Note: Descriptions are shown in the official language in which they were submitted.
CA 02245004 1998-07-28
WO 97/28402 PCT/US97/01619
ICE SEEDING APPARATUS FOR CRYOPRESERVATION SYSTEMS
BACKGROUND OF THE INVENTION
1. Field of the Invention:
This invention relates to an ice seeding apparatus for cryopreservation
= systems for biological samples such as cells, harvested tissue and cellular
biological constructs such as cultured tissue equivalents. Ice seeding in a
cryopreservation protocol initiates the formation of ice that is controllable
to allow
for maximal viability of the tissue or tissue equivalent to be cryopreserved
after
it has been subsequently thawed. By use of the cryopreservation technology,
either cryopreserved harvested tissue or cryopreserved cultured tissue may be
stored for indefinite periods of time prior to use. The cultured tissue is an
in vitro
model of the equivalent human tissue, which, when retrieved from storage, can
be used for transplantation or implantation, in vivo, or for screening
compounds
in vitro.
2. Brief Description of the Background of the Invention:
Heretofore, the cryopreservation of cadaver tissue and cultured tissue
equivalents for the purposes of preserving the viability of the cells in the
tissue
has been achieved, but with limited success. Currently, the storage time of
cellular biological materials is extended by cooling to "cryogenic"
temperatures.
The transition from the liquid into the solid state by lowering the
temperature of the system can take place either as crystallization (ice),
involving
an orderly arrangement of water molecules, or as vitrification or
amorphization
(glass formation), in the absence of such an orderly arrangement of
crystalline
phase. The challenge for a cryobiologist is to bring cells to cryogenic
temperatures and then return them to physiological conditions without injuring
them.
There are two basic approaches to cryopreservation of cells and tissues:
, 30 freeze-thaw and vitrification. In freeze-thaw techniques, the
extracellular solution
is frozen (i.e., in crystalline form), but steps are taken to m;rimize the
intracellular
ice formation. In vitrification procedures, there is an attempt to prevent
crystalline ice formation throughout the cells and tissue. The former approach
is
CA 02245004 1998-07-28
WO 97/28402 I'CT/US97/01619
-2-
problematic in that if ice crystals are formed inside the cells, they are
detrimental
to cell viability upon thawing. However, cells could survive a freeze-thaw
cycle
if they are cooled at controlled rates in the presence of non-toxic levels of
cryoprotectants. The latter approach of vitrification seeks to avoid
potentially 5 damaging affects of intra- and extracellular ice by depressin.g
ice formation by the
addition of very high concentrations of solutes and/or polymers. However, cell
damage may occur from long exposure to toxic levels of these additives
required
for vitri.fication.
Cryoprotectants protect living cells from the stresses involved in the
freezing process. One way cryoprotectants protect cells is by diluting the
salt that
becomes increasingly concentrated in the unfrozen solution as water is
transformed to ice. The amount of ice is dictated by the temperature and
initial
composition of the solution; whereas the amount of unfrozen fraction is a
function
of temperature only. Cryoprotectants have several other functions. An
important
one is that they usually reduce the intracellular ice formation during
freezing and
thawing of a biological sample. Another function is that they stabilize
membranes
and proteins. Once the extracellular ice is seeded and the sample is
surrounded
by the ice phase, it is necessary to cool the sample to a cryopreserved state.
The.
cooling step is one of the most critical steps in a freeze-thaw protocol. Due
to the
formation of ice, that is, pure water, the partially frozen extracellular
solution is
more concentrated than the intracellular compartment. As a consequence, the
cell
will dehydrate by losing water in an attempt to restore thermodynamic
equilibrium. As the system cools, more extracellular ice is generated and the
concentration of solutes rises and forces the cells to dehydrate further.
There are
three characteristics of the cells that control their rate of dehydration. One
is the
cell membrane water permeability; the lower the water permeability, the longer
it takes for the cells to dehydrate. Another is the temperature dependence of
the
cell membrane water permeability; water permeability decreases with decreasing
temperatures. The final is cell size; larger cells take longer to dehydrate
than 30 smaller cells. Given that each cell type may have drastically
different
characteristics, the optimal cryopreservation conditions can vary by orders of
magnitude for different cell types.
CA 02245004 1998-07-28
WO 97/28402 PCT/US97/01619
-3-
All solutions will supercool below their freezing point until they find a
random nucleation site for crystal formation. When cryopreserving by a
freeze-thaw method, ice formation in the extracellular medium should be
deliberately initiated by seeding at low degrees of supercooling. If ice
formation is not induced by seeding, ice will form spontaneously when the
solution is cooled sufficiently far below its equilibrium freezing point.
Because
this process is random in nature, ice formation will occur at random,
unpredictable temperatures. Consequently, survival rates will be highly
variable between repeated trials with the same freezing protocol. Furthermore,
the extremely rapid crystallization which results when ice forms in a highly
supercooled solution can cause damage to cells and tissues. Moreover, it has
been shown that if extracellular ice formation is initiated at high degrees of
supercooling, the probability of intracellular ice formation is drastically
increased. This phenomenon results from the delayed onset of freeze-induced
cell dehydration, which results in increased retention of intracellular water,
and
thus higher likelihood of ice formation in the cell.
Although the exact mechanisms of cell damage during cryopreservation
have not yet been completely elucidated, cell survival as a function of
cooling rate
appear to be qualitatively similar for all cell types and displays an inverted
U-shaped curve. Cell survival is low at very slow and very fast cooling rates,
and
there is an intermediate cooling rate yielding optimal survival. Even though
the
optimal cooling rate and the width of the curve can vary drastically for
different
cell types, the qualitative behavior appears to be universal. Faster cooling
rates
do not allow cells enough time to dehydrate and cells therefore form ice
internally. Cell injury at fast cooling rates is attributed to intracellular
ice
formation. At slow rates of cooling, cell injury is thought to be due to the
effects
of exposure to highly concentrated intra- and extracellular salt and
cryoprotectant
solutions or to the mechanical interactions between cells and the
extracellular ice.
= It is necessary to dehydrate the cells as much as possible before they cross
the intracellular ice nucleation curve. It is at this point that water
remaining in
the cell will nucleate and form ice. The temperature where this will happen is
approximately -40 C to -50 C when the cells are slowly frozen in the presence
of
1M to 2M concentrations of cryoprotectants. It is important to note that the
CA 02245004 1998-07-28
WO 97/28402 PCT/US97/01619
-4-
amount of water that turns to ice inside a cell at this point may be innocuous
when frozen, but if not thawed fast enough, rearrangement of ice may kill the
cell
upon thawing. (The Biophysics of Organ Cryopreservation, Pg. 117-140, edited
by
David E. Pegg and Armand M. Karow, Jr. NATO ASI Series A: Life Sciences Vol.
147 1987 Plenum Press, New York 233 Spring St., New York, NY 10013).
Other cryopreservation systems, particularly those relating to the freezing
of biological samples comprising cells either seed ice by another means, as in
chamber spike methods or by use of electronic or mechanical means, or do not
seed ice at all.
To seed ice using chamber spike methods, a chamber containing biological
samples, such as vials of cells, is quickly cooled to a temperature well below
the
freezing (i.e., liquid-solid equilibrium temperature) point of the
cryopreservation
medium then the temperature is raised quickly to near the equilibrium
temperature. A drawback to this method is that chamber temperature variations
-create problems for uniform ice seeding. In many cases, overseeding of ice in
the
samples occurs resulting in cell damage.
Electronic methods use thermoelectric elements based on semiconductor
thermocouples that can controllably produce local cooling. Interfacing these
thermoelectric elements with the surface of a container or package containing
a
biological sample is difficult and variations in effective cooling of the
container
surface can occur.
Mechanical means of providing localized cooling by use of cold probes,
bars or pins to contact the side of a container is problematic in that the
process
is labor intensive and requires opening the thermal chamber or development of
sophisticated mechanisms for guiding the cold probe.
Accordingly, it has long been desired to provide a better method of
cryopreserving harvested tissue and cultured tissue equivalents to improve
cell
viability after the tissue has subsequently been thawed. The inventors of the
present invention have developed a novel apparatus and method of inducing ice
30 formation in cryopreservation solution, contained in a package with tissue
to be
frozen, that allows for consistent and reliable seeding of ice of a sufficient
amount.
The apparatus is standardized and is expandable with additional fixtures added
to the apparatus.
CA 02245004 2004-11-18
29398-6
SUMMARY OF THE INVENTION
The apparatus and method of the present invention provide for
cryopreservation of biological specimens such as cells, tissues and tissue
5 equivalents, and maintains their viability after subsequent thawing.
Cryopreservation is performed in a freezing chamber at a controlled freezing
rate.
Tissues and tissue equivalents are perfused with a cryoprotective medium while
agitated. Specimens are sealed in a package containing cryoprotective medium
and cooled to or slightly below the liquid-solid equilibrium temperature of
the
medium. At that temperature, the ice seeding is performed, resulting in a seed
of ice in the medium. The temperature is held constant for a sufficient amount
of time to allow equilibration between the liquid and solid phases. The
temperature of the chamber is then lowered at a slow rate to an intermediate
temperature; then rapidly to a cryogenic temperature.
In the preferred embodiment, ice seeding is accomplished by discharging
a liquefied or chilled gas, preferably freon', from the sprayrails to adjacent
racks
containing tissue equivalent samples packaged in cryoprotectant. The freonT'"
discharged contacts the exterior surface of the package and evaporates. The
heat
transfer from the package due to the evaporation of the freon results in local
cooling in the cryoprotective medium at the freon' contact site within the
package.
Sufficient cooling of the medium at that site causes a degree of ice
formation, an
ice seed, in the medium. The apparatus for cooling preferably has a vertically-
oriented sprayrail with nozzles at a position to be dose to containers when
mounted on a removable rack. The system can have multiple sprayrails and
valves for directing the fluid to the desired sprayrail.
An advantage of the ice seeding system is the ability to consistently form
a seed of ice in a plurality of sealed packages containing cryoprotective
medium
and tissue or equivalents thereof. The cryopreservation apparatus and n-ethod
of
the present invention can be used in the manufacturing process for storing and
shipping of these tissues while frozen The tissues are rendered viable when
thawed.
The use of the ice seeding system in the cryopreservation process has
demonstrated an application in the manufacturing process of living tissue
CA 02245004 2004-11-18
29398-6
6
equivalents. Prior to this invention, harvested tissue and
living tissue equivalents had limited shelf-life and,
subsequently, their window of use is short, resulting in
much waste. There is a need to preserve such tissues for
extended periods of time, as in shipping and storage, until
their use. Previous attempts to freeze or freeze dry these
tissues have been met with limited success and have
compromised their use for grafting, in vivo, or for in vitro
testing. The ability to use these tissues in a viable state
represents an exceptional advantage of the present
invention.
According to one aspect of the present invention,
there is provided ice seeding apparatus for the
cryopreservation of biological samples with a fluid held in
a fluid source, the apparatus comprising: a freezing
chamber; a plurality of sprayrails mounted in the chamber,
each sprayrail having a number of nozzles for discharging an
amount of fluid in a horizontal direction, each sprayrail
having a fluid inlet for receiving fluid; a valving system
fluidly coupling the fluid source and the plurality of
sprayrails; a rack for use in the freezing chamber, the rack
for holding containers of biological samples at a number of
different vertical locations on the rack, the sprayrail and
nozzles being positioned such that when the containers are
held in the rack the nozzles are near the containers so that
at least one nozzle is associated with each vertical
location on the rack to direct the fluid close to the
containers of biological samples; and a controller for
controlling the amount of fluid provided from the fluid
source to the sprayrails and for controlling the valving
system for selecting which of the sprayrails will receive
fluid from the fluid source.
CA 02245004 2004-11-18
29398-6
6a
According to another aspect of the present
invention, there is provided ice seeding apparatus for the
cryopreservation of biological samples with a fluid from a
fluid source comprising: a freezing chamber; a rack for use
in the freezing chamber, the rack for holding containers of
biological samples at a number of different locations, the
rack having a top plate, a bottom plate, a plurality of
parallel rails extending perpendicular to the top and bottom
plates and rigidly connected to the top and bottom plates,
the rails having members for supporting containers of
biological samples, and a number of trays oriented in
parallel to the top and bottom plates and rigidly fastened
between each location where a container is held, the trays
serving as a shield to prevent excess liquid from dripping
from one location to a lower location on the rack; and a
sprayrail for receiving fluid from the fluid source and
having a number of nozzles for discharging fluid, the
sprayrail and the nozzles being positioned to be close to
the containers of biological samples when the containers are
held on the rack.
According to still another aspect of the present
invention, there is provided ice seeding apparatus for the
cryopreservation of biological samples with a fluid from a
fluid source comprising: a freezing chamber; a rack for use
in the freezing chamber, the rack for holding containers of
biological samples at a number of different locations, the
rack having a top plate, a bottom plate, a plurality of
parallel rails extending perpendicular to the top and bottom
plates and rigidly connected to the top and bottom plates,
the rails having members for supporting containers of
biological samples, and a removable retaining bar that
extends in parallel to the rails, such that when the bar is
positioned for retaining, the bar helps to keep the
CA 02245004 2004-11-18
29398-6
6b
containers in a desired location on the rack; and a
sprayrail for receiving fluid from the fluid source and
having a number of nozzles for discharging fluid, the
sprayrail and the nozzles being positioned to be close to
the containers of biological samples when the containers are
held on the rack.
According to yet another aspect of the present
invention, there is provided ice seeding apparatus for the
cryopreservation of biological samples with a fluid from a
fluid source comprising: a freezing chamber; a rack for use
in the freezing chamber, the rack for holding containers of
biological samples at a number of different locations, the
rack having a top plate, a bottom plate, and a plurality of
parallel rails extending perpendicular to the top and bottom
plates and rigidly connected to the top and bottom plates,
the rails having members for supporting containers of
biological samples, wherein the top plate and the bottom
plates of the rack have interengaging positioning members
for allowing one rack to be stacked on top of another and
positioned with respect to that other rack; and a sprayrail
for receiving fluid from the fluid source and having a
number of nozzles for discharging fluid, the sprayrail and
the nozzles being positioned to be close to the containers
of biological samples when the containers are held on the
rack.
According to a further aspect of the present
invention, there is provided a method of ice seeding a
biological sample comprising: (a) perfusing a biological
sample with a cryoprotective medium; (b) sealing said
perfused biological sample in a package containing
cryoprotective medium; (c) cooling said package to the
liquid-solid equilibrium temperature of the medium; (d) ice
seeding the cryoprotective medium, wherein said ice seeding
CA 02245004 2004-11-18
29398-6
6c
is accomplished by an apparatus which regulates liquefied or
chilled gas discharge from a pressurized source; (e)
maintaining the temperature of step (c) for a time
sufficient to allow equilibrium between liquid and solid
phases of the medium; (f) cooling said package at a slow
cooling rate to an intermediate temperature; and (g) cooling
said package at a high cooling rate to a cryogenic
temperature.
BRIEF DESCRIPTION OF THE FIGURES
Figure 1 depicts an electronic schematic of the
ice seeding controller.
Figures 2A and 2B show views of a sprayrail
proximal to a rack.
Figures 3A, 3B, and 3C show views and features of
a rack.
Figure 4 shows a schematic of the ice seeding
system and freezing chamber according to one embodiment of
the present invention.
Figure 5 is a part pictorial, part schematic view
of an ice seeding system and freezing chamber according to
another embodiment of the present invention.
DETAILED DESCRIPTION OF THE INVENTION
The purpose of the ice seeding system of the
present invention is to initiate the formation of ice seeds
in packages containing biological samples such as cells,
tissue or tissue equivalents in a cryoprotective medium to
be cryopreserved. Ice seeding must be accomplished within a
temperature range slightly below the equilibrium freezing
point of the cryoprotectant solution used. Ice seeds
CA 02245004 2004-11-18
29398-6
6d
initiated above the freezing point may melt before cooling
proceeds below this point. To preserve cell viability, ice
seeding must be initiated in the cryopreservation medium
outside of the tissue or cells. The invention performs ice
seeding without causing ice to form within the tissue: A
plug of ice is formed primarily in the cryoprotectant
bordering the outside of the tissue.
The ice seeding apparatus comprises three major
elements: A controller, spray rails, the racks. The ice
seeding controller is a mechanism that regulates the flow of
liquefied or chilled gas from a pressurized source to the
chamber
CA 02245004 1998-07-28
WO 97/28402 PCT/1TS97/01619
-7-
containing the items to be frozen for storage. By using a number of switches,
ports, valves, a rack selector, and a timing mechanism, the controller
centrally
manages the operations of the system.
Regulation of liquefied or chilled gas discharge, herein also referred to as
'discharge', from a pressurized source is achieved using timed valves with
which
the timing can be manipulated to control the volume of discharge to be
released
from the controller to the chamber. Contact of the discharge with the
container
containing a biological specimen initiates ice seeding inside the container at
specified temperatures. The longer a valve is open, the larger the volume of
the
discharge. A separate valve serves each spray rail apparatus, thereby allowing
the
apparatus to be standardized for situations involving a plurality of spray
rails and
associated racks.
The ports comprise at least one inlet and at least one outlet for the
pressurized liquefied or chilled gas source. A pressurized source is attached
to
an inlet port associated with the controller. Continuous from the port, the
gas is
ducted to the valves, which regulate the amount of gas discharged to the spray
rails.
The rack selector allows the selection of which valves and associated spray
rails will serve the rack or racks containing product to be seeded with ice.
The
timer regulates the amount of time the valves are open, thus the volume of
discharge through the spray rails to the appropriate racks to be seeded.
The spray rail is a manifold that at one end receives liquefied or chilled gas
from the valves via tubing or other ducting means. The spray rail comprises of
a plurality of orifices to deliver an amount of liquefied or chilled gas at
each level
of a rack. These orifices are located in series along the manifold and each
orifice
contains a nozzle therein. The nozzles may also contain filter screens to
prevent
plugging. At the base of the spray rail there is a mounting foot to position
and
stably secure the spray rail to a baseplate or chamber floor.
The racks provide support during cryopreservation for the items to be
cryopreserved as well as a means for transporting and storing the items in
cold
storage. The racks consist of parallel top and bottom plates supported by
rails
rigidly fixed perpendicularly to the plates therebetween. From the top plate
of the
rack are upwardly extending pins or pegs. In one mode of the invention, there
CA 02245004 2004-11-18
29398-6
8
are at least four pins equally distributed about the periphery of the plate.
One
pair of opposite pins are connected with a rod to form a handle. In the bottom
plate are holes or recessions arranged in the same orientation as the pins on
the
top plate. The holes or recessions will align with the pins for stacking a
rack atop
another rack during cryopreservation or storage or to the pins upwardly
extending from the baseplate along the floor of the chamber to align the rack
proximal to the sprayrail.
In another mode of the invention, a handle is formed in the top plate by
machining two oblong orifices on either side of the midline, or diameter, of
the
top plate.. The top plate can be either grasped by hand or hooked to lift or
maneuver the rack
The rails of the rack are machined to have gaps to form horizontal
planar surfaces equaIly distributed along the length of the rail, at about the
same distances as the orifices of a sprayrail. The gaps are large enough to
accommodate the bulk of an item, such as a petri dish or specialized
container,
to be cryopreserved. The rails, fixed perpendicularly between the top and
bottom plates, are in parallel to each other. The rails are arranged so that
the
gaps are facing inwardly to the center of the rack, at about 90' angles, so
two
are facing opposite each other and the third 'in between In this arrangement
the gaps of the rails provide three points on which an item can be placed and
supported. To maintain the items in position, a retention bar or rod is placed
in the space 90' from the opposing rails, across fronn the central rail. In
one
embodiment, the bar is led down through a hole in the top plate to 'the bottom
plate and is fastened in place to the bottom plate.
In a preferred embodiment, located between the two adjacent rails between
each gap, there is an arc shaped plate, or driptray, oriented in parallel to
the top
and bottom plates. The driptrays are rigidly fastened between each gap to
contain
an item. The driptrays serve as a shield to prevent excess liquefied gas, such
as
freonT ; from dripping from an item to another item below, or overspraying
from
the nozzle onto the top of an item below the item being sprayed, which would
subsequently result in unwanted ice seeding.
When the rack is positioned in the chamber according to the alignment
pins, the gaps to contain items for cryopreservation will about evenly line up
with
CA 02245004 1998-07-28
WO 97/28402 PCT/US97/01619
-9-
the nozzles of the sprayrail. In turn, the drip trays will be aligned proximal
to the
sprayrail. The combination of the rack with the baseplate locating pins and
sprayrails provides a reproducible distance between the spray nozzles and the
~ sides of the items to be cryopreserved. Although this distance is
consistent, the
spray seeding method is tolerant of variations. The baseplate is a planar
surface
that is positioned along the bottom of the freezing chamber. The baseplate
comprises of an organized pattern of pins and holes that allow one to locate
the
proper positions for the parts such as the spray rail, rack, etc. into the
appropriate
places on the baseplate.
Figure 1 is a electronic schematic of the controller and includes a grounded
power entry module 51; a fuse 52; a main power switch 53; a time delay-relay
54;
a start button 55; an output indicator lamp 56; a single pole, multi position
rotary
switch 57; connecting terminals 58; and normally closed solenoid valves 59.
Power for the controller is provided by a grounded alternating current (AC)
power source. Power entry module 51 is connected to the AC power source.
Electrical overload is protected by fuse 52 connected in series adjacent to
the
power entry module 51- Main power switch 53, which controls the electrical
flow
to the controller, is serially connected to a digital time setting and readout
time-delay relay 54 that controls the duration of the controller activation.
Momentary push-button start switch 55 is serially associated with the time-
delay
relay 54 to initiate the action of the controller. Indicator lamp 56 is
connected in
parallel with the output of the relay 54 to indicate that the controller is
activated.
The time-delay relay 54 is serially connected to a single pole, multiposition
switch
57 which is, in turn, serially connected to a plurality of normally closed
solenoid
valves 59. The series is completed by a parallel connection of the solenoid
valves
59 back to the time-delay relay 54 and ultimately to the power entry module.
The amount of freon delivered by the ice seeding system is a function of
the time the delivery valves are open. This duration of time the valves are
open
dictates the amount of freon discharged to the spray rails. This time is set
on the
dme-delay relay on the front panel of the ice seeding controller. Before
beginning
a cryopreservation procedure, the tubing lines of the system should be purged
of
air by discharging liquefied gas from the spray rails for a sufficient
interval of
time.
CA 02245004 1998-07-28
WO 97/28402 PCT/US97/01619
-10-
Modifications and enhancements may be made to the controller by the
skilled artisan by substituting one or more components of the electrical
system
and still achieve essentially the same controller functions of regulating
liquefied
gas flow to the sprayrails.
Figures 2A and 2B depict the association of the sprayrail with the rack. The
top view of Figure 2A shows a nozzle 21; sprayrail 20; mounting foot 22; rack
30;
container 40. Side view of Figure 2B shows a nozzle 21, a segment of sprayrail
20, and container 40. Nozzle 21 is mounted within an orifice of sprayrail 20.
Sprayrail 20 is attached to a mounting foot 22 which is turn stably secured to
a
baseplate. Rack 30 is placed on the baseplate proximal to sprayrail 20,
adjacent
with mounting foot 22. The side of container 40 is centrally aligned with
nozzle
21 to ensure that discharge from the nozzle is directed at the side of the
container.
Figures 3A, 3B, and 3C show views of rack 30. Shown are rack 30; top
plate 31; bottom plate 32; rails 33; driptray 34; locating pins 35; locating
holes 36;
handle 37; and handle for retention bar 38. The frame of rack 30 consists
essentially of three rails 33 securely mounted to top plate 31 and bottom
plate 32
at either ends of the rails. Rails 33 contain gaps equally spaced along the
rails for
placement of containers. In one mode of the invention, driptrays 34 are
mounted
between adjacent rails 33 along the side of rack 30 to be placed adjacent to
the
spray rail. Top plate 31 has iipwardly extending locating pins 35 positioned
along
the periphery of the top plate with two opposing pins connected by a handle 37
therebetween. Bottom plate 32 has holes positioned along the periphery of the
plate for mating with the locating pins 35 of top plate 31 or with those on
the
baseplate. A retention bar closes the large gap through which containers are
placed in rack 30 by inserting the bar through a hole in top plate 31 down to
a
mount located on bottom plate 32 to prevent containers from falling out of the
rack during positioning or storage.
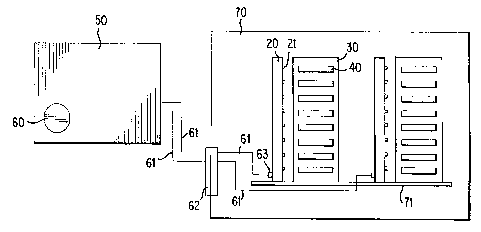
Figure 4 is a schematic of the ice seeding system and freezing chamber.
Shown are controller 50; inlet port for pressurized liquefied or chilled gas
60; lines
61; feedthrough plug 62; sprayrail inlet 63; sprayrail 20; nozzle 21; rack 30;
container 40; chamber 70; and baseplate 71. Controller 50 contains an inlet
port
for pressurized liquefied or chilled gas 60. Discharge of the gas is mediated
by
the solenoid valves of the controller 50 and is delivered to the sprayrails 20
via
CA 02245004 1998-07-28
WO 97/28402 PCT/US97/01619
-91-
lines 61. Feedthrough plug 62 allows passage of lines 61 into chamber 70 while
maintaining the seal of the chamber. Lines 61 are connected to the sprayrails
21,
containing a plurality of nozzles 21 at sprayrail 63 located at the mounting
foot
= of the sprayrail. Liquefied or chilled gas is discharged from nozzles 21 to
containers 40 held proximal in racks 30. Sprayrails 20 and racks 30 are stably
secured to baseplate 71 within chamber 70.
Referring to Figure 5, in another embodiment, a system 100 has a liquid
supply 102, a controller 104, a valving manifold 106, and a sprayrail 108 with
nozzles for directing the liquefied gas from supply 102 to containers on a
rack 110
in a freezing chamber 112. Liquid supply 102 is pressurized from the top of
supply 102 with a source 114 of gas pressure, preferably regulated nitrogen
gas
at 150 psig. While supply 102 can be kept continually under pressure, the
container need only be pressurized once for multiple uses, because only small
quantities of liquid are typically drawn from supply 102. If more fluid were
drawn from the supply, the supply could be pressurized more frequently.
Valving manifold 106 is provided inside of freezing chamber 112 and
preferably has eight valves 116 (only one of which is shown here) for
directing
the liquefied gas to one of eight sprayrails in response to control signals
over
electric signal line 115 from controller 104. Each valve is preferably a three-
way valve having an inlet 119 for receiving the liquefied gas through line
124,
one outlet 118 for providing liquefied gas to sprayrail 108, and a second
outlet
120 vented to the atmosphere inside chamber 112. With a three-way valve,
venting after the system is shut off depressurizes the spray rails without
requiring several seconds of residual gas and liquid to be sprayed to the
containers; rather, it takes less than a second, and the gas and liquid are
immediately shut off from the containers when desired_ By locating valving
manifold 106 inside chamber 112, there is only one liquid line 124 extending
through a feedthrough 126 from outside to inside chamber 112; moreover,
there is better control over the liquid because there is less distance between
the
valves 116 and their respective sprayrails 108. A filter 128 is provided
between
valve 116 and sprayrail 108 to trap particles that can cause clogging at the
nozzles.
CA 02245004 1998-07-28
WO 97/28402 PCT/US97/01619
-12-
While oniy one sprayrail is shown for use with one rack having eight
levels for holding containers, the device of the present invention can be used
with multiple sprayrails, in which case there are multiple valves, filters,
and
sprayrails. In another alternative, a sprayrail can be provided with 16
nozzles
at 16 different vertical levels, in which case, two racks can be stacked one
on
top of the other, and appropriately located relative to each other with
positioning pins.
As an alternative to the nitrogen pressure on the liquefied gas supply, a
gear pump can be used to provide fluid at a pressure well above the phase
transition.
An alternate setup for large volume freezing to enable larger lots of
living tissue or equivalents to be cryopreserved at one time consists of a
large
chamber outfitted with a large area baseplate with a greater number of
positions for the location of spray rails and racks. Additional components can
be incorporated to the existing controller arrangement. Sprayrails can be
extended to a longer length to continue the manifold to another series of
orifices. Racks can be stacked as the bottom plates of the racks contain holes
to accommodate pins of the top plate of the rack below. The controller may
also be integrated within the freezing chamber itself and further modified
with
sensors and controls to initiate the ice seeding when the chamber temperature
reaches a specific point. Other mechanisms can be added to provide chamber
air circulation.
A preferred element or compound of liquefied or chilled gas for use in the
cryopreservation system is one that undergoes a phase change from liquid to
gas
at a temperature below the freezing temperature of the cryopreservation
solution
in the package. The liquefied or chilled gas used must be under pressure.
Pressure can be provided by using the natural expanding qualities of the
liquid
or gas; by providing a propellant mixed with the liquefied or chilled gas; or
by
mechanical means by employment of a pump. When a small amount of the gas
is delivered by way of the apparatus to the side of the package, the phase
change
of the liquefied gas to a vapor, i.e., evaporation, causes cooling of the
package
wall at the point of contact to temperatures below the freezing temperature of
the
. , ... I I.. . .
CA 02245004 2004-11-18
29398-6
13
cryopreservative, sufficiently low to form a seed of ice in the cryoprotectant
within the package.
The freezing temperature of the cryopreservation medium varies as it is
dependent on the. nature of the components contained in the medium. Most
cryopreservatives are water based but also contain salts and glass forming
agents
that affect the freezing temperature of the cryopreservative. Glass forming
agents
prevent the crystallizaation of the cryopreservative to protect the cells or
tissue to
be frozen from cryofracture (cracking of the frozen tissue due to crystal
formation), thus maintaining viability of the cells or tissue. A
cfyoprotectant
medium may contain "cell penetrating glass forming agents" or "non-cell
penetrating glass forming agents" or both. The cell, penetrating glass forming
agent is preferably glycerol, but may include propylene glycol, ethylene
glycol,
dimethylsulfoxide, and'other penetrating glass forming -agents known in the
art.
Non-cell penetrating glass forming agents include high molecular weight forms
of complex carbohydrates, such as chondroitin sulfate, polyvinylpyrrolidone,
polyethylene glycol or hetastarch, such as hydroxyethyl starch. The cell
-~penetrating glass forming agents and/or non-cell penetrating glass forming
agents are diluted in a base of a physiological pH. The base is preferably
DMEM, but
may be substituted with phosphate buffered saline, IDMEM, MEM, M199, RPNII
1640, Ham's F-12 Ham's F-10, NCTC 109, NCTC 135, or combinations - thereof.
The preferred cryoprotectant solution contains 1.SM to 2.5M glycerol,
preferably
2M glycerol, in a base of Dulbecco's Modified Eagle's Medium (DMEM). These
solutions can be modified and optimized by one of skill in the art using known
cryoprotectants and freezing, storing, thawing, and rinsing procedures that
are
compatible with maintaining maximal viability, depending on the particular
application. The selection of a gas source to be used in accordance with the
invention shoiild take into consideration the nature of the cryopreservative
in
which the ice seeding is to be initiated. ' Those skilled in the art will be
able to
determine a proper combination of gas and cryopreservative compatible with the
material to be cryopreserved and the liquid-solid equilibrium temperature of
the
cryopreservative required to perform ice seeding.
A preferred gas source is freonTM compound HFC-134a (1,1,1,2
tetra-fluoroethane) which contains no ozone-depleting chlorine, thus it is not
CA 02245004 2004-11-18
29398-6
14
targeted for phase-out by the Environmental Protection Agency (EPA). The
temperature at which the freonTM compound HFC-134a evaporates is nominally
between -25 to -30'C, sufficiently low to guarantee formation of ice. Other
liquefied gasses that evaporate at a sufficiently low temperature to induce
ice
formation would indude liquid nitrogen (W), isopentane, propane, and other
fluorocarbons such as FreonT" 12 and FreonT" 22; however, some
of these gases have their drawbacks.
As it is well kr-own, isopentane (also known as 2-methyl butane) is a
neurological, respiratory, and hepatic toxin to humarp. Since 'it is toxic to
humans, it presents a.danger of adverse health effects to anynearby persons
who
inhale them, especially those persons performing the freezing of the specimen
Also, isopentane is flammable and poses a danger of fire or explosion.
Isopentane
is denserthan air and can eaaily move some distance from the apparatus in
which
the freezing procedure is being performed. Thus, if the isopentane reaches a
source of ignition, it will~ignite or explode. Additionally, fluorocarbons
pose a
threat as well. FreonTM 12, Freon' 22 and dichtorofluoromethane are
chlorofluorocarbons (hereafter, abbreviated as CFCs). If a CFC is employed as
the
fluid for freezing, a hazard arises in that CFCs are detrimental to the
protective
ozone layer of the earth. As a result, the United States Government has
imposed
regulations requiring the phase-out of several CFCs for all but certain uses
that
are critical to the preservation of human life. Hence, it is desirable to find
a
hazard-free method of cryopreserving specimens with a liquefied gas that not
oniy
will be suitable at extremely low temperatures, but also will be non-toxic to
humans, non-flammable and environmentaily safe.
Rather than a liquefied gas, a chilled gas under pressure may alternately
be discharged from the spray rails. The temperature of the chilled gas would
be
required to be well below the solid liquid equilibrium temperature of the
cryoprotectant in the container. The gas' may beanitrogen, oxygen,
carbon'dioxide
or simply air. Atty gas may be used provided that it be under pressure and
appropriately chilled. Chilling may be obtained through expansion of the
pressurized gas-
In the preferred embodiment, the cryopreservative is Dulbecco's Modified
Eagle's Medium (DMEM), a common cell culture media base, containing glycerol,
CA 02245004 1998-07-28
WO 97/28402 PCT/US97/01619
-35-
a cell penetrating glass forming agent, at a 2M concentration. The equilibrium
freezing point of DMEM/2M glycerol is about -5.2 C. Ice seeding must then be
accomplished at -6 C +/- 0.5'C, a temperature range slightly below the
equilibrium freezing point, -5.2 C, of the cryoprotectant solution used.
Seeding
must occur below -5.2 C, since ice formed above this temperature may melt
before
' cooling proceeds below this point.
Control of the temperature at which the solid phase (ice) begins to form,
as well as the cooling rate after its formation, is required to control the
degree of
dehydration of the tissue. The equilibrium phase diagram for glycerol-water
solutions relates to both the amount of ice and the amount of remaining
solution
over temperature. Since ice, as it forms, rejects solutes, the solution in
equilibrium
with the ice becomes more concentrated as the temperature is lowered. This
increasing solute concentration in the extracellular solution provides an
osmotic
gradient which causes water to be removed from the cells. If sufficient water
is
not removed at relatively high sub-zero temperatures, ice can precipitate
within
the cells, an event associated with cell and/or tissue damage. If seeding of
the
extracellular fluid is not performed deliberately, ice will appear
spontaneously at
temperatures ranging from the equilibrium point for the starting solution, -
5.2"C,
to as low as -40'C. The lowest temperature at which spontaneous seeding has
been observed in the packages is -14 C. The lower the temperature of the
spontaneous event, the greater the likelihood that significant amounts of
water
will be trapped and frozen within the cells or tissue, thus the greater the
degree
of damage. Since the spontaneous seeding can occur over a wide temperature
range and can vary from package to package, survival rates of cells between
similar tissues can vary widely. For the above described situation, the
seeding
operation should achieve a localized region of cooling with the temperature of
the
localized cooling below -15 C to guarantee formation of the seed.
The amount of ice that must be produced by the seeding operation is not
critical to the success of the cryopreservation process as long as some ice is
formed. At -6.0 C, the amount of ice that must be present in equilibrium with
the
remaining solution is about 0.36 g, for a starting solution of 16 mL of 2.0 M
glycerol in DMEM. This quantity of ice should be the total present above and
below the plane of the tissue. It is acceptable for the seeding operation to
produce
CA 02245004 2004-11-18
29398-6
16
less or more than this amount, provided sufficient time is allowed for
equilibrium
to be established by the growth or melting of ice. It is best to control the
process
to produce excess ice, provided the region in which the ice is formed does not
encompass the area of the tissue and is allowed to achieve equilibrium with
the
solution in the package. A rough estimate of the amount of HFC-134a required
to produce this 'amount of ice can be obtained from the HFC-134a properties.
Since the material absorbs approximately 217 joules/g in going from liquid to
gas
at 1, atmosphere, and the latent he t of fusion for water is 333 joules/g,
about 0.56
g of :FreonTM are required to produce about 0.36 g of ice. The volume of
liquefied
gas to be discharged can be determined and optimized by one of skill in the
art
to form an ice seed within an amourit of cryoprotectant.
The following examples are provided to better elucidate the practice of the
present invention and sllould not be interpreted in any way to limit the scope
of
the present invention. Those skilled in the art will recognize that various
modifications can be made to the methods described herein while not departing
from the spirit and scope of the present invention.
EXAMPLES
EXAMPLE 1: Cryopreservation of Living Skin Equivalents (LSE) Using the Ice
Seeding System
Living Skin Equivalent (LSE) constructs were prepared in accordance to US
Patent No. 5,536,656. LSEs
and attached 75 mm carrier inserts (T'tAN SWELLAA, Costar, Cambridge), at 7 to
12 days post air lift, were placed in 100 mm petri dishes (Costar). LSE
constructs
were perfused with cryoprotectant by submerging the constructs and the
transwell
with 25 mL of cryoprotective media, 2M Glycerol in DMEM, in the 100 mm petri
dish for one hour. During perfusion, the constructs were agitated for one hour
on an orbital shaker (Bellco) at 70 rpm in a 10% CO2 gassed chamber. Agitation
allows for more complete perfusion and better reproducibility of the
cryopreservation method. After LSE were perfused, the petri dishes containing
LSE, carrier inserts and extracellular freezing media (2M Glycerol and DMEM)
were placed in a cryopreservation package and heat sealed. Cryopreservation
CA 02245004 2004-11-18
29398-6
17
packages are described in patent publication, no. WO 96/24018,.
The chamber of a programmable freezer (Planar) was outfitted with the
invention described herein. The freezer was set at a starting temperature of
20.0'C. The tubing lines were purged with freon for a single one second
interval
to remove any air in the lines. Packaged LSE units were placed securely into
racks accommodating eight ISE units each. The racks, guided by locating pins,
were placed adjacent to the spray rails. The chaaiber door of the freezer was
closed to seal the chamber from the external environment.
LSE. units were cooled at -10'C/minute to -6'C and the chamber
temperature was held at -6'C for 40 minutes to equilibrate the cryoprotectant
and
perfused constructs to the chamber temperature. . After the 40 minute hold,
extracellular ice was initiated by directly discharging freonTM for one second
at dose
proximity to the side of the package. The freonn", as it evaporated from the
surface
of the package, caused the contact area of the freon to drop in temperature
enough to initiate extracellular ice formation.
After all ISE units were seeded with ice crystals, the units are allowed to
equilibrate for one hour at -6'C. The chamber temperature was then cooled at
-0.0TC/minute to a final temperature of -200C. The chamber was then cooled at
-0.5'C/min to a final temperature of -70'C. Once the LSE units were
cryopreseived, they were transferred to a vapor phase storage tank (Dewar) at
a
temperature of -120' to -150'C.
Although the foregoing invention has been described in some detail by way
of- illustration and example for purposes of darity of understanding, it will
be
obvious to one skilled in the art that certain changes and modifications may
be
practiced within the scope of the appended claims.