Note: Descriptions are shown in the official language in which they were submitted.
CA 02243661 1998-07-20
W O 98n2476 rCT~US96/1&~
--1--
~YNTHE8I8 OF TITANOCENE8
FT~Tn OF TRE TNV~pTlON
This invention relates to the synthesis of
titanocenes including constrained geometry titanocene
catalysts utilizing a unique titanium trichloride
reagent.
BA~-~kGuND OF T~ TNV~NTION
The evolution of metallocene-h~~1 catalysts for the
polymerization of ethylene and higher ~-olefins is
reviewed in H.H. Brintzinger, et al., Anq~ew.
~hem.Int.~.Enal. 34:1143-1170 (1995) and in P.C.
Mohring, et al., J. Orqanomet~l.Chem. 479:1-29 (1994).
The applications of chiral metallocenes in organic
synthesis are reviewed in R.L. Halterman, Chem.Rev.
~:965-994 (1992). These reviews highlight the
applications of state-of-the art metallocenes. Most
often, these applications center on the use of titanium
containing and other Group IV metallocenes.
The early preparation$ of Group IV metallocenes
involved reactions of the metal tetrahalides, typically
the tetrachlorides, with deprotonated ligands, such as
sodium cyclopentadienide, to give the metallocene~ in
good yields. The metallorQn~c of current interest
possess more complicated ligand structures, and their
preparations are not as straightforward. For the
preparation of these metallocenes, the use of titanium
tetrachloride often results in low yields of the desired
metallocenes. Titanium trlchloride (TiC13) is often
specified for use in place of titanium tetrachloride
(TiCl~); subsequent oxidation gives the desired
metallocenes in greatly improved yields.
For some recent examples which specify the use of
titanium trichloride, see L.A. Paquette, et al.,
Orqanome~llics ~:4865-4878 (1995): F. Zaegel, et al.,
Or~anometallics 14:4576-4584 (1995); and M. E.
Huttenloch, et al., Orqanonetallics 11:3600-3607 (lg92).
Halterman, supra, cites references which show the use of
titanium trichloride in several metallocene preparations.
. . .. .. ... . .. ... . ... .
CA 02243661 1998-07-20
W098/22476 PCT~S96118666
-2-
The titanium trichloride so used is produced from
commercial titanium tetrachloride. Titanium trichloride
produced ~y hydrogen reduction of the tetrachloride is
most often used in lab-scale preparations. ~or
commercial-scale preparations, this is impractical due to
cost and the presence of acidic impurities. These
impurities require purification of the titanium
trichloride, typically by preparation and isolation of an
ether complex, usually the tetrahydrofuran complex.
Commercially-available titanium trichloride is
produced by the reduction of the tetrachloride with alkyl
aluminum compounds. The titanium trichloride so produced
contains aluminum chloride, which is not removed.
Typical analyses specify 76-79 weight percent of titanium
trichloride with the remaining weight percent comprised
mostly of aluminum chloride. The use of aluminum-reduced
titanium trichloride in metallocene preparations often
gives products which contain varying amounts of aluminum-
containing impurities. Separation of these impurities
from the product titanocenes is not straightforward in
most cases, especially on a commercial scale. The
pres~nc~ of these impurities can have significant adverse
effects during subseguent uses of the titanocenes,
particularly in olefin polymerizations.
Accordingly, a need exists for a titanium
trichloride reagent useful to produce titanocenes free of
aluminum containing impurities.
D~ ~lNl~ lON8
For the purposes of this invention, the following
terms have the meaning stated:
Tit~noq~ns Compound - A compound comprised of
titanium bonded to one or more cyclopentadienyl rings.
Tit~nocene Ligand - A chemical precursor which
contains cyclopentadienyl or substituted cyclopentadienyl
CA 02243661 1998-07-20
W O 98n2476 PCT~US96/18666
-3-
moieties (including indenyl, fluorenyl, etc.) used to
prepare a titanocene compound.
Constrained ~eometry Catalyst (CGC) - A catalyst in
which the metal center is contained in a ring structure
and covalently bonded to a cyclic group via a delocalized
~-system and covalently bonded via a sigma-bond to
another atom such as carbon, nitrogen, oxygen, etc. A
small ring size induces constraint about the metal atom
center. For titanium-containing CGC's, the incorporated
titanium atom can be in a formal ~4, +3, or +2 oxidation
state. See EP application 90309496.9, WO 95/00526 and
U.S. Patent 5,470,996.
CpSA Ligand - (t-butylamino)(tetramethylcyclo-
pentadienyl)dimethylsilane.
(Cp8A) 2- _ doubly-deprotonated CpSA ligand.
(Cp~A)2-TiClz - [(t-butylamido)(tetramethylcyclo-
pentadienyl)dimethylsilane]titanium dichloride.
8ubstantially 8toichiometric A~ount - An amount not
less than 90% nor more that 110% of stoichiometric.
~UNMARY OF T~ INV~TION
This invention includes a general method for
producing titanium trichloride containing mixtures
suitable for the preparation of titanium-containing
metallocenes including constrained geometry Ti(IV),
Ti(III) and Ti(II) complexes free of aluminum containing
impurities.
The titanium trichloride containing mixtures are
produced by the preferably stoichiometric ~1:1) reaction
of an organometallic compound, such as n-butyl lithium or
n-butyl magnesium chloride, with titanium tetrachloride
in a non-interfering solvent medium. These mixtures are
used directly without isolation of the titanium
trichloride in reactions with appropriate ligands to
produce the desired titanocenes, including constrained
geometry titanium complexes, in good yields. The
CA 02243661 1998-07-20
W 0 9X~2476 PCT~US96/18666
-4-
- resulting titanocene products are specifically free of
aluminum-containing impurities.
D~TA~LED DBSCRI~TION OF THE INVENTION
The invention is a method for producing a titanium-
con~ining metallocene compound which comprisesseparately providing a first reaction mixture containing
titanium trichloride and a second reaction mixture
containing a magnesium or alkali metal or alkaline earth
metal salt of a metallocene compound ligand. The first
and second mixtures are combined for reaction to produce
an intermediate from which an aluminum-free titanocene
useful as an olefin polymerization catalyst may be
synthesized.
The first reaction mixture is produced by reacting
TiCl4 with an alkali metal compound having the formula
~-M or a Grignard reagent having the formula RMgX. In
each formula, R is a straight or branched chain aliphatic
hydrocarbon group, preferably an alkyl group, having 2
to 10 carbon atoms. R may also be an alkaline earth
metal such as calcium, barium or strontium. X is the
value of M. In the formula ~-M, M is an alkali metal
such as sodium, potassium or lithium. In the formula
KMgX, X is a halogen, preferably chlorine. n-butyl
lithium or n-butyl magnesium chloride are preferred. The
reactants are combined in substantially stoichiometric
amounts in a non-interfering, preferably hydrocarbon,
medium.
Useful hydrocarbon media include aliphatic or
aromatic hydrocarbons, such as hexane, heptane,
cycloheY~e, benzene, toluene and xylene. Toluene is
preferred for the specific examples shown here. Useful
ether and polyether solvents include tetrahydrofuran,
diethyl ether, ethylene glycol dimethyl ether, and
dioxane. Mixtures of any hydrocarbon and ether solvents
are useful for the reaction.
CA 02243661 1998-07-20
W098~2476 PCT~S96/18~6
-5-
The reaction is preferably accomplished under dry,
oxygen-free conditions. The temperature at which the
reaction is conveniently conducted is -20~C or 120~C,
with the optimum temperature range being 30-40~C.
The second reaction mixture i~ separately provided
by deprotonating the desired metallocene ligand with the
appropriate base by known methods. See, generally,
Paquette, et al., ~u~ra; Zaegel, et al., su~ra; and
Halterman, supra.
The first reaction mixture, which includes the
medium or solvent, titanium trichloride and a metal
halide such a LiCl or MCl2, is added directly without
isolation of the titanium trichloride to the second
deprotonated ligand reaction mixture to produce a first
titanocene.
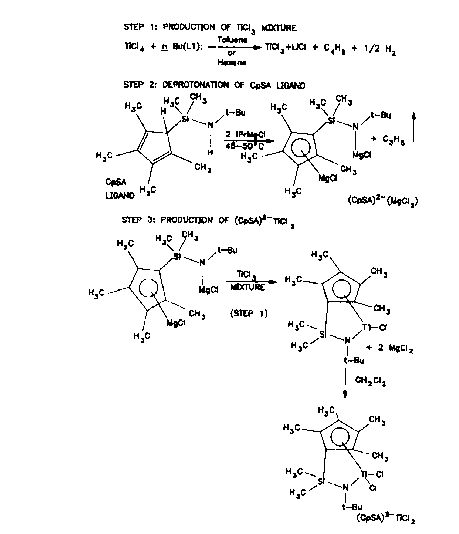
DE~CRIPTION OF THE FIGU~
Figure l is a generalized depiction of steps (l),
(2), and (3) as comprised by one embodiment of the
invention for preparation of a Ti(IV) complex constrained
geometry catalyst.
Ste~ Al~mi~um-Free TiCl3
Step (l~ of Figure l illustrates the reaction of
TiCl4 in substantially stoichiometric amount with n-butyl
lithium or n-butyl magnesium chloride to produce TiCl3 and
lithium or magnesium chloride in a hydrocarbon or ether
medium, or mixed hydrocarbon and ether medium.
Step (2) - Deprotonation
Of a Metallocene Co~pound Liqand
Step (2) of Figure l illustrates the double
deprotonation of the metallocene compound ligand (~-
butylamino)(tetramethylcyclopentadienyl)dimethylsilane
(CpSA ligand) with an organometallic deprotonating agent,
preferably an organolithium or an organomagnesium
CA 02243661 1998-07-20
W 0 98/22476 PCT~USg6/18666
--6--
~u.~oulld (Grignard reagent), in a hydrocarbon medium,
preferably toluene.
The solvent medium and the organometallic compound
may be the same as or different from the solvent medium
and the organometallic compound used in Step (1). The
concentration of the CpSA ligand in the solvent is
appropriately 0.05 to 1.5 M, preferably 0.45 to 0.6 M.
Any Grignard reagent may be used to deprotonate the
metallocene compound ligand, e.g., CpSA. Useful Grignard
reagents have the formula RMgX as defined above.
Isopropyl magnesium chloride is preferred. A practical
range of Grignard concentration in the solvent is 0.5
to 3.0 M, preferably 1.9 to 2.3 M. For CpSA, the
temperature is controlled to be 45-50~C. at the end of
the Grignard feed, and then heated to 85-90~C for the
prescribed time.
The Step (2) reaction mixture is preferably used
directly in Step (3) as the toluene solution present in
the reaction vessel in which it is produced.
Step (3) - Reaction of TiCl3 With
De~rotonated Liqands- Production of (CDSA)2-TiCl2
Step (3) of Figure 1 illustrates one method for
reacting the titanium trichloride containing reaction
mixture of Step (1) with the (CpSA) 2- containing second
reaction mixture of Step (2) to produce [(t-butylamido)
(tetramethylcyclopentadienyl) dimethylsilane]titanium
dichloride, (CpSA) 2- ~iC12.
In this embodiment of the invention, the agitated
Step (1) reaction mixture is transferred directly into
the reactor containing a~itated Step (2) reaction
mixture. Preferably, the vessel which contained the
Step (1) mixture is rinsed with toluene which is then
charged to the Step (3) reactor. The exothermic reaction
mixture becomes reddish brown in color. A temperature
rise of about 15~C. is usually observed.
CA 02243661 1998-07-20
wo9~n~76 PCT~S96/1~6
-7-
A chloride-containing oxidizing agent, such as
dichloromethane or silver chloride, is then charged to
the reaction vessel utilized in Step (3). The resulting
reaction mixture is agitated for a time appropriate,
usually about two hours, for the Step (3) reaction to
occur.
Solvents are removed under reduced pressure,
i.e., 60-80 mm Hg, to about one-half of the starting
volume. Hydrocarbon solvent, e.g., toluene, is added
back, Celite~ filter aid i9 added, and the mixture is
filtered. Solvent~ are distilled to concentrate the
product.
The solid titanocene can be isolated from this
mixture by methods dependent upon the actual compound
being produced. For the (CpSA)2-TiCl2 example shown in
Figure l, the solid product was collected in 75-80% yield
a~ described in Example 6. Additional material of lower
purity can be isolated upon further manipulation of the
mother liquors. Alternatively, the product solution
obtained after removal of the magnesium salts can be used
directly to produce other metallocenes described in
Example 6.
EXEMPLIFICATION OF THE INV~NTION
Preparative Procedures For Titanium Trichloride
25Reactant Mixtures and Resultinq Metallocenes
The general procedure for the preparation of
titanium trichloride-containing mixtures by the reaction
of titanium tetrachloride and an organometallic compound
under an inert atmosphere is first described, followed by
three specific examples. The reaction apparatus
consisted of a 500-mL 3-neck flask equipped with a
mechanical stirrer. On one side-neck was placed a
Claisen adapter with a reflux condenser and a thermometer
inserted into the reaction mixture. This apparatus was
previously dried and then purged with nitrogen after
CA 02243661 1998-07-20
WOg8~76 PCT~S96/18666
--8--
assem~ly. The solvent was added via the other side-neck
of the reaction flask, which was then capped with a
rubber septum. TiC14 (ca. 25 mL, 42-44 g. 0.22-0.23 mol)
was transferred from a weighed bottle to the reaction
flask using a syringe. The rubber septum was replaced
with a dried, nitrogen-purged addition funnel. The THF
and/or the organometallic compound was transferred to the
addition funnel and then added to the TiCl4/solvent
reaction mixture at the de6ired temperature.
~MPLE 1
A solution of n-butyllithium (BuLi) in he~nes (156
mL of a 1.60 M solution, 0.250 mol of BuLi) was added to
TiCl4 (43.0 g, 0.227 mol) in 300 mL of toluene over 30
min. The initial temperature of the reaction mixture
was 10~C, the temperature increased to 40~C during the
addition, and was then maintained at 35-40DC using
external cooling. After the addition of the BuLi, the
reaction mixture was stirred at 35-40-C for 1 hour.
After cooling to room temperature, the addition funnel,
¢ondenser, and Claisen adapter were removed while
maintaining an inert atmosphere of nitrogen over the TiCl3
product mixture. The resulting TiC13 containing mixture
was used directly in reactions with deprotonated
metallocene ligands.
EX~MP1F 2
THF (100 mL) was added to a solution of TiCl4 (43.4
g, 0.229 mol) in 200 mL of toluene over 30 minutes
at 0-15~C. Then BuLi in hexanes (156 mL of a 1.60 M
solution, 0.250 mol) was added over 40 min at 5-lO-C.
The resulting mixture was heated to 35-40~C and stirred
for 1 hour. After cooling, the resulting TiC13 slurry was
used direct~y in reactions with deprotonated ligands.
EXAMP~ 3
THF (75 mL) was added to a solution of TiCl4 (42.0 g,
0.221 mol) in 150 mL of toluene over 20 min at 0-15~C.
CA 02243661 1998-07-20
W O 9&~2476 rCTAUS96/186G6
_g_
Then a solution of butylmagnesium chloride ~BuMgCl) in
THF (115 mL of a 2.10M solution, 0.242 mol of BuMgCl) was
added over 30 min. The initial temperature of the
reaction mixture was O~C; the temperature increased
to 40~C during the addition and was then maintained
at 35-40~C using external cooling. After the BuMgCl
addition, the reaction mixture was stirred for 1 hour
at 35-40-C. The resulting TiCl3 product slurry was used
directly in reactions with deprotonated ligands.
EXAMP~
The D-protonation of Cp8A ~ig~nd
~ith i-Propylmagn-s;um Chloride
The reaction apparatus consisted of a 2000-mL 3-neck
flask e~uipped with a mechAnical stirrer; on one side-
neck was placed a Claisen adapter with a Vigereaux columnand distillation head for solvent distillation. A
thermometer was inserted into the reaction flask through
the Claisen adapter. The glass apparatus was previously
dried and purged with nitrogen after assembly. Toluene
(425 mL) and CpSA ligand (55.0 g, 0.219 mol) were added
to the reaction flask. The temperature of the reaction
mixture was adjusted to 45-50CC. A solution of i-
propylmagnesium chloride (i-PrMgCl) in ether (205 mL of
a 2.30 M solution, 0.472 mol of i-PrMgCl) was added
over l hour using an addition funnel. After the i-PrMgCl
addition, the reaction mixture was gradually heated
to 85-90~C over 2 hours and stirred at this temperature
for an additional 2 hours. The (CpSA)Z~(MgCl)2 formed a
gummy solid at thi~ stage. The heating is removed and
the temperature of the reaction mixture cooled
to 60-65~C. At this temperature, THF (150 mL) was added
dropwise over 15 min, which dissolved the solid (CpSA)2-
(MgCl)2. The reaction mixture is then cooled to room
temperature. The distillation head, Vigereaux column,
and addition funnel are then removed from the reaction
CA 02243661 1998-07-20
W 0 9~22476 PCTrUS96/18666
--10--
apparatus while maintaining an inert atmosphere of
nitrogen over the product mixture. This (CpSA) 2- (MgCl) 2
solution was then used directly in a reaction with a TiCl3
slurry prepared previously.
EXAMP~ 5
The Deprotonation of Cp8A
Liaand wi~ n-Butyl~ithium
The reaction apparatus consisted of a 2000-mL 3-neck
flask equipped with a mechAnical stirrer; on one side-
neck was placed a Claisen adapter with a reflux condenserand a thermometer inserted into the reaction flask. The
glass apparatus was previously dried and purged with
nitrogen after assembly. Ether (300 mL) and CpSA ligand
(62.9 g, 0.250 mol) were added to the reaction flask.
The reaction mixture was cooled to -20-C. A solution of
BuLi in he~Anes (305 mL of a 0.170 ~ solution, 0.518 mol
of BuLi) was added over 1.5 hours; the temperature was
maintained at -20 to -15-C during this addition. The
reaction mixture was then warmed to 0-5-C over 1.5 hours
and stirred at this temperature for 3 hours. The
resulting (CpSA)2-Liz slurry, which consisted of a white
solid with a pale yellow supernatant, was used directly
in a reaction with a TiC13 slurry prepared as described in
Examples 1 to 3.
EX~MP~E 6
The Preparation of (C~A)2-TiCl2
The TiCl3 containing mixture from Example 1 above was
transferred under nitrogen pressure via a wide-bore
cannula to the (CpSA)Z~(MgCl) 2 solution from Example 4
above over 2-3 min. Toluene (100 mL) was added to the
TiCl3 flask which contained some residual TiC13, and this
wash was quickly transferred to the reaction flask. The
initial temperature of the reaction mixture was 22-C: the
temperature increased to 3~~C during the TiCl3 addition.
The reaction mixture was stirred for 15 min at 35~C, at
CA 0224366l l998-07-20
W O 98/22476 PCT~USg6/18666
--11--
- which time dichloromethane (13.5 g) was added over 1 min;
the temperature increased to 38-C. The resulting red-
brown mixture was stirred for 2 hours with gradual
cooling to 25~C. Solvents were removed by simple
distillation under reduced pressure (60-80 mm Hg) to a
final volume of ca. 600 mL; the temperature ranged
from 30 to 60-C during this distillation. After cooling
to 20-C and pressurizing with nitrogen, toluene (400 mL)
was added to the product mixture. Magnesium salts were
removed from this mixture by pressure filtration under
nitrogen using Celite~ filter-aid. The reaction flask
and filter cake were washed with two 200-m~ portions of
fresh toluene. The red-brown filtrate was concentrated
by simple distillation under reduced pressure as before
to a volume of 400 mL. This toluene solution is again
filtered under nitrogen pressure to remove residual
magnesium salts. The filtrate is concentrated again to a
volume of 200 mL by simple distillation under reduced
pressure. Heptanes (400 mL) were added over 30 min with
stirring at 20-25-C. A first crop of orange, crystalline
(CpSA)2-TiCl2 is collected by filtration under nitrogen,
washing with heptanes, to give 61.1 g of product in 76%
yield. A second crop was obtained by concentration of
the mother liquors to ca. 100 mL and dilution with
heptanes.
Alternatively, the product solution in toluene
obtained after removal of the magnesium salts was used
directly to prepare other metallocenes. For example, the
toluene solution of (CpSA)2-TiCl2 was treated with 2
equivalents of methylmagnesium chloride ~THF solution) to
give (CpSA)2-Ti(CH3) 2 in 70-7596 overall yield.