,lule 40. The third
vacuum region 35 contains a portion of the lens stack 60, the ion trap 10 and a
charged pallicle detector 50. The third vacuum region 35 is typically O~la~d at
10-8 to 10-3 Torr.
~E~
CA 02241320 1998-06-24
~ S ~ J ~J 2
P~A/~JO6 AU~1997
EXPERIMENT 1
A series of e~ye~ le.ll~ was pclrolnlcd utili7ing the apparatus described in
the first plefe..~ embodiment. The configuration of the various components is
shown in ~IG. 1. The vacuu_ regions 15,25,3S were O~.dt~ under
conventional conditions as desc~il~ above. The potentials applied to the lens
stack 60 were within the ranges reco.. P~YIP~l by the m~nllf~ rer of the ICP/MS
(Fisons). The first and second ape.lulc~ 20,30 were both grounded. The third
apellule 40 was biased at a DC potential of about -120 V. The potentials on the
10 lens stack plates 70,80 were opth.,i~ for ~..~ a~r~. errlcie~ of ions
into the ion trap 10 and were di~.."lt than the potentials used in con~.,lional
ICP/MS ic~ e.lt~. Ions are gated into the ion trap 10 by switching the po~tial
on plate 80 in the lens stack 60. The pbt~,~lials on plate 80, de~lil~d as lens
element L3 by the ,.U..~ra~ el (Fisons), were ~ hed ~h.~en a ~ati~e value
15 used to adrnit ions into the ion trap 10, in the range ~h.~n about -10 V to about
-500 V, prefelably -35 V, and a positive value used to prevent ions from ~.ltl ~ ;i~g
the ion trap 10, in the range bet~.~n about +10 V to about +500 V, pl~,f~.ably
~- above +10 V, or the kinetic energy of the ions. The ele.,llol~ie gating control (not
shown) used for ~.i~hing the voltage on plate 80 was provided by h.~e.lhlg the
20 s~ddl~ signal provided by the ~h ~ gan MAT ITMS to gate elccllo~s. This
inversion was accoll.plished using an extra hl~e.~r (not shown) on the printed
circuit board (not shown) that pe.ru~ s the gating.
The ion trap 10 is rnanufactured with a port 90 typically used for
introduction of a buffer gas such as helium. Reagent gases were introduced into
25 the ion trap 10 by adding the reagent gases to the helium. Typical helium buffer
gas pres~ .,s were in the range ~h.~n about 10-5 and 10-3 Torr. Reagent to
buffer gas pl~,s~.l.e ratios ranged ~t~ .. about 0.01% to 100~. EA~Ii-le~t~
were performed in this h~ cn~ wL~,.eil~ Ar, H2, Xe, or Kr were introduced as
reagent gases into the ion trap 10.
CA 02241320 1998-06-24
~/US 9 ~ / ~ O 0 2 i3
IPEAll~S O 6 AUG 1997
- 12 -
The effect of these reagent gases on the analyte and ion signals were
observed by recording the ion trap mass ~c~ l. Repl~,se.lLali~e mass spectra
showing the effects of added H2 are shown in ~IG. 2. The upper trace 100 in
FIG. 2 was obtained using pure helium buffer gas and is offset from ~ro for the
sake of clarity in FIG. 2. The lower trace 110 in F~G. 2 was obtained using
about 5% H2 and about 95% helium. The upper trace 100 shows the inle~ily of
various peaks, most notably, H20+ at m/z 1~ 102, H30+ at m/z 19 104, Ar+ at
m/z 40106, ArH+ at m/z 41 108. With the addition of H2 as a reagent gas,
Ar+, H20+, ArH+, and H30+ are ~ ~-ir~lly reduced as indicated by the
10 reduction of peak i.~ n~;l ;es at the a~pl~liate mtz in the lower trace 110,
in.1i~ the near or total elimination of these ch~,~ species.
In addition to the elimination of these charged species, one must also be
co~llled with the effect of any added reagent gases on the analyte ions. The
following CIP~ ; were tèsted as analyte ions for reaction with H2 in the
lS appd~alus of the first plefe,lcd embodiment as desc~il~ above using argon as
carrier gas: Mg, Al, K, Ca, Sc, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, Zn, Ga, As, Se,
Rb, Sr, Ag, Cd, In, Xe, Cs, Ba, Tl, Pb, Bi, and U. In all of the eA~ c~t~, the
red~cti-n in Ar+ il~te~ily was at least 100,000 times greater than the reduction in
any of the ;.-r~-. l;Ps of those analyte ions.
THE SECOND PREFERRED EMBODIMENT
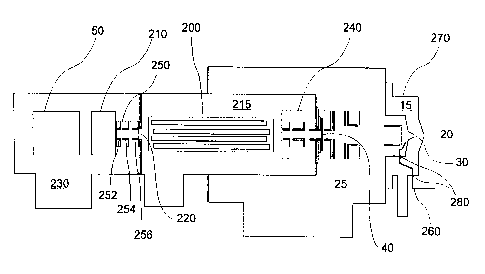
In a second ~lefe~l~d embodiment as shown in ~IG. 3, a con~_~tional
ICPtMS m~nllfactllred by VG Fl~mPnt~l, now Fisons (Winsford, Cheshire,
25 F.l~l~rl-~ model PQ-I) was m~ifiP~ by i~.~osing an RF quadmpole ion trap 210
~t~.~n the linear quadrupole 200 and the charged particle detector SO. ~ltho.lgh,
the electrodes (not shown) used in the ion trap 210 were custom built to be scaled
versions of the IWS electrodes ~ J I~ Jred by Finnigan MAT (San Jose,
California), standard ion trap electrodes would work equally well. The electrodes
30 of the custom built ion trap 210 were 44% larger than the electrodes of the
~E~ SH~
CA 02241320 1998-06-24
~/Us97/Qo02 3
IPEAIUS O 6 AUG t997
Finnigan MAT ITMS and were assembled in a pure quadrupole, or un~ ,t~hed
geometry. The standard ITMS elu;llonics package (not shown) mqnllfa~tllred by
Finnigan MAT was used with the mo~ifi~vqtions as described in the first plefell~d
embo~im~nt using the voltages as desclibcd below.
The ~nd~d lens stack 240 is operated at potentials reco~ .ended by the
mqnllfa~ rer. In addition to the standard lens stack 240, a second lens stack 250 is
interpo~d ~h.een the third apellw~ 220 and the ion trap 210 in the fourth
vacuum region 230. The second lens stack 250 consisted of three plates
252,254,256 taken from ~l~d~-i Fisons lens stacks, specifically two L3 plates and
an L4 plate. The second lens stack 250 was fabricated to provide high ion
transport err~ .~n the linear quadrupole 200 and the ion trap 210. A
potential of ~h..xn about -10 V and about -300 V, pl~fe.lably about -30 V were
applied to plates 252 ~r~ at each end of the second lens stack 250. The center
plate 254 was used to gate ions into the ion trap 210 and the potc.llial applied was
15 varied ~eh.~n about -180 V for the open potential and about +180 volts for the
closed potential. The ele~;L,on~c gating control (not shown) used for the centerplate 254 of the second lens stack 250 was provided by inverting the s~d
'- signal provided by the Finnigan MAT ITMS to gate ele~;L.ol~s. This hl~ ion was
acco,.lplislled using an extra h~ (not shown) on the printed circuit board (not
20 shown) that p-,.Ç~lll.s the gating.
The vacuum system was the ~ Fisons system co~is~ing of four
vacuum regions separated by three ape.lul~s with an additional pump on the fourth
vacuum region 230. These vacuum regions are evacuated by standard ~ ;UW~l
pumps (not shown). The first vacuum region 15 is col~ ed in ~h.een a first
apcl~ul~; 20 and second a~.lul~ 30 and is typically operated at 0.1 to 10 Torr.
The second Va~;UWll region 25 is contained ~h..,en the second a~.t~e 30 and a
third apellul~, 40 and is typically operated at 10-5 to 10-3 Torr. The third
aperture 40 is located within the lens stack 240. The third vacuum region 215 is
contained ~h.~n the third a~.lule 40 and the fourth aperturc 220 and is
30 typically ope.dt~ at 10-8 to 10-4 Torr. The third vacuum region 215 contains the
CA 02241320 1998-06-24
~CT/IIS~ ~ / OO02 3
IP~AIU~ O 6 A.JG 1997
- 14 -
linear quadrupole 200. The fourth vacuum region 230 is separated from the third
vacuum region 215 by the fourth apellu~ 220. The fourth vacuum region 230
contains the ion trap 210 and a charged particle ~le~;lor 50. The fourth vacuum
region 230 is typically operated at 10-8 to 10-3 Torr.
S As illustrated in FIG. 3, a 1/16" ~i~m~ter metal tube 260 was provided toallow the introduction of reagent gases into the second vacuum region 25 throughtwo ports 280 provided in the housing 270 ~lvu~dillg the first vacuum region 15.~ The tube 260 was fashioned into a shape so as to avoid electrical contact with the
lens stack 240 and to position the end of the tube 260 approximately 1 cm behindthe base of the second ape.nl.~, 30 and appro~ 1 cm from the central axis
defined by the four dpC~ S 20,30,40,220. In this way, reagent gases are
introduced into the second vacuum region 2S as close to the second a~.t~ 30 as
possible without i,ltelre~g with the gas dynamics of the s~p'~~ plasma and with
minimql distortion of the electric field ge~lat~d by the lens stack 240.
EXPERIMENT 2
~--- A series of eA~ .,nls was pe.rO,."ed u~ ing various reagent gases and an
argon carrier gas in the above ~ d apparatus shown in ~IG, 3. Reagent
gases, H2, Ar, Xe, Kr and an Ar/Xe/Kr ~iAlul~, were i~ ~ced vh tube 260
into the second vacuum region 25. Mass spectra were obtained for reagent gas
partial ple~;,~.,s in vacuum region 25 ~t~.een zero and about 1 mTorr to about 10
mTorr. Table I lists relative Mtes of reaction for the carrier gas and analyte ions
shown in the first column with i~leashlg p~s~e of the reagent gases listed at
the top of the remqining columns. Thus, by way of example, the values in the
second column under the h~A~ H2" show that as the H2 pl_~e is i~.~,
the Ar+ ion intensity falls about 10 times faster than the In+ ion intensity,
co.~r~ g the selective removal of carrier gas ions.
CA 02241320 1998-06-24
~CT/ls~l/Qoo23
I?L~ G ~ Y9~'
Table I. Relative Reaction Rates of Carrier Gas Ions and Analyte Ions with
Reagent Gases
Ions ¦ H2 Ar¦ Ar/Xe/Kr
Ar+ 0.1 0.6 --
ArH + non-linear 0.35 0.25
Sc+ 0.017 0.23 0. 18
~4Kr+ 0.06 -- 0.26
, 115In+ 0.01 0.24 0.14
lZ~xe+ 0.01 -- 0.15
THE THIRD PREFERRED EMBODIMENT
In a third plcr~ d e.llboiil.l.,n~ shown in FIG. 4, a conventional ICP/MS
15 miqmlfs~ lred by VG F.lPmPntql, now Fisons (Winsford, Cheshire, F.r~g]qn l model
PQ-II+) was mo~ifiP~I by pl~vidillg a 1/16" .li~.... t~,l metal tube 260 to allow the
ud~l~;Lion of reagents into the second vacuum region 25 in a manner identi~ql tothe second ~l~,f.,~l~l embo~imPnt As shown in Fig. 4, the remqin~lP~r of the
ICP/MS WAS not mo~ifi-P~ from that provided by the mqmlfactllrer. A series of
20 e~ ,~ was p~lÇol..led utili7ir~g an argon carrier gas and H2 as a reagent gasinL.ud~d via tube 260 into the seicond ~acuuJJl region 25. Mass spectra were
obtained for H2 p~ ule in the second vacuum region 25 ~h.~n zcro and about
2 mTorr and are ~ .. q.iLed below.
EXPERIMENT 3
The effect of H2 p~s~c on the analyte and ion signals were ob~.~d by
recording the mass S~,LIUII1 in both the Analog and pulse cc~unt.lg modes of
operation of the ICP/MS as provided by the m~mlf.~elllrer. Two mass spectrA
30 recorded will~oul addition of H2 intû the second ~A~;Uulll region 25 are shown in
CA 02241320 1998-06-24
T/US 9 7 ~ 00 0 2 3
FIG. 5. The upper trace 500 in FIG. 5 was obtained using the analog mode of
operation. The lower trace 510 in FIG. 5 was obtained using the pulse counting
mode of operation. The upper trace 500 shows the il,t. n~i~ of various peaks,
most notably, N+ at m/z 14 502, O+ at m/z 16 504, OH+ at m/z 17 506,
H2O+ at m/z 18 508., Ar+ at m/z 40 512, ArH+ at m/z 41 514,H2+ at m/z 2
516, and H3+ at m/z 3 518. Two mass spectra recorded with addition of a
pres~ule of about 2 mTorr H2 into the second vacuum region 25 are shown in
FIG. 6. The upper trace 600 in ~IG. 6 was obtained using the analog mode of
operation. The lower trace 610 in FIG. 6 was obtained using the pulse Cuul~
mode of opcldlion. The vertical and ho.;~ t.l scales of FIG. S and ~IG. 6 are
the same. The same ion peaks arc labeled in ~IG. C as in FIG. 5, namely, N+ at
m/z 14 602, O+ at m/z 16 604, OH+ at m/z 17 60C, H20+ at m/z 18 608.,
Ar+ at m/z 40 612, ArH+ at m/z 41 614,H2+ at m/z 2 616, and H3+ at m/z 3
618.
As the mass spectra in FIG. S and ~IG. 6 show, this method of
il.lpl~ on allows the direct d~;o~- of H3+ produced in the r~c~ll of
,. ~ Ar+ with H2. The fo~ ;o-- of this ion is strongly i~f~ d from the e~
performed in the appdlalu~s of the first two embo~imPntc~ but H3+ could not be
detec~d using the Finnigan MAT ion trap mass ~pe~,l,.,l.le~.~. Tn~ .h as this
mPth~ ploduces a mass ~ in the same way as a convçntion~l ICP/MS
u~ , polyatomic ions which are commonly observed in conventional
ICP/MS, but not by using the meth~ of the first and second plefc,,l~
embo~ , may also be obs~.~ed here. Thus, for example, the effect of
elevated H2 p,~s~ s in Va~;UWll region 25 on Ar+ may be ob~.~d along with
the effects on ArO+ and Ar2+.
The most d~ ic effect of added H2 is an approximately 200 fold iLu~lease
in the h~.lsil~ of the H3 + peak 618. Addition of H2 also causes an
approximately 10-fold decrease in the hlt~nsily of the Ar+ peak 612 and an
approximately 2-fold increase in the intensity of the ArH+ peak 614. These mass
spectra show minimql reduction (less than 10%) in the hltensily of the peaks for
CA 02241320 1998-06-24
O O o 2 3
IPEAIUS O 6 AUG 1997
other analytes (not shown). These mass spectra thus show a selective removal of
Ar+ and an il~lease in H3+ thereby confirming the mPch~nicm of charge
in the reaction of H2 with Ar+.
S EXPERIMENT 4
A series of eA~line.lls was also pe.ro~ ed utili7ing the ICP/MS with no
mo~lifir~ti-~ns other than adjusting the potenlials in the lens stack 240 to reduce the
kinetic energy of the ions from typical values under normal OpC.aLiilg conditions.
10 H2 was introduced as a reagent gas into the second vacuum region 25 via the
vacuum port 400 provided by the ~ f* ~.el for pie;.~ lea~.lll~.lt~. H2
pres~.lres ranged from about 0.1 mTorr to about 1 mTorr. The ~.leas~d Ar+
hllensily was reduced by a factor of two with the introduction of the H2 reagentgas, d~ O~llati~g that introduction of H2 into the second vacuum region 25 of an15 llnm~lifiPd ICPIMS can be used to reduce the Ar+ ion intensity. We further
ob~e,~.,d an hlclease of about a factor of 10 in signal at m/z 41, indicating
formation of ArH+ consistent with the eA~.h~ tal observations from the
' - appal.. lus of the first embo~iimpnt
Table II cor~t~inc ~k~t~J data from the e~ rolled using the
20 apparatus of the first, second, and third plef,ll~d c..lbod;.-.~ deselibed herein.
Each row of the table gives reduction factors for Ar+ and an analyte ion as wellas the ratio of these reduction factors. The ratio is the sel~tivily with which the
Ar+ i~t~.~ily in the mass ~ is reduced relative to the intensity of the
analyte ion. The entries in the first column in Table II lists the p.~fel.ed
25 embo~ ..Pn~ used to obtain the data given in each row. The second column in
Table II lists the reagent gas used. The reagent gas was i ll.~hlc~d into the ion
trap 20 for the results shown in Table II for the first ~lef~ ll. d emboAimPnt above.
The reagent gas was introduced in vacuum region 25 for the results shown in
Table II for the second and third embo~lim~ntc. Thus, by way of e~cample, the
30 third row in Table II shows that the reaction of the carrier gas ion (Ar+) leads to
CA 02241320 1998-06-24
~/IIS'3 ~ ~ Q~ 0 2 3
IPEAll~S 0 6 AUG 19~-
- 18 -
a 30-fold reduction in Ar+ illt~,l~ily under conditions that reduce the int~sily of
Sc+ by a factor of two.
Table II. Selectivity of Ar+ Removal
Embodiment Reagent Reduction Factors
Ar+ Analyte Ion Ratio
Re~lloti~n Re~luction
First H2100,000 (In+)<5% 1,000,000
Second Ar 300 (Sc+) 7 45
Second H2 30 (Sc+) 2 15
Third H2 10 (In+) <10% 100
THE FOURTH PREFERRED EMBODIMENT
~...
In a fourth pl~f~ ed embodiment as shown in FIG. 7, carrier gas ions and
analyte ions ge~.dted from an ion source 700 are dil~t.,d through a first aperture
710 to a cell 720 where the ions are allowed to react with a reagent gas. S~ le
20 ion sources include, but are not limited to thermal ionization sources, electron
impact, laser irradiation, ion spray, ele.;~ , thPrmospray, inductively coupled
plasma sources, arc/spark disch~ges, glow disch~es, hollow cathode dischdrg,es
and microwave plasma sources. While the fourth p-~f~ ,d embo~1imPnt as
described herein is limited to what are considelcd its es~Pntiql CGlllpO~t~, it will
25 be app~ l to those skilled in the art that the fourth plefe,l~,d emb~;..~ could
readily be constructed using conventional ICP/MS colllpon~l~ as des~,lil.ed in
prior plefell~,d embodiments. The cell is coll~iL~ed within a first vacuum region
730. The cell 720 confines ions in a region close to the apellu e 710 through
which the ions are introduced into the first vacuum region 730. In this manner,
CA 02241320 1998-06-24
31/0002
r ~ a7
- 19 -
ions are directed from tke ion source 700 to the cell 720 with minimllm
opportunity for ion dispersion. The first vacuum region 730 is made to contain
the optimal pressure of reagent gas which allows both ion transport through the
cell 720 and sufficient charge transfer between the carrier gas ions and the reagent
5 gas.
The cell 720 also can be made to control the kinetic energy of the ions.
Thus, the cell 720 can be used to il~clease the resi~n~e time the carrier gas ions
- are in contact with the reagent gas and thus to il~clcase the extent of charge
ll~r~,l. Also, the cell 720 can be made to discl lui~dle ag~in~t i.e., not
10 transmit, slow ions by application of velocity or kinetic energy discl;,..i,u~ g
methods, such as the application of suitable DC electric fields. In this manner,charge excll~n~e between fast carrier gas ions and slow reagent gas neutrals can be
used to remove sf~ t~fd carrier gas ions from the ion beam. The kinetic energy of
the ions in the cell 720 is ~ ;n~ as high as possible so as to ~ni--;~ , space
15 charge e~ n~Qn of the ions, but low enough for a given ples~ of reagent gas
to allow ~urr~if-~- charge l,~r~,l. The optimal pl~ of the reagent gas will be
limited by ~e~ept~ble analyte ion scattering losses in the cell and pl~;lical
~~ considerations such as l,~up~g l~uile u~llt~.
As an e~uple, the fourth pl~,f .-~,d embodiment may be operated using
20 argon as the carrier gas. The cell 720 may be provided as any app~atl,s suitable
for courn~g the ions in the first vacuum region 730, including but not limited to,
an ion trap, a long flight tube, a lens stack or an R~ multipole ion guide. For
example, by SClf~ g the cell 720 as an RF multipole ion guide, the cell 720 may
be operated to selectively di~l~ reagcnt gas ions from the ion bea~n. By
25 self~ling a reagent gas having a low mass, such as H2, the RF mllltipole ion guide
may be op_.ated with a low mass cut-off greater than mlz 3. In this manner,
H2+ and H3+, which are formed as charge lr~r~,l products, are sele~ ly
di~ ed from the ion beam by virtue of their low m/z.
The reslllt~nt ion beam may then bc utilized as one of any uulubel of end
30 uses in~ ing but not limited to an ion gun or an ion implanter. Further, the
CA 02241320 1998-06-24
2 3
O 6 AUG 1997
- 20 -
resultant beam may be analyzed in various apparalus including but not limited toan optical ~c~ollleter, mass sp~ ull.eters (MS), including linear quadrupole
MS, ion trap quadrupole MS, ion cyclotron l~,so~lce MS, time of flight MS, and
magnetic and/or electric sector MS. Finally, the resultant ion beam may be
5 directed through any electrical or m~gn~tir ion focusing or ion dhe~,ling appalalus,
including but not limited to, a lens stack, an RF multipole ion guide, an
ele~,l,o~tic sector, or a magn~.tir. sector.
The resultant ion beam thus has an hlcleas~d luropollion of analyte ions
comp~d to carrier gas ions. Thus, in any of the s~lggested uses ~L~.~,.n the
10 res~lt~nt ion beam is dhe~t~d through an dp~ e at the space charge current
limit, the hh;lcased ~lol)ullion of analyte ions cOlllpal~d to carrier gas ions
directed into the a~.~ul., will create an inc~ in the rate at which the analyte
ions pass through the ap.,llule.
While a plefell~d embo~im~nt of the present invention has been shown and
1~ described, it will be app&c.ll to those skilled in the art that many changes and
mc-lif1r ~ions _ay be made without del)a,ling from the invention in its broader
aspects. The alp~n-lPd claims are Ih.,.efol~ t~-~ed to cover all such chq~ees and
modifications as fall within the true spirit and scope of the in~e.llion.
~JIEt~ S~