Note: Descriptions are shown in the official language in which they were submitted.
CA 02243801 1998-07-23
Process and Devices for Detecting Substance Emissions
The present int~ .ntion relates to a process and devices for detecting substance emissions.
The collection and detection of trace substances from the gas phase is extremely important in many
areas of application. Particular difficulties are encountered when ~etecting substance emissions, which
is to say of gases, vapours, or aerosols, that are emitted from the surfaces of articles such as, for
example, park~ging, textiles, joists, p~nelling and the like.
Similar tasks are associated with the analysis of foodstuffs, where the diffusion and/or penetration of
substances through park~ging materials has to be cletectr~, or with environmelltal analysis, when the
off-gassing of injurious substances from articles has to be identified.
Up until now, test-chamber methods (Matthewsj T.G., 1987: Atmospheric Environment 21, 321-329)
have been used in order to quantify substance emissions from articles. The disadvantage of these
methods lies in the costly implementation of sample collection, and subsequent instrumental analysis
that is time-consuming and has to be conducted by practitioners skilled in the art.
DE 195 38 075 describes a procedure that simplifies the concentration of substance emissions. Using
this process, a self-adhesive saunple collection system is affixed to the article from which samples are to
be taken, and this makes it possible to collect substances that are off-gassed from articles in a sorbent.
The analysis samples can be detected by subsequent chemical or biochemical reaction that is then
evaluated in a multi-stage procedure once the analysis samples have been extracted from the sorbent or
after incubation of the sorbent in various solutions.
CA 02243801 1998-07-23
.
Biochemical detection processes have already become established in medical tli~gno~ (Hage, D.S.,
1993: Analytical Chemistry 65, 420r-424r) and in the analysis of foodstuffs. To an ever-increasing
extent, corresponding processes are being used in envh."~ r~,l;ll analysis (Bangs, B.L., Meza, M.B.,
1995: IVD Tec~nology 2). So-called immunoassays, which are based on antibody-antigen reactions,
5 are of great interest because of their broad applicability and great sensitivity.
Procedures for the immunochemical detection of analysis samples from the gas phase are also known
from the prior art. For example, DE 41 21 493 describes processes for the detection of injurious
substances from the gas phase; this describes a competitive reaction between analysis samples or
10 marked tracers and antibodies.
In contrast to this, DE 44 25 963 describes processes in which the gaseous analysis sample is bound to
a bonding partner present in a carrier matrix by means of immunological bonding, and then identified.
Finally, EP 650 054 relates to a processes for detecting analysis samples from the gas phase, in which a
gas is passed through a carrier matrix, whereupon the substances that are to be identified bond to a
bonding partner contained in the matrix.
None of the processes or devices described in the docllm~nt~ referred to above is suitable for detecting
20 substance emissions from articles.
Accordingly, it is the task of the present invention to describe processes and devices that permit the
rapid and simple identification of substance emissions when used as sample-collection and analysis
systems.
CA 02243801 1998-07-23
According to the present invention, this task has been solved by a process in which a device comprising
a self-adhesive layer, a collector matrix, and an indicator matrix is aff1xed to the surface of an article,
when it then detects the analysis sample that has been ~cc lm--l~ted in the collector matrix, a developer
is added, and the analysis sample is converted in a single-stage processes with a bonding partner, when
5 the presence or absence of the analysis sample in the indicator matrix is indicated. As a result of this
one-step process, it is only necessary to add the developer solution, and no other processing or analysis
steps are required, as was the case with the multi-stage processes that have been used up to the present
time.
10 The present invention provides an indication of substance emissions, this surprisingly sensitive and
selective and combines rapid detection with ease of use. For the first time ever, the process according to
the present invention permits in situ indication of substance emissions such as gases, vapours, and/or
aerosols that diffuse from articles. In the shortest possible time, this process can be used by persons
who are not necessarily practitioners skilled in the art. Detection that has been carried out according to
15 the present invention can be assessed, for example, after three to ten minutes.
The process provides for both quantitative and qualitative indication.
The process according to the present invention is suitable for detecting any form of analysis sample that
20 is diffused from articles. Preferred is the detection of odorising or arom~ticing agents, drugs,
explosives, pesticides, or environmP.nt~lly harmful substances. Examples of such substances are
cocaine, tetrahyrdocannabinol, trinitrotoluene, nitroglycerine, hexogene, polyaromatic hydrocarbons,
and polychlorinated biphenyls.
2s The process according to the present invention can also be configured so as to permit the detection of a
variety of analysis samples using only one device.
CA 02243801 1998-07-23
According to one preferred embodiment of the present invention, all of the reaction partners that are
required to detect an analysis sample are present in the device and/or in the developer solution at the
beginning of the detection process. Particular advantages ensue if the bonding partner, which can be
marked, is present in dry form within the collector or indicator matrix.
The detection reaction is initiated by releasing or applying a metered dose of developer. To this end, a
container for the developer can be integrated into the device. The developer can be any solution in
which the analysis sample and the bonding partner can be dissolved. Preferred according to the present
invention are buffered, aqueous solutions that contain up to 50 %-vol of organic solvents and other
10 dissolving intermediaries such as d~ g~
According to a preferred embodiment of the present invention, the analysis sample is accumulated in the
solution during diffusion of the developer through the collector matrix. The result of this is that even
very small quantities of the substance can be detected in the indicator matrix that follows.
According to another embodiment of the present invention, addition of the developer initiates the
reaction between the analysis sample and the bonding partner. According to the present invention,
immunoglobulins or fragments or derivatives thereof are preferred as bonding others. Preferably, these
are antibodies, with monoclonal antibodies being especially preferred.
In keeping with the procedures described in the prior art, the bonding partner can be marked, marking
with enzymes, fluorphores, radioactive isotopes, metal colloids, and/or dyed particles being preferred.
Marking with dyed particles and/or metal colloids is particularly preferred, since this permits direct
visible evaluation or analysis.
CA 02243801 1998-07-23
The prior art describes numerous procedures that permit identification of a reaction between the
analysis sample and a bonding partner. The use of appropriate identification procedures in the process
according to the present invention is limited only by their sensitivity and their amenability to analysis.
5 According to one preferred embodiment of the present invention, detection is based on an
immunochemical solid phase reaction in which a specific bonding partner is immobilised in the
indicator matrix. Coupling to the solid phase can be adsorptive, ionic, covalent, or by bridging the
specific bonding partner with, for example, protein A, avidin, or latex particles.
10 Depending on the format of the immunochemical detection, the solid phase reaction can be in the
formation of the complex from immobilised bonding partner, the substance that is to be detecte-l, and
the bonding partner (bilateral test) or of the complex of immobilised bonding partner and additional
bonding partners (competitive test).
15 In the competitive test, in addition to the specific bonding partners or fragments thereof, the substance
to be detected, or derivatives thereof that can be coupled to macromolecules are used as additional
bonding partners.
The present invention also includes a process for detecting substance emissions in which the release of
20 analysis samples from the article from which samples are to be collected is enhanced by heating the
article or by the use of electrochemical processes, for example, iontophoresis.
In particular, the present invention relates to devices used to carry out the detection process according
to the present invention. Appropriate devices incorporate a self-adhesive layer, a collector matrix, and
25 an indicator matrix, and are so configured that an analysis sample that has become concentrated in the
CA 02243801 1998-07-23
.
collector matrix is converted in a single-stage process by the addition of a developer with a bonding
partner, the reaction then being indicated in the indicator matrix.
According to the present invention, it is preferred that the adhesive layer leaves an enclosed sample
5 space between the article and the collector matrix when the device is affixed to the article from which
samples are to be collected. Environm~nt~l influences such as convection, visible light, and dust
concentrations are thus precluded. The collection of samples is only affected by temperature .
In addition, the self-adhesive layer can also affix the sample collection system to the article itself. Rigid
10 housings that are of various plastics or metals can be used as materials for the self-a&esive layer or
barriers, as well as flexible adhesive films; the latter entail the advantage that they mould themselves to
the shape of the article from which samples are to be collected.
The material for the collector matrix can be any material that permits adsorption and concentration of
15 the particular analysis samples when it is in the dry state. In addition, it must be possible to elute the
analysis sample from the collector matrix by the addition of a developer. To this end, the collector
matrix must also be capable of capillary action. According to the present invention, it has been
established that membranes or random mats are suitable as the collector matrix. Random mats that are
of glass fibres, cellulose, plastics, or silica have been found to be particularly effective.
Preferred according to the present invention are devices in which the collector matrix is of a material
that has a linear water absorption rate between 1 mm/minute and 10 cm/minute and/or a pore diameter
between 0.1 ~lm and 50 ~lm, with materials having a pore diameter between O.S ~m and 10 ~m and/or a
linear water absorption rate between 5 mm/minute and 5 cm/minute being particularly preferred.
CA 02243801 1998-07-23
According to another preferred embodiment of the present invention, the collector matrix is between
100 ~m and 1 cm thick.
All types of materials that can enter into fluid contact with the preceding collector matrix can be used
S as materials for the indicator matrix, these being characterised in that the substances that are to be
indicated do not bond to the material or do so only to an in~ignific~nt degree. The indicator matrix can
be of only one material or can be of a plurality of different materials that are in fluid contact with each
other. Preferred according to the present invention are membranes or random mats that are between 100
!lm and 1 cm thick and/or have a pore diameter between 0.1 ~m and 50 ~lm and/or a linear water
10 absorption rate of 1 mm/minute to 10 cm/minute. The indicator matrix can also be of the same material
as the collector matrix.
The devices according to the present invention can also incorporate a spacer that prevents any direct
contact between the collector matrix and the surface of the article from which samples are to be
15 collected. Especially preferred is a spacer that is of a mesh-like structure with an unobstructed surface
of more than 50 percent and which is between 10 !lm and 100 ~lm thick. Thin metal, textile, or plastic
fabrics can be used as these materials, for example, these adsorbing the substance that is to be indicated
either not at all or only to a very small extent.
20 If the device is to be used for a competitive indicating process, configurations of the devices that
incorporate a catch zone are preferred. This catch zone is used to immobilize bonding partners that are
- not coupled to an analysis sample. This principle is familiar from breakthrough chromatography (cf.
C.R. Lowe, P.D.G. Dean, Affinify Chromafography, Wiley & Sons, New York, 1974; and EP 052
769), and results in the fact that only the bonding partners that break through will reach the indicator
25 zone.
CA 02243801 1998-07-23
According to another preferred embodiment of the present invention, the device is so configured that it
is possible to apply the developer to a specific point in the device.
The present invention will be described in greater detail below on the basis of embodiments shown in
5 the drawings appended hereto. These drawings show the following:
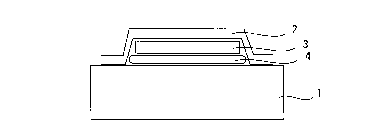
Figure 1: a cross section through the device according to the present invention that is used to detect
substance emissions that are associated with a particular article;
Figure 2a: a view of a device according to the present invention that is based on the unidirectional
movement of liquid;
Figure 2b: a few of an analogous device that is based on the radial movement of liquid;
Figure 3: a preferred embodiment based on the unidirectional movement of liquid, shown
diagrammatically in plan view.
15 The devices shown in Figure 1 to Figure 3 clarify the underlying structural principles of the devices
according to the present invention. These do not, however, preclude the fact that another structure can
be selected and/or that additional components can be integrated into the device.
The device that is shown in Figure 1 consists of a self- adhesive layer 2, the collector matrix 3, and the
20 spacer 4 and is affixed to the surface of an article 1 that is releasing a substance. The substance that is
emitted at the surface of the article 1 passes through the spacer into the collector matrix, where it
concentrates.
The self-adhesive layer or barrier 2 is used to exclude external influences and renders the device
25 gastight with respect to the substance that has been collected.
CA 02243801 1998-07-23
Various substances can be used in the form of l--w--bl~les or random mats to form the collector matrix
3 . The spacer 4 prevents any direct contact between the collector matrix and the surface of the article
from which samples are to be collected if diffusion sampling is used to do this.
5 Figure 2a shows a device according to the present invention that is based on the unidirectional
movement of liquid. After application of the developer to the distal end x of the collector matrix 3 the
sample liquid moves by capillary action into the indicator matrix 4 in which the immunochemical
detection reaction takes place.
10 Figure 2b shows a device that is based on the radial movement of liquid. Because of the lateral
movement of liquid through the collector matrix, the analysis sample conc~ Lt;s in the liquid front.
The result of this is that even very small quantities of substance can be detected in the indicator matrix
5 that follows.
15 The marked bonding partner can be contained in the collector matrix 3 or in the indicator matrix 5 in
dry form. Because of the movement of liquid of the developer the marked bonding partner will be
dissolved out of the matrix and moved into the zone in which an immuno~h~mic~l solid phase reaction
takes place.
20 Figure 3 is a diagrammatic plan view of a preferred embodiment based on the unidirectional movement
of liquid.
The immuno-chromatographic test section that consists of the collector matrix 3 and the indicator
matrix 5 lies on the spacer 4 and is covered by the layer 2. The layer 2 incorporates a cutout 6 through
25 which the developer is applied, and a viewing aperture 7 that permits detection of the immunochemical
CA 02243801 1998-07-23
signal in the indicator matrix. The cutout 6 can be covered over by an adhesive film during the
collection phase, and this film is then removed imm~ tely prior to the application of the developer.
Testing is carried out such that the device is affixed to the article from which samples are to be
5 collected with the adhesive surface that is contained in the layer 2. After a specific collection period, a
metered does of the developer is applied to the cutout 6 or is released, and this initiates immuno-
chromatographic detection of the substance that has been collected. The signal can be seen through the
viewing aperture 7, and then evaluated.
10 The present invention is described below on the basis of a device used to detect the emission of
pentachlorphenyl from various kinds of wood, and a corresponding indication process:
Examples
A) Concentration effficiency of various collector matrices
Various random mats or membranes measuring 2 cm2 were cemented for four hours
USillg an air-impermeable adhesive film to a wood sample that had been treated with 1
g PCP/m2. A spacer (Estal Mono PE 22 HC Super, Schweizer Seidengazefabrik,
Switzerland) prevented any direct contact between the collector matrix with the surface
from which samples were taken.
~0
Next, the sorbents were extracted in 2 ml 0.1 M sodium phosphate buffer at pH 8.0 in
an ultrasonic bath and the PCP content of the extracts analyzed with a microtitation
plate ELISA. This indicated clear differences in the concentration efficiency of the
various collector matrices.
CA 02243801 1998-07-23
Collector matrix Concentrated quantity of PCP
(nglcm2)
LN0038 (Nicolon, Holland) 10.7
F39Z
(Hollingsworth &Vose, UK) 4.0
Nitrocellulose, 3~m
(Schleicher & Schull, Germany) 2.25
F286-13 (Wlhatman, UK) 3.5
F075-14 (Whatman, UK) 15.2
B) Extraction efficiency of various collector matrices
50 ng PCP in methanol was applied to various 2cm2 random mats or membranes by
pipette. After evaporation of the methanol, the sorbents were extracted in 2 ml 0.1 M
sodium phosphate buffer pH 8.0 (PBS buffer) in an ultrasonic bath and the PCP
content was analyzed with the help of a microtitation plate ELISA
There were considerable differences in the extraction efficiency of the various collector
matrices:
Collector matrix Extraction efficiency (%)
LN0038 (Nicolon, Holland) 85
F39Z
(Hollingsworth &Vose, UK) 90
Nitrocellulose, 3~1m
(Schleicher & Schull, Germany) 55
F286- 13 (Wlnatman, UK) 80
F075-14 (Whatman, UK) 85
Immunodyne, 5 ~lm, (Pall, Germany) 25
CA 02243801 1998-07-23
C) Production of the gold marking
0.5 L of distilled and filtered (0.2 llm) water was heated to boiling point in asiliconized beaker whilst being stirred, when 5 ml 1-% auric acid was added to it. The
solution was allowed to boil for a further 5 minutes and then 20 ml 1-% sodium citrate
S solution was added to it. After another 10 minutes a change of colour from blue to red
indicated the end of the reaction. The colloid was cooled to room temperature in an ice
bath and stabilized by the addition of 5 ml 2-% NaN3 solution and 0.5 ml 1-% PEG(polyethyleneglycol) 20000 (all qll~ntitie~ in the section indicate %-wt).
10 D) Production of the gold-marker conjugate
The pH value of the gold colloid solution was adjusted to pH 9 by the addition of 0.2
M K2CO3. 10 mg of a PCP-specific monoclonal antibody was added to the solution
and then incubated for 30 minutes at room temperature. After the addition of 200 mg
protein C to the solution, it was incubated for a further 30 minutes and then
centrifuged for 15 minutes at 40,000 x g. The conjugates of the antibody and the gold
marker were obtained by absorbing the pellet in 0.1 M HEPES (hydroxyethyl
piperazine ethane sulfonic acid)-buffer, pH 7.0, with 0.1% protein C and 0.05 % PEG
20000.
20 E) Production of the PCP conjugate
1 mg 5-(4-hydroxy-2,3,5,5-tetrachlorphenoxy) valeric acid with 1.7 mg N-hydroxy
succinimide (NHS) and 6.2 mg N,N'-dicyclohexylcarbodiimide (DCC) were dissolved
in DMF and incubated for 18 hours at room temperature. The mixture was then added
drop-by-drop to a solution of 2 mg Ziegen-IgG in 2 ml 0.15 M sodium hydrogen
carbonate solution, incubated for a further 3 hours, and dialyzed for 2 days against
PBS buffer.
F) Production of the gold marker zone
F075-14 glass fibre mat material (Whatman, UK) was cut into l-cm wide strips,
soaked in the gold marker conjugate solution (optical density adjusted to 10 at 520 nm)
and dried at 40~C for 20 minutes in a convection oven.
CA 02243801 1998-07-23
G) Production of the collection zone
F-286-13 carboxyl group modified glass fibre mat material (Whatman, UK) was cut
into l-cm wide strips and incubated in a solution of 0.2 mg/ml ) PCP conjugate and
0.1 mg/ml 1-ethyl-3(3-dimethylaminopropyl)-carbodiimide in PBS, pH 7.0, for 2 hours
at room temperature. The mat material was then washed with PBS for 4 hours and
dried in a convection oven for 20 minutes at 40~C.
H) Assembly of the immunochromatographic test strip
As in Figure 2a, an immunochromatographic test strip, of a constant width of 0.5 cm,
was affixed to a l-mm thick plastic laminate with double-sided adhesive tape. The
collector matrix (3) was assembled from a 2-cm long F075-14 glass fibre material(Whatman, UK) and adjacent to it from the gold marker zone (f) The detection matrix
(5), which was in fluid contact with the collector matrix, comprised the collection zone
(g) and an adjacent indicator zone of GFF 1-cm long glass fibre mat (Whatman, IJK).
Fluid contact was effected by overlapping the zones by 1 mm
I) Device for indicating substance emissions
The immunochromatographic test strip discussed in Para. H above was aff1xed withthe plastified side to a plastic strip impermeable to air, in such a way that an a&esive
edges 2 cm long was left on each side. The spacer (Estal Mono PE 220 HC Super,
Schweizer Sei~rng~7~f~brik, Switzerland) was then stretched across the
immunochromatographic test strip, and was secured on the adhesive film by
ov~llap~illg it by 5 cm.
25 J) Production of the developer solution
0.1% sodium azide, 0.5% Triton X-100, and 0.5% polyethyleneglycol 2000 was addedto a PBS base buffer, pH 8.0
K) Sample collection and analysis
The device constructed as in Para. I was c~m~nted onto a wood sarnple that had been
treated with 1 g PCP/m2. A similar device was affixed to a steel plate as a reference.
After an exposure of 10 hours, the sample-collection and analysis systems were
removed from the articles from which samples were collected and laid, non-a&esive
CA 02243801 1998-07-23
side down, on a horizontal surface. The immunochemical detection process for thePCP's ~ccnmlll~ted withing the collector matrix was initiated by applying 200 ~1 of the
developer solution (J) at the start of the collector matrix (see Figure 2a, x) with the
help of a dropping bottle.
In the case of the PCP wood sample, red coloration was observed in the indicator zone
after 5 mimltes7 whereas there was no visible coloration on the steel plate.
14