Note: Descriptions are shown in the official language in which they were submitted.
CA 02241324 2004-02-12
WO 97/25619 1 PCT/US96/16981
DISPLACEMENT ASSAY ON A POROUS MEMBRANE
Background of the Invention
1. Field of the Invention
The present invention relates generally to assays and more
specifically to displacement-type assavs.
2. Description of the Background Art
United States Patent No. 5,183,740
describes a flow immunoassay
system and method for performing displacement immunoassays.
in a displacement assay, unlike a competitive assav, the
antibody is exposed to labelled analyte prior to exposure to
analvte. The analyte is in contact with the antibody and
labelled, bound analyte an insufficient amount of time to
establish equilibrium.
Because no time needs to be dedicated to establishing
equilibrium, displacement assays are faster than competitive
assays. A displacement assay, however, generally provides a
smaller signal than a competitive assay. In a displacement
assay, the available binding sites of the antibody are
saturated or nearly saturated with labelled analyte before the
unlabelled analyte is added. Since equilibrium (with labelled
analyte and unlabelled analyte continually binding, releasing
and competing with each other for rebinding to the available
binding sites on the antibody in a steady state) has not been
achieved, most of the labelled analyte in a displacement assay
remains bound to the antibody and unable to provide a signal.
The relatively small signal provided by the displacement
assay places an additional value on assuring the consistency
of assay conditions. The bead-containing columns described in
USP 5,183,740 for displacement assays must be carefully stored,
prepared, and i.oaded to assure chemical and physical
consistency (i.e., porosity, avoidance of channeling) from test
to test. The need for this careful preparation and testing
increases the labor, skill, and costs needed to perform
accurate displacement assays. Additionally, the problems
CA 02241324 1998-06-24
WO 97/25619 2 PCT/US96/16981
associated with the use of bead - containincT columns limit the
lower detectior, limit for displacement assays.
In studies performed at US Drug Testing, Inc. (Rancho
Cucamonga, Californ:ia) , better results for a displacement assay
were achieved using tall, thin columns of beads coated with an
antibody and labelled antigen than with short, wide columns.
Furthermore, the efficiency with which the labelled antigen
dissociated from antibody in the presence of uniabelled antigen
was greater when flow rates were reduced and the antigen had
more time to interact with the immobilized complex (Wemhoff et
al. J. Immunol. Methods, 223-230, 1992) . Both of these sets
of experiments suggested that immobilization of the antibody
and labelled antigen on a porous membrane would not provide a
suitable matrix for the displacement assay since this geometry
would not allow sufficient time, under flow conditions, for the
antigen to interact efficiently with the ccmplex to displace
detectaple amounts of the labelled antigen.
United States Patent No. 5,369,007, to David A. Kidwell
discloses a displacement assay in which samples pass through
a membrane having an antibody immobilized thereon. The binding
sites of the immobilized antibody are bound to an enzymatically
labelled analyte. Analyte from the sample displaces the
labelled analyte, causing the labelled analyte and the
remainder of the sa-nple to pass into a superabsorbent layer.
The superabsorbent layer contains a subst-ate for the enzymatic
label and any needed indicator. The Kidwell patent, however,
teaches the need for a flow rate of about 0.02 ml/min and
interaction times of about 1 to 5 min to assure a detectable
interaction between the analyte and the antibody. In many
situations, even faster results are desirable. Additionally,
the Kidwell microassay card is not reusable.
Summary of the Invention
Accordingly, it is an object of this invention to perform
bioassays capable of detecting minute quantities of an analyte
in under one minute.
It is another object of the present invention to quickly
perform bioassavs in a format that allows reuse of the matrix
that selectively binds the analyte.
CA 02241324 2004-02-12
- 2a -
According to a first aspect of the invention, there is provided a quantitative
assay method for detecting a target analyte, comprising the steps of:
providing a porous membrane having binding elements immobilized thereon,
each of said binding elements having at least one binding site capable of
specifically
binding to said target analyte;
exposing said binding sites to a labelled analog of the target analyte to form
complexes of membrane-immobilized binding elements and labelled analogs;
pumping a first aqueous liquid sample, suspected of containing the target
analyte, so as to flow said first liquid sample normal to and through said
membrane
having said complexes thereon, at a flow rate allowing the target analyte to
displace
the labelled analog from the complexes under non-equilibrium conditions to
form
downstream of said membrane a flowable liquid effluent including said
displaced
labelled analog, said flow rate also providing an interaction time between
said analyte
and said membrane of about 0.1 sec through about 30 sec;
interrogating said flowable liquid effluent to detect and quantitatively
determine
the amount of the displaced labelled analog, the amount of said displaced
labelled
analog being proportional to the concentration of said target analyte in said
first
sample.
According to a second aspect of the invention, there is provided a device for
the assay of an aqueous sample suspected of containing a target analyte,
comprising:
a porous membrane having binding elements immobilized thereon, each of
said binding elements having at least one binding site capable of specifically
binding
to said target analyte, essentially all of said binding sites on said membrane
being
CA 02241324 2004-02-12
- 2b -
occupied by a labelled analog of the target analyte to form complexes of
membrane-
immobilized binding elements and labelled analogs;
a pump for flowing an aqueous liquid sample, suspected of containing the
target analyte, normal to and through said membrane having said complexes
thereon,
at a flow rate allowing the target analyte to displace the labelled analog
from the
complexes under non-equilibrium conditions to form downstream of said membrane
a
flowable liquid effluent including said displaced labelled analog, said flow
rate also
providing an interaction time between said analyte and said membrane of about
0.1
sec through about 30 sec;
and a detector that interrogates said flowable liquid effluent and the
presence
of said labelled analog therein.
According to a third aspect of the invention, there is provided a device for
the
immunoassay of an aqueous sample suspected of containing a target analyte,
comprising:
a container, said container including an open end and a closed end;
a cap within which said open end of said container may be fitted in a first
position in which the closed end of said container is a first distance from
said cap and
in a second position in which the closed end of said container is a second
distance
from said cap, said second distance being less than said first distance;
a tip for receiving said aqueous sample extending outwardly from said cap;
a hollow needle extending from said cap in a direction opposite to said tip;
a septum extending across the width of said container, said septum being
positioned between said closed end of said container and an end of said needle
distal
CA 02241324 2004-02-12
- GCi -
to said cap when said container is seated in said first position, said septum
being
essentially impermeable to fluid;
a porous membrane positioned between said septum and said closed end of
said container, said membrane extending across the width of said container,
and an
evacuated reservoir between said membrane and said closed end, said membrane
having binding elements immobilized thereon, each of said binding elements
having
at least one binding site capable of specifically binding to said target
analyte,
essentially all of said binding sites on said membrane being either occupied
by a
labelled analog of the target analyte to form complexes of membrane-
immobilized
binding elements and labelled analogues;
a detecting means for detecting the presence of said labelled analog in said
reservoir;
said hollow needle being positioned so as to puncture said septum, but not
said membrane, when said container is in said second position.
CA 02241324 1998-06-24
WO 97/25619 PCTIUS96/16981
3
These and additional objects of the ,nvention are
accomplished by quickly flowing a sample past a non-absorbent
membrane having a binding element covalently bound thereto to
form attachment sites for the analyte. The available
attachment sites are essentially saturated with a labelled form
of the analyte. Nonspecific binding sites are blocked to
prevent nonspecific binding. Additionally, the sample flows
past the membrane at a rate greater than that needed to achieve
equilibrium between the dissociation of labelled analyte from
the bindina sites and the attachment of analyte (labelled or
unlabelled) therezo. The processed sample is then analyzed for
the presence of anv labelled antigen that the unlabelled
analyte has displaced from its binding site. This analysis can
be qualitative or quantitative.
Brief Description of the Drawings
A more complete appreciation of the invention will be
readily obtained by reference to the following Description of
the Preferred Embodiments and the accompanying drawings in
which like r.umerals in different figures represent the same
structures or elements, wherein:
Fig. "_ schematically illustrates a device according to the
present invention.
Fig. 2 schematically illustrates an alternative embodiment
of a device according to the present invention.
Fig. 3 schematically illustrates another alternative
embodiment of a device according to the present invention.
Fig. _ is a graph of data from a membrane assay, in
accordance with the present invention, in which the membrane
was prepared by the test tube incubation method.
Fig. 5 is a graph of data from a membrane assay, in
accordance with the present invention, in which the membrane
was prepared by saturating the immobilized antibody with
labelled analyte in the column as opposed to in a test tube.
CA 02241324 1998-06-24
WO 97/25619 PCT/US96/16981
4
Fig. 6 is a graph of data frcm a single membrane assay,
according to the present invention, prepared by saturating the
antibody directly in the column.
Fig. 7 is a flowchart schematically illustrating an
embodiment of an assay according to the method of the present
invention.
Figs. 8a, 8b, and 8c show the results obtained from assay
performed in accordance with the method flowcharted in Fig.
7.
Description of the Preferred Embodiments
Membranes useful in the present i:.vention are typically
non-absorbent (with respect to aqueous materials) materials.
The non-absorbent membrane assists in providing a fast flow-
through rate. Additionally, the use of a non-absorbent
membrane allows the membrane, once used, to be readily rinsed
of sample and reused. If displacement has occurred, reloading
with labelled analyte is an option.
Typically, membranes useful in the present invention have
thicknesses, e:posed surface areas, and porosities that allow
detection of the analyte with an interaction time ef about 0.7-
sec to about 30 seconds, and typically about 1 sec to about 15
secends, between a sampie suspected of containing of the
analyte and the membrane having a labelled analyte of the
analyte thereon. Generally, the pore sizes in the membrane are
about 0.2-1.0 microns, and are typically about 0.45 microns.
Of course, other pore sizes may be used to achieve the desired
interaction time. Likewise, the thickness and surface area of
the membrane can be adjusted to provide the desired interaction
time.
Any non-absorbent membrane, of appropriate pore size and
density of sites for immobilizing binding elements for the
analyte, may be used. For example, the membrane may be a
polyamide ( e. g., Nylon' membranes such as Immunodyne ABCTM (a
NylonTM 6,6 membrane made by Pall Biosupport, Port Washington,
New York)) or a polyvinylidine fluoride, such as ImmobilonT' or
Durapore7"'membranes made by Millipore, Bedford, Massachusetts.
CA 02241324 1998-06-24
WO 97/25619 PCTIUS96/16981
Other suitable membranes include, but are not limited to,
cellulose, nitrocellulose, silica fiber, aluminum oxide, and
polyvinyl chloride.
Binding elements may be immobilized on the selected
5 membrane in any manner that assures the availability on the
immobilized binding element of at least one binding site for
selectively bindinq the labelled analyte and target analyte in
an aqueous medium. Several methods for attaching binding
elements to the membranes are well-known and therefore will not
be specifically described herein. The binding element may be
immobilized either throughout the thickness of the membrane,
or on only one or both surfaces thereof.
The binding element may be any substance that can be
immobilized on the membrane and that specifically binds the
target analvte and its labelled anaior. Binding elements
include, but are not limited to, --ectins, antibodies,
antibiotics, and binding proteins other than antibodies and
antibiotics.
Once the binding elements have been immobilized on the
membrane, their available binding sites for selectively binding
with the analyte will usually be essentially saturated with a
labelled analog of the analyte (denoted 'nerein as a "labelled
analyte"). Saturation of the available binding sites with the
labelled analyte enhances sensitivity by assuring tnat the
maximum number of analyte molecules wi_il displace labelled
analytes, rather than binding directly to unoccupied bindina
sites.
The membrane may be oriented in any manner with respect
to sample flow that allows the sample to flow past the complex
of binding element and labelled analyte on the membrane over
the desired interaction time. For example, the sample may flow
through and essentially normal to the plane of the membrane.
Alternatively, the membrane may be configured as a dipstick and
the sample allowed to flow laterally through the membrane, for
example by capillary action. In another alternative, the
membrane support may be a hollow fiber configured so the sample
flows along the hollow center before passing through the
membrane. In any embodiment of the present invention, the flow
of the sample through the membrane may be passive (i.e.,
gravitational cr capillary flow) or active (flow resulting
CA 02241324 1998-06-24
WO 97/25619 PCT/US96/16981
6
entirely or partly from the action of a flow pump, manual
pressure, or vacuum).
Any label useful in assays for the analyte may be used tc
label the analyte. Fluorophores are particularly useful
labels. Suitable fluorophores include, but are noz limited to
Fluorescein, Cadaverine, Texas RedTM (Molecular Probes, Eugene,
OR) and Cyanine 57' " (BDS, Pennsylvania). If used, the
fluorophore label is typically one that is detectable in the
visible to near infrared range.
Once the sample has completed its interaction with the
membrane having the immobilized binding element-labelled
analyte thereon, the processed sample (e.g., the e=Fluent from
a samr)le column or the portion of the sample that has passed
through and beyond the labelled portion of a tes-: strip) is
then analyzed t:o determine the concentraticn ..- displaced
labelled analyte. The detection means for t:_s analysis
includes a readout for informing the user that a threshold
amount of the label has been detected in the sample. When the
label is fluorescent, the detection means also =ncludes a light
source for exciting the fluorophore-?abelled analytes. The
detection system can use various methods of optical
measurement, including but not limited to a spectrcphotometer,
infrared spectrometer, fluorimeter, optical biose-:sor, or the
eye.
The present iz-,vention is useful in the uereccion, ,n
aqueous media, of any analyte that specifically binds to the
binding element. The invention may be used, for example, to
detect the presence of analytes in body fluids (blood, semen,
saliva, urine, etc.), water, pharmaceuticai preparations,
environmental samples, aerosols, foods, and beverages. If the
sample suspected of containing the analyte is originally in a
viscous liquid, solid, gaseous state, the sample is preferably
further dissolved in water before being exposed to the
membrane.
Multiple binding elements for multiple analytes can be
immobilized on a single membrane. Membranes containing the
same or a different binding element can be arranged in stacks.
Where multiple binding elements for multiple analytes are used,
different labels on the labelled analytes can be used to
distinguish which analyte is present.
CA 02241324 1998-06-24
WO 97/25619 PCTIUS96/16981
7
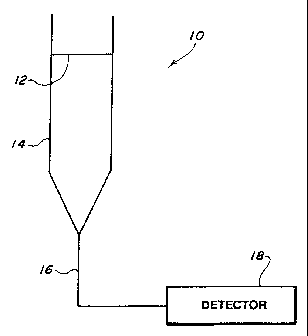
Fic. 1 schematically shows a device 10 according to Lne
present invention where the membrane is normal to sample flow.
Membrane 12, with binding elements covalently bound or
otherwise immobilized thereto and available binding sites
saturated with a labelled analyte of the analyte, is positioned
across column 14. An aqueous sample entering the top of column
14 flows through membrane 12. Analyte in the sample interacts
with membrane 12 and displaces the labelled analyte from
membrane 12. The labelled analyte, if it does not dispiace
another labelled analyte or unlabelled analyte from the
membrane, joins the effluent from column 14. The acrueous
sample effluent from column 14 then enters line 16, which
carries the effluent to detector 18 for detecting the presence
of the labelled analyte in the effluent from column 14.
Fig. 2 shows an alternative embodiment of the tDresent
inventicn, where the membrane is also normal to sample flow.
Porous membrane 102, with binding elements covalently bound or
otherwise immobilized thereto and available binding sites
saturated with a labelled analyte of the analyte, is positioned
across column 104 having an open tip. To prevent the flow of
sample between the outer edge of membrane 102 and the inner
wall of column 104, the membrane typically extends fully across
the width of column 104. The open tip of column 104 is
inserted into the top of container 106 (typically throuah a
septum (not shown)), which holds a sample suspecteu of
containing the analyte. Suction means 105 can apply a vacuum
to pull sample from container 106 through membrane 102 into
column 104. Any label in the column may be detected by a
detection means external to the column. To facilitate this
external detection, column 104 is preferably transparent to,
or includes a suitably placed window transparent to, the energy
used for detection.
Although Fig. 2 shows the suction means as a plunger and
column 104 as the syringe housing the plunger, other vacuum
arrangements are possible. For example, Fig. 3 shows a design
similar to that used by Vacuutainers'. Evacuated tube 204 has
porous membrane 205, with binding elements covalently bound or
otherwise immobilized thereto and available binding sites
saturated with a labelled analyte of the analyte, thereacross.
To prevent the flow cf sample between the outer edge of pcrous
CA 02241324 1998-06-24
WO 97/25619 PCTIUS96/16981
8
membrane 205 and the inner wall cf evacuated tube 204, the
membrane typically extends fully across the width of evacuated
tube 204. The open end of evacuated tube 204 is sealed by cap
206 having flange 208 extending about the rim of open end of
tube 204. Tip 210 extends from cap 206 opposite to hollow
needle 212, which also extends from cap 206. Needle 212
extends to near septum 214 when tube 204 is placed, with only
slight pressure, within flange 208. Septum 214 maintains the
vacuum in the portion 216 of tube 204. Although septum 214 is
essentially impermeable to gas or liquid, it is punctured by
needle 212 once tube 204 is fully inserted into flange 208.
Upon the puncture of septum 214, the vacuum within portion 216
draws liquid from sample container 218 through tip 2-0, into
hollow needle 212, through membrane 205 and into portion 216.
Any label within portion 216 can be detected as with other
embodiments of the invention. To assure that needle 212 does
not puncture membrane 205, the distance between the bottom of
septum 214 and the bottom of membrane 205 should be greater
than the height of needle 212. This embodiment of the
invention assures that the flow across membrane 205 is
consistent from sample to sample.
Having described the invention, the following examples are
given to illustrate specific applications of the invention
including the best mode now known to perform the invention.
These specific exan;ples are not intended to limit the scope of
the invention described in this application.
EXAMPLES
Example 1. TNT Detection
To prepare the membranes, the monoclonal 11B3 antibody
(mouse lgG,) with specificity for TNT (trinitrotoluene) was
immobilized onto the Immunodyne ABC membrane with a pore size
of 0.454m. The 11B3 antibody, 10041 of a 2 nmol/ml solution
in phosphate buffered saline (PBS), was attached to the
membrane by either placing the solution in a test tube, with
subsequent addition of the membrane, or pipetting the antibody
into a column that already contained the membrane. Whether in
a column or a test tube, membranes were incubated with the
CA 02241324 2004-02-12
WO 97/25619 PCT/US96/169$1
9
antibody for four hours at room temperature. Following
incubation, the antibody solution was removed. Membranes
exposed to antibody in a test.tube were placed in a column.
Any unreacted birlding sites on the membrane were blocked with
the addition of 100gl of 1M Tris for approximately 30 minutes.
To reduce nonspecific binding, the membranes weredrained and
washed three times with PBS containing 0.011; Triton X-1000
detergent.
The labelled analyte was prepared by attaching the
fluorophore CYSO' (BDS, Pennsylvania) to trinitrobenzyl
cadaverine (CY5-TNB). To saturate the antibody binding site
with the labelled antigen, a solution of the CY5-TNB (4 nmoles
in SO l PBS) was added to each column, and the columns were
placed on a rocking bed overnight. The columns were connected
to the fluorimeter and, washed briefly. Samples were
introduced at a f low rate of 1 mL/min. Analyze injections were
made in triplicate with concentrations ranging ber.ween 18.75
ng/mL and 1200 ng/mL. Fig. 4 illustrates data obtained for a
membrane assay prepared with the test tube incubation method.
A fluorescence signal peak was obtained at all, analyte-
conceritrations which was proportional to the amount of analyte
added to:the column.
Fig. 5 represents data from a membrane assay prepared by
saturating the immobilized antibody with ?abelled analyte in
the column as opposed to in a test tube. Again, an increase
in signal intensity with increasing analyLe concentration was
observed. However, a plateau was seen between an analyte
concentration of 700 ng/mL and 1200 ng/mL where a negligible
increase in-signal intensity was observed despite a two-fold
increase in analyte concentration suggesting that there is less-
labelled analyte on the membrane available for -displacement,
compared to the membrane prepared in the test tube.
Both Figs. 4 and S; demonstrate reproducible results, with
minimal standard error as indicated by the error bars. Assay
35' time's were fast with-the exact time being simply a function.of
the flow rate (1 mL/'min in this case) and the length of tubing
between the analyte introduction site and the fluorimeter flow
cell. For these experiments, signals were generated les,-3 than
l minute from the time of sample introduction.
CA 02241324 2004-02-12
WO 97/25619 PCT/US96/16981
Exampl:e 2. Detection of RDX
Similar experiments were conducted whereby a monoclonal
antibody with specificity for the explosive, cyclonite (RDX),
was immobilized onto the membrane. The procedure for
5 immobilization was identical to the one used for the anti-TNT
antibody. However, 100 l of 0.5o casein was used instead of
Tris in order to block the remaining binding sites on the
membrane. Fig. 6 represents data from a single membrane assay
prepared by saturating the antibody directly in the column.
10 A linear relationship between signal intensity and analyte
concentration is observed. The lower limit of detection for.
this assay is at 5 ng/m1 which corresponds to part per billion
(ppb) levels.
II. Displacement Dipstick Studies.
The main objective of these experiments was to desigr. a
qualitative membrane-based immunoassay for the detection of a
target analyte in solution. The tests rely the displacement
immunoassay to work on the Immunodyne membranes with the fluid
flowing through them membranes laterally as opposed to
perpendicular to the membrane as described above. Transported
by capillary action, the fluid conducts the analyte in the
sample to the immobilized antibody-labelled analyte complex and
transports the displaced labelled analyte further along the
membrane strip. The dipstick displacement assay is not onlv
dependent upon the ability of the target analyte to displace
the labelled analyte from the immobilized antibody but also on
several other factors such as the rate of the capillary action
of the mobile phase and the rates of transport of analyte and
labelled analyte through the membrane. Fig. 7 provides
a schematic of the, experimental protocol. First, in step (a),
strips 100 were cut from an ABC Immunodyne~ membrane 110 that
were either 30x5 mm or 50x10 mm. A monoclonai antibody specific
for TNT (11B3) in concentrations ranging from 2 to 10 nmol/ml
was placed in .5 L droplets onto the membrane strips. and
allowed to immobilize for thirty minutes. In step (b), strips
100 were then soaked, using test tube 112, in a Tris solution
for about an hour to block any other covalent binding sites.
A washing of membrane strips 100 followed that consisted of
I40 three consecutive exposures to PBS containing 0.01a Triton X-
CA 02241324 2004-11-29
WO 97125619 PCT/US96/16981
11
100 to wash away any excess TNT antibody (step (c)). After a
final wash with PBS, CYS-TNB labelled analyte,. in excess of
five-to-thirty times the molar amount of antibody, was applied
in 6.5 L droplets onto the antibody and incubated overnight
(step (d)). In step (e), strips 100 were then washed in PBS
containing 2.5 t ethanol, and 1% Tween.20T''for ten minutes in
order to remove nonspecifically bound labelled ar.alyte. In
step (f), before drying, strips 100 were put into a solution
of 100mM trehalose dihydrate in phosphate buffer for ten
minutes. Finally, in step (g) the strips were dried at room
temperature. The displacement assay (step (h)) was conducted
by dipping the end of membrane strip 100 in TNT solution 114,
13.6, 118, or 120 (of the concentration specified in Fig. 7,
step (h)), and allowing capillary action to bring the target
analyte up to the 'antibody/labelled analyte- complex for
displacement. A 650 nm laser (not shown) connected 4o a
fluorescence detector was used to look for any displaced
labelled analyte (CyS-TNB) on membrane str'-p 100.
In the first experiment, TNT antibody at a concentration
of 2 nmol/ml was piaced at- the center of a 3 x 0.5 cm,
rectangul=a= membrane strip and was saturated by five times
excess CY5-TNB. The strip was then dipped into a sample
soluticz containing 300 ng/ml TNT. Fig. 8a represents this
strip when the dipped end is held under the laser first (left
side) . The hiaheY plateau indicates the fluorescence from the
CY5-TNB bound to the immobilized antibody. A shoulder is
evident to the .right of the higher plateau, indicating
displacement of the labelled- analyte from the antibody. Fig.
6b shows this.same strip optically interrogated in the reverse
direction where the dipped end is on the right. -Another
membrane strip, also having 2.nmol/ml of.immobilized anti=TNT
antibodv was treated identically and exposed to the same 300
ng/ml TNT solution. After placing it under the.laser with the
dipped end on the right, the data shown in Fig. 8c 'was
obtained. These experiments were conducted by manually moving
the membrane strip along the laser path. "Time" on the x-axis
refers to scanning time and has no relation to assay time.
Obviouslv, many modifications and variations- of the
present invention are possible in light of the above teachings.
CA 02241324 1998-06-24
WO 97/25619 PCT/US96/16981
12
It is therefore to be understood that, within the scope of the
appended claims, the invention may be practiced otherwise than
as specifically described.