Note: Descriptions are shown in the official language in which they were submitted.
-
CA 022427l3 l998-07-07
W O 97/26261 PCT/GB96/02674
- PYRIDO (3,2,1-IJ)-1,3,4-BENZOXADIAZINE
The present invention relates to a novel compound, to processes for its
production, to pharmaceutical form~ tions con-~inin~ it, and to its use in
therapy, particularly in the tre~ nt of microbial in~ections.
~P-A-0 2~9 804 describes the compound of formula (A):
O O
F ~' H
~N~J O~N~
(A)
(9-fluoro-3-methyl- 1 0-(4-methyl- 1 -piperazinyl)-7-oxo-2,3-dihydro-7H-
pyrido~3,2,1-ij~-1,3,4-b~n7~ 7ine-6-carboxylic acid). The compound of forrnula
(A) is reported to have antibacterial activity.
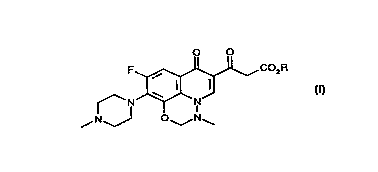
The present invention provides a compound of forrnula (I~:
O O
F ~5~J~CO2R
f N~--N
~N~J O~N~
CA 02242713 1998-07-07
W O 97/26261 PCT/GB96102674
(I)
wherein R is C 1-6 alkyl, such as methyl or ethyl. A particular compound of
formula (I~ is 6-(ethoxycarbonyl)acetyl 9-fluoro-3-methyl-10-(~methyl-1-
piperazinyl)-7-oxo-2,3-dihydro-7H-pyrido[3,2,1-ij~-1,3,~b~n7.o~ ine,
Compounds of formula (I) have antibacterial activity and are therefore of use inthe tre~tmPnt and prophylaxis of b~c teri~l infections in hllm~n.~ and ~nim~}.
The invention in a second aspect, further provides a process for the production of
a compound of formula (I), which comprises treating a compound of formula (~):
O O
F~X
f N~--N
~N~J O~N~
(II)
wherein X is a leaving group, such as imid~7ole or halide, such as chlorine, with a
carbanion derivable by treating a diaL~cyl malonate with a strong base, such as
sodium hydride.
The compound of formula (II) can be obtained by treating the compound of
forrnula (A~ with, for example, an excess of carbonyl ~iiimi~7Ole. This reactionis typically carried out in an aprotic solvent such as chloroform at arnbient orelevated temperature, eg at reflux, under dry conditions, e.g. in an argon,
atmosphere.
Generation of the carbanion is typically carried out at between-70 and 70~ C. for
instance at arnbient temperature, in an aprotic solvent such as THF. A solution ~f t
the compound of forrnula (II) in THF is then added and the mixture is refluxed.
After evaporation of solvent the residue is dissolved in water and the reaction
CA 02242713 1998-07-07
W O 97/26261 PCT/GB96102674
- ~uenched by neutralization to pH7. The product is extracted and purified by
chromatography such as HPLC.
The compound according to the invention'is suitably provided in subst~nti~11y
pure forrn, for e~mple at least 50% pure, suitable at least 6û% pure,
advantageously at least 75% pure, preferably at least 85% pure, more preferably
at !east 9~% pure~esneGiallyatleact98% pUtP-, allpe-rGe-n-tages heingGalGU
weight/weight. An impure or less pure form of the compound according to the
invention may, for example, be used in the preparation of a more pure form of the
same compound.
The compound of the invention has ~ntih~r.trri~1 activity and is useful for the
prophylactic and therapeutic tre~tment of bacterial infections in ~nim~1.c,
especially m~mm~1~, including hl-m~nc, in particular humans and domestic~ted
~nim~1.c (including farm ~nim~l.c). The compound may be used for the tre~tment
of infections caused by, among other or~ni.cmc, species of Staphylococcus,
Streptococcus, Aerococcus, Enterococcus, Micrococcus, Kactob~cm~
Bifidob~rt~.ril1m, Clostridium, Eub~cteri~1m, Peptococcus, Peptostreptococcus,
Propionib~terium, Citrobacter, Campylobacter, Enterobacter, ~1~.bsie11~,
Proteus, Pseudomonas, Serratia, Salmonella, Shigella, Vibrio, Aeromonas,
Haemophi~us, Neisseria, Acinetobacter. Alcaligenes, Bordetella, Bacteroids,
Fusobacterium, Myocoplasma and other microorE~nicm.c
Accordingly a third aspect of the invention provides the compound of formula (I)for use in me~ therapy, in particular for use as an antibacterial agent.
The invention further provides a method of treating a human or animal suffering
from a b~ctrri~l infection by the a-lmini.stration of an effective amount of thecompound of the invention.
A particular method of the invention comprises treating or preventing bacterial
infections in non-human ~nim~1c, more particularly domestir~t~l m~mm~1c and
birds, such as horses. cattle, swine, sheep, companion ~nim~ including dogs and
cats, and poultry including chickens. The method comprises aAmini.ctering to theanimal via the oral route an antibacterially effective amount of a compound of
forrnula ~
CA 02242713 1998-07-07
WO 97/26261 PCTIGB96/02674
-
A further aspect of the invention provides use of a compound of formula (I) in the
m~nllf~qcture of a merlir~ment for use in the tre~tmP-nt or prevention of bacterial
infections in non-human ~nim~lc by ~mini.ctration via the oral route.
The invention further provides a ph~rm~e~lti~ ~l composition cornr ri.cing a
compound of the formula (I~ together with a ph~rm~eutic~lly acceptable diluent
or carrier.
The compound of the invention can be ~dminictered alone, but will generally be
~lmini.ctPred in ~ mixtllre with a ph~rm~elltical carrier selected with regard to
the intPnded route of ~tlminictration and standard pharmaceutical pr~cti~e For
example, it may be ~rlmini.ctPred orally in the form of a tablet co~t~ining suchexcipients as starch or lactose, or in a c~rsule or ovule either alone or in
admixture with excipients, or in the form of an elixir or suspension cont~ining a
flavouring or colouring agent. It may be iniected parenterally, for e~mrlP,
intravenously, intramllcc~ rly or subcutaneously. For parenteral ~tlminictration~
it is best used in the form of a sterile solution which may contain other
substances, for example, enough salt,c or glucose to make the solution isotonic.
For oral and parenteral ~rlminictr~tion, it is expected that the daily dosage level
of the compound of formula (I) will be from 0.5 to ~00, preferably 1 to 300
mg/kg (in divided doses) when ~-lminictPred by either the oral or parenteral route.
No unacceptable toxicological effects are expected when the compound is
a~minictered in the above mentioned dosage ranges.
The compounds and compositions according to the invention may be formulated
for ~1minictration in any convenient way for use in human or veterinary
medicine, by analogy with other antib~teri~l agents.
The tablets and capsules for oral ~-lminictration may be in unit dosage form, and
may contain conventional excipients incl~rling, for example, binding agents, forexample, syrup, acacia, gelatin, sorbitol, tr~g~< ~nth or polyvinylpyrrollidQne
fillers, for example lactose, sugar, maize~starch. calcium phosphate, sorbitol or
glycine; tabletting lubricants, for example m~gnesium stearate, talc, polyethylene
glycol or silica; disintegrants, for example potato starch; and pharm~ceutie~llyacceptable wetting agents, for example sodium lauryl sulrh~t~o The tablets may
be coated according to methods well known in normal pharmaceutical practice.
Oral liquid preparations may be in the form of, for example, aqueous or oily
suspensions, solutions. emulsions, syrups or elixirs, or may be presented as a dry
product for reconstitution with water or another suitable vehicle before use. Such
CA 02242713 1998-07-07
W O 97/26261 PCTIGB96/02674
liquid preparations may contain convention~l additives; in~luf~in~, for example,suspending agents, for e~cample sorbitol, methyl cellulose, gl-lcose syrup, gelatin,
hydroxyet'nyl cellulose, carboxymethyl cellt~l~se. ~ minillm stearate gel or
hydrogen~ted edible fats; emulsifying agents, for example lecithin, sorbitan
monooleate or acacia; non-aqueous vehicles (wnich may include edible oils), for
example almond oil, oily esters (for example glycerine), propylene glycol, or
ethyl alcohol; preservatives, for example methyl or propyl p-hydroxy'oen7o~t~P or
sorbic acid; and, if desired, conventional flavouring and colour agent~s.
Compositions according to the invention intPIlded for topical ~tTminictration may,
for example, be in the form of ointments, creams, lotions, eye ointme~ts~ eye
drops, ear drops, impregnated dr~P-ssing~s, and aerosols, and may contain
applupliate conventional additives, including, for e~r~mplP, preservatives,
solvents to assist d;ug penetration, and emollient~s in ointmPnt~c and creams. Such
topical formulations may also contain compatible conve,ntion~l ca;riers, for
example cream or ointment bases, and ethanol or oleyl alcohol for lotions. Such
carriers may constitute from about 1% to about 98% by weight of the
formulation; more usually they will constitute up to about 80% by weight of the
formulation.
Compositions according to the invention may be formulated as suppositories,
which may contain conventional suppository bases, for example cocoa-butter or
other glycerides.
Compositions according to the invention intended for parenteral ~(lmini~strationmay conveniently be in fluid unit dosage forms, which may be prepared utiii7ing
the compound and a sterile vehicle, propyleneglycol. The compound, depending
on the vehicle and concentration used, may be either suspended or dissolved in
the vehicle. Parenteral suspensions may be prepared in substantially the same
manner except that the compound is suspended in the vehicle instead of being
dissolved and sterilisation cannot be accomplished by filtration. The compound
may instead be sterilised by exposure to ethylene oxide before being suspended in
the sterile vehicle. Advantageously, a st~ t~nt or wetting agent is included in
such suspensions in order to facilitate uniform distribution of the compound.
Compositions according to the invention may also be ~riministp~ed by inhalation.By '~inh~lationll is meant intranasal and oral inh~l~tion ~ministration.
Applopliate dosage forms for such ~minictration such as an aerosol formulation
or a metered dose inhaler, may be prepared by conventional techniques.
Preferably, the compound of formula (I) is ~lminict~red in admixture with the
-
CA 02242713 1998-07-07
W O 97/26261 PCT/GB96/02674
animal's feedstuff or drinking water. Thus, a further aspect of the invention
provides feetlstnff or drinking water having a compound of formula (I) mixed
therewith, as well as a premix comprising a compound of formula (I) together
with a veterinarily acceptable carrier. Suitable carriers include a mi~ture of abinder, such as polyvinylpyrrollidone, and a filler, such as l~t~ se, which can be
extruded, granulated and mixed with or sprinkled on the ~nim~l.c' food. For
addition to <1rinking water, the active is first made up as a con~entrate with aliquid carrier, such as gluconolactone.
The following example serves to illustrate the present invention.
EXAMPLE 1
6-(Ethoxycarbonyl)acetyl 9-fi!wro-3-me~hyl-l o-(4-methyl-l -piperazinyl)-7
2,3-dihydro-7H-pyridol3,2,1-i3]-1.3.4-benzoxn~iA~ine.
Sodium hydride (0.67g, 16.7mmol of a 60% suspension in oil) was added
pOnionwise to a stirred solution of diethyl malonate (2.67g, 16.7mmol) in dry
THF (SOml). The r~slllting effervescent mixture turned pale yellow after Smin
and was allowed to stir at room temperature for 3h (solution A). A solution of
carbonyl ~liimi(l~Qle (1.8g, 11.12mmol) and 9-fluoro-3-methyl-10-(4-methyl-l-
piperazinyl)-7-oxo-2,3-dihydro-7H-pyridor3,2,1-ij]-1,3,4-~e.n7.oY~ .in~.-6-
carboxylic acid (2.0g, 5.56mmol) in chloroform (SOml) was heated under reflux
in an argon atmosphere for 12h. The chloroform was then evaporated in vacuo
and the residue was dissolved in anhydrous THF (50ml) (solution B). Solution B
was added to solution A in one portion under an argon atmosphere and the
resulting mixture was heated under reflux for a period of 16h. The volatiles were
then removed by evaporation in vacuo and the residue was dissolved in water
(SOml). The aqueous solution was neutralised to pH 7 by careful addition of
acetic acid and the reslllting mixture was extracted with ethyl acetate ~3 x 50ml~.
The combined organic extracts were dried (MgS04) and evaporated in vacuo.
The residue was purified by normal phase HPLC using a Dynamax 300A silica
column with a flow rate of l.OmVmin and 5% methanol/dichloromethane as
eluant to give the title compound, retention time 20.7min, m/z (FAB~ 433 (M +
1).
CA 02242713 1998-07-07
W O 97/26261 PCTIGB96102674
O O
-F ~ COzEt
N ~ O~" N ~